Enhanced Resistance of atnigr1 against Pseudomonas syringae pv. tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtNIGR1 Encoding NAD(P)-Binding Rossmann-Fold in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
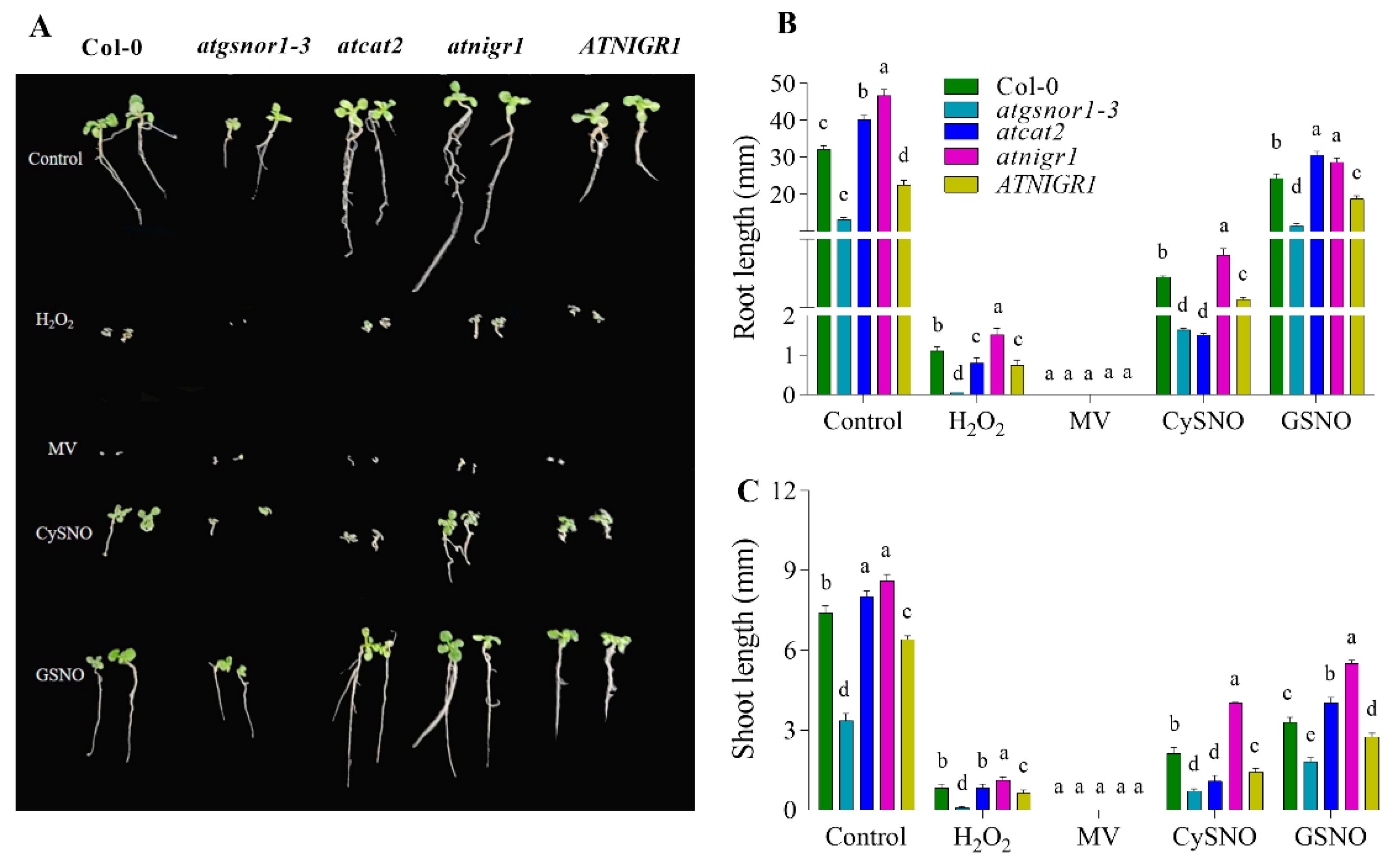
2.2. Redox Stress Assay
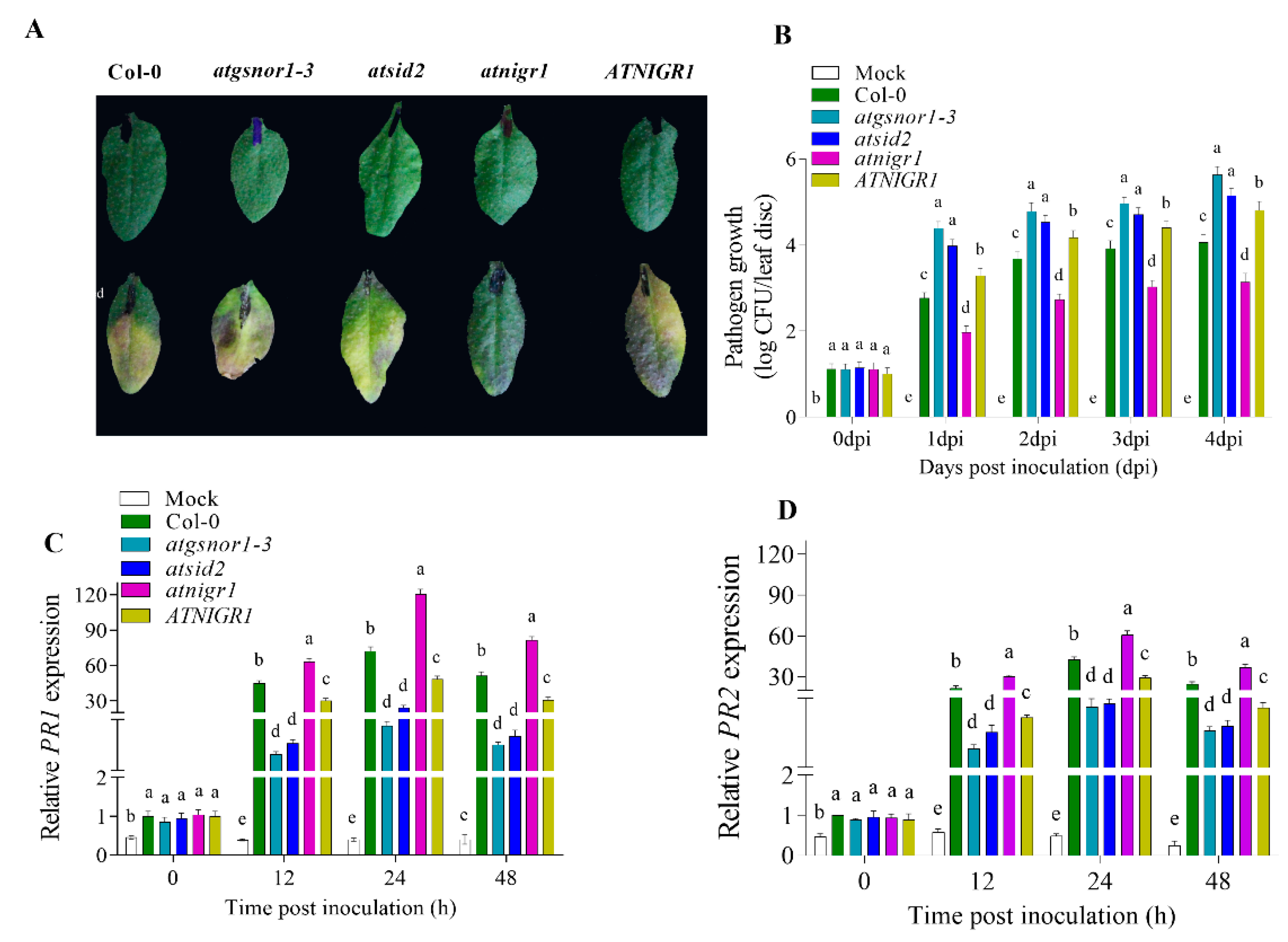
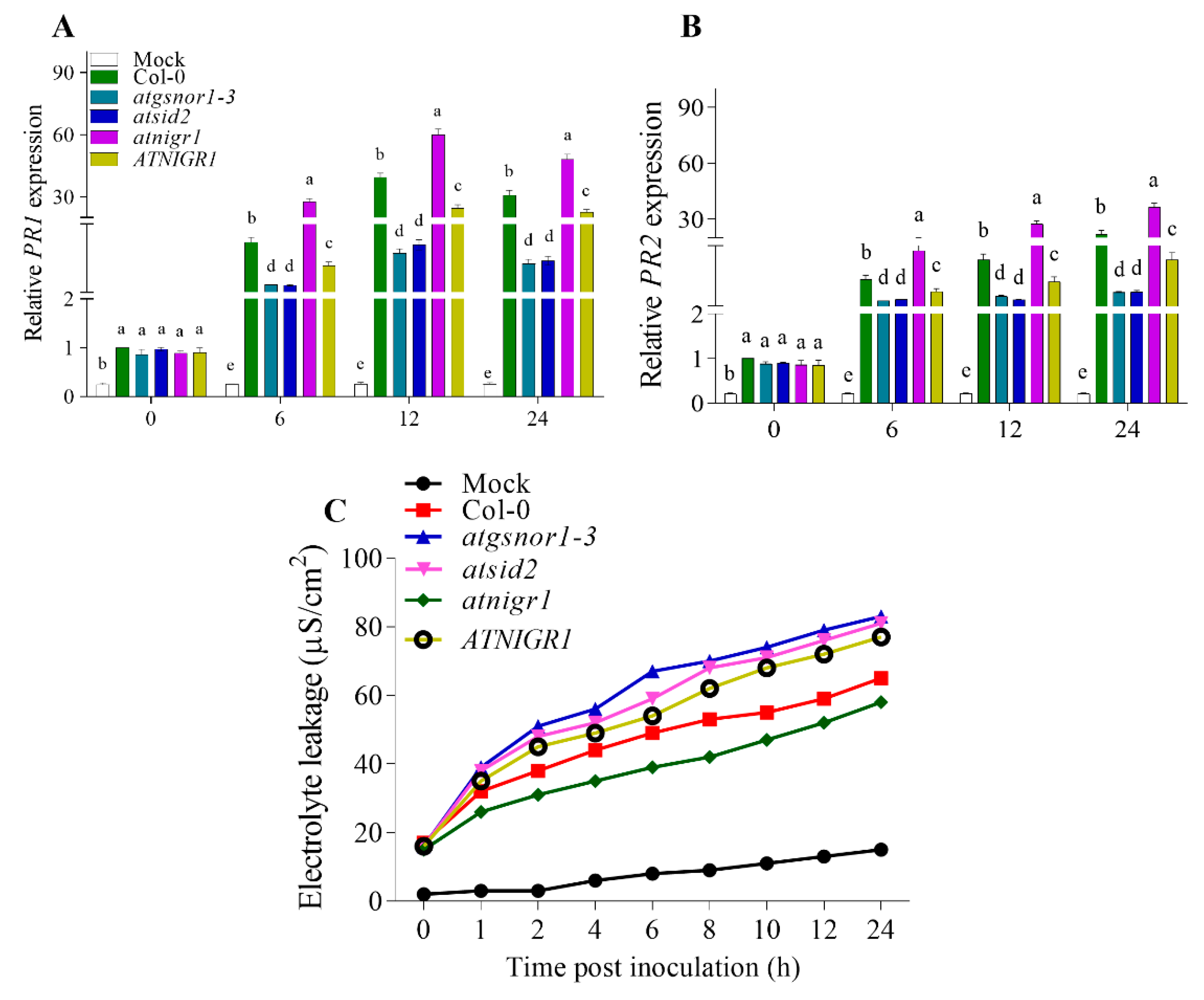
2.3. Pathogenic Growth, Inoculation, and Electrolyte Leakage Assay
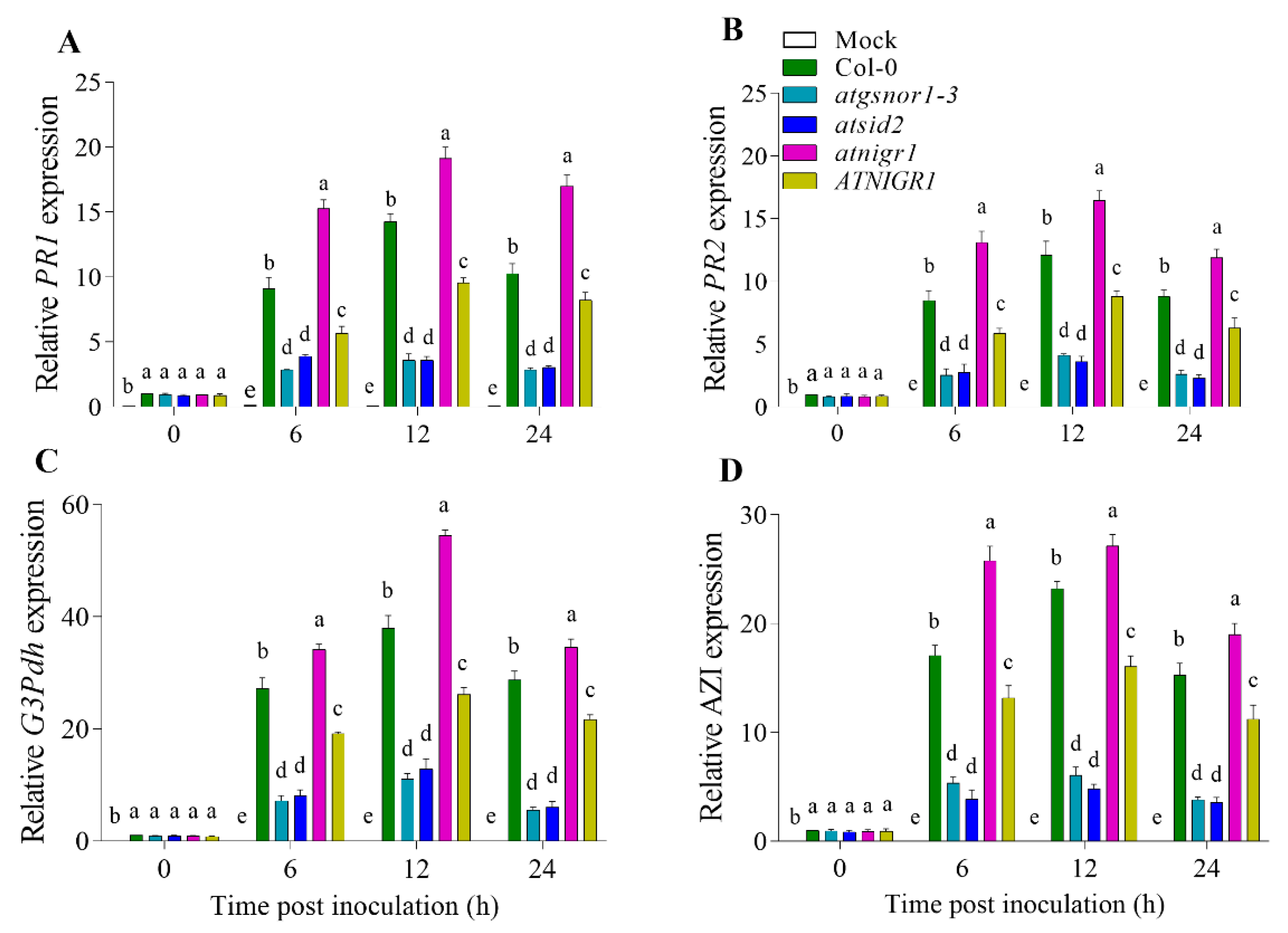
2.4. qRT-PCR (Quantitative Real-Time PCR) Analysis
2.5. Functional Categorization and Gene Ontology Annotation
2.6. Gene Structure and Domain Analysis
2.7. Identification of Plant Homologs and Phylogenetic Analysis
2.8. Expression Pattern of AtNIGR1 in Different Organs
2.9. In Silico Prediction of S-Nitrosylation Sites
2.10. Protein Interaction Analysis
2.11. Statistical Analysis
3. Results
3.1. Root and Shoot Growth Patterns of AtNIGR1 KO and OE
3.2. AtNIGR1 Negatively Regulates Plant Basal Defense
3.3. Negative Regulation of R-Gene-Mediated Resistance by the AtNIGR1 Gene
3.4. AtNIGR1 Negatively Regulates Systemic Acquired Resistance
3.5. In Silico Characterization, Gene Ontology, Predicted Organ-Specific Expression, and Homology
3.6. In Silico Prediction of Protein Interaction and S-Nitrosylation Sites
4. Discussion
4.1. NO-Downregulated AtNIGR1 Gene Negatively Regulates Root and Shoot Lengths of A. thaliana
4.2. NO-Downregulated AtNIGR1 Gene Negatively Regulates Basal Defense, R-Gene-Mediated Resistance, and SAR of A. thaliana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nabi, R.B.S.; Rolly, N.K.; Tayade, R.; Khan, M.; Shahid, M.; Yun, B.W. Enhanced Resistance of atbzip62 against Pseudomonas syringae pv. tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtbZIP62 Transcription Factor. Int. J. Mol. Sci. 2021, 22, 11541. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.S.; Dadalto, S.P.; Gonçalves, A.B.; De Souza, G.B.; Barros, V.A.; Fietto, L.G. Plant bZIP Transcription Factors Responsive to Pathogens: A Review. Int. J. Mol. Sci. 2013, 14, 7815–7828. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Al Azawi, T.N.I.; Pande, A.; Mun, B.-G.; Lee, D.-S.; Hussain, A.; Lee, B.-H.; Yun, B.-W. The Role of Nitric Oxide-Induced ATILL6 in Growth and Disease Resistance in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 685156. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Imran, Q.M.; Hussain, A.; Shahid, M.; Yun, B.-W. Functional Insight of Nitric-Oxide Induced DUF Genes in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin Function and Crosstalk with Other Phytohormones under Normal and Stressful Conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.-G.; Lee, D.-S.; Khan, M.; Lee, G.-M.; Hussain, A.; Yun, B.-W. NO Network for Plant–Microbe Communication Underground: A Review. Front. Plant Sci. 2021, 12, 658679. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in plants—Maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.-G.; Khan, M.; Rahim, W.; Lee, D.-S.; Lee, G.-M.; Al Azawi, T.N.I.; Hussain, A.; Yun, B.-W. Nitric Oxide Signaling and Its Association with Ubiquitin-Mediated Proteasomal Degradation in Plants. Int. J. Mol. Sci. 2022, 23, 1657. [Google Scholar] [CrossRef]
- Turkan, I. ROS and RNS: Key signalling molecules in plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef]
- Tuteja, N.; Chandra, M.; Tuteja, R.; Misra, M.K. Nitric Oxide as a Unique Bioactive Signaling Messenger in Physiology and Pathophysiology. J. Biomed. Biotechnol. 2004, 2004, 227–237. [Google Scholar] [CrossRef]
- Campbell, J.Y.; Hentschel, L. No news is good news: An asymmetric model of changing volatility in stock returns. J. Financ. Econ. 1992, 31, 281–318. [Google Scholar] [CrossRef]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.-U.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar] [CrossRef]
- Rahim, W.; Khan, M.; Al Azzawi, T.N.I.; Pande, A.; Methela, N.J.; Ali, S.; Imran, M.; Lee, D.-S.; Lee, G.-M.; Mun, B.-G.; et al. Exogenously Applied Sodium Nitroprusside Mitigates Lead Toxicity in Rice by Regulating Antioxidants and Metal Stress-Related Transcripts. Int. J. Mol. Sci. 2022, 23, 9729. [Google Scholar] [CrossRef]
- Shahid, M.; Imran, Q.M.; Hussain, A.; Khan, M.; Lee, S.U.; Mun, B.G.; Yun, B.-W. Comprehensive Analyses of Nitric Oxide-Induced Plant Stem Cell-Related Genes in Arab. Thaliana. Genes 2019, 10, 190. [Google Scholar] [CrossRef]
- Khan, M.; Imran, Q.M.; Shahid, M.; Mun, B.-G.; Lee, S.-U.; Khan, M.A.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Nitric oxide- induced AtAO3 differentially regulates plant defense and drought tolerance in Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 602–619. [Google Scholar] [CrossRef]
- Hussain, A.; Mun, B.-G.; Imran, Q.M.; Lee, S.-U.; Adamu, T.A.; Shahid, M.; Kim, K.-M.; Yun, B.-W. Nitric Oxide Mediated Transcriptome Profiling Reveals Activation of Multiple Regulatory Pathways in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 975. [Google Scholar] [CrossRef]
- Kwon, E.; Feechan, A.; Yun, B.-W.; Hwang, B.-H.; Pallas, J.A.; Kang, J.-G.; Loake, G.J. AtGSNOR1 function is required for multiple developmental programs in Arabidopsis. Planta 2012, 236, 887–900. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of A rabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, Z.; Gao, X.; Jin, C.; Wen, L.; Yao, X.; Ren, J. GPS-SNO: Computational Prediction of Protein S-Nitrosylation Sites with a Modified GPS Algorithm. PLoS ONE 2010, 5, e11290. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2017, 46, D493–D496. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef]
- Parani, M.; Rudrabhatla, S.; Myers, R.; Weirich, H.; Smith, B.; Leaman, D.W.; Goldman, S.L. Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol. J. 2004, 2, 359–366. [Google Scholar] [CrossRef]
- Huang, X.; von Rad, U.; Durner, J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 2002, 215, 914–923. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2008, 69, 473–488. [Google Scholar] [CrossRef]
- Ali, M.W.; Kim, I.-D.; Bilal, S.; Shahzad, R.; Saeed, M.T.; Adhikari, B.; Nabi, R.B.S.; Kyo, J.R.; Shin, D.-H. Effects of Bacterial Fermentation on the Biochemical Constituents and Antioxidant Potential of Fermented and Unfermented Soybeans Using Probiotic Bacillus subtilis (KCTC 13241). Molecules 2017, 22, 2200. [Google Scholar] [CrossRef]
- Muday, G.K.; Brown-Harding, H. Nervous system-like signaling in plant defense. Science 2018, 361, 1068–1069. [Google Scholar] [CrossRef]
- Hussain, A.; Yun, B.-W.; Kim, J.H.; Gupta, K.J.; Hyung, N.-I.; Loake, G.J. Novel and conserved functions of S-nitrosoglutathione reductase in tomato. J. Exp. Bot. 2019, 70, 4877–4886. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell 1996, 8, 1809. [Google Scholar] [CrossRef]
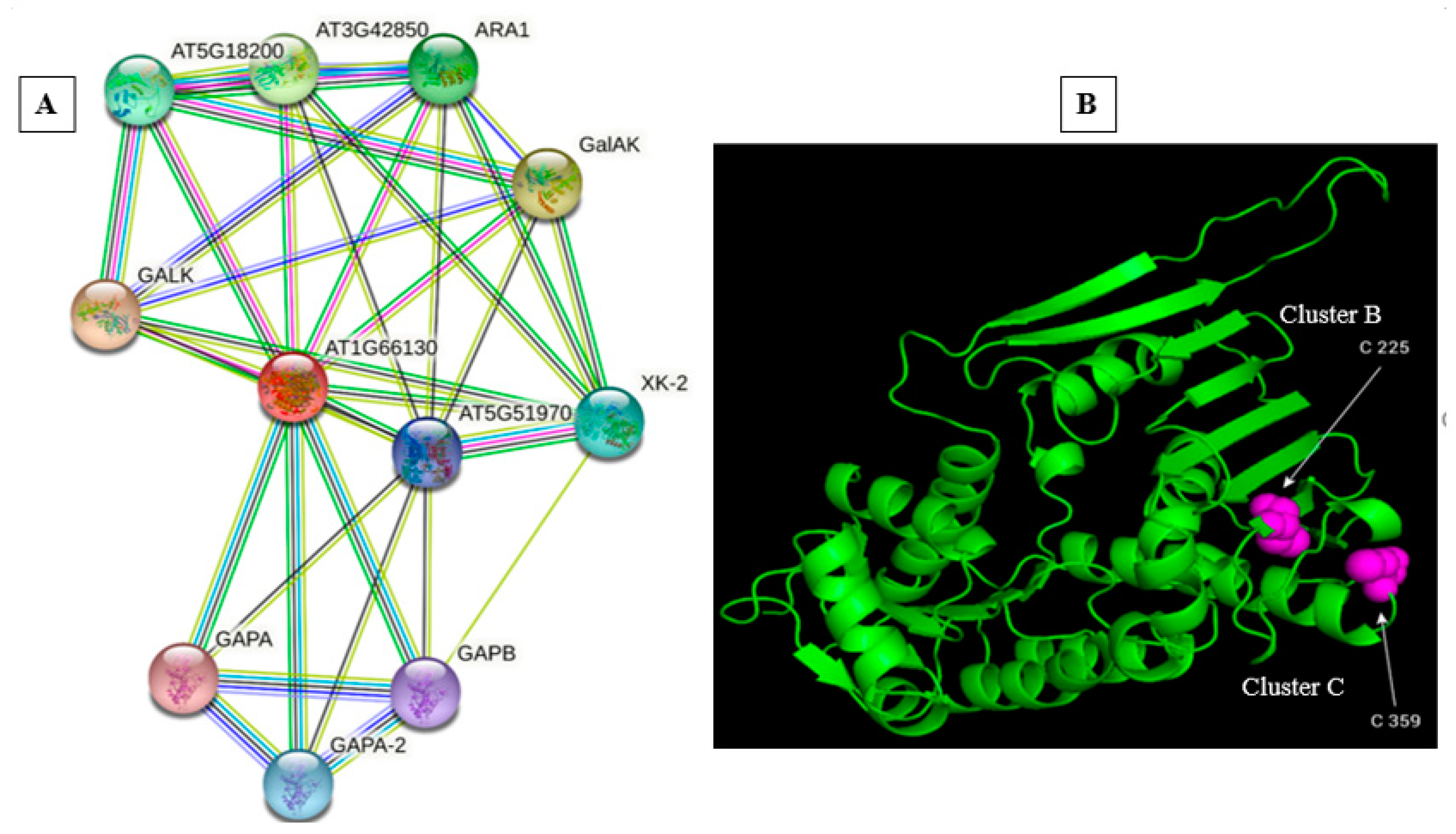
| Symbols | Full Name | Score |
|---|---|---|
| GALK | Mevalonate/galactokinase family protein | 0.829 |
| GalAK | Galacturonic acid kinase | 0.829 |
| AT3G42850 | Mevalonate/galactokinase family protein | 0.829 |
| ARA1 | Arabinose kinase; Arabinose kinase | 0.829 |
| AT5G18200 | UDP glucose-hexose-1-1-phosphate uridylyltransferase | 0.689 |
| XK-2 | Putative xylulose kinase | 0.671 |
| GAPA-2 | Glyceraldehyde-3-phosphate dehydrogenase (NADP+) (phosphorylating) | 0.653 |
| AT5G51970 | GroES-like zinc-binding alcohol dehydrogenase family protein | 0.649 |
| GAPB | Glyceraldehyde-3-phosphate dehydrogenase (NADP+) (phosphorylating) | 0.639 |
| GAPA | Glyceraldehyde-3-phosphate dehydrogenase (NADP+) (phosphorylating) | 0.630 |
| Position | Peptide | Score | Cut Off | Cluster |
|---|---|---|---|---|
| 225 | SVGTILSCTASLQFG | 2.647 | 2.454 | Cluster B |
| 359 | KKSVDIGCEVVHL | 21.547 | 20.743 | Cluster C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Azzawi, T.N.; Khan, M.; Mun, B.-G.; Lee, S.-U.; Imran, M.; Hussain, A.; Rolly, N.K.; Lee, D.-S.; Ali, S.; Lee, I.-J.; et al. Enhanced Resistance of atnigr1 against Pseudomonas syringae pv. tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtNIGR1 Encoding NAD(P)-Binding Rossmann-Fold in Arabidopsis thaliana. Antioxidants 2023, 12, 989. https://doi.org/10.3390/antiox12050989
Al Azzawi TN, Khan M, Mun B-G, Lee S-U, Imran M, Hussain A, Rolly NK, Lee D-S, Ali S, Lee I-J, et al. Enhanced Resistance of atnigr1 against Pseudomonas syringae pv. tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtNIGR1 Encoding NAD(P)-Binding Rossmann-Fold in Arabidopsis thaliana. Antioxidants. 2023; 12(5):989. https://doi.org/10.3390/antiox12050989
Chicago/Turabian StyleAl Azzawi, Tiba Nazar, Murtaza Khan, Bong-Gyu Mun, Sang-Uk Lee, Muhammad Imran, Adil Hussain, Nkulu Kabange Rolly, Da-Sol Lee, Sajid Ali, In-Jung Lee, and et al. 2023. "Enhanced Resistance of atnigr1 against Pseudomonas syringae pv. tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtNIGR1 Encoding NAD(P)-Binding Rossmann-Fold in Arabidopsis thaliana" Antioxidants 12, no. 5: 989. https://doi.org/10.3390/antiox12050989
APA StyleAl Azzawi, T. N., Khan, M., Mun, B.-G., Lee, S.-U., Imran, M., Hussain, A., Rolly, N. K., Lee, D.-S., Ali, S., Lee, I.-J., & Yun, B.-W. (2023). Enhanced Resistance of atnigr1 against Pseudomonas syringae pv. tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtNIGR1 Encoding NAD(P)-Binding Rossmann-Fold in Arabidopsis thaliana. Antioxidants, 12(5), 989. https://doi.org/10.3390/antiox12050989











