Abstract
Oxidative stress contributes to impairment of skin health, the wound healing process, and pathologies such as psoriasis or skin cancer. Five Polynesian medicinal plants, among the most traditionally used for skin care (pimples, wounds, burns, dermatoses) are studied herein for their antioxidant properties: Calophyllum inophyllum, Gardenia taitensis, Curcuma longa, Cordia subcordata, and Ficus prolixa. Plant extracts were submitted to in vitro bioassays related to antioxidant properties and their bioactive constituents were identified by a metabolomic analytical approach. High performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) analysis was performed leading to the characterization of 61 metabolites. Compounds annotated for F. prolixa and C. subcordata extracts were reported for the first time. Antioxidant properties were evaluated by total phenolic content (TPC), free radical scavenging DPPH (1,1-diphenyl-2-picryl-hydrazyl), and Ferric Reducing Antioxidant Power activity (FRAP) assays. F. prolixa extract was the most active one and showed antioxidant intracellular activity on keratinocytes by Anti Oxydant Power 1 assay. Online HPLC-DPPH allowed the identification of phenolic bioactive compounds such as quercetin-O-rhamnoside, rosmarinic acid, chlorogenic acid, procyanidins, epicatechin, 5-O-caffeoylshikimic acid, and curcumin as being responsible for the scavenging properties of these plant extracts. These results highlight the potential of F. prolixa aerial roots as a source of antioxidants for skin care applications.
1. Introduction
The skin is the largest organ of the human body. On the outer layer (epidermis), the skin is composed of keratinocytes, and on the second layer (dermis), it is composed of fibroblasts. It establishes a protective barrier between the organism and the external environment, and against UV radiation, air pollution, chemical components (drug, cosmetics, etc.), and different pathogen threats [1]. In response to these external aggressions, keratinocytes produce reactive oxygen species (ROS) aiming to activate cell proliferation and survival. However, on the other hand, ROS production may damage DNA, collagen structures, and lipid membranes [2]. This oxidative stress is regulated by enzymatic and non-enzymatic antioxidants creating a balance between the ROS production system and the antioxidant system [2]. In case of damaging threats or pathologies such as diabetes, these systems and their natural balance are disturbed, contributing to the generation of impairment in the wound healing process as well as inducing acne, psoriasis, or skin cancer [2,3]. Different therapeutic strategies are possible to help avoid such cutaneous disorders. The most commonly used strategy is to reduce the level of ROS by the use of an additional antioxidant administered orally or topically.
Skin diseases pose an issue in French Polynesia where they are particularly enhanced by the occurrence of higher rates of diabetes [4] and sun exposure. On the other side, the geographical isolation of islanders living far away from city hospitals may reduce their access to healthcare and pharmaceuticals. French Polynesia is a territory with rich plant biodiversity that is used in well-known traditional medicines and practices of pharmacopeia as well as cosmetopeia [5]. Plants have been used for centuries in health and skin care and they can provide a natural source of antioxidant molecules, including mainly glutathione, ascorbic acid, carotenoids, tocopherols, and phenolic compounds (flavonoids, coumarins, xanthones, phenolic acids, tannins) [6].
To address this issue, Polynesian plants used for wound healing and anti-inflammatory and antioxidant properties were studied. The selection of the herein-investigated plants had been achieved following four main criteria. First of all, the selected plants must have ethnobotanical uses in Polynesian traditional medicine (Raau Tahiti) for burns, wounds, dermatoses, pimples, itch, rashes, and other skin treatments. Secondly, their bio-ecological status should be considered to exclude protected, endangered, and rare plants (from the UICN list) to keep only abundant and renewable ones. Thirdly, the biogeographic status had been also used to exclude modern introduced plants (settled after European arrival in Polynesia), as they do not have long-term Polynesian ancestral use, so we are keeping indigenous and Polynesian introduced plants brought by Polynesian first settlements and immigrants in the Oceania region. Then, the importance of the selected plant phytochemical data from the literature was also considered. This network led to a short list of five selected plants, used for skin care, and some of them were known to be incorporated into “Monoi” which is a local coconut oil preparation that includes macerated plants. The plants are are among the most commonly used in French Polynesia in traditional medicine and skin care [5]:
Calophyllum inophyllum L. (Calophyllaceae) is an evergreen tree called “Tamanu” in Tahiti. C. inophyllum nuts are sun-dried for 2–3 months and cold-pressed to yield viscous oil ubiquitously used in Raau Tahiti to treat burns, sunburn, infected wounds, eczemas, and to heal many skin problems [5,7]. C. inophyllum leaves are also used in traditional recipes to cure dermatosis, itch, or inflammation [7,8].
Flowers of Gardenia taitensis DC trees (Rubiaceae) called locally “Tiare” are emblematic and flagship flowers of Tahiti island. They are used as a major cosmetic ingredient, being commonly macerated in coconut oil yielding Monoi which is applied for daily skin and hair care. They are also mentioned in numerous recipes to treat infected wounds, dermatosis, contusion, skin abscesses, and cutaneous allergies [5,8,9].
Curcuma longa L. (Zingiberaceae) named “Rea” is an herbaceous plant with tuberous rhizomes widely used in food, cosmetics, medicine, and as a yellow dye. In Raau Tahiti, they are incorporated into wound healing or skin abscess treatment [8]. They are also added in Monoi.
The two last selected plants for this study are two indigenous trees: Cordia subcordata Lam. (Boraginaceae) called “Tou”, a medium-sized evergreen tree growing in coastal areas, and Ficus prolixa G. Forst (Moraceae) named “Ora”, a sacred banyan with a complex trunk formed of anastomosed filiform aerial roots. Green leaves of C. subcordata and aerial roots of F. prolixa are both traditionally used to treat cutaneous allergy, dermatosis, inflammation, and wounds [5,7,8,9]. The studied plants are presented in Table S1 of Supplementary data.
The present study focuses on the antioxidant capacity of these five Polynesian plants aiming to determine the antioxidant properties of their extracts through in vitro bioassays and to identify their bioactive components by a metabolomics analytical approach.
For this purpose, ultrasound-assisted extractions (UAE), a well-known eco-friendly process saving extraction time and solvent quantity use, were performed on plant materials to obtain crude extracts constituting the studied samples. Then, antioxidant properties were evaluated in samples by, respectively, analysis of total phenolic content (TPC), scavenging free radical ability (DPPH assay), metal-reducing activity (FRAP assay), and cellular-based Antioxidant Power 1 (AOP1) assay. The On-Line-HPLC-DPPH method also was used to assign bioactive radical scavenging constituents. Finally, LC-MS/MS data were used to create molecular networking through MZmine 3 and GNPS to characterize plant extracts metabolite contents.
2. Materials and Methods
2.1. Plant Materials
2.1.1. Collection and Preparation
The five selected plants are some of the most used plants in Polynesian traditional medicine, especially for skin application. All plant parts were collected between January and May 2022 in French Polynesia: leaves of C. inophyllum L. (GPS coordinates: −17.631237; −149.614159), leaves of C. subcordata Lam. (−17.576955; −149.610608), nuts of C. inophyllum L. (−17.4822; −140.4539), aerial roots of F. prolixa G. Forst (−17.678171; −149.587278), and flowers of G. taitensis DC (−17.736932; −149.282326). C. longa L. rhizomes were bought from local markets. Plants were identified by the botanist J-F. Butaud and voucher specimens were deposited at the herbarium of French Polynesia (PAP). They were then oven-dried at 40 °C, except C. inophyllum nuts, which had been sundried for 8 weeks. So dried, plant parts were ground into a powder of 1–3 mm using an IKA MF 10 basic grinder.
2.1.2. Ultrasound-Assisted Extraction
Ultrasound-assisted extraction (UAE) was performed using the PEX 1N-cs (24 kHz, 150 W, Reus France) with the homogenizer HS-50A Witeg. A constant temperature of 39 °C was maintained by the refrigerant system CF30 Julabo. The plant powder (80 g) of each sample was extracted for the first time in 550 mL of ethanol/water (70:30; v/v) for 30 min. This first extracted sample was then filtered and submitted to a second extraction for 30 min in renewed solvent. Solvent was removed from extracts by vacuum rotary evaporation and lyophilisation operations (Martin Christ Beta 2-8 LSCbasic, Osterode am Harz, Germany) to yield crude extract as the starting materials from which were performed all further analytical investigations.
2.1.3. Liquid/Liquid Extraction
Liquid/Liquid extraction was performed by dissolving 1 g of crude extract in 20 mL of water. The obtained solution was submitted to a fractionation using the SPE (LLE/SLE) column CHROMABOND XTR, 70 mL/14,500 mg (Macherey-Nagel 730507, lot 2315.232, Dueren, Germany), and the crude extract constituents were eluted, respectively, with stepwise gradient solvents of 100 mL of hexane, then dichloromethane, ethyl acetate, and finally n-butanol. The solvents were removed from fractions by a vacuum rotary evaporation and lyophilisation operations (Cryotec, Saint-Gély-du-Fesc, France).
2.2. Total Phenolic Content, Radical Scavenging, and Antioxidant Activity
2.2.1. Total Phenolic Content (TPC)
The total phenolic content was determined following the Folin–Ciocalteu colorimetric method adapted from El Hosry et al. [10]. The crude extracts were prepared at a concentration of 3 mg/mL in ethanol/water (50:50, v/v). In a 100 mL volumetric flask, 5 mL of the prepared solutions were mixed with 1 mL of Folin–Ciocalteu reagent (Sigma Aldrich, lot BCBP2077V, Saint-Louis, MO, USA), 4 mL of Na2CO3 7.5% (m/v) (Honeywell Fluka Biochemika, 347579/1 596 lot.71345, Charlotte, NC, USA), and completed with distilled water. Samples were incubated for 2 h 30 in the dark at 30 °C in an oven. Absorbance of the solutions was measured at 760 nm using a UV/Vis spectrophotometer (Thermo Scientific Genesys 10S, Waltham, MA, USA). TPC was expressed as mg of gallic acid equivalent (GAE) per g of extracts.
2.2.2. Determination of DPPH Radical Scavenging Activity
Free radical DPPH (1,1-diphenyl-2-picryl-hydrazyl) scavenging capacity assay was performed according to Blois et al. [11] and adapted for a 96-well plate (Sterilin Ltd., Newport, UK). A fresh DPPH (Sigma Aldrich, D9132-5G lot STBD2362V) solution was prepared everyday by dissolving the reagent (4 mg) in methanol (100 mL), kept at room temperature in the dark for 3 h before use. Then, positive control and samples were diluted with methanol to obtain different final concentrations in the wells: 3–15 µg/mL for ascorbic acid (Sigma Aldrich lot SLBB4446) and 5–100 µg/mL for crude extracts except for F. prolixa which needed lower concentrations, 1.25–50 µg/mL. The plate plan was realized according to Breaud et al. [12] and was composed of a blank row (250 µL of MeOH), a negative control row (50 µL of MeOH with 200 µL of DPPH solution), a positive control, and samples at different concentrations in triplicate (50 µL of positive control or sample with 200 µL of DPPH solution). The 96-well plate was placed in the microplate spectrophotometer (BioTek EON, Providence, RI, USA) and was incubated for 1 h at 30 °C. Absorbance was then read at 517 nm. The percentage of DPPH-H was calculated using the following formula (where Abs stands for absorbance):
% of DPPH-H = [(Abscontrol − Abssample)/Abscontrol] × 100
Then, the concentration providing 50% efficiency (EC50) in µg/mL was calculated using the equation of the polynomial curve expressing the percentage of DPPH-H in relation to the concentration. Statistical analysis was performed by ordinary one-way ANOVA test followed by Dunnett’s multiple comparisons tests.
2.2.3. Determination of Ferric Reducing Antioxidant Power
The ferric reducing antioxidant power (FRAP) was determined using a modified version of the FRAP assay described by Benzie and Strain [13]. The assay was performed in a 96-well plate (Sterilin Ltd., Newport, UK). First, the FRAP solution reagent was prepared by mixing one volume of 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) reagent (Honeywell Fluka lot BCBK6346V, Charlotte, NC, USA) (in solution with 40 mM hydrochloric acid), one volume of 20 mM Iron(III) chloride hexahydrate (Honeywell Riedel-de-Haën lot 02550), and ten volumes of 300 mM sodium acetate buffer (pH 3.6). Then, positive control and samples were diluted with distilled water to obtain different final concentrations in the wells: 0.5–5 µg/mL for ascorbic acid (Sigma Aldrich lot SLBB4446) and 2.5–20 µg/mL of crude extracts. The plate was composed of a blank row (50 µL of distilled water with 200 µL of FRAP solution), a positive control, and samples at different concentrations in triplicate (50 µL of positive control or sample with 200 µL of FRAP solution). The 96-well plate was placed in the microplate spectrophotometer (BioTek EON, Providence, RI, USA) and incubated for 30 min at 40 °C. The absorbance was read at 593 nm. The obtained data were calculated and expressed as the FRAP value in mmol Fe2+/g. Statistical analysis was performed by ordinary one-way ANOVA test followed by Dunnett’s multiple comparisons tests.
2.2.4. Antioxidant Power 1 (AOP1) Assay on Keratinocytes
This assay used a Light-Up Cell System (LUCS) patented approach based on the production of cellular radical species following the addition in the culture medium of a photo-inducible fluorescent nucleic acid biosensor [14]. The effect of light application in the presence of the cellular biosensor triggers the production of singlet oxygen which, in turn, causes the production of ROS in a biochemical cascade linked to an increase in emitted fluorescence. The effect is measured by a delay in the kinetic evolution of fluorescence emission. This method allows the evaluation of intracellular antioxidant activity.
A sample of crude extract of F. prolixa was kept at 4 °C and solubilized at a final concentration of 50 mg/mL in DMEM culture medium. A centrifugation at 8700 rpm for 10 min was added. Experiments were carried out with the supernatants. Human HaCaT cells, from the American Type Cell Collection (catalog number CRL-2404), were seeded in 96-well plates at a density of 40,000 cells/well in DMEM supplemented with Fetal Calf Serum (FCS) and kept in the incubator for 24 h at 37 °C/5% CO2. After the 1 h incubation with the fluorescent biosensor, cells were then incubated in the presence of samples (8 concentrations obtained by serial log2 dilutions) for 1 h at 37 °C/5% CO2. Experiments were realized in DMEM without FCS. At least two independent experiments were realized, each on triplicate wells.
Fluorescence was measured (RFU at 535 nm) according to a recurrent 480 nm LED application procedure (20 iterations) of the whole 96-well plate. Kinetic profiles were recorded. The sample monograph presents raw RFU values recorded during the kinetic analysis for each tested concentration and the corresponding normalized values. Antioxidant cell index (AOP index) is calculated from normalized kinetic profiles according to the formula:
AOP index (%) = 100 − 100 (0∫ 20 RFUsample/0∫ 20 RFUcontrol)
By compiling AOP indices according to logarithm (10) of the sample concentration, dose–response curves were obtained and submitted to a sigmoid fit according to the following formula (SC = sample concentration and HS = Hill slope):
AOP index = AOP indexmin + (AOP indexmax − AOP indexmin)/(1 + 10(Log(EC50-SC)*HS))
EC50 (50% efficacy concentration), EC10, and EC90 are then evaluated.
2.2.5. Online RP-HPLC-DPPH
All method details and the instrumental setup were described by Breaud et al. [12]. This method allowed for the identification of radical scavenging compounds in crude extracts. Compounds were first separated with an HPLC Agilent 1260 system and detected with a DAD UV detector (DAD G7117C) at 325, 280, 254, and 210 nm. Then, compounds reacted with the DPPH solution (80 µg/mL) in a coil (25 m × 0.25 mm, corresponding to a contact time of 1 min 14 s) delivered by another HPLC pump (quaternary pump G1311A) at 60 °C with a flow rate of 0.2 mL/min. The final reaction solution is detected by a DAD UV-Vis detector (DAD G1315B) at 515 nm. The Agilent Zorbax Eclipse Plus C18 column (2.1 × 100 mm, 1.8 µm) at 43 °C was used for chromatographic separation. The mobile phase was composed of ultrapure water (A) and acetonitrile (B) (Carlo Erba, Milan, Italy), both acidified with 0.1% formic acid (Carlo Erba, Italy), and the following gradient was applied: isocratic hold 2 min at 5% B, 5–50% B over 2–17 min, 50–100% B over 17–27 min, then isocratic hold 2 min at 100% B (27–29 min). This was then followed by a decrease to 5% B for the column’s equilibration. Crude extracts were prepared at a concentration of 10 mg/mL. The injection volume was 1 µL and the flow rate was 0.2 mL/min. For annotation peak, the same gradient and column were used for both Online RP-HPLC-DPPH and LC-MS/MS analysis.
2.3. UHPLC-MS/MS Analysis
The high-performance liquid chromatography analyses were performed on a Dionex Ultimate 3000 (Thermo Scientific®) system equipped with a Photo Diode Array detector and coupled to a High-Resolution Mass Spectrometer (Bruker Impact II QToF) equipped with an electrospray ionization source. The sample solutions were prepared by solubilizing 1 mg of dry crude extract in 1 mL of ethanol/water (70:30, v/v). The chromatographic separation was carried on an Agilent Zorbax Eclipse Plus C18 column (2.1 × 100 mm, 1.8 µm) at 43 °C. The mobile phase was composed of ultrapure water (A) and acetonitrile (B), both acidified with 0.1% formic acid, and the following gradient was applied: isocratic hold 2 min at 5% B, 5–50% B over 2–17 min, 50–100% B over 17–27 min, then isocratic hold 2 min at 100% B (27–29 min). For the column’s equilibration, this was then followed by a decrease to 5% B in 1 min and held for 3 min. The injection volume was 1 µL and the flow rate was 0.8 mL/min. A sodium formate acetate calibration solution was injected at the beginning of each run as a calibration. Mass spectra were acquired using both positive and negative modes in a mass range of m/z 50 to 1200 and the following parameters were applied for the quadrupole time-of-flight (Q-TOF): end plate offset at 500 V; nebulizer N2 pressure at 3.5 Bar; dry N2 flow at 12 L/min; drying temperature at 200 °C; acquisition rate at 4 Hz; capillary voltage at 3500 V for positive mode and 3000 V for negative mode; stepped collision energy 20–40 eV.
2.4. Molecular Network
Raw data from UHPLC-MS/MS analysis were calibrated with Bruker Compass Data Analysis 5.0 SR1 (64-bit) and converted into mzXML with GNPS Vendor Conversion master. Data were then imported to MZmine 3.3.0 and processed with the following workflow: mass detection, ADAP chromatogram builder, chromatogram resolving (local minimum resolver), 13C isotope filter, alignment (join aligner), assign MS2 to features, feature list rows filter and blank subtraction. Parameters are described in Table S2 of Supplementary Data. Molecular networks were generated by using GNPS [15]. Annotation was facilitated with Sirius 5.6.3 [16].
3. Results and Discussion
3.1. Extraction
The ultrasound-assisted extraction (UAE) method was performed using 80 g of plant powder in 550 mL of ethanol/water (70:30, v/v). This extraction technique provided satisfying yields ranging from 16.5 to 33.1% (m/m) depending on the studied plant (Table 1). Ultrasound creates cavitation bubbles in the plant tissue that keep growing until they finally collapse. This process destroys plant material structure by breaking the cell wall and thus releasing molecules into the solvent to ease a quicker and better extraction process [17]. Moreover, ethanol can be considered as a biobased solvent for a greener extraction approach.

Table 1.
Ultrasound-assisted extraction yield and total phenolic content.
The UAE method allowed for reduced extraction duration compared to conventional methods, therefore saving time and energy. Indeed, Hughes, et al. [18] applied a 12 h maceration to C. inophyllum nuts that provided, respectively, extract yields of 0.76% with water solvent, 10.32% with ethanol/water (50:50, v/v), and 11.98% with ethanol, whereas one hour of UAE process led to a higher yield of 33.1% with ethanol/water (70:30, v/v). Likewise, the herein UAE method gave a yield of 21.5% for C. inophyllum leaves, whereas a percolation method at room temperature for 24 h with 95% ethanol led to a 12% extract yield [19]. In the same manner, C. longa extract yield was higher with the UAE process (24.6%) than with the Soxhlet extraction method (8.9%) described by Patil et al. using ethanol 99% for 12 h at 60 °C [20]. For these last two examples (C. inophyllum and C. longa), the UAE method allowed for the obtaining of higher extract yields within a shorter extraction time.
The choice of taking abundant plants and renewable parts in Polynesia for the present study aimed to promote environment preservation.
3.2. Total Phenolic Content (TPC)
Natural antioxidants found in plants are mainly composed of phenolic compounds such as flavonoids, coumarins, xanthones, phenolic acids, tannins, etc. Thus, the total phenolic content of crude extracts was assessed following the Folin–Ciocalteu method [10]. The results were expressed in gallic acid equivalent (GAE) and presented in Table 1.
For the G. taitensis flowers extract, a phenolic amount of 75 mg GAE/g was found. C. inophyllum nuts showed a TPC of 71 mg GAE/g, probably due to the high proportion of lipids in this oily extract inducing a dilution effect. Cassien et al. also obtained low TPC in a similar extract (14 mg GAE/g) [21]. C. inophyllum leaves extract showed a higher rate than nuts, with a TPC of 143 mg GAE/g, which was in good agreement with Hapsari et al. who reported a quite similar value of TPC for ethanolic extract (124.89 mg GAE/g) [22]. Indeed, C. inophyllum leaves are known to contain coumarins, xanthones, and flavonoids compounds, which may contribute to this TPC value [23]. A TPC value of 140 mg GAE/g was obtained for C. longa rhizomes, due to their phenolic content being constituted mainly of curcuminoid compounds. A previous study conducted by Singh et al. reported a TPC of 112.50 mg GAE/g for a turmeric extract obtained by UAE using ethanol solvent, thus showing an almost similar TPC value to our studied C. longa rhizomes extract [24]. C. subcordata extract had a TPC value of 139 mg GAE/g. Herein, the highest TPC value was found for F. prolixa aerial roots crude extract, with a value of 148 mg GAE/g. The TPCs of the last two extracts were measured presently as a first report.
Phenolic compounds are characterized by an aromatic ring with –OH or –OCH3 substituents. They have the ability to donate a hydrogen atom or electron because of their capacity to stabilize the formed phenol radical by resonance [6]. That is why a high number of phenolic compounds may suggest relatively radical scavenging and antioxidant capacity, investigated in the present work by conducting DPPH, FRAP, and AOP1 assays on the studied plant extracts.
3.3. DPPH Radical Scavenging Activity
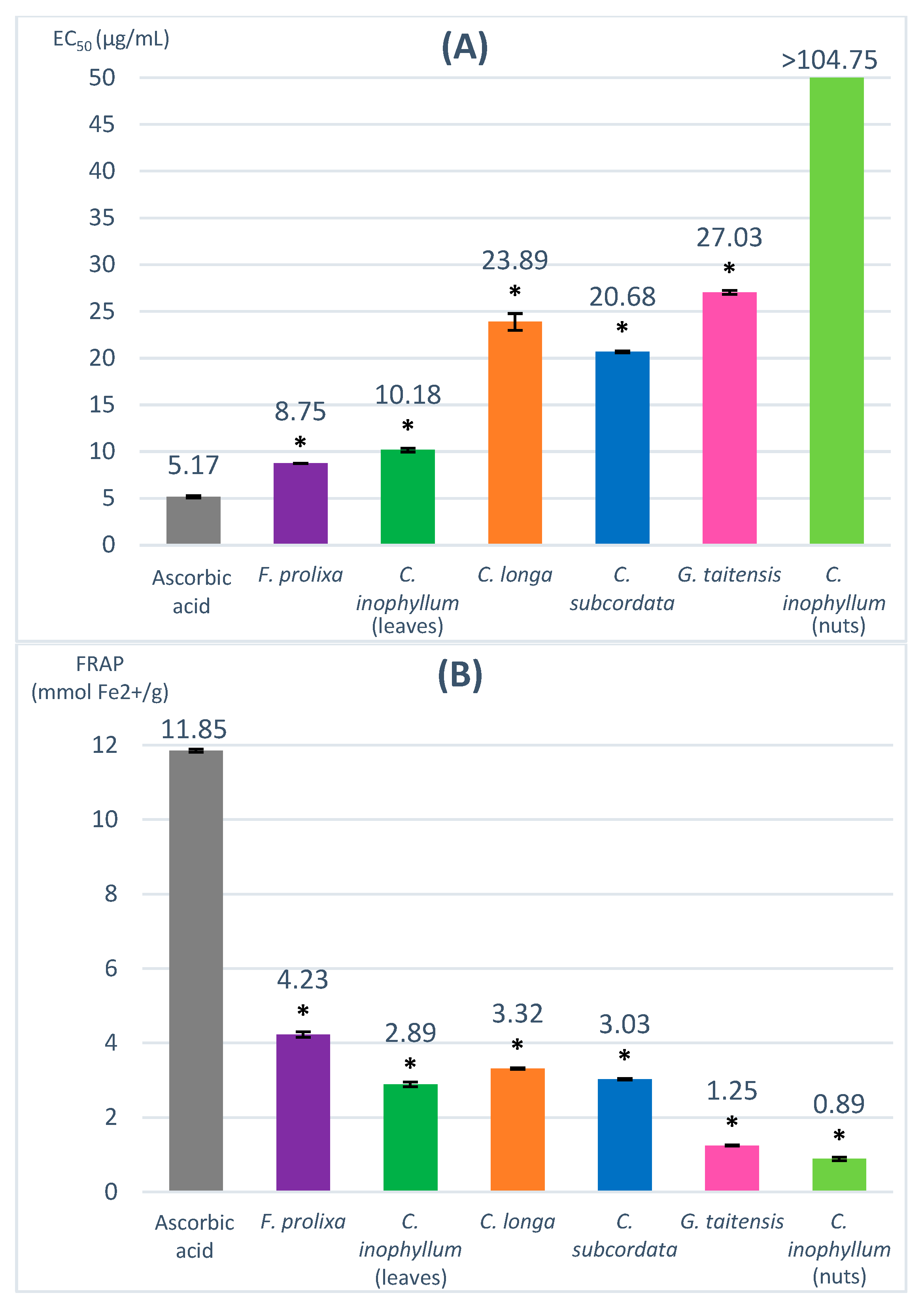
The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay evaluates the capacity to scavenge the nitrogen radical, characterized by its deep violet color corresponding to UV/Vis spectrophotometry absorbance at 517 nm, into DPPH-H (yellow color). In the presence of hydrogen donor compounds, a correlated decrease in absorbance is observed. Results are expressed in EC50 (µg/mL), corresponding to the concentration of sample that decreases the DPPH absorbance by 50%, and compared to a positive control, ascorbic acid (EC50 = 5.17 µg/mL). A lower EC50 value means a more efficient scavenging activity. The obtained EC50 of studied plant extracts ranged from 8.75 to 27.03 µg/mL (Figure 1).
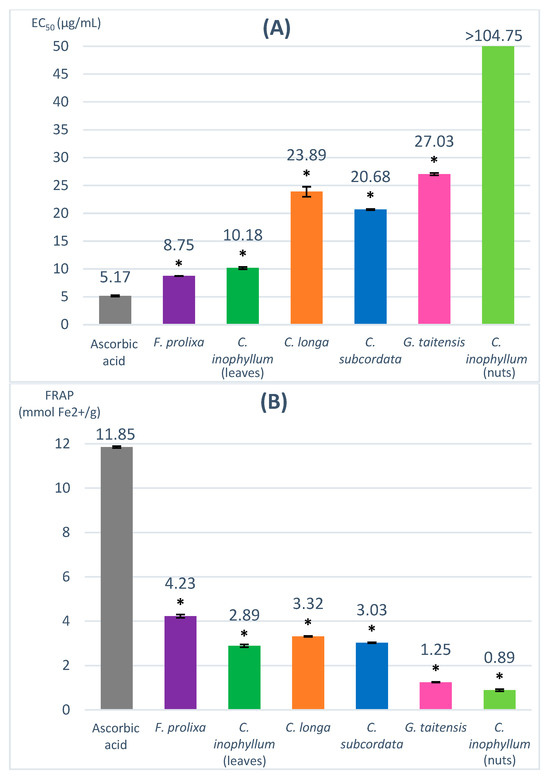
Figure 1.
Radical scavenging and antioxidant capacity evaluated by DPPH (A) and FRAP assay (B). * = ANOVA followed by Dunnett’s multiple comparisons test results (p ≤ 0.01).
The used concentration range (5–100 µg/mL) did not allow us to determine the EC50 of C. inophyllum nuts extract, which may be correlated to a very low scavenging activity of this extract. Cassien et al. reported an EC50 of 432 µg/mL from a DPPH assay for cold-pressed C. inophyllum oil [21]. The leaves of C. inophyllum extract showed a higher scavenging activity with an EC50 of 10.18 µg/mL. The C. longa rhizomes extract had an EC50 of 23.89 µg/mL, in agreement with Sabir et al.’s results which reported similar results for an ethanolic extract of turmeric (27.2 μg/mL) [25]. Despite a low TPC value, G. taitensis flowers extract seemed to possess interesting radical scavenging activity (EC50 = 27.03 µg/mL), which may be due to non-phenolic constituents having radical scavenging capacity. Such properties had been previously reported in the Gardenia genus in G. jasminoides flowers [26,27]. F. prolixa crude extract had the lowest EC50 value (8.75 µg/mL), suggesting its highest scavenging capacity amongst the studied plant extracts.
3.4. Ferric-Reducing Antioxidant Power (FRAP) Assay
The FRAP (ferric-reducing antioxidant power) assay evaluates the capacity to reduce Fe3+ to Fe2+ in complex with TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) inducing a blue color corresponding to UV/Vis spectrophotometry absorbance at 593 nm. The higher the FRAP values, the stronger the antioxidant activity. Ascorbic acid was used as a positive control (11.85 mmol Fe2+/g). The obtained FRAP value for our studied plant extracts ranged from 0.89–4.23 mmol Fe2+/g (Figure 1).
C. inophyllum nuts extract showed the lowest FRAP value (0.89 mmol Fe2+/g) and seemed to have very low antioxidant properties. The FRAP value of C. inophyllum leaves extract was higher (2.89 mmol Fe2+/g) than the obtained result for C. inophyllum nuts extract. Similar differences in antioxidant activity between leaves and nuts was reported by Hughes et al. for ethyl acetate and aqueous extract [18]. C. subcordata extract had a FRAP value of 3.03 mmol Fe2+/g. Only very few data are reported about the properties of this plant, but a previous study mentioned some antioxidant activity of ethanol leaves extracts in rat models [28]. F. prolixa extract showed the highest FRAP value (4.23 mmol Fe2+/g), indicating the best antioxidant capacity among the studied plant extracts.
In this study, the DPPH and FRAP assay results of the studied plant extracts are consistent with the above-obtained data of their TPC contents. Plant crude extracts with a high number of phenolic compounds, such as F. prolixa, seemed to have better antioxidant and scavenging properties. No study had been reported previously regarding these radical scavenging or antioxidant properties of F. prolixa extract. Another Ficus species, namely, Ficus microcarpa, was shown to have some antioxidant and scavenging properties, as reported by Ao et al., with an EC50 DPPH value of 6.8 µg/mL for a methanol extract of its aerial roots extract [29].
As among the presently studied five plant extracts, F. prolixa showed the most promising antioxidant and anti-radical scavenging activities, further investigations were performed on its crude extract.
3.5. Scavenging and Antioxidant Properties of Liquid/Liquid Extracts of F. prolixa
Liquid/liquid (L/L) extraction and fractionation were performed on crude extract of F. prolixa solubilized in water on a column Chromabond XTR using stepwise gradient solvents for elution of, respectively, hexane, dichloromethane, ethyl acetate, and butanol.
Hexane and dichloromethane fractions yields were very low due to the initial polarity of F. prolixa crude extract and so, scavenging and antioxidant properties were assessed only for ethyl acetate and butanol fractions. The obtained EC50 values from DPPH assay of these later fractions, respectively, 3.87 and 3.28 µg/mL for ethyl acetate and butanol extracts, were lower than those of ascorbic acid (Table 2). DPPH scavenging activity of fractions obtained from the methanolic aerial roots crude extract of F. microcarpa, respectively, for ethyl acetate extract (EC50 = 6.0 µg/mL) and butanol extracts (EC50 = 11.2 µg/mL), as reported by Ao et al. [27], suggested lower scavenging properties of F. microcarpa extracts compared to our results regarding F. prolixa fractions. The FRAP assay results of these F. prolixa fractions, respectively, of 9.36 and 9.18 mmol Fe2+/g for ethyl acetate and butanol fractions, were quite in the same range of the FRAP value of ascorbic acid (Table 2). Thus, these F. prolixa fractions should contain polar constituents with strong scavenging and antioxidant properties.

Table 2.
Yields, scavenging, and antioxidant capacity of F. prolixa L/L extracts.
Aiming to evaluate and confirm the antioxidant effect of the F. prolixa extract on a cell model, AOP1 assay was then performed on keratinocytes.
3.6. Antioxidant Power Assay on F. prolixa Extract
Antioxidant Power 1 (AOP1) assay, performed on the HaCaT human keratinocyte model, allowed us to evaluate the antioxidant intracellular activity of tested samples. Using the patented Light-Up Cell System (LUCS) technology, cells were incubated in the culture medium with a photo-inducible fluorescent nucleic acid biosensor. The light application triggers the production of singlet oxygen which, in turn, causes the production of ROS, in a biochemical cascade linked to an increase of emitted fluorescence. Therefore, by measuring the fluorescence, this approach evaluated the ability of plant extracts to neutralize oxidative stress in cells [14].
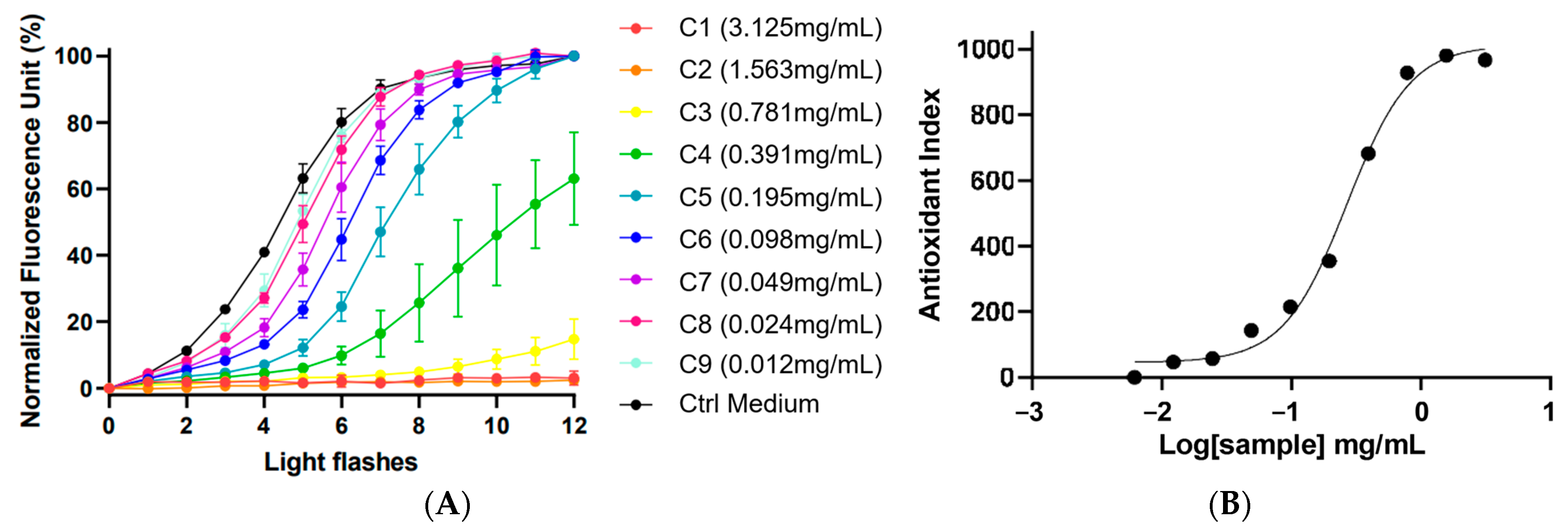
The kinetic graph (Figure 2) showed the % of fluorescence in relation to the number of light flashes at various extract concentrations of F. prolixa crude extract (from 0.012 mg/mL to 3.125 mg/mL). The black curve is the negative control (without added antioxidant), showing the cellular response to the induced production of ROS and the increasing % of fluorescence. Herein, F. prolixa crude extract showed a full direct antioxidant activity by neutralization of intracellular free radicals on human HaCaT cells. Any cytotoxic effects after 1 h were detected for any assayed concentrations below or equal to 25 mg/mL (max concentration tested). Dose–response curves showed a calculated antioxidant index according to logarithm (10) of the sample concentration (Figure 2). Efficacy concentration of F. prolixa crude extract had been measured and showed an EC50 value of 268.0 µg/mL on HaCaT cells. The EC10 of 77.6 µg/mL indicates the needed concentration to have an antioxidant activity. The EC90 of 924.6 µg/mL represents the concentration that neutralized 90% of the produced ROS. Taken as an example for comparison, resveratrol had an EC50 of 0.2621 µg/mL on this AOP1 assay on HaCaT (unpublished data). The obtained results for F. prolixa crude extract showed a good dose–response effect of the extract antioxidant activity on HaCaT cells.
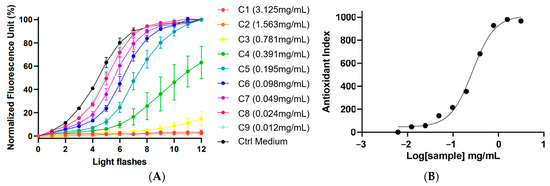
Figure 2.
AOP1 assay of F. prolixa crude extract. (A) kinetic data: normalized fluorescence unit (%) obtained with increasing light flashes at each tested concentrations; (B) Dose–Response graph (EC50 = 268.0 µg/mL).
This AOP1 assay demonstrated the capacity of F. prolixa extract to reduce oxidative stress and to provide a strong antioxidant activity on skin cells at non-cytotoxic concentrations. These data are promising results for potential skin topical applications of this plant extract.
3.7. Online RP HPLC DPPH Assay
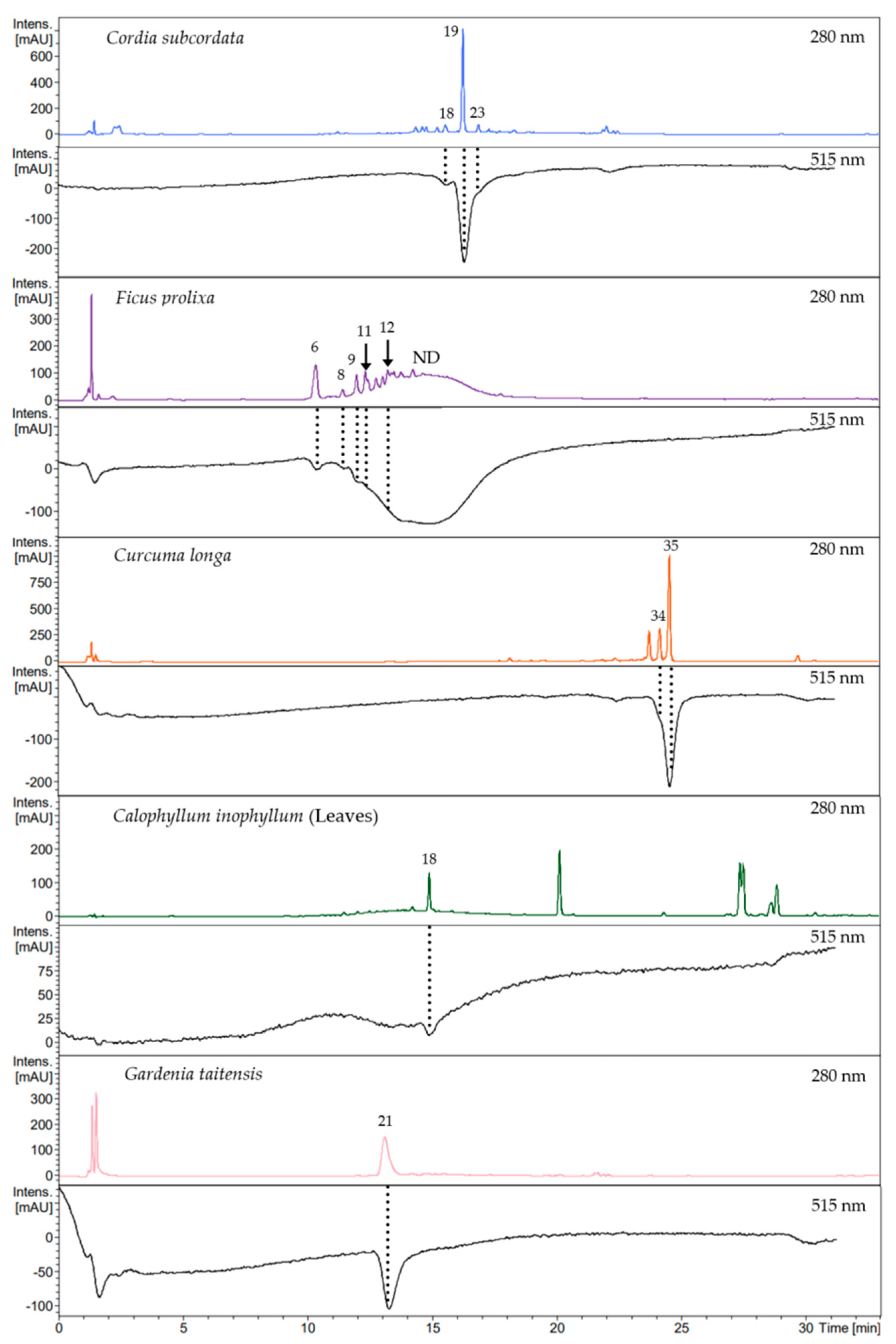
In order to identify compounds responsible for the radical scavenging activity of the studied plant extracts, HPLC analysis was combined with DPPH online assay. Plant extract components were first separated and detected at 280 nm. This was then followed by the reaction of chromatographed components with DPPH solution, incorporated in the analytic system. Then, bleaching of the solution induced by active compounds was detected at 515 nm. Rutin was used as a positive control to determine the elution time shift between both detection systems.
This DPPH online analysis revealed that the scavenging activity of plant extracts, respectively, for C. subcordata, C. longa, C. inophyllum leaves, and G. taitensis was, for each, mostly due to one main compound (Figure 3), except for F. prolixa extract, which presented multiple compounds with scavenging properties. C. inophyllum nuts extract showed no decrease in UV/Vis spectrophotometry absorbance at 515 nm (Figure S1) which means that its constituents had no or very weak radical scavenging properties or the concentration of active compounds was too low to be detected. This finding was in agreement with Cassien et al. regarding scavenging activity of C. inophyllum oil extract. Some neoflavonoids content from C. inophyllum oil, especially inophyllum P, showed an EC50 of 26.2 µM from DPPH assay, but this compound represented only 0.05% of the extract [21].
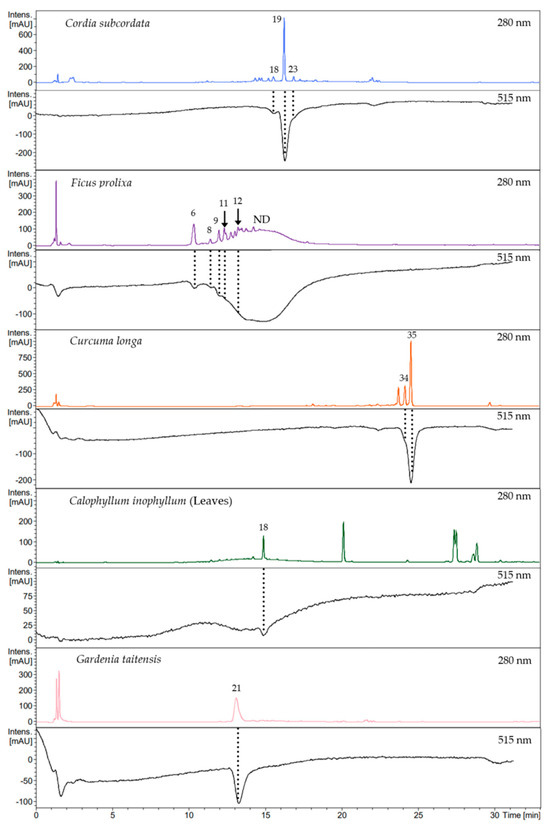
Figure 3.
Chromatograms of Online RP HPLC DPPH assay. Crude extract profiles were recorded at 280 nm and their corresponding active compounds, shown in negative peaks, were recorded at 515 nm: chlorogenic acid (6), procyanidin type B (8), epicatechin (9), 5-O-caffeoylshikimic acid (11), procyanidin type C (12), quercetin-O-rhamnoside (18), rosmarinic acid (19), 3,5-di-O-caffeoyl-4-O-(3-hydroxy, 3-methyl)glutaroylquinic acid (21), lithospermate B (23), demethoxycurcumin enol form (34), curcumin enol form (35), ND: Not Determined.
By using a similar analytical method, those active compounds can be characterized by UHPLC-MS/MS analysis (Table 3).
3.8. UHPLC-MS/MS and Molecular Network
Mass tandem spectrometry was performed to study the phytochemical composition of the crude extracts. Spectral data were acquired in both positive and negative ionization modes using an LC-MS Q-TOF. Data were then processed via MZmine 3 and structured into molecular networks using GNPS. Finally, a total of 61 metabolites had been annotated (Table 3) by using reference standards, previous literature data, mass databases, spectral prediction, and molecular networking. Herein, the identification levels of confidence of Schymanski et al. were applied to classify annotated compounds [30]: starting with level 3 (L3) for tentative candidate, followed by level 2 for probable structure identified by diagnostic evidence (L2b) or by library spectrum match (L2a), and then level 1 (L1) for confirmed structure by the use of reference standard compound or by NMR.
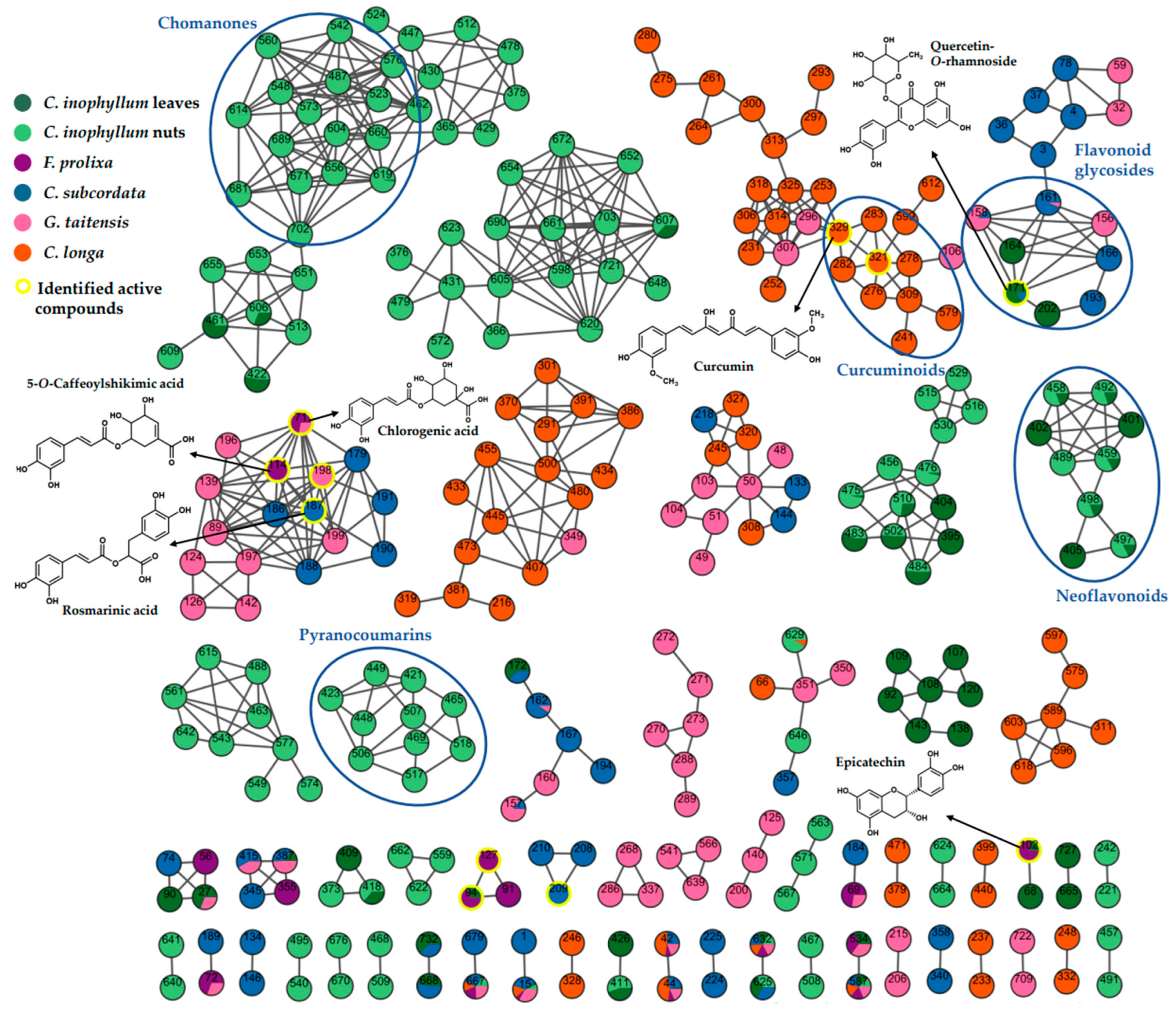
The resulting molecular networks (Figure 4 and Figure S2) revealed big green clusters, suggesting that some chemical classes were specific to C. inophyllum. A difference in composition and amount between leaves and nuts was noticed. Metabolites from C. inophyllum extracts were mainly identified by standards from our internal database. Two light green clusters showed that some pyranocoumarins and chromanones are specific to nuts. Indeed, pyranocoumarins such as tamanolide (53), tamanolide E (44) and C (51), and calanolide D (41) had been identified with a high level of confidence (L2a) thanks to standards from our internal database. Otherwise, calanolide A (42) and B (45) were annotated as tentative for identification (L3). These two isomer compounds had a structure close to calanolide D that only differs from a hydroxyl group due to ketone function. According to these data, other identifications were suggested, 12-oxocalanolide A or B (40), as isomers of calanolide D, and 12-methoxycalanolide A (52) and B (54) [31]. Among chomanones, inocalophyllin B methyl ester (61) was identified, showing the same fragmentation behavior as inocalophyllin B (55) but with a difference of 14 uma due to a methyl group. Likewise, the structural difference between inocalophyllin A (56) and inocalophyllin B was consistent with MS spectral information and expressed by a difference of 34 uma [32]. Caledonic acid (47) is described as a tentative of identification as it is the only known compound in Calophyllaceae with a molecular formula of C27H38O6. Neoflavonoids (4-phenylcoumarins or Ar-C3-Ar) like calophyllolide (49) inophyllum E (48) and its isomer soulattrolone (43) were found in both plant part extracts. Some metabolites were identified in leaves only: jacareubin (36), inophyllum G (37), and tomentolide A (38) [33,34,35]. Flavonoids and derivatives were also identified as major constituents in leaves: quercitrin-O-rhamnoside (18), procyanidin type B (8) [36], amentoflavone (30) [19], and epicatechin (9). These compounds are known for their antioxidant properties and could be responsible for the activity of this extract. As they were only found in leaves extracts, this difference of composition between part of plants could explain the relative absence or very low levels of antioxidant activity in nuts extract.
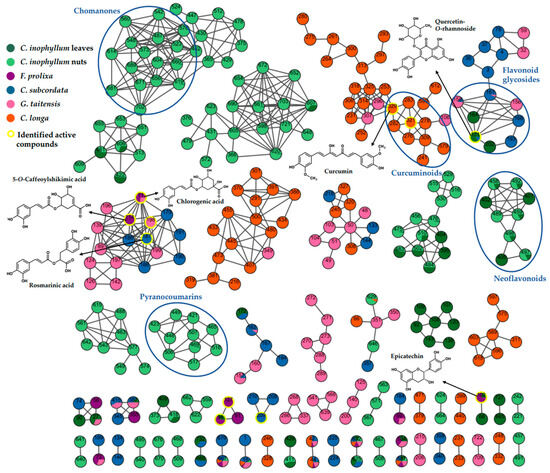
Figure 4.
Molecular Network cluster created with GNPS using spectral data of crude extracts in positive mode (self-loop nodes removed). Node colors represent repartition in plant extracts: C. inophyllum leaves (dark green), C. inophyllum nuts (light green), F. prolixa (purple), C. subcordata (blue), G. taitensis (pink), C. longa (orange). Node numbers correspond to MZmine numbers in positive mode as shown in Table 3 and in LC/MS data at: https://doi.org/10.5281/zenodo.8300733 (accessed on 1 September 2023).
This molecular network also showed an orange cluster composed of curcuminoids. These polyphenol compounds, specific to Curcuma species, are major compounds in C. longa rhizomes, with an amount of up to 2590 mg/100 g for curcumin [37]. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin were identified in both keto and enol forms in C. longa extract. As described by Jia et al., the presence of the β-diketone system in curcuminoid creates a keto–enol tautomerism. The keto form can be distinguished by its lower peak area, earlier retention time, and difference of fragmentation in negative ion mode [38].
In G. taitensis flowers extract, various compounds were identified: some iridoids like gardenoside (5) and geniposide (10), flavonoids such as rutin (14) and quercetin-O-hexose (15), and some phenolic acids as chlorogenic acid (6) and 3,5-di-O-caffeoyl-4-O-(3-hydroxy, 3-methyl)glutaroylquinic acid (21). Spectral MS/MS data of these compounds were consistent with those mentioned by Guo et al. in G. jasminoides flowers [39]. A terpene compound was identified as 7,8,11-trihydroxyguai-4-en-3-one-8-O-β-D-glucopyranoside (16), also reported in G. jasminoides [40].
As few data are available regarding F. prolixa and C. subcordata extract constituents, this molecular network facilitated the identification process. Identification of compounds from well-known plants with phytochemistry LC-MS data helped to confirm the chemical class of compounds in the cluster from plants of unknown composition. Moreover, some metabolites found in various plants, and that have been identified in one of them in previous studies, helped to confirm their identification and occurrence in the other less known plants.
Compounds identified in C. subcordata extract in the present work were described for the first time for this species. Listhospermoside (1) is a cyanoglucoside mentioned by Sosa et al. in other Boraginaceae: Lithospermum purpureo-caeruleum and Lithospermum oficinale [41]. Flavonoid glycosides such as quercetin derivatives were also detected. They had been previously isolated in Cordia species and known to possess radical scavenging properties [42,43]. Rosmarinic acid (19) and lithospermate B (23), a rosmarinic acid dimer, were identified. These polyphenols had also been isolated in various Boraginaceae such as Lithospermum erythrorhizon [44] and Cordia sebestana [45].
Metabolites identified in F. prolixa were mentioned for the first time in this species: chlorogenic acid (6) and cryptochlorogenic acid (7), procyanidin B1 or B2 (8) and type C (12), epicatechin (9), and 5-O-caffeoylshikimic acid (11). In the same way, Ao et al. had isolated epicatechin, procyanidin B1, and chlorogenic acid in another Ficus species, specifically F. microcarpa, and reported their radical scavenging activity by DPPH assay [46].

Table 3.
Metabolites identified in crude extracts (ethanol 70%) of five Polynesian plants, C. inophyllum leaves, C. inophyllum nuts, F. prolixa, C. subcordata, G. taitensis, and C. longa analyzed by UHPLC-MS/MS (Qtof) in both negative and positive ionization modes. Metabolites are sorted by retention times (RT).
Table 3.
Metabolites identified in crude extracts (ethanol 70%) of five Polynesian plants, C. inophyllum leaves, C. inophyllum nuts, F. prolixa, C. subcordata, G. taitensis, and C. longa analyzed by UHPLC-MS/MS (Qtof) in both negative and positive ionization modes. Metabolites are sorted by retention times (RT).
| N° | Annotation | Molecular Formula | RT (min) | IC | MS | MSMS | Ref | Plants | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM N° + | [M+H]+ (Error in ppm) | MM N° − | [M-H]− (Error in ppm) | [M+H]+ (Relative Intensity in %) | [M-H]− (Relative Intensity in %) | |||||||
| 1 | Lithospermoside | C14H19NO8 | 0.62 | L2b | 3 | 330.1184 (+0.2) | 4 | 328.1037 (−0.3) | 168.0653 (100); 122.0599 (33); 105.0333 (20); 330.1186 (15) | 148.0406 (100); 130.0301 (95); 283.2646 (88); 146.0243 (81); 161.0450 (55) | [41,47,48] | C. subcordata |
| 2 | Pantothenic acid | C9H17NO5 | 1.04 | L2b | 21 | 220.1183 (+1.6) | ND | ND | 90.0552 (100); 202.1067 (37); 116.0349 (35); 184.0964 (32); 103.0750 (24); 95.0494 (22) | ND | [49] | F. prolixa C. subcordata |
| 3 | Prunasin amide | C14H19NO7 | 1.39 | L3 | 36 | 314.124 (+1.8) | ND | ND | 152.0708 (100); 107.0501 (15); 194.0799 (10); 296.1128 (10); 134.0601 (9); 314.1247 (7) | ND | [50] | C. subcordata |
| 4 | Sinapic acid | C11H12O5 | 1.80 | L2a | 51 | 225.0761 (+1.6) | ND | ND | 91.0542 (100); 147.0439 (93); 119.0490 (80); 95.0492 (34); 175.0387 (32); 123.0441 (19); 189.0545 (16) | ND | [51] | G. taitensis |
| 5 | Gardenoside | C17H24O11 | 1.80 | L2a | ND | ND | 29 | 403.1245 (−0.2) | ND | 127.0403 (100); 241.0699 (70); 177.0557 (41); 89.0246 (34) | [39,52,53] | G. taitensis |
| 6 | Chlorogenic acid (5-CQA) | C16H18O9 | 3.46 | L1 | 71 | 355.1026 (+0.7) | 46 | 353.0877 (−0.3) | 163.0388 (100); 135.0437 (12); 145.0283 (7); 117.0334 (4) | 191.0559 (100); 85.0294 (4); 127.0403 (2) | [39,46] | F. prolixa G. taitensis |
| 7 | Cryptochlorogenic acid (4-CQA) | C16H18O9 | 4.22 | L2a | 89 | 355.1032 (+2.4) | 58 | 353.0872 (−1.7) | 163.0394 (00); 135.0443 (14); 145.0286 (8); 193.0501 (5) | 173.0452 (100); 135.0446 (88); 179.0359 (81); 191.0552 (65) | [54] | F. prolixa G. taitensis |
| 8 | Procyanidin B1 or B2 | C30H26O12 | 5.06 | L2a | 94 | 579.1505 (+1.4) | 64 | 577.1351 (−0.1) | 127.0390 (100); 139.0390 (43); 287.0551 (35); 163.0390 (34); 289.0712 (33); 291.0855 (33); 271.0605 (30); 275.0543 (24); 247.0592 (23) | 289.0714 (100); 407.0765 (86); 125.0245 (47); 425.0857 (46); 577.1339 (31); 426.0897 (24); 451.1021 (24); 245.0822 (20) | [36,46,55,56] | F. prolixa C. inophyllum leaves |
| 9 | Epicatechin | C15H14O6 | 5.41 | L2a | 102 | 291.0867 (+1.3) | 70 | 289.0716 (−0.6) | 139.0390 (100); 123.0440 (62); 147.0440 (16); 207.0650 (14); 165.0543 (12) | 123.0450 (100); 109.0285 (81); 137.0235 (52); 151.0390 (52); 245.0807 (41); 121.0292 (40); 125.0237 (39); 149.0247 (38); 205.0508 (36) | [46,57,58] | F. prolixa C. inophyllum leaves |
| 10 | Geniposide | C17H24O10 | 5.43 | L2a | ND | [M+NH4]+ 406.1709 | ND | ND | 209.0810 (100); 149.0596 (75); 227.0913 (46); 121.0649 (39); 177.0547 (38) | ND | [39,59] | G. taitensis |
| 11 | 5-O-caffeoylshikimic acid | C16H16O8 | 5.65 | L2a | 114 | 337.0917 (−0.3) | 80 | 335.0771 (−0.4) | 163.0387 (100); 135.0440 (16); 145.0281 (7); 117.0336 (5); 89.0384 (3) | 135.0450 (100); 179.0349 (81); 161.0245 (27); 133.0293 (16); 93.0342 (9) | [60] | F. prolixa |
| 12 | Procyanidin type C | C45H38O18 | 6.26 | L2a | 127 | 867.2144 (1.5) | 96 | 865.1986 (+0.1) | 289.0705 (100); 247.0599 (58); 127.0388 (49); 275.0544 (35); 163.0385 (34); 409.0918 (32); 579.1512 (31) | 865.1976 (100); 287.0559 (78); 407.0757 (67); 289.0716 (65); 577.1346 (60); 575.1208 (48); 425.0879 (48); 125.0242 (43); 451.1039 (32); 413.0849 (30) | [61,62] | F. prolixa |
| 13 | Icariside B5 | C19H32O8 | 6.70 | L2b | 143 | 389.2179 (−0.5) | ND | ND | 209.1531 (100); 191.1436 (55); 149.0962 (35); 173.1327 (27); 163.1470 (23) | ND | [63] | C. inophyllum leaves |
| 14 | Rutin | C27H30O16 | 7.45 | L2a | 158 | 611.1609 (+0.4) | 122 | 609.1465 (+0.6) | 303.0504 (100); 129.0551 (8); 85.0285 (7); 465.1044 (4) | 300.0275 (100); 609.1464 (85); 271.0254 (3); 178.9994 (2); 151.0036 (1); 255.0309 (1) | [39,64,65,66] | C. subcordata G. taitensis |
| 15 | Quercetin-O-hexose | C21H20O12 | 7.58 | L2a | 161 | 465.1031 (+0.7) | 127 | 463.088 (−0.4) | 303.0500 (100); 85.0282 (7); 145.0494 (5); 127.0389 (4); 97.0288 (3); 91.0396 (1) | 300.0272 (100); 463.0879 (54); 271.0243 (25); 255.0299 (10) | [39,67,68] | C. subcordata G. taitensis |
| 16 | 7,8,11-trihydroxyguai-4-en-3-one-8-O-β-D-glucopyranoside | C21H34O9 | 7.86 | L2a | 165 | 431.2282 (+1.5) | 133 | 429.2129 (−0.2) | 269.1851 (100); 251.1644 (57); 233.1533 (44); 163.1117 (23); 137.0962 (15); | 174.9579 (100); 209.1232 (92) | [40,69] | G. taitensis |
| 17 | Quercetin 3-malonylglucoside | C24H22O15 | 8.02 | L2a | 166 | 551.104 (+1.5) | 137 | 549.0888 (+0.4) | 303.0502 (100); 127.0387 (14); 159.0293 (7); 145.0496 (7); 109.0284 (6) | 300.0283 (100); 505.0992 (67); 271.0268 (1) | [70] | C. subcordata |
| 18 | Quercetin- O-rhamnoside | C21H20O11 | 8.34 | L2a | 171 | 449.1081 (+0.6) | 145 | 447.093 (−0.6) | 303.0500 (100); 85.0281 (21); 129.0543 (15); 71.0488 (8) | 300.0271 (100); 447.0929 (51); 271.0242 (26); 255.0295 (13) | [36,71,72] | C. subcordata C. inophyllum leaves |
| 19 | Rosmarinic acid | C18H16O8 | 8.77 | L2a | 187 | 361.0922 (+1.1) | 153 | 359.0772 (−0.1) | 163.0391 (100); 135.0441 (19); 139.0390 (9); 145.0287 (7); 181.0495 (5); 117.0337 (4); 89.0385 (2) | 161.0242 (100); 197.0454 (36); 135.0450 (30); 133.0294 (28); 179.0349 (20); 123.0448 (14); 72.9931 (13) | [45,73,74] | C. subcordata |
| 20 | Kaempferol O-malonylglucoside | C24H22O14 | 8.86 | L2a | 193 | 535.1092 (+1.8) | ND | ND | 287.0553 (100); 127.0391 (14); 145.0495 (7); 159.0287 (6); 109.0287 (6) | ND | [75] | C. subcordata |
| 21 | 3,5-di-O-caffeoyl-4-O-(3-hydroxy, 3-methyl)glutaroylquinic acid | C31H32O16 | 9.17 | L2a | 198 | 661.1764 (+0.1) | 163 | 659.1616 (−0.2) | 163.0393 (100); 301.0927 (5); 135.0445 (2); 355.1032 (2); 337.0919 (2); 145.0286 (2) | 497.1298 (100); 335.0771 (40); 191.0559 (37); 161.0454 (35); 335.0974 (21); 659.1616 (19); 353.0875 (16) | [39,76,77] | G. taitensis |
| 22 | Kaempferol- O-rhamnoside | C21H20O10 | 9.21 | L2a | 202 | 433.1127 (−0.5) | 166 | 431.0977 (−1.6) | 287.0549 (100); 85.0279 (25); 129.0540 (22) | 285.04 (100); 255.0292 (40); 227.0367 (38); 431.0974 (37) | [78] | C. inophyllum leaves |
| 23 | Lithospermate B | C36H30O16 | 9.64 | L2a | 209 | 719.1613 (+0.9) | 167 | 717.1456 (−0.7) | 181.0496 (100); 323.0553 (71); 295.0606 (53); 139.0390 (36); 521.1081 (34) | 321.0399 (100); 519.0931 (97); 339.0509 (48); 295.0600 (17) | [79,80] | C. subcordata |
| 24 | Curcumalongin A | C20H16O6 | 11.63 | L2a | 233 | 353.1024 (+1.2) | 191 | 351.0877 (+0.8) | 353.1022 (100); 147.0446 (22); 153.0546 (18); 166.0260 (16); 149.0233 (9); 121.0287 (8); 150.0313 (7); 338.0804 (6) | 351.0880 (100); 279.0660 (94); 308.0698 (90); 336.0657 (73); 291.0671 (63); 143.0505 (44) | [38] | C. longa |
| 25 | Bisdemethoxycurcumin (keto form) | C19H16O4 | 11.66 | L2a | ND | 309.1126 (+1.5) | 192 | 307.0979 (+1.0) | 147.0442 (100); 119.0490 (22); 91.0543 (6) | 145.0294 (100); 119.0505 (65); 117.0346 (49); 161.0611 (26); 143.0502 (16); 214.9273 (10) | [38] | C. longa |
| 26 | Curcumalongin B | C21H18O7 | 11.98 | L2a | 237 | 383.1129 (+1.0) | 200 | 381.0985 (+1.4) | 383.1129 (100); 153.0546 (13); 149.0233 (7); 177.0550 (6); 163.0385 (6); 294.0881 (5); 145.0287 (5) | 381.0985 (100); 366.0756 (63); 277.0505 (36); 309.0773 (34); 295.0609 (31); 267.0681 (24); 338.005 (23) | [38] | C. longa |
| 27 | Demethoxycurcumin (keto form) | C20H18O5 | 12.06 | L2a | ND | 339.1230 (+0.9) | 202 | 337.1083 (+0.5) | 177.0547 (100); 147.0441 (66); 145.0285 (32); 119.0495 (11) | 145.0293 (100); 175.0404 (77); 160.0161 (57); 119.0501 (55); 117.0353 (45) | [38] | C. longa |
| 28 | Curcumin (keto form) | C21H20O6 | 12.44 | L2a | ND | 369.1337 (+1.2) | 207 | 367.1195 (+2.1) | 177.0550 (100); 145.0287 (39); 117.0336 (12) | 175.0404 (100); 160.0172 (83); 134.0378 (28); 132.0218 (23); | [38] | C. longa |
| 29 | Centaureidin | C18H16O8 | 12.86 | L2b | 250 | 361.0924 (+1.7) | 211 | 359.0773 (+0.2) | 361.0923 (100); 303.0501 (53); 331.0439 (17); 346.0687 (13); 345.0618 (11); 328.0593 (9) | 344.0550 (100); 329.0307 (80); 286.0119 (80); 301.0378 48); 359.0772 (39); 258.0170 (37) | [81] | G. taitensis |
| 30 | Amentoflavone | C30H18O10 | 13.54 | L2a | 257 | 539.0986 (+2.5) | 216 | 537.0834 (+1.3) | 539.0986 (100); 403.0453 (8); 377.0662 (7); 387.0876 (3); 497.0882 (2); 421.0565 (2); 335.0548 (2) | 537.0833 (100); 375.0514 (80); 417.0616 (22); 376.0545 (19); 331.0612 (12) | [19,82] | C. inophyllum leaves |
| 31 | 2,3-dihydro amentoflavone | C30H20O10 | 14.01 | L2a | 274 | 541.1136 (+1.3) | 231 | 539.0983 (+0.2) | 389.1039 (100); 541.1131 (63); 153.0182 (41); 171.0293 (28) | 413.0663 (100); 387.0870 (76); 539.0982 (46); 537.0840 (29); 251.0355 (26); 225.0551 (25) | [83,84] | C. inophyllum leaves |
| 32 | Chikusetsusaponin iva | C42H66O14 | 14.54 | L2a | ND | ND | 239 | 793.4371 (−1.1) | ND | 793.4372 (100); 631.3829 (6); 569.3832 (2) | [85] | G. taitensis |
| 33 | Bisdemethoxycurcumin (enol form) | C19H16O4 | 15.69 | L2a | 309 | 309.1129 (+2.5) | 280 | 307.0978 (+0.7) | 147.0445 (100); 225.0918 (46); 119.0497 (39); 91.0546 (12) | 119.0505 (100); 143.0504 (25); 187.0401 (7) | [38,86] | C. longa |
| 34 | Demethoxycurcumin (enol form) | C20H18O5 | 16.11 | L2a | 321 | 339.1238 (+3.2) | 292 | 337.1087 (+1.6) | 147.0446 (100); 177.0553 (85); 255.1026 (68); 145.0291 (41); 119.0497 (29); 117.0341 (18); 223.0763 (16) | 119.0505 (100); 134.0375 (12); 158.0374 (11); 173.0611 (10); 143.0503 (9); 217.0509 (6); 149.0609 (6); 202.0272 (4) | [38,86] | C. longa |
| 35 | Curcumin (enol form) | C21H20O6 | 16.53 | L2a | 329 | 369.134 (+2.0) | 300 | 367.1187 (0) | 177.0549 (100); 145.0287 (54); 285.1127 (30); 117.0338 (18); 161.0603 (12) | 134.0374 (100); 149.0609 (55); 173.0609 (24); 158.0375 (22); 217.0509 (12) | [38,86] | C. longa |
| 36 | Jacareubin | C18H14O6 | 16.80 | L2a | 335 | 327.0869 (+1.8) | 305 | 325.0721 (+1.0) | 327.0871 (100); 273.0407 (33); 257.0460 (13); 285.0403 (11) | 325.0720 (100); 309.0405 (23); 295.0257 (9); 310.0466 (8); 267.0306 (4) | [87,88] | C. inophyllum leaves |
| 37 | Inophyllum G | C25H24O5 | 20.27 | L3 | 396 | 405.1701 (+1.6) | 350 | 403.155 (−0.2) | 387.1601 (100); 349.1072 (33); 405.1701 (27) 311.0548 (19); 345.1122 (18) | 403.1554 (100); 347.0925 (46); 348.0968 (11); 303.1034 (9) | [34] | C. inophyllum leaves |
| 38 | Tomentolide A | C25H22O5 | 20.43 | L3 | 401 | 403.1547 (+1.7) | ND | ND | 403.1547 (100); 347.0914 (77); 365.1015 (12); 293.0432 (11); 171.0448 (10) | ND | [35] | C. inophyllum leaves |
| 39 | Calophyllic acid | C25H24O6 | 20.55 | L2a | 409 | 421.1653 (+1.7) | 353 | 419.1496 (−1.0) | 403.1538 (100); 347.0913 (56); 377.1746 (46); 321.1121 (31) | 375.1592 (100); 319.0958 (12); 419.1489 (11) | [34] ID | C. inophyllum nuts C. inophyllum leaves |
| 40 | 12-oxocalanolide A or B | C22H24O5 | 20.64 | L3 | 423 | 369.1695 (−0.7) | ND | ND | 369.1695 (100); 285.1121 (45); 341.1746 (19); 313.1056 (14); 257.1165 (9); 243.0637 (8) | ND | [21] | C. inophyllum nuts |
| 41 | Calanolide D | C22H24O5 | 21.06 | L2a | 448 | 369.1694 (−0.7) | ND | ND | 369.1695 (100); 285.1121 (29); 341.1739 (21); 313.1075 (9); 189.1273 (9); 257.1155 (7) | ND | [21] ID | C. inophyllum nuts |
| 42 | Calanolide A | C22H26O5 | 21.15 | L3 | 456 | 371.1847 (−1.6) | ND | ND | 353.1741 (100); 371.1847 (17); 311.1270 (12); 283.0966 (7); 325.1800 (6) | ND | [21,31] | C. inophyllum nuts |
| 43 | Soulattrolone | C25H22O5 | 21.16 | L2b | 458 | 403.154 (0) | ND | ND | 403.1534 (100); 347.0911 (64); 365.1017 (8); 293.0439 (5); 319.0950 (5) | ND | [89] | C. inophyllum nuts C. inophyllum leaves |
| 44 | Tamanolide E | C23H26O5 | 21.28 | L2a | 469 | 383.1855 (+0.5) | ND | ND | 383.149 (100); 327.1226 (51); 299.1277 (27); 355.1902 (25); 328.1254 (11); 269.0804 (9); 281.1163 (7) | ND | ID | C. inophyllum nuts C. inophyllum leaves |
| 45 | Calanolide B | C22H26O5 | 21.33 | L3 | 476 | 371.1855 (+0.5) | ND | ND | 353.1745 (100); 371.1849 (42); 311.1278 (16); 325.1794 (8); 283.1321 (7) | ND | [21,31] | C. inophyllum nuts |
| 46 | Inophyllum A or D | C25H24O5 | 21.47 | L2b | 484 | 405.1691 (−1.4) | ND | ND | 387.1595 (100); 405.1701 (17); 345.1129 (15); 317.0821 (21) | ND | [34] | C. inophyllum nuts C. inophyllum leaves |
| 47 | Caledonic acid | C27H38O6 | 21.50 | L3 | 487 | 459.2737 (−0.9) | 389 | 457.2578 (−3.9) | 275.1275 (100); 335.1485 (75); 317.1380 (69); 233.0804 (24); 336.1520 (15) | 457.2573 (100); 315.1587 (80); 301.1425 (52); 413.2671 (22) | [90] | C. inophyllum nuts |
| 48 | Inophyllum E | C25H22O5 | 21.54 | L2a | 492 | 403.154 (0) | ND | ND | 403.1537 (100); 347.0911 (61); 387.1580 (7); 293.0443 (7); 365.1018 (6); 319.0961 (5) | ND | [21,34,91] ID | C. inophyllum nuts C. inophyllum leaves |
| 49 | Calophyllolide | C26H24O5 | 21.64 | L2a | 497 | 417.1696 (−0.1) | ND | ND | 417.1692 (100); 361.1066 (57); 331.0599 (14); 362.1099 (13); 329.0803 (11) | ND | [34,91] ID | C. inophyllum nuts C. inophyllum leaves |
| 50 | Inophyllum P | C25H24O5 | 21.70 | L2a | 510 | 405.1688 (−2.1) | ND | ND | 405.1676 (100); 387.1594 (33); 345.1129 (6); 317.0815 (4) | ND | [21,34,91] ID | C. inophyllum nuts C. inophyllum leaves |
| 51 | Tamanolide C | C23H26O5 | 21.69 | L2a | 506 | 383.1851 (−0.5) | ND | ND | 383.1851 (100); 355.1904 (56); 299.1277 (43); 281.1175 (15); 287.1278 (14) | ND | ID | C. inophyllum nuts |
| 52 | 12-Methoxycalanolide A | C23H28O5 | 21.81 | L3 | 516 | 385.2007 (−0.7) | ND | ND | 367.1905 (100); 385.2007 (23); 339.1952 (19); 295.1326 (14) | ND | [31] | C. inophyllum nuts |
| 53 | Tamanolide | C24H28O5 | 21.85 | L2a | 517 | 397.2007 (−0.6) | ND | ND | 397.2008 (100); 369.2056 (35); 313.1432 (26); 341.1381 (23); 370.2091 (10); 339.1590 (8); 283.0964 (6); 245.0806 (6) | ND | [21] ID | C. inophyllum nuts |
| 54 | 12-Methoxycalanolide B | C23H28O5 | 21.97 | L3 | 530 | 385.2006 (−0.9) | ND | ND | 367.1901 (100); 385.2006 (59); 339.1950 (25); 295.1327 (13) | ND | [31] | C. inophyllum nuts |
| 55 | Inocalophyllin B 1 | C32H46O6 | 23.61 | L2a | 604 | 527.3363 (−0.8) | 461 | 525.3205 (−3.2) | 335.1487 (100); 275.1276 (84); 317.1382 (73); 318.1415 (16); 276.1310 (15); 233.0805 (10) | 525.3205 (100); 383.2219 (36); 369.2061 (20); 481.3322 (18) | [32] ID | C. inophyllum nuts |
| 56 | Inocalophyllin A | C35H44O6 | 23.64 | L2b | 606 | 561.3209 (−0.3) | 466 | 559.3056 (−1.6) | 369.1333 (100); 351.1227 (64); 309.1122 (56); 221.0808 (53); 233.0809 (27) | 559.3049 (100); 355.1907 (19); 323.1283 (16); 446.2451 (13); 471.3243 (10); | [32] | C. inophyllum nuts C. inophyllum leaves |
| 57 | Inocalophyllin B 2 | C32H46O6 | 23.88 | L2a | 619 | 527.3373 (+1.1) | 473 | 525.3212 (−1.8) | 317.1385 (100); 335.1496 (76); 275.1281 (75); 336.1526 (30); 276.1314 (25); 69.0697 (24); 233.0810 (20) | 525.3206 (100); 383.2216 (56); 369.2057 (33) | [32] ID | C. inophyllum nuts C. inophyllum leaves |
| 58 | Linoleic acid | C18H32O2 | 24.03 | L2a | 629 | 281.2477 (+0.7) | 484 | 279.2322 (−2.7) | 97.1011 (100); 83.0851 (69); 95.0857 (64); 109.1017 (55) | 279.2325 (100); 146.9580 (1) | [92] | C. inophyllum nuts |
| 59 | Inocalophyllin B 3 | C32H46O6 | 24.60 | L2a | 660 | 527.3368 (+0.2) | 498 | 525.3218 (−0.7) | 335.1492 (100); 275.1279 (92); 317.1384 (89); 276.1313 (20); 318.1418 (18); 459.2743 (17); 233.0807 (13); 69.0698 (13) | 525.3209 (100); 333.1338 (50); 387.1805 (13); 334.1376 (12); 219.0658 (12) | [32] ID | C. inophyllum nuts |
| 60 | Pheophorbide A | C35H36N4O5 | 24.73 | L2b | 668 | 593.2764 (+0.9) | ND | ND | 593.2761 (100); 533.2560 (18); 534.2571 (26); 460.2277 (3); | ND | [93] | C. inophyllum leaves C. subcordata |
| 61 | Inocalophillin B methyl ester | C33H48O6 | 25.15 | L3 | 689 | 541.3519 (−0.9) | 520 | 539.3368 (−1.9) | 349.1642 (100); 331.1539 (87); 289.1434 (86) | 539.3375 (100); 347.1490 (51); 348.1534 (14); 303.1585 (14) | [32] | C. inophyllum nuts |
IC: Identification confidence. L1: reference standard or NMR. L2a: library spectrum match. L2b: diagnostic evidence. L3: tentative candidate. MM N°: MZmine Number. ND: Not detected. ID: Internal Database.
Using the same analytical method on both Online RP HPLC DPPH assay and LC-MS/MS analysis, the obtained chromatograms were consistent and the previously annotated compounds led to the identification of active metabolites. Thus the scavenging activity of crude extracts was mainly due to: quercetin-O-rhamnoside (18) in C. inophyllum leaves; rosmarinic acid (19) in C. subcordata leaves; chlorogenic acid (6), procyanidins type B (8) and C (12), epicatechin (9), and 5-O-caffeoylshikimic acid (11) in aerial roots of F. prolixa; curcumin (35) in C. longa rhizomes; 3,5-di-O-caffeoyl-4-O-(3-hydroxy, 3-methyl)glutaroylquinic acid (21) in G. taitensis flowers (Figure 3). According to the structure–activity relationships reported in the literature by Truzzi et al., hydroxycinnamic acids, especially caffeic acid derivatives, and flavonoids such as epicatechin and quercetin, are among the strongest scavengers [94]. This fact may explain the high scavenging capacity of F. prolixa, C. inophyllum leaves, and C. subcordata extracts within such compounds. Their strong scavenging activity could also be related to the amount of these compounds in these latter plant extracts. Identification of these bioactive phenolic molecules highlighted the antioxidant properties of these plant extracts.
The identified active compounds in F. prolixa extract could be correlated with its intracellular antioxidant activity on HaCaT cells. Indeed, various studies evaluated activities of these well-known antioxidant molecules on skin cells. Protective effects on HaCaT human keratinocytes against UV-induced oxidative damage were demonstrated by procyanidin fractions from Vitis vinifera [95]. In the same way, epicatechin increased the viability of UVB-irradiated HaCaT cells [96]. Chlorogenic acid and other caffeoyl derivatives extracted from Ficus dubia showed radical scavenging activity on keratinocytes [97]. Moreover, chlorogenic acid also reduced ROS production and HaCaT cell deaths when exposed to airborne particulate matters [98]. The pool of bioactive compounds with antioxidant potential in F. prolixa aerial roots make this extract a promising ingredient for skin care.
4. Conclusions
UHPLC-MS/MS and a molecular network approach enabled the characterization of the chemical composition of five Polynesian plants used in traditional medicine and skin care. This network led to the identification of 61 metabolites. Compounds annotated for F. prolixa and C. subcordata were described for the first time in these two indigenous Polynesian trees. As far as we are aware, no previous study had been reported regarding their phytochemical content.
Despite some limits of the analytical method, interesting phytochemical results were obtained on the studied plant extracts. Actually, common MS databases used for annotation are not exhaustive and do not allow for the identification of all detected compounds with high confidence levels. Moreover, characterization of isomer compounds, and especially stereoisomers, is limited by the use of LC-MS/MS data analysis, as their mass spectra fragmentation patterns can hardly be differentiated.
Further investigation could be performed to identify more active compounds in F. prolixa by the use of different HPLC analytical methods aiming at a better separation of analytes or allowing for checks of different classes of metabolites which were not found in the present work.
The performed DPPH and FRAP assays on the five studied plant extracts revealed the radical scavenging activity and the antioxidant activity of, respectively, C. inophyllum leaves, F. prolixa aerial roots, C. subcordata leaves, G. taitensis flowers, and C. longa rhizomes. DPPH online assay allowed the identification of phenolic active compounds such as quercetin-O-rhamnoside, rosmarinic acid, chlorogenic acid, procyanidin type B and C, epicatechin, 5-O-caffeoylshikimic acid, and curcumin as responsible for the antiradical scavenging properties of the plant extracts. Further investigations were performed on F. prolixa extract, considered as the most active one from the five studied plant extracts. AOP1 assay confirmed its intracellular antioxidant activity on a HaCaT human keratinocyte model. Moreover, DPPH and FRAP assays performed on L/L extracts revealed antioxidant activities similar to or higher than ascorbic acid. To our knowledge, no previous studies have been reported regarding the antioxidant or scavenging properties and a phytochemical assessment of F. prolixa extract.
These results highlight the potential of F. prolixa aerial roots as a source of antioxidants for skin care topical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12101870/s1, Figure S1. On-Line RP HPLC DPPH assay chromatogram profiles of inactive extract; Figure S2. Molecular Network cluster created with GNPS using spectral data of crude extracts in negative mode; Table S1. Studied plant presentation; Table S2. MZmine parameters.
Author Contributions
Conceptualization, M.C., R.H., G.H., E.G. and P.R.; Data curation, M.C. and E.G.; Formal analysis, M.C., G.H. and E.G.; Funding acquisition, R.H. and P.R.; Methodology, B.B., G.H., S.-S.B.-L. and E.G.; Project administration, P.R.; Resources, G.H. and P.R.; Software, E.G.; Supervision, R.H., G.H., E.G. and P.R.; Validation, R.H., B.B., G.H., S.-S.B.-L., E.G. and P.R.; Visualization, S.-S.B.-L.; Writing—original draft, M.C.; Writing—review and editing, R.H., B.B., G.H., S.-S.B.-L., E.G. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Action 3 «Programme de Recherche et d’Innovation de la Convention d’application relative à la stratégie de la Polynésie française en matière d’Enseignement Supérieur, de Recherche et d’Innovation» (2021–2023) under the project “CAMELIA” (Etude des propriétés du Calophyllum inophyllum en MELange avec des plantes bIoActives polynésiennes) and supported by PhD grant co-financed by UPF and MedEx (Convention October 2021) attributed to Marion Chambon.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors ensure that this manuscript adheres to the transparency guidelines by disclosing all noteworthy aspects of the study being reported. The data presented in this study are openly available. Data are available in a publicly accessible repository. Raw LC/MS data are available at: ftp://massive.ucsd.edu/MSV000092788/ (accessed on 1 September 2023) and and treated LC/MS data at: https://doi.org/10.5281/zenodo.8300733 (accessed on 1 September 2023). GNPS Feature-Based Molecular Networking Jobs are available online at: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=b6c3f78c95864bd7b32dc3d9c718048e (accessed on 1 September 2023) and https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=807ba58b73a34ace9b3aea96f9361e4c (accessed on 1 September 2023), for positive and negative modes, respectively.
Acknowledgments
We are grateful to Herve Vergeaud and Olivier Marrec from MedEx for providing plant materials (Calophyllum inophyllum kernels and Curcuma longa roots) for the study. The authors are thankful to Jean-François Butaud for botanical identification and validation. We would like to thank Charlotte Simmler and Stéphane Greff for the access to the MALLABAR platform and LC/MS/MS realized analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, M.-G. Oxidative Stress and Antioxidant Strategies in Dermatology. Redox Rep. Commun. Free Radic. Res. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Ndwiga, D.W.; MacMillan, F.; McBride, K.A.; Simmons, D. Lifestyle Interventions for People with, and at Risk of Type 2 Diabetes in Polynesian Communities: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 882. [Google Scholar] [CrossRef]
- Pétard, P. Plantes Utiles de Polynésie et Raau Tahiti; Edition Haere po no Tahiti: Papeete, French Polynesia, 1986; ISBN 978-2-904171-06-21. [Google Scholar]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Pétard, P. Raau Tahiti, Plantes Médicinales Polynésiennes et Remèdes Tahitiens; (Technical Document); South Pacific Commission: Nouméa, New Caledonia, 1972; Volume 167. [Google Scholar]
- Girardi, C.; Butaud, J.F.; Ollier, C.; Ingert, N.; Weniger, B.; Raharivelomanana, P.; Moretti, C. Herbal Medicine in the Marquesas Islands. J. Ethnopharmacol. 2015, 161, 200–213. [Google Scholar] [CrossRef]
- Moretti, C.; Butaud, J.F.; Girardi, C.; Ollier, C.; Ingert, N.; Raharivelomanana, P.; Weniger, B. Médecine et pharmacopée végétale traditionnelles aux Iles Marquises (Polynésie française). J. Ethnopharmacol. 2015, 53, 7–27. [Google Scholar]
- Hosry, L.E.; Boyer, L.; Garayev, E.E.; Mabrouki, F.; Bun, S.-S.; Debrauwer, L.; Auezova, L.; Cheble, E.; Elias, R. Chemical Composition, Antioxidant and Cytotoxic Activities of Roots and Fruits of Berberis libanotica. Nat. Prod. Commun. 2016, 11, 1934578X1601100523. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Breaud, C.; Lallemand, L.; Mares, G.; Mabrouki, F.; Bertolotti, M.; Simmler, C.; Greff, S.; Mauduit, M.; Herbette, G.; Garayev, E.; et al. LC-MS Based Phytochemical Profiling towards the Identification of Antioxidant Markers in Some Endemic Aloe Species from Mascarene Islands. Antioxidants 2023, 12, 50. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gironde, C.; Rigal, M.; Dufour, C.; Furger, C. AOP1, a New Live Cell Assay for the Direct and Quantitative Measure of Intracellular Antioxidant Effects. Antioxidants 2020, 9, 471. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, E96. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Ho, R.; Butaud, J.-F.; Filaire, E.; Ranouille, E.; Berthon, J.-Y.; Raharivelomanana, P. A Selection of Eleven Plants Used as Traditional Polynesian Cosmetics and Their Development Potential as Anti-Aging Ingredients, Hair Growth Promoters and Whitening Products. J. Ethnopharmacol. 2019, 245, 112159. [Google Scholar] [CrossRef]
- Prasad, J.; Shrivastava, A.; Khanna, A.K.; Bhatia, G.; Awasthi, S.K.; Narender, T. Antidyslipidemic and Antioxidant Activity of the Constituents Isolated from the Leaves of Calophyllum inophyllum. Phytomedicine 2012, 19, 1245–1249. [Google Scholar] [CrossRef]
- Patil, S.S.; Bhasarkar, S.; Rathod, V.K. Extraction of Curcuminoids from Curcuma longa: Comparative Study between Batch Extraction and Novel Three Phase Partitioning. Prep. Biochem. Biotechnol. 2019, 49, 407–418. [Google Scholar] [CrossRef]
- Cassien, M.; Mercier, A.; Thétiot-Laurent, S.; Culcasi, M.; Ricquebourg, E.; Asteian, A.; Herbette, G.; Bianchini, J.-P.; Raharivelomanana, P.; Pietri, S. Improving the Antioxidant Properties of Calophyllum inophyllum Seed Oil from French Polynesia: Development and Biological Applications of Resinous Ethanol-Soluble Extracts. Antioxidants 2021, 10, 199. [Google Scholar] [CrossRef]
- Hapsari, S.; Yohed, I.; Kristianita, R.A.; Jadid, N.; Aparamarta, H.W.; Gunawan, S. Phenolic and Flavonoid Compounds Extraction from Calophyllum inophyllum Leaves. Arab. J. Chem. 2022, 15, 103666. [Google Scholar] [CrossRef]
- Febrilliant Susanto, D.; Wirawasista Aparamarta, H.; Widjaja, A.; Firdaus; Gunawan, S. Calophyllum inophyllum: Beneficial Phytochemicals, Their Uses, and Identification. In Phytochemicals in Human Health; Rao, V., Mans, D., Rao, L., Eds.; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Singh, K.; Srichairatanakool, S.; Chewonarin, T.; Prommaban, A.; Samakradhamrongthai, R.S.; Brennan, M.A.; Brennan, C.S.; Utama-ang, N. Impact of Green Extraction on Curcuminoid Content, Antioxidant Activities and Anti-Cancer Efficiency (In Vitro) from Turmeric Rhizomes (Curcuma longa L.). Foods 2022, 11, 3633. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.M.; Zeb, A.; Mahmood, M.; Abbas, S.R.; Ahmad, Z.; Iqbal, N. Phytochemical Analysis and Biological Activities of Ethanolic Extract of Curcuma longa Rhizome. Braz. J. Biol. 2020, 81, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wei, Y. A Study on the Antioxidant Activities in Different Parts of Gardenia jasminoides. Front. Soc. Sci. Technol. 2019, 1, 25–29. [Google Scholar]
- Yu, R.; Li, Y.; Si, D.; Yan, S.; Liu, J.; Si, J.; Zhang, X. Identification, Quantitative and Bioactivity Analyses of Aroma and Alcohol-Soluble Components in Flowers of Gardenia jasminoides and Its Variety during Different Drying Processes. Food Chem. 2023, 420, 135846. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, R.; Saravana Kumar, A. Evaluation of Antioxidant Activity of Cordia subcordata Lam. Against Carbon Tetrachloride (CCl4) Induced Erythrocyte Damage in Rats. Pharmacol. 2019, 2, 720–727. [Google Scholar]
- Ao, C.; Deba, F.; Tako, M.; Tawata, S. Biological Activity and Composition of Extract from Aerial Root of Ficus microcarpa L. fil. Int. J. Food Sci. Technol. 2009, 44, 349–358. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Kashman, Y.; Gustafson, K.R.; Fuller, R.W.; Cardellina, J.H.I.; McMahon, J.B.; Currens, M.J.; Buckheit, R.W., Jr.; Hughes, S.H.; Cragg, G.M.; Boyd, M.R. HIV Inhibitory Natural Products. Part 7. The Calanolides, a Novel HIV-Inhibitory Class of Coumarin Derivatives from the Tropical Rainforest Tree, Calophyllum lanigerum. J. Med. Chem. 1992, 35, 2735–2743. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Hung, M.-C.; Wang, L.-T.; Chen, C.-Y. Inocalophyllins A, B and Their Methyl Esters from the Seeds of Calophyllum inophyllum. Chem. Pharm. Bull. 2003, 51, 802–806. [Google Scholar] [CrossRef]
- Li, Z.-L.; Liu, D.; Li, D.-Y.; Hua, H.-M. A Novel Prenylated Xanthone from the Stems and Leaves of Calophyllum inophyllum. Nat. Prod. Res. 2011, 25, 905–908. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R. The Inophyllums, Novel Inhibitors of HIV-1 Reverse Transcriptase Isolated from the Malaysian Tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Parmar, S.S.; Bhatnagar, S.C.; Misra, G.; Nigam, S.K. Anticonvulsant and Antiinflammatory Activity of Natural Plant Coumarins and Triterpenoids. Res. Commun. Chem. Pathol. Pharmacol. 1974, 9, 11–22. [Google Scholar] [PubMed]
- Hughes, K.; Ho, R.; Greff, S.; Herbette, G.; Filaire, E.; Ranouille, E.; Berthon, J.-Y.; Raharivelomanana, P. Feature-Based Molecular Networks Identification of Bioactive Metabolites from Three Plants of the Polynesian Cosmetopoeia Targeting the Dermal Papilla Cells of the Hair Cycle. Molecules 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Filho, J.G.; De Almeida, M.J.; Sousa, T.L.; Dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma Longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–22. [Google Scholar]
- Jia, S.; Du, Z.; Song, C.; Jin, S.; Zhang, Y.; Feng, Y.; Xiong, C.; Jiang, H. Identification and Characterization of Curcuminoids in Turmeric Using Ultra-High Performance Liquid Chromatography-Quadrupole Time of Flight Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1521, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, X.; Jiang, Y.; He, J.; Ge, W.; Hao, H.; Huang, T.; He, Y.; Wen, J.; Zhou, T. Characterization and Quantification of the Chinese Medical Formula Zhi-Zi-Chi Decoction, a Systematic Strategy for the Attenuation and Synergy of Compatibility Mechanism. J. Pharm. Biomed. Anal. 2023, 223, 115130. [Google Scholar] [CrossRef]
- Machida, K.; Oyama, K.; Ishii, M.; Kakuda, R.; Yaoita, Y.; Kikuchi, M. Studies of the Constituents of Gardenia Species. II. Terpenoids from Gardeniae Fructus. Chem. Pharm. Bull. 2000, 48, 746–748. [Google Scholar] [CrossRef]
- Sosa, A.; Winternitz, F.; Wylde, R.; Pavia, A.A. Structure of a Cyanoglucoside of Lithospermum purpureo-caeruleum. Phytochemistry 1977, 16, 707–709. [Google Scholar] [CrossRef]
- Fouseki, M.M.; Damianakos, H.; Karikas, G.A.; Roussakis, C.; Gupta, M.P.; Chinou, I. Chemical Constituents from Cordia alliodora and C. colloccoca (Boraginaceae) and Their Biological Activities. Fitoterapia 2016, 115, 9–14. [Google Scholar] [CrossRef]
- Al-Musayeib, N.; Perveen, S.; Fatima, I.; Nasir, M.; Hussain, A. Antioxidant, Anti-Glycation and Anti-Inflammatory Activities of Phenolic Constituents from Cordia sinensis. Molecules 2011, 16, 10214–10226. [Google Scholar] [CrossRef]
- Murata, T.; Oyama, K.; Fujiyama, M.; Oobayashi, B.; Umehara, K.; Miyase, T.; Yoshizaki, F. Diastereomers of Lithospermic Acid and Lithospermic Acid B from Monarda fistulosa and Lithospermum erythrorhizon. Fitoterapia 2013, 91, 51–59. [Google Scholar] [CrossRef]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Williams, P.G. Sebestenoids A–D, BACE1 Inhibitors from Cordia sebestena. Phytochemistry 2010, 71, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Higa, T.; Ming, H.; Ding, Y.; Tawata, S. Isolation and Identification of Antioxidant and Hyaluronidase Inhibitory Compounds from Ficus microcarpa L. fil. bark. J. Enzyme Inhib. Med. Chem. 2010, 25, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Jiang, B.; Mei, S.; Ding, G.; Sun, H.; Xie, J.; Liu, Y. Constituents from the Roots of Semiaquilegia adoxoides. Fitoterapia 2001, 72, 86–88. [Google Scholar] [CrossRef]
- Lithospermoside CFM-ID Spectrum Prediction. Available online: https://cfmid.wishartlab.com/queries/c3c2dbf85618324c8cfa3f3adefb5bb98b3e7108 (accessed on 27 June 2023).
- Pantothenic Acid [M+H]+ MassBank Record: MSBNK-RIKEN-PR100400. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100400&dsn=RIKEN (accessed on 29 June 2023).
- Sendker, J.; Ellendorff, T.; Hölzenbein, A. Occurrence of Benzoic Acid Esters as Putative Catabolites of Prunasin in Senescent Leaves of Prunus Laurocerasus. J. Nat. Prod. 2016, 79, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Sinapic Acid [M+H]+ MassBank Record: MSBNK-RIKEN-PR101042. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR101042 (accessed on 27 June 2023).
- Zhu, H.; Bi, K.; Han, F.; Guan, J.; Zhang, X.; Mao, X.; Zhao, L.; Li, Q.; Hou, X.; Yin, R. Identification of the Absorbed Components and Metabolites of Zhi-Zi-Da-Huang Decoction in Rat Plasma by Ultra-High Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Gardenoside [M-H]- MoNA Spectrum Bruker_HCD_library000119. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/Bruker_HCD_library000119 (accessed on 23 June 2023).
- Cryptochlorogenic Acid (4-CQA) [M-H]- GNPS Library Spectrum CCMSLIB00004701970. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00004701970 (accessed on 29 June 2023).
- Procyanidin B1 [M-H]- MassBank Record: MSBNK-BS-BS003943. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-BS-BS003943 (accessed on 27 June 2023).
- Procyanidin B1 [M+H]+ MassBank Record: MSBNK-RIKEN-PR100266. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100266&dsn=RIKEN (accessed on 27 June 2023).
- (+)-Epicatechin [M+H]+ MassBank Record: MSBNK-RIKEN-PR100263. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100263&dsn=RIKEN (accessed on 27 June 2023).
- (+)-Epicatechin [M-H]- MassBank Record: MSBNK-RIKEN-PR100688. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100688&dsn=RIKEN (accessed on 27 June 2023).
- Geniposide Spectrum [M+NH4]+ VF-NPL-QEHF003622. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/VF-NPL-QEHF003622 (accessed on 23 June 2023).
- 5-O-Caffeoylshikimic Acid [M+H]+ GNPS Library Spectrum CCMSLIB00000848306. Available online: https://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000848306 (accessed on 27 June 2023).
- Procyanidin C1 [M-H]- MassBank Record: MSBNK-RIKEN-PR101005. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR101005 (accessed on 27 June 2023).
- Andrade, C.; Ferreres, F.; Gomes, N.G.M.; Duangsrisai, S.; Srisombat, N.; Vajrodaya, S.; Pereira, D.M.; Gil-Izquierdo, A.; Andrade, P.B.; Valentão, P. Phenolic Profiling and Biological Potential of Ficus curtipes Corner Leaves and Stem Bark: 5-Lipoxygenase Inhibition and Interference with NO Levels in LPS-Stimulated RAW 264.7 Macrophages. Biomolecules 2019, 9, 400. [Google Scholar] [CrossRef]
- Icariside B5 [M+H]+ GNPS Library Spectrum CCMSLIB00000578418. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000578418 (accessed on 29 June 2023).
- Wang, L.; Liu, S.; Xing, J.; Liu, Z.; Song, F. Characterization of Interaction Property of Multi-Components in Gardenia jasminoides with Aldose Reductase by Microdialysis Combined with Liquid Chromatography Coupled to Mass Spectrometry: Characterization of Interaction Property of Multi-Components in Gardenia jasminoides. Rapid Commun. Mass Spectrom. 2016, 30, 87–94. [Google Scholar]
- Rutin [M+H]+ Spectrum VF-NPL-QTOF009582. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/VF-NPL-QTOF009582 (accessed on 27 June 2023).
- Rutin [M-H]- Spectrum VF-NPL-QTOF009580. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/VF-NPL-QTOF009580 (accessed on 27 June 2023).
- Hyperoside [M-H]- MassBank Record: MSBNK-RIKEN-PR100676. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100676 (accessed on 27 June 2023).
- Hyperoside [M+H]+ MassBank Record: MSBNK-RIKEN-PR100253. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100253 (accessed on 27 June 2023).
- 2(1H)-Azulenone [M+H]+ GNPS Library Spectrum CCMSLIB00000854577. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000854577 (accessed on 27 June 2023).
- Quercetin 3-(6’’-Malonyl-Glucoside) [M+H]+ HMDB0037368. Available online: https://hmdb.ca/spectra/ms_ms/2238378 (accessed on 27 June 2023).
- Quercitrin [M+H]+ MassBank Record: MSBNK-Fiocruz-FIO00579. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-Fiocruz-FIO00579 (accessed on 27 June 2023).
- Quercitrin [M-H]- MassBank Record: MSBNK-Fiocruz-FIO00585. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-Fiocruz-FIO00585 (accessed on 27 June 2023).
- Rosmarinic Acid [M+H]+MassBank Record: MSBNK-RIKEN-PR040220. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR040220 (accessed on 27 June 2023).
- Rosmarinic Acid [M-H]-MassBank Record: MSBNK-RIKEN-PR100686. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100686 (accessed on 27 June 2023).
- Flavone Base + 4O, O-MalonylHex GNPS Library Spectrum CCMSLIB00005740573. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00005740573 (accessed on 28 June 2023).
- GNPS Library Spectrum CCMSLIB00005721208. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00005721208 (accessed on 27 June 2023).
- Spectrum VF-NPL-QTOF000303. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/VF-NPL-QTOF000303 (accessed on 27 June 2023).
- Kaempferol-3-O-Alpha-L-Rhamnoside [M-H]- MassBank Record: MSBNK-RIKEN-PR100970. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR100970 (accessed on 27 June 2023).
- Zhu, J.; Yi, X.; Zhang, J.; Chen, S.; Wu, Y. Chemical Profiling and Antioxidant Evaluation of Yangxinshi Tablet by HPLC–ESI-Q-TOF-MS/MS Combined with DPPH Assay. J. Chromatogr. B 2017, 1060, 262–271. [Google Scholar] [CrossRef]
- GNPS Library Spectrum CCMSLIB00000845756. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000845756 (accessed on 27 June 2023).
- Centaureidin [M+H]+ GNPS Library Spectrum CCMSLIB00000845642. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000845642 (accessed on 28 June 2023).
- Amentoflavone [M+H]+ MassBank Record: MSBNK-IPB_Halle-PB006306. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-IPB_Halle-PB006306 (accessed on 27 June 2023).
- Zheng, J.-X.; Zheng, Y.; Zhi, H.; Dai, Y.; Wang, N.-L.; Fang, Y.-X.; Du, Z.-Y.; Zhang, K.; Li, M.-M.; Wu, L.-Y.; et al. New 3′,8′′-Linked Biflavonoids from Selaginella uncinata Displaying Protective Effect against Anoxia. Molecules 2011, 16, 6206–6214. [Google Scholar] [CrossRef]
- 2,3-Dihydroamentoflavone [M+H]+ GNPS Library Spectrum CCMSLIB00000848211. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000848211 (accessed on 28 June 2023).
- Wang, J.; Lu, J.; Lv, C.; Xu, T.; Jia, L. Three New Triterpenoid Saponins from Root of Gardenia jasminoides Ellis. Fitoterapia 2012, 83, 1396–1401. [Google Scholar] [CrossRef]
- Quirós-Fallas, M.I.; Vargas-Huertas, F.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A.; Navarro-Hoyos, M. Polyphenolic HRMS Characterization, Contents and Antioxidant Activity of Curcuma longa Rhizomes from Costa Rica. Antioxidants 2022, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Nagafuji, S.; Okabe, H.; Akahane, H.; Estrada-Muñiz, E.; Huerta-Reyes, M.; Reyes-Chilpa, R. Trypanocidal Constituents in Plants 3. Leaves of Garcinia intermedia and Heartwood of Calophyllum brasiliense. Biol. Pharm. Bull. 2004, 27, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.D.; Salger, M.; Frank, O.; Balemba, O.B.; Wakamatsu, J.; Hofmann, T. Antioxidative Compounds from Garcinia buchananii Stem Bark. J. Nat. Prod. 2015, 78, 234–240. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Bokesch, H.R.; Fuller, R.W.; Cardellina, J.H.; Kadushin, M.R.; Soejarto, D.D.; Boyd, M.R. Calanone, a Novel Coumarin from Calophyllum teysmannii. Tetrahedron Lett. 1994, 35, 5821–5824. [Google Scholar] [CrossRef]
- Hay, A.E.; Guilet, D.; Morel, C.; Larcher, G.; Macherel, D.; Le Ray, A.M.; Litaudon, M.; Richomme, P. Antifungal Chromans Inhibiting the Mitochondrial Respiratory Chain of Pea Seeds and New Xanthones from Calophyllum caledonicum. Planta Med. 2003, 69, 1130–1135. [Google Scholar]
- Charles, L.; Laure, F.; Raharivelomanana, P.; Bianchini, J.-P. Sheath Liquid Interface for the Coupling of Normal-Phase Liquid Chromatography with Electrospray Mass Spectrometry and Its Application to the Analysis of Neoflavonoids. J. Mass Spectrom. 2005, 40, 75–82. [Google Scholar] [CrossRef]
- Linoleic Acid [M+H]+ MassBank Record: MSBNK-BGC_Munich-RP029503. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-BGC_Munich-RP029503 (accessed on 27 June 2023).
- Pheophorbide A [M+H]+ GNPS Library Spectrum CCMSLIB00000076748. Available online: http://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00000076748 (accessed on 28 June 2023).
- Truzzi, E.; Marchetti, L.; Gibertini, G.; Benvenuti, S.; Cappellozza, S.; Giovannini, D.; Saviane, A.; Sirri, S.; Pinetti, D.; Assirelli, A.; et al. Phytochemical and Functional Characterization of Cultivated Varieties of Morus Alba L. Fruits Grown in Italy. Food Chemistry 2024, 431, 137113. [Google Scholar] [CrossRef]
- Matito, C.; Agell, N.; Sanchez-Tena, S.; Torres, J.L.; Cascante, M. Protective Effect of Structurally Diverse Grape Procyanidin Fractions against UV-Induced Cell Damage and Death. J. Agric. Food Chem. 2011, 59, 4489–4495. [Google Scholar] [CrossRef]
- Mittraphab, Y.; Amen, Y.; Nagata, M.; Matsumoto, M.; Wang, D.; Shimizu, K. Anti-Phototoxicity Effect of Phenolic Compounds from Acetone Extract of Entada phaseoloides Leaves via Activation of COX-2 and INOS in Human Epidermal Keratinocytes. Molecules 2022, 27, 440. [Google Scholar] [CrossRef]
- Chansriniyom, C.; Nooin, R.; Nuengchamnong, N.; Wongwanakul, R.; Petpiroon, N.; Srinuanchai, W.; Chantarasuwan, B.; Pitchakarn, P.; Temviriyanukul, P.; Nuchuchua, O. Tandem Mass Spectrometry of Aqueous Extract from Ficus dubia Sap and Its Cell-Based Assessments for Use as a Skin Antioxidant. Sci. Rep. 2021, 11, 16899. [Google Scholar] [CrossRef]
- Ha, J.W.; Boo, Y.C. Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress. Antioxidants 2021, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).