Featured Application
The adequate conditions (temperature, pressure, space time, and molar ratios in the feed of H2/COx and CO2/CO) for the production of dimethyl ether are described, whose commercialization would partially compensate the costs of the process.
Abstract
The direct synthesis of dimethyl ether (DME) is an ideal process to achieve the environmental objective of CO2 conversion together with the economic objective of DME production. The effect of the reaction conditions (temperature, pressure, space time) and feed composition (ternary mixtures of H2 + CO + CO2 with different CO2/CO and H2/COx molar ratios) on the reaction indices (COx conversion, product yield and selectivity, CO2 conversion) has been studied by means of experiments carried out in a fixed-bed reactor, with a CuO-ZnO-MnO/SAPO-18 catalyst, in order to establish suitable ranges of operating conditions for enhancing the individual objectives of CO2 conversion and DME yield. The optimums of these two objectives are achieved in opposite conditions, and for striking a good balance between both objectives, the following conditions are suitable: 275–300 °C; 20–30 bar; 2.5–5 gcat h (molC)−1 and a H2/COx molar ratio in the feed of 3. CO2/CO molar ratio in the feed is of great importance. Ratios below 1/3 are suitable for enhancing DME production, whereas CO2/CO ratios above 1 improve the conversion of CO2. This conversion of CO2 in the overall process of DME synthesis is favored by the reverse water gas shift equation, since CO is more active than CO2 in the methanol synthesis reaction.
1. Introduction
Dimethyl ether (DME) is receiving increasing attention as alternative fuel and raw material. Its properties (low toxicity and ease of storage, transport, and distribution, high cetane index) are suitable for its use as fuel in different sectors (domestic, automotive, and electric power generation) [1,2,3]. Besides, DME is a potential raw material for the production of diesel fuels (such as dimethoxymethane and polyoxymethylene dimethyl ethers, via oligomerization) [4], and chemicals (methyl acetate, formaldehyde, ethanol, among others) [5], replacing methanol. Moreover, it can replace methanol for light olefin production [6,7] and it is a H2 vector through steam reforming [8,9,10]. In 2015 a DME production market of US$5.2 billion was reported, with an annual growth projection of 9.9% until 2024 [11], produced by methanol dehydration. The demand corresponds in a 65% to the consumption in Asia (mainly in China) as domestic fuel, replacing liquefied petroleum gases (LPG), being its price dependent on the market of methanol and of LPG, which can be estimated as the 75 to 90% of the price per unit mass of LPG [12].
The synthesis of dimethyl ether (DME) in a single-stage (STD process) is more effective than the conventional route in two-stages, and is of great interest for the utilization on a large-scale of the CO2 co-fed with syngas, which can be obtained from sources alternative to oil and with greater availability (natural gas, coal, biomass, and wastes) [13,14,15].
The STD process co-feeding CO2 together with syngas involves the following reactions:
Methanol synthesis:
CO + 2H2 ↔CH3OH (ΔH0 = −90.56 kJ mol−1)
CO2 + 3H2 ↔CH3OH+ H2O (ΔH0 = −49.43 kJ mol−1)
Water gas shift (WGS):
CO + H2O ↔CO2 + H2 (ΔH0 = −41.12 kJ mol−1)
Methanol dehydration to DME:
2CH3OH ↔CH3OCH3 + H2O (ΔH0 = −23.56 kJ mol−1)
Secondary reactions of paraffin formation (mainly methane):
CO + 3H2 ↔CH4 + H2O (ΔH0 = −206.30 kJ mol−1)
It is well established that the direct synthesis of DME is thermodynamically more favored than the synthesis of methanol and than the synthesis of DME in two-stages, because the in situ conversion of methanol into DME (Equation (4)) displaces the thermodynamic equilibrium of the reactions of methanol synthesis (Equations (1) and (2)), favoring the conversion of CO and CO2 [16,17,18,19]. Therefore, the STD process is an attractive route to accomplish two objectives, that is, the production of DME, and the utilization of CO2. Combining both targets, the economy of DME would partially fund the CO2 capture costs, which condition the implementation of the different technologies for its utilization [20]. This economic compensation would be achieved by replacing fossil fuels by DME in different industries (iron and steel, cement, oil refining, pulp, and paper) in which CO2 capture is a priority [21]. The effort of the refineries to reduce CO2 emissions is also to be highlighted [22], and the investment costs could be offset by the commercialization of DME as green fuel (without emissions of particulate matter and post-combustion shoot).
In the studies on the direct synthesis of DME incorporating CO2 in the feed together with syngas, the attention has been focused on the effect of the reaction conditions and the composition of the catalyst on the yield and selectivity to DME (economic objective) [23,24,25,26,27,28,29,30,31,32,33], but without studying in detail the effect on the conversion of CO2 (environmental objective) and without analyzing the relation between both targets. As to the complex reaction scheme concerns (Equations (1)–(5)), it is observed that CO2 is both a reactant (Equation (2)), and a product of the WGS reaction (Equation (3)); and therefore, CO2 conversion does not have a direct relationship with the yield of DME, it depends on the reaction conditions, which must be determined experimentally.
The main objective of this work is to determine the appropriate conditions (temperature, pressure, space time, H2/COx, and CO2/CO molar ratios in the feed) to avoid the emission of CO2 and attain its effective conversion, as well as achieving a good compromise between DME production and CO2 conversion. The study has been conducted using a CuO-ZnO-MnO/SAPO-18 bifunctional catalyst, selected based on its good kinetic performance (activity, selectivity, and stability) in the direct synthesis of DME co-feeding CO2 [34].
2. Experimental
2.1. Catalyst Preparation and Characterization
The hybrid catalyst (CZMn/S-18) is composed of a CuO-ZnO-MnO (CZMn) metallic function, for methanol synthesis, and a SAPO-18 (S-18) acid function, for the selective dehydration of methanol to DME, with a mass ratio between the metallic and acid function of 2/1. The CZMn/S-18 hybrid catalyst is prepared by physical mixture of the metallic and acid functions, and it is then finely powdered, pelletized, crushed and sieved to the desired particle size (125–500 µm). The preparation conditions of both functions and their properties, along with the properties of the hybrid catalyst, have been detailed in previous works [34,35], and the most relevant properties have been summarized in Table 1. The regenerability of this catalyst by coke combustion with air has also been assessed [36]. Prior to each run, the catalyst is subjected to an in situ treatment in the reactor, aimed at reducing CuO species to Cu0. This treatment consists of a reduction in hydrogen atmosphere, in two steps: (i) reduction with H2 (10% diluted in N2) at 200 °C, for 14 h; (ii) reduction with H2 (20% diluted in N2) at 300 °C, for 1.5 h.

Table 1.
Physical and acid properties of the individual metallic and acid functions and of the bifunctional catalyst.
2.2. Reaction Equipment and Product Analysis
The automated reaction equipment used (PID Eng. and Tech. Microactivity) (Figure 1) is illustrated elsewhere [35]. It is provided with a high-pressure isothermal 316 stainless steel reactor, with an internal diameter of 9 mm and 10 cm of effective length. The reactor is located inside a ceramic chamber, heated by an electric resistance, and can operate up to 100 atm, 700 °C, and 5 g of catalyst. The catalyst is mixed with an inert solid (carborundum with 0.035 mm average particle size), to attain a sufficient bed height under low space time conditions, as well as to ensure the isothermality of the fixed-bed.
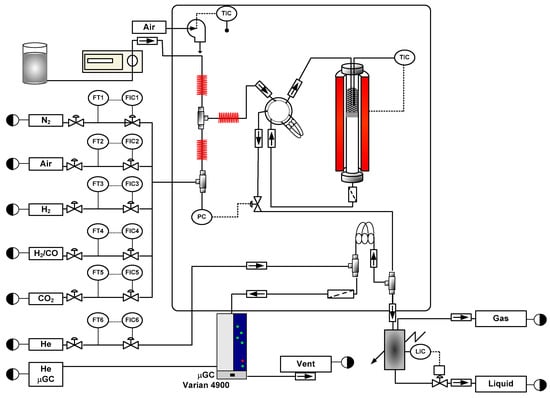
Figure 1.
Reaction equipment; FIC: Flow Indicator Controller; TIC: Temperature Indicator Controller; LIC: Level Indicator Controller; PC: Pressure Controller; FT: Flow Transmiter.
Reactant and reaction product samples (diluted in a He stream of 25 cm3 min−1) are continuously analyzed in a Varian-CP4900 gas micro-chromatograph provided with three analytical modules: (i) Porapak Q (PPQ) (10 m × 20 µm), where light products (CO2, methane, ethane, propane, methanol, DME, water and butanes) are separated; (ii) a molecular sieve (MS-5) (10 m × 12 µm) to separate H2, CO, O2 and N2; (iii) 5CB (CPSiL) (8 m × 2 µm), to identify the presence of possible C5–C10 fraction (not observed).
Runs have been carried out in a wide range of reaction conditions: temperature, 250–350 °C; pressure, 10–40 bar; space time, 1.25–20 gcat h (molC)−1; H2 + CO + CO2 feeds, with a H2/COx molar ratio of 3–4, and a CO2/CO molar ratio of 0–1.
2.3. Reaction Indices
The conversion of COx (CO + CO2) has been defined as the fraction of COx converted:
where and are the molar flow rates of COx in the feed and in the product stream, respectively.
The yield of each product has been determined as follows:
where ni is the number of C atoms in the i product, and Fi is the molar flow rate of the i product in the outlet stream.
Product selectivity (by mass unit of carbon) has been calculated as the ratio between the molar flow rate of the i compound and the sum of the molar flow rates of the organic compounds (DME, MeOH and C1–C3 hydrocarbons) in the outlet stream:
CO2 conversion has been defined as the mole fraction of CO2 converted into products:
where and are the molar flow rates of CO2 in the feed and in the outlet stream, respectively.
It should be noted that CO2 is a product of the WGS reaction (Equation (3)), and with the definition of Equation (9), a positive CO2 conversion indicates that a fraction of the CO2 co-fed together with syngas is converted into DME and methanol, and other by-products, avoiding CO2 emissions.
3. Results and Discussion
To simplify the exposition of the experimental results, those corresponding to certain conditions providing more useful information have been selected.
3.1. Effect of Temperature
The experiments focused on studying the effect of temperature on the reaction indices have been conducted feeding ternary mixtures of H2 + CO + CO2 with CO2/CO molar ratios between 1/3 and 1 under the following conditions: pressure, 30 bar; space time, 10 gcat h (molC)−1; H2/COx molar ratio, 3. Figure 2 shows the results of the effect of temperature over COx conversion (Figure 2a), product yield (Figure 2b) and CO2 conversion (Figure 2c). These results are shown as an example and correspond to a CO2/CO molar ratio of 1/3 in the feed. It is observed that the maximum COx conversion and DME yield (Figure 2a,b, respectively) are reached at 300 °C. These results differ from those obtained with the same catalyst feeding syngas (without CO2), where the maximum is attained within the 275–300 °C temperature range, and COx conversion and DME yield are higher, while methanol yield is lower than that shown in Figure 2b [35]. Moreover, it is also remarkable that methanol yield (Figure 2b) remains almost constant within the whole temperature range studied. This result is characteristic of a CO2/CO ratio of 1/3, as it has been proven (results not shown) that for higher values of this ratio, the yield of methanol shows a growing trend with temperature. On the other hand, when comparing these results with those obtained using syngas feeds [35], it is observed that paraffin yield diminishes slightly co-feeding CO2, being the decrease more evident for feeds richer in CO2.
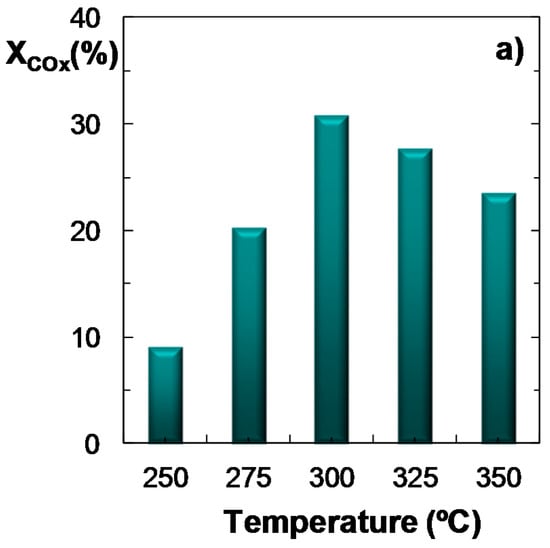
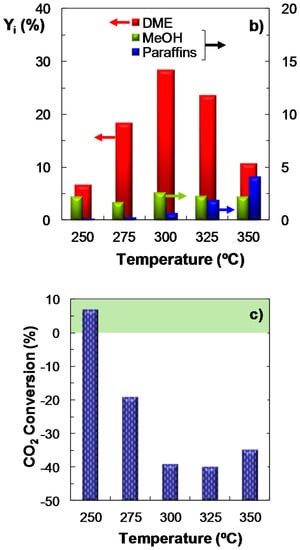
Figure 2.
Effect of temperature on the conversion of COx; (a) Product yield; (b) CO2 conversion (or CO2 yield, for negative values); (c) Reaction conditions: Feed, H2 + CO + CO2 with H2/COx molar ratio of 3, and CO2/CO molar ratio of 1/3; 30 bar; 10 gcat h (molC)−1.
When CO2 utilization is analyzed in Figure 2c, it is necessary to distinguish two regions: (i) the area where the net CO2 conversion is negative, that is, where there is formation of CO2, due to the predominant role of the reverse WGS reaction (reverse Equation (3)). In this case, the value will correspond (in absolute terms) to CO2 yield; and (ii) the area of positive CO2 conversion. In general, the effect of temperature over CO2 conversion shows the opposite trend to that of DME yield (Figure 2b). The results plotted in Figure 2c show a positive value of CO2 conversion at 250 °C (7.5%), while above this temperature there is a net formation of CO2, with a maximum at 325 °C. Regarding the study of the capability of the process for the utilization of CO2, it should be noted that temperatures below 250 °C have not been studied, as under these reaction conditions both COx conversion and DME yield are very low. On the other hand, it was observed that for CO2/CO ratios above 1/3 (results not shown) the effect of temperature on CO2 conversion is attenuated: (i) at 250 °C, the conversion of CO2 is similar to that obtained with a CO2/CO molar ratio of 1/3; (ii) at 275 °C, the CO2 conversion value is low; (iii) at 300 °C, the net CO2 formation is lower than that of CO2/CO = 1/3. It must also be noted, that the results correspond to per-pass conversion values. This conversion can therefore be increased operating on a larger scale, by recycling the (CO + CO2 + H2) gas stream, which are easily separated from the products (DME, methanol and water) by condensation. Note that this limitation of the per-pass conversion by thermodynamics is greater in the reaction of CO2 hydrogenation to methanol, which therefore, also requires the recycling of the unconverted reactant gases to improve the economics of the process [37].
3.2. Effect of Pressure
Figure 3 shows the influence of pressure (between 10–40 bar) on the reaction indices. The remaining operating conditions have been fixed as follows: Feed, ternary mixtures of H2 + CO + CO2 with CO2/CO molar ratios between 1/3 and 1; temperature, 275 °C; space time, 10 gcat h (molC)−1; H2/COx molar ratio, 3. The results indicate that COx conversion increases with pressure (Figure 3a), which is characteristic of the DME synthesis reaction, given that it involves a reduction in mole number. It is observed that, for the CO2/CO molar ratio used in the feed (2/3), an increase in pressure from 20 to 40 bar results in a very low improvement of the reaction indices. Hence, DME yield rises from 11.0% to 12.3% (Figure 3b). Moreover, under the operating conditions studied, the conversion of CO2 is positive (Figure 3c) in the whole pressure range, with a minimum within the 20–30 bar range. Nevertheless, it should be noted that for a CO2/CO ratio lower than that plotted in Figure 3 (that is, for CO2/CO molar ratios of 1/3), the effect of pressure on the reaction indices is more pronounced (results not shown). In consequence, it can be concluded that an increase in the CO2 content in the feed attenuates the favorable influence of pressure on the reaction indices.
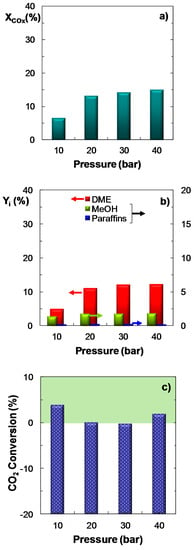
Figure 3.
Effect of pressure on the conversion of COx; (a) Product yield; (b) CO2 conversion (or CO2 yield, for negative values); (c) Reaction conditions: Feed, H2 + CO + CO2 with H2/COx molar ratio of 3, and CO2/CO molar ratio of 2/3; 275 °C; 10 gcat h (molC)−1.
The good kinetic performance of the catalyst for all the CO2/CO feeds studied (between 1/3 and 1) and under moderate pressures (20–30 bar) should be highlighted, as the results of COx conversion and DME yield are only scarcely lower than those obtained at 40 bar, with positive CO2 conversion. On the other hand, despite CO2 conversion is greater at 10 bar, as previously discussed, the other reaction indices are significantly lower under these conditions, in particular the yield of DME. In view of these results, it is concluded that a good balance among COx conversion, DME yield and selectivity and CO2 conversion is achieved within the 20–30 bar range.
3.3. Effect of Space Time
The effect of space time (in the 1.25–20 gcat h (molC)−1 range) on COx conversion, product yield and CO2 conversion has been studied under the following reaction conditions: Feed, ternary mixtures of H2 + CO + CO2 with CO2/CO molar ratios between 1/3 and 1; temperature, 275 °C; pressure, 30 bar; H2/COx molar ratio, 3. Results in Figure 4 show the influence of space time on the reaction indices for a ternary feed with a CO2/CO molar ratio of 2/3, as an example of the data obtained for the different feeds studied. It is observed that an increase in space time has a favorable effect on COx conversion (Figure 4a) and DME yield (Figure 4b). It should be highlighted that at these conditions CO2 conversion is positive within almost the entire range of space time studied (Figure 3c), with a maximum value (10%) at 2.5 gcat h (molC)−1.
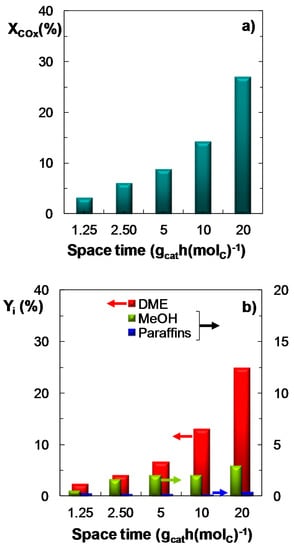
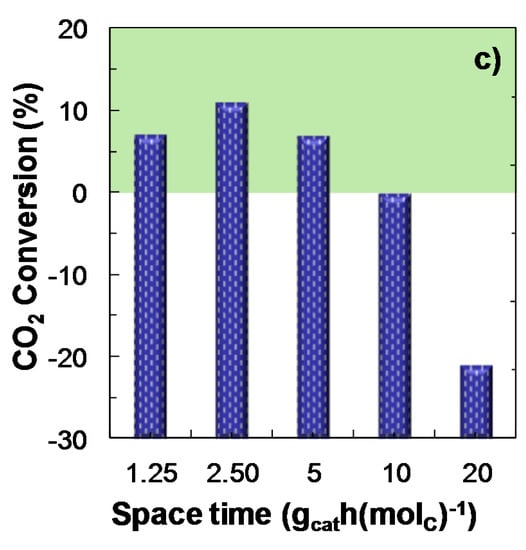
Figure 4.
Effect of space time on the conversion of COx; (a) Product yield; (b) CO2 conversion (or CO2 yield, for negative values); (c) Reaction conditions: Feed, H2 + CO + CO2 with H2/COx molar ratio of 3, and CO2/CO molar ratio of 2/3; 275 °C; 30 bar.
On the other hand, the comparison of these results in Figure 4 with those regarding the syngas feed [35] reveal that significantly higher values of space time are required when co-feeding CO2, to obtain similar values of COx conversion and DME yield. In addition, as observed when studying the influence of reaction temperature (Figure 2), pressure (Figure 3) and space time (Figure 4), under the conditions corresponding to the maximum value of CO2 conversion, low DME yield is attained. The explanation is that the unfavorable effect of the presence of CO2 in the feed over the WGS reaction (Equation (3)), leads to the attenuation of the methanol synthesis reactions (Equations (1) and (2)), and its subsequent dehydration to DME (Equation (4)), due to the increase of the water content in the reaction medium.
3.4. Effect of H2/COx Molar Ratio in the Feed
The influence of H2/COx molar ratio over the reaction indices has been studied, maintaining constant the remaining operating conditions in the following values: Feed, ternary mixtures of H2 + CO + CO2 with CO2/CO molar ratios between 1/3 and 1; temperature, 275 °C; pressure, 30 bar; space time, 10 gcat h (molC)−1. The main target is to assess the possible improvement in the reaction indices with a higher H2/COx molar ratio than that used in the preceding sections (3).
The results of COx conversion, DME yield and selectivity in Figure 5a (corresponding to feeds with a CO2/CO molar ratio of 1, taken as an example) point out that the improvement in these reaction indices when increasing H2 concentration in the feed from H2/COx = 3 to 4 is negligible. The same behavior was observed for the rest of the feeds studied (CO2/CO molar ratios of 1/3 and 2/3). However, the increase of H2/COx ratio above 3 is effective for increasing CO2 conversion (Figure 5b), since the transformation of CO2 to CO through the reverse WGS reaction (reverse Equation (3)), is favored.

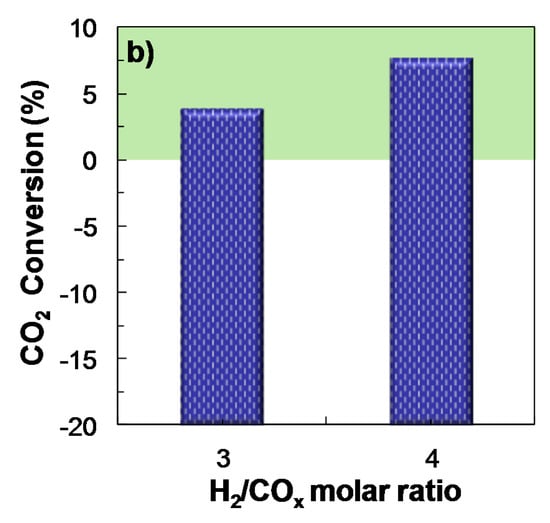
Figure 5.
Effect of H2/COx molar ratio in the feed on the conversion of COx and DME yield (a); CO2 conversion (or CO2 yield, for negative values) (b). Reaction conditions: Feed, H2 + CO + CO2 with CO2/CO molar ratio of 1; 275 °C; 30 bar; 10 gcat h (molC)−1.
3.5. Effect of CO2/CO Molar Ratio in the Feed
Figure 6 displays the effect of CO2/CO molar ratio in the feed (between 1/3 and 1) on COx conversion (Figure 6a), product yield (Figure 6b) and CO2 conversion (Figure 6c). The other operating conditions are: feed, ternary mixtures of H2 + CO + CO2 with H2/COx molar ratio of 3; temperature, 275 °C; pressure, 30 bar; space time, 10 gcat h (molC)−1. The results are consistent with those reported in the previous sections and reinforce the fact that an increase in the concentration of CO2 in the feed leads to a decrease in COx conversion and in DME yield, in good agreement with the results reported in the literature [25]. The upgrade of CO2 conversion with increasing CO2/CO ratio is also confirmed in Figure 6c.
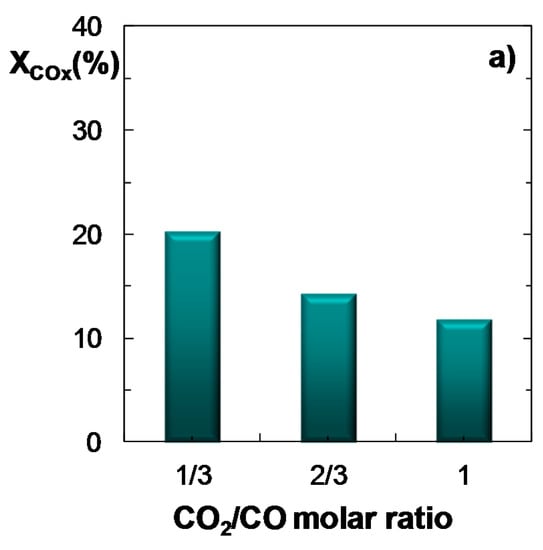
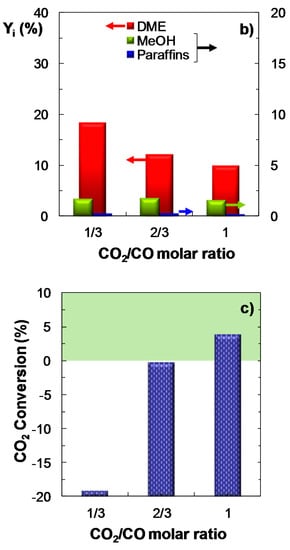
Figure 6.
Effect of CO2/CO molar ratio in the feed on the conversion of COx (a); Product yield (b); CO2 conversion (or CO2 yield, for negative values) (c). Reaction conditions: Feed, H2 + CO + CO2 with H2/COx molar ratio of 3; 275 °C; 30 bar; 10 gcat h (molC)−1.
When analyzing the effect of CO2/CO molar ratio on the conversion of CO2 for different values of space time (within the 2.5 and 10 gcat h (molC)−1 range), (Figure 7), it is observed that the importance of the favorable effect of decreasing space time on the conversion of CO2 depends on the CO2/CO ratio in the feed. Indeed, for low values of this ratio (1/3), the conversion of CO2 is significantly favored using low space time values. Nevertheless, this effect decreases when increasing CO2/CO ratio, that is, under operating conditions of higher CO2 conversion.
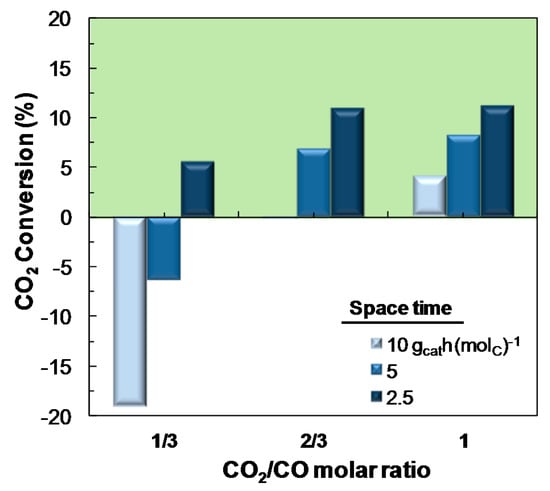
Figure 7.
Effect of CO2/CO molar ratio in the feed on the CO2 conversion (or CO2 yield, for negative values), for different space time values. Reaction conditions: Feed, H2 + CO + CO2 with H2/COx molar ratio of 3; 275 °C; 30 bar.
4. Conclusions
The results obtained with the CuO-ZnO-MnO/SAPO-18 catalyst evidence the capacity of the DME synthesis process in a single-step for the utilization of the CO2 co-fed with syngas. Given the complexity of the reaction system, wherein CO2 is both reactant and product, the suitable operating conditions for the conversion of CO2 correspond to those of minimum DME production. Thus, CO2 conversion in enhanced by decreasing temperature and increasing H2/COx molar ratio and CO2 content in the feed, while it goes through a minimum for intermediate pressure, and through a maximum for a relatively low space time value. These conditions favor (by means of the reverse WGS reaction) the formation of CO, more active than CO2 in the methanol synthesis reaction.
The catalyst used enables co-feeding CO2 under the suitable operating conditions for striking a good balance between the individual and opposing targets of CO2 conversion and DME production. In this study, we have determined the operating conditions ranges in which both objectives are achieved, producing DME with a negative balance of net CO2 emission (positive CO2 conversion) and under moderate reaction conditions (pressure and H2/COx ratio). This compromise is reached within the 275–300 °C range at moderate pressure (20–30 bar), which is interesting concerning the economic viability of the process. Under these conditions both objectives (environmental and economic) are attained using space time values between 2.5 and 5 gcat h (molC)−1 and a H2/COx molar ratio in the feed of 3. On the other hand, CO2/CO molar ratio in the feed is of great importance. Indeed, ratios below 1/3 are suitable for enhancing DME production, whereas CO2/CO ratios above 1 improve the conversion of CO2.
Author Contributions
A.A., J.E. and A.T.A. have conceived and designed the experimentes; A.A., M.S.-C. and P.P.U. performed the experiments; A.A., J.E., A.T.A. and J.B. analyzed the data and wrote the paper.
Acknowledgments
This work has been carried out with the financial support of the Ministry of Economy and Competitiveness of the Spanish Government (CTQ2013-46173-R), the FEDER funds, the Basque Government (Project IT748-13). Ainara Ateka and Miguel Sánchez-Contador are grateful for the Ph.D. grants from the Department of Education, University and Research of the Basque Government (BFI09.69 and PRE_2013_1_841, respectively).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| B/L | Brönsted/Lewis sites ratio. |
| , | COx (CO + CO2) and CO2 molar flow rates in the feed, respectively, mol h−1. |
| Fi, | Molar flow rates of the i component and COx (CO + CO2) in the reactor outlet stream, respectively, molC h−1. |
| ni | Number of carbon atoms of the i component. |
| SBET | BET specific surface area, m2 g−1. |
| Si | Selectivity of product i, Equation (8). |
| STD | Syngas to DME process. |
| Vmicropore, Vp | Micropore volume and total pore volume, respectively, cm3 g−1. |
| , | COx (CO + CO2) and CO2 conversions, respectively, Equations (6) and (9). |
| Yi | Yield of the i component, Equation (7). |
References
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yoon, S.H.; Ryu, B.W.; Lee, C.S. Combustion and emission characteristics of DME as an alternative fuel for compression ignition engines with a high pressure injection system. Fuel 2008, 87, 2779–2786. [Google Scholar] [CrossRef]
- Marchionna, M.; Patrini, R.; Sanfilippo, D.; Migliavacca, G. Fundamental investigations on di-methyl ether (DME) as LPG substitute or make-up for domestic uses. Fuel Process. Technol. 2008, 89, 1255–1261. [Google Scholar] [CrossRef]
- Liu, H.; Iglesia, E. Selective one-step synthesis of dimethoxymethane via methanol or dimethyl ether oxidation on H3+nVnMo12-nPO40 Keggin structures. J. Phys. Chem. B 2003, 107, 10840–10847. [Google Scholar] [CrossRef]
- Li, X.; San, X.; Zhang, Y.; Ichii, T.; Meng, M.; Tan, Y.; Tsubaki, N. Direct synthesis of ethanol from dimethyl ether and syngas over combined H-Mordenite and Cu/ZnO catalysts. ChemSusChem 2010, 3, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Al-Dughaither, A.S.; de Lasa, H. Neat dimethyl ether conversion to olefins (DTO) over HZSM-5: Effect of SiO2/Al2O3 on porosity, surface chemistry, and reactivity. Fuel 2014, 138, 52–64. [Google Scholar] [CrossRef]
- Pérez-Uriarte, P.; Ateka, A.; Gamero, M.; Aguayo, A.T.; Bilbao, J. Effect of the Operating Conditions in the Transformation of DME to olefins over a HZSM-5 Zeolite Catalyst. Ind. Eng. Chem. Res. 2016, 55, 6569–6578. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Shimoda, N.; Viriya-empikul, N.; Kikuchi, R.; Eguchi, K. Limiting mechanisms in catalytic steam reforming of dimethyl ether. Appl. Catal. B Environ. 2010, 97, 21–27. [Google Scholar] [CrossRef]
- Vicente, J.; Gayubo, A.G.; Ereña, J.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Improving the DME steam reforming catalyst by alkaline treatment of the HZSM-5 zeolite. Appl. Catal. B Environ. 2013, 130–131, 73–83. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Remiro, A.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Effect of Operating Conditions on Dimethyl Ether Steam Reforming over a CuFe2O4/γ-Al2O3 Bifunctional Catalyst. Ind. Eng. Chem. Res. 2015, 54, 9722–9732. [Google Scholar] [CrossRef]
- Dimethyl Ether (DME) Market Size, Share, Price, Report. 2024. Available online: https://www.gminsights.com (accessed on 4 April 2018).
- International DME Association. Available online: https://www.aboutdme.org/index.asp?bid=23 (accessed on 4 April 2018).
- Bhattacharya, S.; Kabir, K.B.; Hein, K. Dimethyl ether synthesis from Victorian brown coal through gasification—Current status, and research and development needs. Prog. Energy Combust. Sci. 2013, 39, 577–605. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Eden, M.R. Toward the development and deployment of large-scale carbon dioxide capture and conversion processes. Ind. Eng. Chem. Res. 2016, 55, 3383–3419. [Google Scholar] [CrossRef]
- Jia, G.; Tan, Y.; Han, Y. A comparative study on the thermodynamics of dimethyl ether synthesis from CO hydrogenation and CO2 hydrogenation. Ind. Eng. Chem. Res. 2006, 45, 1152–1159. [Google Scholar] [CrossRef]
- Sierra, I.; Ereña, J.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Deactivation kinetics for direct dimethyl ether synthesis on a CuO-ZnO-Al2O3/g-Al2O3 Catalyst. Ind. Eng. Chem. Res. 2010, 49, 481–489. [Google Scholar] [CrossRef]
- De Falco, M.; Capocelli, M.; Centi, G. Dimethyl ether production from CO2 rich feedstocks in a one-step process: Thermodynamic evaluation and reactor simulation. Chem. Eng. J. 2016, 294, 400–409. [Google Scholar] [CrossRef]
- Ateka, A.; Pérez-Uriarte, P.; Gamero, M.; Ereña, J.; Aguayo, A.T.; Bilbao, J. A comparative thermodynamic study on the CO2 conversion in the synthesis of methanol and of DME. Energy 2017, 120, 796–804. [Google Scholar] [CrossRef]
- van der Spek, M.; Sanchez Fernandez, E.; Eldrup, N.H.; Skagestad, R.; Ramirez, A.; Faaij, A. Unravelling uncertainty and variability in early stage techno-economic assessments of carbon capture technologies. Int. J. Greenh. Gas Control 2017, 56, 221–236. [Google Scholar] [CrossRef]
- Leeson, D.; Mac Dowell, N.; Shah, N.; Petit, C.; Fennell, P.S. A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources. Int. J. Greenh. Gas Control 2017, 61, 71–84. [Google Scholar] [CrossRef]
- Escudero, A.I.; Espatolero, S.; Romeo, L.M. Oxy-combustion power plant integration in an oil refinery to reduce CO2 emissions. Int. J. Greenh. Gas Control 2016, 45, 118–129. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Ereña, J.; Sierra, I.; Olazar, M.; Bilbao, J. Deactivation and regeneration of hybrid catalysts in the single-step synthesis of dimethyl ether from syngas and CO2. Catal. Today 2005, 106, 265–270. [Google Scholar] [CrossRef]
- Ereña, J.; Sierra, I.; Aguayo, A.T.; Ateka, A.; Olazar, M.; Bilbao, J. Kinetic modelling of dimethyl ether synthesis from (H2 + CO2) by considering catalyst deactivation. Chem. Eng. J. 2011, 174, 660–667. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lee, H.M.; Huang, M.H. One-step synthesis of dimethyl ether from the gas mixture containing CO2 with high space velocity. Appl. Energy 2012, 98, 92–101. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis chemistry of dimethyl ether synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Zhang, M.H.; Liu, Z.M.; Lin, G.D.; Zhang, H.B. Pd/CNT-promoted CuZrO2/HZSM-5 hybrid catalysts for direct synthesis of DME from CO2/H2. Appl. Catal. A 2013, 451, 28–35. [Google Scholar] [CrossRef]
- Liu, R.W.; Qin, Z.Z.; Ji, H.B.; Su, T.M. Synthesis of dimethyl ether from CO2 and H2 using a Cu-Fe-Zr/HZSM-5 catalyst system. Ind. Eng. Chem. Res. 2013, 52, 16648–16655. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Arena, F.; Frusteri, F. The changing nature of the active site of Cu-Zn-Zr catalysts for the CO2 hydrogenation reaction to methanol. Appl. Catal. B Environ. 2014, 152–153, 152–161. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Mezzapica, A.; Spadaro, L.; Arena, F.; Frusteri, F. Catalytic behaviour of a bifunctional system for the one step synthesis of DME by CO2 hydrogenation. Catal. Today 2014, 228, 51–57. [Google Scholar] [CrossRef]
- Qin, Z.; Su, T.; Ji, H.; Jiang, Y.; Liu, R.; Chen, J. Experimental and theoretical study of the intrinsic kinetics for dimethyl ether synthesis from CO2 over Cu-Fe-Zr/HZSM-5. AIChE J. 2015, 61, 1613–1627. [Google Scholar] [CrossRef]
- Frusteri, F.; Cordaro, M.; Cannilla, C.; Bonura, G. Multifunctionality of Cu-ZnO-ZrO2/H-ZSM5 catalysts for the one-step CO2-to-DME hydrogenation reaction. Appl. Catal. B Environ. 2015, 162, 57–65. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176–177, 522–531. [Google Scholar] [CrossRef]
- Ateka, A.; Sierra, I.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Performance of CuO-ZnO-ZrO2 and CuO-ZnO-MnO as metallic functions and SAPO-18 as acid function of the catalyst for the synthesis of DME co-feeding CO2. Fuel Process. Technol. 2016, 152, 34–45. [Google Scholar] [CrossRef]
- Ateka, A.; Pérez-Uriarte, P.; Sánchez-Contador, M.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Direct synthesis of dimethyl ether from syngas on CuO-ZnO-MnO/SAPO-18 bifunctional catalyst. Int. J. Hydrogen Energy 2016, 41, 18015–18026. [Google Scholar] [CrossRef]
- Ateka, A.; Pérez-Uriarte, P.; Sierra, I.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Regenerability of the CuO-ZnO-MnO/SAPO-18 catalyst used in the synthesis of dimethyl ether in a single step. React. Kinet. Mech. Catal. 2016, 119, 655–670. [Google Scholar] [CrossRef]
- Lange, J.P. Methanol synthesis: A short review of technology improvements. Catal. Today 2001, 64, 3–8. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).