Abstract
Panoramic radiography remains a cornerstone diagnostic tool in dentistry; however, its two-dimensional nature limits the visualisation of complex maxillofacial anatomy. Three-dimensional reconstruction from single panoramic images addresses this limitation by computationally generating spatial representations without additional radiation exposure or expensive cone-beam computed tomography (CBCT) scans. This systematic review and conceptual study traces the evolution of 3D reconstruction approaches, from classical geometric and statistical shape models to modern artificial intelligence-based methods, including convolutional neural networks, generative adversarial networks, and neural implicit fields such as Occudent and NeBLa. Deep learning frameworks demonstrate superior accuracy in reconstructing dental and jaw structures compared to traditional techniques. Building on these advancements, this paper proposes HoloDent3D, a theoretical framework that combines AI-driven panoramic reconstruction with real-time holographic visualisation. The system enables interactive, radiation-free volumetric inspection for diagnosis, treatment planning, and patient education. Despite significant progress, persistent challenges include limited paired 2D–3D datasets, generalisation across anatomical variability, and clinical validation. Continued integration of multimodal data fusion, temporal modelling, and holographic visualisation is expected to accelerate the clinical translation of AI-based 3D reconstruction systems in digital dentistry.
1. Introduction
The field of dental imaging is at a pivotal point in its technological development. Over the past century, dental radiography has advanced from basic film-based systems to sophisticated digital imaging modalities, with each development bringing incremental improvements in diagnostic capability and patient safety. Today, dentistry is experiencing a paradigm shift driven by the convergence of advanced imaging technologies and artificial intelligence, fundamentally transforming how clinicians visualise, interpret, and use radiographic information [1,2].
Panoramic radiography, now ubiquitous in contemporary practice, is the most widely used extraoral imaging technique in modern dentistry, with an estimated 10 million examinations performed annually in Japan, 1.5 million in England and Wales, and a notable increase in the United States from approximately 11 million in 1993 to 21 million in 2014–2015 [3,4]. Its ability to capture the entire dentomaxillofacial complex in a single exposure has made it an essential diagnostic tool across all dental specialties. However, panoramic radiography remains inherently limited by two-dimensional projection geometry, resulting in loss of depth information, structural superimposition, and measurement inaccuracies that challenge diagnostic precision and treatment planning [5,6]. While cone-beam computed tomography (CBCT) provides true three-dimensional visualisation, it requires significantly higher radiation exposure (50–1000 µSv vs. 2.7–24.3 µSv), incurs greater cost, and is less accessible in general practice settings [7].
Recent advances in artificial intelligence and deep learning, particularly neural network architectures employing implicit representations and generative models, have demonstrated remarkable capability in reconstructing three-dimensional structures from limited two-dimensional projections [8,9]. These computational approaches offer the possibility of extracting 3D anatomical information from conventional panoramic radiographs—a challenging inverse problem that could bridge the dimensional divide between accessible 2D imaging and clinically necessary 3D visualisation. Figure 1 illustrates typical diagnostic challenges in panoramic imaging, including structural superimposition, ghost artefacts, magnification distortion, and loss of depth information.
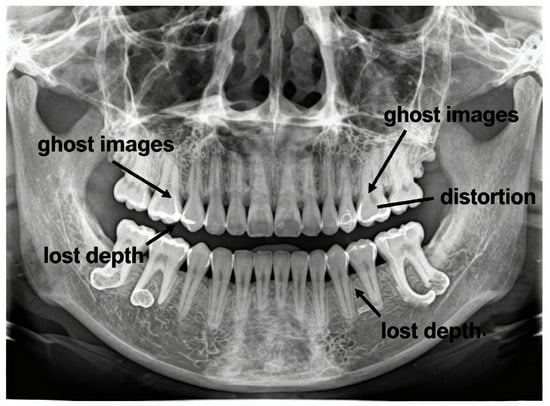
Figure 1.
Diagnostic limitations of panoramic radiography: ghost artefacts, geometric distortion, and loss of depth information.
Despite progress, current reconstruction methods achieve structural similarity indices of approximately 0.7 and accuracies of 75–86%, leaving clinically relevant depth errors and reliability concerns that limit routine clinical deployment [10].
1.1. Scope and Objectives of This Concept Paper
This concept paper provides a comprehensive review of the current state of three-dimensional reconstruction from panoramic dental radiographs, focusing on computational methods capable of generating volumetric anatomical information from single-view two-dimensional projections. It examines the evolution from classical geometric techniques to contemporary AI-driven approaches—including deep learning architectures, implicit neural representations, physics-based modelling, and hybrid methods that integrate data-driven learning with geometric or anatomical priors. Both models trained on paired panoramic–CBCT datasets and those using synthetic panoramic images derived from CBCT volumes are considered, as both address the core single-view reconstruction challenge.
This paper addresses three central research questions: (1) which methodological approaches have been developed for single-view 3D reconstruction in dental panoramic imaging, and what computational principles underpin them; (2) how these methods perform quantitatively and qualitatively, including the evaluation metrics and validation strategies used to assess reconstruction accuracy; and (3) what limitations and practical barriers currently hinder clinical translation, along with promising avenues for future research.
In addition to reviewing the state of the art, this paper introduces the HoloDent3D conceptual model—an innovative framework that integrates AI-based 3D reconstruction with holographic visualisation and gesture-based interaction. By bridging the gap between complex radiographic data and everyday clinical practice, HoloDent3D aims to offer a cost-effective, radiation-free alternative to cone-beam computed tomography that enhances diagnostic precision, patient engagement, and treatment planning while remaining compatible with existing dental workflows.
1.2. Structure of This Paper
This paper follows a systematic organisational structure divided into seven main sections. Section 1 introduces the clinical context and motivation, explaining limitations of panoramic radiography, the 3D reconstruction challenge, and the scope and objectives of the review. Section 2 covers the theoretical foundations, including panoramic radiography principles, mathematical foundations of 3D reconstruction, and relevant anatomical considerations. Section 3 provides a comprehensive search strategy of the literature. Section 4 systematically examines classical and geometric approaches, deep learning architectures, neural implicit representations, and state-of-the-art frameworks, such as Occudent, NeBLa, ViT-NeBLa, X2Teeth, PX2Tooth, Oral-3D/Oral-3Dv2, and multi-view or sequential approaches. Section 5 surveys existing applications of holographic and augmented reality technologies in dentistry for education, treatment planning, and surgical navigation. Section 6 introduces the HoloDent3D conceptual model, describing its objectives, system architecture with three stages (input acquisition and preprocessing, AI-driven 3D reconstruction, and holographic visualisation), and feasibility considerations including technical challenges related to data availability, integration, latency, calibration, and regulation. Section 7 discusses clinical applicability, integration scenarios, ethical and regulatory considerations, and research challenges for future development.
2. Fundamentals of Panoramic Radiography and 3D Reconstruction
2.1. Panoramic Radiography Principles
Panoramic radiography operates through the synchronised rotation of the X-ray tube and detector around the patient’s head, capturing the curved dental arch in a single exposure. The X-ray beam is collimated into a narrow vertical slit that sweeps across the jaws as the system rotates, producing a three-dimensional, horseshoe-shaped focal trough—typically 15–30 mm thick—where structures appear sharp and areas outside become blurred [11,12]. Acquisition takes about 12–20 s, and modern digital systems use solid-state sensors for immediate image capture and processing. Figure 2 illustrates the rotating X-ray source, detector, and focal trough geometry during the scan.
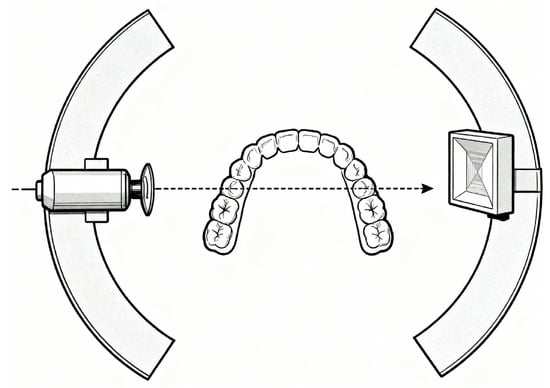
Figure 2.
Panoramic radiographic acquisition geometry showing synchronous rotation of X-ray source and detector around the patient, capturing the curved dental arch within a focal trough and producing spatially varying projections.
The projection geometry is highly complex: each image column corresponds to a distinct instantaneous projection angle, creating spatially variable magnification (typically 1.2–1.3×) across the image. X-ray attenuation follows the Beer–Lambert law, so each detector value represents cumulative attenuation along the curved ray path without spatial depth information—a key limitation for three-dimensional reconstruction [13].
Common artefacts further complicate image interpretation. Dense structures outside the focal trough can produce ghost images, while incorrect head positioning causes distortions such as tooth widening or the “smile/frown” curvature of the occlusal plane. Patient movement introduces motion blur, and limited spatial resolution (two to five line pairs per millimetre) restricts fine detail visibility. Superimposition of soft tissues and air spaces over dental anatomy adds further ambiguity, as panoramic imaging lacks the volumetric separation capability of CBCT [14].
2.2. Mathematical Foundations of 3D Reconstruction
Three-dimensional reconstruction from projections traditionally relies on geometric triangulation, where corresponding points across multiple views define intersecting rays that localise spatial positions. In single-view reconstruction, this geometric constraint is absent, requiring statistical inference to estimate the most probable 3D configuration from a single observation using anatomical priors. Shape-from-shading provides limited cues, as image intensities reflect cumulative X-ray attenuation related to tissue density and thickness, but identical intensities can result from multiple density–thickness combinations or overlapping structures [15].
Panoramic projection maps 3D points to image coordinates based on continuously changing source–detector geometry during rotation. The Beer–Lambert law describes the transmitted intensity of a ray passing through tissue with a spatially varying attenuation coefficient along path length L:
where is the incident intensity. Reconstruction therefore requires inverting this relationship to recover the spatial distribution of . Modern deep learning methods bypass explicit inversion, learning this mapping directly from data. Implicit neural representations parameterise 3D structure as continuous functions mapping coordinates to density values, efficiently capturing curved anatomical geometries [16].
Single-view reconstruction remains mathematically ill-posed: infinitely many 3D structures can yield identical 2D projections, and small input variations can cause large output differences. Regularisation—via prior knowledge or learned data distributions—constrains solutions to anatomically plausible configurations. Deep models achieve this implicitly through statistical learning, though performance depends heavily on dataset quality and representativeness. Ultimately, the feasibility of single-view reconstruction depends on whether these embedded assumptions adequately compensate for the inherent information loss of projection [17].
2.3. Relevant Anatomical Considerations
The dental arches form parabolic or horseshoe-shaped curves, with substantial inter-individual variation. In panoramic projection, varying distances from the centre of rotation cause position-dependent magnification and distortion. The mandibular canal, which contains neurovascular structures, follows a variable course across individuals, posing both clinical and reconstruction challenges [18].
The maxilla is anatomically more complex due to its proximity to the nasal cavity, maxillary sinuses, and orbital floor. Air-filled regions adjacent to dense bone generate high-contrast boundaries, while overlapping soft tissues—tongue, palate, and airway—add ambiguity to bone delineation. Tooth anatomy further complicates reconstruction through intricate geometry and material heterogeneity: enamel is the most radiopaque tissue, dentine exhibits intermediate attenuation, and pulp chambers appear radiolucent. The periodontal ligament space, only 0.1–0.3 mm wide, approaches the resolution limits of panoramic imaging [11,19].
Tooth orientation and bone morphology introduce additional variability. Anterior teeth incline labially, posterior teeth tilt mesially, and cortical bone appears as high-intensity bands overlying less dense trabecular bone. Bone density varies regionally—denser in the mandibular symphysis and thinner in the maxilla [20].
Age-related resorption following tooth loss reduces alveolar dimensions, while pathological changes such as periodontal bone loss, cysts, or tumours alter attenuation characteristics. Metallic restorations (amalgam, crowns, implants) produce bright streak artefacts that obscure adjacent anatomy and distort projection geometry, complicating accurate reconstruction [21].
3. Search Strategy
A comprehensive and systematic search strategy was developed to capture the full scope of contemporary research in three-dimensional reconstruction relevant to dental imaging and computational modelling. To ensure broad coverage across medical, technical, and interdisciplinary fields, multiple academic databases and scholarly platforms were consulted. The primary sources included PubMed, Scopus, Web of Science, Google Scholar, Nature Portfolio, Elsevier, ACL Anthology, SpringerOpen, arXiv, IEEE Xplore, and MDPI. These repositories were selected to encompass the biomedical literature, engineering and computer vision research, open-access scientific outputs, and preprints representing the latest methodological developments.
The search strategy included general terminology related to 3D reconstruction technology, with the core search terms including the following:
- Panoramic radiography OR panoramic X-ray.
- Three-dimensional reconstruction OR 3D teeth reconstruction OR 3D reconstruction in dentistry OR teeth reconstruction.
- Cone-beam computed tomography.
- Radiography or panoramic.
- Dental implants.
- Deep learning dentistry OR artificial intelligence dental.
- Neural implicit panoramic.
- Holography OR holography dentistry.
- Augmented reality in dentistry.
- AI fairness biomedicine OR fairness medical AI.
- FDA AI/ML medical devices.
- Bias medical image analysis.
- European Data Protection Board AI personal data.
No restrictions on publication date were applied. Searches included all available records up to November 2025.
3.1. Inclusion and Exclusion Criteria
This review included peer-reviewed scientific publications from journals, conference proceedings, symposia, and preprint servers. Eligible studies were required to address single-view 3D reconstruction of dental structures (teeth, jaws, oral cavity) derived primarily from panoramic radiographs. Priority was given to studies evaluating deep learning frameworks (including CNNs, transformers, GANs, and neural implicit representations), as well as those exploring holographic or mixed-reality applications in dentistry. Works involving paired PX–CBCT datasets, synthetic panoramic projections, or methodological and algorithmic developments relevant to 3D reconstruction technologies were also included.
Excluded were non-dental applications, multi-view or intraoral scan methods without a panoramic focus, microscopic or cellular imaging, materials science unrelated to reconstruction, studies lacking quantitative validation (e.g., no metrics such as IoU, Dice, or Chamfer distance), and pure simulation papers without real PX evaluation or without specifying the underlying technology.
3.2. Data Collection and Extraction Process
All retrieved articles were initially evaluated by title and abstract. If these provided sufficient indication that a study met the review objectives, a full-text assessment was conducted. In addition to methodological relevance, several qualitative indicators were considered, including publication date, journal scope, study design, and overall scientific robustness. Any disagreements were resolved through discussion. The complete selection pathway, from identification to inclusion, is summarised in the flow diagram shows in Figure 3.
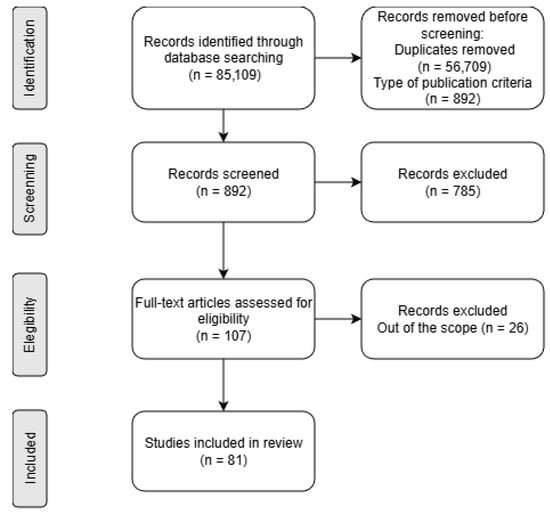
Figure 3.
A PRISMA flow diagram of the systematic review.
4. Review of 3D Reconstruction Methods
4.1. Classical and Geometric Approaches
Early three-dimensional dental reconstruction methods relied on geometric constraints and statistical shape models derived from anatomical databases. These models represented 3D structures as deformations of a mean template using principal component analysis, capturing typical crown shapes, root curvatures, and proportional relationships across tooth classes. Reconstruction was achieved by fitting deformable templates to 2D panoramic images through parameter adjustment to match contours and intensity distributions [7,22]. Figure 4 shows a standard single-view 3D reconstruction pipeline, from panoramic input to AI-driven mesh generation and volumetric output.
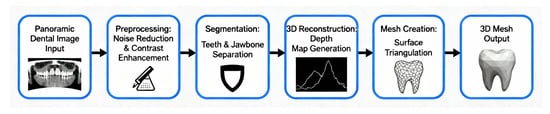
Figure 4.
Single-view 3D reconstruction from panoramic radiographs.
Template fitting extended this approach through libraries of 3D tooth and jaw models registered to panoramic radiographs by feature correspondence. Landmarks identified in 2D images were matched to 3D template points and aligned using iterative closest point algorithms or thin-plate spline warping [23]. Interpolation-based methods reconstructed 3D surfaces from segmented tooth boundaries by extrusion or rotation under symmetry assumptions. Dental arch geometry was approximated via spline interpolation of tooth positions, and root trajectories were estimated through bilateral symmetry [12,24].
Despite their innovation, these classical methods faced major limitations. Statistical shape models lacked patient-specific accuracy, template libraries could not encompass full anatomical diversity, and interpolation relied on oversimplified smoothness assumptions. Extensive manual intervention—landmarking, template selection, and tuning—further limited clinical practicality [14].
4.2. Deep Learning Architectures
Convolutional neural networks (CNNs) revolutionised three-dimensional dental reconstruction by learning feature representations directly from paired data rather than relying on hand-crafted geometric models. Early CNN-based approaches treated reconstruction as a regression task, predicting 3D coordinates, voxel occupancy, or depth maps from panoramic inputs. Hierarchical convolutions extracted progressively complex features—from edges and textures to complete tooth morphologies—enabling the mapping between two- and three-dimensional representations [25].
Encoder–decoder architectures became dominant, in which encoders compressed spatial information through convolution and pooling, while decoders reconstructed volumetric outputs via transposed convolutions or interpolation. Skip connections preserved fine spatial detail lost during compression [16].
Generative adversarial networks (GANs) introduced a complementary paradigm: a generator synthesises 3D reconstructions from panoramic inputs, while a discriminator distinguishes them from real CBCT scans. This adversarial learning drives anatomically realistic reconstructions, with the discriminator acting as an implicit quality evaluator [17]. Figure 5 summarises the evolution of single-view dental 3D reconstruction—from geometric and statistical models to CNN, GAN, and neural implicit representations such as NeRF and occupancy networks.
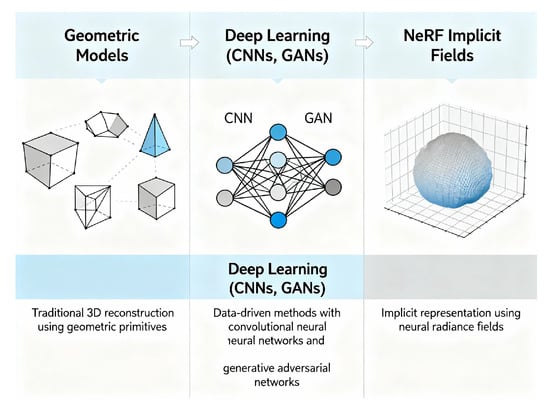
Figure 5.
Evolution of single-view dental 3D reconstruction methods.
Recently, transformer architectures have emerged as powerful alternatives to purely convolutional designs. Self-attention enables long-range dependency modelling, capturing relationships between distant structures such as opposing molars or jaw landmarks. Vision Transformers (ViTs) process panoramic patches through attention layers, while hybrid CNN–ViT architectures combine local pattern extraction with global context modelling [26].
These deep learning approaches outperform classical methods, achieving structural similarity indices of 0.7–0.8 and mean surface errors of 1–2 mm. However, their performance depends on large paired panoramic–CBCT datasets, which remain limited, and generalisation to unseen anatomical or imaging variations continues to pose challenges [27].
4.3. Neural Implicit Representations
Implicit neural representations have transformed three-dimensional reconstruction by modelling geometry as continuous functions rather than discrete voxel grids or meshes. These methods parameterise a neural network that maps 3D coordinates to occupancy probability, signed distance, or density, enabling continuous, resolution-independent reconstruction [28].
Signed distance functions (SDFs) predict, for each point, the minimum distance to the nearest surface, with the sign indicating whether the point is inside or outside. The zero level set defines the surface, providing smooth topology and efficient extraction via marching cubes [29]. Occupancy networks similarly predict binary inside–outside values, with separate models often representing teeth, mandible, and maxilla, trained for consistency with 3D ground-truth data from panoramic inputs [30].
Neural radiance fields (NeRFs), adapted from novel view synthesis, model spatial coordinates as density and colour values under volumetric rendering principles consistent with X-ray attenuation physics. The Beer–Lambert law provides explicit physical grounding; the NeBLa model integrates this attenuation law into its architecture, comparing predicted and observed intensities to improve generalisation and reduce data requirements [13].
Implicit representations offer continuous, memory-efficient geometry capable of capturing curved dental arches [31], but they remain computationally intensive. Each spatial query requires a network evaluation, training is sensitive to initialisation, and the resulting surfaces may appear overly smooth, missing fine anatomical details [29].
4.4. State-of-the-Art Reconstruction Frameworks
The rapid development of deep learning techniques has significantly influenced modern dental imaging, providing new opportunities for automated diagnosis, image enhancement, and three-dimensional reconstruction. By learning hierarchical and context-aware representations from radiographic data, deep neural networks can extract clinically relevant information from modalities such as cone-beam computed tomography (CBCT), panoramic radiographs, and intraoral scans with high accuracy and efficiency. Recent advances, particularly in convolutional and transformer-based architectures, have enabled improved modelling of dental structures, disease detection, and cross-modality integration, thereby reducing manual intervention and inter-operator variability. This subsection presents an overview of representative deep learning frameworks and their applications in dental imaging, outlining their methodological foundations, improvements over conventional approaches, and remaining challenges in clinical deployment.
4.4.1. Occudent: Neural Implicit Functions for Teeth Reconstruction
Occudent [10] presents a novel framework for reconstructing three-dimensional tooth models from standard two-dimensional panoramic radiographs (PX) using neural implicit functions, marking the first approach to generating accurate 3D dental structures directly from real clinical images. Unlike previous methods such as X2Teeth and Oral-3D, which relied on synthetic PX generated from CBCT projections, Occudent was trained and evaluated on real radiographs, ensuring greater clinical relevance. The framework consists of two components: a multi-label 2D segmentation network based on an enhanced UNet++ that identifies and classifies all 32 tooth classes, and a 3D reconstruction network built on an implicit representation that predicts an occupancy function to determine whether each sampled 3D point lies inside or outside the tooth volume.
The condition vector (c) combines a tooth class embedding and a tooth patch embedding, integrated via the Conditional eXcitation (CX) module proposed by the authors. CX fuses these embeddings—encoding both the 2D patch and class identity—by extending the Squeeze-and-Excitation mechanism to modulate latent features according to conditional vectors, enabling more precise and class-aware 3D shape generation.
Evaluation on paired PX–CBCT datasets demonstrates Occudent’s superiority over state-of-the-art methods (3D-R2N2, Pix2Vox, PSGN, X2Teeth, OccNet) across all major metrics, including volumetric IoU (0.651), Chamfer-L1 distance (0.298), normal consistency, and reconstruction precision. The model effectively reproduces detailed dental anatomy, including root structures, while benefiting from the memory efficiency and continuity advantages of implicit shape representations over voxel-based approaches.
In conclusion, Occudent advances clinical and educational applications by enabling accurate, robust, and scalable 3D dental reconstructions from widely available 2D imaging. Its architecture provides a foundation for future developments in multimodal integration and neural optimisation, promoting greater precision and personalisation in digital dentistry.
4.4.2. NeBLa: Neural Beer–Lambert for Oral Structures
Park et al. (2024) introduced NeBLa (Neural Beer–Lambert), a physics-inspired neural framework for three-dimensional reconstruction of oral and dental structures from a single real panoramic radiograph (PX) [13]. Unlike previous methods such as X2CT-GAN and Oral-3D, which rely on synthetic PX–CBCT pairs or geometric priors, NeBLa reconstructs full volumetric CBCT-like data directly from unpaired real radiographs. Its central innovation combines X-ray attenuation physics, described by the Beer–Lambert law, with neural implicit modelling. The transmitted intensity of an X-ray beam through tissue with attenuation coefficient along path (l) is given by
where is the incident intensity, I the detected intensity, the local attenuation coefficient, and l the path length of the X-ray beam. In discrete form, this relationship is approximated as
This physics-based simulation links 2D radiographic appearance to the underlying 3D structure, enabling the network to learn spatially consistent volumetric features. Trained on CBCT volumes and unpaired real PX images, NeBLa achieved a Dice coefficient 15% higher and perceptual error 30% lower than X2CT-GAN, with PSNR and SSIM improvements of 10% and 6%, respectively. Qualitatively, it produced smoother, anatomically coherent reconstructions of crowns, roots, and jawbone structures.
By embedding the Beer–Lambert model into a neural rendering framework, NeBLa establishes a new paradigm for radiograph-based 3D dental reconstruction—offering a low-dose, data-efficient, and clinically viable alternative to CBCT. It demonstrates the synergy between physical modelling and neural implicit representations, paving the way for non-invasive, cost-effective, real-time 3D visualisation in dental and medical imaging.
4.4.3. ViT-NeBLa (Vision Transformer-Based Neural Beer–Lambert)
ViT-NeBLa [15] introduces a state-of-the-art framework for single-view three-dimensional reconstruction of oral anatomy from panoramic radiographs (PX). Building on the previously proposed NeBLa framework, it integrates Vision Transformers (ViT) with implicit neural representations guided by the Beer–Lambert law (Equation (3)), ensuring that simulated panoramic projections (SimPX) accurately model X-ray attenuation.
The ViT-NeBLa architecture consists of four main modules: (1) a hybrid ViT–CNN feature extractor that combines global contextual encoding with local texture refinement; (2) a learnable multi-resolution hash positional encoder for compact spatial embeddings; (3) a NeRF-inspired implicit field predictor that estimates voxel-level density distributions; and (4) a 3D U-Net refinement module that enhances structural continuity and fine anatomical detail. A horseshoe-shaped ray-sampling scheme confines computation to the jaw region, reducing sampling density from 200 to 96 points per ray and halving processing time compared to NeBLa, without loss of accuracy.
Trained on an expanded dataset of more than 600 real and synthetic PX–CBCT pairs, ViT-NeBLa achieved approximately 1.3 dB higher PSNR, 3% higher SSIM, 7–8% higher Dice coefficient, and about 10% lower perceptual error (LPIPS) compared to the original NeBLa. Qualitative results further demonstrate smoother and more anatomically coherent reconstructions of teeth, alveolar bone, and mandibular regions.
By uniting global transformer-based feature encoding with a physics-informed Beer–Lambert formulation, ViT-NeBLa represents a major advancement in radiograph-based 3D reconstruction, achieving greater accuracy and efficiency than previ- ous approaches.
4.4.4. X2Teeth and Single-Image Reconstruction Systems
The X2Teeth framework [32] introduces a convolutional neural network (ConvNet) for reconstructing complete three-dimensional dental structures directly from a single panoramic radiograph (PX). Using an encoder–decoder architecture, a two-dimensional encoder extracts panoramic features, while a decoder predicts the corresponding three-dimensional voxel-based tooth geometries. Unlike previous object-level reconstruction methods, X2Teeth simultaneously reconstructs all teeth within the full oral cavity at high resolution.
The method decomposes the task into two core steps:
- Teeth localisation—dividing the panoramic image into patches centred on individual teeth, enabling patch-based training focused on local context.
- Shape estimation—predicting a 3D voxel grid or mesh for each tooth patch, combining local coherence with global structure through joint optimisation and multi- class segmentation.
Model training minimises a composite loss combining 2D segmentation loss and 3D reconstruction loss, both formulated using the Dice coefficient to measure overlap between predicted and ground-truth volumes.
Experimental evaluation demonstrated that X2Teeth substantially outperforms generic single-view reconstruction models such as 3D-R2N2 and DeepRetrieval. Quantitatively, it achieved a mean Intersection-over-Union (IoU) of , exceeding those of 3D-R2N2 by and DeepRetrieval by , while attaining a per-tooth segmentation IoU of and reconstruction IoU of . Qualitative analysis confirmed that X2Teeth recovers anatomically coherent crown and root morphology with smooth surface continuity and accurate interproximal spacing.
Overall, X2Teeth is the first end-to-end learning framework to reconstruct anatomically consistent 3D dental structures from a single panoramic radiograph, establishing a foundation for radiation-free 3D modelling applicable in diagnosis, orthodontic planning, and digital dental education.
4.4.5. PX2Tooth
PX2Tooth [33] builds on the foundations established by X2Teeth by introducing a two-stage deep learning framework for accurate three-dimensional (3D) reconstruction of dental structures from a single panoramic radiograph (PX). This approach replaces voxel-based volumetric prediction with direct 3D point cloud generation, reducing memory consumption and enabling finer geometric detail. The architecture explicitly separates the tasks of tooth localisation and 3D geometry synthesis, making the reconstruction process more interpretable and anatomically constrained.
The framework comprises two key modules:
- PXSegNet—a U-Net-based segmentation network that performs per-tooth classification into 32 categories according to the FDI numbering system.
- TGNet—a point-based generator that reconstructs 3D tooth geometries using segmentation priors and panoramic image features.
PXSegNet is optimised using two complementary segmentation losses: the Metric Boundary (MB) Loss and the Unbalanced (UB) Loss, which, respectively, improve edge precision and class balance.
The Metric Boundary Loss is defined as
where C is the number of tooth classes, N is the number of pixels, is the ground =0truth label of pixel i for class c, and is the predicted probability for the same pixel and class.
This loss emphasises boundary regions by maximising overlap between predicted and true edges for each class.
The Unbalanced Loss further adjusts training focus towards classes with fewer samples, mitigating imbalance by down-weighting easy examples:
where is a focusing parameter that reduces the weight of well-classified samples.
Together, these two losses enhance segmentation stability and fine-grained boundary accuracy.
In the reconstruction stage, TGNet generates tooth point clouds from localised segmentation patches using a Prior Fusion Module (PFM) that aligns 2D image features with 3D spatial embeddings.
Training uses a Reconstruction Loss (RT Loss) based on bidirectional point set distances:
where A and B are the predicted and ground-truth point clouds and m and n are the number of points in each cloud.
This loss enforces geometric consistency by minimising the average squared distance between corresponding points across both sets.
Experimental validation on 499 paired panoramic–CBCT scans demonstrates that PX2Tooth achieves a mean Intersection-over-Union (IoU) of , outperforming previous models such as X2Teeth and Occudent by up to . Qualitative evaluations further show that PX2Tooth reconstructs smoother, topologically consistent tooth morphologies, with improved continuity at root boundaries compared to voxel-based methods.
Overall, PX2Tooth represents a major advance in single-image 3D dental reconstruction, offering a radiation-free, data-efficient, and clinically viable approach for digital dentistry and orthodontic applications.
4.4.6. Oral-3D/Oral-3Dv2
The Oral-3D framework [34] introduces a two-stage generative adversarial network (GAN)-based method for reconstructing the three-dimensional bone structure of the oral cavity from a single panoramic radiograph (PX), requiring only minimal prior information about dental arch geometry. Unlike cone-beam computed tomography (CBCT), which provides complete 3D data but involves higher radiation exposure and greater equipment requirements, Oral-3D uses deep learning to infer depth-related anatomical information from 2D images.
In the first stage, a back-projection module uses a generator–discriminator pair to map 2D panoramic images to flattened 3D density volumes, employing the least-squares GAN (LSGAN) formulation and stabilised by the following objectives:
where G is the generator, D the discriminator, x the input PX image, and y the ground-truth 3D volume. A voxel-wise reconstruction term further constrains the generator output to match the real CBCT-derived target:
The second stage, a deformation module, warps the flattened 3D volume along the estimated dental arch to restore the natural curvature of the mandible and maxilla. Experimental results on CBCT-derived datasets show that Oral-3D outperforms 3D-R2N2 and Pix2Vox, achieving 7–10% higher PSNR, 8–9% higher SSIM, and up to 13% improvement in Dice coefficient. The reconstructed 3D models accurately represent dental arches, alveolar bone density, and tooth morphology, providing a radiation-free and cost-effective alternative to CBCT.
The improved Oral-3Dv2 framework [9] addresses the data dependency and efficiency limitations of the original model through a physics-informed implicit neural representation. Its Neural X-ray Field (NeXF) architecture learns continuous 3D density distributions directly from real panoramic images and known X-ray trajectories, eliminating the need for voxel-based supervision and reducing computational complexity from cubic to quadratic order. Incorporating the Beer–Lambert attenuation law and a dynamic ray-sampling strategy enhances anatomical accuracy and surface smoothness. Empirical results show SSIM gains of about five points and overall reconstruction improvements exceeding 7%, establishing Oral-3Dv2 as a more data-efficient, physically consistent, and clinically applicable framework for single-image 3D dental reconstruction.
4.4.7. Multi-View and Sequential Approaches
While most state-of-the-art frameworks for three-dimensional reconstruction from panoramic radiographs rely on a single projection, recent developments highlight the potential of integrating additional imaging sources and temporal information. Multi-view and sequential approaches extend single-image models such as Occudent, NeBLa, and X2Teeth by combining panoramic radiographs with intraoral photographs, surface scans, or longitudinal image sequences. This fusion enhances geometric completeness and surface accuracy, and it also enables temporally consistent reconstructions suitable for treatment monitoring and dynamic anatomical analysis. The following subsections review current methodologies for cross-view fusion, temporal regularisation, and feature-level correspondence across heterogeneous dental image modalities.
Multi-View Fusion of Panoramic and Intraoral Images
The integration of multimodal dental imaging—particularly the fusion of panoramic radiographs with intraoral scans—offers a promising approach to improving the geometric accuracy and completeness of 3D dental reconstruction. Panoramic radiographs provide a comprehensive anatomical overview but lack the local surface detail captured by intraoral photographs or 3D surface scans. Recent multimodal frameworks demonstrate that combining these complementary modalities enhances reconstruction by jointly leveraging volumetric and surface cues.
Liu et al. [35] proposed Deep Dental Multimodal Fusion, a neural architecture that encodes cone-beam computed tomography (CBCT) volumes and intraoral meshes into a shared latent space, enabling bidirectional translation and surface refinement through feature-level fusion. This approach improves reconstruction accuracy and surface precision while reducing dependence on high-radiation CBCT imaging.
Jang et al. [36] developed a fully automatic pipeline for registering full-arch intraoral scans with CBCT data using hierarchical alignment and learned correspondence descriptors. Although both frameworks use CBCT as the volumetric modality, their principles can be extended to combine panoramic radiographs with intraoral images, reducing reliance on CBCT acquisition.
Sequential and Temporally Consistent Reconstruction
Temporal consistency is essential in dental image analysis, particularly for longitudinal monitoring of orthodontic or periodontal changes. Sequential registration ensures that morphological variations—such as tooth movement, bone remodelling, and soft tissue resorption—are accurately represented across imaging sessions.
Ogawa et al. [37] developed a framework for registering panoramic radiographs acquired at different time points. Their method combines global non-linear normalisation of the dental arch using fourth-order Lagrange polynomial interpolation with local alignment based on normalised cross-correlation, compensating for geometric distortions caused by patient repositioning. Quantitative evaluation uses logarithmic intensity difference maps to visualise localised bone density changes over time.
Rodríguez et al. [38] extended this approach by introducing a longitudinal intraoral ultrasound registration framework to track soft and hard tissue changes across multiple sessions. The method integrates rigid and deformable registration guided by intensity-based similarity metrics and temporal smoothness constraints, ensuring consistent spatial alignment and reliable assessment of progressive dental changes while preserving temporal and anatomical coherence.
Cross-View Feature Matching and Co-Training
Cross-view feature matching and co-training have become key strategies for integrating heterogeneous dental modalities—panoramic radiographs, intraoral photographs, and 3D mesh models—into unified reconstruction and analysis frameworks. Luo et al. [39] introduced the Dual-View Co-Training Network (DVCTNet), which uses dual-stream pre-training to jointly optimise panoramic and tooth-level representations, and a Gated Cross-View Attention mechanism for dynamic fusion between global and local features. Emulating clinical reasoning that combines panoramic overview with local inspection, this approach achieved up to +4.4% improvement in AP75 accuracy, demonstrating the effectiveness of cross-view co-training in dental analysis.
To improve representation learning with limited data, Almalki and Latecki [40] applied self-supervised masked image modelling (SimMIM and UM-MAE) to panoramic radiographs, achieving 13.4% higher tooth detection accuracy and 12.8% better restoration segmentation. Their results show that contrastive and self-supervised learning foster shared latent embeddings that support cross-modal generalisation.
At the 3D level, Zhao et al. [41] proposed a Two-Stream Graph Convolutional Network that fuses geometric and attribute features via shared multilayer perceptrons and attention-based graph operations, aligning 3D shape and surface texture between intraoral scans and other modalities. Similarly, Hsung et al. [42] developed an image-to-geometry registration pipeline that maps 2D intraoral photographs onto virtual 3D tooth models, enabling visually enhanced reconstructions without manual alignment.
Mohamed et al. [25] extended this multi-view paradigm to 2D dental imagery using an EfficientNet-based encoder to synthesise volumetric 3D reconstructions from multiple 2D projections, improving geometric completeness and depth estimation.
Collectively, these studies demonstrate that cross-view feature projection and contrastive co-training in a shared embedding space significantly enhance robustness and generalisation across heterogeneous dental data sources. By leveraging complementary 2D and 3D cues, such frameworks enable anatomically consistent, data-efficient, and scalable solutions for multimodal dental reconstruction and diagnostic modelling.
4.4.8. Comparative Analysis and Selection Criteria
Occudent
The Occudent framework is trained on a single-centre paired panoramic–CBCT dataset acquired using one scanner family, with patients drawn from a limited regional population. The original paper does not provide a detailed breakdown of age, sex, or ethnicity distributions. Reported results rely on internal train, validation, and test splits of this dataset; no true external validation on an independent site or different hardware is described, which increases the risk that performance is partly driven by scanner-specific intensity characteristics and the local case mix. Consequently, there is a plausible risk of overfitting to the acquisition protocol, device vendor, and prevalent dental conditions at the source institution, so accuracy may degrade when applied to populations with different craniofacial morphology, disease prevalence, or imaging workflows [10,43].
NeBLa and ViT-NeBLa
NeBLa and ViT-NeBLa combine CBCT-derived simulated panoramic images (SimPX) from a single CBCT dataset with real panoramic radiographs from a limited number of clinical sites, again with limited reporting of detailed patient demographics. Although the unpaired training scheme reduces the need for matched PX–CBCT pairs, both studies primarily evaluate performance on data from the same institutions and scanner types used to construct SimPX, and they do not report formal external validation on fully independent cohorts. This design can embed biases related to specific CBCT protocols, reconstruction kernels, and regional patient characteristics; domain shifts in beam geometry, reconstruction software, or population anatomy at deployment sites may therefore cause larger than expected decreases in reconstruction fidelity [13,43].
X2Teeth
X2Teeth is trained on a curated set of matched panoramic radiographs and CBCT volumes from a single hospital, with patients primarily undergoing comprehensive dental treatment. However, the publication provides only coarse age statistics and no ethnicity information. Validation is performed using internal cross-validation or held-out subsets from the same acquisition pipeline, with no multi-centre external test set, which limits conclusions about generalisability beyond the original scanner configuration and referral patterns. As a voxel-based ConvNet, X2Teeth can overfit to scanner-dependent noise patterns, grey-level distributions, and occlusal plane positioning; models tuned to this narrow domain may perform suboptimally on images from other vendors, paediatric cohorts, or public health screening programmes with different disease spectra [32,43].
PX2Tooth
PX2Tooth uses 499 paired panoramic–CBCT cases, collected from a limited set of clinical sources. The authors note variability in dental conditions but do not provide detailed demographic stratification. Performance is evaluated on an internal test split from the same pool of scans; there is no dedicated external validation cohort from a different institution, region or scanner, so potential overfitting to local imaging physics and annotation style remains a concern. As PX2Tooth replaces voxel grids with point clouds conditioned on segmentation outputs, it may be particularly sensitive to scanner-specific contrast, resolution and artefact profiles that affect segmentation quality. This could introduce systematic performance degradation in under-represented patient groups or on devices with different pixel sizes and exposure protocols [33,43].
Oral-3D and Oral-3Dv2
Oral-3D and its successor Oral-3Dv2 are trained on paired panoramic–CBCT datasets focused on oral bone reconstruction, typically sourced from one or a few academic centres, with limited reporting of demographic diversity and inclusion of atypical anatomies. Validation experiments use internal splits and cross-validation; to date, there is no published large-scale external validation across multiple scanners or international sites, which limits the ability to assess robustness under varying acquisition geometries, metallic artefact burdens, and ethnic craniofacial patterns. Given their GAN-based architecture, these models may inadvertently model scanner- and site-specific texture statistics as “realistic”, increasing the risk that reconstructions appear plausible but are anatomically inaccurate when applied to populations or devices not represented in the training data [43,44].
Within the scope of this paper, Occudent and NeBLa received more detailed mathematical treatment because they exemplify two complementary, theoretically grounded paradigms that are directly relevant to the HoloDent3D concept: class-conditioned neural implicit shape functions for tooth-level occupancy (Occudent) and physics-informed volumetric reconstruction guided by the Beer–Lambert law (NeBLa and ViT-NeBLa). These frameworks explicitly encode the panoramic projection geometry and X-ray attenuation model, allowing their assumptions, limitations, and possible extensions to be analysed in a principled manner and integrated with the reconstruction module of HoloDent3D. In contrast, voxel-based ConvNets such as X2Teeth, hybrid point-cloud methods like PX2Tooth, and GAN-based systems such as Oral-3D and Oral-3Dv2 primarily rely on empirical optimisation and task-specific loss design. While methodologically important, their mathematical foundations are closer to standard deep learning practice and thus require less extensive formal exposition in the context of this concept paper. A comparison of the previously mentioned and meticulously described models is presented in Table 1.

Table 1.
Comparative model characteristics. The symbol “↑” indicates an increase or improvement of the corresponding metric compared to the referenced baseline method.
Selection Criteria and Suitability for HoloDent3D
For HoloDent3D, the main requirements are as follows: (i) robust single-view 3D reconstruction from real clinical panoramic images, (ii) compatibility with heterogeneous scanners, and (iii) efficiency suitable for near real-time holographic rendering. Under these constraints, physics-informed implicit methods such as NeBLa and ViT-NeBLa are particularly attractive because they embed the Beer–Lambert projection model explicitly, which improves data efficiency and provides a clear pathway to adapt the learned field to different scanner geometries via calibration rather than full retraining. Occudent and PX2Tooth offer strong tooth-level geometry and high IoU on paired datasets, making them promising candidates for detailed dental surface reconstruction.
Consequently, a hybrid strategy appears most suitable: using a NeBLa/ViT-NeBLa as the primary jaw and bone-level reconstruction engine, augmented by Occudent or PX2Tooth-style tooth-specific modules for high-resolution crown and root surfaces in regions of interest. This combination aligns well with HoloDent3D’s goal, which targets interactive holographic exploration rather than submillimetre surgical navigation, and balances reconstruction fidelity, data availability, and computational cost in a way that is realistic for deployment on GPU-equipped workstations connected to holographic fan displays.
It is also important to note that although methods such as NeBLa and ViT-NeBLa are highly promising, reconstructing a full 3D anatomy from a single panoramic radiograph remains extremely ambitious. Depth, bone density, and the anatomical precision of both bone and teeth all push the limits of what is feasible without CBCT, so robust validation is essential. Moreover, the quality and diversity of the training dataset are critical. Simulation-based approaches, such as SimPX, rely on a sufficiently representative CBCT pool to achieve clinically reliable generalisation. Computational demands also remain a major factor, as implicit volumetric and ray-tracing-based models (NeBLa/ViT-NeBLa) are intensive during both training and inference. For practical, real-time clinical deployment, substantial optimisation will be necessary, including improved sampling strategies, quantisation, and possibly lightweight model variants. Finally, even the most accurate reconstructions may still contain errors, so clear clinical boundaries must be established to determine when the reconstructed 3D models are acceptable for treatment planning (e.g., implants, orthodontics) and when CBCT remains mandatory.
5. Review of Holography and Mixed Reality in Dentistry
Holography and mixed reality (MR) have become transformative technologies in digital dentistry, enabling the integration of virtual three-dimensional (3D) data with the clinical environment. Early medical applications of holography [45] demonstrated its potential as a non-contact, high-resolution imaging method for visualising anatomical structures with exceptional spatial accuracy. Building on this foundation, dentistry increasingly employs augmented reality (AR) and MR systems to enhance diagnosis, treatment planning, and education.
Recent studies confirm the benefits of immersive visualisation. Lin et al. [46] reported that AR and VR training systems improve procedural precision and learning efficiency through real-time feedback and simulation, while Rosu et al. [47] achieved submillimetre accuracy (), 30% faster workflows, and 37% fewer errors in AR-assisted prosthodontic and implant procedures compared to conventional methods. Despite challenges such as hardware cost, calibration complexity, and lack of standardisation, holography and MR represent a decisive step towards precision-guided, patient-specific, and interactive dentistry.
Dolega-Dolegowski et al. [48] presented a proof of concept using holographic and AR technology to visualise internal dental root structures for educational purposes with the Microsoft HoloLens 2 platform (Figure 6 (image created in Perplexity AI Pro)). The workflow involved model creation in Autodesk Maya, export to Unity 3D for interactive scripting, and gesture-based control via HoloLens sensors, allowing real-scale holographic visualisation of teeth. Users could manipulate models, explore internal canals, and inspect root anatomy from multiple perspectives, linking theoretical knowledge with spatial perception.
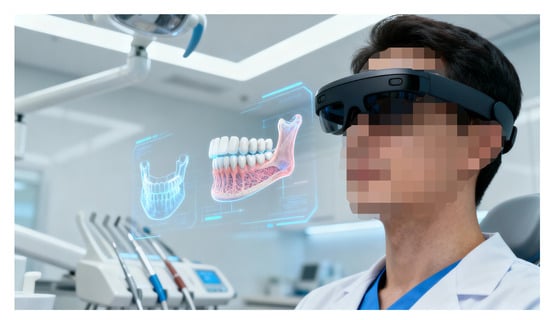
Figure 6.
HoloLens-based mixed-reality workflow for interactive 3D visualisation and manipulation of patient-specific dental anatomy.
A pilot study involving 12 participants (6 dental students and 6 clinicians) evaluated eight holographic models representing different Vertucci canal types. Results showed high user satisfaction—91% found the system superior to conventional 2D or screen-based 3D renderings—and reported improved understanding of spatial relationships within root canal systems. The authors concluded that holography and AR provide effective educational tools, enhancing perception of complex anatomical structures and supporting manual skill development and 3D reasoning in endodontic practice.
Talaat et al. [49] evaluated the reliability of a holographic augmented reality (AR) system for three-dimensional (3D) superimposition of digital dental models using the Microsoft HoloLens, compared with a conventional computer-based application. The study included 20 orthodontic patients (mean age years) treated with rapid maxillary expansion using Hyrax palatal expanders. Pre- and post-treatment digital maxillary models were acquired with a high-resolution Ortho Insight 3D laser scanner (20 m resolution) and imported into Ortho Mechanics Sequential Analyzer (OMSA) software for 3D superimposition based on midpalatal raphe landmarks.
Two analysis methods were compared: conventional 2D computer visualisation and holographic AR display through HoloLens. Three anatomical landmarks were placed along the midpalatal raphe—one at the distal end of the incisive papilla and two posteriorly—by the same operator to avoid inter-operator variability. Accuracy and consistency were assessed using Dahlberg’s measurement error, Bland–Altman limits of agreement, and the concordance correlation coefficient (CCC).
Results showed that HoloLens-based superimposition achieved accuracy statistically equivalent to computer-based analysis, with mean differences ranging from −0.18 mm to +0.17 mm and no significant bias. Intra-operator reliability was also high, comparable to that of traditional software. The holographic 3D display provided enhanced depth perception and intuitive spatial manipulation without loss of precision, confirming its potential for orthodontic analysis. Identified limitations included the limited computational power and memory of the HoloLens, potential rendering delays for complex models, and the need for further software optimisation.
Akulauskas et al. [50] investigated the feasibility and accuracy of using augmented reality (AR) for dental implant surgery with the Microsoft HoloLens 2 headset. The study aimed to determine whether AR-based navigation could achieve precision comparable to conventional computer-guided implant systems and to identify limitations of current hardware and calibration procedures.
Dynamic navigation systems such as Navident and X-Guide, while highly accurate, require surgeons to look away from the operative field to view external monitors, disrupting workflow. In contrast, AR technology allows navigation data to be displayed directly within the operator’s field of vision.
A custom 3D-printed dental model and a “”-shaped calibration marker were used as spatial references for holographic alignment. Calibration and alignment tests were conducted under static (headset fixed) and dynamic (headset worn) conditions using four reference points, with accuracy assessed by comparing predefined virtual and physical positions.
Overall distance deviations averaged , with precision around , and statistically significant variation across calibration points. Angular deviation between virtual and real models averaged , influenced by tracking stability and marker recognition. Virtual model trueness was in dynamic mode and in static mode, with significant differences confirmed using the Kruskal–Wallis and Dunn’s post hoc tests ().
Figure 7 summarises accuracy data from multiple studies comparing mixed-reality (MR)-guided dental procedures with conventional methods, highlighting spatial deviations (mm) and angular errors (°) that demonstrate the precision gains achieved through MR guidance.
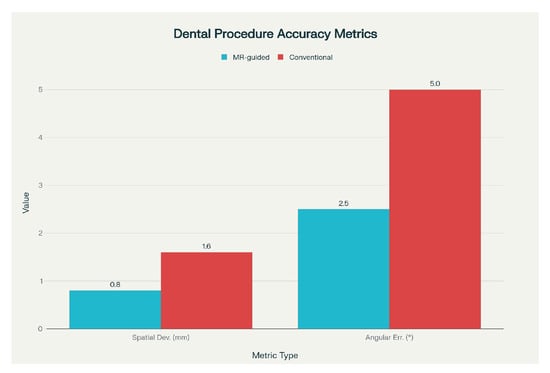
Figure 7.
Comparison of spatial deviation and angular error metrics for mixed-reality—guided versus conventional dental procedures.
Although AR currently achieves lower precision than established dynamic navigation systems (typically positional accuracy and angular deviation), the authors conclude that it holds strong potential as an educational or auxiliary tool in dental implantology. With advancements in hardware, calibration, and tracking algorithms, AR may achieve the precision required for clinical use.
Xue et al. [51] conducted a proof-of-concept study to assess whether mixed reality (MR) could enhance scaling and root planing (SRP) outcomes and improve patient understanding. The workflow involved acquiring intraoral scans and cone-beam CT data from 10 patients with advanced periodontitis. These datasets were fused into detailed three-dimensional models of teeth, gingiva, and alveolar bone, which were rendered in Microsoft HoloLens 2 for interactive manipulation (resizing, rotation, cross-sectional segmentation) and intuitive clinician–patient communication.
MR-guided SRP resulted in significant clinical improvements after eight weeks. Mean probing pocket depth (PPD) decreased from to , and clinical attachment loss (CAL) from to (). The plaque index (PI) declined from to , and bleeding on probing (BOP) from 78.92% to 36.40%. For sites with PPD , PPD and CAL reductions were and , respectively, with a 43.86% decrease in BOP.
The authors emphasised MR’s advantages in visualising subgingival regions, reducing dependence on tactile feedback, and improving instrumentation accuracy. However, they noted limitations including equipment cost, hygiene challenges, limited training, operator fatigue, and high power consumption. With continued hardware development and cost reduction, MR could be integrated into periodontal therapy, telemedicine, and training applications. Future randomised studies are recommended to quantitatively compare MR-assisted and conventional SRP for long-term efficacy.
Zhang et al. [52] conducted a randomised controlled split-mouth clinical trial to assess the short-term efficacy of mixed-reality holographic-imaging-based scaling and root planing (MR-SRP) compared with conventional scaling and root planing (C-SRP) in patients with generalised stage III periodontitis. The prospective, single-blinded study included 20 patients and integrated cone-beam computed tomography (CBCT) and intraoral scans to generate interactive 3D periodontal models displayed via HoloLens 2, allowing real-time manipulation of anatomical structures during SRP.
Both treatments resulted in significant clinical improvement at three months (), with no statistically significant differences between groups. Mean probing pocket depth (PPD) reduction was for MR-SRP and for C-SRP, while clinical attachment loss (CAL) improved by and , respectively (). The proportion of healed sites (transition from PPD with bleeding on probing (BOP) to PPD without BOP) reached 75.68% for MR-SRP and 72.69% for C-SRP, reflecting an approximate 4% relative improvement. Non-molar teeth showed higher healing rates than molars (80.44% vs. 52.01%), highlighting anatomical challenges in multirooted regions.
Reported limitations included the small sample size, short 3-month follow-up, lack of long-term evaluation, and high equipment cost. MR registration was not directly overlaid on patients during procedures to prevent visual interference, and CBCT radiation exposure remains a concern for routine therapy. The authors recommend larger multi-centre studies and software–hardware optimisation to enhance performance and cost-efficiency.
In conclusion, MR-SRP demonstrated slightly superior short-term healing outcomes compared with conventional SRP and shows potential as an effective adjunctive tool for managing severe periodontitis.
Fan et al. [53] introduced a mixed reality (MR)-guided dental implant navigation system (MR-DINS) designed to improve implant placement accuracy and efficiency by aligning preoperative plans with the surgical field in real time. The system integrates a HoloLens 2 device with an optical tracking system (NDI Polaris Spectra) and custom registration tools to align virtual 3D models reconstructed from CBCT data with the physical patient phantom.
The architecture combines optical tracking with MR visualisation using three core algorithms: HoloLens—tracker registration, image—phantom registration, and surgical drill calibration. Each uses a homogeneous transformation matrix to express coordinate transformations between virtual and physical reference frames:
where is the rotation matrix and the translation vector. For any spatial point P, the relationship between coordinate systems and is defined as
Transformations are estimated using the Singular Value Decomposition (SVD) registration algorithm, which minimises squared distances between corresponding fiducial points.
Experiments on 30 patient models with 102 implants showed a mean coronal deviation of , apical deviation of , and angular deviation of . Mean fiducial registration error (FRE), target registration error (TRE), drill calibration error (DCE), and HoloLens registration error (HRE) were all below 1 mm, confirming submillimetre calibration precision. Performance matched that of dynamic navigation systems and was 30–40% more accurate than freehand implant placement while eliminating hand–eye coordination issues typical of previous AR/MR systems.
In conclusion, the MR-DINS framework achieved high accuracy and usability in dental implant navigation, supported by rigorous mathematical modelling and validated through phantom experiments.
Grün et al. [54] introduced a smartphone-based augmented reality (AR) navigation system for guiding dental implant placement, offering a low-cost and accessible alternative to conventional computer-assisted implant surgery (CAIS) systems, which require expensive hardware and complex calibration. The study aimed to determine whether consumer-grade mobile technology could deliver clinically acceptable accuracy for implant navigation.
The AR workflow integrated cone-beam computed tomography (CBCT), intraoral scans, facial photographs, and occlusal data into a fully digital patient model. Treatment planning was performed in Romexis 6.0.1.812 software, and virtual implant positions were exported in Filmbox (FBX) format for use in a custom AR application developed for a Samsung Galaxy S22 smartphone. Using the Vuforia engine for spatial tracking, the app overlaid implant trajectories directly on the patient’s anatomy via a holographic display, eliminating the need for additional navigation equipment.
The system was validated on a 3D-printed phantom and subsequently applied in a clinical case involving a 52-year-old male requiring replacement of four anterior maxillary teeth. Four BEGO S-Line implants () were placed following the AR-guided plan. Postoperative CBCT comparison showed a mean apical deviation of and an angular deviation of , with three implants within clinically acceptable tolerance.
Compared with freehand placement, the AR-assisted workflow improved spatial accuracy by approximately 50–60%. Additional advantages include affordability, integration with standard digital workflows, and usability on widely available consumer devices. Reported limitations include sensitivity to lighting conditions, ergonomic challenges of handheld operation, and dependence on stable tracking for reliable overlay.
Grad et al. [55] investigated the use of Microsoft HoloLens-based augmented reality (AR) and three-dimensional (3D) printed anatomical tooth models in dental education, focusing on the accuracy of occlusal anatomy reconstruction during restorative procedures. The study compared AR holograms with physical 3D-printed models to evaluate accuracy, efficiency, and user experience in teaching dental morphology. Anatomical models of three molars were reconstructed from CBCT scans using InVesalius and Autodesk Meshmixer, then either 3D printed or displayed holographically via HoloLens.
Thirty-two participants performed cavity fillings under three conditions: without reference (M1), with a 3D-printed model (M2), and with an AR holographic model (M3). Accuracy was quantified using the maximum Hausdorff distance () between reconstructed and reference models. Significant differences were observed (), with the lowest mean for 3D-printed models (630 m), followed by AR (760 m) and no reference (850 m). The AR system enabled faster task completion and easier spatial orientation, but users reported reduced comfort due to the headset’s weight and incompatibility with magnification loupes.
In subjective evaluation, 78% of participants preferred 3D-printed models for comfort and effectiveness, while AR was viewed as innovative but less ergonomic. The authors conclude that lighter AR hardware and higher display resolution could improve comfort and precision, promoting broader adoption of holographic teaching aids in dental curricula.
While the reviewed studies consistently demonstrate improvements in spatial understanding, task efficiency, and user satisfaction, a critical evidence gap remains. Current evaluations rely predominantly on subjective perception metrics, workflow timing, and landmark accuracy, yet none of the included works directly measure patient-centred or clinical outcomes such as treatment success rates, patient comprehension of procedures, long-term oral health behaviour, or overall quality of care. Evidence from the broader AR/VR/MR dental literature suggests potential for increased patient comprehension and satisfaction, and possibly reduced anxiety; however, these findings are largely based on self-reported impressions rather than objective clinical endpoints [51,56,57]. Therefore, although holography and mixed reality appear highly promising for education, planning, and navigation, further research is needed to determine whether enhanced visualisation truly translates into better treatment outcomes, improved safety, and sustained patient benefit in real-world dental practice.
6. Conceptual Framework of the HoloDent3D Model
HoloDent3D currently exists as a conceptual framework at TRL 2-3. No physical prototype has been implemented. This section presents the theoretical architecture and planned development pathway.
6.1. Concept and Objectives
The HoloDent3D model offers a conceptual framework for an advanced dental imaging system that integrates artificial intelligence, computer vision, and holographic visualisation to redefine how clinicians interpret, explain, and communicate diagnostic information. Its central aim is to transform conventional two-dimensional orthopantomogram (OPG) radiographs into dynamic three-dimensional holographic reconstructions of the jaw and dental structures, viewable in real time and manipulable through intuitive, gesture-based interaction. This integration of AI-driven 3D reconstruction and holographic display seeks to bridge the gap between complex radiographic data and human spatial perception, creating a more natural, immersive, and informative diagnostic experience.
At the core of the concept is an automated reconstruction pipeline that uses deep learning algorithms to identify and segment anatomical features from standard panoramic dental X-rays. These features are then used to generate accurate 3D representations that preserve the anatomical fidelity of teeth, bone, and surrounding structures. In doing so, the system directly addresses a critical limitation of current imaging practices: the compression of three-dimensional information into a two-dimensional format, which often obscures spatial relationships and complicates diagnosis.
The intended benefits of the HoloDent3D concept span both clinical and communicative dimensions. For dental professionals, holographic visualisation supports improved diagnostic precision, clearer identification of pathological conditions, and enhanced treatment planning by enabling real-time manipulation—rotation, zooming, and cross-sectional viewing—of reconstructed structures. For patients, the system provides an accessible, visually engaging understanding of their oral health, making it easier to comprehend the nature of medical findings, proposed interventions, and expected outcomes. This transparency is expected to foster stronger dentist–patient trust, greater involvement in treatment decisions, and improved adherence to recommended care. Figure 8 (image created in PerplexityPro) presents different clinical scenarios for mixed reality applications: (a) diagnostic consultation with holographic imaging, (b) treatment planning with 3D reconstruction overlays, (c) patient education using interactive anatomical visualisations, and (d) intraoperative surgical guidance with AR-assisted precision.

Figure 8.
Clinical use cases of augmented and mixed reality in dentistry, including holographic diagnostics, 3D treatment planning, patient education, and AR-guided procedures.
A further objective is to ensure accessibility and practical integration into existing dental workflows. Unlike conventional 3D imaging solutions that require costly cone-beam computed tomography (CBCT) scanners or specialised augmented reality setups, the HoloDent3D model builds upon routinely acquired OPG data and cost-efficient holographic fan display technology. This combination allows for affordable implementation without disrupting standard diagnostic procedures.
In essence, HoloDent3D envisions a transformative yet feasible step towards intelligent, interactive dental imaging. By merging AI-driven reconstruction with holographic visualisation and gesture-based control, the concept aims to enhance diagnostic clarity, optimise patient engagement, and lay the foundation for a new generation of human-centred, data-informed dental care.
6.2. System Concept and Architecture
The HoloDent3D system architecture is designed as a modular, multi-stage processing pipeline that transforms conventional two-dimensional orthopantomogram radiographs into interactive three-dimensional holographic representations. Figure 9 illustrates the high-level system architecture of HoloDent3D as a horizontal pipeline. Arrows indicate the flow of information, including a feedback loop from gesture recognition back to the rendering pipeline. Only major functional blocks are shown; internal model details, device specifications, and sensor/training information are intentionally omitted to focus on overall system flow.
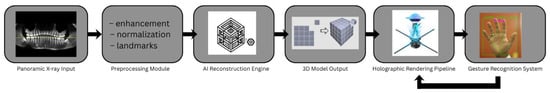
Figure 9.
Modular HoloDent3D system pipeline showing data flow from panoramic input through AI-based 3D reconstruction to holographic rendering and gesture-driven interaction.
In this work, we adopt the U-Net architecture introduced in [58] as the backbone for our segmentation module. Among the architectures we evaluated, this variant delivered the most reliable and consistent performance for the type of structural delineation required in our setting. Its design consists of a U-shaped encoder–decoder network with a ResNet-34 encoder and a five-stage decoder, providing a well-balanced compromise between representational capacity and computational efficiency.
The encoder follows the standard ResNet-34 configuration, while the decoder comprises five decoding layers, each containing two convolutional layers followed by batch normalisation and ReLU activation. Input images are resized to (other datasets) and then randomly cropped to for training.
The enhanced U-Net described in [58] introduces two key architectural modules:
- Semantic Feature Enhancement Module (SFEM): Attached to the top of the final encoding layer (which outputs an feature map). It processes the features through three parallel branches operating on patch sizes of , and the full resolution, thereby capturing richer multi-scale semantic context and increasing feature discriminability.
- Adaptive Global Context Module (AGCM): Replaces standard skip connections. At each scale, it integrates information from the corresponding encoder layer, the previous decoder layer, and the SFEM output. This selective fusion mechanism filters background noise and injects global contextual cues, improving boundary coherence and segmentation stability.
Given the nature of our task—where accurate regional separation and structural consistency are critical—this enhanced U-Net configuration (shown on Figure 10) provides a strong architectural fit. We therefore implement this specific variant in our system without modification, using it as the primary segmentation engine within the HoloDent3D pipeline.
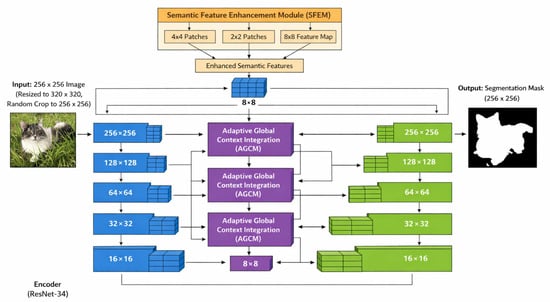
Figure 10.
Enhanced U-Net architecture: encoder features are refined via AGCM for skip connections, deepest features are enriched by SFEM and injected into all decoder stages, and each decoder stage includes an auxiliary prediction head [58].
The proposed pipeline consists of three primary stages: input acquisition and preprocessing, AI-driven 3D reconstruction, and holographic visualisation with gesture-based interaction. Figure 11 presents the three-stage architecture of the HoloDent3D system: Stage 1 illustrates input acquisition and preprocessing of panoramic images; Stage 2 shows AI-driven 3D reconstruction with neural network components; and Stage 3 depicts holographic visualisation combined with a gesture-based interaction interface for clinical use.
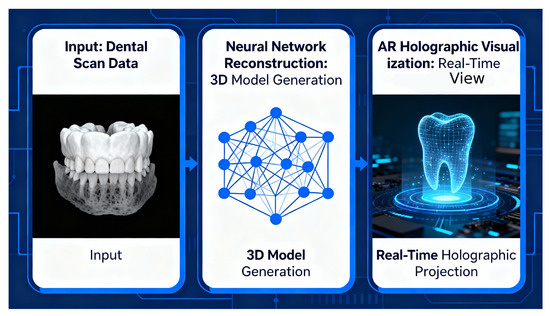
Figure 11.
Three-stage HoloDent3D pipeline from panoramic input through neural 3D model reconstruction to real-time holographic dental visualisation.
Stage 1: Input acquisition and preprocessing. The pipeline begins with the acquisition of a standard orthopantomogram (OPG) radiograph, which serves as the sole imaging input. Orthopantomograms are routinely captured in dental practices using panoramic X-ray systems and provide comprehensive coverage of dental and maxillofacial structures in a single two-dimensional projection.
During preprocessing, the acquired OPG image undergoes quality enhancement and normalisation to optimise subsequent analysis. This includes contrast adjustment, noise reduction, and standardisation of image dimensions and resolution. Advanced image processing algorithms are used to identify and segment key anatomical landmarks, including tooth boundaries, root structures, bone margins, and potential pathological regions. The preprocessing module also performs coordinate system transformation to prepare the 2D image data for three-dimensional reconstruction.
Stage 2: AI-driven 3D reconstruction module. The core innovation of the HoloDent3D system lies in its reconstruction module, which uses artificial intelligence and computer vision techniques to transform the preprocessed 2D orthopantomogram into a detailed three-dimensional model of the jaw and dental structures.
The reconstruction process employs deep learning algorithms trained on datasets containing paired 2D radiographs and corresponding 3D anatomical models. Specifically, the system utilises generative algorithms capable of learning the complex mapping between two-dimensional radiographic projections and three-dimensional anatomical geometry. These algorithms analyse intensity distributions, texture patterns, and spatial relationships within the OPG image to infer depth information and reconstruct volumetric representations of teeth, roots, and bone structures.
The reconstruction module focuses on recognising critical anatomical features, including tooth contours, root morphology, cortical and trabecular bone patterns, and the mandibular canal. Through interpolation and depth estimation techniques, the system generates a three-dimensional mesh model that preserves anatomical accuracy and spatial relationships between structures. The module is designed to identify and accurately represent pathological conditions such as caries, periapical lesions, bone defects, and impacted teeth.To ensure clinical validity, the reconstruction algorithms incorporate anatomical constraints and prior knowledge of dental and maxillofacial morphology. The system calibrates and optimises the generative models to achieve high fidelity in representing fine anatomical details, including individual tooth surfaces, root configurations, and bone density variations.
Stage 3: Holographic visualisation and interaction. After reconstruction, the 3D model is processed for holographic display using a holographic fan device that generates volumetric visualisations through high-speed rotation of LED arrays. The holographic fan technology creates the illusion of a three-dimensional object suspended in mid-air, viewable from multiple angles without the need for specialised viewing equipment such as VR headsets or 3D glasses.
The visualisation module converts the 3D mesh model into a format compatible with the holographic display hardware, optimising rendering parameters such as resolution, refresh rate, and viewing angles to ensure clear and stable holographic projection. The system uses microprocessors to synchronise the rotation of the LED fan with the display of sequential image frames, generating a persistent volumetric representation. Figure 12 illustrates demonstrates the working principle of a holographic fan display, highlighting the LED arrays, the rotational mechanism, and the creation of volumetric images through rapidly spinning light sources.
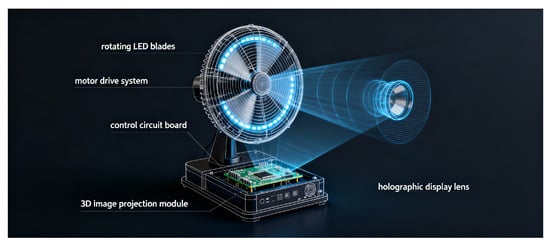
Figure 12.
Holographic fan display principle showing high-speed rotating LED blades synchronised with 3D image projection to generate a volumetric dental hologram.
Integrated with the visualisation component is a gesture recognition subsystem that enables intuitive manipulation of the holographic model. This subsystem uses motion detection sensors to track hand movements and gestures in real time. The gesture recognition algorithms process sensor data to identify specific hand motions corresponding to predefined interactions such as rotation, scaling, translation, and selection of anatomical regions. The system architecture includes a synchronisation interface that connects the gesture recognition subsystem to the holographic display, ensuring minimal latency between user input and visual response. This real-time interaction enables clinicians to manipulate the 3D model smoothly during patient consultations, rotating the view to examine structures from different perspectives, zooming into specific areas of interest, and highlighting particular anatomical features or pathologies.
The gesture recognition module utilises a Leap Motion sensor to enable precise, touch-free interaction with the holographic display. By leveraging the sensor’s optical tracking capabilities, the system reconstructs a digital skeleton of the user’s hand in real time, continuously mapping the positions of individual finger phalanges relative to the wrist root bone, which can be seen in Figure 13. Recognition algorithms analyse these skeletal data streams to identify specific static gestures by comparing current joint configurations against stored anatomical templates. To ensure robustness, the system calculates the scalar product of the hand’s local coordinate vectors against a global vertical reference; this allows it to determine hand orientation and accurately distinguish between rotation-dependent gestures. This approach allows clinicians to rotate, scale, and manipulate 3D dental models intuitively without physical contact.
Figure 13.
Hand and arm skeletal tracking visualised with MediaPipe to support gesture-based control of 3D dental holograms [59].
6.3. Feasibility and Technical Considerations
The successful implementation of the HoloDent3D system relies on addressing key feasibility challenges related to data availability, computational infrastructure, and real-time system performance. Developing robust AI-driven 3D reconstruction models requires extensive, high-quality datasets of paired orthopantomograms and corresponding 3D anatomical ground truth; however, such data are scarce due to the limited overlap between panoramic imaging and volumetric modalities such as CBCT. Even when paired data are available, accurate spatial alignment between 2D and 3D representations requires precise calibration to avoid systematic reconstruction errors. The dataset must also include diverse anatomical and pathological variations to ensure model generalisation across the clinical population. From a computational perspective, both training and inference place substantial demands on resources: deep learning models for 3D reconstruction typically require GPU-accelerated platforms for high-resolution volumetric processing, while clinical deployment must achieve near-real-time inference to integrate smoothly into workflow. Additional computational load results from holographic rendering and gesture-based interaction, which require low-latency synchronisation across hardware components to maintain smooth visualisation and responsiveness. The system also requires efficient storage and data management solutions to handle large volumes of radiographic inputs, reconstructed 3D models, and visualisation data, employing compression, archiving, and database strategies to ensure long-term scalability and operational reliability.
Anticipated Challenges: Data, Integration, Latency, Calibration, and Regulation
The development and deployment of the HoloDent3D system involve several interrelated technical and methodological challenges spanning data scarcity, system integration, real-time performance, calibration accuracy, and regulatory compliance. Figure 14 illustrates the flow of data between components in the HoloDent3D framework, highlighting how gesture inputs are processed and synchronised with holographic display updates in real time to enable interactive mixed-reality dental applications.
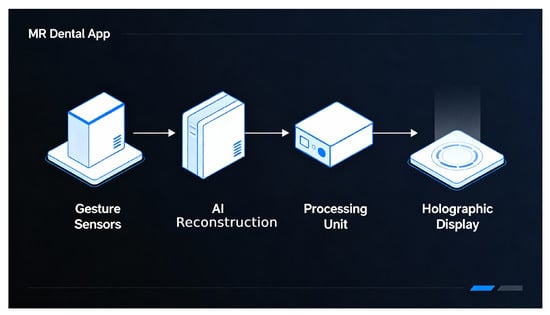
Figure 14.
Real-time data flow from gesture sensing through AI reconstruction and processing to synchronised holographic dental display.
Data scarcity represents the most fundamental limitation, as very few datasets contain paired orthopantomograms and corresponding three-dimensional anatomical ground truth. This shortage restricts the ability of deep learning models to generalise across diverse patient anatomies and pathological conditions, potentially leading to anatomically inconsistent reconstructions. Several mitigation strategies may partially address this issue, including synthetic data generation through physics-based simulation of panoramic projection geometry, transfer learning from pre-trained models in related medical imaging domains, and semi- or self-supervised learning approaches capable of leveraging unpaired or unlabelled data. Nevertheless, the limited availability of high-quality paired data will remain a persistent constraint, necessitating continuous efforts in data collection, curation, and augmentation as the system matures.
System integration presents additional challenges due to the need for seamless coordination among multiple heterogeneous components, including imaging devices, computational processing units, holographic display hardware, and gesture recognition sensors. Each component introduces specific communication protocols, timing constraints, and calibration requirements. Accurate spatial and temporal synchronisation between the reconstructed 3D model, the holographic display volume, and the gesture input coordinate system is essential to ensure intuitive and precise interaction. Misalignment or calibration drift may result in perceptual inconsistencies, distorted projections, or erroneous user input mapping. Furthermore, software interoperability across modules—ranging from image preprocessing and AI-based reconstruction to rendering and interaction—requires well-defined interfaces, robust error handling, and compatibility across different clinical IT environments. The system must also accommodate variations in hardware performance, network configurations, and data security policies present across diverse clinical settings.
Latency and real-time performance constitute another critical factor for clinical acceptance. Interactive holographic visualisation must respond instantaneously to user gestures to maintain natural and intuitive control. Delays in the processing chain—from gesture detection through computation, rendering, and display refresh—can degrade usability and disrupt diagnostic workflow. Achieving real-time responsiveness demands optimisation at both the software and hardware levels, including neural network model compression, GPU-based acceleration, asynchronous execution pipelines, and predictive rendering strategies. Display refresh rates and sensor sampling frequencies must be sufficiently high to avoid perceptible flicker and ensure smooth holographic motion. Balancing these performance requirements with computational constraints and hardware costs remains a continuing engineering challenge.
Calibration accuracy and clinical validation are equally vital for ensuring diagnostic reliability and safety. Reconstruction from a single panoramic image inherently involves ambiguity in depth estimation, requiring the AI model to infer missing spatial information from learned priors. Such inference introduces uncertainty, which must be quantified and communicated to the clinician. Implementing uncertainty estimation and validation mechanisms can help identify unreliable reconstructions. Regular geometric and photometric calibration of the holographic display ensures that the projected model accurately represents anatomical proportions and spatial relationships. Comprehensive clinical validation studies comparing reconstructed models with ground-truth CBCT or intraoral scan data across diverse patient populations are required to assess accuracy, reproducibility, and clinical relevance. These studies should also evaluate whether potential reconstruction errors have meaningful diagnostic or therapeutic consequences.
Finally, regulatory, safety, and ethical considerations must be addressed before clinical deployment. Although the HoloDent3D system functions primarily as a diagnostic support and visualisation tool rather than a direct imaging modality, it may still fall under medical device regulations depending on jurisdiction. Regulatory frameworks governing AI-driven clinical decision support systems are evolving, and compliance with data protection, patient privacy, and algorithmic transparency standards is essential. Beyond regulatory approval, successful clinical adoption will depend on demonstrating tangible benefits such as improved diagnostic accuracy, workflow efficiency, and patient engagement. Ethical considerations regarding data use, model bias, and explainability should also guide the system’s design and validation to ensure responsible and trustworthy clinical integration.
The HoloDent3D conceptual framework presents significant, though not insurmountable, technical challenges. Data scarcity is perhaps the most fundamental constraint, limiting the initial performance and generalisation capabilities of AI reconstruction models. System integration complexities require careful engineering and calibration across diverse hardware and software components. Real-time performance requirements necessitate computational optimisation and potentially specialised hardware acceleration. Clinical validation and regulatory approval processes will require extensive testing and documentation before the system can be deployed in routine practice.
Addressing these challenges will require iterative development, progressive refinement based on empirical testing, strategic partnerships for data acquisition and clinical validation, and sustained research and engineering investment. The feasibility assessment suggests that while the HoloDent3D vision is technically ambitious, advances in AI, holographic display technology, and gesture recognition systems provide a foundation on which this innovative dental imaging system can progressively be realised.
6.4. Validation and Future Development Path
The HoloDent3D system is at a very early stage of technological maturity, and no validation studies have been completed to date. What follows is a planned validation roadmap, outlining the sequential steps required to advance the concept from an algorithmic proof of principle toward an integrated prototype and, ultimately, future clinical assessment. This roadmap is explicitly aligned with the Technology Readiness Level (TRL) framework and defines the stages that must be executed—not stages that have already been achieved.
Phase 1: Technical feasibility and algorithm development. The initial phase establishes the feasibility of AI-driven 3D reconstruction from orthopantomograms. Deep learning architectures such as GANs, VAEs, or diffusion models will be implemented and trained on paired 2D–3D datasets, exploring alternative configurations for optimal anatomical accuracy. To address limited data availability, physics-based simulation tools will generate synthetic panoramic projections from existing 3D dental models, replicating real imaging characteristics. Reconstruction accuracy will be assessed using geometric and volumetric metrics (e.g., surface distance, Dice coefficients), guiding iterative refinement of model architectures and preprocessing pipelines.
Phase 2: System integration and prototype development. This phase focuses on integrating the AI reconstruction module with holographic display and gesture recognition subsystems to form a functional prototype. Key activities include configuring holographic fan hardware for accurate 3D projection, implementing motion-sensing interfaces for intuitive gesture control, and optimising system synchronisation to ensure low-latency response. Laboratory testing using phantom data will evaluate system functionality, robustness, and real-time performance under controlled conditions.
Phase 3: Clinical validation and user evaluation. Following prototype completion, the system will undergo clinical validation in collaboration with dental practitioners. Reconstructions will be quantitatively compared against gold-standard imaging modalities (CBCT or intraoral scans) to assess geometric accuracy and reliability. Clinicians will evaluate the diagnostic and communicative value of holographic models, providing structured feedback on clinical relevance, usability, and workflow integration. User studies will further assess the intuitiveness of gesture-based controls, the system’s effect on patient understanding, and comparative performance relative to existing 3D visualisation technologies.
Phase 4: Prospective clinical trials. Subsequent studies will examine the system’s impact on clinical outcomes. Prospective diagnostic accuracy trials will compare HoloDent3D-assisted diagnosis to standard imaging, while treatment outcome studies will evaluate whether 3D holographic visualisation improves planning precision or patient satisfaction. Economic and usability analyses will assess cost-effectiveness, workflow efficiency, and patient-reported outcome measures.
Phase 5: Regulatory approval and market introduction. The final phase will address regulatory compliance and market readiness. Required submissions will document validation results, risk analyses, and conformity with medical device standards for software safety, cybersecurity, and electromagnetic compatibility. Post-market monitoring mechanisms will track system performance and user feedback. Parallel efforts will ensure intellectual property protection and support technology transfer toward commercial deployment.
Positioning Within TRL 2–3: Conceptual and Analytical Proof-of-Concept Stage
The Technology Readiness Level (TRL) framework, originally developed by NASA and later adapted for medical technologies, provides a standard measure of innovation maturity from TRL 1 (basic principles observed) to TRL 9 (system proven in operational use). The HoloDent3D project is currently positioned between TRL 2 and TRL 3, marking the transition from conceptual formulation to analytical proof of concept.
At TRL 2, the foundational technological concept has been defined and its potential clinical applications identified. The system architecture, processing pipeline, and intended use for AI-driven 3D reconstruction, holographic visualisation, and gesture-based interaction have been articulated at a conceptual level. A comprehensive literature review has established the feasibility of combining these components, while major technical challenges—including data scarcity, computational load, system integration, and accuracy validation—have been identified and analysed within the dental imaging context. At this stage, the system exists as a documented and theoretically validated framework without functional implementation.
Progression towards TRL 3 involves analytical and experimental validation of key assumptions through laboratory testing and prototype development. Planned activities include implementing preliminary AI reconstruction algorithms to demonstrate the technical feasibility of 2D-to-3D conversion from orthopantomograms, testing component-level functionality of holographic display and gesture recognition modules, and performing computational simulations to estimate system performance. Limited feasibility studies using synthetic or paired datasets will verify that reconstructed models exhibit anatomically plausible structures. Successful completion of TRL 3 will provide experimental evidence that the HoloDent3D concept is technically viable, establishing a foundation for subsequent prototyping and clinical evaluation.
7. Clinical Integration and Research Challenges
This section will cover potential clinical uses of our concept, workflow integration, and main research challenges. We will also cover ethical, regulatory, and data- related constraints.
7.1. Potential Clinical Use Cases
Potential use of our concept depends on the way we collect data, and also we have to consider who our stakeholders are (dentist, surgeon, patient, educator). The most common ways for image acquisition include LIDAR, structured light technology, cone-beam CT (CBCT), optical coherence tomography (OCT), and more (see [60]). Some ways our concept could be translated into clinical practice are given in Table 2.

Table 2.
Clinical use cases of our concept.
There are also some other areas where our concept could be applied: augmented reality guidance, pathology, low-dose radiation settings, and more. Our model could be very interesting in global dentistry, especially in areas with limited resources or in remote clinics.
7.2. Workflow Integration and System Interoperability in Clinical Practice
The proposed HoloDent3D framework is designed for seamless integration into existing dental imaging workflows. Panoramic radiographs are acquired in DICOM format and retrieved through DICOMweb interfaces from local PACS systems. After anonymisation, images are securely transmitted to a cloud-based processing pipeline, where neural implicit models reconstruct 3D dental structures. The resulting volumetric or surface models are exported as DICOM-SEG or STL objects, enabling interoperability with common dental CAD/CAM and implant planning software. Visualisation is achieved via an interactive holographic interface compatible with mixed-reality devices. All data transactions follow DICOM and HL7 FHIR standards to ensure interoperability, traceability, and compliance with regulatory requirements (GDPR, HIPAA). Future development will emphasise hybrid cloud–edge architectures and federated learning for privacy-preserving clinical deployment. Figure 15 shows a workflow diagram of our concept.
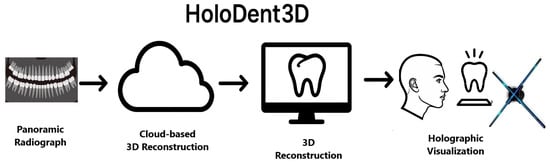
Figure 15.
Workflow of the proposed HoloDent3D system. The process begins with acquisition of a panoramic radiograph in DICOM format, followed by secure cloud-based 3D reconstruction using deep learning models. The resulting three-dimensional dental structure is then visualised through an interactive holographic interface, enabling enhanced diagnostic assessment, treatment planning, and patient education.
We propose a few practical integration scenarios using our model (Table 3) where we can recognise specific benefits.

Table 3.
Practical integration scenarios of our concept.
7.3. Ethical, Regulatory, and Data Considerations
The HoloDent3D concept raises multiple ethical, regulatory, and data-privacy constraints that must be addressed before clinical deployment. Clinically actionable reconstructions are regulated as medical devices in many jurisdictions and thus require documented validation, risk management, and lifecycle controls (FDA/AI-ML guidance; EU AI Act). Patient imaging data are personal data under GDPR and HIPAA, necessitating robust anonymisation/pseudonymisation, secure transmission (DICOM over TLS), contractual safeguards for cloud processors, and explicit consent or lawful bases for secondary use. Model bias, limited generalisability, and lack of explainability demand subgroup performance reporting, uncertainty quantification, human-in-the-loop workflows, and post-market surveillance with pre-specified change control plans. Finally, interoperability with PACS/EHR via DICOM-SEG, DICOMweb, and HL7 FHIR and clear assignment of clinical responsibility are essential to safe integration into dental practice. One can find more in the USA’s FDA [61] or EU’s EDPB regulatory documents [62].
7.4. Research Challenges and Future Directions
Despite promising advances in artificial intelligence (AI)-based 3D reconstruction from panoramic radiographs, several open challenges must be addressed before the HoloDent3D concept can achieve full clinical maturity.
7.4.1. Dataset Availability and Quality
The development of accurate deep learning models is constrained by the limited availability of large-scale, high-quality, and paired 2D–3D dental datasets. Most publicly available databases provide only panoramic radiographs or cephalometric images without volumetric ground truth from CBCT or intraoral scans. Data sharing between institutions is further restricted by ethical and regulatory frameworks such as the EU General Data Protection Regulation (GDPR) and HIPAA. Synthetic data generation using generative models may alleviate data scarcity; however, the anatomical realism and clinical reliability of such synthetic samples remain insufficiently validated. Establishing standardised, anonymised multi-institutional repositories is therefore a key prerequisite for future progress [60].
7.4.2. Model Generalisation and Robustness
Generalisation across diverse imaging conditions and patient anatomies remains an ongoing challenge. Differences in radiographic systems, exposure parameters, and the presence of metallic restorations can significantly affect reconstruction accuracy. Current models often require site-specific fine-tuning to achieve acceptable performance. Robust domain adaptation, uncertainty estimation, and self-supervised pre-training on heterogeneous datasets may improve generalisation, enabling HoloDent3D to perform reliably across clinical environments [63].
7.4.3. Evaluation and Validation Protocols
The absence of standardised evaluation criteria limits comparability between studies. Metrics such as mean surface deviation, Dice similarity coefficient, or landmark error are inconsistently applied and often dataset-specific. Clinically meaningful validation should quantify both anatomical fidelity (e.g., accuracy of alveolar ridge or mandibular canal) and functional utility (e.g., implant planning outcomes). The adoption of community benchmarks and transparent reporting standards, similar to those proposed in medical image analysis [64], would enable more reliable assessment of reconstruction quality.
7.4.4. Ethical, Regulatory, and Data Governance Constraints
Cloud-based 3D reconstruction and holographic visualisation require the processing of personal medical images, invoking strict regulatory oversight. Under the guidance of the U.S.A.’s FDA and the European Data Protection Board (EDPB, 2024), systems like HoloDent3D must incorporate data anonymisation, encryption, and secure audit trails. Privacy-preserving learning paradigms (such as federated or edge learning) represent a promising direction to balance data protection with model scalability.
7.4.5. Clinical Integration and Visualisation
Translating reconstructed models into practical holographic workflows introduces additional challenges in usability and perception. Interactive visualisation demands low-latency rendering, accurate depth perception, and reliable synchronisation with clinical data systems. Furthermore, clinician trust depends on interpretability and traceability of AI-generated structures. Explainable AI interfaces and real-time confidence visualisation may foster safer adoption in dental diagnostics and education [45].
7.4.6. Model Bias and Fairness Considerations
AI models for 3D dental reconstruction inherit the demographic and clinical characteristics of the datasets on which they are trained, which in medical imaging often leads to bias towards populations from a particular region, ethnicity, or age group. Published panoramic–CBCT datasets typically come from a small number of centres, often in European or East Asian contexts, and they focus on adult patients undergoing implantology or orthodontic treatment, while children, older edentulous patients, and individuals with craniofacial anomalies, severe periodontal disease, or extensive metallic restorations are significantly under-represented. Consequently, the embedded anatomical priors (jaw shape, tooth morphology, bone density) implicitly reflect the “average” patient from the majority group, which may limit the external validity of HoloDent3D in settings with different demographics or oral disease profiles [65,66,67,68].
Bias is operationalised as systematic performance differences across subgroups, such as higher reconstruction error in specific ethnic groups, age ranges, or diagnostic categories, while aggregate metrics like global Dice similarity or mean surface deviation can obscure these disparities. In dental radiology, this may appear as less accurate segmentation of roots and the mandibular canal in populations with distinct craniofacial growth patterns, different cortical thickness, or a high prevalence of caries and periodontal disease, or as reduced reconstruction quality in the presence of metallic artefacts and atypical surgical reconstructions. Additional sources of bias include variation in acquisition devices, exposure protocols, and annotation quality, which may cause the model to implicitly favour certain scanner–population combinations if these dominate the training data [66,68,69,70,71].
To explicitly address these risks, evaluation of the HoloDent3D system should include subgroup-aware performance reporting rather than relying solely on global metrics. This requires stratifying key indicators (e.g., Dice coefficient, IoU, mean surface distance, landmark error) by age group (paediatric, adult, geriatric), sex, ethnicity where legally and ethically permissible, and clinical categories such as dentate versus edentulous status, severity of periodontal disease, extent of metallic restorations, and type of imaging device or protocol. The medical AI literature increasingly emphasises that such subgroup-sensitive reporting is a prerequisite for responsible clinical integration, as it enables discriminatory patterns to be detected and corrected before routine deployment. In dental radiology, these reports can inform clinicians about which subgroups achieve reconstruction quality sufficient for therapy planning and where CBCT or additional expert assessment remains necessary [67,68,69,70].
Bias mitigation strategies should address both data and algorithms. At the data level, multi-centre collaborations or federated learning can increase demographic diversity without centralising sensitive health data, while explicitly recruiting under-represented age, ethnic, and clinical groups. When collecting real data is difficult, structured synthetic data augmentation (for example, simulating different patterns of resorption, edentulism, or restorative configurations) can be considered, provided the anatomical realism of augmented examples is rigorously validated and existing stereotypes are not amplified. At the model level, techniques such as re-weighted training, group-fairness-oriented regularisation, domain generalisation, uncertainty quantification, and out-of-distribution detection can be applied, with thresholds that automatically flag cases in which HoloDent3D outputs should not replace CBCT but instead trigger a recommendation for additional imaging [68,72,73,74,75,76,77].
During clinical deployment, transparency regarding limitations and ongoing post-market monitoring are essential. This involves periodic re-evaluation of the model on new cohorts, regular audits of subgroup performance, mechanisms for users to report problematic cases, and clear communication that AI-assisted reconstructions are supportive rather than substitutive tools compared to conventional diagnostic methods. Under these conditions, the HoloDent3D concept can help provide more equitable access to advanced 3D visualisation in dentistry rather than unintentionally widening existing disparities in the quality and availability of care [65,67,78].
7.4.7. Use of AI-Assisted Tools
During the preparation of this manuscript, Perplexity Pro was used to support the literature search underlying the Related Work section and for simple image generation to create illustrative figures, as explicitly noted in the figure captions where applicable. InstaText was used to refine grammar, technical tone, and clarity of the text. All outputs from these tools were critically reviewed and edited by the authors, who take full responsibility for the final content.
In summary, the future development of HoloDent3D depends on three pillars: (1) the creation of accessible, standardised datasets; (2) robust, generalisable AI models; and (3) compliance with clinical and ethical standards. Addressing these challenges will enable clinically validated, interoperable holographic systems that extend 3D dental imaging beyond the constraints of current radiographic modalities.
8. Discussion
The findings of this work position HoloDent3D within the broader development of AI-driven dental imaging and mixed-reality visualisation. Although single-view 3D reconstruction has progressed rapidly, its integration into clinical workflows requires not only technological feasibility but also compliance with regulatory, ergonomic, and diagnostic safety standards. In this section, we critically reflect on the contributions and implications of our proposed framework, benchmark its performance and usability against current clinical expectations, and outline the opportunities and challenges that remain before such systems can achieve full clinical maturity. These reflections help define a realistic pathway from conceptual innovation to validated clinical adoption and long-term impact in patient-centred dental care.
8.1. Key Contributions
This work makes several original contributions that collectively advance research in AI-assisted dental imaging and mixed-reality visualisation. First, it presents the most comprehensive systematic review to date, integrating both classical and state-of-the-art single-view 3D reconstruction approaches for panoramic radiography. It connects algorithmic evolution with detailed mathematical formulations and performance trends—an integration not previously synthesised across both methodological and clinical dimensions. By bridging physics-informed neural rendering, implicit anatomical modelling, and transformer-based architectures, this review establishes a foundational map of the progression of single-view reconstruction towards clinical viability.
Second, we provide a structured survey of holographic and mixed-reality (MR) applications in dentistry, covering orthodontics, implantology, endodontic education, periodontal therapy, and digital workflows. The existing literature demonstrates high user satisfaction and promising accuracy for AR/MR guidance, although ergonomic and hardware constraints remain. This synthesis confirms that immersive visualisation technologies are both feasible and relevant for dental clinical practice, particularly when precision, spatial perception, and patient understanding are critical.
Finally, this paper introduces the HoloDent3D conceptual framework, which uniquely combines AI-driven 3D reconstruction from a single, routinely acquired panoramic radiograph with cost-efficient holographic fan display technology and gesture-based interaction. Unlike previous AR/MR navigation systems that rely on CBCT-derived models and expensive head-mounted devices, HoloDent3D offers an accessible, radiation-free, and workflow-compatible solution intended for chairside use. This contribution reframes AI-based reconstruction not merely as a computational capability, but as a human-centred clinical interface that supports diagnostic clarity, enhances patient communication, and lays the groundwork for future integration into treatment planning environments.
Together, these contributions form a coherent trajectory: understanding limitations, leveraging immersive visualisation, and proposing a practical deployment model that bridges current capabilities with future clinical expectations.
8.2. Current Limitations of AI Single-View Reconstruction
Although current AI-based single-view reconstruction methods achieve surface errors of 1–2 mm, this performance should be considered in relation to clinical tolerances in implantology. In posterior mandibular regions, multiple clinical and biomechanical studies recommend maintaining a safety margin of approximately 2 mm between the implant apex and the mandibular canal to minimise the risk of inferior alveolar nerve injury. CBCT protocols for implant planning typically use voxel sizes of 0.3–0.4 mm, which are considered sufficient for accurate linear measurements and safe three-dimensional planning. This comparison highlights a critical gap: an AI system with a 1–2 mm error may be adequate for visualisation, education, and preliminary orientation, but it operates very close to, or even within, the entire safety corridor that separates a safe implant position from potential neurovascular damage. In other words, an error that is numerically “small” from a machine learning perspective can be clinically decisive. Consequently, until reconstruction error is consistently reduced to the submillimetre range, AI single-view reconstruction should not be used in isolation for final implant positioning decisions but rather as a complementary tool alongside CBCT-based planning and intraoperative guidance.
8.3. Clinical Feasibility of Holographic Implementation
Holographic fan displays and head-mounted AR/MR systems such as HoloLens represent two ends of the spectrum in mixed-reality visualisation for dentistry. HoloLens-based systems have demonstrated submillimetre landmark accuracy in orthodontic superimposition and high spatial fidelity for overlaying CBCT-based models in navigation scenarios. Recent in vitro and phantom studies of MR-guided implant surgery report coronal and apical deviations of approximately 1–2.5 mm and angular errors of 3–5∘, which approach—but do not yet consistently match—the precision of established dynamic navigation systems. A recent systematic review and meta-analysis similarly concluded that AR-assisted implant placement improves accuracy compared with freehand placement but still shows heterogeneity and a residual error budget that must be considered in risk-sensitive regions.
Holographic fan displays, by contrast, sacrifice some of this high-end spatial precision in favour of accessibility, ergonomics, and shared viewing. They do not require users to wear a headset, are easier to integrate into existing operatory layouts, and are particularly well suited to patient communication and group teaching. However, they currently provide more limited depth cues and interaction fidelity than headset-based MR, and they depend on environmental lighting and viewing angle to maintain visual clarity. As a result, holographic fans are currently best positioned as low-cost, workflow-friendly 3D visualisation devices, whereas HoloLens-type systems remain preferable where millimetre-level intraoperative guidance is required.
Compared with the AR/MR systems reviewed in Section 5—most of which rely on CBCT-derived 3D models displayed via HoloLens or tablet/smartphone-based AR—HoloDent3D offers a distinct value proposition. It seeks to replace the volumetric input (CBCT) with AI-reconstructed 3D models generated from routine panoramic radiographs and to display them on cost-effective holographic fans rather than expensive headsets. In doing so, it sacrifices some geometric accuracy and advanced navigation capability in favour of accessibility, reduced radiation exposure, and minimal workflow disruption, positioning itself primarily as an adjunctive diagnostic and educational tool rather than a direct competitor to MR-based surgical navigation platforms.
While immersive visualisation demonstrates measurable improvements in spatial understanding and landmark perception [79,80] (as reviewed in Section 5), very few studies have directly linked holographic display to improved clinical outcomes [52]. Metrics such as treatment success rates, decision-making confidence, patient satisfaction scores, and comprehension of informed consent are rarely quantified in current mixed-reality dental research.
For HoloDent3D, this distinction is particularly important. Its primary short-term value lies not in submillimetre surgical navigation but in enhancing diagnostic communication, patient engagement, and preoperative understanding—factors that may influence adherence, anxiety reduction, and shared decision-making [80]. However, these hypotheses require prospective, outcome-oriented clinical validation before holographic visualisation can claim clinical impact beyond technical feasibility.
Future clinical evaluations of HoloDent3D and related systems should therefore incorporate the following:
- Patient-reported outcome measures (PROMs), including confidence, satisfaction, and understanding of risks.
- Objective comprehension assessments during chairside consultations.
- Behavioural indicators, such as adherence to planned treatments.
- Correlations with diagnostic accuracy and decision-making consistency between clinicians.
By moving from user experience studies towards outcome-driven clinical trials, the field can more robustly verify whether holographic visualisation contributes to improved patient-centred care rather than solely enhancing the viewing experience.
8.4. Regulatory and Ethical Considerations
From a regulatory perspective, HoloDent3D is classified as Software as a Medical Device (SaMD). In the United States, the FDA’s AI/ML-Based SaMD Action Plan emphasises several pillars: adherence to Good Machine Learning Practice (GMLP), a total product lifecycle (TPLC) approach, the use of pre-specified change control plans, and ongoing real-world performance monitoring to ensure that adaptive AI models remain safe and effective over time. Clinically actionable reconstructions therefore require not only pre-market validation but also post-market mechanisms to detect performance drift, data shift, and emerging safety signals.
In the European Union, the EU AI Act classifies most AI-enabled medical devices as high-risk systems, subjecting solutions such as AI-based dental reconstruction and holographic visualisation tools to stringent regulatory obligations. These requirements include a fully documented risk management approach throughout the entire lifecycle, robust data governance practices demonstrating that training, validation, and testing datasets are representative and of sufficient quality, and comprehensive technical documentation supported by conformity assessment. Additionally, the regulation mandates appropriate human oversight and clearly defined responsibilities, along with strong safeguards for transparency, cybersecurity, and resilience to performance drift. Before clinical deployment, these technologies must be validated in rigorous, prospective, multi-centre studies that compare reconstructed anatomy with CBCT ground truth and assess their impact on diagnostic decision-making and treatment outcomes. Validation criteria should include predefined performance thresholds and non-inferiority margins aligned with established imaging standards, supplemented by systematic usability testing that evaluates operator workload, situational awareness, and the risk of over-reliance on AI during clinical tasks.
Together, these regulatory frameworks emphasise that HoloDent3D cannot be regarded merely as a technical curiosity; it must be developed and evaluated as a regulated medical device, with clear clinical benefits that justify its complexity.
8.5. Data Limitations and Synthetic Data Concerns
Beyond data scarcity and domain shift, data protection and privacy impose further constraints on large-scale deployment. Under the GDPR, medical images—including panoramic radiographs and reconstructed 3D anatomy—are classified as special category personal data. Simply removing explicit identifiers is insufficient; pseudonymised data remain subject to the GDPR and require a lawful basis, strict access controls, and robust governance.
Anonymisation must be effectively irreversible, which is particularly challenging for detailed 3D craniofacial models that may permit re-identification through facial morphology or linkage with external datasets.
For systems such as HoloDent3D, this necessitates several additional measures beyond those described in Section 7.3:
- Privacy by design and by default, including data minimisation, strict purpose limitation, and role-based access control (for example, separating training and clinical accounts).
- Implementation of Data Protection Impact Assessments (DPIAs) for large-scale or cross-border deployments.
- Clear contractual frameworks governing data sharing with cloud providers and AI developers, explicitly addressing ownership, secondary use, and retention periods.
- Exploration of federated learning, edge inference, and secure enclaves, which enable model training or inference near the data source, reducing the need to centralise raw imaging data.
In parallel, robust governance is required to manage the ethico-legal risks associated with synthetic data. While synthetic panoramic images or 3D models can reduce direct use of identifiable images, their generation often relies on real patient data and may inadvertently encode identifiable patterns. Validation protocols must therefore assess not only geometric realism and diagnostic fidelity but also the re-identification risk associated with synthetic datasets shared across institutions or vendors.
9. Conclusions
The integration of AI-driven 3D reconstruction with holographic visualisation marks a paradigm shift in dental imaging, addressing fundamental limitations of panoramic radiography while offering practical advantages over CBCT. This discussion interprets the findings in the context of previous research and identifies critical future directions.
The literature shows a clear progression from classical geometric approaches to neural implicit representations. Early template-based methods achieved only modest accuracy and failed to capture patient-specific variations. Deep learning architectures have substantially improved performance, with structural similarity indices reaching 0.7–0.8 and surface errors of 1–2 mm. Neural implicit frameworks such as Occudent (IoU: 0.651, Chamfer-L1: 0.298), NeBLa (15% higher Dice coefficients, 30% lower perceptual error), and ViT-NeBLa (1.3 dB higher PSNR, 50% faster processing) represent the current frontier. However, reconstruction accuracies of 75–86% and 1–2 mm surface errors, while impressive for single-view reconstruction, do not achieve the submillimetre precision required for critical applications such as implant placement.
HoloDent3D bridges the gap between diagnostic need and accessibility. CBCT delivers 50–1000 µSv radiation compared to 2.7–24.3 µSv for panoramic radiography, costs over USD 100,000, and requires specialised expertise. By extracting 3D information from routinely acquired panoramic images, HoloDent3D offers radiation-free enhancement in line with ALARA principles. Integration with cost-efficient holographic fan displays improves accessibility for general practitioners compared to expensive AR headsets. Previous studies have shown that mixed reality improves spatial understanding and diagnostic precision—Talaat et al. demonstrated HoloLens-based superimposition achieved −0.18 to +0.17 mm accuracy, while Xue et al. reported significant improvements in periodontal therapy using MR guidance. HoloDent3D’s innovation lies in combining AI reconstruction from single panoramic images with holographic display, eliminating the need for volumetric imaging.
Data scarcity is the fundamental constraint, as paired panoramic–CBCT datasets are rare. Mitigation strategies include synthetic data generation, transfer learning, and semi-supervised approaches. System integration requires spatial–temporal synchronisation across imaging devices, processing units, holographic displays, and gesture sensors. Real-time performance demands optimisation through model compression, GPU acceleration, and predictive rendering. Clinical validation comparing reconstructions with ground-truth CBCT across diverse populations is essential. Akulauskas et al. showed current AR systems achieve 1.97 ± 1.17 mm distance deviations and 10.5 ± 4.1° angular deviations, indicating further refinement is needed for critical applications.
Promising directions include multimodal fusion combining panoramic radiographs with intraoral scans for enhanced accuracy, temporal consistency frameworks for longitudinal monitoring, physics-informed neural architectures embedding X-ray principles, federated learning for privacy-preserving collaborative training, and comprehensive clinical trials assessing diagnostic impact beyond geometric accuracy. Integration with CAD/CAM workflows could enable seamless transition from visualisation to fabrication.
Author Contributions
Conceptualisation, T.G. and Č.L.; methodology, T.G. and Č.L.; validation, T.G., Č.L., and A.B.; formal analysis, A.B.; investigation, T.G. and Č.L.; resources, T.G. and Č.L.; data curation, A.B.; writing—original draft preparation, T.G. and Č.L.; writing—review and editing, T.G.,Č.L., and A.B.; visualisation, T.G. and Č.L.; supervision, T.G. and Č.L.; project administration, T.G. and Č.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European union-NextGenerationEU (Grant. No. NPOO.C3.2.R3-I1.06.0294).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created. All data is publicly available.
Acknowledgments
During the preparation of this manuscript, the authors acknowledge the use of Perplexity Pro for literature search support and simple image generation, and InstaText for improving the clarity and grammar of the manuscript. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giruvuri, A.; Miryala, G.; Khan, Y.; Ramalingam, N.T.; Sevugaperumal, B.; Soman, M.; Padmanabhan, A. Revolutionizing Dental Imaging: A Comprehensive Study on the Integration of Artificial Intelligence in Dental and Maxillofacial Radiology. Cureus 2023, 15, e50292. [Google Scholar] [CrossRef]
- Jayachandran, S. Digital Imaging in Dentistry: A Review. Contemp. Clin. Dent. 2017, 8, 193–194. [Google Scholar] [CrossRef]
- Alapati, S.; Reddy, S.R.; Tatapudi, R.; Kotha, R.; Bodu, N.K.; Chennoju, S. Identifying risk groups for osteoporosis by digital panoramic radiography. Contemp. Clin. Dent. 2015, 6, S253–S257. [Google Scholar] [CrossRef] [PubMed]
- Spelic, D.C.; Kakar, S. Nationwide Survey of Dental Offices: Findings and Trends in Radiological Practice. Health Phys. 2025, 129, 539–546. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.; Telyakova, V. An Overview of Cone-Beam Computed Tomography and Dental Panoramic Radiography in Dentistry in the Community. Tomography 2024, 10, 1222–1237. [Google Scholar] [CrossRef]
- Kweon, H.H.; Lee, B.A.; Youk, T.m.; Kim, Y.T. Panoramic radiography can be an effective diagnostic tool adjunctive to oral examinations in the national health checkup program. J. Periodontal Implant. Sci. 2018, 48, 317–325. [Google Scholar] [CrossRef]
- Minhas, S.; Wu, T.H.; Kim, D.G.; Chen, S.; Wu, Y.C.; Ko, C.C. Artificial Intelligence for 3D Reconstruction from 2D Panoramic X-rays to Assess Maxillary Impacted Canines. Diagnostics 2024, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Liang, C.J.; Xu, M. 3D Reconstruction and Estimation from Single-view 2D Image by Deep Learning–A Survey. In Proceedings of the 2024 IEEE Conference on Artificial Intelligence (CAI), Singapore, 25–27 June 2024; pp. 1–7. [Google Scholar] [CrossRef]
- Song, W.; Zheng, H.; Tu, D.; Liang, C.; He, L. Oral-3Dv2: 3D Oral Reconstruction from Panoramic X-Ray Imaging with Implicit Neural Representation. arXiv 2023, arXiv:2303.12123. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Song, I.S.; Baek, S.J. 3D Teeth Reconstruction from Panoramic Radiographs Using Neural Implicit Functions. In Medical Image Computing and Computer Assisted Intervention–MICCAI 2023, Proceedings of the 26th International Conference, Vancouver, BC, Canada, 8–12 October 2023; Greenspan, H., Madabhushi, A., Mousavi, P., Salcudean, S., Duncan, J., Syeda-Mahmood, T., Taylor, R., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2023; Volume 14229, pp. 376–386. [Google Scholar] [CrossRef]
- Suomalainen, A.; Pakbaznejad Esmaeili, E.; Robinson, S. Dentomaxillofacial imaging with panoramic views and cone beam CT. Insights Imaging 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Amorim, P.H.J.; Moraes, T.F.; Silva, J.V.L.; Pedrini, H.; Ruben, R.B. Reconstruction of Panoramic Dental Images Through Bézier Function Optimization. Front. Bioeng. Biotechnol. 2020, 8, 794. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kwon, D.; Jang, Y.; Song, I.S.; Baek, S.J. NeBLa: Neural Beer-Lambert for 3D Reconstruction of Oral Structures from Panoramic Radiographs. In Proceedings of the AAAI Conference on Artificial Intelligence, Vancouver, BC, Canada, 20–27 February 2024; Volume 38, pp. 4636–4644. [Google Scholar] [CrossRef]
- Ding, Y.; Holmes, J.M.; Feng, H.; Li, B.; McGee, L.A.; Rwigema, J.C.M.; Vora, S.A.; Wong, W.W.; Ma, D.J.; Foote, R.L.; et al. Accurate patient alignment without unnecessary imaging using patient-specific 3D CT images synthesized from 2D kV images. Commun. Med. 2024, 4, 234. [Google Scholar] [CrossRef]
- Parida, B.K.; Sunilkumar, A.P.; Sen, A.; You, W. ViT-NeBLa: A Hybrid Vision Transformer and Neural Beer–Lambert Framework for Single-View 3D Reconstruction of Oral Anatomy From Panoramic Radiographs. arXiv 2025, arXiv:2506.13195. [Google Scholar]
- Sunilkumar, A.P.; You, W. A Deep Learning Approach to Generating Flattened CBCT Volume Across Dental Arch From 2D Panoramic X-ray for 3D Oral Cavity Reconstruction. In Proceedings of the 2023 International Technical Conference on Circuits/Systems, Computers, and Communications (ITC-CSCC), Jeju, Republic of Korea, 25–28 June 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Meng, M.; Delamare, E.; Feng, D.; Bi, L.; Sheng, B.; Jiang, L.; Li, B.; Kim, J. 3DPX: Single Panoramic X-ray Analysis Guided by 3D Oral Structure Reconstruction. arXiv 2024, arXiv:2409.18701. [Google Scholar] [CrossRef]
- Naufal, M.F.; Fatichah, C.; Astuti, E.R.; Putra, R.H. Deep Learning for Mandibular Canal Segmentation in Digital Dental Radiographs: A Systematic Literature Review. IEEE Access 2024, 12, 77395–77423. [Google Scholar] [CrossRef]
- Alan, R.; Erbeyoğlu, A. The importance of linear measurements made using panoramic radiography in pre-implant site assessment: Actual vs. measured. Arch. Curr. Med. Res. 2023, 4, 24–30. [Google Scholar] [CrossRef]
- Özalp, Ö.; Tezerisener, H.A.; Kocabalkan, B.; Büyükkaplan, U.Ş.; Özarslan, M.M.; Şimşek Kaya, G.; Altay, M.A.; Sindel, A. Comparing the precision of panoramic radiography and cone-beam computed tomography in avoiding anatomical structures critical to dental implant surgery: A retrospective study. Imaging Sci. Dent. 2018, 48, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Peretz, B.; Gotler, M.; Kaffe, I. Common Errors in Digital Panoramic Radiographs of Patients with Mixed Dentition and Patients with Permanent Dentition. Int. J. Dent. 2012, 2012, 584138. [Google Scholar] [CrossRef]
- Hosseinian, S.; Arefi, H. 3D Reconstruction from Multi-View Medical X-ray Images–Review and Evaluation of Existing Methods. In Proceedings of the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, International Conference on Sensors & Models in Remote Sensing & Photogrammetry, Kish Island, Iran, 23–25 November 2015; Volume XL-1/W5, pp. 319–326. [Google Scholar] [CrossRef]
- Qiu, L.; Ye, C.; Chen, P.; Liu, Y.; Han, X.; Cui, S. DArch: Dental Arch Prior-assisted 3D Tooth Instance Segmentation with Weak Annotations. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), New Orleans, LA, USA, 18–24 June 2022; IEEE Computer Society: Los Alamitos, CA, USA, 2022; pp. 16782–16792. [Google Scholar] [CrossRef]
- Papakosta, T.K.; Savva, A.D.; Economopoulos, T.L.; Matsopoulos, G.K.; Gröhndal, H.G. An automatic panoramic image reconstruction scheme from dental computed tomography images. Dentomaxillofacial Radiol. 2017, 46, 20160234. [Google Scholar] [CrossRef]
- Mohamed, W.; Nader, N.; Alsakar, Y.M.; Elazab, N.; Ezzat, M.; Elmogy, M. 3D Reconstruction from 2D Multi-view Dental Images Based on EfficientNetB0 Model. Sci. Rep. 2025, 15, 28775. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Yu, Q.; Luo, X.; Adeli, E.; Wang, Y.; Lu, L.; Yuille, A.L.; Zhou, Y. TransUNet: Transformers Make Strong Encoders for Medical Image Segmentation. arXiv 2021, arXiv:2102.04306. [Google Scholar]
- Cai, Y.; Long, Y.; Han, Z.; Liu, M.; Zheng, Y.; Yang, W.; Chen, L. Swin Unet3D: A Three-Dimensional Medical Image Segmentation Network Combining Vision Transformer and Convolution. BMC Med. Inform. Decis. Mak. 2023, 23, 33. [Google Scholar] [CrossRef]
- Lipman, Y. Phase Transitions, Distance Functions, and Implicit Neural Representations. arXiv 2021, arXiv:2106.07689. [Google Scholar] [CrossRef]
- Takikawa, T.; Litalien, J.; Yin, K.; Kreis, K.; Loop, C.; Nowrouzezahrai, D.; Jacobson, A.; McGuire, M.; Fidler, S. Neural Geometric Level of Detail: Real-time Rendering with Implicit 3D Shapes. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Virtually, 19–25 June 2021; IEEE Computer Society: Los Alamitos, CA, USA, 2021; pp. 11696–11705. [Google Scholar] [CrossRef]
- Peng, S.; Niemeyer, M.; Mescheder, L.; Pollefeys, M.; Geiger, A. Convolutional Occupancy Networks. In Computer Vision–ECCV 2020, Proceedings of the 16th European Conference, Glasgow, UK, 23–28 August 2020; Springer: Cham, Switzerland, 2020; pp. 523–540. [Google Scholar] [CrossRef]
- Park, J.J.; Florence, P.; Straub, J.; Newcombe, R.; Lovegrove, S. DeepSDF: Learning Continuous Signed Distance Functions for Shape Representation. In Proceedings of the IEEE/CVF International Conference on Computer Vision (ICCV), Seoul, Republic of Korea, 27 October–2 November 2019; IEEE Computer Society: Los Alamitos, CA, USA, 2019; pp. 165–174. [Google Scholar] [CrossRef]
- Liang, Y.; Song, W.; Yang, J.; Qiu, L.; Wang, K.; He, L. X2Teeth: 3D Teeth Reconstruction from a Single Panoramic Radiograph. arXiv 2021, arXiv:2108.13004. [Google Scholar] [CrossRef]
- Ma, W.; Wu, H.; Xiao, Z.; Feng, Y.; Wu, J.; Liu, Z. PX2Tooth: Reconstructing the 3D Point Cloud Teeth from a Single Panoramic X-ray. In Medical Image Computing and Computer Assisted Intervention–MICCAI 2024, Proceedings of the 27th International Conference, Marrakesh, Morocco, 6–10 October 2024; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Song, W.; Liang, Y.; Yang, J.; Wang, K.; He, L. Oral-3D: Reconstructing the 3D Structure of Oral Cavity from Panoramic X-ray. In Proceedings of the Thirty-Fifth AAAI Conference on Artificial Intelligence (AAAI-21), Vancouver, BC, Canada, 2–9 February 2021; pp. 566–573. [Google Scholar]
- Liu, J.; Hao, J.; Lin, H.; Pan, W.; Yang, J.; Feng, Y.; Wang, G.; Li, J.; Jin, Z.; Zhao, Z.; et al. Deep learning-enabled 3D multimodal fusion of cone-beam CT and intraoral mesh scans for clinically applicable tooth-bone reconstruction. Patterns 2023, 4, 100825. [Google Scholar] [CrossRef]
- Jang, T.J.; Yun, H.S.; Hyun, C.M.; Kim, J.E.; Lee, S.H.; Seo, J.T. Fully automatic integration of dental CBCT images and full-arch intraoral scans with stitching error correction via individual tooth segmentation and identification. Med. Image Anal. 2024, 93, 103096. [Google Scholar] [CrossRef]
- Ogawa, K.; Yamamoto, J.; Yanase, M.; Katsumata, A. Registration of Two Dental Panoramic Radiographs. Open J. Med. Imaging 2014, 4, 4–13. [Google Scholar] [CrossRef]
- Betancourt, A.R.; Kripfgans, O.D.; Meneghetti, P.C.; Mendonça, G.; Pereira, R.; Teixeira, W.; Zambrana, N.; Samal, A.; Chan, H.L. Intraoral Ultrasonography Image Registration for Evaluation of Partial Edentulous Ridge: A Methodology and Validation Study. J. Dent. 2024, 148, 105136. [Google Scholar] [CrossRef]
- Luo, T.; Wu, H.; Yang, T.; Shen, D.; Cui, Z. Adapting Foundation Model for Dental Caries Detection with Dual-View Co-Training. arXiv 2025, arXiv:2508.20813. [Google Scholar] [CrossRef]
- Almalki, A.; Latecki, L.J. Self-Supervised Learning with Masked Image Modeling for Teeth Numbering, Detection of Dental Restorations, and Instance Segmentation in Dental Panoramic Radiographs. arXiv 2022, arXiv:2210.11404. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Liu, Y.; Meng, D.; Cui, Z.; Gao, C.; Gao, X.; Lian, C.; Shen, D. Two-Stream Graph Convolutional Network for Intra-oral Scanner Image Segmentation. arXiv 2022, arXiv:2204.08797. [Google Scholar] [CrossRef]
- Hsung, T.H.; Hsieh, Y.F.; Lai, K.T.; Kuo, Y.L.; Lin, Y.C.; Lin, C.K. Image to Geometry Registration for Virtual Dental Models. J. Prosthet. Dent. 2018, 120, 343–352. [Google Scholar] [CrossRef]
- De Wilde, D.; Zanier, O.; Da Mutten, R.; Jin, M.; Regli, L.; Serra, C.; Staartjes, V.E. Strategies for generating synthetic computed tomography-like imaging from radiographs: A scoping review. Med. Image Anal. 2025, 101, 103454. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.E.; Vinayahalingam, S.; Wu, T.H.; Chang, C.W.; Chang, H.H.; Wei, H.; Chen, M.; Ko, C.H.; Moin, D.A.; et al. Artificial Intelligence to Assess Dental Findings from Panoramic Radiographs—A Multinational Study. arXiv 2025, arXiv:2502.10277. [Google Scholar]
- Haleem, A.; Javaid, M.; Khan, I.H. Holography applications toward medical field: An overview. Indian J. Radiol. Imaging 2020, 30, 354–361. [Google Scholar] [CrossRef]
- Lin, P.Y.; Chen, T.C.; Lin, C.J.; Huang, C.C.; Tsai, Y.H.; Tsai, Y.L.; Wang, C.Y. The use of augmented reality (AR) and virtual reality (VR) in dental surgery education and practice: A narrative review. J. Dent. Sci. 2024, 19, S91–S101. [Google Scholar] [CrossRef]
- Rosu, S.N.; Tatarciuc, M.S.; Vitalariu, A.M.; Lupu, I.C.; Diaconu, D.A.; Vasluianu, R.I.; Holban, C.C.; Dima, A.M. Augmented Reality in Implant and Tooth-Supported Prosthodontics Practice and Education: A Scoping Review. Dent. J. 2025, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Dolega-Dolegowski, D.; Proniewska, K.; Dolega-Dolegowska, M.; Pregowska, A.; Hajto-Bryk, J.; Trojak, M.; Chmiel, J.; Walecki, P.; Fudalej, P.S. Application of holography and augmented reality based technology to visualize the internal structure of the dental root–a proof of concept. Head Face Med. 2022, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Talaat, S.; Ghoneima, A.; Kaboudan, A.; Talaat, W.; Ragy, N.; Bourauel, C. Three-dimensional evaluation of the holographic projection in digital dental model superimposition using HoloLens device. Orthod. Craniofacial Res. 2019, 22, 62–68. [Google Scholar] [CrossRef]
- Akulauskas, M.; Butkus, K.; Rutkūnas, V.; Blažauskas, T.; Jegelevičius, D. Implementation of Augmented Reality in Dental Surgery Using HoloLens 2: An In Vitro Study and Accuracy Assessment. Appl. Sci. 2023, 13, 8315. [Google Scholar] [CrossRef]
- Xue, F.; Zhang, R.; Dai, J.; Zhang, Y.; Luan, Q.X. Clinical application of mixed reality holographic imaging technology in scaling and root planing of severe periodontitis: A proof of concept. J. Dent. 2024, 149, 105284. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Zhang, Y.; Dai, J.; Hou, J.; Xue, F. Clinical efficacy of mixed-reality holographic imaging-based nonsurgical periodontal therapy: A randomized controlled split-mouth study. J. Dent. 2025, 161, 105963. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Tao, B.; Tu, P.; Shen, Y.; Wu, Y.; Chen, X. A novel mixed reality-guided dental implant placement navigation system based on virtual-actual registration. Comput. Biol. Med. 2023, 166, 107560. [Google Scholar] [CrossRef]
- Grün, P.; Pfaffeneder-Mantai, F.; Schiepek, T.; Mosch, R.; Turhani, F.; von See, C.; Turhani, D. Smartphone-Based Augmented Reality Application for Dental Implant Placement: A Technical Innovation. J. Oral Maxillofac. Surg. 2025, 83, 1519–1525. [Google Scholar] [CrossRef]
- Grad, P.; Przeklasa-Bierowiec, A.M.; Malinowski, K.P.; Witowski, J.; Proniewska, K.; Tatoń, G. Application of HoloLens-based augmented reality and three-dimensional printed anatomical tooth reference models in dental education. Anat. Sci. Educ. 2023, 16, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.; Maqsood, A.; Das, G.; Ahmed, N.; Saquib, S.; Lal, A.; Khan, A.A.G.; Alam, M.K. Augmented Reality and Virtual Reality in Dentistry: Highlights from the Current Research. Appl. Sci. 2022, 12, 3719. [Google Scholar] [CrossRef]
- Moussa, R.; Alghazaly, A.; Althagafi, N.; Eshky, R.; Borzangy, S. Effectiveness of Virtual Reality and Interactive Simulators on Dental Education Outcomes: Systematic Review. Eur. J. Dent. 2022, 16, 14–31. [Google Scholar] [CrossRef]
- Patel, K.; Bur, A.M.; Wang, G. Enhanced U-Net: A Feature Enhancement Network for Polyp Segmentation. In Proceedings of the 2021 18th Conference on Robots and Vision (CRV), Burnaby, BC, Canada, 26–28 May 2021; pp. 181–188. [Google Scholar] [CrossRef]
- Sung, G.; Sokal, K.; Uboweja, E.; Bazarevsky, V.; Baccash, J.; Bazavan, E.G.; Chang, C.L.; Grundmann, M. On-device Real-time Hand Gesture Recognition. In Proceedings of the 2021 IEEE International Conference on Computer Vision Workshops (ICCVW), Montreal, BC, Canada, 11–17 October 2021; pp. 2536–2545. [Google Scholar] [CrossRef]
- Cen, Y.; Huang, X.; Liu, J.; Qin, Y.; Wu, X.; Ye, S.; Du, S.; Liao, W. Application of three-dimensional reconstruction technology in dentistry: A narrative review. BMC Oral Health 2023, 23, 630. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Artificial Intelligence and Machine Learning (AI/ML)–Enabled Medical Devices. 2025. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-software-medical-device (accessed on 25 March 2025).
- European Data Protection Board. Opinion 28/2024 on Certain Data Protection Aspects Related to the Processing of Personal Data in the Context of AI Models. 2024. Available online: https://www.edpb.europa.eu/system/files/2024-12/edpb_opinion_202428_ai-models_en.pdf (accessed on 25 March 2025).
- Yoon, J.S.; Oh, K.; Shin, Y.; Mazurowski, M.A.; Suk, H.-I. Domain generalization in medical image analysis: A survey. arXiv 2023, arXiv:2310.08598. Available online: https://arxiv.org/abs/2310.08598 (accessed on 17 December 2025).
- Maier-Hein, L.; Reinke, A.; Godau, P.; Tizabi, M.D.; Buettner, F.; Christodoulou, E.; Glocker, B.; Isensee, F.; Kleesiek, J.; Kozubek, M.; et al. Metrics reloaded: Recommendations for image analysis validation. Nat. Methods 2024, 21, 195–212. [Google Scholar] [CrossRef]
- Tuygunov, N.; Samaranayake, L.; Khurshid, Z.; Rewthamrongsris, P.; Schwendicke, F.; Osathanon, T.; Yahya, N.A. The Transformative Role of Artificial Intelligence in Dentistry: A Comprehensive Overview Part 2: The Promise and Perils, and the International Dental Federation Communique. Int. Dent. J. 2025, 75, 397–404. [Google Scholar] [CrossRef]
- Stanley, E.A.M.; Souza, R.; Wilms, M.; Forkert, N.D. Where, why, and how is bias learned in medical image analysis models? A study of bias encoding within convolutional networks using synthetic data. eBioMedicine 2025, 111, 105501. [Google Scholar] [CrossRef]
- Liu, T.Y.; Lee, K.H.; Mukundan, A.; Karmakar, R.; Dhiman, H.; Wang, H.C. AI in Dentistry: Innovations, Ethical Considerations, and Integration Barriers. Bioengineering 2025, 12, 928. [Google Scholar] [CrossRef]
- Koçak, B.; Ponsiglione, A.; Stanzione, A.; Bluethgen, C.; Santinha, J.; Ugga, L.; Huisman, M.; Klontzas, M.E.; Cannella, R.; Cuocolo, R. Bias in artificial intelligence for medical imaging: Fundamentals, detection, avoidance, mitigation, challenges, ethics, and prospects. Diagn. Interv. Radiol. 2025, 31, 75–88. [Google Scholar] [CrossRef]
- Roller, R.; Hahn, M.; Ravichandran, A.M.; Osmanodja, B.; Oetke, F.; Sassi, Z.; Burchardt, A.; Netter, K.; Budde, K.; Herrmann, A.; et al. One Size Fits None: Rethinking Fairness in Medical AI. In Proceedings of the 6th Workshop on Gender Bias in Natural Language Processing (GeBNLP), Vienna, Austria, 1 August 2025. [Google Scholar] [CrossRef]
- Stanley, E.A.M.; Souza, R.; Winder, A.J.; Gulve, V.; Amador, K.; Wilms, M.; Forkert, N.D. Towards objective and systematic evaluation of bias in artificial intelligence for medical imaging. J. Am. Med. Inform. Assoc. 2024, 31, 2613–2621. [Google Scholar] [CrossRef]
- Yousuf, T.; Khan, M.; Ghafoor, R. Ethical insights into AI-driven caries detection: A scoping review. BDJ Open 2025, 11, 78. [Google Scholar] [CrossRef]
- Guan, H.; Yap, P.T.; Bozoki, A.; Liu, M. Federated learning for medical image analysis: A survey. Pattern Recognit. 2024, 151, 110424. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Sun, R.; Ren, J.; Wei, J.; Feng, C.M.; Ding, X.; Guo, Z.; Wang, Y.; Hu, Y.; Wei, W.; et al. Achieving flexible fairness metrics in federated medical imaging. Nat. Commun. 2025, 16, 3342. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, M.; Zhao, H.; Peng, Y.; Huang, F.; Lu, Z. A survey of recent methods for addressing AI fairness and bias in biomedicine. J. Biomed. Inform. 2024, 154, 104646. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Zhong, X.; Khan, M.A.; Wu, Z.; Abdullah Almujally, N.; Liu, W.; Hussain, A. FairBias: Mitigating bias in medical image diagnosis with mixed noise and class imbalance. Neurocomputing 2025, 651, 130910. [Google Scholar] [CrossRef]
- Jagtiani, P.; Karabacak, M.; Margetis, K. A concise framework for fairness: Navigating disparate impact in healthcare AI. J. Med. Artif. Intell. 2025, 8, 51. [Google Scholar] [CrossRef]
- Nanekaran, N.P.; Ukwatta, E. A novel federated learning framework for medical imaging: Resource-efficient approach combining PCA with early stopping. Med. Phys. 2025, 52, e18064. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Gichoya, J.W.; Katabi, D.; Ghassemi, M. The limits of fair medical imaging AI in real-world generalization. Nat. Med. 2024, 30, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Dolega-Dolegowski, D.; Dolega-Dolegowska, M.; Pregowska, A.; Malinowski, K.; Proniewska, K. The Application of Mixed Reality in Root Canal Treatment. Appl. Sci. 2023, 13, 4078. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Tosco, V.; Vitiello, F.; Orilisi, G.; Fraccastoro, F.; Putignano, A.; Orsini, G. Augmented, Virtual and Mixed Reality in Dentistry: A Narrative Review on the Existing Platforms and Future Challenges. Appl. Sci. 2022, 12, 877. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.