Abstract
Artichoke by-products are rich in bioactive compounds and could be valorized for use as flour. Blanching is a critical pretreatment in the production of flour from artichokes, as it helps preserve bioactive and nutritional compounds before cutting, drying and milling. However, studies on the blanching of artichoke by-products for flour production are scarce in contrast to those studies on edible artichoke parts. In this article, the effect of different blanching treatments (steam or immersion; 3–15 min) on the bioactive compounds (total phenolic content, TPC; total antioxidant capacity, TAC; and inulin) and color quality of flours of artichoke by-products (obtained after cutting, drying and milling of stems and bracts) was studied. Blanching treatments induced increases in TPC, TAC and inulin, although those increments varied greatly depending on the treatment type and artichoke part. In particular, steaming (15 min) induced the highest TPC increment in artichoke hearts and stems (94 and 46%, respectively); TAC increment in hearts, stems and bracts (987, 1107 and 1660%, respectively); and inulin increment in hearts and stems (106 and 14%, respectively). Steaming (3 min) induced the highest inulin increment in bracts (40%). Immersion (15 min) induced the highest TPC increment in bracts (632%). In addition, the short blanching treatment (3 min) was not enough to inactivate browning enzymes with browning index values of 75, 52 and 67, which were similar, or even higher, to control samples (unblanched). In conclusion, steam blanching for 15 and 3 min induced the highest bioactive contents in stems and hearts and inulin and TAC contents in bracts.
1. Introduction
The artichoke plant (Cynara cardunculus var. Scolymus) is a plant belonging to the Asteraceae family, native to the Mediterranean region and commonly consumed in this area since at least the fourth century BC []. The commercially valuable part of the artichoke corresponds to the immature plant inflorescence, known as the ‘head’. Depending on the cultivar, the artichoke head can weigh from 150 to 600 g. The head should be harvested unripe; otherwise, it loses its culinary value. However, the conventionally edible part corresponds to the inner part of the head, known as ‘heart’, and the inner bracts. The heart represents 10–18% of the total head weight, while the heart + innermost bracts sum represents around 40% of the head weight. This implies that 60–82% of the total head weight is biomass, which is discarded as waste. Hence, artichoke stems, leaves and outer bracts are also commonly discarded []. However, all these by-products are highly rich in nutrients and bioactive compounds with health-promoting properties, such as vitamins, minerals, polyphenols and inulin, among others []. Currently, there is an important trend of searching for numerous ways to valorize by-products from the food industry, as they pose an important environmental issue [,,]. The problem with artichoke is that its by-products are usually very fibrous and inedible. Many options have been proposed to valorize them, though they tend to rely on difficult, hardly scalable techniques [,]. Nevertheless, artichoke by-products could be easily and efficiently valorized through a drying process that would allow their incorporation into flour in many different forms []. However, artichoke is rich in endogenous enzymes (mainly peroxidase and polyphenol oxidase), which can cause an off-flavor and off-color when the artichoke is cut, peeled and comes into contact with polyphenols []. Hence, to inactivate the endogenous enzymes, mild thermal treatments (commonly known as blanching) are conducted before the artichoke is processed, as previously studied in the literature [,,]. Similarly, it is highly important to blanch artichoke by-products to ultimately obtain a high-quality flour (after milling and drying). The different tissue structure and composition of artichoke by-products (stems and outer bracts) compared to the edible part (heart and inner bracts) lead to different thermal properties (i.e., different thermal treatment efficiency) due to (i) less water content, which has higher conductivity than the fibrous structures (mainly composed of lignin and cellulose); and (ii) harder and more compact fibers, which behave as thermal insulators. Therefore, it is very important to study the effects of blanching to optimize the blanching + drying process to obtain a high-quality artichoke by-product flour.
Regarding blanching of artichoke by-products, Ruiz-Cano et al. [] studied the effect of blanching (5–30 min, 96 °C) on artichoke by-products, mainly stems and bracts. Regarding the edible part of the artichoke, Guida et al. [] studied the effect of immersion + ohmic blanching (0–5 min at 80 °C). Şahin et al. [] also studied the blanching effect (steaming for 1 min at 98 °C and immersion for 1 min at 98 °C) on artichoke hearts, followed by drying (vacuum drying (10–25 kPa vacuum pressure) at 70 °C for 240–375 min). Muştu & Eren [] also considered alternative drying techniques, such as microwave drying (450–800 W, for 1–25 min), on artichoke heart slices that did not undergo prior blanching. Other studies have investigated the effect of drying on the nutritional and bioactive contents of artichoke by-products (bracts and stems) [], but without studying the effect of previous blanching. Finally, regarding blanching + drying of artichoke by-products, only Icier [] studied the effect of different blanching treatments (5–9.5 min immersion at 85–100 °C; 3.3 min ohmic at 85 °C) and subsequent fluidized bed drying (60–80 °C for 30–70 min) of artichoke bracts and stems. As observed, the literature regarding the study of the effects of blanching and subsequent drying of artichoke by-products is very scarce. Therefore, research on the effect of different blanching methods (e.g., immersion, steam) on the subsequent dried artichoke by-products is needed, as these treatments have a critical effect on the content of bioactive compounds of interest considered as quality indicators, such as polyphenols or inulin. Hence, understanding the effect of these treatments on artichoke by-products and on the edible part of the artichoke is key for optimal valorization.
The objective of this study was to investigate the effect of blanching (immersion or steam; 3–15 min) on the nutritional/bioactive contents of artichoke by-products (bracts and stems) and edible parts (hearts), which were subsequently dried (hot-air drying at 60 °C), for the production of artichoke flour with high content of bioactive components.
2. Materials and Methods
2.1. Plant Material
Artichokes (Cynara cardunculus var. scolymus cv. Blanca de Tudela) were purchased from a local supermarket (Murcia, Spain) in March 2024 and processed the same day. Artichokes were produced in open parcels in the Fuente-Álamo area (Murcia, Spain). Intact artichoke heads (i.e., including the inedible part (by-products: stem and outer bracts) and the edible part (heart and inner bracts)) were washed with cold tap water before blanching and drying treatments.
2.2. Blanching Treatments
Immersion (hot water) and steam blanching treatments were studied. Immersion blanching was carried out in a stainless-steel domestic pot (10 L capacity) with water at 99 °C (heated at maximum power in an electric hot plate (Severin Elektrogeräte; Sundern, Germany)) for 3 or 15 min. The artichoke heads were kept completely submerged during the immersion blanching treatments at an artichoke:blanching-water ratio of 0.15:1 (weight (w):volume (v)). For steam blanching, the same pot was used, but it was partially filled with tap water at the pot bottom, and a perforated stainless-steel grid was placed on the water to avoid product contact with water. Steam blanching was conducted at atmospheric pressure, with the pot lid semi-open. After blanching treatments, the blanched product was drained with a domestic vegetable drainer and left to cool at room temperature (approximately 5 min). Finally, the artichoke heads were vacuum-packed (vacuum sealer SFS 110 B2, Silvercrest; Hamburg, Germany) in polypropylene-embossed bags (15 × 30 cm) using vacuum packaging equipment and stored at −20 °C until drying treatments were conducted (<2 weeks).
2.3. Drying Treatments
The frozen blanched samples were thawed at 4 °C for 24 h in the dark and then manually cut (using a ceramic knife) into the edible part (heart and inner bracts) and their by-products (stems and outer bracts) before the drying treatments. In the case of stems and hearts, they were cut into 0.5 cm slices. The weights of the different artichoke parts were as follows: head, 224.2 ± 74.3 g; hearts, 31.47 ± 16.6 g; outer bract (1 unit), 1.65 ± 0.67 g; and stems, 16.46 ± 8.48 g (4.4 ± 0.2 cm length).
Drying was conducted using a prototype of a forced-air convection drying tunnel with an integrated precision balance (±0.01 g, model AH-300, Gram Precisión S.L.; Barcelona, Spain) to allow continuous product weight monitoring (Figure 1). As the heat source, the prototype included an electric air heater (electric fan heater 2000 W, King d’home; Rosny-sous-Bois, France) at 90% of its total power (2000 W), which maintained a temperature of 60 ± 2 °C inside the drying tunnel (measured with a thermohygrometer; PCE-555, PCE Ibérica S.L.; Albacete, Spain). The relative humidity inside the drying tunnel was 5.5 ± 0.5% (measured with the thermohygrometer), and the air velocity was 1.0 ± 0.1 m s−1 (measured with an anemometer; PCE-AM 81, PCE Ibérica S.L.; Albacete, Spain). These drying conditions were adjusted to those commonly used in the food industry for vegetable and artichoke drying []. Samples were arranged (in a 1–2 cm thick layer) on a perforated stainless-steel grid placed over the precision balance of the drying tunnel. Samples were dried until product weight variations were below 0.5%. In brief, the approximate drying times were 135, 210–270 and 300–360 min for bracts, stems and hearts, respectively. Drying trials were performed in triplicate for each of the factors (blanching treatment and artichoke part). Finally, dried samples were ground (Bosch TSM6A013B, Bosch; Stuttgart, Germany), vacuum-packed in bags (as described above) and stored at room temperature in the dark until further analyses.
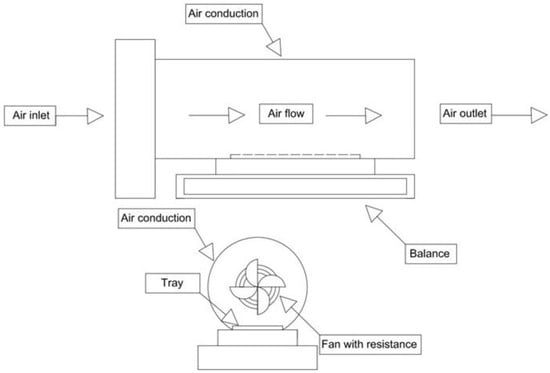
Figure 1.
Diagram of the forced-air convection drying tunnel prototype.
2.4. Phenolic Compounds and Antioxidant Capacity Analyses
Extracts for the analysis of phenolic compounds and total antioxidant capacity were obtained using ISO 14502-1 methodology, with slight modifications (International Standard Organization [ISO], []). For analysis, 1 g of dried sample (previously milled, <0.5 mm pore size) was added to 55 mL of hot water at 52 °C and allowed to extract at room temperature for 30 min. Subsequently, 10 mL of MeOH was added and, finally, distilled water was added until the mixture reached 100 mL. It was then centrifuged at 2030× g for 10 min at 22 °C (Mixtasel p 540, JP Selecta K; Barcelona, Spain). The obtained supernatant was used as the phenolic/total antioxidant extract.
Analyses of the total phenolic content (TPC) of the above extracts were made by the Folin–Ciocalteu method, with slight modifications []. In brief, 1 mL of the extract (or blanching water) was mixed with 5 mL of Folin–Ciocalteu reagent (0.2 N) and allowed to react for 8 min, after which 4 mL of a 0.7 M sodium carbonate solution was added. Distilled water was used as a blank. Then, the mixture was incubated for 1 h at room temperature in the dark, and finally, the absorbance was measured at 765 nm in a spectrophotometer (Nicolet Evolution 300, Thermo; Waltham, MA, USA). The results were expressed as gallic acid equivalents (GAE) per dry weight (DW) of flour (mg g−1).
Analysis of the total antioxidant capacity (TAC) of the above extracts was performed according to the DPPH (2,2-diphenyl-1-picrylhydrazyl) method []. In brief, 1.2 mL of adjusted DPPH solution (adjusted to 1.10 ± 0.02 absorbance at 517 nm) was added to 0.4 mL of the extract and incubated in the dark at room temperature for 10 min. Finally, the absorbance at 517 nm was measured in the spectrophotometer. The results were expressed as ascorbic acid equivalents (AAE) per dry weight of flour (mg g−1).
2.5. Inulin Analysis
The determination of inulin content was performed following the method of El Sayed et al. [], with slight modifications. In brief, 1 g of dried sample was extracted with 90 mL of hot (85 °C) ultrapure water in a water bath with stirring at 85 °C for 25 min and allowed to cool to room temperature. Then, it was filled up to 100 mL with ultrapure water and centrifuged (10,000× g, 20 min), and finally, the supernatant was filtered through a 0.2-µm PTFE syringe filter. This extract was used as the inulin extract.
Inulin analysis of the previous extract was conducted using an HPLC-RID device (DGU-20 A degasser, LC-170 30AD quaternary pump, SIL-30AC autosampler, CTO-10AS column heater, refractive index detector (RID); Shimadzu, Kyoto, Japan). A Luna NH2 column (150 × 4.6 mm, 5 µm, Phenomenex, Macclesfield, UK) was used for chromatographic separation using 40:60 (v:v) water:acetonitrile mixture in isocratic mode for 50 min. Chromatographic conditions consisted of a column temperature of 40 °C, flow rate of 0.6 mL min−1 and injection volume of 20 µL. The refractive index was recorded, and inulin content was quantified with commercial HPLC-grade standard (Sigma-Aldrich; Berlin, Germany) prepared at 5, 2.5, 1, 0.5 and 0.25 mM. The results were expressed in mg g−1 dry weight of flour.
2.6. Color
Color was measured using a colorimeter (TCD-100, Beijing TIME High Technology; Beijing, China) based on the CIELAB color space. This system is based on three coordinates, where L* represents the lightness of the color component (from 0 to 100, with zero being black and 100 being white). The coordinates a* and b* represent the green–red and blue–yellow axes, respectively (from −60 to +60, in both cases). Additionally, the color indices total color difference (TCD), hue angle (h°) and browning index (BI) were calculated, as shown in Equations (1)–(3). For color determination, 3 measurements of the artichoke powder (placed on a surface forming a 0.5 cm thick layer) were taken and automatically averaged by the colorimeter.
where L0*, a0* and b0* are the values of the control artichoke (i.e., not blanched), while L*, a* and b* refer to the blanched samples.
2.7. Statistical Analysis
The effect of the different blanching and drying treatments on TPC, TAC and inulin, as well as the drying behavior, was tested by performing one-way ANOVA. Fisher’s least significant difference (LSD) test was performed to find statistically significant differences, p < 0.05. All determinations were made in triplicate, and data were expressed as mean ± SD. The tests were performed using STATGRAPHICS Centurion v15.2 software (2025 Statgraphics Technologies. Inc.; The Plains, VA, USA). Correlation (R2) of color indexes with TPC, TAC and inulin was evaluated using Excel software 365 (Microsoft, Redmont, WA, USA).
3. Results and Discussion
3.1. Drying Behavior
The drying trend of artichoke samples can be observed in Figure 2. In general, the drying behavior of all samples was similar, reaching final moisture values of 20–25%. In other fruit and vegetables, it is possible to reach lower moisture values of approximately 5–10% in industrial conditions []. However, the structure of artichoke tissue, rich in fiber, makes the moisture-loss process during drying very difficult, as samples are very hygroscopic after drying []. Carbohydrate polymers are known to form complex structures with water through chemical linking. Particularly, it is known that the hydroxyl groups from lignin are enough to act as a reaction site for hydrogen bond formation with water molecules, binding them. Therefore, water diffusion is strongly affected by these carbohydrate-water complexes present in plant tissue []. Thus, Lutz et al. [] observed moisture levels of 20% in artichoke (cv. Green Globe) hearts that were blanched (pressure cooker for 10 min) and dried (forced air at 50 °C for 5 days).
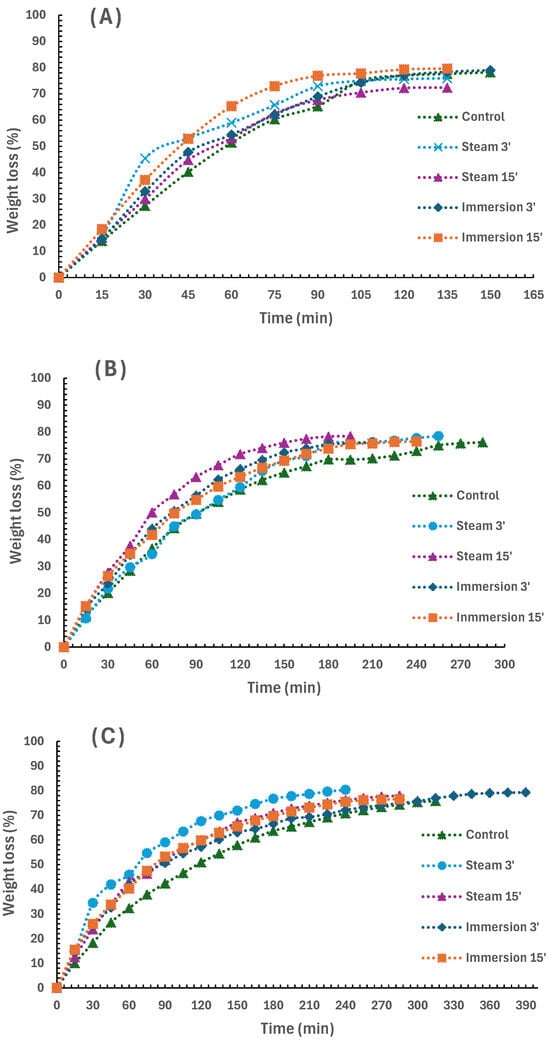
Figure 2.
Effect of drying process on the weight loss of bracts (A), stems (B) and hearts (C) of artichoke heads previously blanched by immersion (3 and 15 min) and steam (3 and 15 min), or unblanched (control) (mean (n = 3)).
The drying trends were different depending on the artichoke part and the blanching treatment used. First, the drying time was considerably shorter in the case of the bracts, ranging from 135 to 150 min in all cases. This is probably due to the tissue structure of the bracts, since, being a wide and thin sheet, the surface volume ratio is much higher, making the effective area of heat transmission greater. Therefore, moisture diffusion increased in the bracts, facilitating drying []. In contrast, hearts and stems required longer drying times of 195–285 min and 240–390 min, respectively. Moreover, differences were observed depending on the treatment, while in the case of bracts, all treatments required a similar time.
Another important trend observed was that, in all cases, the drying rate of control samples was lower than that of the blanched samples, except for the case of the 15 min steam-treated bracts. This indicates the importance of blanching for more efficient subsequent drying of the samples. For bracts and hearts, significant differences (p < 0.05) were observed between control final weight loss and all four treatments. In the case of stems, no significant differences (p > 0.05) were found between control final weight loss and immersion treatments. However, there was a difference between the control and both steam treatments. The fact that steam treatment is more efficient for the case of stems and hearts may be because, after blanching treatment, plant tissues become more permeable to moisture and, therefore, increase water absorption []. Furthermore, these same authors observed how the moisture content after an immersion blanching process (95 °C, 5 min) was significantly higher in the more internal bracts. This could be especially relevant in the case of artichoke hearts, since their structure is composed of numerous sheets that are very tightly packed together and highly retain moisture. Thus, steam blanching leads to less water absorption than blanching by immersion in water and, therefore, subsequent drying would be facilitated. The fact that the most effective drying in the hearts was obtained with the 3 min steam treatment seems to support this. In contrast, the outer bracts of the artichoke are mature structures very rich in cellulose, lignin and other heteropolysaccharides []. It is therefore possible that a more intense blanching treatment, such as immersion, is necessary to induce structural changes in bract tissues that would favor the observed better moisture diffusion during drying []. In summary, the best treatments for each artichoke part, defined as the fastest drying treatment until final weight, were 15 min immersion for bracts (79.65 ± 0.49% weight reduction in 135 min), 15 min steaming for stems (78.48 ± 0.67% weight reduction in 195 min) and 3 min steaming for hearts (80.38 ± 0.29% weight reduction in 240 min). Particularly in the case of hearts, the 3 min steaming treatment was the best, with a significant difference (p < 0.05), compared with the rest of the treatments.
3.2. Total Phenolic Content
The TPC results of the samples after treatment are shown in Figure 3. The TPC values of the different artichoke parts of unblanched samples (control) were 23.5, 23.0 and 2.22 mg GAE g−1 DW for stems, hearts and bracts, respectively. The literature shows that the polyphenol content in the edible parts of artichokes is extremely variable depending on the cultivar and part of the artichoke []. Thus, the artichoke heart generally has a higher polyphenol content than the stem, but different results have been observed in other artichoke cultivars [,,,,]. However, there is a consensus regarding the lower content of the outer bracts of artichokes compared to the rest of the parts, which agrees with our data [,].
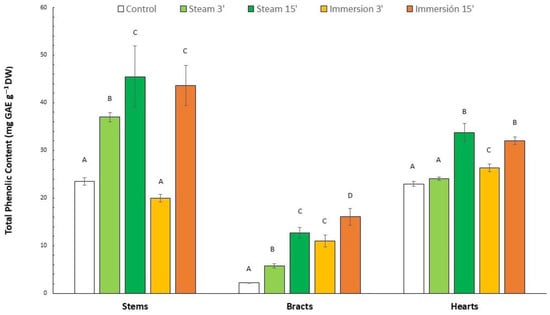
Figure 3.
Total phenolic content of different artichoke parts after different blanching treatments by immersion (3 and 15 min) and steam (3 and 15 min), or unblanched (control), and subsequent drying to obtain flours (mean (n = 3) ± SD). Different letters indicate significant differences (p < 0.05) among blanching treatments for each fraction.
The TPC was 20.0–43.6, 24.1–33.7 and 5.7–16.1 mg GAE g−1 DW for stems, hearts and bracts, respectively. Hence, a TPC increase was observed in all the blanched samples compared to the unblanched control samples. Nevertheless, no differences (p > 0.05) were observed for the 3 min steamed hearts and the 3 min immersion stems. This phenomenon could be explained because intense thermal treatments would cause a rupture or softening of cellular tissues, releasing antioxidant compounds into the medium and thus increasing their bioavailability. In addition, such thermal treatment could break down other more complex compounds, releasing individual phenolic compounds capable of reacting with the Folin–Ciocalteu reagent []. This effect is particularly prominent in the case of bracts, where an increase in the extractability of these phenolic compounds of up to 632% is observed for 15 min immersion blanching []. According to Domínguez-Fernández et al. [], this may be because the intense heat treatment can induce the release of a certain amount of bound phenolic compounds, thus increasing their extractability and bioavailability. These bound phenolic compounds are generally not identified with traditional phenolic compound assays, such as the Folin–Ciocalteu, which is employed in most studies. Most of the phenolic compounds present in the tissues may be in their bound form, probably due to the very fibrous nature of the bracts, and are released after plant tissue disruption due to thermal treatment. This may explain the TPC increase in bracts after thermal treatment, especially since immersion blanching is a more intensive treatment than steam blanching.
Although no studies on the blanching and subsequent drying of the phenolic content of artichoke by-products have been found, there is existing literature on other types of heat treatments. Thus, Kayahan & Saloglu [] found increased TPC in edible artichoke parts (hearts) of the cv. Bayrampasa (from 212 to 2447 mg GAE 100 g−1 fresh weight (FW)) and cv. Sakiz (from 844 to 1836 mg GAE 100 g−1 FW) after a different cooking treatment (boiling in hot water at 95 °C for 18 min). Similarly, Lutz et al. [] observed a 50% increment of TPC of artichoke (cv. Green Globe) edible parts (hearts) after blanching with a pressure cooker (10 min), followed by drying (50 °C for 5 days in an oven). Rinaldi et al. [] also observed increments of 800% and 1140% in the TPC of artichoke (cv. Violetto) edible parts (hearts) after blanching by immersion and steam blanching (conditions not described), respectively. However, Guida et al. [] reported a 27% reduction of the TPC of artichoke edible parts (hearts) after blanching (immersion for 8 min in water at 100 °C). Guida et al. [] explained that these contradictory data resulted from the artichoke cultivar used, which had fewer bound phenolic compounds than the artichokes used in the studies that showed the opposite trend (increments), as similarly reported by Domínguez-Fernández et al. [].
The 15-min blanching treatments (steam or immersion) showed a greater increase in the bioavailability of phenolic compounds compared to their 3-min counterparts. This may be explained by the short blanching period, since the 3 min blanching was not enough for the release of all the bound phenolic compounds described above or for a complete inactivation of polyphenol oxidase. The inactivation of oxidative enzymes is one of the fundamental reasons for applying blanching treatments []. For example, Abdulaziz et al. [] observed traces of peroxidase and catalase activity in artichoke hearts blanched by immersion for 10 min. Guida et al. [] described that blanching by immersion for 8 min was enough to completely inactivate peroxidase in artichoke hearts, but 3 min of blanching still led to peroxidase activity of approximately 45%. It has been suggested that optimal blanching should reach between 3–10% residual peroxidase activity []. However, we observed that even a blanching pretreatment of only 3 min has a positive effect on the bioavailability of phenolic compounds, except in the case of stems treated by immersion for 3 min. In addition, we observed that the blanching effectiveness varies depending on the artichoke part. For example, in the case of bracts, the 15-min immersion induced the highest bioavailability increment of the TPC (632%) (Figure 3).
In contrast, for hearts and stems, blanching by steaming was more effective than immersion. In both cases, steaming for 15 min obtained the highest TPC increments (94% for stems and 46% for hearts) when compared to the control. It has been suggested on many occasions that immersion blanching causes greater leaching of bioactive compounds into the environment (blanching wastewater) than steaming []. This phenomenon has been observed in other vegetables such as spinach, carrots, mushrooms and peas []. Specifically, Rinaldi et al. [] observed by optical microscopy a higher plant cell disruption in artichoke hearts after immersion compared to steaming (conditions not described). After subjecting several artichoke hearts to an immersion and steam blanching treatment, they observed a higher TPC in the steam-cooked hearts (6.5 vs. 4.5 mg GAE g−1 DW). Though the increments reported are much higher (1140 and 800%, respectively, compared to the control), the final TPC is much lower than the TPC obtained in our study (Figure 3). Similarly, Ferracane et al. [] observed a TPC increase (68%) in artichoke hearts after steam blanching (22 min) compared to immersion (98 °C, 15 min; 44%). However, in our study, no significant differences (p > 0.05) were observed between blanching by steaming and immersion, both at 15 min (Figure 3).
After the immersion treatment, TPC values of 12.9 and 18.9 mg GAE L−1 were observed in the immersion water after 3 min and 15 min, respectively. To our knowledge, no previous studies have analyzed the TPC of artichoke blanching water. Considering that the cooking volume corresponding to one artichoke head was 1.5 L, this is equivalent to a total of 19.35 and 28.35 mg GAE released into 1 L of the blanching water, respectively. The average weight of the artichoke heads used was 224.2 ± 74.3 g. Hence, this would be equivalent to 0.08 and 0.12 mg GAE released into the environment per gram of FW. Therefore, in the case of artichoke hearts (and also artichoke stems), the utilization of steam blanching is preferable to immersion to avoid excessive wastewater effluents.
3.3. Total Antioxidant Capacity
The TAC results for both the fresh control and the samples after blanching and drying treatments are shown in Figure 4. The TAC values of the control artichoke were similar (p > 0.05) in both stems and hearts (22.95 and 23.99 mg AAE g−1 DW, respectively), and considerably lower in the case of bracts (13.76 mg AAE g−1 DW) (Figure 4). Therefore, the same trend was observed as for the TPC. An increase in TAC was observed in all cases compared to the control, after the application of all treatments.
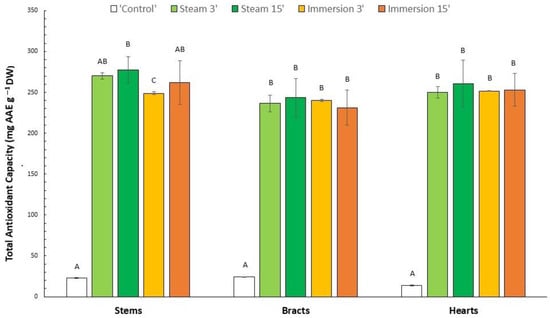
Figure 4.
Total antioxidant capacity of different artichoke parts after different blanching treatments by immersion (3 and 15 min) and steam (3 and 15 min), or unblanched (control), and subsequent drying to obtain flours (mean (n = 3) ± SD). Different letters indicate significant differences (p < 0.05) among blanching treatments for each fraction.
Furthermore, it is noteworthy that the increase in TAC recorded is considerably large. This increase ranged from 942% (250.2 mg AAE g−1 DW) in 3 min steamed hearts to 1660% (243.4 mg AAE g−1 DW) in 15 min steamed bracts. These results agree with those obtained by Ferracane et al. [], who observed high TAC increases (1018–1423%) in artichokes after steam and immersion blanching treatments (without subsequent drying, unlike our study). Lutz et al. [] described a TAC increase (up to 1200%) in artichoke hearts (cv. Green Globe) after immersion blanching (10 min, 98 °C) followed by drying (50 °C in an oven, 5 days). In contrast, Borsini et al. [] observed TAC decreases in stems and bracts (81–85% reduction) after drying (60 °C, 400–600 min), but without previous blanching. Jiménez-Monreal et al. [] also observed that TAC of artichoke hearts increased after different cooking treatments (boiling, microwaving, pressure-cooking, griddling, frying and baking), while such TAC (determined by lipoperoxyl and hydroxyl radical scavenging, not DPPH) enhancement was not observed by those authors in twenty other studied vegetables (asparagus, broccoli, eggplant, maize, onion and spinach, among others).
Comparing the different blanching treatments for stems, hearts and bracts, we observed that the highest TAC increase was observed after the 15 min steam blanching for stems, hearts and bracts (1107, 987 and 1660%, respectively). This treatment also obtained the best results for TPC for stems and hearts. However, for bracts, the best treatment for TPC was the 15 min immersion. Contrary to TPC, no TAC differences (p > 0.05) were observed between the four different blanching treatments for bracts and hearts. In the case of stems, the 15 min steam blanching induced a TAC increment. On the other hand, the 3 min immersion treatment for stems induced the lowest TAC of the four blanching treatments. Coincidentally, this treatment also showed the lowest TPC in stems, as previously observed.
The TAC correlation with TPC was very low for hearts, stems and bracts with R2 values of 0.53, 0.32 and 0.33, respectively (Table 1). It has been proposed that this could be due to the hydrolysis and transesterification phenomena that caffeoylquinic acids, the main phenolic compounds in artichoke, undergo when subjected to high temperatures, while other antioxidant compounds may be more resistant to thermal degradation. These well-known phenomena lead to significant redistributions of the phenolic profile []. The spatial distribution of functional groups in phenolic compounds has been observed to have significant effects on TAC []. A study on the TAC of individual caffeoylquinic acids in bamboo found that isomeric compounds exhibited significant TAC differences when tested in comparison with the overall DPPH method []. Caffeoylquinic acids constitute by far the largest proportion of phenolic compounds found in artichokes. In fact, caffeoylquinic acid content can represent up to 8% on a dry matter basis in artichoke young tissues []. Therefore, blanching-induced isomerization of antioxidant compounds may lead to enhanced TAC.

Table 1.
Correlations (R2) of color indexes (TCD, total color difference; BI, browning index) with total phenolic content (TPC) and total antioxidant capacity (TAC) of different artichoke parts after blanching by immersion (3 and 15 min) and steam (3 and 15 min), and in the unblanched control, after subsequent drying.
3.4. Inulin
The inulin contents for both the fresh control and the samples after blanching and drying treatments are shown in Figure 5. The inulin contents of control samples were 170.9, 73.7 and 81.4 mg g−1 DW for stems, bracts and hearts, respectively. Lattanzio et al. [] found higher inulin contents in fresh artichoke hearts of nine different cultivars, which ranged from 189 to 362 mg g−1 DW. This may be explained by the fact that the cultivar used in our study may contain less inulin than those studied by that author, apart from other factors like production zone, cultural practices, etc. In addition, Lattanzio et al. [] clarified that large inulin variations may be due to different physiological states of samples, since it is impossible to accurately determine the age of a series of artichoke heads.
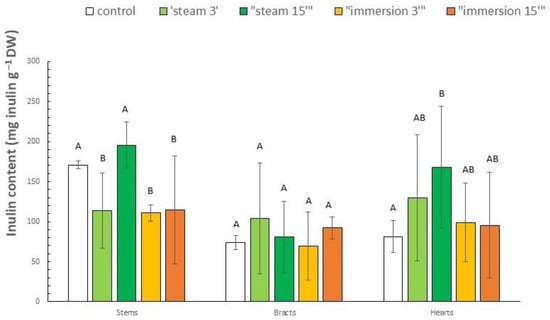
Figure 5.
Inulin content of different artichoke parts after different blanching treatments by immersion (3 and 15 min) and steam (3 and 15 min), or unblanched (control), and subsequent drying to obtain flours (mean (n = 3) ± SD). Different letters indicate significant differences (p < 0.05) among blanching treatments for each fraction.
Regarding the different blanching treatments, in the stems, inulin contents ranged from 111 (3 min immersion) to 195.5 mg g−1 DW (15 min steam). Except for the 15 min steam, the other three blanching treatments recorded similar losses of around 33%. The 15 min steam remained like the control (p > 0.05). For the bracts, the range was between 69.3 (3 min immersion) and 103.8 mg g−1 DW (3 min steam). In this case, all four treatments also remained (p > 0.05) like the control. In the hearts, values between 95.3 (15 min immersion) and 168.1 mg g−1 DW (3 min steam) were recorded. The 15 min steam treatment showed an increase compared to the control (106%), and the other three treatments remained similar. Overall, the highest contents were observed in the steam treatments compared to the immersion treatments. Specifically, the most effective treatments were 15 min steam (14% increment), 3 min steam (40% increment) and 15 min steam (106% increment) for stems, bracts and hearts, respectively. The previous results may be explained by inulin being a highly water-soluble compound [,]. Immersion blanching treatments may cause greater inulin loss through leaching. Furthermore, the observed highest inulin increase in the heart could be explained by it being the innermost fraction. Hence, this would make it difficult for inulin to leach into the blanching medium. This could also explain why it is the only fraction in which all treatments, to a greater or lesser extent, show an increase compared to the control. In turn, this increase compared to the control observed in almost all treatments could be due to changes in the tissues and in the inulin chains that favor greater extractability, despite possible losses due to leaching, although no previous literature has addressed this.
In general, the inulin content of artichokes has not been widely studied beyond the edible fraction. El Sayed et al. [] reported inulin contents of 41.5 mg g−1 DW in a mixture of artichoke (cv. Balady) leaves, bracts, stems and hearts. This mixture was dried and ground using forced air, although the working conditions are not described, and was not subjected to prior blanching. This result is considerably lower than the lowest value obtained in this study in pure bract samples, which are the fraction with the lowest inulin content. Likewise, Francavilla et al. [] analyzed the inulin content of a series of artichoke cv. Madrigal hearts and stems. Again, the samples were dried (no pre-blanching was performed) at 60 °C (no convective forced-air drying oven) and extracted following a procedure like that used in this work, except for the use of microwaves (microwave-assisted extraction with water at 80 °C for 5 min; artichoke weight:water ratio of 1:10 (w/v)). The same authors ([]) observed an inulin content of hearts of 93 mg g−1 DW. This value is very similar to our data for the 3 min immersion (99 mg g−1 DW) and 15 min immersion (95 mg g−1 DW) treatments. However, Francavilla et al. [] also reported an inulin content in stems of 29 mg g−1 DW, which was well below the lowest values obtained in this work (111 mg g−1 DW for the 3 min immersion treatment) (Figure 5). Meanwhile, Ruiz-Cano et al. [] also analyzed the inulin content in artichoke bracts (cv. Blanca de Tudela) from industrial waste that had been subjected to a blanching process (5 min immersion, 96 °C) or blanching + cooking (30 min immersion, 96 °C), and finally oven-dried (70 °C for 24 h; non-convective forced-air drying). Surprisingly, they observed that the inulin content of the cooked bracts was higher than the blanched bracts, the same trend observed in our study for the 3 min immersion and 15 min immersion treatments (Figure 5). The inulin values observed by those authors for the control bract samples (fresh, unblanched) were similar to those in our study (100 vs. 73 mg g−1 DW, respectively). Finally, Noriega-Rodríguez et al. [] analyzed the inulin content of a mixture of industrial waste composed mainly of bracts (cultivar and pretreatment unknown). The plant material samples were freeze-dried, ground and subjected to a hydroalcoholic extraction process (40 °C, ethanol:water 75:25 (v:v), solid:solvent ratio 1:20 (w:v)). The results obtained were 70 mg g−1 DW, very similar to those obtained in our work for the 3 min immersion treatment (69 mg g−1 DW), and somewhat lower than those obtained for the other treatments (between 80 and 103 mg g−1 DW).
3.5. Color
The color data of artichoke flours is shown in Table 2. Longer blanching treatments, regardless of immersion or steaming, induced lower BI changes than shorter ones when compared to control samples. However, the 3 min treatments (both immersion and steaming) showed similar or even slightly higher BI than the longer treatments. This may be explained by a higher thermal inactivation of polyphenol oxidase, leading to lower enzymatic browning []. This is consistent with TPC data, since longer blanching treatments led to higher TPC. It could also be due to increased thermal-induced extractability of the polyphenols and enzymatic inactivation, which led to lower biosynthesis of quinones and other related polyphenol oxidation enzymatic products responsible for artichoke browning.

Table 2.
Color of different artichoke parts after different blanching treatments by immersion (3 and 15 min) and steam (3 and 15 min), or unblanched (control), and subsequent drying to obtain flours (mean (n = 3) ±SD). Different letters indicate significant differences (p < 0.05) among blanching treatments for each fraction.
The correlation of the different color indexes is shown in Table 1 to elucidate the most representative color index during blanching + drying for the different artichoke parts. Overall, TCD showed the highest correlations (R2 = 0.93–0.94) with TPC for all three studied artichoke parts. The BI was inversely correlated with TPC due to the enzymatic oxidation of polyphenols into colored quinones and related oxidation products, as previously commented. Interestingly, the BI of stems and bracts showed lower correlations with TPC. This may be explained by the lower BI of stems and bracts, which may be due to a higher efficiency of the thermal enzymatic inactivation in these thinner plant parts compared to the artichoke hearts. However, h° showed a correlation with hearts TAC (R2 > 0.6) and a strong correlation with hearts TPC (R2 > 0.9).
There is no previous literature studying the effects of different blanching treatments followed by drying on the color of different artichoke parts to obtain artichoke flour. Nevertheless, focusing on available literature on drying treatments of artichokes (without prior blanching), Canale et al. [] studied the effect of drying treatments (oven drying at 40 ± 5 °C for 24 h or 48 h) to obtain flour from the hearts + stems of artichokes (cv. Violetto di Ramacca). The BI data reported by those authors [,] were very similar to those of the long blanching treatments of our study. Interestingly, the much longer thermal treatment times (24–48 h) from those authors, compared to our study, led to lower BI, probably due to a higher enzymatic inactivation as previously discussed. Mustu & Eren [] also studied the effects of microwave drying on the color of artichoke hearts (without prior blanching), observing a h° reduction (increased redness compared to yellowness increment, indicating greater browning).
Regarding the literature related to the effects of blanching treatments of different artichoke parts (without subsequent drying), Ferracane et al. [] reported L* and b* decreases in stems after steam (15 min) and immersion (22 min) blanching treatments, while those color parameters increased after those treatments for bracts. They also reported an a* increase in bracts after immersion blanching, which agrees with our study. Ihl et al. [] also observed the same behavior, explaining that it could be due to a lower chlorophyll degradation or a different chlorophyll conversion pattern. Likewise, the rapid expulsion of intercellular air, as well as its replacement by water (and other cellular fluids), during immersion blanching treatments could affect other optical properties, such as surface reflectance or the ability of light to penetrate the tissue. Guida et al. [] reported that L* of artichoke hearts decreased after a combined blanching treatment (immersion + ohmic), which is in agreement with our data.
4. Conclusions
This investigation studied different blanching treatments of the edible parts of an artichoke, as well as of artichoke by-products (stems and bracts), prior to drying to obtain artichoke flours. Hence, this process method to obtain flour from artichoke by-products with high nutritional/bioactive quality can be easily scaled at an industrial level, aiming for a circular economy. The parameters studied in this work, especially total phenolic content, total antioxidant capacity and color, are easy determinations conventionally considered as quality indexes. Hence, knowing how these industrial treatments affect the different parts of the artichoke is key to allowing large-scale valorization. Steam blanching enhanced total antioxidant capacity and phenolic and inulin contents, especially for the 15 min steam blanching, as observed in the total antioxidant capacity of the hearts, stems and bracts. In addition, the 15 min steam blanching also induced high phenolic and inulin enhancements in stems and hearts. On the other hand, dried samples without prior blanching showed a lower nutritional/bioactive quality. In particular, among blanching methods, steaming was more effective than immersion in inducing the subsequent fastest drying time in stems and hearts (15 min and 3 min, respectively), while the 15 min immersion was more effective in bracts. Nevertheless, steam blanching treatments performed worse in terms of color compared to immersion treatments. Overall, steam blanching allows for, in general terms, the best nutritional and bioactive properties, even though the different parts studied behave differently. Color is a fundamental property of food. However, most artichokes blanched in the industry are used for canning. They could also be used to obtain flour that would be formulated into other products, as has been proposed in many studies. In this way, color might not be such an important factor. Furthermore, steam blanching, unlike the immersion blanching currently used in the artichoke industry, would significantly reduce wastewater effluents and the environmental impact of the process.
Author Contributions
Conceptualization, L.T.-D., G.B.M.-H. and F.M.-I.; methodology, L.T.-D. and G.B.M.-H.; validation, G.B.M.-H.; investigation, L.T.-D., F.J.L.-A., J.S.-M. and M.J.-M.; resources, G.B.M.-H. and F.M.-I.; writing—original draft preparation, L.T.-D.; writing—review and editing, L.T.-D., F.M.-I. and G.B.M.-H.; visualization, L.T.-D.; supervision, F.M.-I. and G.B.M.-H.; project administration, F.M.-I.; funding acquisition, F.M.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant ALICHAR CPP2022-009740, funded by MCIN/AEI/10.13039/501100011033/ and by the “European Union NextGenerationEU/PRTR”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets generated during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Abbreviations
The following abbreviations are used in this manuscript:
| TPC | Total Phenolic Content |
| TAC | Total Antioxidant Capacity |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| BI | Browning Index |
| TCD | Total Color Difference |
| DW | Dry Weight |
| FW | Fresh Weight |
| GAE | Galic Acid Equivalents |
| AAE | Ascorbic Acid Equivalents |
References
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Nutritional and functional properties of cynara crops (Globe Artichoke and Cardoon) and their potential applications: A Review. Int. J. Appl. Sci. 2012, 2, 64–70. [Google Scholar]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Tortosa-Díaz, L.; Saura-Martínez, J.; Taboada-Rodríguez, A.; Martínez-Hernández, G.B.; López-Gómez, A.; Marín-Iniesta, F. Influence of industrial processing of artichoke and by-products on the bioactive and nutritional compounds. Food Eng. Rev. 2025, 17, 384–407. [Google Scholar] [CrossRef]
- Sedlar, T.; Čakarević, J.; Tomić, J.; Popović, L. Vegetable by-products as new sources of functional proteins. Plant Foods Hum. Nutr. 2021, 76, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Dhumal, S.; Singh, S.; Pandiselvam, R.; Rais, N.; Natta, S.; Senapathy, M.; Sinha, N.; et al. Onion (Allium cepa L.) peel: A review on the extraction of bioactive compounds, its antioxidant potential, and its application as a functional food ingredient. J. Food Sci. 2022, 87, 4289–4311. [Google Scholar] [CrossRef]
- Quispe, M.A.; Valenzuela, J.A.P.; de la Cruz, A.R.H.; Silva, C.R.E.; Quiñonez, G.H.; Cervantes, G.M.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols From Globe Artichoke (Cynara scolymus L.) Bracts Residues Using Response Surface Methodology. Acta Sci. Pol. Technol. Aliment. 2021, 20, 277–290. [Google Scholar] [CrossRef]
- Benkhoud, H.; Baâti, T.; Njim, L.; Selmi, S.; Hosni, K. Antioxidant, antidiabetic, and antihyperlipidemic activities of wheat flour-based chips incorporated with omega-3-rich fish oil and artichoke powder. J. Food Biochem. 2021, 45, e13297. [Google Scholar] [CrossRef]
- Santos, D.; Lopes da Silva, J.A.; Pintado, M. Fruit and vegetable by-products’ flours as ingredients: A review on production process, health benefits and technological functionalities. LWT 2022, 154, 112707. [Google Scholar] [CrossRef]
- Sergio, L.; Gatto, M.A.; Spremulli, L.; Pieralice, M.; Linsalata, V.; Di Venere, D. Packaging and storage conditions to extend the shelf life of semi-dried artichoke hearts. LWT 2016, 72, 277–284. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A. The effect of cooking on the chemical composition of artichoke (Cynara scolymus L.). Afr. J. Food Sci. 2013, 4, 182–187. [Google Scholar] [CrossRef]
- Guillén-Ríos, P.; Burló, F.; Martínez-Sánchez, F.; Carbonell-Barrachina, Á.A. Effects of processing on the quality of preserved quartered artichokes hearts. J. Food Sci. 2006, 71, 176–180. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- Guida, V.; Ferrari, G.; Pataro, G.; Chambery, A.; Di Maro, A.; Parente, A. The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT 2013, 53, 569–579. [Google Scholar] [CrossRef]
- Şahin, K.; Özcan Sinir, G.; Durmus, F.; Çopur, Ö. The effect of pretreatments and vacuum drying on drying characteristics, total phenolic content and antioxidant capacity of artichoke (Cynara cardunculus Var. scolymus L.) slices. Gıda 2020, 45, 699–709. [Google Scholar] [CrossRef]
- Muştu, C.; Eren, I. Drying kinetics, heating uniformity and quality changes during the microwave vacuum drying of artichokes (Cynara scolymus L.). Ital. J. Food Sci. 2019, 31, 681–702. [Google Scholar] [CrossRef]
- Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Icier, F. Ohmic blanching effects on drying of vegetable byproduct. J. Food Process Eng. 2010, 33, 661–683. [Google Scholar] [CrossRef]
- ISO 14502-1:2005; Determination of Substances Characteristics of Green and Black Tea. Part 1: Content of Total Polyphenols in Tea-Colorimetric Method Using Folin-Ciocalteu Reagent. International Standard Organization: Geneve, Switzerland, 2005.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Hussein, R.; Motaal, A.A.; Fouad, M.A.; Aziz, M.A.; El-Sayed, A. Artichoke edible parts are hepatoprotective as commercial leaf preparation. Rev. Bras. Farmacogn. 2018, 28, 165–178. [Google Scholar] [CrossRef]
- Afolabi, I.S. Moisture migration and bulk nutrients interaction in a drying food systems: A Review. Food Nutr. Sci. 2014, 5, 692–714. [Google Scholar] [CrossRef]
- Femenia, A.; Robertson, J.A.; Waldron, K.W.; Selvendran, R.R. Cauliflower (Brassica oleracea L), globe artichoke (Cynara scolymus) and chicory witloof (Cichorium intybus) processing by-products as sources of dietary fibre. J. Sci. Food Agric. 1998, 77, 511–518. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. In Biopolymers. Advances in Polymer Science; Abe, A., Dusek, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 232. [Google Scholar] [CrossRef]
- Lutz, M.; Henríquez, C.; Escobar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]
- Domingo, C.S.; Soria, M.; Rojas, A.M.; Fissore, E.N.; Gerschenson, L.N. Protease and hemicellulase assisted extraction of dietary fiber from wastes of Cynara cardunculus. Int. J. Mol. Sci. 2015, 16, 6057–6075. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Total polyphenol content and antioxidant activity among clones of two sicilian globe artichoke landraces. Acta Hortic. 2013, 983, 95–102. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crops Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauro, R.; Mauromicale, G. Variation of phenolic content in globe artichoke in relation to biological, technical and environmental factors. Ital. J. Agron. 2009, 4, 181–189. [Google Scholar] [CrossRef]
- Dosi, R.; Daniele, A.; Guida, V.; Ferrara, L.; Severino, V.; Di Maro, A. Nutritional and metabolic profiling of the globe artichoke (Cynara scolymus L. cv. capuanella heads) in province of Caserta, Italy. Aust. J. Crop Sci. 2013, 7, 1927–1934. [Google Scholar]
- Kayahan, S.; Saloglu, D. Comparison of phenolic compounds and antioxidant activities of raw and cooked turkish artichoke cultivars. Front. Sustain. Food syst. 2021, 5, 761145. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara Cardunculus L. var. Scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Galieni, A.; Stagnari, F.; Pisante, M.; Platani, C.; Ficcadenti, N. Biochemical characterization of artichoke (Cynara cardunculus var scolymus L.) spring genotypes from marche and abruzzo regions (central Italy). Adv. Hort. Sci. 2019, 33, 23–31. [Google Scholar] [CrossRef]
- Domínguez-Fernández, M.; Irigoyen, Á.; de los Angeles Vargas-Alvarez, M.; Ludwig, I.A.; De Peña, M.P.; Cid, C. Influence of culinary process on free and bound (poly)phenolic compounds and antioxidant capacity of artichokes. Int. J. Gastron. Food Sci. 2021, 25, 100389. [Google Scholar] [CrossRef]
- Rinaldi, M.; Littardi, P.; Cavazza, A.; Santi, S.; Grimaldi, M.; Rodolfi, M.; Ganino, T.; Chiavaro, E. Effect of different atmospheric and subatmospheric cooking techniques on qualitative properties and microstructure of artichoke heads. Food Res. Int. 2020, 137, 109679. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Abdulaziz, L.; Yaziji, S.; Azizieh, A. Effect of preliminarily treatments on quality parameters of artichoke with different preservation methods. Int. J. Chemtech Res. 2015, 7, 2565–2572. [Google Scholar]
- Bureau, S.; Mouhoubi, S.; Touloumet, L.; Garcia, C.; Moreau, F.; Bédouet, V.; Renard, C.M.G.C. Are folates, carotenoids and vitamin C affected by cooking? Four domestic procedures are compared on a large diversity of frozen vegetables. LWT 2015, 64, 735–741. [Google Scholar] [CrossRef]
- Ferracane, R.; Pellegrini, N.; Visconti, A.; Graziani, G.; Chiavaro, E.; Miglio, C.; Fogliano, V. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J. Agric. Food Chem. 2008, 56, 8601–8608. [Google Scholar] [CrossRef]
- Jiménez-Monreal, A.M.; García-Diz, L.; Martínez-Tomé, M.; Mariscal, M.; Murcia, M.A. Influence of cooking methods on antioxidant activity of vegetables. J. Food Sci. 2009, 74, 97–103. [Google Scholar] [CrossRef]
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J. Agric. Food Chem. 2001, 49, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2004, 52, 4090–4096. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: From food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Noriega-Rodríguez, D.; Soto-Maldonado, C.; Torres-Alarcón, C.; Pastrana-Castro, L.; Weinstein-Oppenheimer, C.; Zúñiga-Hansen, M.E. Valorization of globe artichoke (Cynara Scolymus) agro-industrial discards, obtaining an extract with a selective eect on viability of cancer cell lines. Processes 2020, 8, 715. [Google Scholar] [CrossRef]
- Canale, M.; Sanfilippo, R.; Strano, M.C.; Amenta, M.; Allegra, M.; Proetto, I.; Papa, M.; Palmeri, R.; Todaro, A.; Spina, A. Artichoke industrial waste in durum wheat bread: Effects of two different preparation and drying methods of flours and evaluation of quality parameters during short storage. Foods 2023, 12, 3419. [Google Scholar] [CrossRef]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from artichoke processing industry: Reuse in bread-making and evaluation of the physico-chemical characteristics of the final product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef]
- Ihl, M.; Monsalves, M.; Bifani, V. Chlorophyllase inactivation as a measure of blanching efficacy and colour retention of artichokes (Cynara scolymus L.). LWT 1998, 31, 50–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).