Abstract
Seed priming with Pulsed Electric Fields (PEF) is a promising strategy to enhance early plant development and crop quality. This study evaluated PEF priming effects on Beetroot (Beta vulgaris L.), Arugula (Eruca vesicaria), and Basil (Ocimum basilicum L.) microgreens by assessing the effects of three distinct monopolar PEF protocols (PEFA: 2 kV/cm; PEFB: 3 kV/cm; PEFC: 4 kV/cm). PEFB and PEFC treatments significantly influenced imbibition. Germination Indexes (DGI, CGI, and SVI) were positively and significantly affected, with radicle length increasing up to 33% and DGI improving from 40 to 66 on the 1st day (Beetroot, PEFC). Chlorophylls and the Total Carotenoid concentration increased in Basil but decreased in Beetroot and Arugula. Fat and Protein increased in Beetroot (Fat: +41%; Protein: +34%) and Arugula (Fat: +91%; Protein: +11%) treated with PEFC. PEFB led to an increase in Starch in all species. Crude Fibre and Neutral Detergent Fibre decreased amongst all species. Methionine rose by 100% in Beetroot treated with PEFC. Sensory analysis showed slight increases in Sweet (Beetroot) and Aroma Intensity (Basil and Arugula), although these changes were not statistically significant. Species-specific responses to different PEF protocols were observed: optimal protocols seem to be PEFC for Beetroot, PEFB for Arugula, and PEFA/B for Basil.
1. Introduction
Global demographic trends and societal awareness of the need for sustainable, healthier, and functional foods paint a pressing picture for the future of food systems [1,2]. The United Nations projects that the world population will reach 10.4 billion people by 2100 [3]. Simultaneously, the FAO estimates that by 2030, 8% of the global population, representing 670 million people, will suffer from undernourishment, making the Zero Hunger goal proposed in the UN’s Agenda 2030 increasingly difficult to achieve [4]. These prospects, together with present and future challenges linked to industrialisation, land use conflicts, environmental issues, food security disparities, and healthcare cost increases associated with problematic dietary and lifestyle choices [5,6], urge the necessity of a combination of heritage farming practices and innovative technologies and strategies to overcome these challenges.
Humans have been consuming germinated seedlings since the dawn of time. For this study, microgreen production was selected, with four main considerations.
Firstly, the economic factor: Although the first mention of microgreens utilisation dates back to the 1980s, when high cuisine chefs primarily valued them for culinary reasons (such as being more palatable than other germinated seedlings and for being colourful, which sometimes earns them the name “food confetti”), their presence remained relatively low profile [7,8]. However, recently, the market has been revitalised and, according to Research and Markets, is expected to grow from 2.14 billion in 2024 to.93 billion USD by 2029, representing a rapid compound annual growth rate (CAGR) of 13.1% [9].
Secondly, their nutritional richness: Microgreens contain a high concentration of essential minerals, such as Ca, Mg, Mn, and Zn, and generally exhibit lower nitrate content when compared to their mature versions. In addition, recent studies highlight their abundance of bioactive metabolites, such as β-carotene, which earned them the title of functional foods or even superfoods [10,11,12,13,14].
Thirdly, Food Safety advantages: Compared with other germinated seedlings, particularly sprouts, microgreens represent a safer alternative from a food security perspective. While sprouts are grown under ideal conditions for microbial development, which, unfortunately, has already been linked to foodborne disease outbreaks, microgreens are typically less prone to such risks, considering that only the aerial organs of the seedlings are consumed [8].
Fourthly, and perhaps the most decisive factor, their suitability for assessing the potential of new technologies and strategies to improve the germination rate and potentially enhance the nutritional value of these foods at a fast pace: Given the short cycle between sowing and harvesting, microgreens offer the advantage of rapid experimentation, allowing for conclusions to be reached more quickly, which can be extrapolated and further studied in other agricultural contexts.
Pulsed Electric Fields (PEF) have emerged as a technology with several potential applications in the AgriFood industry, some of which have been widely studied and already approved by regulatory organisms for specific applications, such as the International Organisation of Vine and Wine, and extrapolated to legislation, i.e., the European Commission, for yield and extraction optimisation in red and white winemaking [15,16]. PEF is considered a nonthermal, sustainable, and scalable process, capable of working at continuous flow and with low energetic requirements [17] and consisting of the application of short high-voltage pulses to biological materials, capable of inducing a permanent or transient increase in the cell’s membrane permeability by inducing electroporation and/or electropermeabilisation phenomena. The level of permeability can be modulated depending on the PEF protocol used (electrical field strength , specific energy , pulse polarity, shape, width, and number), equipment, biological properties of the cell (i.e., shape, size, and cell membrane type), physicochemical conditions of the medium (i.e., pH and electrical conductivity), and position/angle of the cell centre relative to the electrical field vector [18,19,20]. This versatility and modulation capacity allow for the application of PEF for a vast array of applications with distinct objectives, such as mass transfer optimisation [21,22], inactivation or stimulation of microorganisms [23,24,25], and, more recently, seed electropriming [11,26,27].
Although the first documented work on the use of electric stimuli on plants dates back to 1746, being the subject of discussion amongst pioneer scholars, such as Dr Van Maimbray of Edinburgh, Jan Ingenhousz (the “father” of photosynthesis), and Abbé Pierre Bertholon de Saint-Lazare [28,29], the stimulation of plant growth and development through electricity is still not widely applied in agricultural practices today. Earlier approaches to electrical stimulation in plants often involved long and time-consuming treatments, which limited their practical application. In contrast, PEF technology may be capable of overcoming these limitations by enabling short treatments and allowing for easy and fast application in seeds.
Published research on PEF electropriming of seeds is quite limited, as demonstrated by the exhaustive review conducted by Attri et al. (2022) [26]. Published research has explored the application of Pulsed Electric Fields (PEF) with various protocols on a range of plant species, including Leaf Lettuce, Barley, Kale, Wheat, Chickpea, Mung bean, Bitter gourd, Tomato, Medicago sativa, Chilli, Morning glory, and Green Foxtail [26]. Dymek et al. (2012) reported that while treatments up to = 1.2 kV/cm and 910 J/kg did not disrupt metabolic activities, more intense negatively affected radicle elongation [30]. Wheat (Triticum aestivum L.) was studied by various teams. Ahmed et al. (2020), subjected wheat seeds to PEF and obtained the best results with a protocol of 6 kV/cm [27]. Conversely, Leong et al. (2016) [11] reported that an application of 0.5 kV/cm did not affect seed growth, whereas treatment at 1.4 kV/cm slightly increased seedling size. However, exposure to 2 kV/cm caused a marked reduction in coleoptile length (−6 mm) and primary leaf growth (−10 mm) [11]. In other species, namely Leaf Lettuce, growth was stimulated at 0.2 kV/cm but inhibited at > 1 kV/cm [31]. In contrast, Scutellaria baicalensis seeds pre-imbibed for 12 h at 40 °C and exposed to fields ranging from 0 to 2.5 kV/cm showed the highest germination potential (+29.25%) under a protocol of 0.5 kV/cm with 99 pulses of 120 μs [32]. These findings show that the impact of PEF follows a threshold-dependent pattern, dependent on species and environmental/processing conditions.
In this context, the present work explores the application of PEF as a seed priming strategy for microgreens, aiming to improve our understanding of the optimal protocols to enhance germination and growth and to modulate the nutritional and bioactive profiles of each species under study, with the potential to improve Food Quality and optimise production efficiency and economic returns.
2. Materials and Methods
2.1. Plant Material
Wild Arugula (Arugula vesicaria var. sativa), Basil (Ocimum basilicum L.), and Beetroot “Detroit 2” (Beta vulgaris subsp. vulgaris var. conditiva) seeds were purchased (Flora Lusitana, Cantanhede, Portugal) to perform this assay.
2.2. PEF Equipment, Protocols, and Application
Considering the required output power and the PEF protocols for this assay, a high-voltage, solid-state Marx Generator (SSMG) EPULSUS® PM1A-12, designed and produced by EnergyPulse Systems (Lisbon, Portugal), was selected. Coupled to this SSMG, a static batch transducer with parallel plate design, consisting of two stainless-steel electrodes encased in acrylic, with variable distance between the electrodes () and a maximum load capacity of 1 L, was used. This equipment delivers positive square-shaped pulses, with a maximum capacity of 12 kV/250 A and 3 kW average output power. For this specific assay, the selected gap between electrodes was . Due to differences in seed size and imbibition capacity, Beetroot and Basil required 60 mL of water, giving an electrode area () of 30 cm2, while Arugula required 50 mL ().
Three distinct monopolar PEF protocols were selected: PEFA, consisting of ; PEFB, with ; and PEFC, with . This was accomplished by only varying the applied pulse voltage amplitude ()— for PEFA, for PEFB, and for PEFC—as demonstrated in Equation (1):
The remaining input PEF parameters were equal in all assays: 10 pulses () with a pulse width (τ) of +10 μs at . To be able to determine the energy per pulse and the specific energy () applied in each protocol, it is necessary to use Equations (2)–(4):
where m is the adimensional mass of the product, which was, in this case, directly extrapolated from the volume of the samples, estimating a density of .
Pulse Current (), given in A, is an SSMG output value, dependent on the electrical conductivity of the load (σ), pulse voltage (), and as per Ohm’s law. The PEF protocols applied are summarised in Table 1.

Table 1.
PEF protocol parameters for each subject assay of Arugula, Basil, and Beetroot.
It is important to bear in mind that the selected range of protocols represented a compromise between ensuring sublethal electropriming conditions [11,27] and maintaining technological and economic feasibility. More intense protocols require an increase of the equipment capacity, thereby raising costs and ultimately affecting the potential adoption of this technology by the industry.
2.3. Germination, Growth, and Harvest Conditions
Sowing rates are crop specific, dependent on average seed weight, estimated germination rate, and desired crop density rate, and are essential not only to maximise fresh yield per unit area and profitability but also to maintain product quality [33,34]. Thus, the selected seed density for Arugula was 12 seeds/cm2, 5 seeds/cm2 for Basil, and 1.75 seeds/cm2 for Beetroot.
For each species, seeds were sown in four perforated aluminium trays (20 cm × 50 cm) divided into three equal areas (20 cm × 16.67 cm), to use each section for each replicate of the same treatment. The trays were previously prepared with ±560 g of germination substrate (h = ±2 cm) (Siro® Germinação Bio, Leal & Soares, S.A., Mira, Portugal). In each tray, 450 mL of distilled water was added to guarantee an optimal environment for germination. Trays were covered and stacked to create a germination-friendly environment, free of light and highly humid. After 2 days, they were placed in an environmental simulation plant growth chamber (FitoClima 1200, Aralab, Lisbon, Portugal), equipped with programmable controls for temperature, relative humidity, airflow, and photoperiod. The programmed conditions were 23 °C, 50% relative humidity, and 50% airflow. Regarding the photoperiod, a total blackout was maintained for the first 5–6 days, after which it was adjusted to a 12 h light/12 h dark cycle.
Parallelly, 10 seeds were counted and separately sown in substrate-filled Petri dishes for each replicate, with each seed being numbered. These seeds were used to assess germination and radicle length evaluation during the first 5 days after sowing, by visual assessment of germination events and the determination of radicle length (seeds were considered successfully germinated if the radicle ≥ 1 mm).
Each replicate was irrigated with 50 mL of distilled water per day, while seeds allocated to Petri dishes received 10 mL per day.
2.4. Evaluation of Electroprimed Seed Germination and Growth
One of the most relevant indices used to assess germination development is the Germination Index (GI). There are several formulas to calculate the GI. In fact, the GI can be used to assess information directed to individual days—Daily Germination Index (%) (DGI, Equation (2))—or in a cumulative form: Cumulative Germination Index (CGI) [27]. While there are different variations of the CGI, for this study, the index formula based on Reddy (1985) and adapted by Walker-Simmons (1987) was selected [35,36]. This decision was made since a maximum weight is attributed to embryos and seeds that develop earlier, at the detriment of later-germinated seeds. These indices are still widely used and remain relevant in the recent literature [32,37], as they are considered some of the most comprehensive parameters, since they both account the number of germinated seeds and germination speed, as supported by recent studies such as Kader (2005) and Al-Ansari & Ksiksi (2016) [38,39]. This occurs since the CGI’s used formula is as in Equation (3):
where tf is the last day of germination (i.e., if germination lasts 5 days, tf = 5), and n is the number of seeds germinated (i.e., n1 = number of seeds germinated on day 1).
Kotowski’s Coefficient of Velocity Index (CV) is possibly the oldest index still in use nowadays, largely due to its higher sensitivity to early germination [40,41]. This happens given that the CV increases when the number of germinated seedlings rises and/or when the time required for germination decreases [42,43]. Therefore, CV serves as an indicator of the germination velocity and can be calculated using Equation (7):
where the total number of germinated seeds (ntotal) is divided by the weighted sum of the number of germinated seeds by the day they germinated. Theoretically, the maximum possible CV is 1, which would occur if all seeds germinated on the first day [38].
Seed Vigour Indexes (SVI-I and SVI-II) were developed by Abdul-Baki & Anderson (1973) and comprise both the length and Dry Weight of the radicles or roots and shoots and the standard germination percentage [44]. This classic non-destructive approach of this index (SVI-I) considers the radicle lengths, which are ideal for assessing seed development without compromising assays, therefore being widely used in plant development assessment assays [45,46]. The formula used is as demonstrated in Equation (8):
Growth evaluation can also be assessed by the determination of Fresh and Dry Weights and their relation. These kinds of parameters are essential to monitor the impact of different agricultural practices and to estimate species’ standard growing behaviours.
2.5. Post-Harvest: Nutritional and Physicochemical Evaluation
2.5.1. Microgreen Sample Preparation
Extract Preparation
To assess physicochemical parameters, such as bioactive compounds, namely Chlorophylls a and b, Total Phenolic Content, and antioxidant capacity (ABTS and DPPH), it is essential to select an adapted protocol compatible with different compound extractions. Considering that dietary Chlorophylls a and b are mainly considered as lipophilic pigments [47] while most plant phenolics possess a hydrophilic nature [48], two different types of solvents were selected.
For the determination of photosynthetic pigments, the protocol based on the traditional methodology of Lichtenthaler (1987) and as demonstrated by Falcioni et al. (2023) and Xu et al. (2024) was used, with the formula variation adapted to the selected solvent, Diethyl Ether [49,50,51]. The process consisted of the collection of healthy microgreen leaves, followed by their weighing and joining at a ratio of 1:10 to Diethyl Ether (Fisher Scientific, Loughborough, UK) [51]. This was followed by grinding using a stainless-steel pestle and mortar. The resulting “pulp” was separated from the liquid by filtration. Immediately after processing, spectroscopic analysis was performed to avoid the degradation of Chlorophylls.
For DPPH and ABTS Radical Scavenging assays, Total Phenolic Content, and Total Soluble Solids, a hydroethanolic solution with a ratio of 70:30 ethanol/water was used. For such, two extraction protocols were selected, both with a sample-to-solvent ratio of 1:10. One protocol comprised Magnetic Stirring at 700 rpm (Variomag® Multipoint HP, Daytona Beach, FL, USA), while the other underwent ultrasonic treatment at 37 kHz, resorting to an S60 H Elmasonic ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany). The extraction time and temperature were equal for both: 30 min at 20 °C. Afterwards, the samples were subjected to filtration using Whatman nº 3 filter paper and a vacuum pump, with this process being finalised by the refrigeration of the samples, protected from light, until analysis.
2.5.2. UV–Vis Spectrophotometric Analysis
Chlorophyll a, Chlorophyll b, and Total Carotenoids
Leaf pigments were assessed by spectrophotometric analysis (UV-1280 Spectrophotometer, Shimadzu, Kyoto, Japan) via direct measurements of the Diethyl Ether extract absorbance at λ = 470 nm, λ = 642.2 nm, and λ = 660.6 nm. The respective readings were applied in the following formulas (Equations (9)–(12)):
where Ca refers to Chlorophyll a, Cb to Chlorophyll b, C(a+b) to Total Chlorophylls, and C(x+c) to Total Carotenoids, in μg/mL of plant extract [51].
Total Phenolic Content
Total polyphenolic content (TPC) was analysed based on spectrophotometric analysis, using the Folin–Ciocalteau method [52]. Initially, a calibration curve was prepared using Gallic Acid as the standard (y = 3.6214x + 0.1881; R2 = 0.9961). The determination of TPC was based on an optimised Singleton & Rossi methodology and performed as described by Dulyanska et al. (2022) [53,54,55]. Succinctly, it consists of the addition of 125 μL of the extract sample, 125 μL of Folin-Ciocalteau reagent, and 750 μL of Milli-Q water to a vial. The mixture was incubated in the dark for 6 min, followed by the addition of 2 mL of 5% Sodium Carbonate (Na2CO3). The solution was then vigorously shaken to ensure proper homogenisation. The tubes were protected from light and left to react for 60 min. After this period, the absorbance of the standards was measured at λ = 750 nm. A blank sample was prepared by replacing the extract with 125 μL of Milli-Q water, and an auto-zero adjustment was performed using Milli-Q water before sample readings. The results are expressed in mg of Gallic Acid per gram of Fresh Weight (mg GAE/g FW).
Antioxidant Capacity
- (a)
- DPPH Scavenging Assay
The following protocol was described by Dulyanska et al. (2022), being adapted from Brand-Williams [53,56]: In the 1st assessment, DPPH was used by preparation of an ethanolic solution (6 × 105 M), which was immediately protected from light. A blank reading was performed at λ = 517 nm with ethanol to avoid its interference with the measurements. This was followed by the adjustment of the DPPH solution to Abs ≈ 0.700 (Abs0) to ensure a standardised starting reading for reliable results. Next, 100 μL of the sample, together with 2 mL of the DPPH solution, was collected into a test tube, shaken, and left to react for 30 min, protected from light. The absorbance of the prepared sample is then read at λ = 517 nm (Abssample). The absorbance is adapted to the previously prepared calibration curve (y = −0.2933x + 0.6395; R2 = 0.995) with Trolox, and the results are given in % of DPPH inhibition (Equation (13)) and in μg Trolox Equivalents per gram of Fresh Weight (μg TE/g FW).
- (b) ABTS Scavenging Assay
The evaluation of the antioxidant capacity through the methodology is performed thanks to the reagent’s ability to oxidise and produce a stable free cationic radical ABTS•+. The process began with the preparation of a calibration based on standardised Trolox solutions (y = −0.2117 + 0.5905; R2 = 0.999). The selected protocol requires, as the 1st step, the addition of 2.45 mmol/L potassium persulfate (K2S2O8) to the ABTS radical aqueous solution (7 mmol/L) [57,58]. This will turn an otherwise colourless solution into a green-blue-toned one. The ABTS solution is incubated at room temperature for 12–16 h, achieving oxidative stability. Finalised this period, the solution is diluted in ethanol at a rate of 1:80, which is followed by the determination of the solution’s absorbance at λ = 734 nm (autozero), being adjusted if necessary (Abs ≈ 0.700). The microgreen extract preparation consisted of its addition (0.1 mL) to 2.0 mL of the ABTS•+ solution, followed by homogenisation and a resting period of 15 min to allow for chemical reactions to take place [59]. In the end, the absorbance at λ = 734 nm was determined, with the results being expressed in % ABTS inhibition and μg TE/g FW.
2.5.3. Total Soluble Solids
Total Soluble Solids, TSS, expressed in °Brix, were measured using a digital refractometer (95200-001 model, Alla France, Chemillé-en-Anjou, France) to estimate the concentration of TSS, constituted mainly of sugars, but not exclusively, including other refractive compounds present in the sample, in this case the extract, such as organic acids [60].
2.5.4. Near-Infrared Reflectance Spectroscopy
Fourier Transform Near-Infrared Reflectance (FT-NIR) was the selected spectroscopy technique to quantify the nutritional profile of the obtained microgreens. These analyses were performed using a NIRMaster™ (BÜCHI Labortechnik, Flawil, Switzerland), which has product-specific internal calibration curves and can assess several nutritional parameters, as well as relevant amino acids, including Humidity, Fat, Protein, Crude Fibre, Ash, Starch, NDF (Neutral Detergent Fibre), Lysine, Cystine, Methionine, and Phosphorus. The results are presented as g/100 g of dry matter.
The sample processing followed this protocol: Freshly harvested microgreens were weighed and placed in an oven at 65 °C for 12 h. After this period, dried microgreens were pulverised to assess the Dry Weight using a stainless-steel pestle and mortar. The final sample was collected into a vial and used to perform the readings.
2.6. Post-Harvest: Sensory Analysis
A descriptive sensory test was performed based on the guidelines of the Quantitative Descriptive Analysis (QDA) methodology developed by Sidel & Stone [61,62]. Twelve sensory descriptive attributes were selected and categorised into three groups:
- Visual (Integrity, Colour Intensity, and Tonality);
- Mouthfeel and Flavour (Bitter, Hot, Crispy, Sweet, Fibrous, Moist, Astringent, and Aftertaste);
- Aroma (Intensity).
All sensory attributes were assessed using a Likert-type ordinal numerical scale from 1 to 10 [63]. A score of 1 indicated that the attribute was undetected/weak, while a score of 10 indicated that it was easily detected/excellent. Additionally, an extra parameter—Global Evaluation—was included, also rated on a scale of 1 to 10.
The tasting panel consisted of 9 panellists (6 women and 3 men) with ages ranging from 23 to 59 years and was composed of ESAV academics, researchers, and students, all from the field of Food Technology and/or with knowledge in sensory evaluation of other food categories. The sample was prepared using the same methodology presented by Michell et al. (2020): microgreens were kept in the germination chamber at 23 °C until harvest, followed by washing and refrigeration at 4 °C [64]. This was performed 1 h before tasting, and the sample was served in randomised three-digit coded glass containers.
2.7. Data Analysis
All analyses were performed in triplicate in a randomised block design. The collected data for each parameter are presented as the mean ± standard deviation (SD). All data were subjected to statistical analysis using IBM SPSS Statistics, Version 28.0.1.0 (142) (SPSS Inc., Chicago, IL, USA). A significance level of p = 0.05 was applied in all statistical analyses. Partial Eta-Squared (ηp2) expresses the effect size associated with parametric analysis of variance (MANOVA or ANOVA). Higher values indicate greater proportion of difference between subjects that can be attributed to the factor understudy (i.e., ηp2 = 0.50 can be interpreted as the treatment factor being able to explain 50% of the assessed differences among subjects) [65,66].
Graphics were generated using GraphPad Prism, Version 8.0.2 (GraphPad Software Inc., San Diego, CA, USA) and Microsoft® Excel®, for Microsoft 365, version 2312, Build 16.0.17126.20132 (Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Germination and Plant Growth Evaluation
3.1.1. Imbibition Process: Water Uptake and Electrical Conductivity
The results obtained from the assessment of seed imbibition were monitored through the evaluation of water uptake, electrical conductivity, and temperature.
The results obtained for Beetroot seeds are summarised in Table 2.

Table 2.
Evaluation of water uptake and electrical conductivity during Beetroot seed imbibition.
Before imbibition, the goal was to ensure the most consistent initial conditions across all groups to eliminate any potential differences that could influence the impact of the treatment on seed imbibition. Tap water was used to kickstart seed imbibition. Before imbibition, the σ and temperature of the tap water were determined. Although all three parameters showed statistically significant differences between groups, these differences can be considered negligible from a practical standpoint, given the minimal variations between groups and the very low standard deviations. At 1 h after imbibition and 10 min before PEF application, it is possible to see that the seeds were subjected to similar temperature conditions; in addition, we can also see a slight σ difference, considered statistically significant, which we can attribute to seed variability. This difference was diluted immediately post-PEF treatment. At 4 h after PEF treatment, seeds were separated from water, dried with a paper towel, and weighed.
PEF treatment significantly affected the water uptake capacity of seeds in relation to the Control for protocols PEFB and PEFC, with 3 and 4 kV/cm, respectively, given that the weight was significantly higher when compared with Control subjects. The ηp2 value indicates a large effect, with 45.9% of the variability attributed to the PEF treatment. As expected, σ was higher for all treated subjects, indicating that electroporation was successful, to different degrees, as it indicates the release of ionic content to the imbibition solution.
The results for Arugula are presented in Table 3.

Table 3.
Evaluation of water uptake and electrical conductivity during Arugula seed imbibition.
The evaluation of imbibition in Arugula presented some challenges stemming from the seed morphology. Arugula seeds have a reduced size, which hinders the capacity of drying the excess water from them before the final weighing, given that many seeds can be lost in the process. Consequently, seed weight was only measured prior to imbibition, while the electroporation effect was monitored via σ. Once again, the initial conditions are found to be identical to all groups, considering that significant differences were not found for the Initial Dry Weight, nor for imbibition, water uptake, or electrical conductivity. Immediately post-PEF treatment, differences in terms of σ became evident, where PEFA and PEFC exhibited an increase in conductivity 21% superior to the Control, being this difference attributed to the variable treatment by 72.7%. However, this difference diminished over time. At the 4 h mark, only PEFA presented a significantly higher σ. This suggests that over time, the diffused ions transferred from the seeds to the solution reached the same equilibrium between the Control, PEFB, and PEFC.
Table 4 presents the results obtained for the evaluation of imbibition parameters for Basil.

Table 4.
Evaluation of water uptake and electrical conductivity during Basil seed imbibition.
Basil’s initial samples and condition statistics for initial weight and water conductivity were homogeneous. Immediately before PEF treatment, two groups were formed: the Control and PEFC presented significantly higher σ values than PEFA and PEFB. Despite this, after treatment, the highest σ-value groups switched to PEFB and PEFC, presenting significantly higher σ values in comparison to the Control and PEFA, with 92.6% of this difference attributed to PEF effect. At 4 h post-PEF, the weight and σ were significantly higher in seeds treated with protocols PEFB and PEFC, which indicates that water imbibition was modulated by electroporation of the seed tissues.
While the analysis denotes that statistically there were significant temperature differences, these can be discarded, as the differences are very small, which renders them irrelevant in this context.
The evaluation of water uptake and electrical conductivity during the imbibition of Basil, Beetroot, and Arugula seeds under different PEF treatments revealed several trends and species-specific responses. For instance, the magnitude of the change in membrane permeability varied between species, as observed by the different behaviours observed for σ and water uptake. While Basil presented the highest increase in σ and Final Seed Weight for PEFC, σ was not altered for Beetroot immediately post-PEF treatment. For Beetroot, differences were only found 4 h post-PEF, with PEFB presenting the highest increase in Final Seed Weight, which contrasts with σ values, considering the highest was obtained for protocol PEFA. In opposition, Arugula presented the largest difference in σ immediately post-PEF (PEFA and PEFC); however, after 4 h, PEFA was the only group significantly higher.
Some studies corroborate these results, while others contradict this, which displays the complexity of the protocol selection. For instance, a treatment of 3 kV/cm, when accompanied by a specific energy of 19.8 kJ/kg, represented an increase in water uptake of 25% in wheat seeds before malting; however, at a lower energy protocol (9.9 kJ/kg), a decrease in moisture absorption was observed [67]. Other authors reported that wheat seeds subjected to PEF pre-treatment at an electric field strength below 6 kV/cm (100 µs pulse width, 25 pulses) did not exhibit increased water uptake; however, when treated at 6 kV/cm with 50 pulses, the seeds showed a significant increase in water content, rising from 50.93% to 56.56%. [27]. This suggests that both field strength and specific energy play a critical role in modulating water absorption during early seed hydration.
These results indicate that PEF effects are species dependent, possibly due to differences in seed coat structure and cell membrane composition.
3.1.2. Germination Indexes and Radicle Length
The results obtained for radicle length and the number of germinated seeds for Beetroot, Arugula, and Basil over five days under different treatment conditions are summarised in Table 5.

Table 5.
Radicle length (mm) and number of germinated seeds.
A Linear Mixed Model (LMM) was conducted to examine the effects of the day, treatment, and their interaction, concluding that for Beetroot, a significant main effect associated with treatment was found (F(3, 338) = 4.575, p = 0.004), meaning that statistically, treatments did influence radicle length. The same was observed for Arugula (F(3, 468) = 6.188, p < 0.001) and for Basil (F(3, 268) = 8.292, p < 0.001). For all species, differences regarding both radicle length and number of germinated seeds per subject were clear from day 1. Besides this, observing the table implied that treatment effects were consistent over time for all species, which means that the results were not diluted throughout the assay. This is corroborated by statistical analysis, considering that the interaction day x treatment was not significant for all species (Beetroot: F(12, 338) = 0.482, p = 0.925; Arugula: F(12, 468) = 0.399, p = 0.964; Basil: F(12, 268) = 0.594, p = 0.847).
With this information, information growing indices were determined. The Daily Germination Index (DGI), Cumulative Germination Index (CGI), for Kotowski’s Coefficient of Velocity Index (CV), and Seed Vigour Index (SVI) are presented in Table 6.

Table 6.
Results of the Germination and Growth Indexes.
The effect of PEF treatments is evident for all species, with the optimal protocol varying for each species. For instance, while the best results were observed with the 4 kV/cm protocol (PEFC) for Basil and Beetroot, a 3 kV/cm protocol (PEFB) seemed to be the most suitable for Arugula. This conclusion is supported by both the average radicle length and the number of seeds germinated, further corroborated by the Germination Indexes. This is consistent with previous work on wheat seeds, in which PEF priming improved water uptake, accelerated germination, and increased juice yield (%) [27]. Mechanistically, the electroporation effect of the PEF treatment facilitates faster and more efficient imbibition, which impacts the activation of metabolic enzymes, enhances embryo growth potential, and possibly weakens the outer protective layers, which might contribute to more efficient radicle protrusion. Water uptake by the embryo is crucial for radicle emergence, as it drives its expansion and the pressure required for radicle protrusion [68,69]. As said, imbibition also kickstarts the mobilisation of seed reserves during germination. Amylase activation leads to the degradation of Starch molecules into simple sugars (i.e., glucose and maltose), while proteases release amino acids (essential for protein synthesis), and lipases mobilise stored lipids into usable energy [70]. Thus, it can be hypothesised that an optimisation of the imbibition process will accelerate and/or increase metabolite availability while also improving cellular hydration, resulting in higher turgor pressure that positively influences cell expansion, elongation, and division, promoting faster tissue growth [71]. A recent study performed by Song et al. (2024) supports this theory, as they reported an increase in α-amylase, antioxidant enzymes, soluble proteins, and soluble sugars [32].
The observed species-specific responses to different protocols are consistent with the fact that pore formation during PEF application depends strongly on three main factors: (a) PEF protocol (electrical field strength (), specific energy (), pulse shape, polarity, width and number, and treatment time), (b) physicochemical characteristics of the medium (e.g., conductivity (), temperature, and pH), and (c) biological properties of the cell (e.g., cell radius (), shape, and cell membrane type) [18,19,72]. Therefore, if the PEF protocol is too intense for the morphological characteristics of the seeds and its cells, it may negatively affect embryo development, ultimately leading to delayed germination or even seed inactivation and death. Excessive electroporation can cause severe or irreversible membrane damage, compromising cell viability [73]. Thus, optimising PEF parameters for each species is essential.
3.2. Post-Harvest: Nutritional and Physicochemical Evaluation
3.2.1. UV–Vis Spectrophotometric Analysis
Chlorophyll a, Chlorophyll b, and Total Carotenoids
The effects of the different PEF treatments on Chlorophyll a (Ca), Chlorophyll b (Cb), Total Chlorophylls (C(a+b)), and Total Carotenoids are summarised in Figure 1.
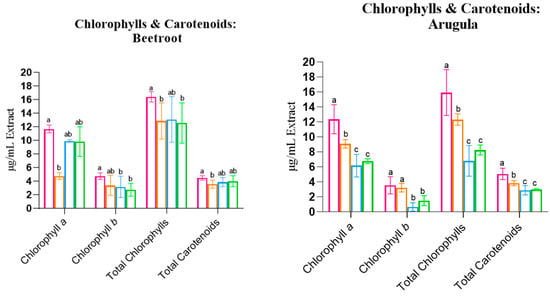
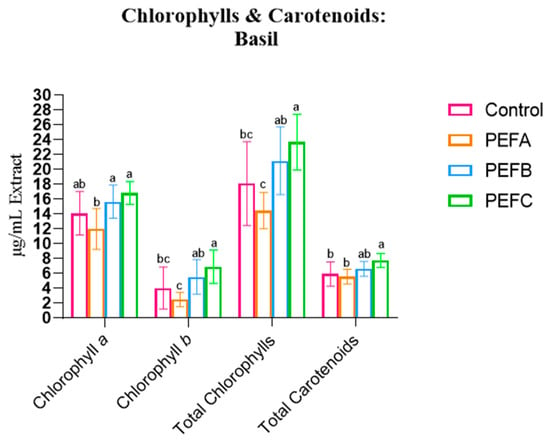
Figure 1.
Chlorophyll a, Chlorophyll b, and Total Carotenoids of the three studied species. Statistical comparisons were conducted between different groups within each species. Statistically significant differences (Tukey’s p < 0.05) are indicated by different letters assigned to the means. PEFA: 2 kV/cm, PEFB: 3 kV/cm, PEFC: 4kV/cm.
For Beetroot and Arugula, PEF treatments led to a significant reduction in Ca, Cb, C(a+b), and Carotenoids. However, for Beetroot, the effect size of the treatment was considered relatively low, with a maximum of 30.6% of the variability attributed to the PEF. In contrast, for Arugula, the treatment had a large effect size: at least 73.1% of the differences could be attributed to PEF application.
Basil contradicted this trend. While PEFA also resulted in a decrease in Chlorophyll and Carotenoid concentrations, more intense protocols (PEFB and PEFC, at 3 and 4 kV/cm, respectively) led to an increase in all pigment concentrations: Ca, Cb, C(a+b), and Carotenoids. However, in this case, the statistical effect of the treatment was lower, with a maximum of 42.6% of the variability attributed to PEFs. These findings align with Ahmed et al. (2020), who reported that higher electrical field strengths and a greater number of pulses led to a nearly proportional increase in Total Chlorophylls and the Carotenoid concentration [27].
In summary, pigments were significantly affected by the application of PEF, exhibiting decreased levels of Chlorophylls and Carotenoids, except for Basil species. PEF significantly affected pigment concentrations, generally leading to a decrease in Chlorophylls and Carotenoids. The observed species and protocol-specific responses might reflect differences in seed coat permeability and seed and cellular morphology, which can influence PEF effects and affect electroporation, leading to different impacts on seed imbibition, hydrolytic enzymes, and ion fluxes, affecting the germination kickstart and influencing metabolic processes, growth, and baseline pigment biosynthesis [19]. In species where the pigment concentration decreased, faster tissue growth may have diluted pigments within expanding tissues, reducing the concentration per unit of mass. Thus, while Basil may benefit from higher-intensity PEF due to threshold-stimulated biosynthesis, Beetroot and Arugula may experience dilution effects. Despite its potential, little is known about how electroporation affects seed germination and plant physiology. This knowledge gap emphasises the novelty of the area and the need for further mechanistic research.
Total Phenolic Content
In order to better understand the impact of PEF treatments across species and extraction methods, multivariate analyses were conducted under two perspectives: (i) globally, by species, to assess possible differences between the selected extraction methods and (ii) separately, by species and extraction type, to determine the impact of each PEF treatment on the antioxidative content, Total Phenolic Contend, and Total Soluble Solids of each extract.
With the (i) approach, it was possible to conclude that the extraction methodology presents considerable effects on the concentration of compounds within the extracts:
Effect: extraction method:
- Beetroot: F(5, 60) = 5.084, p < 0.001, ηp2 = 0.770;
- Arugula: F(5, 60) = 4.777, p < 0.001, ηp2 = 0.285;
- Basil: F(5, 60) = 12.109, p < 0.001, ηp2 = 0.502.
Interaction: extraction method x treatment:
- Beetroot: F(15, 186) = 3.081, p < 0.001, ηp2 = 0.321;
- Arugula: F(15, 186) = 2.182, p = 0.008, ηp2 = 0.150;
- Basil: F(15, 186) = 2.657, p = 0.001, ηp2 = 0.176.
Therefore, it is possible to conclude that the type of extraction methodology—Magnetic Stirring (MS) and Ultrasound (US)—significantly affected the extraction capacity of the compounds under study, as was observed by the results obtained by effect analysis of both the extraction method and the interaction of extraction methodology x treatment. This is easily corroborated by the data tables displayed over the course of this subchapter, where in general, higher extraction rates are found in extracts subjected to US treatment when compared with MS.
In addition, across all species, in a general way, it is viable to conclude that all PEF treatments present a strong and significant antioxidant activity of the plant extracts, considering that for the condition treatment, the MANOVA results show:
Effect: treatment:
- Beetroot: F(15, 77 697) = 34.498, p < 0.001, ηp2 = 0.429;
- Arugula: F(15, 77 697) = 11.045, p < 0.001, ηp2 = 0.441;
- Basil: F(15, 77 697) = 3.136, p < 0.001, ηp2 = 0.514.
Thus, it was viable to advance with the (ii) approach to allow for the detection of differences caused by the different PEF protocols applied.
Starting with the comparison between Total Phenolic Content (TPC), it was possible to find some key differences between its concentration on microgreens subjected to different PEF-priming protocols. The results for TPC are shown in Figure 2.
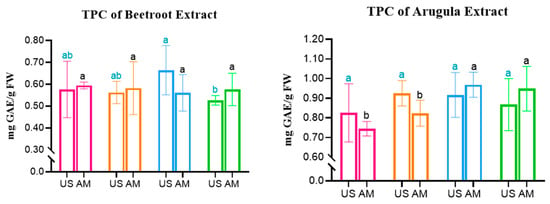
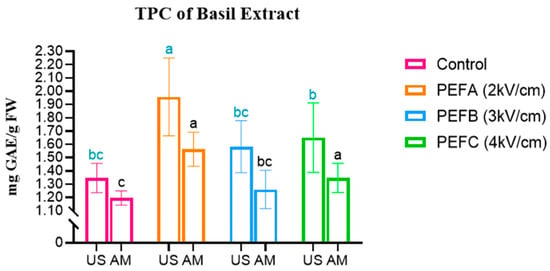
Figure 2.
Total Phenolic Content in Beetroot, Arugula, and Basil microgreens. Statistical comparisons were conducted within each colour group. MS: Magnetic Stirring; US: Ultrasound. Statistically significant differences (Tukey’s p < 0.05) are indicated by different letters assigned to the means.
Regarding Beetroot, it is possible to observe that for extracts obtained by MS, no significant differences were observed between all subjects. However, for the same species but in extracts obtained with the aid of Ultrasound, PEFB (3 kV/cm) presented a significantly higher concentration of TPC, representing a 15% increase in TPC when compared to the Control. For Arugula, the opposite trend is observed, with no statistically significant differences between subjects obtained with US. In contrast, for MS extraction, a significant increase of up to 30% TPC concentration was obtained for more intense PEF treatments, namely the PEFB and PEFC protocols.
Finally, for Basil, all protocols represented an increase in the TPC of both extract types. The highest TPC concentration was obtained with US extraction, when it increased 45%, from 1.345 to 1.956 mg GAE/g FW, with a protocol of 1 kV/cm. Regarding MS extraction, the peak was also observed for the PEFA protocol, which allowed for an increase from 1.196 to 1.562 mg GAE/g FW, representing 30.5% more TPC in this extract.
Antioxidant Capacity
The results of the DPPH and ABTS assays are presented by species in the tables below. The data for Beetroot are shown in Table 7.

Table 7.
Antioxidant capacity (DPPH and ABTS) of Beetroot in μg TE/g FW.
Consistent with the trends observed for TPC, US proved more effective for the recovery of bioactive compounds from microgreens. In this species, the most effective seed priming protocols for enhancing the antioxidant potential were those involving higher electric field intensities, 3 to 4 kV/cm. Statistically significant increases in antioxidant capacity, as measured by DPPH, were observed in PEF-treated samples, ranging from 10.8% (PEFB, US) to 12.4% (PEFC, AM) inhibition increases when compared to the respective Controls. A similar pattern was found with the ABTS assay, where all PEF-primed samples exhibited significantly higher antioxidant capacities than the Controls. This reinforces the conclusion that seed priming with more intensive Pulsed Electric Fields can effectively enhance the synthesis or accumulation of antioxidant compounds in Beetroot microgreens.
The results for Arugula are presented in Table 8:

Table 8.
Antioxidant capacity (DPPH and ABTS) of Arugula.
In contrast to what was observed for Beetroot, no clear advantage was detected for US extraction over MS in enhancing antioxidant capacity, as both extracts exhibited similar trends. The highest DPPH inhibition values were obtained with the PEFC protocol, indicating that the most intense electric field protocol might enhance the antioxidant potential of this species. Specifically, for US extracts, microgreens whose seeds were subjected to PEFC exhibited a statistically significant increase in DPPH inhibition (+5% vs. Control). A similar pattern was observed for MS extracts, reinforcing this trend.
Regarding ABTS, no statistically significant differences were found among US extracts (p = 0.796), suggesting that this method may not be sensitive enough to detect variations in antioxidant capacity in Arugula under these extraction conditions. However, for MS extracts, a significant effect was observed (p = 0.002), with PEFC treatment again yielding the highest inhibition percentage (40.68%), significantly surpassing the Control and PEFA. These results suggest that for Arugula, the ABTS method is more responsive to differences in seed priming treatments when AM is used and that PEFC can be the most suitable to enhance the extraction of ABTS-reactive compounds.
Basil DPPH and ABTS results are displayed in Table 9:

Table 9.
Antioxidant capacity (DPPH and ABTS) of Basil.
Overall, DPPH inhibition and concentration values were high across all subjects, which demonstrates the antioxidant potential of this species. While no statistically significant differences were observed among treatments in US extracts when using MS, PEFC treatment presented a statistically significant enhancement in antioxidant activity, with a large effect size of 52.7% attributed to the PEF treatment. However, realistically, this only represents an increase of 2.6% in antioxidant capacity.
Contrary to what was observed in the DPPH assay, ABTS results were not significantly different between treatments in any of the extraction modalities. This might indicate one of two conclusions: there is a less pronounced impact of PEF priming on ABTS-reactive compounds under this extraction method, or there was no influence of the treatment on antioxidant activity. Considering the results obtained for DPPH, the first hypothesis might be the correct one, as there is the possibility that each assay—DPPH and ABTS—might target different antioxidant compounds.
Based on the present results, PEF treatments, particularly the PEFB and PEFC protocols, induced significant changes in the antioxidant profile of microgreens, with Beetroot showing the most pronounced effects. When exposed to field strengths of 3 to 4 kV/cm, Beetroot microgreens exhibited a 15% increase in TPC and a 12.4% enhancement in antioxidant capacity (ABTS). Ahmed et al. (2020) also reported significant increases in concentration of antioxidant compounds and TPC in wheat plantlet juice after applying 2 to 6 kV/cm to the seeds [27]. Curiously, no impact on DPPH inhibition was found when PEF treatments of 0.5 to 2 kV/cm were applied to wheatgrass seeds as a pre-sowing technique [11]. These findings are consistent with previous studies indicating that PEF-induced membrane permeabilisation can trigger the generation of reactive oxygen species (ROS), a response to abiotic stress in plant cells [11,74]. The transient oxidative burst caused by ROS accumulation is believed to activate antioxidant defence pathways, often resulting in the biosynthesis of phenolic compounds and other secondary metabolites with protective functions [75].
3.2.2. Total Soluble Solids
The results obtained for Total Soluble Solids (TSS), by direct measurement of °Brix of the prepared hydroalcoholic extract, are represented in Figure 3.
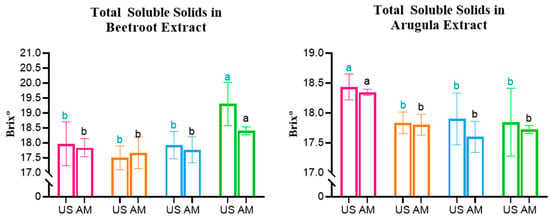
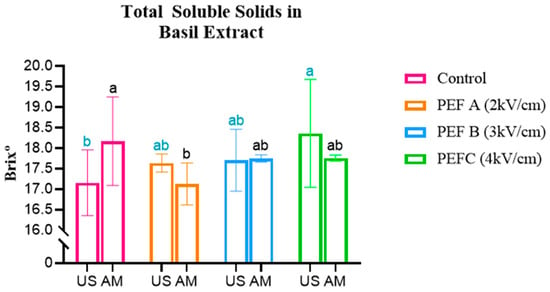
Figure 3.
Total Soluble Solids in Beetroot, Arugula, and Basil microgreens. Statistically significant differences are indicated by different letters assigned to the means. Statistical comparisons were conducted within each colour group. MS: Magnetic Stirring; US: Ultrasound.
The analysis of Beetroot extracts presented consistent behaviour, with both the MS and US extraction methods displaying significantly higher °Brix values for extracts obtained from microgreens derived from seeds treated with the PEFC protocol (AM—Control: 17.8 °Brix vs. PEF3: 18.4 °Brix; US—Control: 17.96 °Brix vs. PEF3: 19.30 °Brix). This corresponds to an increase in TSS present in microgreens of approximately 3.4 and 7.5%, respectively.
The opposite was observed for Arugula, considering that the °Brix values were significantly higher in Control samples—both US and MS—compared to all PEF-treated groups, presenting a maximum decrease in TSS of −3.2% and −4% for US and MS, respectively.
Basil presented intermediate results: in MS extracts, the highest TSS was found in the Control sample (18.43 °Brix), whereas in US extracts, the PEFC treatment yielded the highest value (18.34 °Brix).
Beetroot results are in line with a study conducted on Scutellaria baicalensis seeds, which showed that PEF treatment of 0.5 kV/cm significantly increased α-amylase activity, leading to the conversion of Starch into soluble sugars during early growth [32]. This suggests that PEF can accelerate cellular metabolism and promote sugar accumulation in seedlings. However, given the contrasting results observed in Basil and Arugula, the effect of PEF on sugar content appears to be species specific and dependent on the applied treatment parameters.
3.2.3. % Dry Matter
Dry matter content (%) was assessed and is shown in Figure 4.
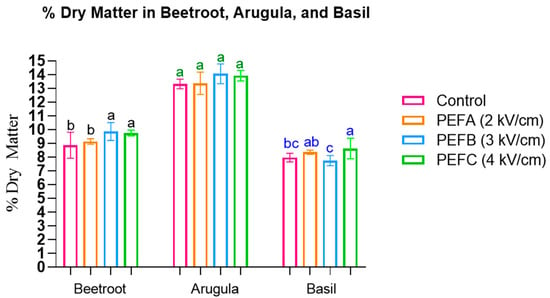
Figure 4.
The % Dry Matter in Beetroot, Arugula, and Basil microgreens. Statistically significant differences are indicated by different letters assigned to the means. Statistical comparisons were conducted within each colour group.
The results demonstrate that significant increases in the % dry matter were observed for Basil seeds treated with 4 kV/cm (F(3, 32) = 6.789, p = 0.001, η2p = 0.246) and for the most intense PEF treatments in Beetroot (3 and 4 kV/cm) (F(3, 32) = 5.902, p = 0.003, η2p = 0.389). Similarly, an increase for these protocols was also reported in Arugula, albeit not statistically significant.
3.2.4. Near-Infrared Reflectance Spectroscopy
After analysis, the sample humidity was standardised to ensure a consistent basis for comparison. This allows the results to be expressed either as a percentage or in g/100 g of dried sample. The results were analysed, and the final data are presented in Table 10:

Table 10.
Nutritional parameters of the different PEF protocols for Beetroot, Arugula, and Basil.
There are clear trends regarding the global results of nutritional profiles. For instance, Starch increased in all three species when subjected to PEFB protocols (Beetroot: +17%; Arugula: +12%; Basil: +9.4%). Protein presented an increase of up to 34% and 11% in Beetroot and Arugula when exposed to 4 kV/cm. The same intensity was also responsible for the significant increase in Fat (vs. Control): 41% in Beetroot, 91% in Arugula, and +14% in Basil.
The impact on Fibre Content is particularly interesting. Crude Fibre generally decreased in comparison with the Control: −24.7% in Beetroot, −5.7% in Basil (PEFC), and 30.6% in Arugula (PEFB). The concentration of Neutral Detergent Fibre (NDF) was also diminished, with the most relevant differences being −17.9% in Beetroot, −5.5% in Arugula, and −4.8% in Basil, all observed when seeds were subjected to 4 kV/cm.
Since Crude Fibre mainly consists of cellulose and lignin, a reduction in its concentration may enhance digestibility. These compounds are concentrated in cell walls, and their reduction can increase microgreen nutrient density and decrease “fill”, thereby increasing intake and better digestibility rates. In contrast, NDF also includes hemicellulose, which humans have more difficulty degrading, but ruminants can utilise through degradation and fermentation [76]. NDF decreased in all species, treated with PEFC (Beetroot: −17.9%; Arugula: −5.5%; Basil: −4.8%).
In addition, the CF/NDF ratio in Beetroot decreased from 39% to 36% when subjected to a 4 kV/cm protocol, which might be interpreted as an improvement in digestibility, since a higher relative proportion of hemicellulose implies that vegetable cell walls present a more soluble composition. While this is less relevant regarding human consumption, it would be interesting to extrapolate to other agronomic systems and study the possible positive impacts on ruminant feed. Arugula and Basil presented opposite trends, where CF/NDF increased from 33.5% to 34% in Arugula and from 21% to 27% in Basil with the PEFC protocol.
Regarding amino acids, Lysine decreased in PEF-treated samples for all species by up to 13%, while Methionine increased in all species: up to 100% in Beetroot, 72% in Arugula, and 5.8% in Basil. Cystine concentration was species dependent, increasing by 42% in Beetroot treated with 4 kV/cm but decreasing in Arugula (−46%) and Basil (−10%).
With the exception of Beetroot, where the Phosphorus content decreased under PEFC treatment (−45%), its concentration increased by up to 28% in Arugula and 16.6% in Basil.
Across all species, PEFB and PEFC treatments generally had the most significant impacts on nutritional parameters.
3.3. Post-Harvest: Sensory Analysis
The results of the comparative sensory analysis performed on the three species under study—Beetroot, Arugula, and Basil—to assess possible organoleptic differences are represented in radar charts, one for each of the species. The results for Beetroot are presented in Figure 5.
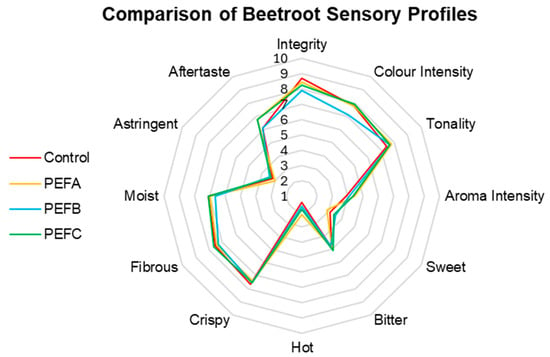
Figure 5.
Comparison of Beetroot sensory profiles.
For instance, Beetroot’s sensory profile was not heavily affected by any of the PEF treatments, with the most pronounced differences being reported for Colour Intensity, with PEFB subjects demonstrating a slight reduction compared to other treatments. Sweet is possibly one of the most appealing and considered attributes when discussing Beetroot; PEF treatments above 3 kV/cm seem to enhance this attribute, as PEFC and PEFB received the highest scores. In contrast, Tonality remained relatively stable across all groups. Aroma Intensity was enhanced in all Beetroot microgreens treated with PEFs, with this difference being more prominent in PEFA and PEFC. Crispy, Hot, Fibrous, Moisture, and Astringency attributes showed minor variations but remained consistent across treatments. In addition, the lingering effect regarding Aftertaste seemed to persist for longer in microgreens obtained from PEFA- and PEFC-treated seeds. The conducted statistical analysis supports the conclusion that neither PEF treatment affected the sensory profile significantly (F(36, 69) = 0.559, p = 0.971, ηp2 = 0.226).
Regarding Arugula’s comparative sensory analysis (Figure 6), panellists identified the most noticeable differences in Aroma Intensity, with PEFB receiving the highest score, followed by PEFC, while subjects of the Control and PEFA groups exhibited lower similar values. One of the primary interests in terms of curiosity was determining if electropriming had any effect on Arugula’s Hot attribute. In fact, a reduction in the score for this attribute was found for all PEF subjects, as the Control received the highest score. Similarly, Aftertaste presented the same behaviour. In contrast, Astringency was slightly more noticeable in PEFC. Like Beetroot, Colour Intensity and Tonality presented small fluctuations but remained generally consistent across treatments. Statistical analysis demonstrates that while these differences are noticed, they are not statistically significant; however, the medium effect size of the treatment was the highest amongst the three species, being able to explain 41.5% of the assessed differences between treatments (F(36, 57) = 1.124, p = 0.341, ηp2 = 0.415).
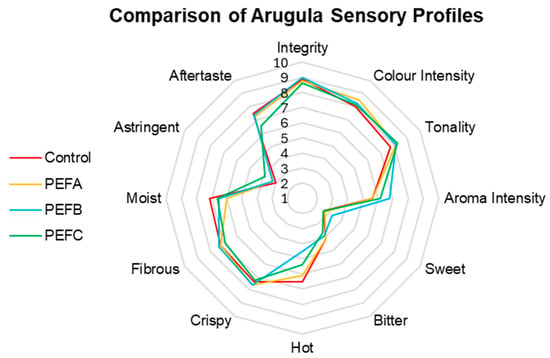
Figure 6.
Comparison of Arugula sensory profiles.
The sensory results of Basil are expressed in Figure 7.
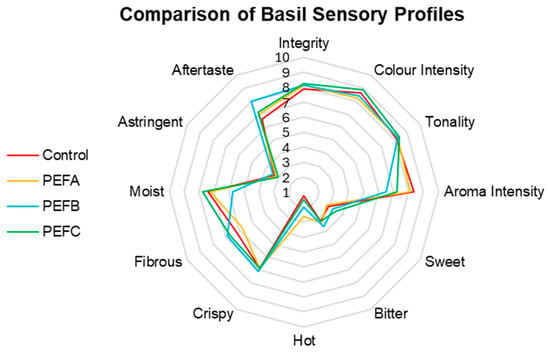
Figure 7.
Comparison of Basil sensory profiles.
The comparative sensory analysis of Basil also revealed interesting results (Figure 7). Different treatments appeared to affect Aroma Intensity, considering that higher scores were attributed to the Control, while PEFB presented the least fragrant sample. In contrast, a more intense PEF protocol seems to increase the perception of Sweet, considering its successive increase of scores up to PEFC. Astringency, Bitter, and Crispy attributes seem not to be affected by the pre-sowing treatments. Equally to the other counterparts, statistical analysis indicated that the observed differences are not statistically different, with 32.4% of the detected variation explained by the different PEF treatments (F(36, 57) = 0.760, p = 0.809, ηp2 = 0.324).
Overall, while some findings suggest that some sensory attributes can be influenced by the different PEF protocols applied as a pre-sowing treatment, statistical analysis reveals that these differences are not significant. However, these results suggest that minor effects can take place for specific sensory attributes, such as Sweet for Beetroot, Hot for Arugula, and Aroma Intensity for Basil, which highlights a potential need for further research on this subject, ideally focusing on future studies with a tasting panel specifically trained for these descriptors and niche types of food products.
4. Conclusions
Electroporation induced by PEF promoted faster seed hydration, enhancing reserve mobilisation, optimised germination and growth, and significantly affected nutritional content, antioxidant capacity, % dry matter, °Brix, and photosynthetic pigment concentration. A significant impact on the sensory profiles was undetected. Species-specific responses to different PEF protocols were observed: PEF application seems to be the most suitable for Beetroot at 4 kV/cm, 3 kV/cm for Arugula, and 2–3 kV/cm for Basil. These results show the potential of PEF to tailor microgreen characteristics according to desired objectives, while further studies should address long-term effects, underlying mechanisms, and scalability for agricultural applications.
Author Contributions
Conceptualisation, M.A.-M., L.M.R., D.V.T.A.C. and R.P.F.G.; methodology, M.A.-M.; formal analysis, M.A.-M. and Y.D.; investigation: M.A.-M.; writing, M.A.-M.; review and editing, M.A.-M., Y.D., L.M.R., D.V.T.A.C. and R.P.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to express their sincere gratitude to Rui Coutinho, Cláudia B. Neves, Bárbara Martins, and Volodymyr Goncharuk for their valuable support, guidance, and assistance during the laboratory trials and microgreen production.
Conflicts of Interest
This manuscript includes results generated within the framework of a dissertation submitted in partial fulfilment of the requirements for a master’s degree. EnergyPulse Systems develops and commercialises PEF equipment for research and industry applications. Author M. Aguiar-Macedo was employed by the company Energypulse Systems. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Demirtas, B. Assessment of the Impacts of the Consumers’ Awareness of Organic Food on Consumption Behavior. Food Sci. Technol. 2019, 39, 881–888. [Google Scholar] [CrossRef]
- Rezai, G.; Teng, P.K.; Mohamed, Z.; Shamsudin, M.N. Consumers’ Awareness and Consumption Intention towards Green Foods. Afr. J. Bus. Manag. 2012, 6, 4496–4503. [Google Scholar] [CrossRef]
- Dorling, D. World Population Prospects at the UN: Our Numbers Are Not Our Problem? In The Struggle for Social Sustainability; Policy Press: Bristol, UK, 2021; pp. 129–154. ISBN 978-1-4473-5612-7. [Google Scholar]
- FAO. The State of Food Security and Nutrition in the World 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136499-4. [Google Scholar]
- Reisch, L.; Eberle, U.; Lorek, S. Sustainable Food Consumption: An Overview of Contemporary Issues and Policies. Sustain. Sci. Pract. Policy 2013, 9, 7–25. [Google Scholar] [CrossRef]
- Premanandh, J. Factors Affecting Food Security and Contribution of Modern Technologies in Food Sustainability. J. Sci. Food Agric. 2011, 91, 2707–2714. [Google Scholar] [CrossRef]
- Treadwell, D.D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop: HS1164/HS1164, 4/2010. EDIS 2010, 2010. [Google Scholar] [CrossRef]
- Ebert, A. Sprouts, Microgreens, and Edible Flowers: The Potential for High Value Specialty Produce in Asia. In Proceedings of the Regional Symposium on High Value Vegetables in Southeast Asia, Chiang Mai, Thailand, 24 January 2012. [Google Scholar]
- Research and Market. Microgreen Market Report; Research and Market: Dublin, Ireland, 2025. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-Scale Vegetable Production and the Rise of Microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Electropriming of Wheatgrass Seeds Using Pulsed Electric Fields Enhances Antioxidant Metabolism and the Bioprotective Capacity of Wheatgrass Shoots. Sci. Rep. 2016, 6, 25306. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.; Aguiar, A.; Ferreira, I. Comparison between the Mineral Profile and Nitrate Content of Microgreens and Mature Lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Márton, M.; Mándoki, Z.; Csapóné Kiss, Z.; Csapó, J. The Role of Sprouts in Human Nutrition. A Review. Acta Univ. Sapientiae 2010, 3, 81–117. [Google Scholar]
- International Organization of Vine and Wine Resolution OIV-OENO 634-2020; OIV: Paris, France, 2020.
- European Commission. Commission Delegated Regulation (EU) 2022/68 of 27 October 2021 Amending Delegated Regulation (EU) 2019/934 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Authorised Oenological Practices; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Aguiar-Macedo, M.; Redondo, L.M.; Pereira, M.T.; Silva, C. Pulsed Electric Fields vs. Pectolytic Enzymes in Arinto Vinification: Effects on Yield and Oenological Parameters. Appl. Sci. 2023, 13, 8343. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-Efficient Biomass Processing with Pulsed Electric Fields for Bioeconomy and Sustainable Development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Pulsed-Electric-Fields-Induced Effects in Plant Tissues: Fundamental Aspects and Perspectives of Applications. In Electrotechnologies for Extraction from Food Plants and Biomaterials; Food Engineering Series; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Schwan, H.P. Electrical Properties of Tissue and Cell Suspensions. In Advances in Biological and Medical Physics; Elsevier: Amsterdam, The Netherlands, 1957; Volume 5, pp. 147–209. ISBN 978-1-4832-3111-2. [Google Scholar]
- Aguiar-Macedo, M.; Redondo, L.M.; Silva, C.; Correia, E.; Vilela, A. Impact of Monopolar and Bipolar Pulsed Electric Fields on the Quality of Tinta Roriz Wines. In IVES Conference Series; OIV: Dijon, France, 2024. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed Electric Field Treatment of Citrus Fruits: Improvement of Juice and Polyphenols Extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Aguiar-Macedo, M.; Pereira, M.T.; Redondo, L.M.; Silva, C.; Controlling, B. Bruxellensis with Pulsed Electric Fields: Optimization of Industrial Protocols and Impact on the Wine Profile. BIO Web Conf. 2023, 68, 02041. [Google Scholar] [CrossRef]
- Mattar, J.R.; Turk, M.F.; Nonus, M.; Lebovka, N.I.; El Zakhem, H.; Vorobiev, E. Stimulation of Saccharomyces Cerevisiae Cultures by Pulsed Electric Fields. Food Bioprocess. Technol. 2014, 7, 3328–3335. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed Electric Fields Inactivation of Wine Spoilage Yeast and Bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef]
- Attri, P.; Okumura, T.; Koga, K.; Shiratani, M.; Wang, D.; Takahashi, K.; Takaki, K. Outcomes of Pulsed Electric Fields and Nonthermal Plasma Treatments on Seed Germination and Protein Functions. Agronomy 2022, 12, 482. [Google Scholar] [CrossRef]
- Ahmed, Z.; Manzoor, M.F.; Ahmad, N.; Zeng, X.-A.; Din, Z.U.; Roobab, U.; Qayum, A.; Siddique, R.; Siddeeg, A.; Rahaman, A. Impact of Pulsed Electric Field Treatments on the Growth Parameters of Wheat Seeds and Nutritional Properties of Their Wheat Plantlets Juice. Food Sci. Nutr. 2020, 8, 2490–2500. [Google Scholar] [CrossRef]
- Sitzmann, W.; Vorobiev, E.; Lebovka, N. Applications of Electricity and Specifically Pulsed Electric Fields in Food Processing: Historical Backgrounds. Innov. Food Sci. Emerg. Technol. 2016, 37, 302–311. [Google Scholar] [CrossRef]
- Benham, C. The Electro-Vegetometer. Nature 1911, 88, 41. [Google Scholar] [CrossRef]
- Dymek, K.; Dejmek, P.; Panarese, V.; Vicente, A.A.; Wadsö, L.; Finnie, C.; Galindo, F.G. Effect of Pulsed Electric Field on the Germination of Barley Seeds. LWT-Food Sci. Technol. 2012, 47, 161–166. [Google Scholar] [CrossRef]
- Sonoda, T.; Takamura, N.; Wang, D.; Namihira, T.; Akiyama, H. Growth Control of Leaf Lettuce Using Pulsed Electric Field. In Proceedings of the 2013 19th IEEE Pulsed Power Conference (PPC), San Francisco, CA, USA, 16–21 June 2013; pp. 1–5. [Google Scholar]
- Song, Y.; Zhao, W.; Su, Z.; Guo, S.; Du, Y.; Song, X.; Shi, X.; Li, X.; Liu, Y.; Liu, Z. Effect of Pulsed Electric Field Treatment on Seed Germination and Seedling Growth of Scutellaria baicalensis. Agriculture 2024, 14, 158. [Google Scholar] [CrossRef]
- Nolan, D.A. Effects of Seed Density and Other Factors on the Yield of Microgreens Grown Hydroponically on Burlap; Major Report; Virginia Tech: Blacksburg, VA, USA, 2019; Available online: https://hdl.handle.net/10919/86642 (accessed on 21 November 2025).
- Di Gioia, F.; Santamaria, P. The Nutritional Properties of Microgreens. In Microgreens: Novel, Fresh and Functional Food to Explore all the Value of Biodiversity; ECO-logica SRL: Bari, Italy, 2015; pp. 41–50. ISBN 978-88-909289-3-2. [Google Scholar]
- Reddy, L.V.; Metzger, R.J.; Ching, T.M. Effect of Temperature on Seed Dormancy of Wheat. Crop Sci. 1985, 25, 455–458. [Google Scholar] [CrossRef]
- Walker-Simmons, M. ABA Levels and Sensitivity in Developing Wheat Embryos of Sprouting Resistant and Susceptible Cultivars 1. Plant Physiol. 1987, 84, 61–66. [Google Scholar] [CrossRef]
- Zuo, J.; Liu, J.; Gao, F.; Yin, G.; Wang, Z.; Chen, F.; Li, X.; Xu, J.; Chen, T.; Li, L.; et al. Genome-Wide Linkage Mapping Reveals QTLs for Seed Vigor-Related Traits Under Artificial Aging in Common Wheat (Triticum aestivum). Front. Plant Sci. 2018, 9, 1101. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Al-Ansari, F.; Ksiksi, T. A Quantitative Assessment of Germination Parameters: The Case of Crotalaria Persica and Tephrosia Apollinea. Open Ecol. J. 2016, 9, 13–21. [Google Scholar] [CrossRef]
- Jakusek, M.; Brennensthul, M.; Markowska, J.; Wolski, K.; Sobol, Ł. Effect of a Micronutrient Fertilizer and Fungicide on the Germination of Perennial Ryegrass Seeds (Lolium perenne L.) in Field Conditions. Agronomy 2020, 10, 1978. [Google Scholar] [CrossRef]
- Aliyar, Z.B.; Shafiei, A.B.; Seyedi, N.; Rezapour, S.; Moghanjugi, S.M. Effect of Traffic-Induced Air Pollution on Seed Germination of Arizona Cypress (Cupressus arizonica Green) and Black Pine (Pinus nigra Arnold). Urban For. Urban Green. 2020, 55, 126841. [Google Scholar] [CrossRef]
- Brown, R.F.; Mayer, D.G. Representing Cumulative Germination. 1. A Critical Analysis of Single-Value Germination Indices. Ann. Bot. 1988, 61, 117–125. [Google Scholar] [CrossRef]
- Kotowski, F. Temperature Alternation and Germination of Vegetable Seed. Acta Soc. Bot. Pol. 1927, 5, 71–78. [Google Scholar] [CrossRef][Green Version]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Pentoś, K.; Wondołowska-Grabowska, A.; Gajda, G.; Babij, M.; Chohura, P.; Zaleski, A.; Szpunar-Krok, E.; Jobczyk, W.; Romaniuk, A.; Gajda, D. The Effect on the Germination Vigour of Cucumber Seeds after Receiving Magnetic Field Treatment Pre-Sowing. Appl. Sci. 2022, 12, 5490. [Google Scholar] [CrossRef]
- Barik, S.R.; Pandit, E.; Sanghamitra, P.; Mohanty, S.P.; Behera, A.; Mishra, J.; Nayak, D.K.; Bastia, R.; Moharana, A.; Sahoo, A.; et al. Unraveling the Genomic Regions Controlling the Seed Vigour Index, Root Growth Parameters and Germination per Cent in Rice. PLoS ONE 2022, 17, e0267303. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, Absorption, and Cancer Preventative Activity of Dietary Chlorophyll Derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.-N.; López-Camarillo, C.; Salas, E. Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yu, J.; Ma, R.; Ji, Y.; Hu, Q.; Mao, Y.; Ding, C.; Li, Z.; Ge, S.; Deng, W.-W.; et al. Chlorophyll and Carotenoid Metabolism Varies with Growth Temperatures among Tea Genotypes with Different Leaf Colors in Camellia Sinensis. Int. J. Mol. Sci. 2024, 25, 10772. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. A Novel Method for Estimating Chlorophyll and Carotenoid Concentrations in Leaves: A Two Hyperspectral Sensor Approach. Sensors 2023, 23, 3843. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Dulyanska, Y.; Cruz-Lopes, L.; Esteves, B.; Ferreira, J.; Domingos, I.; Lima, M.J.; Correia, P.; Ferreira, M.; Fragata, A.; Barroca, M.; et al. Evaluation of the Antioxidant Activity of Extracts Obtained Form Cherry Seeds. Hyg. Eng. Des. 2022, 40, 221–226, UDC 634.233-157.63:615.272(469). [Google Scholar]
- Santos, S.C.R.V.L.; Guiné, R.; Barros, A. Effect of Drying Temperatures on the Phenolic Composition and Antioxidant Activity of Pears of Rocha Variety (Pyrus communis L.). J. Food Meas. Charact. 2014, 8, 105–112. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F.; Serafini, M. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dulyanska, Y.; Cruz-Lopes, L.P.; Esteves, B.; Ferreira, J.V.; Domingos, I.; Lima, M.J.; Correia, P.M.R.; Ferreira, M.; Fragata, A.; Barroca, M.J.; et al. Extraction of Phenolic Compounds from Cherry Seeds: A Preliminary Study. Agronomy 2022, 12, 1227. [Google Scholar] [CrossRef]
- Paul, V.; Singh, A.; Pandey, R. Estimation of Total Soluble Solids (TSS). In Laboratory Manual on “Post-Harvest Physiology of Fruits and Flowers”; Indian Agricultural Research Institute, Division of Plant Physiology: New Delhi, India, 2010; pp. 41–43. [Google Scholar]
- Sidel, J.; Stone, H. Sensory Science: Methodology. In Handbook of Food Science, Technology, and Engineering; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-8493-9848-3. [Google Scholar]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices; Academic Press: Cambridge, MA, USA, 2004; ISBN 978-0-12-672690-9. [Google Scholar]
- Likert, R. A Technique for the Measurement of Attitudes. Arch. Psychol. 1932, 22, 55. [Google Scholar]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer Sensory Perception and Acceptance of an Emerging Functional Food Crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Norouzian, R.; Plonsky, L. Eta- and Partial Eta-Squared in L2 Research: A Cautionary Review and Guide to More Appropriate Usage. Second Lang. Res. 2018, 34, 257–271. [Google Scholar] [CrossRef]
- Marôco, J. Análise de Equações Estruturais: Fundamentos Teóricos, Software & Aplicações; ReportNumber, Lda: Pêro Pinheiro, Portugal, 2011; ISBN 978-989-96763-1-2. [Google Scholar]
- Polachini, T.C.; Norwood, E.-A.; Le-Bail, P.; Le-Bail, A.; Cárcel, J.A. Pulsed Electric Field (PEF) Application on Wheat Malting Process: Effect on Hydration Kinetics, Germination and Amylase Expression. Innov. Food Sci. Emerg. Technol. 2023, 86, 103375. [Google Scholar] [CrossRef]
- Bagarinao, N.C.; King, J.; Leong, S.Y.; Agyei, D.; Sutton, K.; Oey, I. Effect of Germination on Seed Protein Quality and Secondary Metabolites and Potential Modulation by Pulsed Electric Field Treatment. Foods 2024, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of Seed Protein Reserves. Physiol. Plant 2012, 145, 140–153. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The Biomechanics of Seed Germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Mittal, G.S. Electroporation of Cell Membranes: A Review. Crit. Rev. Biotechnol. 1996, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.P.; Schoenbach, K.H. Electroporation Dynamics in Biological Cells Subjected to Ultrafast Electrical Pulses: A Numerical Simulation Study. Phys. Rev. E 2000, 62, 1025–1033. [Google Scholar] [CrossRef]
- Li, Z.; Burritt, D.J. The Influence of Cocksfoot Mottle Virus on Antioxidant Metabolism in the Leaves of Dactylis glomerata L. Physiol. Mol. Plant Pathol. 2003, 62, 285–295. [Google Scholar] [CrossRef]
- Sabri, N.; Pelissier, B.; Teissié, J. Electropermeabilization of Intact Maize Cells Induces an Oxidative Stress. Eur. J. Biochem. 1996, 238, 737–743. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).