Safety and Functional Properties of Rapeseed Honey Regarding Its Geographical Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
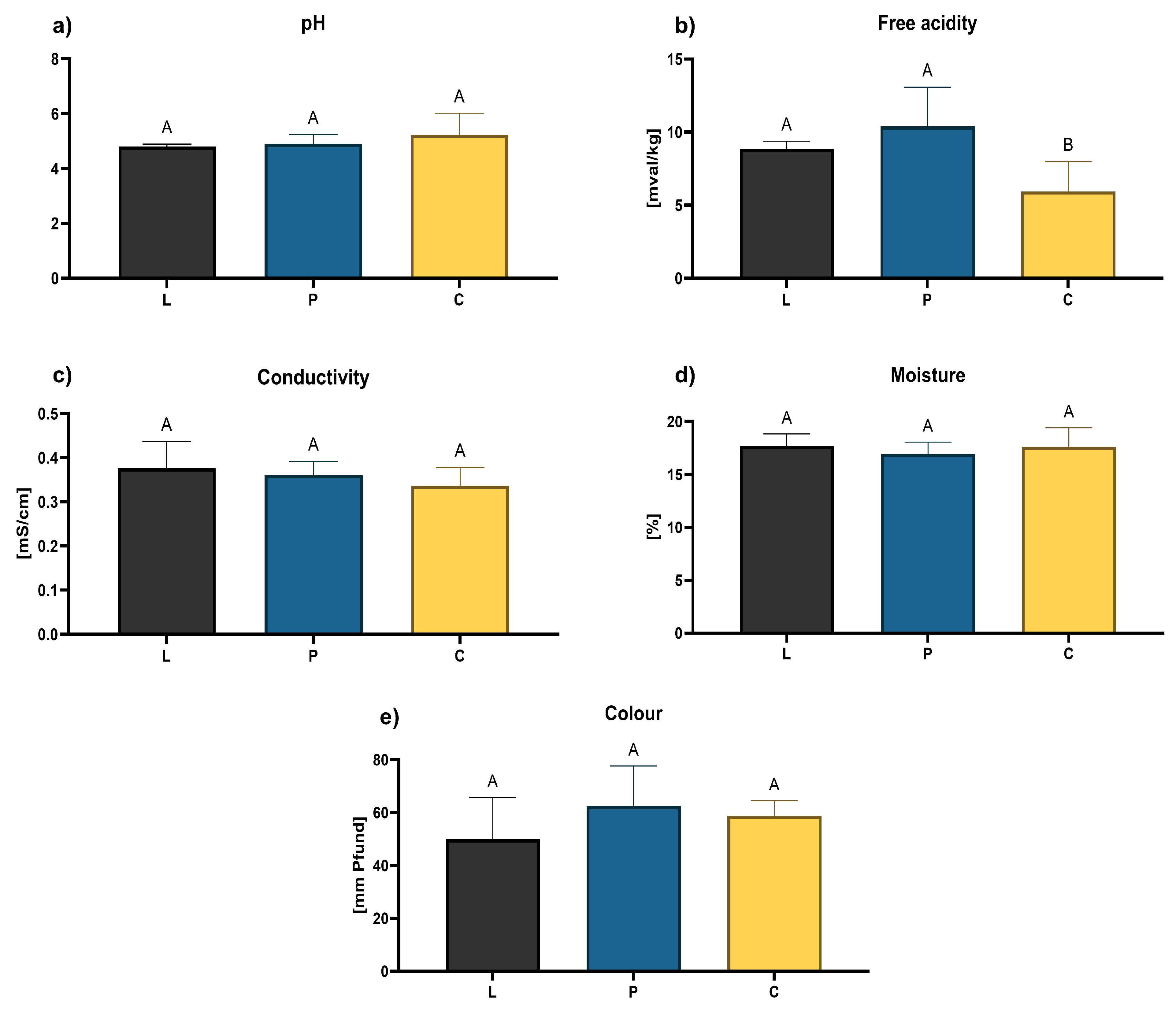
2.3. Quality Evaluation Regarding Legal Requirement for Honey
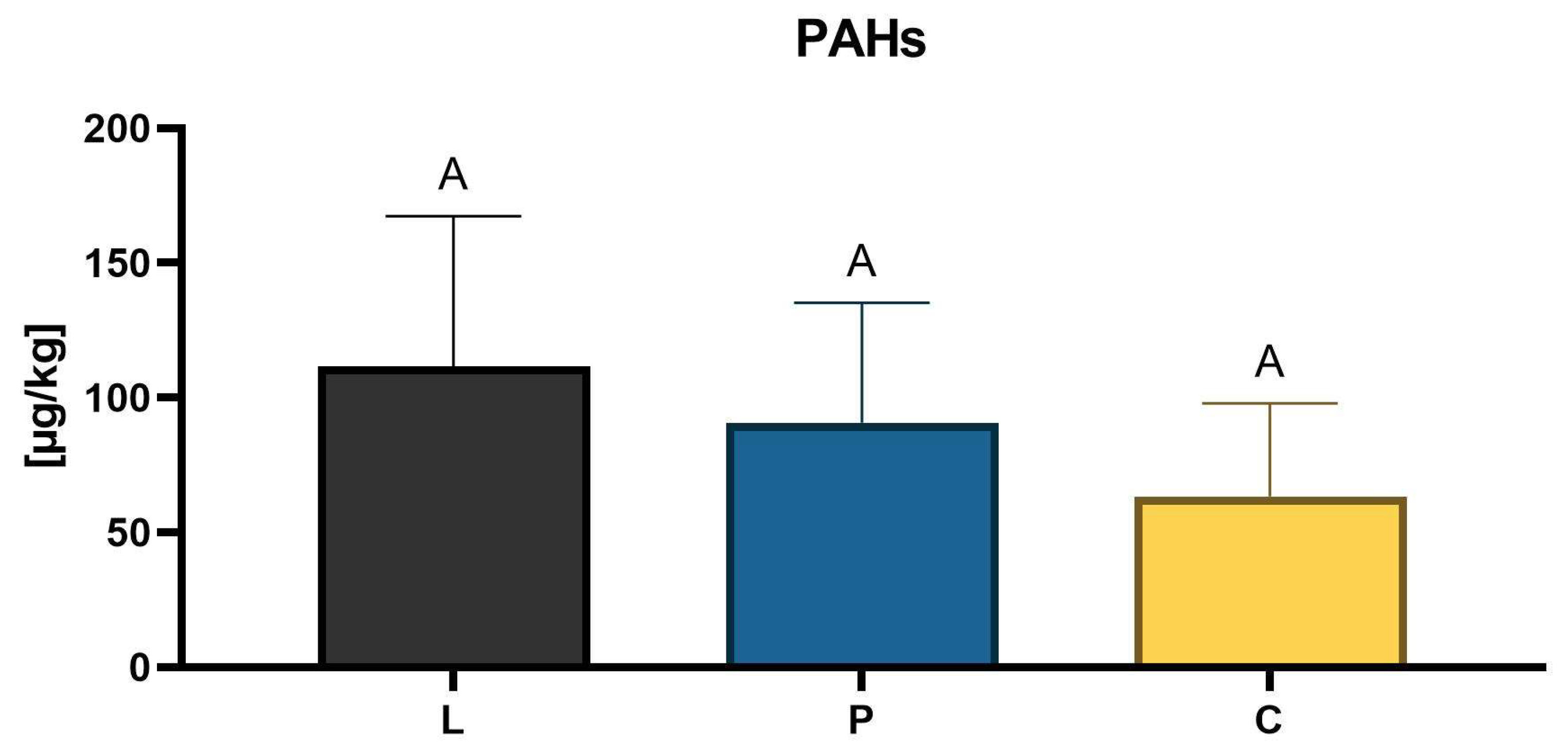
2.4. Detection of Pesticide Residues and PAHs
2.4.1. Sample Preparation
2.4.2. GC-MS Analyses
2.4.3. HPLC-DAD Analysis
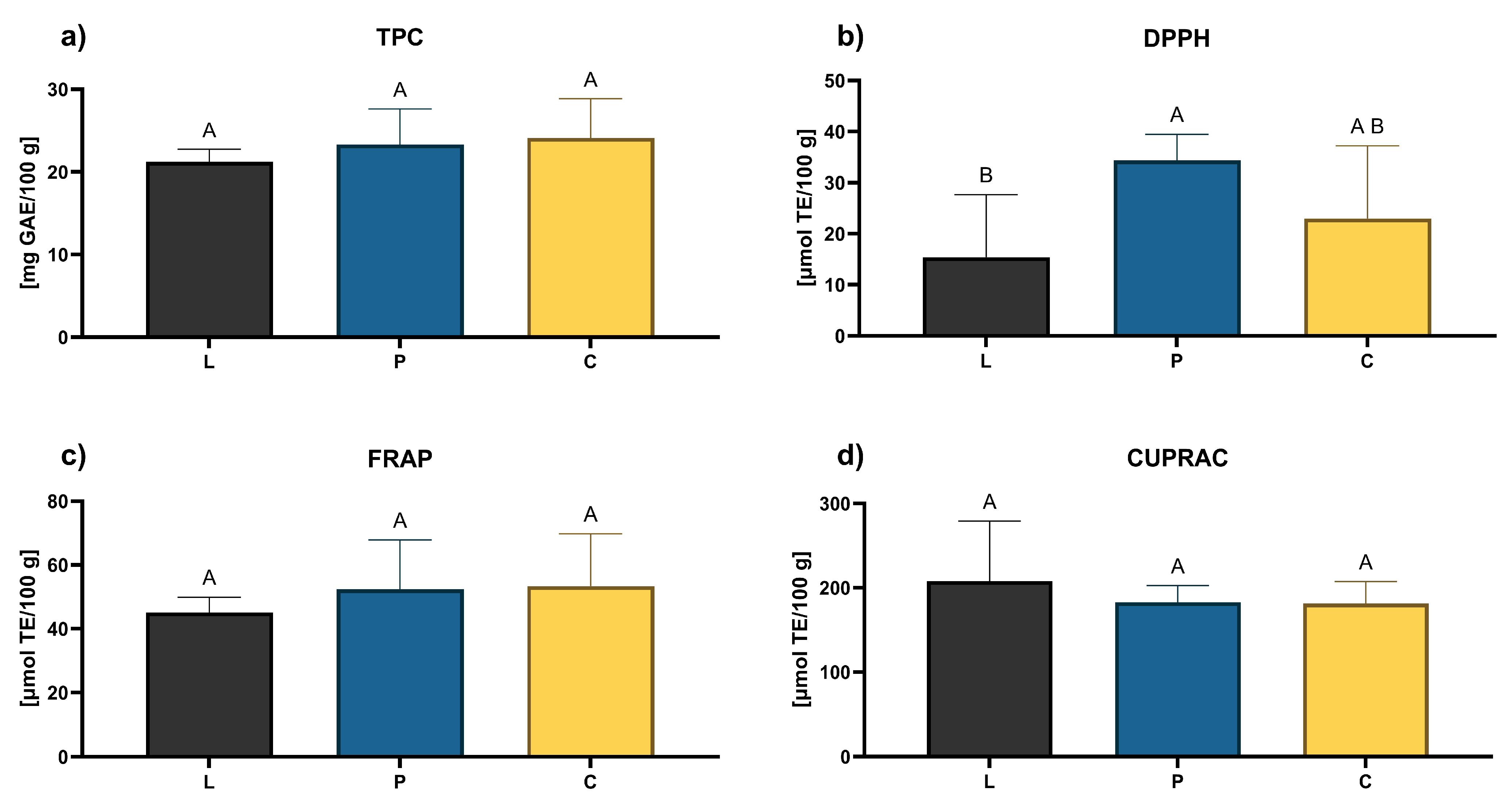
2.5. Antioxidant Activity and Total Phenolic Content in Honey
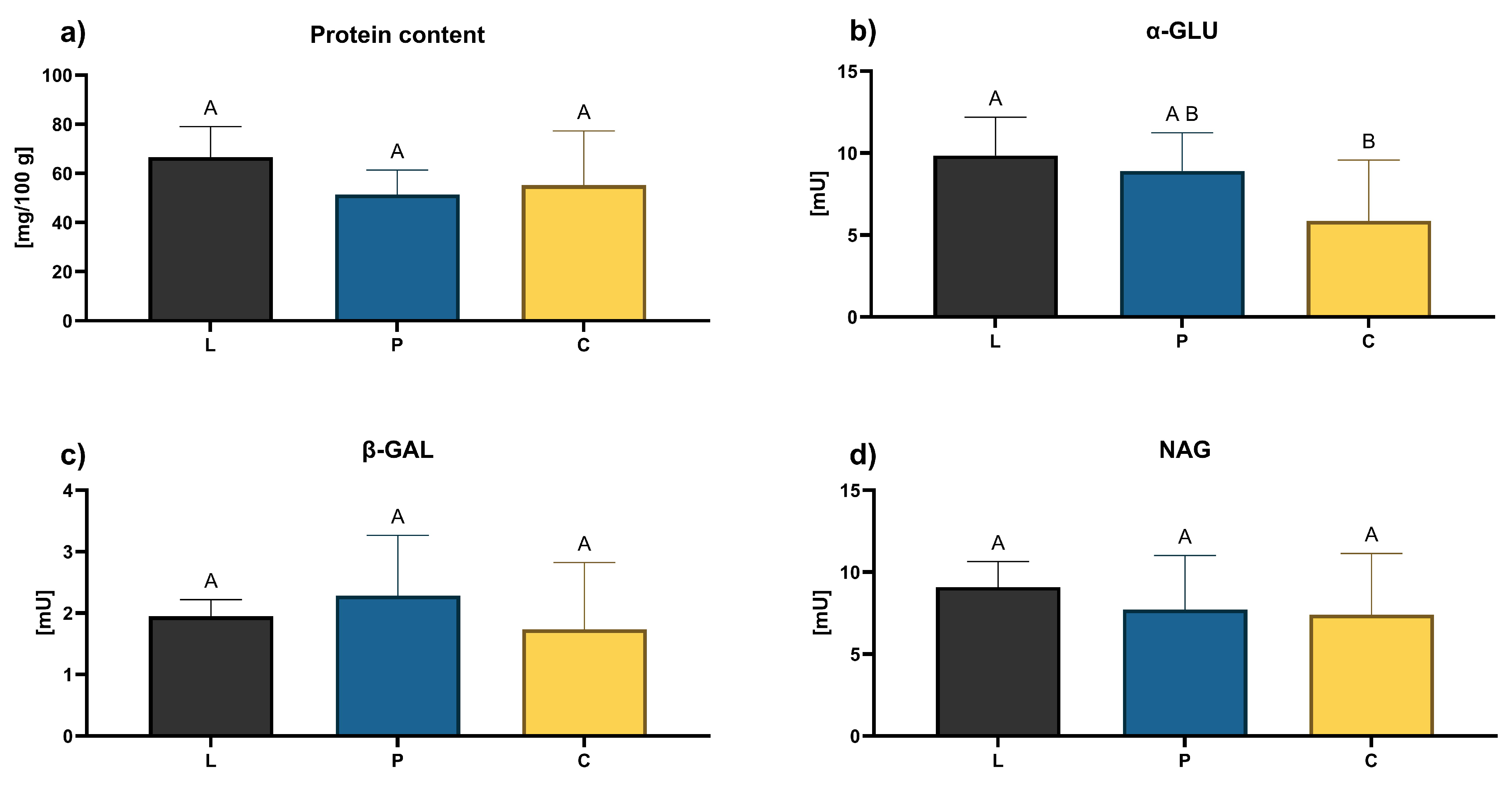
2.6. Honey Protein and Enzymes
2.7. HPTLC Polyphenolic Profile
2.8. Statistical Analysis
3. Results
3.1. Honey Quality Evaluation Regarding Legal Requirements
3.2. Pesticide Residues and PAHs Detection
3.3. Functional Properties of Honey
3.4. Searching for Rapeseed Honey Fingerprint
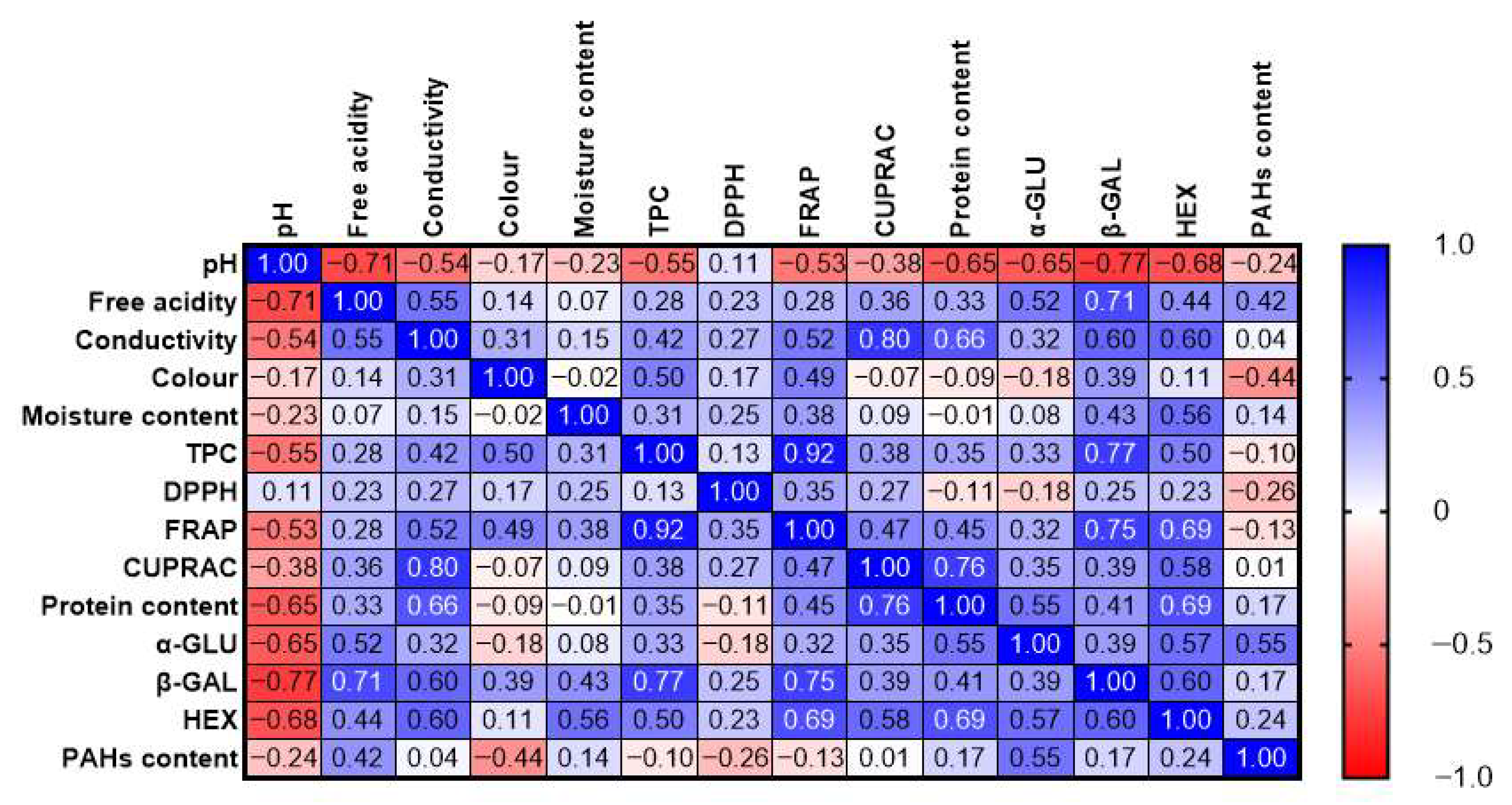
3.5. Correlation Between PAHs Content and Functional Properties of Honey
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAHs | Polycyclic aromatic hydrocarbons |
| HPTLC | High performance thin layer chromatography |
| Rf | Retardation factor |
| GC-MS | Gas chromatography coupled with mass spectrometry |
| HPLC-DAD | High-performance liquid chromatography with diode array detection |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| OCP | Organochlorine pesticides |
| OP | Organophosphate |
| POPs | Persistent organic pollutants |
| DDT | Dichlorodiphenyltrichloroethane |
| CCD | Colony collapse disorder |
| IARC | International agency for research on cancer |
| MRLs | Maximum residue limits |
| PSA | Primary-secondary amine |
| PP | Polypropylene |
| SIM | Selected ion monitoring |
| DPPH• | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric-reducing antioxidant power |
| CUPRAC | Cupric reducing antioxidant capacity |
| TPC | Total phenolic compounds |
| GAE | Gallic acid equivalents |
| TE | Trolox equivalents |
| NAG | N-acetyl-β-D-glucosaminidase |
| α-GLU | α-glucosidase |
| β-GAL | β-galactosidase |
References
- Queirós, J.; Malça, J.; Freire, F. Environmental life-cycle assessment of rapeseed produced in Central Europe: Addressing alternative fertilization and management practices. J. Clean. Prod. 2015, 99, 266–274. [Google Scholar] [CrossRef]
- Pound, M.; Dalgleish, A.; McCoy, J.; Partington, J. Melissopalynology of honey from Ponteland, UK, shows the role of Brassica napus in supporting honey production in a suburban to rural setting. Palynology 2018, 42, 400–405. [Google Scholar] [CrossRef]
- Puvača, N.; Bursić, V.; Brkić, I.; Vapa Tankosić, J.; Prodanović, R.; Vuković, G.; Vještica, S.; Lekić, S.; Vapa, I.; Gvozdenac, S. Toxicological assessment of honey from conventional and organiproduction and risk assessment for public health. J. Cent. Eur. Agric. 2024, 25, 554–566. [Google Scholar] [CrossRef]
- Gizaw, G.; Kim, Y.; Moon, K.; Choi, J.B.; Kim, Y.H.; Park, J.K. Effect of environmental heavy metals on the expression of detoxification-related genes in honey bee Apis mellifera. Apidologie 2020, 51, 664–674. [Google Scholar] [CrossRef]
- Tahir, F.; Goblirsch, M.; Adamczyk, J.; Karim, S.; Alburaki, M. Honey bee Apis mellifera L. responses to oxidative stress induced by pharmacological and pesticidal compounds. Front. Bee Sci. 2023, 1, 1275862. [Google Scholar] [CrossRef]
- Ahsan, Z.; Wu, Z.; Lin, Z.; Ji, T.; Wang, K. The Sublethal Effects of Neonicotinoids on Honeybees. Biology 2025, 14, 1076. [Google Scholar] [CrossRef]
- Shepherd, S.; Park, Y.G.; Krupke, C.H. Effects of common co-occurring pesticides (a neonicotinoid and fungicide) on honey bee colony health in a semi-field study. Heliyon 2024, 10, e29886. [Google Scholar] [CrossRef]
- Naccari, C.; Ferrantelli, V.; Cammilleri, G.; Barbaccia, G.; Riolo, P.; Ferrante, M.C.; Procopio, A.; Palma, E. Study of Toxic Metals and Microelements in Honey as a Tool to Support Beekeeping Production and Consumer Safety. Foods 2025, 14, 1986. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Wesołowska, M.; Zaguła, G.; Kaczmarski, M.; Czernicka, M.; Puchalski, C. Honeybees (Apis mellifera) as a biological barrier for contamination of honey by environmental toxic metals. Environ. Monit. Assess. 2018, 190, 101. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Sadowska-Rociek, A.; Draszanowska, A. Levels of Contamination by Pesticide Residues, Polycyclic Aromatic Hydrocarbons (PAHs), and 5-Hydroxymethylfurfural (HMF) in Honeys Retailed in Europe. Arch. Environ. Contam. Toxicol. 2023, 84, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Zárate, M.J.; Londoño-Giraldo, L.M. Pesticides in honey: Bibliographic and bibliometric analysis towards matrix quality for consumption. Braz. J. Food Technol. 2023, 26, e2022112. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Teter, A.; Skałecki, P.; Topyła, B.; Domaradzki, P.; Poleszak, E.; Florek, M. Residues of Pesticides and Heavy Metals in Polish Varietal Honey. Foods 2022, 11, 2362. [Google Scholar] [CrossRef]
- Corredera, L.; Bayarri, S.; Pérez-Arquillué, C.; Lázaro, R.; Molino, F.; Herrera, A. Multiresidue determination of carcinogenic polycyclic aromatic hydrocarbons in honey by solid-phase extraction and high-performance liquid chromatography. J. Food Prot. 2011, 74, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Ciemniak, A.; Witczak, A.; Mocek, K. Assessment of honey contamination with polycyclic aromatic hydrocarbons. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 993–998. [Google Scholar] [CrossRef]
- Végh, R.; Csóka, M.; Mednyánszky, Z.; Sipos, L. Pesticide residues in bee bread, propolis, beeswax and royal jelly—A review of the literature and dietary risk assessment. Food Chem. Toxicol. 2023, 176, 113806. [Google Scholar] [CrossRef]
- Ligor, M.; Bukowska, M.; Ratiu, I.A.; Gadzała-Kopciuch, R.; Buszewski, B. Determination of Neonicotinoids in Honey Samples Originated from Poland and Other World Countries. Molecules 2020, 25, 5817. [Google Scholar] [CrossRef] [PubMed]
- Scripcă, L.A.; Amariei, S. The influence of chemical contaminants on the physicochemical properties of unifloral and multifloral honey. Foods 2021, 10, 1039. [Google Scholar] [CrossRef]
- Fasasi, K.A.; Awodiran, O.F.; Ayeni, D.J.; Awoniyi, O.I.; Awojide, S.H. Assessment of Bee Honey in some Districts in South-Western Nigeria for Agricultural Pesticide Residues and Polycyclic Aromatic Hydrocarbons (PAHs). J. Agric. Sci. 2024, 19, 142–156. [Google Scholar] [CrossRef]
- Hungerford, N.L.; Fletcher, M.T.; Tsai, H.H.; Hnatko, D.; Swann, L.J.; Kelly, C.L.; Anuj, S.R.; Tinggi, U.; Webber, D.C.; Were, S.T.; et al. Occurrence of environmental contaminants (pesticides, herbicides, PAHs) in Australian/Queensland Apis Mellifera honey. Food Addit. Contam. Part B Surveill. 2021, 14, 193–205. [Google Scholar] [CrossRef]
- Witczak, A.; Ciemniak, A. Evaluation of contamination of some types of honey with selected persistent organic pollutants (POPs). Ocena zanieczyszczenia wybranych gatunków miodu związkami z grupy trwałych zanieczyszczeń organicznych. Rocz. Panstw. Zakl. Hig. 2012, 63, 359–366. [Google Scholar]
- Montano, L.; Baldini, G.M.; Piscopo, M.; Liguori, G.; Lombardi, R.; Ricciardi, M.; Esposito, G.; Pinto, G.; Fontanarosa, C.; Spinelli, M.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment: Occupational Exposure, Health Risks and Fertility Implications. Toxics 2025, 13, 151. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Li, W. Polycyclic Aromatic Hydrocarbons (PAHs): Environmental Persistence and Human Health Risks. Nat. Prod. Commun. 2025, 20, 1–8. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Panseri, S.; Nobile, M.; Ceriani, F.; Arioli, F. Distribution of POPs, pesticides and antibiotic residues in organic honeys from different production areas. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Bonerba, E.; Nobile, M.; Cesare, F.D.; Mosconi, G.; Cecati, F.; Arioli, F.; Tantillo, G.; Chiesa, L. Pesticides and environmental contaminants in organic honeys according to their different productive areas toward food safety protection. Foods 2020, 9, 1863. [Google Scholar] [CrossRef]
- Piechowicz, B.; Grodzicki, P.; Podbielska, M.; Tyrka, N.; Śliwa, M. Transfer of active ingredients from plant protection products to a honeybee (Apis mellifera F.) hive from winter oilseed rape crops protected with conventional methods. Pol. J. Environ. Stud. 2018, 27, 1219–1228. [Google Scholar] [CrossRef]
- Minister of Agriculture and Rural Development. Regulation of 14 January 2009 on methods of analysis related to honey quality assessment. J. Laws 2009, 17, 94. [Google Scholar]
- Sawicki, T.; Surma, M.; Sadowska-Rociek, A. Characteristics of contaminants in the Polish-origin bee products and cancer risk assessment. Food Chem. Toxicol. 2023, 175, 113693. [Google Scholar] [CrossRef]
- Dżugan, M.; Miłek, M.; Kielar, P.; Stępień, K.; Sidor, E.; Bocian, A. SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey. Foods 2022, 11, 2390. [Google Scholar] [CrossRef]
- Dżugan, M.; Miłek, M.; Sidor, E.; Buczkowicz, J.; Hęclik, J.; Bocian, A. The Application of SDS-PAGE Protein and HPTLC Amino Acid Profiling for Verification of Declared Variety and Geographical Origin of Honey. Food Anal. Methods 2023, 16, 1157–1171. [Google Scholar] [CrossRef]
- Szczesna, T.; Rybak-Chmielewska, H.; Waś, E.; Kachaniuk, K.; Teper, D. Characteristics of Polish unifloral honeys. I. Rape honey (Brassica napus L. Var. oleifera Metzger). J. Apic. Sci. 2011, 55, 111–119. [Google Scholar]
- Marić, A.; Sakač, M.; Jovanov, P.; Đermanović, B.; Teslić, N.; Plavšić, D.; Jakimov, D. Functional Properties of Rapeseed Honey Enriched with Lyophilized Fruits. Agriculture 2024, 14, 2117. [Google Scholar] [CrossRef]
- Kowalski, S.; Ciesarová, Z.; Kukurová, K.; Tobolková, B.; Polovka, M.; Skoczylas, Ł.; Tabaszewska, M.; Mikulec, K.; Mikulec, A.; Buksa, K. Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys. Appl. Sci. 2025, 15, 5810. [Google Scholar] [CrossRef]
- Minister of Agriculture and Rural Development. Regulation of 3 October 2003 on Detailed Requirements for the Commercial Quality of Honey. J. Laws 2003, 181, 1773. (In Poland) [Google Scholar]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are pesticide residues in honey related to oilseed rape treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer; WHO. Agents Classified by the IARC Monographs. Volumes 1–125. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (accessed on 17 October 2025).
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 5, 562813. [Google Scholar] [CrossRef]
- Kowalska, J.; Stanisławek, M.; Latoch, A.; Marzec, A.; Galus, S.; Kowalska, H.; Ciecierska, M. Polycyclic Aromatic Hydrocarbons in Polish Traditionally and Industrially Smoked Meats as an Element of Monitoring and PAH Reduction Strategies. Foods 2025, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A.; Żak, N.; Stasiuk, E. Content of Selected Harmful Metals (Zn, Pb, Cd) and Polycyclic Aromatic Hydrocarbons (PAHs) in Honeys from Apiaries Located in Urbanized Areas. Foods 2024, 13, 3451. [Google Scholar] [CrossRef] [PubMed]
- Batelková, P.; Borkovcová, I.; Čelechovská, O.; Vorlová, L.; Bartáková, K. Polycyclic aromatic hydrocarbons and risk elements in honey from the South Moravian region (Czech Republic). Acta Vet. Brno 2012, 81, 169–174. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Luchese, R.H.; Prudêncio, E.R.; Guerra, A.F. Honey as a Functional Food. In Honey Analysis; de Alencar Arnaut de Toledo, V., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Pakosz, P. Comparison of Antioxidant Properties and Color of Selected Polish Honeys and Manuka Honey. Foods 2024, 13, 2666. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef] [PubMed]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of polish honey—A short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Flanjak, I.; Strelec, I.; Kenjerić, D.; Primorac, L. Croatian produced unifloral honey characterized according to the protein and proline content and enzyme activities. J. Apic. Sci. 2016, 60, 39–48. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical Composition and Antimicrobial Activity of New Honey Varietals. Int. J. Environ. Res. Public Health. 2023, 20, 2458. [Google Scholar] [CrossRef]
- Nazarian, H.; Taghavizad, R.; Majd, A. Origin of honey proteins and method for its quality control. Pak. J. Bot. 2010, 42, 3221–3228. [Google Scholar]
- Stanek, N.; Teper, D.; Kafarski, P.; Jasicka-Misiak, I. Authentication of phacelia honeys (Phacelia tanacetifolia) based on a combination of HPLC and HPTLC analyses as well as spectrophotometric measurements. LWT 2019, 107, 199–207. [Google Scholar] [CrossRef]
- Stanek, N.; Kafarski, P.; Jasicka-Misiak, I. Development of a high performance thin layer chromatography method for the rapid qualification and quantification of phenolic compounds and abscisic acid in honeys. J. Chromatogr. A 2019, 1598, 209–215. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Karpińska, E.; Moskwa, J.; Socha, K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, X.; Du, X.; Cheng, N.; Chen, L.; Zhao, J.; Zheng, J.; Cao, W. Identification of Acacia Honey Adulteration with Rape Honey Using Liquid Chromatography–Electrochemical Detection and Chemometrics. Food Anal. Meth. 2014, 7, 2003–2012. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 496, 485–496. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
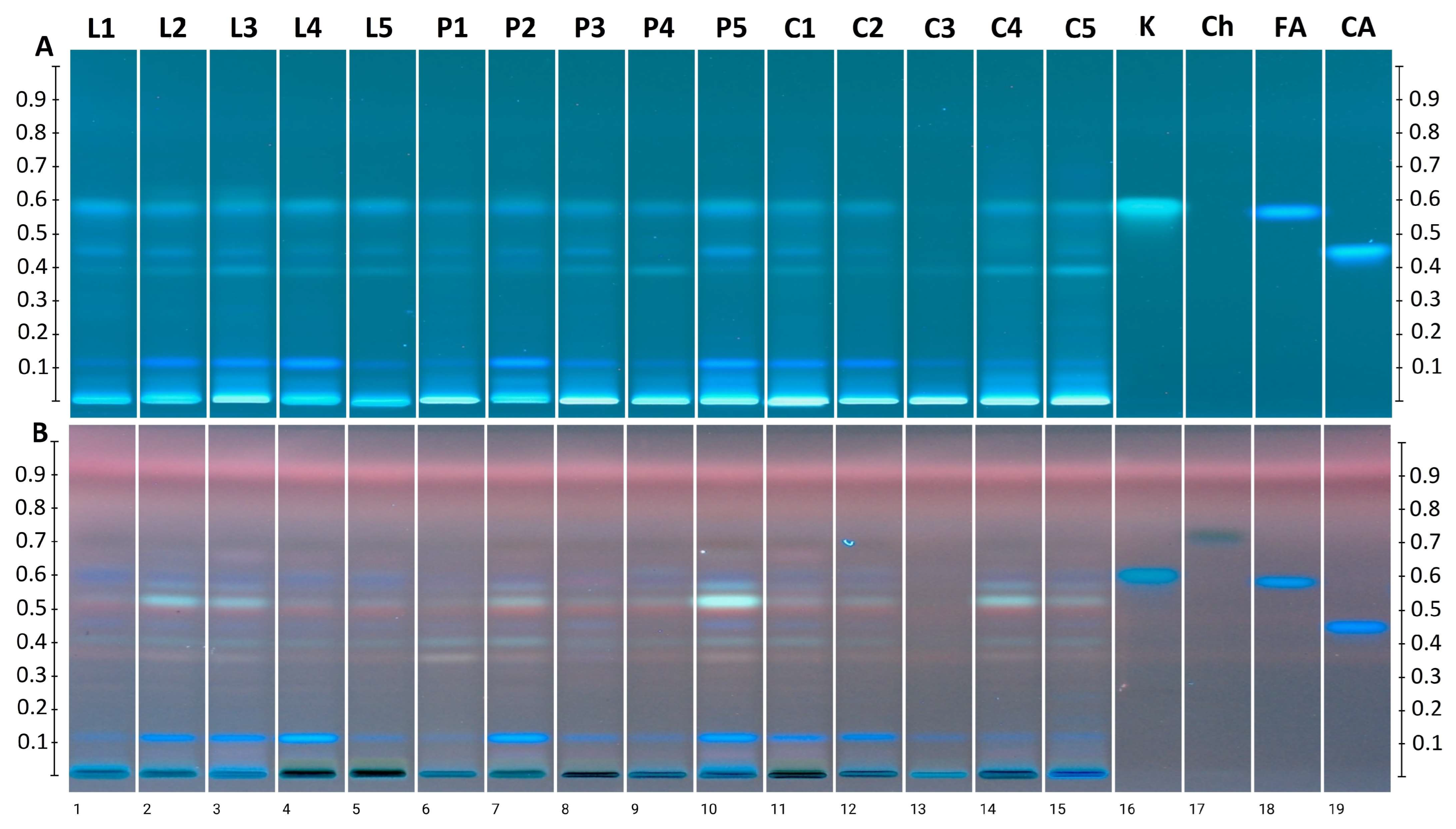
| Compound | L1 | L2 | L3 | L4 | L5 | P1 | P2 | P3 | P4 | P5 | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaP | <LOD | 2.2 ± 0.2 b | 3.4 ± 0.3 a | 2.9 ± 0.2 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 4.0 ± 0.8 a | <LOD | 3.3 ± 0.4 a | <LOD | <LOD |
| MeNaP2 | 7.9 ± 1.0 e,f | 12.7 ± 1.6 b,c,d | 11.7 ± 1.0 c,e | 13.5 ± 2.1 c,d | 4.4 ± 0.4 f,g | 10.6 ± 0.7 d,e | 12.6 ± 1.4 b,c,d | <LOD | <LOD | 8.0 ± 1.0 e,f | 18.8 ± 1.9 a,b | 13.7 ± 0.9 b,c,d | 21.0 ± 2.2 a | 16.3 ± 2.2 b,c | 2.8 ± 0.2 g |
| MeNaP1 | <LOD | 6.8 ± 0.2 a | 4.6 ± 0.3 c | 4.3 ± 0.3 d | <LOD | <LOD | <LOD | < LOQ | <LOD | <LOD | 5.9 ± 0.4 b | <LOD | <LOD | <LOD | <LOD |
| Acp | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | < LOQ | <LOD | <LOD | <LOD | < LOQ | <LOD | <LOD | <LOD | <LOD |
| Ace | <LOD | <LOD | <LOD | < LOQ | 2.9 ± 0.4 e | <LOD | 21.2 ± 2.7 a | 15.8 ± 1.4 b | 12.4 ± 1.3 c | <LOD | 6.6 ± 0.7 d | 5.9 ± 1.0 d | <LOD | 15.5 ± 1.4 b | <LOD |
| Flu | 92.4 ± 4.0 a | <LOD | <LOD | 28.1 ± 2.3 c | <LOD | <LOD | 45.9 ± 2.8 b | <LOD | <LOD | <LOD | < LOQ | <LOD | <LOD | <LOD | <LOD |
| Phen | 61.6 ± 3.5 c | 78.9 ± 3.0 b | 24.6 ± 1.9 f,g | 97.7 ± 3.8 a | 38.8 ± 2.6 d,e | 37.4 ± 3.9 d,e | 43.0 ± 2.3 d | 55.2 ± 3.6 c | 35.8 ± 2.7 e,f | 23.1 ± 1.4 g,h | 86.2 ± 3.3 b | 26.8 ± 2.9 f,g | 21.2 ± 2.7 g,h | 20.9 ± 2.0 g,h | 12.6 ± 1.1 h |
| Ant | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Fla | 10.2 ± 1.3 e | 15.1 ± 1.7 d,e | 6.7 ± 0.9 f | 13.8 ± 0.7 d,e | 13.1 ± 1.5 d,e | 34.5 ± 2.2 a | 40.6 ± 1.8 a | 22.0 ± 1.8 b | 22.5 ± 2.8 b,c | 13.1 ± 0.7 e | <LOQ | <LOD | <LOQ | 15.0 ± 1.2 d,e | 20.6 ± 2.0 c,d |
| Pyr | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[a]a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Chr | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[b]f | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[k]f | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[a]f | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[e]p | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[a]p | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| I[cd]p | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| D[ah]a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| B[ghi]P | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| PAHs total: | 172.0 | 115.7 | 51.0 | 160.2 | 59.2 | 82.5 | 163.3 | 93.0 | 70.7 | 44.2 | 121.6 | 46.4 | 45.5 | 67.6 | 36.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.; Lewczuk, M.; Miłek, M.; Surma, M.; Sadowska-Rociek, A.; Dżugan, M. Safety and Functional Properties of Rapeseed Honey Regarding Its Geographical Origin. Appl. Sci. 2025, 15, 12146. https://doi.org/10.3390/app152212146
Tomczyk M, Lewczuk M, Miłek M, Surma M, Sadowska-Rociek A, Dżugan M. Safety and Functional Properties of Rapeseed Honey Regarding Its Geographical Origin. Applied Sciences. 2025; 15(22):12146. https://doi.org/10.3390/app152212146
Chicago/Turabian StyleTomczyk, Monika, Monika Lewczuk, Michał Miłek, Magdalena Surma, Anna Sadowska-Rociek, and Małgorzata Dżugan. 2025. "Safety and Functional Properties of Rapeseed Honey Regarding Its Geographical Origin" Applied Sciences 15, no. 22: 12146. https://doi.org/10.3390/app152212146
APA StyleTomczyk, M., Lewczuk, M., Miłek, M., Surma, M., Sadowska-Rociek, A., & Dżugan, M. (2025). Safety and Functional Properties of Rapeseed Honey Regarding Its Geographical Origin. Applied Sciences, 15(22), 12146. https://doi.org/10.3390/app152212146







