Ignition Delay Times of Conventional and Green Hypergolic Propellants at Ambient Conditions: A Comparative Review
Abstract
1. Introduction
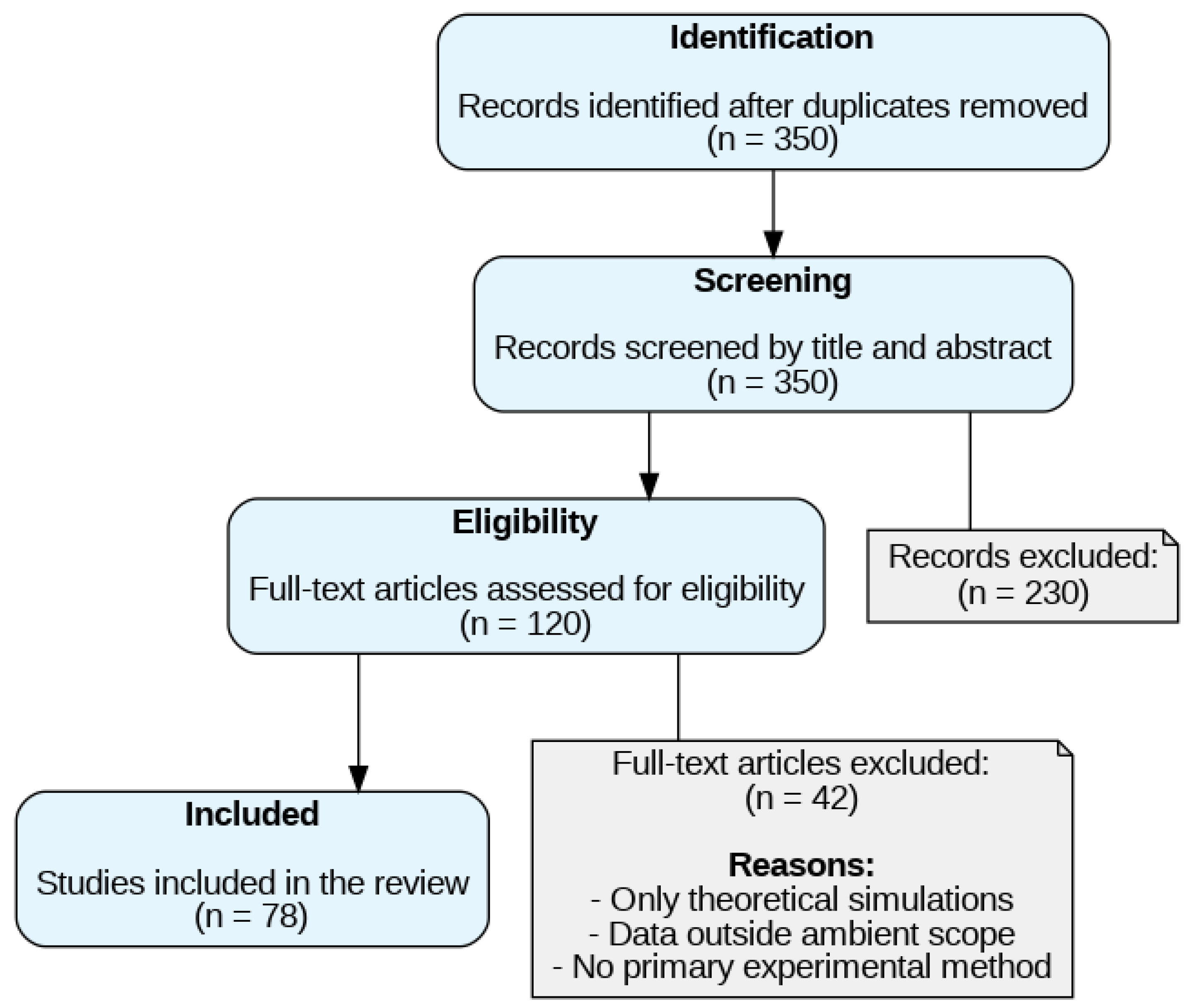
2. Methods
3. Background
3.1. Conventional Hypergolic Propellants and Established Ignition Delay Data
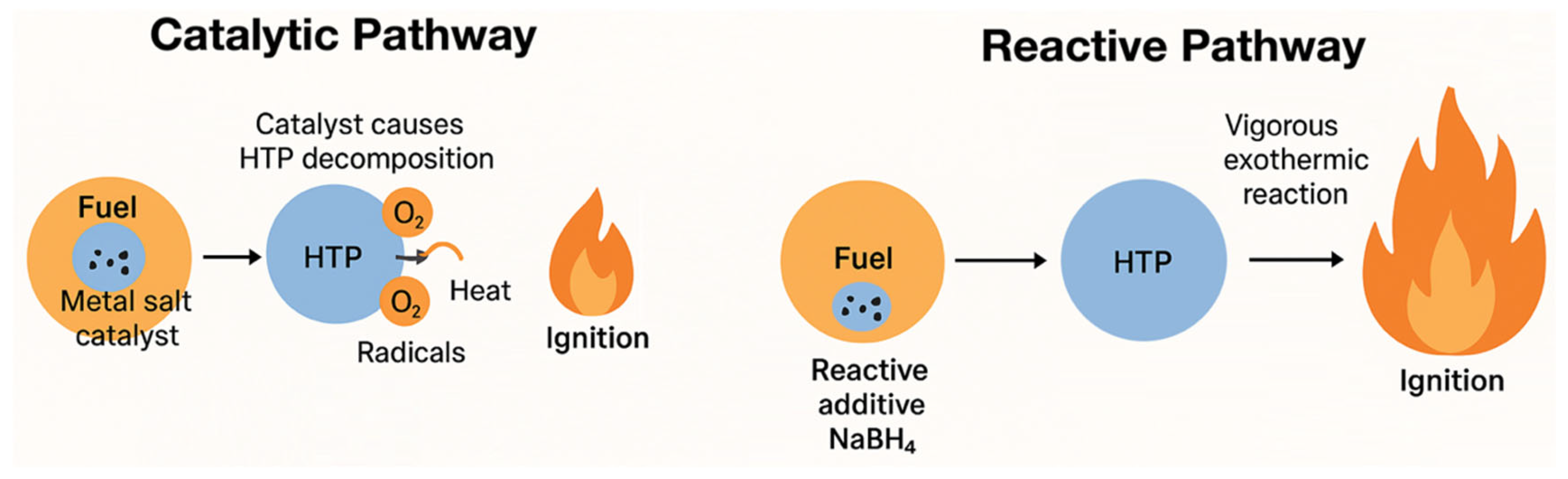
3.2. Green Hypergolic Propellants: Advances in Self-Igniting Catalytic and Reactive Systems
3.2.1. Catalytic Pathway
3.2.2. Reactive Pathway
4. Results
4.1. Conventional Propellants
4.2. Self-Igniting Ionic Liquids and Amine-Based Fuels
4.3. Catalytically Promoted Systems
4.4. Reactively Promoted Systems
4.5. Methodological Sensitivity and Diagnostic Effects
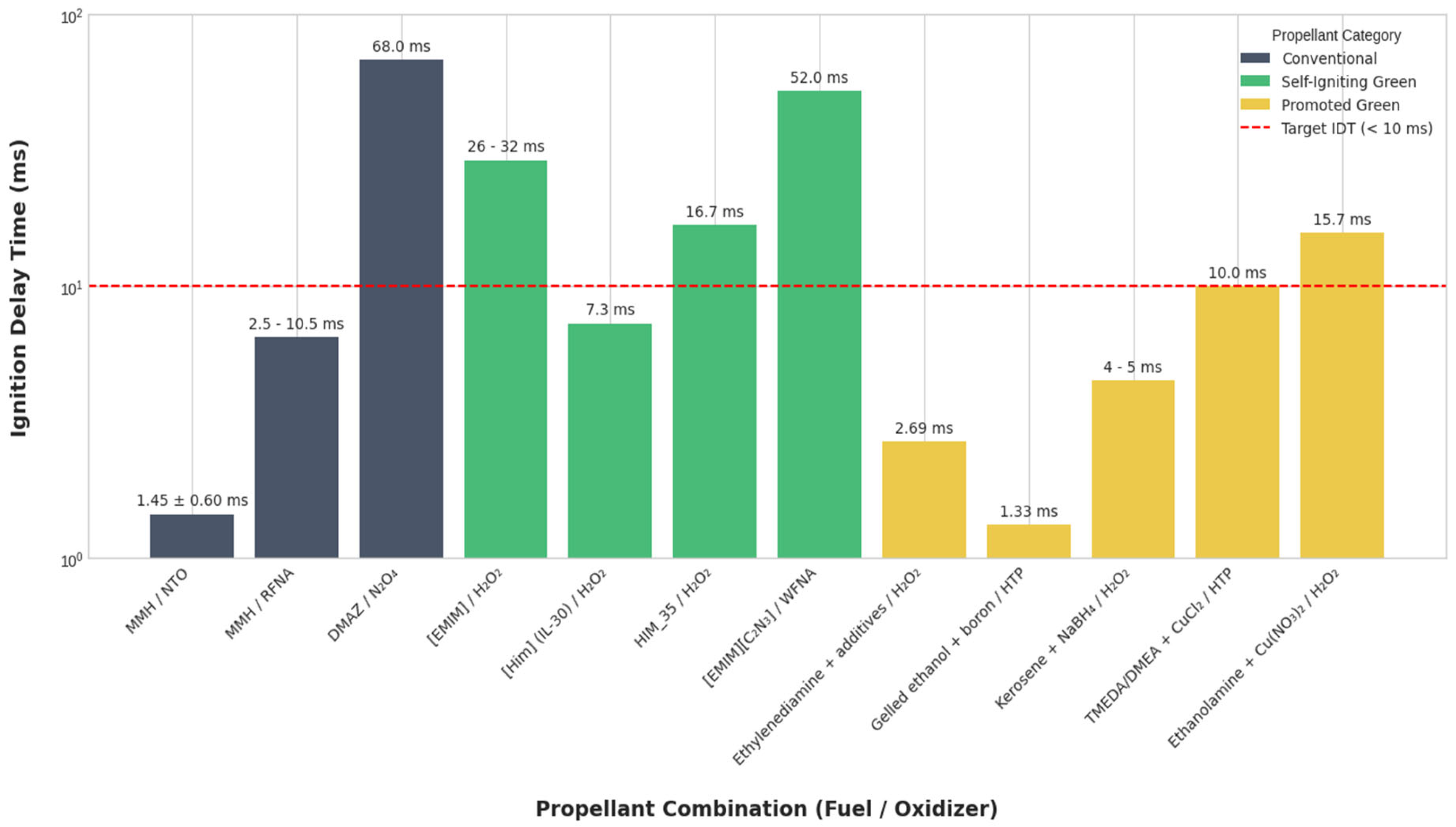
4.6. Quantitative Synthesis and Comparative Ranges
4.7. Data Gaps and Priorities
5. Discussion
5.1. Interpretation of Trends
5.2. Trade-Offs and Limitations
Balancing Performance and Safety in Future Design
5.3. Research Gaps and R&D Directions
6. Conclusions and Outlook
- Performance Benchmark: Conventional MMH/NTO systems reliably achieve IDTs in the 1–3 ms range, establishing the target for green alternatives.
- Promoters are Key: Reactive promoters (e.g., NaBH4, boron particles) are the most effective strategy for achieving sub-5 ms IDTs in green fuels, successfully transforming non-hypergolic base fuels into high-performance options.
- Ionic Liquids Show Promise: Molecular tuning of ionic liquids offers a pathway to intrinsically hypergolic fuels with reduced toxicity, although many current formulations require blending or additives to meet the most aggressive performance targets.
- Methodology Matters: Reported IDT values are highly sensitive to experimental methodology. Standardised testing and reporting protocols are urgently needed for meaningful cross-study comparisons.
- New Challenges Emerge: The transition to green propellants introduces new challenges, including storage stability of reactive additives, combustion residues, and unknown long-term environmental impacts, which must be addressed for flight readiness.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ADN | Ammonium Dinitramide |
| HTP | High-Test Peroxide |
| HAN | Hydroxylammonium Nitrate |
| IDT | Ignition Delay Time |
| IL | Ionic Liquid |
| MMH | Monomethylhydrazine |
| NTO | Nitrogen Tetroxide (Dinitrogen Tetroxide) |
| RFNA | Red Fuming Nitric Acid |
| UDMH | Unsymmetrical Dimethylhydrazine |
| WFNA | White Fuming Nitric Acid |
References
- Henry, G.N.; Humble, R.W.; Larson, W.J. Space Propulsion Analysis and Design; College custom series; McGraw-Hill: New York, NY, USA, 1995; ISBN 9780070313293. [Google Scholar]
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1118174208. [Google Scholar]
- Catoire, L.; Chaumeix, N.; Paillard, C. Chemical Kinetic Model for Monomethylhydrazine/Nitrogen Tetroxide Gas-Phase Combustion and Hypergolic Ignition. J. Propuls. Power 2004, 20, 87–92. [Google Scholar] [CrossRef]
- Frank, I.; Hammerl, A.; Klapötke, T.M.; Nonnenberg, C.; Zewen, H. Processes during the Hypergolic Ignition between Monomethylhydrazine (MMH) and Dinitrogen Tetroxide (N2O4) in Rocket Engines. Propellants Explos. Pyrotech. 2005, 30, 44–52. [Google Scholar] [CrossRef]
- Dennis, J.D.; Son, S.F.; Pourpoint, T.L. Critical Ignition Criteria for Monomethylhydrazine and Red Fuming Nitric Acid in an Impinging Jet Apparatus. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Wang, S.Q.; Thynell, S.T. An Experimental Study on the Hypergolic Interaction between Monomethylhydrazine and Nitric Acid. Combust. Flame 2012, 159, 438–447. [Google Scholar] [CrossRef]
- Williams, E.S.; Panko, J.; Paustenbach, D.J. The European Union’s REACH regulation: A review of its history and requirements. Crit. Rev. Toxicol. 2009, 39, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Sackheim, R.L.; Masse, R.K. Green Propulsion Advancement: Challenging the Maturity of Monopropellant Hydrazine. J. Propuls. Power 2014, 30, 265–276. [Google Scholar] [CrossRef]
- Thomas, J.C.; Rodriguez, F.A.; Teitge, D.S.; Petersen, E.L. Lab-Scale Ballistic and Safety Property Investigations of LMP-103S. Combust. Flame 2023, 253, 112810. [Google Scholar] [CrossRef]
- Neff, K.; King, P.; Anflo, K.; Möllerberg, R. High Performance Green Propellant for Satellite Applications. In Proceedings of the 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Denver, CO, USA, 2–5 August 2009. [Google Scholar] [CrossRef]
- Masse, R.; Allen, M.; Spores, R.; Driscoll, E.A. AF-M315E Propulsion System Advances and Improvements. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016; p. 4577. [Google Scholar]
- Li, S.; Gao, H.; Shreeve, J.M. Borohydride Ionic Liquids and Borane/Ionic-Liquid Solutions as Hypergolic Fuels with Superior Low Ignition-Delay Times. Angew. Chem. Int. Ed. 2014, 53, 2969–2972. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, H.; Joo, Y.H.; Shreeve, J.M. Ionic Liquids as Hypergolic Fuels. Angew. Chem. Int. Ed. 2011, 50, 9554–9562. [Google Scholar] [CrossRef]
- Kim, C.; Kang, H.; LEE, J. Experimental Study on Ignition Behaviors of Green Hypergolic Propellants Depending on Fuel Properties: Nabh4-Promoted Fuels/H2O2 Oxidzier. Fuel 2025, 406, 136983. [Google Scholar] [CrossRef]
- Bhosale, V.K.; Jeong, J.; Kwon, S. Ignition of Boron-Based Green Hypergolic Fuels with Hydrogen Peroxide. Fuel 2019, 255, 115729. [Google Scholar] [CrossRef]
- Boruc, Ł.; Kapusta, Ł.J.; Kindracki, J. Selection of the Method for Determination of Ignition Delay of Hypergolic Propellants. Combust. Engines 2024, 199, 104–111. [Google Scholar] [CrossRef]
- Pourpoint, T.L.; Anderson, W.E. Hypergolic reaction mechanisms of catalytically promoted fuels with rocket grade hydrogen peroxide. Combust. Sci. Technol. 2007, 179, 2107–2133. [Google Scholar] [CrossRef]
- Schneider, S.; Hawkins, T.; Ahmed, Y.; Rosander, M.; Mills, J.; Hudgens, L. Green Hypergolic Bipropellants: H202/Hydrogen-Rich Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 5886–5888. [Google Scholar] [CrossRef] [PubMed]
- Gohardani, A.S.; Stanojev, J.; Demairé, A.; Anflo, K.; Persson, M.; Wingborg, N.; Nilsson, C. Green Space Propulsion: Opportunities and Prospects. Prog. Aerosp. Sci. 2014, 71, 128–149. [Google Scholar] [CrossRef]
- Dennis, J.D.; Pourpoint, T.L.; Son, S.F. Ignition of Gelled Monomethylhydrazine and Red Fuming Nitric Acid in an Impinging Jet Apparatus. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar] [CrossRef]
- Kasbi, Y.; Remissa, I.; Toshtay, K.; Mabrouk, A.; Bachar, A.; Azat, S.; Nosseir, A.E.S.; Tiwari, A.; Sabbar, E.M.; Amrousse, R. H2O2 and HAN Green Monopropellants—A State-of-the-Art Review on Their Recent Development, Corresponding Synthesized Catalysts, and Their Possible Use as Thrusters. Catalysts 2025, 15, 183. [Google Scholar] [CrossRef]
- Dambach, E.M.; Solomon, Y.; Heister, S.D.; Pourpoint, T.L. Investigation into the Hypergolic Ignition Process Initiated by Low Weber Number Collisions. JPP 2013, 29, 331–338. [Google Scholar] [CrossRef]
- Pourpoint, T.L. Hypergolic Ignition of a Catalytically Promoted Fuel with Rocket Grade Hydrogen Peroxide, Purdue University. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2005. [Google Scholar]
- Chambreau, S.D.; Schneider, S.; Rosander, M.; Hawkins, T.; Gallegos, C.J.; Pastewait, M.F.; Vaghjiani, G.L. Fourier Transform Infrared Studies in Hypergolic Ignition of Ionic Liquids. J. Phys. Chem. A 2008, 112, 7816–7824. [Google Scholar] [CrossRef]
- Catoire, L.; Chambreau, S.D.; Vaghjiani, G.L. Chemical Kinetics Interpretation of Hypergolicity of Dicyanamide Ionic Liquid-Based Systems. Combust. Flame 2012, 159, 1759–1768. [Google Scholar] [CrossRef]
- Zaseck, C.R.; Son, S.F.; Pourpoint, T.L. Combustion of Micron-Aluminum and Hydrogen Peroxide Propellants. Combust. Flame 2013, 160, 184–190. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheah, K.H.; Wu, M.H.; Koh, K.S.; Sun, D.; Meng, H. A Review on Hydroxylammonium Nitrate (HAN) Decomposition Techniques for Propulsion Application. Acta Astronaut. 2022, 196, 194–214. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Yi, Z.; Song, D.; Cheng, Y.; Li, Y. Sodium Azotetrazolate: A Novel Environmental-Friendly Hydrogen-Free Gas-Generating Pyrotechnics. Chem. Eng. J. 2021, 413, 127442. [Google Scholar] [CrossRef]
- Dias, G.S.; da Silva Mota, F.A.; Fei, L.; Wu, Y.; Liu, M.; Tang, C.; de Souza Costa, F. Hypergolic Fuel Impacting a Gelled Oxidiser Wall: Droplet Dynamics, Heat Release, Ignition, and Flame Analysis. Exp. Therm. Fluid Sci. 2025, 160, 111322. [Google Scholar] [CrossRef]
- Nath, S.; Laso, I.; Mallick, L.; Sobe, Z.; Koffler, S.; Blumer-Ganon, B.; Borzin, E.; Libis, N.; Lefkowitz, J.K. Comprehensive Ignition Characterization of a Non-Toxic Hypergolic Hybrid Rocket Propellant. Proc. Combust. Inst. 2023, 39, 3361–3370. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Li, L.; Tang, G. Thermal Performance of MMH/NTO Rocket Thrust Chamber Based on Pintle Injector by Using Liquid Film Cooling. Appl. Therm. Eng. 2023, 223, 120035. [Google Scholar] [CrossRef]
- Rarata, G.; Rokicka, K.; Surmacz, P. Hydrogen Peroxide as a High Energy Compound Optimal for Propulsive Applications. Cent. Eur. J. Energetic Mater. 2016, 13, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Florczuk, W.; Rarata, G. Assessment of Various Fuel Additives for Reliable Hypergolic Ignition with 98%+ HTP. In Proceedings of the 66th International Astronautical Congress; International Astronautical Federation Jerusalem, Jerusalem, Israel, 12–16 October 2015. [Google Scholar]
- Lauck, F.; Balkenhohl, J.; Negri, M.; Freudenmann, D.; Schlechtriem, S. Green Bipropellant Development—A Study on the Hypergolicity of Imidazole Thiocyanate Ionic Liquids with Hydrogen Peroxide in an Automated Drop Test Setup. Combust. Flame 2021, 226, 87–97. [Google Scholar] [CrossRef]
- Lauck, F.; Balkenhohl, J.; Negri, M.; Freudenmann, D.; Schlechtriem, S. Ignition Investigations of A Novel Hypergolic Ionic Liquid with Hydrogen Peroxide in Drop Tests. In Proceedings of the 7th Space Propulsion Conference 2020+1, Amaral, Portugal, 5–9 October 2020. [Google Scholar]
- Ricker, S.C.; Freudenmann, D.; Schlechtriem, S. The Impact of Cation Structures on Hypergolicity of Thiocyanate Ionic Liquids with Hydrogen Peroxide. Energy Fuels 2021, 35, 16128–16133. [Google Scholar] [CrossRef]
- Ricker, S.C.; Brüggemann, D.; Freudenmann, D.; Ricker, R.; Schlechtriem, S. Protic Thiocyanate Ionic Liquids as Fuels for Hypergolic Bipropellants with Hydrogen Peroxide. Fuel 2022, 328, 125290. [Google Scholar] [CrossRef]
- Stölzle, S.C.; Kruse, L.; Freudenmann, D. Trialkylsulfonium Thiocyanate Ionic Liquids: Investigation on Temperature-Dependent Ignition Behavior of Green Hypergolic Propellants. Propellants Explos. Pyrotech. 2024, 49, e202400151. [Google Scholar] [CrossRef]
- Bhosale, V.K.; Lee, K.; Yoon, H.; Kwon, S. Green Bipropellant: Performance Evaluation of Hypergolic Ionic Liquid-Biofuel with Hydrogen Peroxide. Fuel 2024, 376, 132688. [Google Scholar] [CrossRef]
- Wang, Z.; Fei, L.H.; Xia, H.L.; Jin, Y.H.; Zhang, Q.H. Organic Superbase-Mediated Synthesis of Borohydride Ionic Liquids as Novel Composite Hypergolic Fuels. Energetic Mater. Front. 2023, 4, 77–84. [Google Scholar] [CrossRef]
- Shin, K.S.; Jang, H.G.; Park, S.H.; Cho, S.J. Characteristics of Ignition Delay of Hypergolic Ionic Liquids Combined with 1-Amino-4-Methylpiperazine. RSC Adv. 2023, 13, 18960. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, V.; Simon, D.; Marée, T.; Moerel, J.-L. Economic Benefits of the Use of Non-Toxic Mono-Propellants For Spacecraft Applications. AIAA 2003. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003. [Google Scholar] [CrossRef]
- Swami, U.; Kumbhakarna, N.; Chowdhury, A. Green Hypergolic Ionic Liquids: Future Rocket Propellants. J. Ion. Liq. 2022, 2, 100039. [Google Scholar] [CrossRef]
- Sippel, T.R.; Shark, S.C.; Hinkelman, M.C.; Pourpoint, T.L.; Son, S.F.; Heister, S.D. Hypergolic Ignition of Metal Hydride-Based Fuels with Hydrogen Peroxide. In Proceedings of the 7th US National Combustion Meeting, Atlanta, GA, USA, 20–23 March 2011; Georgia Institute of Technology. pp. 1–15. [Google Scholar]
- Jyoti, B.V.S.; Naseem, M.S.; Baek, S.W. Hypergolicity and Ignition Delay Study of Pure and Energized Ethanol Gel Fuel with Hydrogen Peroxide. Combust. Flame 2017, 176, 318–325. [Google Scholar] [CrossRef]
- Chand, D.; Zhang, J.; Shreeve, J.M. Borohydride Ionic Liquids as Hypergolic Fuels: A Quest for Improved Stability. Chem.-A Eur. J. 2015, 21, 13297–13301. [Google Scholar] [CrossRef]
- Jyoti, B.V.S.; Naseem, M.S.; Baek, S.W.; Lee, H.J.; Cho, S.J. Hypergolicity and Ignition Delay Study of Gelled Ethanolamine Fuel. Combust. Flame 2017, 183, 102–112. [Google Scholar] [CrossRef]
- Hsia, Y.; Kuo, T.; Chang, K.L.; Chang, C.S.; Wei, S.S.; Wu, J.S.; Tan, Z.P. Experimental Study of Hypergolic Ignition by H2O2 Droplets Impacting a Deep Pool of NaBH4-Based Fuel. Exp. Fluids 2025, 66, 153. [Google Scholar] [CrossRef]
- Hsia, Y.; Kuo, T.; Chang, K.L.; Chang, C.S.; Wei, S.S.; Wu, J.S.; Tan, Z.P. Ignition-delay measurement for drop test with hypergolic propellants: Reactive fuels and hydrogen peroxide. Combust. Flame 2020, 217, 306–313. [Google Scholar] [CrossRef]
- Bian, H.Y.; Yan, Y.F.; Cui, M.; Song, T.T.; Guo, X.D.; Xu, J.G.; Zheng, F.K.; Guo, G.C. Energetic Isostructural Metal Imidazolate Frameworks with a Bridging Dicyanamide Linker towards High-Performance Hypergolic Fuels. Inorg. Chem. Front. 2023, 10, 5468–5474. [Google Scholar] [CrossRef]
- Lousada, C.M.; Yang, M.; Nilsson, K.; Jonsson, M. Catalytic Decomposition of Hydrogen Peroxide on Transition Metal and Lanthanide Oxides. J. Mol. Catal. A Chem. 2013, 379, 178–184. [Google Scholar] [CrossRef]
- Tizaoui, C.; Karodia, N.; Aburowais, M. Kinetic Study of the Manganese-Based Catalytic Hydrogen Peroxide Oxidation of a Persistent Azo-Dye. J. Chem. Technol. Biotechnol. 2010, 85, 234–242. [Google Scholar] [CrossRef]
- Liao, S.S.; Liu, T.L.; Zhou, Z.Y.; Wang, K.C.; Zhang, Q.H. Auto-Ignition of Ionic Liquid Fuels with Hydrogen Peroxide Triggered by Copper-Containing Liquid Promoter. Energetic Mater. Front. 2024, 5, 41–46. [Google Scholar] [CrossRef]
- Bhosale, V.K.; Gwak, J.; Kim, K.S.; Churchill, D.G.; Lee, Y.; Kwon, S. Rapid Ignition of “Green” Bipropellants Enlisting Hypergolic Copper (II) Promoter-in-Fuel. Fuel 2021, 297, 120734. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.M.; Duan, Z.; Sun, G.; Liu, X.F.; Zang, S.Q. High-Performance Hypergolic Fuels Based on Copper Hydride Clusters. J. Am. Chem. Soc. 2025, 147, 17574–17578. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; McAnally, M.; Chambreau, S.D.; Schneider, S.; Sun, R.; Kaiser, R.I. Atmospheric Ignition Chemistry of Green Hypergolic Bipropellant 1-Ethyl-3-Methylimidazolium Cyanoborohydride—Hydrogen Peroxide in an Acoustic Levitator: Exploring a Potent Universal Propellant. Chem.–A Eur. J. 2025, 31, e202500593. [Google Scholar] [CrossRef] [PubMed]
- Shul’Pin, G.B.; Kozlov, Y.N.; Shul’Pina, L.S.; Strelkova, T.V.; Mandelli, D. Oxidation of Reactive Alcohols with Hydrogen Peroxide Catalyzed by Manganese Complexes. Catal. Lett. 2010, 138, 193–204. [Google Scholar] [CrossRef]
- Oberndorfer, J.; Teuffel, P.; Stölzle, S.C.; Freudenmann, D.; Kirchberger, C.U. Investigation of the Combustion Products of Ionic Liquid-Based Green Propellants Using Infrared Spectroscopy. Aerospace 2025, 12, 507. [Google Scholar] [CrossRef]
- He, B.; Nie, W.; He, H. Unsteady Combustion Model of Nonmetalized Organic Gel Fuel Droplet. Energy Fuels 2012, 26, 6627–6639. [Google Scholar] [CrossRef]
- Zhu, H.; Tong, M.; Zhang, Y.; Hu, K.; Zhang, Y.; Mu, C.; Tian, H.; Cai, G. Combustion Characteristics of Hypergolic Solid Fuel Using NaBH4-Embedded Polyethylene Wax for Hydrogen Peroxide Hybrid Rocket. Acta Astronaut. 2025, 236, 1316–1326. [Google Scholar] [CrossRef]
- Bansal, L.; Jindal, P.; Bharti, M.K. Investigations on the Modifications in Ignition Delay Time of Shellac-Based Pyrotechnic Igniter Using Additives of Varying Particle Size. J. Aerosp. Technol. Manag. 2020, 12, e3820. [Google Scholar] [CrossRef]
- Kapusta, Ł.J.; Boruc, Ł.; Kindracki, J. Pressure and Temperature Effect on Hypergolic Ignition Delay of Triglyme-Based Fuel with Hydrogen Peroxide. Fuel 2021, 287, 119370. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, P.; Yuan, Y.; Zhang, T. Hypergolic Ignition by Head-on Collision of N,N,N′,N′−tetramethylethylenediamine and White Fuming Nitric Acid Droplets. Combust. Flame 2016, 173, 276–287. [Google Scholar] [CrossRef]
- He, C.; He, Z.X.; Zhang, P. Droplet Collision of Hypergolic Propellants. Droplet 2024, 3, e116. [Google Scholar] [CrossRef]
- Jiang, J.; Fei, L.; Song, Y.; Huang, Z.; Tang, C. Hypergolic Ignition Behaviors and Propulsive Performance of a Mixture of [AMIm][DCA] with Methanol, Ethanol, and n-Propanol Reacting with White Fuming Nitric Acid. ACS Omega 2025, 10, 16548–16558. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.M.; Deans, M.C. Recommended Figures of Merit for Green Monopropellants. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, San Jose, CA, USA, 14–17 July 2013. 1 PartF. [Google Scholar] [CrossRef]
- McCrary, P.D.; Chatel, G.; Alaniz, S.A.; Cojocaru, O.A.; Beasley, P.A.; Flores, L.A.; Kelley, S.P.; Barber, P.S.; Rogers, R.D. Evaluating Ionic Liquids as Hypergolic Fuels: Exploring Reactivity from Molecular Structure. Energy Fuels 2014, 28, 3460–3473. [Google Scholar] [CrossRef]
- Zhang, Q.; Shreeve, J.M. Energetic Ionic Liquids as Explosives and Propellant Fuels: A New Journey of Ionic Liquid Chemistry. Chem. Rev. 2014, 114, 10527–10574. [Google Scholar] [CrossRef]
- Guseinov, S.L.; Fedorov, S.G.; Kosykh, V.A.; Vaulin, V.A. Hypergolic Propellants Based on High-Test Hydrogen Peroxide and Organic Compounds. Russ. J. Appl. Chem. 2023, 96, 873–888. [Google Scholar] [CrossRef]
- Nosseir, A.E.S.; Cervone, A.; Pasini, A. Review of State-of-the-Art Green Monopropellants: For Propulsion Systems Analysts and Designers. Aerospace 2021, 8, 20. [Google Scholar] [CrossRef]
- Mota, F.A.S.; Fei, L.; Liu, M.; Jiang, J.; Tang, C. Novel Hypergolic Green Fuels with Hydrogen Peroxide for Propulsion Systems. J. Propuls. Power 2024, 40, 207–219. [Google Scholar] [CrossRef]
- Masse, R.K.; Spores, R.A.; Allen, M.; Kimbrel, S.; McLean, C. Enabling High Performance Green Propulsion for SmallSats; Salt Palace Convention Center: Salt Lake City, UT, USA, 2015. [Google Scholar]
- McLean, C.H.; Marotta, B.; Tennant, S.; Smith, T.A.; Sheehy, J.A. Green Propellant Infusion Mission: Program Construct, Technology Development, and Mission Results. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Virtual, 24–28 August 2020; Volume 35812, pp. 1–23. [Google Scholar] [CrossRef]
- Amrousse, R.; Yan, Q.-L. Recent Advancements in Green Propulsion; Springer: Berlin/Heidelberg, Germany, 2024; ISBN 3031625749. [Google Scholar]
- Ju, Y.; Song, C.; Lee, B.J. Guidelines for the Safe Handling of Hypergolic Propellants in Development of Space Propulsion Systems. J. Propuls. Energy 2024, 4, 42–57. [Google Scholar] [CrossRef]

| Propellant Combination | IDT (ms) | Conditions (Temp, Pressure, Method) | Source | Notes |
|---|---|---|---|---|
| MMH/NTO | 1.45 ± 0.60 | Ambient (drop test, oxidiser dropped from 2.5 inches height) | [3,31] | Liquid phase induction delay: 10–40 µs |
| MMH/NTO | 1–3 (typical range) | General (various methods) | [2,4,32,33] | Widely used in US spacecraft |
| UDMH/NTO | Few milliseconds (typical) | General (various methods) | [1,2] | Widely used in Russian rockets |
| Aerozine 50/NTO | Short and repeatable | General (various methods) | [1,2] | Widely used (Apollo, Space Shuttle, Dragon) |
| MMH/RFNA | 2.5–10.5 | Ambient (drop test, varied by impact point) | [5] | Liquid phase induction delay: 30–100 µs. Note: Impinging jet tests reported no ignition |
| DMAZ/N2O4 | 68 | Ambient (open cup test) | [1,2] | Considered a less toxic alternative to hydrazine derivatives |
| (a) Ionic Liquids with H2O2 (HTP) | ||||
| Propellant Combination | IDT (ms) | Conditions (Temp, Pressure, Method) | Original Source | Notes |
| [EMIM]/96.1% H2O2 | 26–32 | Ambient (drop test) | [34] | Thiocyanate anion-based IL |
| [EMIM] + 5 wt% Copper Thiocyanate/96.1% H2O2 | ~13 | Ambient (drop test, impinging injector) | [34] | Copper additive significantly reduces IDT |
| Alkyl-substituted thiocyanate ILs/H2O2 | ~45 | Ambient (drop test) | [35] | Longer alkyl chains increase IDT |
| [EPy] (IL-24)/97.4% H2O2 | 26.8 | Ambient (drop test) | [36] | Pyridinium-based thiocyanate IL, shorter alkyl chains improve IDT |
| [HIM] (IL-30)/H2O2 | 7.3 | Ambient (drop test) | [37] | Protic thiocyanate IL, solid at ambient conditions |
| HIM-35 (35 wt% [HIM] + 65 wt% [EMIM])/H2O2 | 16.7 | Ambient (drop test) | [37] | Liquid blend, avoids metallic additives |
| (IL-38)/H2O2 | 30.8 | Ambient (drop test) | [38] | Trialkylsulfonium thiocyanate IL, IDT increases at lower temperatures |
| [EMIM] (IL-17)/95% H2O2 | 18.5 | Ambient (drop test) | [39] | Borohydride anion-based IL, solid at ambient conditions |
| (IL-2)/90–98% H2O2 | <30 | Ambient (drop test) | [18] | Borohydride anion-based IL |
| Novel Borohydride ILs with Organic Super-bases/90% H2O2 | 28.3 | Ambient (drop test) | [40] | Hybrid borohydride design |
| (b) Ionic Liquids with WFNA | ||||
| Propellant Combination | IDT (ms) | Conditions (Temp, Pressure, Method) | Original Source | Notes |
| [EMIM] [C2N3]/WFNA | 52 | Ambient (drop test) | [41] | Dicyanamide anion-based IL |
| [EMIM] [C2N3]/WFNA | 35 | Ambient (drop test) | [41] | Dicyanamide anion-based IL |
| [EMIM] [C2N3] or [C2N3] + AMPZ/WFNA | ~20 | Ambient (drop test) | [41] | AMPZ additive significantly reduces IDT |
| Propellant Combination | IDT (ms) | Conditions (Temp, Pressure, Method) | Original Source | Notes |
|---|---|---|---|---|
| Ethanolamine + 9% Cu(NO3)2·3H2O/90% H2O2 | 15.7 | Ambient (drop test) | [41] | Amine-based catalytic system |
| TMEDA/DMEA + CuCl2/HTP | ~10 | Ambient (impinging jet) | [41] | Amine-based fuel with Cu catalyst |
| Gel fuel + catalyst/90% H2O2 | 10–50 | Ambient (drop test) | [47] | Widespread due to mixing sensitivity |
| Propellant Combination | IDT (ms) | Conditions (Temp, Pressure, Method) | Original Source | Notes |
|---|---|---|---|---|
| Ethylenediamine + 10 wt.% NaBH4: NaI/95% H2O2 | 2.75 | Ambient (drop test) | [48] | Hybrid reactive/catalytic additives |
| Ethylenediamine + 10 wt.% NaBH4:NH4I/95% H2O2 | 2.69 | Ambient (drop test) | [48] | Hybrid reactive/catalytic additives |
| Kerosene-based gel fuel + NaBH4/90% H2O2 | 4–5 | Ambient (drop-on-drop test) | [14,49] | Metal hydride additive |
| Gelled ethanol + boron particles/HTP | 1.33 | Ambient (drop test) | [15] | Boron particle additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindal, P.; Botchu Vara Siva, J. Ignition Delay Times of Conventional and Green Hypergolic Propellants at Ambient Conditions: A Comparative Review. Appl. Sci. 2025, 15, 11165. https://doi.org/10.3390/app152011165
Jindal P, Botchu Vara Siva J. Ignition Delay Times of Conventional and Green Hypergolic Propellants at Ambient Conditions: A Comparative Review. Applied Sciences. 2025; 15(20):11165. https://doi.org/10.3390/app152011165
Chicago/Turabian StyleJindal, Prakhar, and Jyoti Botchu Vara Siva. 2025. "Ignition Delay Times of Conventional and Green Hypergolic Propellants at Ambient Conditions: A Comparative Review" Applied Sciences 15, no. 20: 11165. https://doi.org/10.3390/app152011165
APA StyleJindal, P., & Botchu Vara Siva, J. (2025). Ignition Delay Times of Conventional and Green Hypergolic Propellants at Ambient Conditions: A Comparative Review. Applied Sciences, 15(20), 11165. https://doi.org/10.3390/app152011165







