Abstract
Polydeoxyribonucleotides (PDRN), highly purified DNA-derived polymers, were approved by the Italian Medicines Agency (AIFA) in 1994 to treat superficial wounds, skin ulcers, and dystrophic connective tissue disorders. Since then, PDRN have gained considerable attention as regenerative biomaterials. Beyond their established role in wound healing, they have also been approved as dermal fillers in several countries, with growing clinical evidence supporting their benefits for facial skin health. Recent clinical and preclinical studies suggest that PDRN may improve various skin conditions, including wrinkles, dryness, hyperpigmentation, hair loss, and barrier dysfunction. These findings have generated interest in their broader dermatological applications beyond traditional indications. This review aims to explore the therapeutic potential of PDRN for the treatment of skin disorders. We examine the efficacy and safety of PDRN-based drugs and medical devices in dermatology, with a focus on their clinical applications, pharmacological effects, and underlying molecular mechanisms. Given that PDRN consists of over 90% purified DNA, we further examine the biological functions of extracellular DNA (exDNA) and propose potential mechanisms by which PDRN may function as exDNA, beyond its classical action via the A2A receptor pathway. Collectively, current evidence highlights PDRN as safe and effective biopolymers with promising potential as DNA-based therapeutics in dermatology.
1. Introduction
Skin diseases rank as the fourth most prevalent health conditions worldwide, affecting approximately 30% of the global population [1]. Common disorders include fungal infections, dermal and subcutaneous tissue disorders, acne, pruritus, alopecia, dermatitis, impetigo, warts, and scabies [2]. The global burden of these conditions carries substantial social and economic consequences [1], as they markedly impair quality of life (QoL) and are closely associated with psychological distress, social stigma, sleep disturbances, and reduced occupational functioning [3]. In the United States alone, the annual economic burden of skin diseases—including direct medical costs, mental health comorbidities, and productivity losses—has been estimated to exceed USD 86 billion [4].
In January 2022, the World Health Organization (WHO) adopted the 11th revision of the International Classification of Diseases (ICD-11), which catalogs more than 2000 distinct dermatological entities [5]. Despite this extensive classification, fewer than 200 drugs have been approved by the U.S. Food and Drug Administration (FDA) for dermatological indications, with only 33 new approvals between 2020 and 2023 [6,7,8]. This stark mismatch between disease burden and therapeutic availability underscores the urgent need for novel and effective interventions. Moreover, the current widely used treatment options are often limited by high costs, accessibility, and their adverse effects. For example, biologics such as rituximab have shown substantial efficacy in psoriasis; however, their annual costs often exceed USD 50,000, posing major barriers to accessibility [9]. Topical treatments including corticosteroids, retinoids, and calcineurin inhibitors are widely prescribed but are frequently associated with adverse reactions such as erythema, dryness, burning, and pruritus [10,11,12]. These economic and safety limitations highlight the need for alternative therapeutic strategies, particularly those capable of promoting skin regeneration and modulating cellular activity without inducing immunosuppression.
Polydeoxyribonucleotides (PDRN) are deoxyribonucleic acid (DNA)-based biopolymers that are primarily derived from the sperm of chum salmon (Oncorhynchus keta) and rainbow trout (Oncorhynchus mykiss), respectively. Structurally, each deoxyribonucleotide unit within PDRN comprises three essential components: a phosphate group, a pentose sugar (deoxyribose), and a nitrogenous base (purine or pyrimidine) linked through a β-N-glycosidic bond at the C1′ position of the sugar [13,14]. Since the 1990s, these substances have garnered attention as regenerative biomaterials in both clinical and aesthetic dermatology. Clinically, PDRN-based drugs have been utilized for skin tissue regeneration, particularly for epithelial repair after burns and surgical procedures [15]. In South Korea, PDRN-based injectable formulations are increasingly used to improve facial skin conditions, such as fine lines, dryness, and rough texture [16,17]. Moreover, recent studies have demonstrated that PDRN exert antioxidant, anti-inflammatory, anti-melanogenic, and pro-collagen biosynthetic effects in cellular models, while in murine and human skin models, they have been shown to improve psoriasis, hyperpigmentation, scarring, alopecia, and wrinkles [18,19,20,21,22].
Despite encouraging preclinical and clinical evidence, research investigating the therapeutic potential of PDRN in dermatology beyond their currently approved indications for ulcer and wound treatment remains limited. To advance PDRN as novel therapeutics for skin diseases, it is essential to elucidate their precise mechanisms of action. Given that PDRN are predominantly composed of DNA fragments, it is particularly important to explore mechanisms beyond the well-characterized A2AR signaling pathway, which is primarily associated with salvage signaling and indirect activation. Identifying DNA-specific pathways that PDRN may directly engage with therefore represents a critical research priority.
Accordingly, this review aims to provide a comprehensive overview of: (1) the clinical effects of PDRN on skin; (2) their pharmacological activities in skin cell and murine models; and (3) putative modes of action as DNA-based biomaterials. Through this analysis, we highlight the potential of PDRN as therapeutic candidates for a broader range of skin diseases and propose alternative mechanisms of action beyond A2AR signaling.
2. Methods
Information on PDRN was collected through a comprehensive literature search of PubMed/MEDLINE and Google Scholar databases. The following search terms were used: (“PDRN” OR “PN” OR “polydeoxynucleotide” OR “polynucleotide”), (“PDRN” AND “cosmetic”), (“PN” AND “cosmetic”), (“PDRN” AND “dermatology”), (“PN” AND “dermatology”), (“PDRN” AND “pharmacology”), (“PN” AND “pharmacology”), (“PDRN” AND “filler”), (“PN” AND “filler”), (“PDRN” AND “skin disease”), (“PN” AND “skin disease”), (“PDRN” AND “mechanism”), and (“PN” AND “mechanism”).
This review includes all relevant studies reporting the clinical or pharmacological effects of PDRN in the field of dermatological science, irrespective of the experimental model used (in vitro, in vivo, or clinical trials). No restrictions were applied regarding publication dates. A total of 175 studies published until the end of August, 2025, were retrieved and reviewed.
3. Clinical Applications of PDRN as a Wound Healing Agent
3.1. Scientific and Commercial Definitions of PDRN/PN
Both PDRN and polynucleotides (PN) are nucleic acid-derived polymers characterized by a DNA backbone [22,23]. Terminologically, the expression “PN” became established earlier, with its meaning clarified in the 1950s; for example, Weed and Courtenay (1954) described deoxyribonucleotide polymers obtained by acid hydrolysis of bacteriophage and sperm DNA as PN [24,25]. Following the initial use of PN to denote DNA fragments, the term PDRN emerged in the 1960s. Notably, Birnie & Fox (1967) showed that DNA polymerase in mouse-embryo extracts could synthesize DNA polymers (PDRN) from defined substrates, and Sarkar (1967) likewise referred to DNA-derived polymers as PDRN [26,27].
As evidence accumulated for the anti-ischemic, anticoagulant, and tissue-regenerative effects of nucleic acid-derived polymers (particularly DNA fragments), pharmacological investigations intensified [28,29,30,31,32]. In 1996, Tonello et al. reported that a defined DNA fraction isolated from placenta exhibited strong tissue-regenerative activity, with fragment sizes ranging from 50 to 2000 base pairs (bp) [32]. Likewise, defibrotide—DNA fragments extracted from mammalian lung or porcine intestinal mucosa—demonstrated anti-inflammatory, antithrombotic, and anti-ischemic properties, with fragment sizes of approximately 45–90 nucleotides [33]. Since the 1990s, PDRN has thus often been defined as DNA fragments within the 50–2000 bp (≈50–1500 kDa) range, although a firm scholarly consensus remains lacking.
The reported size range of pharmacologically active PDRN varies widely. Tonello et al. (1996) identified ~50–2000 bp [32]; Lee et al. (2025) <500 bp [34]; Hwang et al. (2018) [35] <50 kDa, 50–1500 kDa, and >1500 kDa; Chakraborty et al. (2014) [36]; Lee et al. (2023) <100 bp [37]; and Chae et al. (2025) <100 bp [38]. Some reports describe PN as larger fragments (>1500 kDa) than PDRN, whereas others define PN as averaging ~400 bp [19,39,40]. Importantly, most studies do not substantiate these claims with direct experimental determination of DNA fragment size (e.g., gel electrophoresis, HPLC), limiting the robustness of such assertions [39,41,42].
In clinical practice, the distinction between PDRN and PN often follows product content rather than a scientifically rigorous definition, reflecting branding and regulatory conventions more than standardized scientific taxonomy. Although both refer to DNA-based polymers, the commercial differentiation in naming appears to stem from differences in regulatory pathways and market positioning. Accordingly, in this article, given the absence of a universally accepted size-based distinction, we treat both PDRN and PN as DNA-derived fragments and uniformly adopt the term PDRN throughout.
3.2. Clinical Development for Wound Healing and Dermatological Potential
Although DNA is primarily known for its genetic role, early studies have highlighted its extracellular functions. Notably, Fiedel et al. (1979) and Dorsch and Killmayer (1983) reported that high concentrations of double-stranded DNA (dsDNA) could stimulate serotonin (5-HT) release from platelets, indicating that released DNA at injury sites may function as a signaling molecule [43,44]. Extracellular DNA (exDNA) can be passively released from damaged cells or actively secreted by extracellular vesicles (EVs), initiating downstream biological effects through receptors such as Toll-like receptors (TLRs, such as TLR9), which recognize CpG-rich motifs [45]. Also, exDNA—including PDRN—can undergo enzymatic degradation by DNase and 5′-nucleotidase in the salvage pathway, generating nucleosides such as adenosine that are known to promote tissue regeneration and modulate inflammation [15,46,47].
Recognizing these biological properties, the Italian pharmaceutical company Mastelli Srl (Sanremo, Italy) developed Placentex®, the first commercial PDRN formulation, in the early 1990s using DNA extracted from human placenta [32]. Using a bioactivity-guided approach, researchers have identified DNA-enriched fractions with the greatest regenerative capacity and established a novel extraction process to increase the PDRN yield. This led to the successful commercialization of Placentex®. Since then, several PDRN-based formulations, such as Rejuvenex® (PharmaResearch Co., Ltd., Gyeonggi-do, Republic of Korea), have been developed and utilized clinically as regenerative and wound-healing agents [48,49].
PDRN-based therapeutics such as Placentex® and Rejuvenex® have been developed for various clinical indications, with a primary focus on wound healing and tissue regeneration. Placentex® (Mastelli Srl) was the first PDRN drug to be commercialized and is commonly used for managing diabetic foot ulcers [50]. Due to the delayed wound healing in diabetic patients—linked to chronic inflammation, impaired angiogenesis, reduced collagen synthesis, and elevated protease activity—Placentex® is thought to promote repair by stimulating cell proliferation, neovascularization, and matrix remodeling. A clinical study by Squadrito et al. (2014) [50] involving 216 patients with diabetic foot ulcers reported complete wound closure in 37.3% of patients treated with PDRN compared to 18.9% in the placebo group after 8 weeks of treatment (p = 0.0027). These findings indicate that intramuscular and perilesional administration of PDRN (5.625 mg) enhances wound healing by a 3.48-fold increase. Notably, the treatment was well tolerated, with only mild adverse events, such as localized pruritus [50]. Based on its clinical performance, Placentex® has been approved by the AIFA in four pharmaceutical forms [41]:
- 0.08% cream for superficial wounds and minor skin ulcers;
- 0.75 mg/3 mL topical solution for dystrophic and ulcerative connective tissue disorders;
- 5.625 mg/3 mL or 2.25 mg/3 mL injectable solution for dystrophic and ulcerative connective tissue disorders;
- 0.75 mg/mL ophthalmic solution for dystrophic and ulcerative conditions of the conjunctiva and cornea.
In South Korea, Rejuvenex®, derived from O. keta sperm, has been approved in two forms—a 0.08% cream for wound healing and skin nourishment and a 5.625 mg/3 mL injectable solution indicated for post-grafting wound repair. A case report by Shin et al. (2018) described successful treatment of chronic wounds refractory to negative pressure wound therapy and growth factor therapy using Rejuvenex® injections, further supporting its therapeutic value [51].
PDRN-based pharmaceuticals continue to receive regulatory approval in countries such as Italy and South Korea. Although current indications remain centered on wound healing and ulcer management, accumulating preclinical evidence supports broader therapeutic potential in musculoskeletal injuries, ischemic disorders, and neurological damage [52,53,54]. Furthermore, off-label use in conditions such as arthritis and lichen sclerosis has also been reported [55,56]. Mechanistically, PDRN primarily acts by activating the adenosine A2A receptor (A2AR), which mediates cell proliferation, anti-inflammatory responses, collagen synthesis, and tissue remodeling [57]. These properties indicate that PDRN may provide pharmacological benefits in various dermatological conditions, including skin atrophy, inflammatory skin diseases, and alopecia.
4. Clinical Applications of PDRN as a Dermal Filler
Although initially developed for the treatment of wound healing in dystrophic and ulcerative conditions, PDRN has recently been repurposed and approved in several countries as a medical device, particularly as a dermal filler [17,58,59]. Because of its regenerative potential, PDRN is increasingly recognized as a bioremodeling filler, providing volumization as well as functional improvement of the skin [17]. This paradigm shift has broadened its application in aesthetic dermatology, addressing the limitations associated with traditional fillers.
Hyaluronic acid (HA)-based fillers are the standard treatment for wrinkle correction and facial volume enhancement. However, their role remains largely mechanical because HA lacks intrinsic bioactivity. Moreover, the high crosslinking densities necessary for structural durability are associated with an increased risk of adverse events [60]. Synthetic crosslinkers such as 4-butanediol ether (BDDE) and polyethylene glycol diglycidyl ether (PEGDE), which are commonly used in HA fillers, have shown cytotoxicity, oxidative stress induction, and upregulation of pro-inflammatory cytokines at concentrations as low as 100 ppm [61]. Clinical reports have also associated HA fillers containing synthetic crosslinkers with complications such as hypersensitivity reactions, vascular occlusion, nodules, and granulomas [61,62]. These challenges have prompted growing interest in biologically active fillers that promote tissue regeneration and volume restoration. PDRN-based fillers provide mechanical support as well as activate adenosine A2A receptors, which mediate anti-inflammatory effects and tissue repair [42]. In some regions, these agents are classified as skin boosters owing to their regenerative profile [63].
Several PDRN-based medical devices have entered the aesthetics market, supported by growing clinical evidence [42]. Commercially available examples are Rejuran® (PharmaResearch Co., Ltd.), Nucleofill® (Promoitalia, Milano, Italy), and HP Cell VITARANi® (BR PHARM Co., Ltd., Gangwon-do, Republic of Korea). Rejuran®, a purified polynucleotide (20 mg/mL) injectable, has benefits including increased skin thickness, wrinkle reduction, and improved skin texture, with only minor adverse events such as local bruising and pain reported [17]. In a randomized split-face clinical trial involving 27 participants, Rejuran® was compared with an HA filler. The PDRN (PN in the original report)-treated side showed significantly higher Global Aesthetic Improvement Scale (GAIS) scores at 16 weeks, and a greater reduction in skin roughness by week 28 [42]. In another study involving 218 Asian subjects, Rejuran® led to a 61.4% reduction in periorbital wrinkles after 12 weeks, with adverse events limited to minor swelling, pain, bruising, pruritus, and erythema, reinforcing the product’s favorable safety profile [42,64]. Similarly, Kim et al. (2022) evaluated HP Cell VITARANi®, which demonstrated notable improvements in periorbital wrinkles, pore size, skin texture, and tone, further supporting the efficacy and tolerability of PDRN-based fillers [65].
Despite their clinical benefits, PDRN-based fillers tend to exhibit a delayed onset of action, with visible improvements typically emerging after several weeks [42,64]. To address this limitation, recent studies have explored hybrid formulations combining PDRN (PN in the original report) with other filler agents such as HA, aiming to harness the immediate volumizing effect of HA alongside the long-term regenerative activity of PDRN [42,64]. For example, Kim et al. (2020) demonstrated that HA-PDRN (PN in the original report) composites exhibit higher viscosity, elasticity, and in vivo durability than HA alone, while also promoting fibroblast proliferation and tissue regeneration [66]. Furthermore, Oh et al. (2021) reported that hybrid fillers composed of HA, PDRN (PN in the original report), and poly-L-lactic acid (PLLA) enhanced cell migration and collagen synthesis, while reducing pain and irritation, effectively overcoming certain drawbacks of PN-only formulations [67]. A more recent clinical study (2025) confirmed the efficacy of HA-PDRN (PN in the original report) hybrid fillers. Using a multiple micro-aliquot injection technique, investigators observed significant improvements in periorbital wrinkles, skin texture, and brightness, along with faster clinical onset and shorter downtime [18]. Although these findings present promising strategies to overcome the inherent limitations of PDRN-only formulations, further well-controlled clinical trials comparing hybrid formulations with monocomponent fillers are warranted.
Although the approved clinical application of PDRN remain limited to wound healing and dermal fillers, several preliminary clinical studies suggest broader therapeutic potential in dermatology. In hair disorders, Lee et al. demonstrated that intradermal injections of PDRN significantly increased hair density (17.9%) and thickness (13.5%) in women with female pattern hair loss [68]. Similarly, Cho et al. further reported that PDRN in conjunction with 1927 nm Thulium fractional laser therapy markedly improved both hair density and thickness [69], while Choi et al. found that the same regimen enhanced hair shaft caliber and reduced hair graying in male pattern hair loss [70]. Beyond alopecia, Laino et al. described the adjuvant efficacy of PDRN dermal infiltration in male genital lichen sclerosis, where its addition to topical therapy resulted in improved clinical outcomes [71]. Although these studies remain limited in scale and duration, they collectively underscore the potential of PDRN in conditions beyond wound healing and fillers, warranting further controlled clinical investigation.
In summary, PDRN-based dermal fillers have been widely adopted across global markets, including Asia, with accumulating clinical data confirming their safety and efficacy profiles for skin rejuvenation. Unlike HA fillers, which offer predominantly mechanical effects, PDRN-based fillers provide biostimulatory activity, promoting endogenous tissue repair and regeneration. These advantages support the development of combination strategies, incorporating HA or adjunctive modalities such as microneedling, ultrasound, or laser therapy, to further enhance clinical outcomes in aesthetic dermatology [72,73,74].
5. Pharmacological Effects and Molecular Mechanisms of PDRN in Dermatology
5.1. Pharmacological Activities of PDRN in Skin Cell Models
PDRN exerts a range of regenerative effects in dermatology, including enhanced skin texture, wrinkle reduction, and accelerated wound healing. Although its current regulatory approval is limited to use as wound healing agents and dermal fillers, numerous preclinical studies have highlighted its broader dermatological potential. Specifically, PDRN has been shown to enhance skin cell proliferation, stimulate collagen and elastin fiber synthesis, and reduce inflammation and hyperpigmentation [37,75,76,77,78,79,80,81].
In vitro studies have consistently shown that PDRN promotes the proliferation of human dermal fibroblasts (HDFs), increasing cell viability by approximately 25% compared with the control group [75]. This effect is significantly inhibited (>50%) by ZM241385, a selective A2AR antagonist, indicating a key role for A2AR in mediating PDRN-induced proliferation. Similarly, plant-derived PDRN has been shown to promote keratinocyte growth and accelerate wound closure in 3D skin models—this effect was reduced by 7.5% with DMPX (p < 0.05), another A2AR antagonist [37].
Supporting these findings, Jeong et al. (2017) reported that in vivo PDRN treatment in a rat excisional wound model led to reduced inflammation, enhanced collagen deposition, and full re-epithelialization [82]. On postoperative day 7, type I and III collagen levels were elevated by 1.36 ± 0.07-fold and 3.07 ± 0.31-fold, respectively, compared to placebo [82]. These regenerative effects align with the upregulation of COL1A1 and COL3A1, reinforcing PDRN’s role in dermal remodeling at the molecular level [37,75].
PDRN also exhibits strong anti-inflammatory properties. In diabetic models, it significantly downregulated TNF-α, IL-6, IL-1β, while enhancing IL-10, TGF-β, and VEGF expression [83], thereby promoting wound healing. Topical PDRN formulations have similarly enhanced wound contraction, re-epithelialization, and angiogenesis via upregulation of VEGF and TGF-β [84].
PDRN has also demonstrated anti-pigmentation and photoprotective effects. In skin cells and HRM-2 mice models, 1 mM PDRN—alone or with niacinamide and vitamin C—significantly reduced melanin content and UVB-induced damage, while boosting collagen and elastin expressions and lowering oxidative stress markers [76]. Comparable reductions in melanin production and skin damage-related markers were observed in PDRN-treated human melanocytes (Mel-Ab) and mouse melanocytes (B16F10) models [77,81]. Treatment with 1000 µg/mL PDRN effectively suppressed pigmentation, especially in B16F10 melanocytes, and also enhanced antioxidant activity, and stimulated mitochondrial biogenesis [77]. In addition, PDRN effectively suppressed UVB or H2O2-induced cellular senescence by upregulating SIRT1 expression and reducing nuclear autophagy in keratinocytes [78]. These effects are related to the downregulation of senescence-associated markers, including p53, p21, and p16 [78].
Collectively, these studies suggest that PDRN exerts multiple beneficial effects—including skin regeneration, anti-aging, anti-melanogenic, and skin barrier-enhancing effects—through various mechanisms [37,75,76,77,78,79,80,81]. These cellular and molecular actions are consistent with clinical improvements observed in dermatological settings and support the continued development of PDRN as a therapeutic agent in aesthetic and regenerative dermatology.
5.2. Molecular Mechanisms Underlying the Effects of PDRN in Skin Cell Models
Multifaceted pharmacological actions of PDRN are driven by signal transduction and metabolic mechanisms. A growing body of research highlights the selective activation of the A2AR as a key pathway through which PDRN mediates its effects [52]. A2AR, a G protein-coupled receptor (GPCR), plays a central role in regulating inflammatory resolution, angiogenesis, and fibroblast proliferation—three fundamental processes in skin regeneration [85]. Although PDRN itself does not directly bind to A2AR, it undergoes enzymatic degradation in vivo by nucleases into smaller fragments, including the nucleoside adenosine, which serves as the endogenous ligand for A2AR [52]. Upon adenosine binding, A2AR activates intracellular G proteins that stimulate adenylyl cyclase, leading to elevated intracellular cyclic adenosine monophosphate (cAMP) levels [86]. Increased cAMP levels subsequently activate downstream pathways, including protein kinase A (PKA) and Epac2, which regulate transcriptional programs involved in tissue homeostasis and repair [86].
Also, A2AR activation leads to suppression of inflammation, primarily through inhibition of the nuclear factor-κB (NF-κB) signaling pathway—a master regulator of pro-inflammatory cytokine expression [80,87]. By downregulating these inflammatory mediators, PDRN helps to restore a favorable microenvironment for wound healing. Simultaneously, A2AR activation upregulates anti-inflammatory cytokines such as IL-10, further supporting a dual-mode anti-inflammatory mechanism [83,88]. The regulation of inflammation is a critical prerequisite for the subsequent processes of angiogenesis, matrix remodeling, and tissue regeneration.
A2AR signaling also promotes angiogenesis, which is a vital component of dermal repair. Activation of A2AR increases cAMP levels, which subsequently induce hypoxia-inducible factor-1 alpha (HIF-1α) and VEGF, promoting endothelial cell proliferation and migration [89,90]. This enhances microvascular density and local perfusion, as evidenced by the increased expression of PECAM-1/CD31 in regenerating tissues [74]. The angiogenic response triggered by PDRN is closely linked to increased fibroblast activity and collagen deposition, which contribute to improvements in skin elasticity and wrinkle reduction [86].
In addition to its receptor-mediated effects, PDRN contributes to nucleotide salvage metabolism by providing the precursors for nucleic acid synthesis. Upon degradation, PDRN yields deoxynucleotides and nucleosides, which are recycled by cells for DNA and RNA biosynthesis [57]. This salvage pathway is particularly important for metabolically stressed or damaged cells, which favors energy-efficient means of restoring genomic integrity and cellular function [91]. This metabolic support enhances the capacity of regenerating cells to proliferate and repair structural damage, complementing the effects induced by A2AR signaling. Collectively, these studies revealed that PDRN acts through both receptor-mediated signaling cascades and metabolic support mechanisms to orchestrate skin regeneration. By modulating inflammation, promoting angiogenesis, and enhancing structural remodeling, PDRN provides a comprehensive molecular foundation for application in regenerative dermatology.
One study reported that PDRN’s effects are diminished—but not completely blocked—in the presence of A2AR antagonists [80]. This suggests that PDRN may also act through additional signaling pathways independent of A2AR activation. Since the precise molecular mechanisms underlying these alternative pathways remain unclear, further investigations are needed to clarify the potential roles of non-A2AR-mediated mechanisms in PDRN-induced skin regeneration (Table 1).

Table 1.
Summary of PDRN studies in preclinical models of skin-related conditions.
6. Putative Modes of Action of PDRN as an exDNA-Based Material
6.1. PDRN as Extracellular DNA: Potential Mechanisms of Action
6.1.1. Structural Properties of PDRN as exDNA
As previously mentioned, PDRN is a polymer composed of DNA fragments. Most commercial PDRN products exhibit ≥ 90–95% DNA purity with minimal endotoxin or protein contamination [52,92]. High-purity PDRN can be obtained by autoclaving, ultracentrifugation, enzymatic proteolysis, and depurination. Purity was validated using analytical tools, including HPLC, UV–vis spectroscopy, gel electrophoresis, NMR, IR spectroscopy, and fluorescence assays [32,36].
Because of its exceptional purity, it is reasonable to hypothesize that PDRN exerts its pharmacological effects as exDNA. exDNA refers to released DNA into the extracellular space from autologous or non-autologous sources—including host cells, bacteria, and viruses—following lysis by enzymes, cytotoxic agents, antibiotics, or other soluble factors [93,94]. Beyond cell lysis, exDNA may also originate from dietary sources or the administration of DNA-based therapeutics such as PDRN and defibrotide [52,95,96,97]. The presence of exDNA was first reported in human plasma in 1948 [98], and it has since attracted growing interest for its role as a diagnostic biomarker in various diseases [99,100]. The biological activity of exDNA is influenced by its sequence, length, tertiary structure, protein interactions, and subcellular localization [101,102,103]. These properties enable exDNA to modulate diverse physiological processes, including oxidative stress, inflammation, coagulation, and cell cycle regulation [103,104].
Although many of PDRN’s effects—such as anti-inflammatory, adipogenic, and cartilage-regenerative actions—have been linked to A2AR activation, as demonstrated using antagonists like ZM241385, these effects are not fully abolished by receptor blockade [88,105,106,107]. Furthermore, the mechanisms underlying skin-related effects, such as wrinkle reduction and collagen synthesis, remain poorly explained by A2A receptor signaling alone, indicating that additional exDNA-related mechanisms may contribute to PDRN’s bioactivity [35].
6.1.2. Biological Roles of exDNA Beyond Genetic Information
The double-helical structure of DNA, first described by Watson and Crick, is remarkably stable [108]. Within the nucleus, DNA serves as a repository of genetic information and epigenetic modifications, such as methylation [102,108]. Given its essential role in cell viability, complex systems have evolved to detect and repair DNA damage [109].
When DNA damage exceeds repair capacity, various forms of cell death—including apoptosis, necrosis, and NETosis—can be triggered, resulting in the release of DNA into the extracellular space [102,110,111]. exDNA can also originate from infections, tissue injury, ingestion, inhalation, and the administration of DNA-based therapeutics such as PDRN [112,113,114]. Once in the extracellular space, exDNA—including PDRN—is rapidly degraded by DNases, a component of the innate immune system, producing smaller fragments or nucleotides [115,116]. As a result, exDNA exists in three main forms: free DNA fragments, DNA–protein complexes, and DNA enclosed within extracellular vesicles [117]. Its polyanionic nature allows for interactions with proteins such as albumin [95].
Recent studies have shown that exDNA can enter the cells and interact with membrane-bound receptors. Its bioactivity is influenced by a range of variables, including the nucleotide sequence, length, tertiary structure, protein binding, subcellular localization, methylation status, CpG content, and oxidation level [101,102,103]. For instance, CD34 and CD84, membrane glycoproteins found in hematopoietic stem cells, can bind to exDNA [118], and the procoagulant proteases FXII and FXI are activated through such interactions [103]. Intracellularly, exDNA acts as a damage-associated molecular pattern (DAMP) recognized by cytosolic pattern recognition receptors (PRRs) such as RIG-I, AIM2, and DAI, triggering innate immune responses [119]. This is biologically intuitive because cytosolic DNA frequently signals viral infections. Thus, the exDNA functions as follows:
- Generation of nucleotides and metabolites through salvage pathways;
- Interaction with membrane-bound receptors;
- Intracellular activation of cytosolic DNA sensors. These pathways provide potential mechanisms independent of A2A receptor signaling, through which PDRN may exert its effects [120,121,122].
6.2. Proposed Mechanisms by Which PDRN May Exert exDNA-like Biological Effects
6.2.1. TLR9 Activation
exDNA derived from bacteria and mycobacteria is well recognized for its potent immunostimulatory properties, primarily owing to unmethylated CpG motifs that are identified as pathogen-associated molecular patterns (PAMPs) by DNA sensors, such as TLR9 [123,124]. These CpG motifs, defined as cytosine followed by guanine in an unmethylated state, are significantly more abundant in prokaryotic genomes than in eukaryotic genomes [125]. In contrast, eukaryotic DNA tends to exhibit CpG suppression because of extensive cytosine methylation, rendering it largely immunologically inert [126]. In addition, mammalian exDNA may reduce immune recognition of CpG motifs via G-rich sequences at the chromosomal termini, which are known to suppress immune activation [127]. In vivo studies have shown that bacterial DNA acts as a strong immunogen, inducing sequence-specific antibody production, whereas mammalian DNA typically fails to elicit such responses in vitro or in vivo [102,128]. Considering that PDRN is extracted from eukaryotic organisms, such as salmon or rainbow trout, this may explain its reported immunological safety and low immunogenicity [50,129].
However, exDNA can form complexes with extracellular proteins such as albumin, facilitating internalization through endocytosis [130]. Studies employing transfection agents, such as lipofectamine, have shown that intracellular delivery of naked DNA markedly enhances its immunostimulatory activity [101,131]. Moreover, cellular uptake of DNA has been shown to activate macrophage responses regardless of the CpG content [131]. These findings suggest that although CpG-suppressed mammalian DNA is immunologically inert in the extracellular space, it may trigger immune response by activating endosomal DNA sensor (TLR9) and cytosolic DNA sensors—such as DAI, AIM2, PYHIN, and interferon-inducible protein 16 (IFI16)—once internalized [121]. Because DNA readily forms molecular complexes and that membrane-associated proteins can facilitate its uptake, subcutaneous administration of PDRN may activate DNA sensor-related signaling pathways under certain conditions.
Since the identification of TLR4 as a sensor for Gram-negative bacterial lipopolysaccharide (LPS) in 1998, 10 TLRs (TLR1–TLR10) have been characterized in humans [132,133]. TLR9 is typically localized within the endoplasmic reticulum membranes or endosomes of innate immune cells such as dendritic cells and macrophages. However, recent studies have revealed its expression in non-immune cells, including keratinocytes and fibroblasts [133,134,135]. TLR9 possesses two DNA-binding domains—one specific for CpG motifs and another for 5′-terminal cytosine-rich sequences (5′-xCx DNA). Ligand binding induces TLR9 dimerization and activates downstream signaling through MyD88, leading to the induction of IRF and NF-κB pathways [133]. Accumulating evidence has revealed that exDNA-mediated TLR9 activation can promote cellular proliferation [136,137], particularly by advancing cell cycle progression through the TLR9/NF-κB/Cyclin D1 axis [138]. Notably, exDNA has also been shown to stimulate TGF-β secretion in a TLR9-dependent, endocytosis-mediated manner. For instance, stimulation of A549 cells with the TLR9 agonist ODN M362, a class C oligodeoxynucleotide, resulted in an approximately four-fold increase in TGF-β secretion relative to control cells [139]. Similar trends were observed in plasma samples from patients with SLE, where elevated levels of TLR9, TGF-β1, and PDGF-B correlated with enhanced mesangial cell proliferation through the TLR9/TGF-β1/PDGF-B axis [140]. Given the established efficacy of PDRN in dermatological and aesthetic applications—including anti-aging, wrinkle reduction, hydration, and improvement of skin texture—as well as its well-documented stimulatory effects on fibroblast proliferation and collagen synthesis, its therapeutic actions have largely been attributed to adenosine A2A receptor signaling [50,75,79]. However, considering that PDRN is composed of fragmented DNA chains and may act as a form of exDNA, and that exDNA has been reported to activate the TLR9/TGF-β1 axis, it is reasonable to hypothesize that PDRN could exert similar regenerative effects through this alternative pathway.
6.2.2. Cytosolic DNA Sensor Activation
PRRs detect both extracellular pathogens and intracellular damage signals, with several PRRs serving as DNA sensors [141,142]. Endosomal DNA sensors like TLR9 detect DNA within endosomes, while other cytosolic sensors—such as cGAS–STING, AIM2, and IFI16—detect aberrant cytoplasmic DNA and trigger downstream immune signaling, including interferon (IFN) production and inflammasome activation [142,143,144].
The cGAS–STING pathway represents a central cytosolic DNA-sensing mechanism that orchestrates innate immune responses. Upon recognition of cytoplasmic DNA, cGAS catalyzes the synthesis of the secondary messenger 2′3′-cGAMP, which binds to and activates STING located on the endoplasmic reticulum. Activated STING recruits and phosphorylates TBK1, subsequently leading to IRF3 activation and the induction of type I IFNs as well as various inflammatory mediators. Although primarily antiviral, growing evidence shows that cGAS–STING also contributes to tissue regeneration [145,146]. For instance, in full-thickness murine skin wound models, topical application of cGAMP significantly accelerated wound closure by day 6 compared to controls [145]. It also enhanced fibroblast migration, as demonstrated by a scratch assay, and markedly upregulated CXCL10 and CCL2 expression, key mediators that facilitate early inflammatory responses necessary for wound healing. Similarly, in highly regenerative organisms such as salamanders, early injury stages involve reduced STING inhibitors and increased cGAS activity, amplifying IFN signaling that may support blastema formation and limb regeneration [146]. Additionally, several studies have also reported that cGAS–STING activation correlates with increased TGF-β signaling and ECM protein synthesis in fibrosis models [147,148,149].
AIM2 is a member of the PYHIN protein family and a key cytosolic DNA sensor that assembles inflammasomes in response to DNA detection [150]. Upon binding DNA, AIM2 recruits the adaptor ASC, leading to caspase-1 activation and subsequent secretion of pro-inflammatory cytokines such as IL-1β and IL-18. This cascade creates an inflammatory milieu within damaged tissues, which supports immune cell recruitment and modulates tissue repair processes. Notably, AIM2 signaling extends beyond inflammation, influencing ECM remodeling and cell proliferation. In fibroblast models under inflammatory stimuli, AIM2 activation increased TGF-β, as well as proteins such as FN1 [151]. These molecular changes contribute to ECM reorganization and may enhance TGF-β-mediated pathways involved in tissue repair. In addition, AIM2 activation has been linked to enhanced cell proliferation, as observed in head and neck squamous cell carcinoma through IL-17/MAPK signaling [152]. These observations highlight the diverse roles of AIM2 in regulating proliferative processes.
Another member of the PYHIN family, IFI16 detects DNA in both the nucleus and cytoplasm [153]. It participates in STING-dependent type I IFN responses and also contributes to inflammasome activation. Upon binding intracellular DNA, IFI16 can activate STING signaling or induce caspase-1-mediated inflammatory responses similar to AIM2. Within skin cells, IFI16 serves as a pivotal regulator of immune responses and tissue homeostasis. In human keratinocytes, it cooperates with cGAS to fully activate STING signaling, leading to robust type I IFN production and enhanced antiviral defenses [154]. Moreover, IFI16, regarded as the human ortholog of murine p204, has been implicated in the regulation of cell proliferation and differentiation [155]. Given the central role of type I IFN in early wound-healing processes, IFI16 activity may facilitate tissue repair by modulating the local inflammatory environment and promoting regenerative responses.
Collectively, the cGAS–STING, AIM2, and IFI16 pathways are key cytosolic DNA sensors that not only initiate immune responses but also regulate ECM remodeling and TGF-β signaling to support skin repair. Since PDRN is known to promote fibroblast proliferation, collagen synthesis, and skin regeneration, it is plausible that its therapeutic effects may be partly mediated through cytosolic DNA sensor-dependent immune and regenerative mechanisms.
6.2.3. Contribution Through the Nucleotide Salvage Pathway
The nucleotide salvage pathway, originally elucidated in soil microorganisms, describes the cellular recycling of extracellular DNA into metabolically useful intermediates that can be utilized for de novo DNA synthesis. This pathway is initiated by the DNase-mediated cleavage of DNA into oligonucleotides ranging from several tens to hundreds of base pairs. These are further degraded into deoxynucleotides, nucleosides, ribose sugars, and nitrogenous bases [122].
In mammalian systems, this pathway has been implicated in the pathophysiology of various diseases including cancer [156]. Antimetabolites such as methotrexate and 5-fluorouracil (5-FU), which inhibit key enzymes in purine and pyrimidine biosynthesis, are commonly used chemotherapeutics [157]. However, cancer cells may upregulate the salvage pathway to compensate for the inhibition of de novo synthesis, thereby sustaining DNA replication and proliferation [157,158]. The salvage pathway of DNA derives substrates from three primary sources: diet, intracellular nucleic acids, and exogenous DNA-based materials [95]. Exogenous DNA obtained through dietary or therapeutic means is degraded by extracellular enzymes, including nucleases, phosphodiesterases, and 5′-nucleotidases, yielding nucleosides. They are subsequently transported into cells through nucleoside transporters and phosphorylated to regenerate nucleotides. Alternatively, nucleosides may be further catabolized into their constituent bases and sugars, which can then be rephosphoribosylated or phosphorylated by nucleoside kinases to produce nucleotide mono-, di-, and triphosphates. Deoxynucleotide triphosphates (dNTPs), in particular, are essential precursors for DNA repair and synthesis [46].
Among the key enzymes involved in PDRN metabolism, ecto-5′-nucleotidase (CD73) plays a critical role [159]. CD73, which functions as a homodimer on the cell surface, catalyzes the dephosphorylation of the extracellular AMP, produced from ATP and ADP through CD39, into adenosine. Adenosine may act through two primary routes: it can bind to cell-surface adenosine receptors, thereby promoting angiogenesis and anti-inflammatory responses through cAMP elevation, or it may be internalized through nucleoside transporters to enter the adenosine salvage pathway [46,159]. Several studies have proposed that the pharmacological activity of PDRN is partially mediated by adenosine generated through this pathway [47,160]. In diabetic wound models, PDRN was found to enhance wound healing, an effect that was reversed by co-treatment with the A2A receptor antagonist DMPX [161]. Similarly, in models of periodontitis and colitis, PDRN attenuated inflammatory markers and histological damage, and DMPX co-treatment reversed these effects [162,163].
However, considering the broad spectrum of metabolites produced through the nucleotide salvage pathway, which includes diverse nucleosides and nitrogenous bases, it remains unclear why PDRN acts predominantly through the A2A receptor [164]. Moreover, because PDRN is composed of highly purified DNA (>90–95%), its metabolic breakdown may yield deoxyadenosine instead of adenosine. Although RNA salvage and adenosine production have been reported in yeast and certain plant models, evidence for adenosine generation from DNA remains limited [165,166]. Therefore, further research is required to delineate the metabolic fate of PDRN-derived products and explore alternative signaling pathways beyond the A2A receptor, which may contribute to its pharmacological actions (Figure 1).
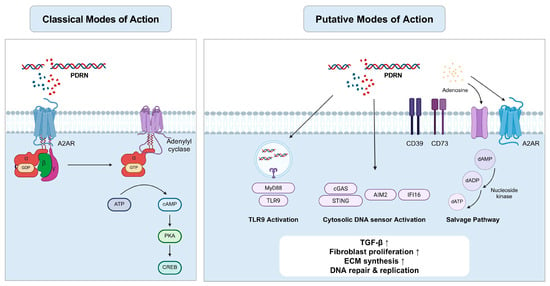
Figure 1.
Classical and putative modes of action of PDRN: Activation of TLR9, cytosolic DNA sensor and the salvage pathway (Created using BioRender, https://www.biorender.com/).
7. Challenges, Controversies, and Future Perspectives
Since the early 1990s, following the development of the first placenta-derived PDRN (Placentex®), its dermatological applications have expanded to include wrinkle reduction, anti-aging, hair regeneration, wound healing, tissue repair, skin hydration, whitening, and erythema reduction [17,19,20,41,167,168]. Although PDRN has not yet received FDA approval, it has been authorized as a pharmaceutical agent in several countries. For instance, Placentex® has been approved by the AIFA in four pharmaceutical forms for treating superficial wounds, skin ulcers, and connective tissue disorders [41]. In South Korea, Rejuvenex® has obtained regulatory approval from the Ministry of Food and Drug Safety (MFDS) for two indications related to tissue regeneration and nutritional support [51]. Additionally, PDRN-based medical devices such as Nucleofill® and Rejuran® are commercially available as dermal fillers in various countries [17,64].
Given the growing clinical and commercial interest in PDRN for dermatology and aesthetics, further research into its potential for treating additional skin diseases is warranted. Beyond approved indications, PDRN has been explored for off-label applications, including the treatment of arthritis and lichen sclerosis [55,56]. These efforts are aligned with the broader strategy of drug repositioning (DR), a well-established approach in drug development [169]. With the acceleration of drug discovery during the COVID-19 pandemic and the rise in artificial intelligence (AI)-driven computational methods, DR is now regarded as an effective means of reducing development time and cost [170,171]. Because repurposed drugs often have well-established pharmacokinetic and preclinical safety profiles, they can frequently proceed directly to late-phase clinical trials, bypassing early-stage toxicity studies [172].
Owing to its favorable safety and well-characterized pharmacokinetics, PDRN is a strong candidate for expanding its therapeutic indications in dermatology. For example, a study involving intradermal facial injections of PDRN-based filler (n = 5) reported no adverse effects [17], whereas another study using scalp injections (n = 28) showed significant hair regrowth without complications such as anaphylaxis, infection, bleeding, or scarring [20]. In a larger clinical trial involving 216 individuals, Squadrito et al. (2014) observed mild pruritus at the injection site in 18 participants with no serious systemic adverse events, further supporting the safety and tolerability of the compound [50]. Pharmacokinetic studies have shown that PDRN exhibits 80–90% bioavailability and a half-life of approximately 3–3.5 h following intraperitoneal or intramuscular administration in both animals and humans [52].
Despite these promising results, several mechanistic challenges remain to be addressed. Although several studies have shown that the anti-inflammatory, adipogenic, and neuroregenerative effects of PDRN are mediated through A2AR signaling, the inhibitory effects of selective A2AR antagonists (such as ZM241385 and DMPX) are often incomplete [54,88,105,106,107]. These findings reveal the involvement of additional noncanonical pathways. Because PDRN is composed of highly purified DNA (≥90–95%), it may also act through exDNA-mediated mechanisms, including cytosolic DNA sensing and nucleotide salvage pathways, both of which remain underexplored.
The biological activity of exDNA is modulated by several factors, including nucleotide sequence, GC content, fragment length, oxidation state, CpG motif frequency, tertiary structure, and protein-binding affinity [101,102,103]. Although CpG methylation generally renders mammalian DNA immunologically inert, endocytosis of exDNA can markedly increase its immunostimulatory potential [101,102,131]. Moreover, the affinity of DNA for extracellular proteins such as albumin might facilitate cellular uptake through endocytosis [130], indicating that PDRN fragments may gain intracellular access. These observations highlight the need for studies investigating whether PDRN can activate cytosolic DNA sensors, such as TLR9, AIM2, and cGAS, after cellular internalization.
Another critical area of investigation is the metabolic fate of the PDRN-derived fragments. Although previous studies have proposed that AMP generated from PDRN is converted into adenosine through CD73 [46,159], evidence supporting the direct conversion of deoxyribonucleotides (instead of ribonucleotides) into adenosine is sparse. Although adenine can be converted to AMP through adenine phosphoribosyltransferase (APRT) activity, few studies have verified the formation of adenosine from adenine, particularly in the context of DNA-derived deoxyadenosine [86]. Although APRT activity has been detected in erythrocytes and blood lysates, extracellular conversion of DNA-derived metabolites to AMP or adenosine has not been conclusively revealed [173,174]. Because of the complexity of nucleotide metabolism, including both the de novo and salvage pathways, reductionist views focusing solely on adenosine may oversimplify the pharmacodynamics of PDRN. Future research should seek to elucidate how PDRN fragments are metabolized intracellularly and whether they contribute to processes such as DNA repair, dNTP recycling, or immunomodulation independent of A2AR signaling [164,175].
Looking ahead, the clinical development of PDRN in dermatology will require several coordinated advances. Establishing efficacy in disease-relevant preclinical models—such as psoriasis-like, atopic dermatitis-like, and dihydrotestosterone-induced alopecia—represents a logical first step, provided that standardized end-points and harmonized protocols are implemented to ensure reproducibility and translational relevance. At the clinical level, randomized trials directly comparing PDRN with established standards of care, including corticosteroids, retinoids, growth-factor preparations, minoxidil, and cyclosporine, will be essential to define its relative efficacy and safety. From a regulatory perspective, precise characterization of critical quality attributes (CQAs)—including GC content, CpG frequency and methylation, residual protein, and DNA fragment-length distribution—will be required, with these parameters mechanistically linked to pharmacological activity through structure–activity relationships to enable indication-specific specifications. In parallel, disease-tailored mechanistic studies should clarify the respective contributions of adenosine A2AR-mediated signaling and extracellular DNA-dependent pathways, using approaches such as pharmacological inhibition and genetic perturbation. Finally, formulation science for PDRN-based injectables and topical agents remains underdeveloped; advances in AI and machine learning (ML)-driven design may accelerate progress by predicting physicochemical behavior, optimizing in vivo residence time, identifying stabilizing excipients, and creating hybrid formulations with biocompatible polymers such as HA and PLLA. Collectively, these priorities outline a translational roadmap that integrates biological insight with regulatory rigor and formulation innovation, paving the way for broader and more precise clinical applications of PDRN in dermatology.
In summary, PDRN-based agents, initially developed for wound healing, are now used globally as regenerative therapies and dermal fillers. As clinical and preclinical evidence grows for their benefits in skin health, interest is increasing in repositioning these agents for conditions such as psoriasis, alopecia, melasma, atopic dermatitis, and rosacea. Understanding alternative mechanisms—particularly those involving exDNA—may further broaden PDRN’s therapeutic potential and clinical applications in dermatology (Figure 2).
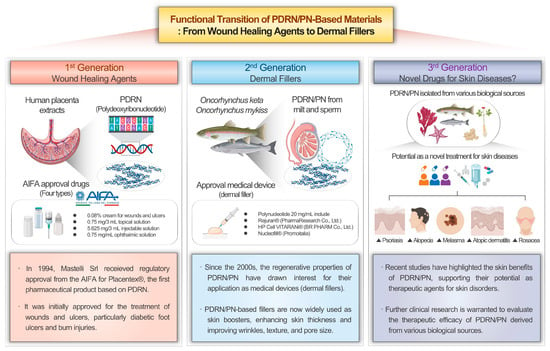
Figure 2.
Functional transition of PDRN-based materials: from wound healing agents to dermal fillers (Created using BioRender, https://www.biorender.com/).
8. Conclusions
This review highlights the emerging therapeutic potential of PDRN in dermatology, with emphasis on their clinical applications, pharmacological effects, and underlying molecular mechanisms. A significant body of evidence supports their safety, with adverse effects limited primarily to minor symptoms such as transient pruritus at the injection site. Numerous in vitro, in vivo, and clinical studies have consistently revealed their efficacy in enhancing skin hydration, improving wrinkles, promoting hair growth, modulating pigmentation, and stimulating cellular proliferation, indicating strong potential for broader dermatological applications.
Current pharmacological research has predominantly focused on A2AR-mediated mechanisms; however, further investigation of intracellular pathways, particularly those involving DNA sensors and nucleotide metabolism, is required. Because PDRN is composed of high-purity DNA, characterizing its molecular interactions with cytosolic DNA sensors and mapping its downstream metabolites are critical to fully understand its mechanism of action. In addition, comparative studies examining the influence of DNA characteristics, such as fragment length, nucleotide sequence, GC content, and oxidative modifications, on biological activity may enhance mechanistic insights as well as support the optimization of PDRN-based therapeutics in both medical and aesthetic dermatology.
Author Contributions
Conceptualization: S.P., S.B. (Seyeol Baek), S.B. (Seunghee Bae) and S.A.; methodology: S.P., S.B. (Seyeol Baek), S.B. (Seunghee Bae) and S.A.; software: S.P. and S.B. (Seyeol Baek); validation: S.P., S.B. (Seyeol Baek), H.-J.S., J.-S.K., H.-I.G., S.B. (Seunghee Bae) and S.A.; investigation: S.P., S.B. (Seyeol Baek), H.-J.S., J.-S.K., H.-I.G., S.B. (Seunghee Bae) and S.A.; resources: S.B. (Seunghee Bae) and S.A.; data curation: S.P., S.B. (Seyeol Baek), H.-J.S., J.-S.K., H.-I.G., S.B. (Seunghee Bae) and S.A.; writing—original draft preparation: S.P., S.B. (Seyeol Baek), S.B. (Seunghee Bae) and S.A.; writing—review and editing: S.P., S.B. (Seyeol Baek), H.-J.S., J.-S.K., H.-I.G., S.B. (Seunghee Bae) and S.A.; resources: S.B. (Seunghee Bae) and S.A.; visualization: S.P. and S.B. (Seyeol Baek); supervision: S.B. (Seunghee Bae) and S.A.; and project administration: S.B. (Seunghee Bae) and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Authors Seokmuk Park, Ji-Seon Kim and Sungkwan An were employed by the company Dermato Bio, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| QoL | Quality of Life |
| WHO | World Health Organization |
| ICD-11 | International Classification of Diseases, 11th Revision |
| FDA | U.S. Food and Drug Administration |
| TCSs | Topical Corticosteroids |
| TCIs | Topical Calcineurin Inhibitors |
| PDRN | Polydeoxyribonucleotides |
| PN | Polynucleotides |
| A2AR | Adenosine A2A Receptor |
| kDa | Kilodalton |
| bp | Base Pairs |
| exDNA | Extracellular DNA |
| EVs | Extracellular Vesicles |
| TLRs | Toll-Like Receptors |
| TLR9 | Toll-Like Receptor 9 |
| CpG | Cytosine-phosphate-Guanine motif |
| 5-HT | 5-Hydroxytryptamine (Serotonin) |
| AIFA | Agenzia Italiana del Farmaco |
| HA | Hyaluronic Acid |
| BDDE | 1,4-Butanediol Diglycidyl Ether |
| PEGDE | Polyethylene glycol Diglycidyl Ether |
| GAIS | Global Aesthetic Improvement Scale |
| PLLA | Poly-L-lactic Acid |
| DMPX | 3,7-Dimethyl-1-propargylxanthine |
| COL1A1 | Collagen Type I Alpha 1 |
| COL3A1 | Collagen Type III Alpha 1 |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| IL-10 | Interleukin-10 |
| TGF-β | Transforming Growth Factor-beta |
| VEGF | Vascular Endothelial Growth Factor |
| HRM-2 | Hairless HRM-2 Mouse |
| SIRT1 | Sirtuin 1 |
| SA-β-gal | Senescence-Associated β-Galactosidase |
| GPCR | G Protein-Coupled Receptor |
| cAMP | Cyclic Adenosine Monophosphate |
| PKA | Protein Kinase A |
| Epac2 | Exchange Protein Directly Activated by cAMP 2 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| HIF-1α | Hypoxia-Inducible Factor-1 alpha |
| PECAM-1/CD31 | Platelet Endothelial Cell Adhesion Molecule-1/CD31 |
| ECM | Extracellular Matrix |
| dNTPs | Deoxyribonucleoside Triphosphates |
| RNA | Ribonucleic Acid |
| HEKs | Human Epidermal Keratinocytes |
| HDFs | Human Dermal Fibroblasts |
| ERK | Extracellular signal-Regulated Kinase |
| AKT | Protein Kinase B |
| MAPK | Mitogen-Activated Protein Kinase |
| FAK | Focal Adhesion Kinase |
| TEER | Transepithelial Electrical Resistance |
| E-cadherin | Epithelial Cadherin |
| NHEMs | Normal Human Epidermal Melanocytes |
| MITF | Microphthalmia-associated Transcription Factor |
| TYR | Tyrosinase |
| HPLC | High-Performance Liquid Chromatography |
| UV–vis | Ultraviolet–visible Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| IR | Infrared Spectroscopy |
| FXII | Coagulation Factor XII |
| FXI | Coagulation Factor XI |
| RIG-I | Retinoic acid-Inducible Gene I |
| DAI | DNA-dependent Activator of IFN-regulatory factors (ZBP1) |
| PYHIN | Pyrin and HIN domain protein family |
| IFI16 | Interferon Gamma Inducible Protein 16 |
| ODN | Oligodeoxynucleotide |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| IRF | Interferon Regulatory Factor |
| SLE | Systemic Lupus Erythematosus |
| PDGF-B | Platelet-Derived Growth Factor-B |
| LPS | Lipopolysaccharide |
| cGAS | Cyclic GMP-AMP Synthase |
| STING | Stimulator of Interferon Genes |
| cGAMP | Cyclic GMP-AMP |
| TBK1 | TANK-Binding Kinase 1 |
| IRF3 | Interferon Regulatory Factor 3 |
| IFN | Interferon |
| FN1 | Fibronectin 1 |
| 5-FU | 5-Fluorouracil |
| APRT | Adenine Phosphoribosyltransferase |
| CD73 | Ecto-5′-Nucleotidase |
| CD39 | Ectonucleoside Triphosphate Diphosphohydrolase-1 |
| ATP | Adenosine Triphosphate |
| ADP | Adenosine Diphosphate |
| AMP | Adenosine Monophosphate |
| MFDS | Ministry of Food and Drug Safety |
| DR | Drug Repositioning |
| AI | Artificial Intelligence |
| COVID-19 | Coronavirus Disease 2019 |
References
- Flohr, C.; Hay, R. Putting the burden of skin diseases on the global map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Trakatelli, M.; Richard, M.A.; Rouillard, A.; Paul, C.; Röcken, M.; Stratigos, A. The burden of skin disease in Europe. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Collins, S.A.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e952. [Google Scholar] [CrossRef]
- White, J.; Lui, H.; Chute, C.G.; Jakob, R.; Chalmers, R.J. The WHO ICD-11 Classification of Dermatological Diseases: A new comprehensive online skin disease taxonomy designed by and for dermatologists. Br. J. Dermatol. 2022, 186, 178–179. [Google Scholar] [CrossRef]
- Sikder, B.; Sil, A. Fresh Baked: An Overview of Newly FDA-Approved Drugs for Dermatological Usage. Indian J. Dermatol. 2023, 68, 707–720. [Google Scholar] [CrossRef]
- Mazmudar, R.S.; Tripathi, R.; Ezaldein, H.H.; Scott, J.F. United States stock market response to FDA approval of new dermatologic drugs. J. Drugs Dermatol. JDD 2020, 19, 639–645. [Google Scholar]
- Eaglstein, W.H.; Corcoran, G. New drugs and new molecular entities in dermatology. Arch. Dermatol. 2011, 147, 568–572. [Google Scholar] [CrossRef]
- Fathi, R.; Armstrong, A.W. The role of biologic therapies in dermatology. Med. Clin. 2015, 99, 1183–1194. [Google Scholar] [CrossRef]
- Pariser, D. Topical corticosteroids and topical calcineurin inhibitors in the treatment of atopic dermatitis: Focus on percutaneous absorption. Am. J. Ther. 2009, 16, 264–273. [Google Scholar] [CrossRef]
- Hanna, S.; Zip, C.; Shear, N.H. What is the risk of harm associated with topical calcineurin inhibitors? J. Cutan. Med. Surg. 2019, 23, 19S–26S. [Google Scholar] [CrossRef]
- Kapała, J.; Lewandowska, J.; Placek, W.; Owczarczyk-Saczonek, A. Adverse events in isotretinoin therapy: A single-arm meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 6463. [Google Scholar] [CrossRef]
- Kim, T.-H.; Heo, S.-Y.; Oh, G.-W.; Heo, S.-J.; Jung, W.-K. Applications of marine organism-derived polydeoxyribonucleotide: Its potential in biomedical engineering. Mar. Drugs 2021, 19, 296. [Google Scholar] [CrossRef]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Stefano, V.D.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc. Hematol. Agents Med. Chem. (Former.) 2009, 7, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Jang, B.-C. Polydeoxyribonucleotide as a Regenerative Agent in Dermatology and Wound Healing: Mechanisms, Clinical Applications, and Safety. Keimyung Med. J. 2025, 44, 9–18. [Google Scholar]
- Rho, N.K.; Han, K.H.; Cho, M.; Kim, H.S. A survey on the cosmetic use of injectable polynucleotide: The pattern of practice among Korean Dermatologists. J. Cosmet. Dermatol. 2024, 23, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Seok, J.; Rho, N.K.; Kim, B.J.; Kim, M.N. Long-chain polynucleotide filler for skin rejuvenation: Efficacy and complications in five patients. Dermatol. Ther. 2016, 29, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Abuyousif, H.S.; Porcello, A.; Cerrano, M.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Chemali, M.; Raffoul, W.; Hirt-Burri, N. In vitro evaluation and clinical effects of a regenerative complex with non-cross-linked hyaluronic acid and a high-molecular-weight polynucleotide for periorbital treatment. Polymers 2025, 17, 638. [Google Scholar] [CrossRef]
- Oh, S.; Jeon, H.-D.; Rho, N.-K.; Son, K.H.; Byun, K. Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation. Int. J. Mol. Sci. 2025, 26, 6399. [Google Scholar] [CrossRef]
- Thanasarnaksorn, W.; Limsuchaiwat, N.; Sirithanabadeekul, P.; Charoensuksira, S.; Suwanchinda, A.; Meephansan, J. Polynucleotides as a novel therapeutic approach in androgenetic alopecia: An analysis of effectiveness and safety. Arch. Dermatol. Res. 2025, 317, 399. [Google Scholar] [CrossRef]
- Kim, M.J.; Wan, J.; Oksana, L.; Yuliia, L.; Chugay, O.; Platonova, O.; Sydorchuk, O.; Yi, K.-H. Polynucleotide-based treatments for various facial scars including combat injuries. J. Dermatol. Treat. 2024, 35, 2426626. [Google Scholar] [CrossRef]
- Kim, S.T. Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives. Pharmaceutics 2025, 17, 1024. [Google Scholar] [CrossRef]
- Lampridou, S.; Bassett, S.; Cavallini, M.; Christopoulos, G. The Effectiveness of Polynucleotides in Esthetic Medicine: A Systematic Review. J. Cosmet. Dermatol. 2025, 24, e16721. [Google Scholar] [CrossRef] [PubMed]
- Weed, L.L.; Courtenay, T.A. A new nucleotide and a new polynucleotide from bacteriophage nucleic acid. J. Bioi. Ohem 1954, 206, 735–740. [Google Scholar] [CrossRef]
- Grunberg-Manago, M.; Ortiz, P.J.; Ochoa, S. Enzymatic synthesis of nucleic acidlike polynucleotides. Science 1955, 122, 907–910. [Google Scholar] [CrossRef]
- Birnie, G.; Fox, S.M. The synthesis of polydeoxyribonucleotide by cell-free extracts of embryonic mouse tissue. Biochem. J. 1967, 104, 239. [Google Scholar] [CrossRef]
- Sarkar, N. Effects of actinomycin D and mitomycin C on the degradation of deoxyribonucleic acid and polydeoxyribonucleotide by deoxyribonucleases and venom phosphodiesterase. Biochim. Et Biophys. Acta (BBA)-Nucleic Acids Protein Synth. 1967, 145, 174–177. [Google Scholar] [CrossRef]
- Vilcek, J.; Ng, M.H.; Friedman-Kien, A.; Krawciw, T. Induction of interferon synthesis by synthetic double-stranded polynucleotides. J. Virol. 1968, 2, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Field, A.K.; Young, C.W.; Krakoff, I.H.; Tytell, A.A.; Lampson, G.P.; Nemes, M.M.; Hilleman, M.R. Induction of interferon in human subjects by poly I: C. Proc. Soc. Exp. Biol. Med. 1971, 136, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Pescador, R.; Capuzzi, L.; Mantovani, M.; Fulgenzi, A.; Ferrero, M. Defibrotide: Properties and clinical use of an old/new drug. Vasc. Pharmacol. 2013, 59, 1–10. [Google Scholar] [CrossRef]
- Thiemermann, C.; Löbel, P.; Schrör, K. Usefulness of defibrotide in protecting ischemic myocardium from early reperfusion damage. Am. J. Cardiol. 1985, 56, 978–982. [Google Scholar] [CrossRef]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef]
- Baker, D.E.; Demaris, K. Defibrotide. Hosp. Pharm. 2016, 51, 847–854. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.-J.; Jang, H.-J.; Park, H.; Kim, G.J. Human Placenta MSC-Derived DNA Fragments Exert Therapeutic Effects in a Skin Wound Model via the A2A Receptor. Int. J. Mol. Sci. 2025, 26, 1769. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.H.; Kim, J.H.; Park, E.Y.; Cha, S.K. An effective range of polydeoxyribonucleotides is critical for wound healing quality. Mol. Med. Rep. 2018, 18, 5166–5172. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.D.; Goswami, S.; Bera, S.; Mukhopadhyay, I. Quantitation of polydeoxyribonucletides (PDRNs) in human placental extract by fluorescence spectroscopy using ethidium bromide. Am. J. Anal. Chem. 2014, 5, 784–795. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, S.; Wang, H.; Lee, G.; Kim, S.; Ryu, Y.-H.; Chang, N.H.; Kang, Y.-W. Analysis of skin regeneration and barrier-improvement efficacy of polydeoxyribonucleotide isolated from Panax ginseng (CA Mey.) adventitious root. Molecules 2023, 28, 7240. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.; Oh, S.-W.; Choi, Y.-S.; Kang, D.-J.; Park, C.-W.; Lee, J.; Seo, W.-S. First report on microbial-derived polydeoxyribonucleotide: A sustainable and enhanced alternative to salmon-based polydeoxyribonucleotide. Curr. Issues Mol. Biol. 2025, 47, 41. [Google Scholar] [CrossRef]
- Kuppa, S.S.; Kim, H.-K.; Kang, J.-Y.; Lee, S.-C.; Yang, H.-Y.; Sankaranarayanan, J.; Seon, J.-K. Polynucleotides suppress inflammation and stimulate matrix synthesis in an in vitro cell-based osteoarthritis model. Int. J. Mol. Sci. 2023, 24, 12282. [Google Scholar] [CrossRef]
- Jose, D.; Porschke, D. Dynamics of the B–A transition of DNA double helices. Nucleic Acids Res. 2004, 32, 2251–2258. [Google Scholar] [CrossRef]
- Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules 2025, 15, 148. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.T.; Lee, Y.J.; Paik, S.H.; Moon, Y.S.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Jung, J.M. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: A randomized, double-blind, split-face trial. J. Dermatol. Treat. 2022, 33, 254–260. [Google Scholar] [CrossRef]
- Dorsch, C.A.; Killmayer, J. The effect of native and single stranded DNA on the platelet release reaction. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1983, 26, 179–185. [Google Scholar] [CrossRef]
- Fiedel, B.A.; Schoenberger, J.S.; Gewurz, H. Modulation of platelet activation by native DNA. J. Immunol. 1979, 123, 2479–2483. [Google Scholar] [CrossRef]
- Liu, B.; Jin, Y.; Yang, J.; Han, Y.; Shan, H.; Qiu, M.; Zhao, X.; Liu, A.; Jin, Y.; Yin, Y. Extracellular vesicles from lung tissue drive bone marrow neutrophil recruitment in inflammation. J. Extracell. Vesicles 2022, 11, e12223. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. 5′-Nucleotidases and their new roles in NAD+ and phosphate metabolism. New J. Chem. 2010, 34, 845–853. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The Effects of Polydeoxyribonucleotide on Wound Healing and Tissue Regeneration: A Systematic Review of the Literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.W.; Byun, J.H.; Lee, W.M.; Kim, M.H.; Wu, W.H. Comparison of wound healing effects between Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and Oncorhynchus mykiss-derived PDRN. Arch. Craniofacial Surg. 2018, 19, 20. [Google Scholar] [CrossRef]
- Kim, T.H.; Heo, S.Y.; Han, J.S.; Jung, W.K. Anti-inflammatory effect of polydeoxyribonucleotides (PDRN) extracted from red alga (Porphyra sp.)(Ps-PDRN) in RAW 264.7 macrophages stimulated with Escherichia coli lipopolysaccharides: A comparative study with commercial PDRN. Cell Biochem. Funct. 2023, 41, 889–897. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Altavilla, D.; Arcoraci, V.; De Caridi, G.; De Feo, M.E.; Corrao, S.; Pallio, G.; Sterrantino, C.; Minutoli, L. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: Results of a clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, E746–E753. [Google Scholar]
- Shin, J.; Park, G.; Lee, J.; Bae, H. The effect of polydeoxyribonucleotide on chronic non-healing wound of an amputee: A case report. Ann. Rehabil. Med. 2018, 42, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Veronesi, F.; Dallari, D.; Sabbioni, G.; Carubbi, C.; Martini, L.; Fini, M. Polydeoxyribonucleotides (PDRNs) from skin to musculoskeletal tissue regeneration via adenosine A2A receptor involvement. J. Cell. Physiol. 2017, 232, 2299–2307. [Google Scholar] [CrossRef]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P. Activation of A2A receptor by PDRN reduces neuronal damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal cord injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef]
- Seo, S.-S.; Lee, I.-S.; Lee, G.-H. Intra-articular injection therapy and biologic treatment. In A Strategic Approach to Knee Arthritis Treatment: From Non-Pharmacologic Management to Surgery; Springer: Singapore, 2021; pp. 171–212. [Google Scholar]
- Zucchi, A.; Cai, T.; Cavallini, G.; D’Achille, G.; Pastore, A.L.; Franco, G.; Lepri, L.; Costantini, E. Genital lichen sclerosus in male patients: A new treatment with polydeoxyribonucleotide. Urol. Int. 2016, 97, 98–103. [Google Scholar] [CrossRef]
- Galeano, M.; Pallio, G.; Irrera, N.; Mannino, F.; Bitto, A.; Altavilla, D.; Vaccaro, M.; Squadrito, G.; Arcoraci, V.; Colonna, M.R. Polydeoxyribonucleotide: A promising biological platform to accelerate impaired skin wound healing. Pharmaceuticals 2021, 14, 1103. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Wang, F. An injectable hyaluronic acid-polydeoxyribonucleotides (HA-PDRN) crosslinked hydrogel as a dermal filler. Eur. Polym. J. 2024, 219, 113395. [Google Scholar]
- Yi, K.-H.; Park, M.-S.; Ree, Y.-S.; Kim, H.M. A review on “skin boosters”: Hyaluronic acid, poly-L-lactic acid and pol-D-lactic acid, polydeoxyribonucleotide, polynucleotides, growth factor, and exosome. Aesthetics 2023, 4, 1–5. [Google Scholar] [CrossRef]
- Keizers, P.H.; Vanhee, C.; van den Elzen, E.M.; de Jong, W.H.; Venhuis, B.J.; Hodemaekers, H.M.; Schwillens, P.; Lensen, D.G. A high crosslinking grade of hyaluronic acid found in a dermal filler causing adverse effects. J. Pharm. Biomed. Anal. 2018, 159, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Yune, J.H.; Kwon, H.C.; Shin, D.-M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; Kim, E.K. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef]
- Abduljabbar, M.H.; Basendwh, M.A. Complications of hyaluronic acid fillers and their managements. J. Dermatol. Dermatol. Surg. 2016, 20, 100–106. [Google Scholar] [CrossRef]
- Yi, K.H.; Winayanuwattikun, W.; Kim, S.Y.; Wan, J.; Vachatimanont, V.; Putri, A.I.; Hidajat, I.J.; Yogya, Y.; Pamela, R. Skin boosters: Definitions and varied classifications. Skin Res. Technol. 2024, 30, e13627. [Google Scholar] [CrossRef]
- Choi, S.Y.; Koh, Y.G.; Yoo, K.H.; Han, H.S.; Seok, J.; Kim, B.J. A Randomized, Participant-and Evaluator-Blinded, Matched-Pair, Prospective Study Comparing the Safety and Efficacy Between Polycaprolactone and Polynucleotide Fillers in the Correction of Crow’s Feet. J. Cosmet. Dermatol. 2025, 24, e16576. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, E.S.; Kim, S.W.; Hong, S.P.; Kim, J. Effects of Polynucleotide Dermal Filler in the Correction of Crow’s Feet Using an Antera Three-Dimensional Camera. Aesthetic Plast. Surg. 2022, 46, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwon, T.-R.; Lee, S.E.; Jang, Y.N.; Han, H.S.; Mun, S.K.; Kim, B.J. Comparative evaluation of the effectiveness of novel hyaluronic acid-polynucleotide complex dermal filler. Sci. Rep. 2020, 10, 5127. [Google Scholar] [CrossRef]
- Oh, H.; Lee, S.; Na, J.; Kim, J.H. Comparative evaluation of safety and efficacy of a novel hyaluronic acid-polynucleotide/poly-L-lactic acid composite dermal filler. Aesthetic Plast. Surg. 2021, 45, 1792–1801. [Google Scholar] [CrossRef]
- Lee, S.H.; Zheng, Z.; Kang, J.S.; Kim, D.Y.; Oh, S.H.; Cho, S.B. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015, 23, 30–36. [Google Scholar] [CrossRef]
- Cho, S.B.; Zheng, Z.; Kang, J.-S.; Kim, H. Therapeutic efficacy of 1927-nm fractionated thulium laser energy and polydeoxyribonucleotide on pattern hair loss. Med. Lasers 2016, 5, 22–28. [Google Scholar] [CrossRef]
- Choi, Y.J.; Cho, S.; Kim, Y.K.; Kim, D.S. Improvement of hair graying during a treatment of male pattern hair loss using 1927-nm fractionated thulium laser energy and polydeoxyribonucleotide injections. Med. Lasers 2017, 6, 37–40. [Google Scholar] [CrossRef]
- Laino, L.; Suetti, S.; Sperduti, I. Polydeoxyribonucleotide dermal infiltration in male genital lichen sclerosus: Adjuvant effects during topical therapy. Dermatol. Res. Pract. 2013, 2013, 654079. [Google Scholar] [CrossRef]
- Gulfan, M.C.B.; Wanitphakdeedecha, R.; Wongdama, S.; Jantanapornchai, N.; Yan, C.; Rakchart, S. Efficacy and safety of using noninsulated microneedle radiofrequency alone versus in combination with polynucleotides for the treatment of melasma: A pilot study. Dermatol. Ther. 2022, 12, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.R.; Moon, Y.S.; Kwon, D.Y. Combination Therapy of Polydeoxyribonucleotide and Microcurrent in Muscle Regeneration on Cast-Induced Muscle Atrophy in Rabbit. BioMed Res. Int. 2022, 2022, 7469452. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lee, J.Y. Polydeoxyribonucleotide improves wound healing of fractional laser resurfacing in rat model. J. Cosmet. Laser Ther. 2017, 19, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Baek, E.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Polydeoxyribonucleotide exerts opposing effects on ERK activity in human skin keratinocytes and fibroblasts. Mol. Med. Rep. 2023, 28, 148. [Google Scholar] [CrossRef]
- Kim, H.M.; Byun, K.-A.; Oh, S.; Yang, J.Y.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. A mixture of topical forms of polydeoxyribonucleotide, vitamin C, and niacinamide attenuated skin pigmentation and increased skin elasticity by modulating nuclear factor erythroid 2-like 2. Molecules 2022, 27, 1276. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, M.-J.; Kweon, D.-K.; Lim, S.-T.; Lee, S.-J. Polydeoxyribonucleotide activates mitochondrial biogenesis but reduces MMP-1 activity and melanin biosynthesis in cultured skin cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Shi, J.; Huang, W.; Zhao, C.; Han, Q. PDRN prevents SIRT1 degradation by attenuating autophagy during skin aging. PLoS ONE 2025, 20, e0321005. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: Involvement of A2 purinergic receptor subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M. PDRN, a bioactive natural compound, ameliorates imiquimod-induced psoriasis through NF-κB pathway inhibition and Wnt/β-Catenin signaling modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef]
- Noh, T.K.; Chung, B.Y.; Kim, S.Y.; Lee, M.H.; Kim, M.J.; Youn, C.S.; Lee, M.W.; Chang, S.E. Novel anti-melanogenesis properties of polydeoxyribonucleotide, a popular wound healing booster. Int. J. Mol. Sci. 2016, 17, 1448. [Google Scholar] [CrossRef]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound-healing model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef]
- Dananjaya, S.; Bandara, N.; Molagoda, I.M.N.; Sandamalika, W.G.; Kim, D.; Ganepola, N.; Attanayake, A.P.; Choi, D. Multifunctional alginate/polydeoxyribonucleotide hydrogels for promoting diabetic wound healing. Int. J. Biol. Macromol. 2024, 257, 128367. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-K.; Yoon, H.-E.; Park, J.-K.; Kim, M.R.; Kim, D.-I. Wound healing effect of low molecular PDRN on experimental surgical excision rat model. J. Soc. Cosmet. Sci. Korea 2015, 41, 401–411. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, K.W.L.; Lee, A.; Lee, C.H.; Wan, J.; Wong, S.; Yi, K.-H. Polynucleotides in aesthetic medicine: A review of current practices and perceived effectiveness. Int. J. Mol. Sci. 2024, 25, 8224. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Hwang, J.; Kang, M.S.; Yoo, C.-Y.; Kwak, M.; Han, D.-W. Versatile and marvelous potentials of polydeoxyribonucleotide for tissue engineering and regeneration. Biomater. Res. 2025, 29, 0183. [Google Scholar] [CrossRef]
- Ceravolo, I.; Mannino, F.; Irrera, N.; Minutoli, L.; Arcoraci, V.; Altavilla, D.; Cavallini, G.M.; Guarini, S.; Squadrito, F.; Pallio, G. Beneficial effects of polydeoxyribonucleotide (PDRN) in an in vitro model of fuchs endothelial corneal dystrophy. Pharmaceuticals 2022, 15, 447. [Google Scholar] [CrossRef]
- Castellini, C.; Belletti, S.; Govoni, P.; Guizzardi, S. Anti inflammatory property of PDRN—An in vitro study on cultured macrophages. Adv. Biosci. Biotechnol. 2017, 8, 13–26. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, S.; Wang, J.; Xu, Y.; Wang, Y.; Zhang, S.; Xu, X.; Yang, Q.; Zeng, X.; Zhou, Y. Endothelial adenosine A2a receptor-mediated glycolysis is essential for pathological retinal angiogenesis. Nat. Commun. 2017, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Ernens, I.; Léonard, F.; Vausort, M.; Rolland-Turner, M.; Devaux, Y.; Wagner, D.R. Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem. Biophys. Res. Commun. 2010, 392, 351–356. [Google Scholar] [CrossRef]
- Tran, D.H.; Kim, D.; Kesavan, R.; Brown, H.; Dey, T.; Soflaee, M.H.; Vu, H.S.; Tasdogan, A.; Guo, J.; Bezwada, D. De novo and salvage purine synthesis pathways across tissues and tumors. Cell 2024, 187, 3602–3618.e3620. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L. Recent advances on polydeoxyribonucleotide extraction and its novel application in cosmeceuticals. Int. J. Biol. Macromol. 2024, 282, 137051. [Google Scholar] [CrossRef]
- Yang, K.; Wang, L.; Cao, X.; Gu, Z.; Zhao, G.; Ran, M.; Yan, Y.; Yan, J.; Xu, L.; Gao, C. The origin, function, distribution, quantification, and research advances of extracellular DNA. Int. J. Mol. Sci. 2022, 23, 13690. [Google Scholar] [CrossRef] [PubMed]
- Liaw, P.C.; Ito, T.; Iba, T.; Thachil, J.; Zeerleder, S. DAMP and DIC: The role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev. 2016, 30, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.X.; Wang, C.Y.; Zhang, J.S.; Wang, Z.Y.; Wei, Y.; Li, Y.t.; Dai, S.Q.; Tay, F.R.; Niu, L.n. DNA-Based Materials Inspired by Natural Extracellular DNA. Adv. Funct. Mater. 2023, 33, 2211669. [Google Scholar] [CrossRef]
- Carbajal-Valenzuela, I.A.; Medina-Ramos, G.; Caicedo-Lopez, L.H.; Jiménez-Hernández, A.; Ortega-Torres, A.E.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Guevara-González, R.G. Extracellular DNA: Insight of a signal molecule in crop protection. Biology 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.J.; Goa, K.L. Defibrotide: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in vascular disorders. Drugs 1993, 45, 259–294. [Google Scholar]
- Mandel, P. Les acides nucleiques du plasma sanguin chez 1 homme. CR Seances Soc Biol Fil 1948, 142, 241–243. [Google Scholar]
- Aarthy, R.; Mani, S.; Velusami, S.; Sundarsingh, S.; Rajkumar, T. Role of circulating cell-free DNA in cancers. Mol. Diagn. Ther. 2015, 19, 339–350. [Google Scholar] [CrossRef]
- Stein, C.; Castanotto, D.; Krishnan, A.; Nikolaenko, L. Defibrotide (Defitelio): A new addition to the stockpile of food and drug administration-approved oligonucleotide drugs. Mol. Ther. Nucleic Acids 2016, 5, e346. [Google Scholar]
- Pisetsky, D.S.; Gauley, J.; Ullal, A.J. Microparticles as a source of extracellular DNA. Immunol. Res. 2011, 49, 227–234. [Google Scholar]
- Pisetsky, D.S. The origin and properties of extracellular DNA: From PAMP to DAMP. Clin. Immunol. 2012, 144, 32–40. [Google Scholar] [CrossRef]
- Fernández-Domínguez, I.J.; Manzo-Merino, J.; Taja-Chayeb, L.; Dueñas-González, A.; Pérez-Cárdenas, E.; Trejo-Becerril, C. The role of extracellular DNA (exDNA) in cellular processes. Cancer Biol. Ther. 2021, 22, 267–278. [Google Scholar] [CrossRef]
- Wen, F.; Shen, A.; Choi, A.; Gerner, E.W.; Shi, J. Extracellular DNA in pancreatic cancer promotes cell invasion and metastasis. Cancer Res. 2013, 73, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Picciolo, G.; Mannino, F.; Irrera, N.; Altavilla, D.; Minutoli, L.; Vaccaro, M.; Arcoraci, V.; Squadrito, V.; Picciolo, G.; Squadrito, F. PDRN, a natural bioactive compound, blunts inflammation and positively reprograms healing genes in an “in vitro” model of oral mucositis. Biomed. Pharmacother. 2021, 138, 111538. [Google Scholar] [CrossRef]
- Baek, A.; Baek, D.; Kim, S.H.; Kim, J.; Notario, G.R.; Lee, D.W.; Kim, H.J.; Cho, S.-R. Polydeoxyribonucleotide ameliorates IL-1β-induced impairment of chondrogenic differentiation in human bone marrow-derived mesenchymal stem cells. Sci. Rep. 2024, 14, 26076. [Google Scholar]
- Mannino, F.; Pallio, G.; Bitto, A.; Altavilla, D.; Minutoli, L.; Squadrito, V.; Arcoraci, V.; Giorgi, D.A.; Pirrotta, I.; Squadrito, F. Targeting adenosine receptor by polydeoxyribonucleotide: An effective therapeutic strategy to induce white-to-brown adipose differentiation and to curb obesity. Pharmaceuticals 2021, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Potlapalli, R.; Quan, H.; Chen, L.; Xie, Y.; Pouriyeh, S.; Sakib, N.; Liu, L.; Xie, Y. Exploring DNA damage and repair mechanisms: A review with computational insights. BioTech 2024, 13, 3. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Fairhurst, A.-M. The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 2007, 40, 281–284. [Google Scholar] [CrossRef]
- Wang, J. DNA damage and apoptosis. Cell Death Differ. 2001, 8, 1047–1048. [Google Scholar] [CrossRef]
- Peddu, V.; Bradley, B.T.; Casto, A.M.; Shree, R.; Colbert, B.G.; Xie, H.; Santo, T.K.; Huang, M.-L.; Cheng, E.Y.; Konnick, E. High-resolution profiling of human cytomegalovirus cell-free DNA in human plasma highlights its exceptionally fragmented nature. Sci. Rep. 2020, 10, 3734. [Google Scholar] [CrossRef]
- Kowarsky, M.; Camunas-Soler, J.; Kertesz, M.; De Vlaminck, I.; Koh, W.; Pan, W.; Martin, L.; Neff, N.F.; Okamoto, J.; Wong, R.J. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc. Natl. Acad. Sci. USA 2017, 114, 9623–9628. [Google Scholar]
- Aucamp, J.; Bronkhorst, A.J.; Badenhorst, C.P.; Pretorius, P.J. The diverse origins of circulating cell-free DNA in the human body: A critical re-evaluation of the literature. Biol. Rev. 2018, 93, 1649–1683. [Google Scholar]
- Hawes, M.C.; Wen, F.; Elquza, E. Extracellular DNA: A bridge to cancer. Cancer Res. 2015, 75, 4260–4264. [Google Scholar] [CrossRef]
- Unno, K.; Taguchi, K.; Fujita, M.; Sutoh, K.; Nakamura, Y. Stress Reduction Potential in Mice Ingesting DNA from Salmon Milt. Biology 2023, 12, 978. [Google Scholar] [CrossRef]
- Peters, D.L.; Pretorius, P.J. Origin, translocation and destination of extracellular occurring DNA—A new paradigm in genetic behaviour. Clin. Chim. Acta 2011, 412, 806–811. [Google Scholar] [PubMed]
- Ruzanova, V.; Oshikhmina, S.; Proskurina, A.; Ritter, G.; Kirikovich, S.; Levites, E.; Efremov, Y.; Karamysheva, T.; Meschaninova, M.; Mamaev, A. A concept of natural genome reconstruction. Part 2. Effect of extracellular double-stranded DNA fragments on hematopoietic stem cells. Vavilov J. Genet. Breed. 2024, 28, 993. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-K.; Pietersz, G.A. Intracellular detection and immune signaling pathways of DNA vaccines. Expert Rev. Vaccines 2009, 8, 1161–1170. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Lou, H.; Pickering, M.C. Extracellular DNA and autoimmune diseases. Cell. Mol. Immunol. 2018, 15, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Campbell, R.G.; Gulden, R.H.; Hart, M.M.; Powell, J.R.; Klironomos, J.N.; Pauls, K.P.; Swanton, C.J.; Trevors, J.T.; Dunfield, K.E. Cycling of extracellular DNA in the soil environment. Soil Biol. Biochem. 2007, 39, 2977–2991. [Google Scholar] [CrossRef]
- Wagner, H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr. Opin. Microbiol. 2002, 5, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Senaratne, R.H.; Hoppstädter, J.; Diesel, B.; Riley, L.W.; Tabeta, K.; Bauer, S.; Beutler, B.; Zuraw, B.L. Attenuated activation of macrophage TLR9 by DNA from virulent mycobacteria. J. Innate Immun. 2008, 1, 29–45. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Cancer immunosuppression and autoimmune disease: Beyond immunosuppressive networks for tumour immunity. Immunology 2006, 119, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Gursel, I.; Gursel, M.; Yamada, H.; Ishii, K.J.; Takeshita, F.; Klinman, D.M. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J. Immunol. 2003, 171, 1393–1400. [Google Scholar] [CrossRef]
- Goodman, S.D. Extracellular DNA-protein interactions. Curr. Opin. Struct. Biol. 2024, 89, 102943. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, R.K.; In, Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef]
- Zhu, F.-G.; Reich, C.F.; Pisetsky, D.S. Effect of cytofectins on the immune response of murine macrophages to mammalian DNA. Immunology 2003, 109, 255–262. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Huffel, C.V.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Kumar, V. The Trinity of cGAS, TLR9, and ALRs Guardians of the Cellular Galaxy Against Host-Derived Self-DNA. Front. Immunol. 2021, 11, 624597109. [Google Scholar] [CrossRef]
- Fang, F.; Marangoni, R.G.; Zhou, X.; Yang, Y.; Ye, B.; Shangguang, A.; Qin, W.; Wang, W.; Bhattacharyya, S.; Wei, J.; et al. Toll-like Receptor 9 Signaling Is Augmented in Systemic Sclerosis and Elicits Transforming Growth Factor β–Dependent Fibroblast Activation. Arthritis Rheumatol. 2016, 68, 1989–2002. [Google Scholar] [CrossRef] [PubMed]
- Lebre, M.C.; van der Aar, A.M.G.; van Baarsen, L.; van Capel, T.M.M.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; de Jong, E.C. Human Keratinocytes Express Functional Toll-Like Receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef]
- Anunobi, R.; Boone, B.A.; Cheh, N.; Tang, D.; Kang, R.; Loux, T.; Lotze, M.T.; Zeh, H.J. Extracellular DNA promotes colorectal tumor cell survival after cytotoxic chemotherapy. J. Surg. Res. 2018, 226, 181–191. [Google Scholar] [CrossRef]
- Speranskii, A.I.; Kostyuk, S.V.; Kalashnikova, E.A.; Veiko, N.N. Erichment of extracellular DNA from the cultivation medium of human peripheral blood mononuclears with Genomic CpG rich fragments results in increased cell production of IL-6 and TNFα via activation of the NF-κB signaling pathway. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2015, 9, 174–184. [Google Scholar] [CrossRef]
- Wang, W.; Kong, P.; Ma, G.; Li, L.; Zhu, J.; Xia, T.; Xie, H.; Zhou, W.; Wang, S. Characterization of the release and biological significance of cell-free DNA from breast cancer cell lines. Oncotarget 2017, 8, 43180–43191. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Zank, D.; Buendia-Roldán, I.; Fiedler, K.; Mays, B.G.; Alvarez, D.; Sembrat, J.; Kimball, B.; Bullock, J.K.; Martin, J.L.; et al. PINK1 attenuates mtDNA release in alveolar epithelial cells and TLR9 mediated profibrotic responses. PLoS ONE 2019, 14, e0218003. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, M.; Wang, K.; Sun, J.; Song, L.; Diao, X.; Jiang, Z.; Cheng, G.; Wang, X. Excessive activation of the TLR9/TGF-β1/PDGF-B pathway in the peripheral blood of patients with systemic lupus erythematosus. Arthritis Res. Ther. 2017, 19, 70. [Google Scholar] [CrossRef]
- Briard, B.; Place, D.E.; Kanneganti, T.-D. DNA sensing in the innate immune response. Physiology 2020, 35, 112–124. [Google Scholar] [CrossRef]
- Zahid, A.; Ismail, H.; Li, B.; Jin, T. Molecular and structural basis of DNA sensors in antiviral innate immunity. Front. Immunol. 2020, 11, 613039. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef]
- Mizutani, Y.; Kanbe, A.; Ito, H.; Seishima, M. Activation of STING signaling accelerates skin wound healing. J. Dermatol. Sci. 2020, 97, 21–29. [Google Scholar] [CrossRef]
- Mathavarajah, S.; Thompson, A.W.; Stoyek, M.R.; Quinn, T.A.; Roy, S.; Braasch, I.; Dellaire, G. Suppressors of cGAS-STING are downregulated during fin-limb regeneration and aging in aquatic vertebrates. J. Exp. Zool. Part B Mol. Dev. Evol. 2024, 342, 241–251. [Google Scholar] [CrossRef]
- Sun, S.-C.; Han, R.; Hou, S.-S.; Yi, H.-Q.; Chi, S.-J.; Zhang, A.-H. Juglanin alleviates bleomycin-induced lung injury by suppressing inflammation and fibrosis via targeting sting signaling. Biomed. Pharmacother. 2020, 127, 110119. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wu, X.; Zhao, D.; Liu, Y.; Du, Q.; Li, Y.; Xu, Y.; Li, Y.; Qiu, Y.; Yang, Y. Fluvoxamine alleviates bleomycin-induced lung fibrosis via regulating the cGAS-STING pathway. Pharmacol. Res. 2023, 187, 106577. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Wang, H.; Hu, W.; Sun, L. Qingfei xieding prescription ameliorates mitochondrial DNA-initiated inflammation in bleomycin-induced pulmonary fibrosis through activating autophagy. J. Ethnopharmacol. 2024, 325, 117820. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, L.; Byrd, K.M.; Ko, C.-C.; Zhao, Z.; Fang, J. AIM2 inflammasome’s first decade of discovery: Focus on oral diseases. Front. Immunol. 2020, 11, 1487. [Google Scholar] [CrossRef]
- Zanini, G.; Bertani, G.; Di Tinco, R.; Pisciotta, A.; Bertoni, L.; Selleri, V.; Generali, L.; Marconi, A.; Mattioli, A.V.; Pinti, M. Dental pulp stem cells modulate inflammasome pathway and collagen deposition of dermal fibroblasts. Cells 2024, 13, 836. [Google Scholar] [CrossRef]
- Ding, D.; Liu, H.; Zhang, L.; Zhang, G.; Wei, Y.; Zhang, W.; Yang, X.; Li, M.; Yin, G.; Guo, W. AIM2 promotes the progression of HNSCC via STAT1 mediated transcription and IL-17/MAPK signaling. Cell. Signal. 2025, 127, 111545. [Google Scholar] [CrossRef]
- Jakobsen, M.R.; Paludan, S.R. IFI16: At the interphase between innate DNA sensing and genome regulation. Cytokine Growth Factor Rev. 2014, 25, 649–655. [Google Scholar] [CrossRef]
- Almine, J.F.; O’Hare, C.A.; Dunphy, G.; Haga, I.R.; Naik, R.J.; Atrih, A.; Connolly, D.J.; Taylor, J.; Kelsall, I.R.; Bowie, A.G. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 2017, 8, 14392. [Google Scholar] [CrossRef]
- Zhao, H.; Gonzalezgugel, E.; Cheng, L.; Richbourgh, B.; Nie, L.; Liu, C. The roles of interferon-inducible p200 family members IFI16 and p204 in innate immune responses, cell differentiation and proliferation. Genes Dis. 2015, 2, 46–56. [Google Scholar] [CrossRef]
- Alegre, B.F.; Alegre, M.M.; Hardy, M.S.; Robison, R.A.; O’Neill, K.L. Abstract 905: Localization of several potential biomarkers in various types of cancer. Cancer Res. 2011, 71, 905. [Google Scholar] [CrossRef]
- Kinsella, A.R.; Smith, D.; Pickard, M. Resistance to chemotherapeutic antimetabolites: A function of salvage pathway involvement and cellular response to DNA damage. Br. J. Cancer 1997, 75, 935–945. [Google Scholar] [CrossRef]
- Weber, G.; Lui, M.S.; Natsumeda, Y.; Faderan, M.A. Salvage capacity of hepatoma 3924A and action of dipyridamole. Adv. Enzym. Regul. 1983, 21, 53–69. [Google Scholar] [CrossRef]
- Kruszewska, H.; Misicka, A.; Chmielowiec, U. Biodegradation of DNA and nucleotides to nucleosides and free bases. Il Farm. 2004, 59, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Urso Pitassi, L.H.; Pearson, K.; Antônio de Assis, L.; Biesman, B.; Calomeni, M.; Bay-Aguilera, S.; Wyles, S.P. Polynucleotides in Skin Regeneration: Targeting the Adenosine A2A Receptor and Salvage Pathway. Dermatol. Surg. 2024, 50, S131–S134. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Squadrito, F.; Squadrito, G.; Pallio, S.; Anastasi, G.P.; Cutroneo, G.; et al. Adenosine Receptor Stimulation by Polydeoxyribonucleotide Improves Tissue Repair and Symptomology in Experimental Colitis. Front. Pharmacol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Ben-Sahra, I. Regulation of nucleotide metabolism in cancers and immune disorders. Trends Cell Biol. 2023, 33, 950–966. [Google Scholar] [CrossRef]
- Welter, E.; Elazar, Z. Autophagy mediates nonselective RNA degradation in starving yeast. EMBO J. 2015, 34, 131–133. [Google Scholar] [CrossRef]
- Ashihara, H.; Stasolla, C.; Loukanina, N.; Thorpe, T.A. Purine metabolism during white spruce somatic embryo development: Salvage of adenine, adenosine, and inosine. Plant Sci. 2001, 160, 647–657. [Google Scholar] [CrossRef]
- Lee, D.; Kim, M.J.; Park, H.J.; Rah, G.C.; Choi, H.; Anh, S.T.; Ji, G.H.; Kim, M.S.; Kim, G.; Shin, D.W. Current practices and perceived effectiveness of polynucleotides for treatment of facial erythema by cosmetic physicians. Skin Res. Technol. 2023, 29, e13466. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Kwon, S.H.; Kim, J.W.; Jeong, W.-J.; Cha, W.; Jung, Y.H.; Na, J.-I.; Huh, C.-H.; Shin, J.-W. Early postoperative injections of Polydeoxyribonucleotide prevent hypertrophic scarring after thyroidectomy: A randomized controlled trial. Adv. Wound Care 2023, 12, 361–370. [Google Scholar] [CrossRef]
- Sahragardjoonegani, B.; Beall, R.F.; Kesselheim, A.S.; Hollis, A. Repurposing existing drugs for new uses: A cohort study of the frequency of FDA-granted new indication exclusivities since 1997. J. Pharm. Policy Pract. 2021, 14, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef]
- Abushaaban, E.; Alhajj, R. A survey on computational methods used for drug repositioning. Netw. Model. Anal. Health Inform. Bioinform. 2025, 14, 8. [Google Scholar] [CrossRef]
- Athul, H.; Shaji, S.; Shabik, K.; Joseph, A. Harnessing the power of drug repurposing: A promising strategy for drug discovery and development. Natl. J. Pharmacol. Ther. 2024, 2, 123–128. [Google Scholar] [CrossRef]
- Jacomelli, G.; Micheli, V.; Peruzzi, L.; Notarantonio, L.; Cerboni, B.; Sestini, S.; Pompucci, G. Simple non-radiochemical HPLC-linked method for screening for purine metabolism disorders using dried blood spot. Clin. Chim. Acta 2002, 324, 135–139. [Google Scholar] [CrossRef]
- Di Pietro, V.; Perruzza, I.; Amorini, A.M.; Balducci, A.; Ceccarelli, L.; Lazzarino, G.; Barsotti, P.; Giardina, B.; Tavazzi, B. Clinical, biochemical and molecular diagnosis of a compound homozygote for the 254 bp deletion–8 bp insertion of the APRT gene suffering from severe renal failure. Clin. Biochem. 2007, 40, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, T.; Goyama, S. Nucleic acid metabolism: The key therapeutic target for myeloid tumors. Exp. Hematol. 2025, 142, 104693. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).