Abstract
Purpose: To evaluate the clinical and histological outcomes of patients that receive implant-supported crowns after vacuum plasma surface treatment (VPST) of biomaterials used in socket preservation (SP) and guided bone regeneration (GBR). Materials and methods: This study was designed as a case series. Patients in need of tooth extraction and socket preservation or guided bone regeneration were enrolled. The socket preservation technique was performed after tooth extraction using a heterologous collagen bone graft and a collagen xenomatrix, both activated with vacuum plasma. Meanwhile, a two-stage horizontal ridge augmentation was performed using a customized titanium mesh and a mix of autologous (untreated) and heterologous (treated) bone grafts, along with a treated collagen membrane. ACTILINK Reborn with Universal Vortex Holder (Plasmapp Co., Ltd., Daejeon, Republic of Korea) was used to treat all biomaterials. The outcome measures were implant and prosthesis failures, complications, and histological examination. Soft and hard tissue samples were collected at the time of implant placement only in patients treated with SP. Results: A total of six patients were treated—three with socket preservation and delayed implant placement, and three with staged GBR. No implant or prosthesis failed. One customized titanium mesh broke after plasma treatment, requiring replacement with a pericardium membrane. No other complications occurred. Histological analysis at three months post-surgery revealed well-vascularized newly formed bone at different stages of maturation with integrated bone graft particles, while the soft tissue appeared to be physiologically structured. Conclusion: VPST may enhance the hydrophilicity of biomaterials, supporting favorable healing outcomes in SP and GBR. Further randomized controlled trials with appropriate sample size calculations are needed to confirm these preliminary results.
1. Introduction
Oral implantology is recognized as a safe and effective clinical methodology capable of ensuring long-term success in oral rehabilitation [1]. The origins of this medical field trace back to the early 1950s when Dr. Per-Ingvar Brånemark, a Swedish orthopedic surgeon, made groundbreaking discoveries while conducting orthopedic experiments on rabbit legs [2]. Since then, the field has rapidly evolved, driven by advances in biomaterials and surgical techniques, leading to improved outcomes and increased patient satisfaction.
Currently, an implant is considered osseointegrated when there is no progressive relative movement between the implant and the surrounding bone, indicating direct contact and stability [3]. Primary stability is largely influenced by mechanical interlocking between the implant surface and the host bone, which is dependent on factors such as bone density, implant design, and surgical technique [4].
Achieving and maintaining long-term osseointegration is complex and can be affected by various biological and mechanical factors. These include the patient’s systemic health, local bone quality, implant loading conditions, and surface characteristics of the implant. Recent studies suggest that modifying implant surfaces to enhance their biological compatibility can significantly improve osseointegration outcomes [5,6]. Numerous surface treatment methods have been investigated and implemented to enhance the biological surface characteristics of implants, supporting the osseointegration process for both short-term and long-term success [5,6]. Among these, surface roughness and hydrophilicity are particularly significant in achieving high bone-to-implant contact (BIC). These surface characteristics are designed to provide better stability during the healing process and enable faster implant loading by accelerating the osseointegration mechanism and promoting stronger, faster bone formation [7,8].
To enhance the osteoconductivity and osteoinductivity of biomaterials for bone regeneration, modification of their surface has been demonstrated to promote cell adhesion, proliferation, and new bone formation [8]. These treatments include additive modifications such as coatings or subtractive modifications including etching, sand blasting, and others [8]. Among the various biomaterial treatments aimed at enhancing osseointegration, plasma treatment has demonstrated significant improvements in cell adhesion by modifying surface roughness and wettability [9,10]. Plasma activation modifies the surface by incorporating functional groups, increasing surface energy, and enhancing hydrophilicity. This leads to a reduction in the contact angle between biological fluids and the implant surface, facilitating osteoblastic cell diffusion without leaving any residues after treatment. Additionally, plasma treatment induces changes in physicochemical properties, including the surface free energy, hydrocarbon content, and the presence of functional hydroxyl groups [11]. Recent advances in plasma technology have enabled the development of low-pressure plasma systems capable of uniformly treating complex implant geometries. These systems offer precise control over treatment parameters such as power, gas composition, and exposure time, enabling tailored surface modifications to enhance biocompatibility. However, despite promising in vitro results [12], clinical evidence on plasma-treated biomaterials remains limited, needing further investigation. Despite the promising results from in vitro studies, clinical evidence on the effectiveness of plasma-treated biomaterials remains limited, necessitating further investigation.
The aim of this clinical audit is to evaluate the clinical and histological outcomes of patients that receive implant-supported crowns after VPST of biomaterials used in socket preservation (SP) and guided bone regeneration (GBR). The findings from this research are intended to inform and potentially refine existing clinical protocols, contributing to a more accurate and predictable approach in oral rehabilitation. As such, this clinical audit may serve as a proof-of-concept study.
2. Materials and Methods
This study was designed as a phase IV case series study, with CE-approved mate-rials and devices used as part of usual professional practice and with no adjunctive risks. All cases were performed in a private clinic in Rome, Italy, and the data were evaluated at the Department of Medicine, Surgery, and Pharmacy, University of Sassari, Italy. The study adhered to the principles of the 2013 Helsinki Declaration and Good Medical Practice principles. Medical data were anonymized so that patients could not be identified, and the ethics committee’s waiver for consent for a case series was obtained (Report number 41/1.6; released on 21 May 2025).
Patients in need of an atraumatic tooth extraction of a hopeless tooth and socket preservation procedure as well as patients in need of a guided bone regeneration procedure prior to implant placement were enrolled in this research.
Inclusion criteria:
- Patients with at least one hopeless tooth in the mandible or maxilla, with intact post-extractive alveolus, able to understand and provide informed consent were considered eligible for inclusion for socket preservation; or any patients who required single implant-supported restoration and staged horizontal guided bone regeneration (defect of class IV according to Cawed and Howell) in both the mandible or maxilla. A hopeless tooth was defined as a tooth with severe structural, periodontal, or endodontic damage that could not be predictably treated or maintained and was recommended for extraction. Common criteria for classifying a tooth as hopeless include: severe periodontal disease with ≥75% bone loss, mobility (Grade III), or class III furcation involvement; extensive decay or fractures that make the tooth non-restorable; unsuccessful endodontic treatment with persistent infection; and trauma leading to non-viable roots or severe displacement.
- Aged 18 years or older and able to provide informed consent.
- Smokers were categorized as: (1) non-smokers; (2) moderate smokers (smoking up to 10 cigarettes/day); and (3) heavy smokers (smoking more than 11 cigarettes/day). Heavy smokers were excluded, only categories 1 and 2 were included in this study.
- Biotype was categorized as: thin (≤1 mm), medium (>1–<2 mm), or thick (≥2 mm).
Exclusion criteria:
- General contraindications to implant surgery
- Patients irradiated in the head and neck area.
- Immunosuppressed or immunocompromised patients.
- Patients treated or under treatment with intravenous amino-bisphosphonates.
- Patients with untreated periodontitis.
- Patients with poor oral hygiene and motivation.
- Uncontrolled diabetes.
- Heavy smokers (more than 11 cigarettes/day).
- Pregnancy or nursing.
- Substance abusers.
- Psychiatric problems or unrealistic expectations.
- Lack of antagonist occluding dentition in the area intended for implant placement.
- Patients with infection and or inflammation in the area intended for implant placement.
- Patients participating in other studies if the present protocol cannot be properly adhered to.
- Patients referred only for implant placement and cannot be followed at the treatment center.
- Patients unable to be followed for 5 years.
2.1. Socket Preservation Procedure
Patients who met the inclusion/exclusion criteria (Figure 1) were thoroughly clinically examined for the assessment of dental caries and periodontal health. Prior to the socket preservation procedure, patients underwent a preoperative CBCT scan to quantify bone volume. Once eligibility was confirmed and informed consent was obtained, a comprehensive oral examination was conducted to evaluate overall oral health and identify any pathologies requiring treatment prior to socket preservation and implant rehabilitation. Approximately 10 days before socket preservation and implant placement, all patients received professionally delivered oral hygiene, including debridement, as necessary. Prophylactic antibiotic administration included 2 g of amoxicillin one hour before surgery, or 600 mg of clindamycin in cases of penicillin allergy. Patients also rinsed with 0.2% chlorhexidine mouthwash for one minute before any surgical procedure. All surgical interventions were performed under local anesthesia with articaine and adrenaline (1:100,000). Intravenous sedation was optional. Atraumatic tooth extraction without flap elevation was performed, followed by curettage of the alveolus, assessment of alveolar integrity, and socket preservation (Figure 2). Post-extraction sockets were filled with heterologous bone substitute using natural equine bone granules (Bioactiva, Arcugnano, Italy) and covered with XC Collagen Xenomatrix (Bioteck S.p.A, Vicenza, Italy). The vacuum plasma surface treatment was performed using the ACTILINK reborn machine (Activelink reborn, Plasmapp Co., Ltd., Daejeon, Republic of Korea) with a customized holder (Figure 3), according to a published protocol [12]. The cycle time of the vacuum plasma surface treatment, named Vortex Plasma mode, was 30 s.
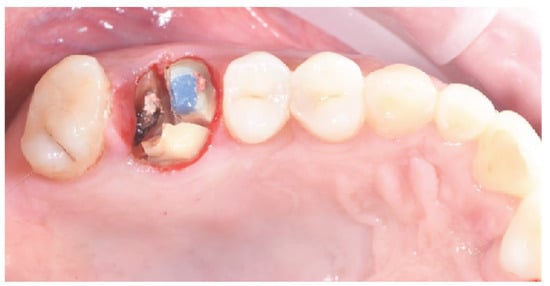
Figure 1.
Hopeless tooth after crown removal.
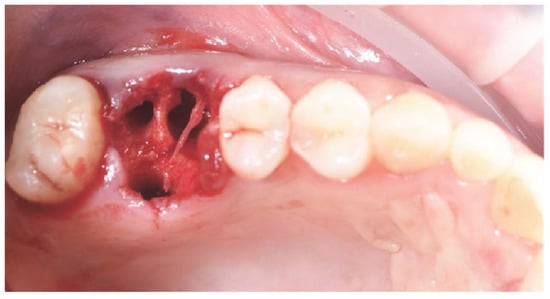
Figure 2.
Atraumatic tooth extraction, without flap.

Figure 3.
Heterologous bone substitute treated with plasma.
The surgical sites were then closed with Vicryl 4.0 simple sutures (Revello S.p.A, Verona, Italy) (Figure 4). After a healing period of three months, implant placement (Osstem Implant, Seoul, Republic of Korea) was performed according to the manufacturer’s instructions. Histological tissue samples were collected immediately before implant site preparation (see Section 2.3 and Section 2.4). The implant sites were prepared freehand, without a surgical template, using drills of increasing diameter. Bone quality was subjectively categorized as hard, medium, or soft. The motor torque was set to 35 Ncm during implant insertion. Implants were placed 1.5 mm below the intact buccal bone, and an immediate loading protocol was adopted with a minimum insertion torque of 35 Ncm. Post-surgical analgesic treatment with ibuprofen 600 mg was prescribed as needed. All patients were strictly followed to evaluate complete healing. Three to four months after immediate loading, definitive impressions were taken, and within one month, a screw-retained, implant-supported, single metal-free crown was delivered. Periapical radiographs were obtained at the time of implant placement (Figure 5).
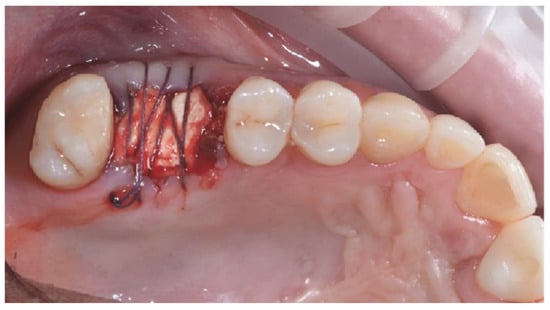
Figure 4.
Post-extractive socket filled with xenograft, covered with collagen xenomatrix.
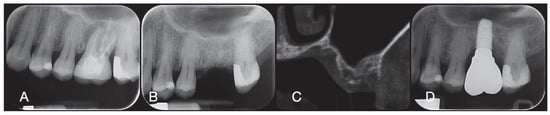
Figure 5.
Periodical radiography before the treatment (A), after socket preservation (B), at crown delivery (D), and CBCT scan before implant placement (C).
2.2. Post-Surgical Instructions and Prosthetic Phase After Socket Preservation
A soft diet was recommended for 30 days in the case of immediate loading. Ibuprofen (400 mg) was prescribed to be taken 2–4 times a day during meals, but patients were instructed not to take it in the absence of pain (in cases of allergy or stomach problems, 1 g paracetamol was prescribed instead). Additionally, 0.2% chlorhexidine mouthwash was recommended for 1 min twice a day for 14 days. Patients were not allowed to wear any removable denture that could have loaded the study implants. Sutures were removed after 7 to 10 days, and oral hygiene instructions were delivered.
At the time of implant insertion, immediate loading temporary restorations were delivered. After osseointegration (8 weeks), the temporary restorations were modified according to the soft tissue management. After 3 to 4 months, a definitive, digital or analog impression was taken. Definitive restorations were delivered within one month after having tested the stability of the individual implants. The occlusal surface was in slight contact with the opposite dentition. Periapical radiographs and clinical pictures of the study implants were taken (Figure 5). If the peri-implant marginal bone levels were not readable, a new radiograph was taken, and oral hygiene instructions were delivered.
2.3. Biopsy Retrieval
After three months, implant placement (Osstem Implant, Seoul, Republic of Korea) was performed according to the manufacturer’s instructions. During implant site preparation, soft and hard tissue biopsies were obtained using a 5 mm mucotome (outer diameter) for soft tissue and a 3.0 mm (outer diameter) trephine drill for bone samples. Hard tissue samples were fixed in 10% neutral buffered formalin and soft tissues in 4% neutral buffered formalin for histological examination.
2.4. Sample Processing and Histological Analysis
Hard tissue samples were dehydrated using an ascending ethanol series. Following dehydration, samples were infiltrated with methacrylic resin and subsequently embedded. The blocks were sectioned using a diamond blade and further reduced to a thickness of about 100 μm. The sections were mounted on plastic slides, glued, and stained with Toluidine Blue and Yellow Pyronin.
Formalin-fixed soft tissue samples were paraffin-embedded. Histological slides were obtained and stained with Hematoxylin and Eosin, Mallory’s trichrome, and Sirius Red. Slides were examined qualitatively under a bright-field optical microscope (Nikon Eclipse 80i, Nikon, Tokyo, Japan) and a high-resolution scanner at different magnifications (Hamamatzu NanoZoomer Series S60, Hamamatzu Photonics, Hamamatsu, Japan). Additionally, Sirius Red-stained samples were observed under polarized light to evaluate collagen fiber organization (Nikon Eclipse 80i, Nikon, Tokyo, Japan).
2.5. GBR Procedure
Patients were assessed for eligibility for the study. A preoperative CBCT scan was obtained for each patient to quantify bone volume. Patients lacking sufficient bone volume for implant placement were informed about the study details and signed the research-informed consent form. Once eligibility was confirmed and informed consent was obtained, a thorough oral examination was conducted to assess overall health and identify any oral pathologies requiring treatment before guided bone regeneration (GBR) and implant rehabilitation. Ten days prior to GBR and implant placement, all patients underwent professional oral hygiene procedures, including debridement, as necessary. Failed teeth in the operative sites were extracted eight weeks before GBR and implant placement. Patients received 2 g of amoxicillin (Zimox, Pfizer, Rome, Italy) one hour before surgery, followed by 1 g twice daily for two weeks. In cases of penicillin allergy, 600 mg of clindamycin was administered with the same postoperative regimen. Immediately before surgery, patients rinsed with a 0.2% chlorhexidine solution (Curasept, Curaden Healthcare, Saronno, Italy) for one minute. The surgical procedure was performed under conscious sedation (Diazepam, Hoffmann-La Roche, Basel, Switzerland) and local anesthesia (Septanest with adrenaline 1/100,000, Septodont, Saint-Maur-des-Fossés, France). A mid-crestal incision (slightly buccally advanced) was made into the residual keratinized tissue using a no. 15 surgical blade, followed by two bilateral vertical incisions one or two teeth away. A full-thickness flap was elevated beyond the mucogingival junction, at least 5 mm beyond the bone defect, and the recipient site was thoroughly cleared of soft tissue remnants. Autogenous bone was harvested from the planned implant site or the mandibular retromolar region using a minimally invasive cortical bone harvester (Micross, Meta, Reggio Emilia, Italy). The bone defect was then filled with a 50:50 mixture of autogenous bone and heterologous bone substitute (natural equine bone granules, Bioactiva, Arcugnano, Italy), pre-treated with Vortex Plasma using the material holder. A plasma-treated, CAD/CAM-designed, titanium mesh (Exocad DentalCAD, Exocad, Darmstadt, Germany), customized based on the contralateral region’s outline and shape (New Ancorvis Srl, Bargellino, Calderara di Reno, Italy), was then placed to cover and protect the grafted site. The customized titanium mesh was fixed with two to three pre-planned bone screws and covered with a plasma-treated pericardium membrane (Bioactiva, Arcugnano, Italy) (Figure 6 and Figure 7). Tension-free flap closure was achieved using Vicryl 4.0 sutures. Postoperatively, patients received 80 mg of ketoprofen (Oki, Dompé, Milan, Italy) and 4 mg/day of betamethasone (Bentelan, Glaxo, Verona, Italy) for two days. They were instructed to rinse with 0.2% chlorhexidine (Curasept) three times daily for two weeks and consume only soft foods for 30 days. Sutures were removed after 14 to 21 days. Baseline periapical radiographs were taken at the end of the procedure, during reopening, at prosthesis delivery, and annually thereafter. The ACTILINK Reborn with Universal Vortex Holder (Plasmapp Co., Ltd., Republic of Korea) was used to treat all biomaterials, as previously reported [12].
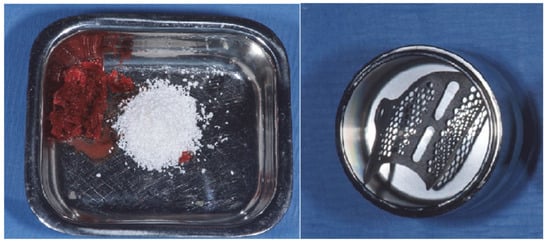
Figure 6.
A Autologous bone and heterologous bone substitute after plasma treatment; 5b Virtually designed CAD/CAM titanium mesh treated with Vortex Plasma.
Figure 7.
Titanium mesh positioned and fixed in place with a collagen membrane upon it.
Six months after GBR, implants were placed following the manufacturer’s standard protocol. After flap elevation, implant sites were prepared using drills of increasing diameter, with bone quality subjectively categorized as hard, medium, or soft. The motor torque was set to 25 Ncm during implant insertion. Implants (Osstem TSIII SOI, Osstem Implant CO., LTD., Seoul, Republic of Korea) were placed in prosthetically planned positions. Since the implants had an activated surface, no additional Vortex Plasma treatment was performed. Implants were placed at crestal level or slightly below, either freehand or using a CAD/CAM surgical template. Four months post-implant placement, the implants were exposed, and soft tissue was managed as needed. Digital or analog impressions were then taken, and temporary restorations were delivered according to the soft tissue management requirements. Two to four months later, definitive monolithic zirconia crowns bonded on Ti-link were delivered after confirming individual implant stability. The occlusal surfaces were designed to be in light contact with the opposing dentition. Periapical radiographs and clinical images were taken at the GBR procedure, implant placement, temporary and definitive restoration stages (Figure 8). Oral hygiene instructions were provided at each follow-up visit. Professional maintenance hygiene was scheduled every six months following definitive loading and as needed during the prosthetic phases. Dental occlusion was evaluated at each follow-up appointment.
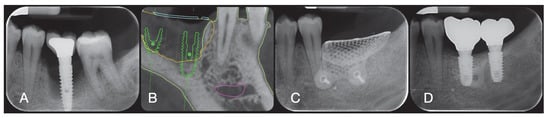
Figure 8.
Periodical radiography before the treatment (A), at the preliminary virtual implant planning (B), after the GBR procedure (C), at crown delivery (D), and CBCT scan before GBR with simulated implants to evaluate the amount of bone to be grafted and to design the customized mesh.
2.6. Outcome Measures
The primary outcomes were:
- Implant failure defined as mobility, infection, fracture, and/or any other mechanical or biological issue that determined its removal.
- Prosthesis failure was considered at any time it had to be replaced.
- Any biological (e.g., drug-resistant pain, swelling, excessive MBL, suppuration, etc.) and/or technical (e.g., fracture of the veneering material and or framework, screw loosening, etc.) complications were recorded during follow-up.
The secondary outcome was to assess bone regeneration through qualitative histological analysis by evaluating the degree of bone mineralization.
3. Results
3.1. Clinical Outcomes
A total of six patients were treated. Three patients with hopeless teeth and intact sockets after tooth extraction received socket preservation procedure and delayed (three months) implant placement, while the other three patients with class IV horizontal defects received implant placement in combination with guided bone regeneration. Six histological samples in three patients were collected at the time of implant placement in the socket preservation group. From each patient, soft and hard tissues were harvested three months after the socket preservation procedure, at the same time of implant surgery, as described. A total of six samples were collected and sent for histological analysis (three soft tissue samples and three bone samples).
A total of six patients were treated. All of the patients were female with a mean age of 50 years old (range 37 to 63). None of the patients were smokers and all of the patients were in good health (ASA 1). All of the patients were followed for at least 1 year after prosthesis delivery, with no drop-outs.
No implants or prostheses failed up to one year after implant placement. In one patient that underwent guided bone regeneration, the titanium mesh broke after plasma treatment. In this patient, a pericardium membrane was used and fixed with tags. No other biological or technical complications occurs.
In one patient in the sock preservation group, at the time of implant placement, the primary stability was around 15–20 Ncm. However, the implant osseointegrated and the final crown was delivered.
3.2. Histological Evaluation
Three months after socket preservation surgery, no histological samples showed signs of inflammation or necrosis.
All hard tissue samples evidenced newly formed bone undergoing mineralization at different stages (Figure 9) that surrounded several bone graft particles, characterized by numerous surface lacunae consistent with osteoclast-resorption lacunae (Figure 10). The newly formed bone appeared to be rich in bone active cells, namely osteoblast-like cells, aligned in deposition lines and secreting osteoid matrix, and immature osteocyte-like cells inhabiting woven bone lacunae (Figure 9). Loose connective tissue was observed around the hard tissue areas and appeared to be rich in cells and blood vessels (Figure 11).
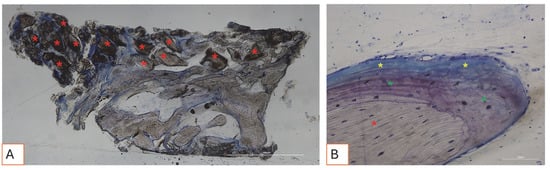
Figure 9.
(A,B). Histological photomicrograph of hard tissue samples collected three months after socket preservation surgery, stained with Toluidine Blue and Yellow Pironin. (A) Overview of the sample. The portion of regenerated tissue is shown, rich in graft material (red asterisks), surrounded by newly formed bone. Below the red line, native bone is visible. Total magnification: 40X. (B) Higher magnification view showing a graft granule surrounded by bone undergoing different stages of mineralization, including woven bone (green asterisks) and osteoid matrix (yellow asterisks). Total magnification: 200X.
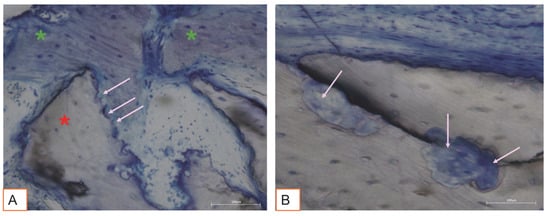
Figure 10.
(A,B). Pink arrows indicate bone graft surface lacunae. (A) The lacunae are adjacent to newly formed woven bone (green asterisks). Total magnification 200X, Toluidine Blue and Yellow Pironin staining.
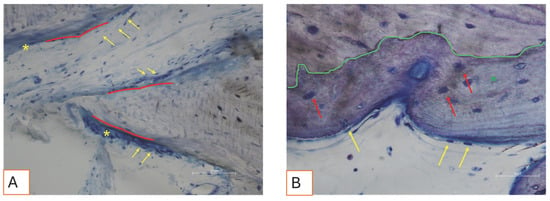
Figure 11.
(A,B). Photomicrograph showing active bone cells. (A) Bone deposition lines (red lines) are visible, characterized by osteoblast-like cells (yellow arrows) actively secreting new osteoid matrix (yellow asterisks). Total magnification 200X. (B) A green line marks the interface between the graft material and newly formed woven bone (green asterisk). Red arrows indicate cells resembling immature osteocytes embedded within their lacunae in the woven bone tissue. On the surface, osteoblast-like cells are aligned along the bone margin (yellow arrows). Total magnification: 400X, Toluidine Blue and Yellow Pironin staining.
The soft tissues exhibited a physiological morphology. The connective component was slightly fibrotic, consistent with post-surgical healing (Figure 12 and Figure 13).
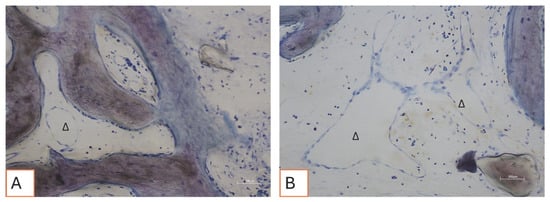
Figure 12.
(A,B) Loose connective tissue surrounds the bone tissue. Delta “∆” symbols indicate blood vessels. Total magnification 200X, Toluidine Blue and Yellow Pironin staining.
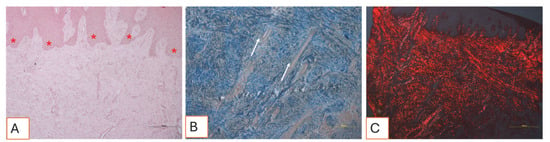
Figure 13.
(A–C). Histological sections of soft tissue collected three months after the surgical procedure. (A) The tissue exhibits a physiological architecture. The connective papillae interfacing with the epithelium (red asterisks) display normal morphology. No signs of inflammation, necrosis, or tissue distress are observed. Total magnification: 100X, Hematoxylin and Eosin staining. (B) View of the dense connective tissue, characterized by tightly packed collagen fiber bundles and numerous small blood vessels (white arrows). Total magnification: 100X, Mallory’s Trichrome staining. (C) Under polarized light, the tissue appears to be slightly fibrotic: collagen fibers appear to be well defined, organized, and oriented in parallel, forming bundles consistent with post-surgical mature healing. Total magnification: 100X, Sirius Red staining.
4. Discussion
This study investigated the impact of VPST on biomaterials used in SP and GBR procedures, assessing its influence on surface hydrophilicity and healing outcomes. The results indicate that plasma treatment enhances the biological compatibility of the biomaterials, promoting effective osseointegration and favorable healing responses.
Histological analysis at three months exhibited no signs of necrosis, inflammation, or scarring. Both soft and hard tissues showed physiological architectures with good vascularization. Post-surgery regenerated hard tissues revealed the presence of active bone cells secreting osteoid matrix and newly formed bone tissue, consistent with the expected healing timelaps. These findings align with previous studies reporting that early bone formation is characterized by the deposition of an osteoid matrix, which progressively mineralizes over time [13,14,15,16]. Particles of bone graft were well integrated into the newly formed bone. Interestingly, many of these particles displayed surface lacunae compatible with osteoclastic resorption. Human osteoclasts cultured for 21 days on the surface of deproteinized equine bone granules (DEBGs) and deproteinized bovine bone granules (DBBGs) resorbed the biomaterials, exhibiting typical osteoclastic markers and morphological features more actively on DEBGs than DBBGs. These findings suggested that DEBGs support osteoclast differentiation and resorptive activity [16]. Furthermore, Di Stefano et al. [17], in a clinical and histomorphometric study conducted six months after socket grafting, reported that DEBGs were associated with a significantly higher amount of newly formed bone and a lower percentage of residual biomaterial compared to DBBGs. In light of our findings, the presence of resorption lacunae observed on the surface of DEBGs might be attributed to the intrinsic properties of the material itself, which appear to be further enhanced by VPST treatment. In addition, VPST enhances surface roughness and wettability, promoting better cell differentiation and apatite formation, which are crucial for successful bone regeneration [18]. Hard tissue analysis, indeed, revealed numerous bone deposition lines, characterized by the presence of osteoblast-like cells actively secreting osteoid matrix. The secretory activity of osteoblasts plays a critical role in the formation of new bone and in the successful integration of the biomaterial within the host tissue. These findings confirmed that VPST treatment did not impair the osteoconductive properties of the DEBG graft, thereby supporting its compatibility with physiological bone regeneration processes. Furthermore, the simultaneous action of osteoblast-mediated matrix deposition and osteoclast-mediated resorption forms the foundation of physiological bone remodeling. This dynamic process is tightly regulated through complex cellular cross-talk between these two cell types.
Concerning the soft tissue, the histological analysis evidenced a physiological architecture with a slightly fibrotic connective component, consistent with post-surgical healing. These results align with those of Pellegrini et al. [19], who described the progression of soft tissue healing following bone grafting procedures in socket preservation techniques. The presence of slight fibrosis in the connective tissue can be interpreted as part of the normal remodelling phase, where fibroblasts contribute to extracellular matrix deposition and tissue maturation.
Clinically, no implant or prosthesis failures were recorded up to one year after implant placement, underscoring the clinical reliability of plasma-treated biomaterials. This is consistent with prior studies that reported improved implant stability and survival rates associated with plasma-activated surfaces [20,21]. The absence of biological or technical complications, except for the isolated titanium mesh breakage, further supports the clinical safety and effectiveness of the proposed treatment protocol [22]. Vacuum plasma interaction with biological objects can also have adverse effects that have to be considered [23]. A possible explanation for the titanium mesh breakage was that the titanium mesh was treated immediately after being tested on the bone defect. The presence of biological fluids may have caused hyperactivation of the surface.
To the best of authors’ knowledge, this is the first study to evaluate VPST on biomaterials. Despite these promising results, this study has some limitations. The small sample size and the nature of a clinical audit limit the generalizability of the findings. Additionally, the lack of a control group prevents a direct comparison between plasma-treated and non-treated biomaterials. Future studies should include larger, randomized controlled trials with long-term follow-up to validate these preliminary findings and establish robust clinical guidelines.
5. Conclusions
Considering the limited sample size, this preliminary case series demonstrates that VPST may enhance the biological compatibility of biomaterials used in SP and GBR procedures. These preliminary findings may suggest that plasma treatment could be safely integrated into clinical protocols to potentially improve oral rehabilitation outcomes. Further randomized controlled trials with sample size calculation are needed to confirm these preliminary results and to explore the long-term impact on implant survival and patient satisfaction.
Author Contributions
Conceptualization, M.T. (Marco Tallarico) and M.T. (Michele Troia); Methodology, M.T. (Marco Tallarico) and M.T. (Michele Troia); Validation, S.M.M.; Histological analyses, C.D.V. and D.H.; Data curation, F.M.C. and C.C.; Writing—original draft, M.T. (Marco Tallarico) and D.M.; Writing—review & editing, S.M.M. and M.P.; Supervision, C.C. and A.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. All graft materials were donated by Bioteck S.p.A, and Plasmapp donated the Activelink reborn machine. Plasmapp also supported the costs for the histological analyses.
Institutional Review Board Statement
This study was design as a phase IV case series study, with CE-approved materials and devices used as part of usual professional practice and with no adjunctive risks. All of the cases were performed in a private clinic in Rome, Italy, and the data were evaluated at the Department of Medicine, Surgery, and Pharmacy, University of Sassari, Italy. The study adhered to the principles of the 2013 Helsinki Declaration and Good Medical Practice principles. Medical data were anonymized so that patients could not be identified, and the ethics committee’s waiver for consent for a case series was obtained (Report number 41/1.6; released on 21 May 2025).
Informed Consent Statement
All patients provided dedicated informed consent.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Bioteck S.p.A (Vicenza, Italy) and Plasmapp Co., Ltd. (Daejeon, Republic of Korea) donated the implants and the biomaterials; however, the data belong to the authors and by no means did the companies interfere with the conduct of the trial or the publication of the results.
References
- Busenlechner, D.; Fürhauser, R.; Haas, R.; Watzek, G.; Mailath, G.; Pommer, B. Long-term implant success at the Academy for Oral Implantology: 8-year follow-up and risk factor analysis. J. Periodontal Implant. Sci. 2014, 44, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I. A tribute to Dr. Per-Ingvar Brånemark. J. Periodontal Implant. Sci. 2014, 44, 265. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 106–134. [Google Scholar] [CrossRef]
- Wong, M.; Eulenberger, J.; Schenk, R.; Hunziker, E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J. Biomed. Mater. Res. 1995, 29, 1567–1575. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 172–184. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Chinigò, G.; Iacono, R.; Pesce, P.; Menini, M.; Mussano, F. Vacuum Plasma Treatment Device for Enhancing Fibroblast Activity on Machined and Rough Titanium Surfaces. Dent. J. 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int. J. Oral Maxillofac. Implant. 2002, 17, 771–777. [Google Scholar]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Meloni, S.M.; Troia, M.; Cacciò, C.; Lumbau, A.I.; Gendviliene, I.; Ceruso, F.M.; Pisano, M. The Use of Vacuum Plasma Surface Treatment to Improve Hydrophilicity and Wettability of Biomaterials: An In-Vitro Study. Dent. J. 2025, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Pellegrini, G.; Canciani, E.; Heinemann, F.; Galliera, E.; Dellavia, C. Alveolar socket preservation technique: Effect of biomaterial on bone regenerative pattern. Ann. Anat. Anat. Anz. 2016, 206, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Alla, I.; Gehrke, S.A.; Carmine, M.D.; Tari, S.R.; Scarano, A. Effect of Different Dental Implant Prosthetic Joints on Marginal Bone Loss: Emerging Findings from a Bayesian Network Meta-Analysis (NMA) and Systematic Review. Prosthesis 2024, 6, 186–205. [Google Scholar] [CrossRef]
- Udeabor, S.E.; Heselich, A.; Al-Maawi, S.; Alqahtani, A.F.; Sader, R.; Ghanaati, S. Current Knowledge on the Healing of the Extraction Socket: A Narrative Review. Bioengineering 2023, 10, 1145. [Google Scholar] [CrossRef]
- Perrotti, V.; Nicholls, B.M.; Piattelli, A. Human osteoclast formation and activity on an equine spongy bone substitute. Clin. Oral Implant. Res. 2009, 20, 17–23. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Zaniol, T.; Cinci, L.; Pieri, L. Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study. Dent. J. 2019, 7, 70. [Google Scholar] [CrossRef]
- Le, P.T.M.; Shintani, S.A.; Takadama, H.; Ito, M.; Kakutani, T.; Kitagaki, H.; Terauchi, S.; Ueno, T.; Nakano, H.; Nakajima, Y.; et al. Bioactivation Treatment with Mixed Acid and Heat on Titanium Implants Fabricated by Selective Laser Melting Enhances Preosteoblast Cell Differentiation. Nanomaterials 2021, 11, 987. [Google Scholar] [CrossRef]
- Pellegrini, G.; Rasperini, G.; Obot, G.; Farronato, D.; Dellavia, C. Soft tissue healing in alveolar socket preservation technique: Histologic evaluations. Int. J. Periodontics Restor. Dent. 2014, 34, 531–539. [Google Scholar] [CrossRef]
- Kahm, S.H.; Lee, S.H.; Lim, Y.; Jeon, H.J.; Yun, K.I. Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo. J. Funct. Biomater. 2024, 15, 278. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Santori, G.; Giovanni, E.; Bagnasco, F.; Canullo, L. Photo and Plasma Activation of Dental Implant Titanium Surfaces. A Systematic Review with Meta-Analysis of Pre-Clinical Studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Ghizzoni, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Poli, P.P.; Maiorana, C.; Spadari, F. Full-Digital Customized Meshes in Guided Bone Regeneration Procedures: A Scoping Review. Prosthesis 2023, 5, 480–495. [Google Scholar] [CrossRef]
- Berger, M.B.; Bosh, K.B.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Benchtop plasma treatment of titanium surfaces enhances cell response. Dent. Mater. 2021, 37, 690–700. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).