Abstract
Graphitic carbon nitride g-C3N4 has been modified using platinum and platinum oxide (0.5–5 wt.%) and studied in photocatalytic H2 evolution reactions with ethanol aqueous solution under visible light irradiation (λ = 409 nm). An analysis of the by-products of the reaction (CO2, CH4, C2H6 etc.) was also carried out. The morphology, particle size distribution, and optical properties of the photocatalysts, and the chemical states of platinum cations were examined using various methods. The photocatalysts were investigated using a wide range of methods to clarify the morphology, particle size distribution, optical properties, and the chemical states of platinum cations. Factors affecting not only the activity, but also the selectivity of the photocatalyst in the target process of hydrogen production, have been established. The highest rate of H2 evolution achieved over 0.5 wt.% Pt/g-C3N4 photocatalyst is 0.6 mmol h−1 g−1 (selectivity 98.9%), which exceeds the activity of pristine g-C3N4 by 250 times. Increasing the Pt or PtO content up to 5 wt.% leads to an increase in the rate of formation of by-products (CH4, C2H6, and CO2) and a decrease in the selectivity of H2 evolution. The study also delves into the role of platinum and the mechanism of charge transfer in PtO/g-C3N4 and Pt/g-C3N4 photocatalysts due to light irradiation.
1. Introduction
The development of alternative energy sources is attracting considerable attention due to the growing demand for energy carriers, as well as the negative impact of greenhouse gases on the climate [1,2]. Hydrogen is one of the most promising energy carriers owing to its high calorific value, wide distribution, and absence of toxic products during combustion [3]. However, traditional methods for producing hydrogen are based on the use of fossil fuels, in particular natural gas [4]. The development of alternative methods of hydrogen production, using only renewable energy sources, will allow the transition to «green» hydrogen [5].
One of the most promising methods is the photocatalytic hydrogen evolution from aqueous solutions on semiconductor materials, since this technology allows one to convert the energy of sunlight into the energy of chemical bonds. Under the action of light irradiation with a certain wavelength, photogenerated charge carriers are formed in the semiconductor—an electron and a hole—and then participate in redox reactions on the surface of the semiconductor, which in this case is a photocatalyst [6,7,8,9]. The first semiconductor material used as a photocatalyst was titanium dioxide, and photocatalysts based on it are still widely used today, despite the wide band gap of TiO2 (3.0–3.2 eV). Significant progress has been made in the field of materials for photocatalytic hydrogen production. Among others, photocatalysts with high activity under the action of visible radiation have been developed: metal organic frameworks [10,11,12], metal sulfides [13,14,15,16,17,18], metal-doped perovskite [19,20], and (oxy)nitrides of Ti and Ta [21,22,23]. However, sulfides and nitrides tend to undergo photocorrosion and decrease in activity during long-term photocatalytic tests. At the same time, the synthesis of MOFs or the doping of perovskites is not always attractive for practical applications due to the complexity and cost of their synthesis.
A promising semiconductor material of interest to researchers is graphitic carbon nitride g-C3N4 due to its band structure, stability, and lack of toxicity [24,25]. The narrow band gap (2.7 eV) provides g-C3N4 with activity under visible light irradiation, which makes up a significant part of the solar spectrum [26,27,28]. Another advantage of g-C3N4 is the strongly negative position of the conduction band (−1.3 V vs. NHE), which provides the significant reduction potential of photogenerated electrons [29,30]. Thus, the band structure of g-C3N4 is favorable for the effective occurrence of reduction reactions under visible light. In addition, g-C3N4 can be produced in one step from available precursors such as melamine, urea, and other nitrogen-containing organic compounds.
However, pristine g-C3N4 has low activity in photocatalytic reactions due to the high rate of recombination of photogenerated electrons and holes, as well as poor adsorption of reagents on the photocatalyst surface [31]. The g-C3N4 performance in the photocatalytic H2 evolution reaction can be improved in several ways. The change of morphology, leading to the breakage of interlayer hydrogen bonds, allows the lifetime of photogenerated charge carriers [32] to increase. The increased surface area allows more reactant to adsorb, which also increases the reaction rate [33]. The coating of the semiconductor surface with g-C3N4 quantum dots allows the shortening of the distance for electrons and holes to migrate to the surface, reducing the rate of charge recombination [34].
A widely used method for modifying g-C3N4 is the deposition of particles of metals or their compounds on the surface of the photocatalyst. Due to the spatial separation of charges occurring at the interface between a semiconductor and a metal, or between two different semiconductors, the lifetime of photogenerated electrons and holes increases significantly [35,36]. Platinum and its compounds are most often used as such cocatalysts in photocatalytic hydrogen evolution reactions [37,38,39,40,41,42,43]. It is known that platinum in the metallic state is the most active cocatalyst due to the high Schottky barrier, formed in this case at the metal–semiconductor interface [44]. This phenomenon allows platinum particles to act as effective traps of electrons, significantly increasing their lifetime and, consequently, the rate of hydrogen production. However, in the case of H2 evolution from solutions of organic compounds, for example, alcohols, it is important to monitor not only the rate of H2 formation, but also the formation rate of oxidation products of organic substrates in the gas phase.
Nevertheless, the selectivity of the H2 evolution process from aqueous solutions of organic substances is rarely studied [45], and the effect of the state of platinum on the activity and selectivity of the process has not been studied at all. At the same time, the influence of the oxidation state of platinum on the rate of photocatalytic destruction of dyes, as well as the oxidation of methanol, phenol and formic acid, has been shown in the literature, which confirms the significant influence of the state of platinum on photocatalytic processes [46,47].
In the current work, we have presented for the first time the effect of the platinum valence state on the activity and selectivity of g-C3N4-based photocatalysts in H2’s evolution from ethanol aqueous solution under light irradiation (409 nm), and, therefore, reveal the key factors that determine the activity and selectivity of photocatalysts based on g-C3N4 modified with Pt and PtOx. The novelty of the study is provided by the establishment of correlations between the platinum oxidation state and the selectivity of the target process—hydrogen evolution from aqueous ethanol solution. The possibility of controlling the selectivity for H2 evolution over g-C3N4-based photocatalysts by changing the platinum state has been shown for the first time.
2. Materials and Methods
2.1. Photocatalyst Synthesis
Melamine was chosen as a precursor for the synthesis of g-C3N4. A sample of melamine (Sigma-Aldrich, St. Louis, MO, USA, 99%) was placed in a crucible and then in an oven at 500 °C for 2 h with a heating rate of 10° per minute. The obtained yellow powder was ground in a mortar and modified via a platinum or platinum oxide deposition method.
For the deposition of metallic platinum, the method of reduction of platinum ions with sodium borohydride was used. The required amount of 0.02 M aqueous solution of H2PtCl6 (Reakhim, Moscow, Russia, 98%) was added to a suspension of g-C3N4 (300 mg) and stirred for 1 h; then, freshly prepared 0.1 M solution of NaBH4 (Acros Organics, Geel, Belgium, 99%) was added and the resulting suspension was stirred for 1 h. The Pt/TiO2 photocatalyst thus obtained was washed several times with distilled water and dried in air at 60 °C for 5 h.
For PtO deposition, 300 mg of g-C3N4 was added to 20% ethanol aqueous solution in a photocatalytic reactor. Then, the reactor with the obtained suspension was purged with argon to remove oxygen for 30 min, and kept under a 380-nm LED (30 V, 1 A) for 3 h. The obtained powder was washed several times with distilled water and dried in air at 60 °C for 5 h.
The amount of deposited Pt or PtO on g-C3N4 was 0.5, 2, and 5 wt.%.
2.2. Photocatalyst Characterization
The photocatalysts were characterized via X-ray diffraction (XRD), UV-vis spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy, and high-resolution transmission electron microscopy (HR TEM), including the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) technique.
The chemical state of cations and surface composition of the catalyst were studied via XPS using a photoelectron spectrometer (SPECS Surface Nano Analysis GmbH, Berlin, Germany) using non-monochromatized Al Kα radiation (hυ = 1486.6 eV). The spectrometer was equipped with a PHOIBOS-150 hemispherical analyzer and an XR-50 X-ray source with a double Al/Mg anode. The calibration of the binding energy scale was performed by setting the C 1s peak at 288.1 eV, which corresponded to carbon in the g-C3N4.
The chemical state of platinum in the bulk of the catalysts was studied via X-ray absorption spectroscopy at the EXAFS spectroscopy beamline of Siberian Synchrotron and Terahertz Radiation Center (SSTRC, Budker Institute of Nuclear Physics, Novosibirsk, Russia). The X-ray absorption near-edge structure (XANES) spectra of Pt L3-edge were recorded via transmission geometry. Energy calibration was performed using the first inflection point in the Pt L3-edge spectrum of Pt foil at 11,564 eV. The spectra were processed using standard procedures for subtracting the background and normalizing to the magnitude of the L3-absorption edge jump using the DEMETER 0.9.25 software package [48].
The morphology of the photocatalysts was studied via HR TEM using a ThemisZ electron microscope (Thermo Fisher Scientific, Waltham, MA, USA) at an accelerating voltage of 200 kV. The microscope was equipped with a SuperX energy-dispersive spectrometer and a spherical aberration corrector. The maximum resolution of the microscope was 0.06 nm. For the HR TEM analysis, the samples were ultrasonically dispersed onto perforated carbon substrates attached to aluminum grids.
The diffuse reflectance UV-vis spectra were measured using a UV-2501 PC spectrophotometer with an ISR-240A diffuse reflectance unit (Shimadzu, Kyoto, Japan).
The X-ray diffraction pattern was recorded using a D8 ADVANCE diffractometer equipped with a LYNXEYE linear detector (Bruker AXS GmbH, Karlsruhe, Germany). The diffraction patterns were obtained at room temperature in the 2θ range from 10 to 70° with a step of 0.05° using Ni-filtered Cu Kα radiation (λ = 1.5418 Å).
2.3. Photocatalytic Activity Test
The activity of photocatalysts was tested in H2 evolution reactions in a batch reactor using ethanol as a substrate (Figure 1a). The reactor with an aqueous suspension (V = 50 mL) containing 20% ethanol aqueous solution and 30 mg of photocatalyst was ultrasonicated for 10 min and then purged with argon for 30 min to remove oxygen. After that, the suspension was irradiated with an LED with a maximum intensity at a wavelength of 409 nm (power density 70 mW·cm–2) through a quartz window (S = 21 cm2). The emission spectrum of the diode is shown in Figure 1b.
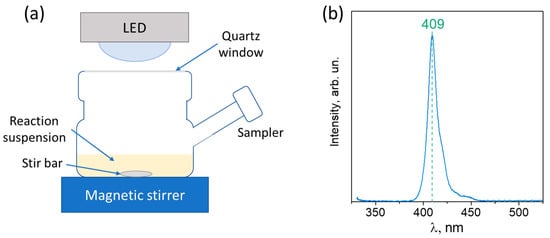
Figure 1.
(a) Scheme of reactor for photocatalytic study; (b) spectrum of LED with the wavelength at maximum intensity.
The gas probe was analyzed with a gas chromatograph “GH-1000” (Chromos, Moscow, Russia) equipped with a flame ionization detector and thermal conductivity detector to identify the products of C1-C2 and H2, respectively. Argon was used as a carrier gas. The hydrogen formation rate was determined using a linear fitting of the dependence of the hydrogen amount on time (a 60 min interval was used).
The selectivity of H2 evolution was defined as
where n is amount of compound, μmol.
3. Results and Discussion
In this work, a series of Pt/g-C3N4 and PtO/g-C3N4 photocatalysts with different mass contents of cocatalyst (Pt, PtO) were synthesized. The structure of g-C3N4 obtained from melamine was examined via XRD. Due to the low content (0.5%) and small particle size, the state of the metal platinum and platinum oxide cocatalysts was investigated using XPS and XANES methods. The activity of photocatalysts was studied in the reaction of H2 production from an aqueous solution of ethanol under visible light irradiation (409 nm). In addition, an analysis of reaction by-products (CO, CO2, CH4, C2H4, C2H6) in the gas phase was carried out to estimate the selectivity of the target process of H2 evolution.
3.1. Photocatalyst Characterization
The synthesized g-C3N4 was characterized via X-ray diffraction to confirm the structure of the obtained material (Figure 2a). One can see in the diffraction pattern of g-C3N4 two main peaks at ≈12.8° and ≈27.7°, corresponding to intraplanar and interplanar distances of g-C3N4, respectively. Based on the presence of additional peaks at ≈17.7° and ≈21.7°, the structure was indexed in the orthorhombic P21212 cell. Interplanar distances d002 = 3.23 Å and d210 = 6.93 Å are in accordance with the literature data [49,50]. The 5% Pt/g-C3N4 and 5% PtO/g-C3N4 photocatalysts were also characterized via XRD (Figure S1). The formation of platinum metal particles was confirmed for the photocatalyst prepared via the chemical reduction method. No peaks related to platinum or its oxide are observed in the XRD of the photocatalyst 5% PtO/g-C3N4, confirming the partial reduction of platinum ions. It is known that platinum oxide is generally poorly crystallized and hardly seen via XRD [51,52].
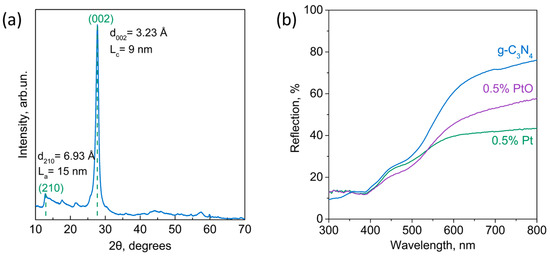
Figure 2.
(a) XRD pattern of synthesized g-C3N4, La—average crystallite size in the plane of the layer calculated using the Scherrer equation; Lc—average crystallite size in the direction perpendicular to the layer calculated using the Scherrer equation; (b) diffuse reflectance spectra of g-C3N4, 0.5% Pt/g-C3N4, and 0.5% PtO/g-C3N4.
The diffuse reflectance spectra are presented in Figure 2b. One can see that the platinum deposition leads to an increase in light absorption beyond the 450 nm region. The increase in light absorbance is due to the surface plasmon resonance of Pt nanoparticles. The shift in the absorption edge leads to a broadening of the action spectrum of the photocatalyst to the visible light. The obtained band gap value of g-C3N4 (2.7 eV) agrees well with the literature data (Figure S2).
To confirm the formation of metallic platinum or PtO nanoparticles, we applied a combination of surface- and volume-sensitive methods—XPS and XANES spectroscopy. The Pt 4f core-level spectra of 0.5% Pt/g-C3N4, and 0.5% PtO/g-C3N4 are presented at Figure 3a. One can see that the Pt4f core-level spectrum of 0.5% Pt/g-C3N4 could be well fitted by one Pt 4f7/2-Pt 4f5/2 doublet with a Pt 4f7/2-binding energy of 70.7 eV. In the case of 0.5% PtO/g-C3N4, the Pt4f core-level spectrum of 0.5% Pt/g-C3N4 is fitted by one Pt 4f7/2-Pt 4f5/2 doublet with a Pt 4f7/2-binding energy of 72.9 eV. According to the literature data, the peak at 70.7 eV could be attributed to the platinum in the metallic state, and the peak at the higher binding energy (72.9 eV) to the platinum in the oxidized state, Pt2+ in PtO [40,53,54,55]. The surface atomic ratios [Pt]/[C3N4] for 0.5% Pt/g-C3N4 and 0.5% PtO/g-C3N4 are equal to 0.002 and 0.005 (Table 1). The observed difference in the surface ratios could be due to the formation of larger metallic platinum nanoparticles more than the deposition of PtO nanoparticles, which will be further confirmed via TEM.
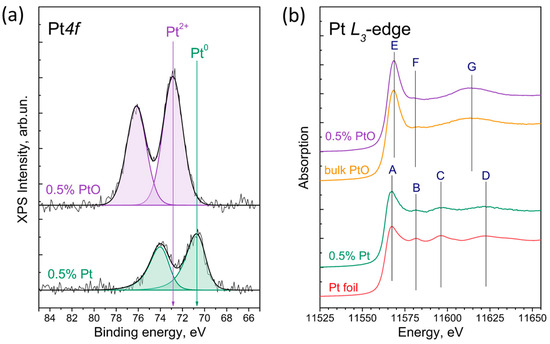
Figure 3.
Pt 4f core-level spectra (a) and XANES Pt L3-edge spectra (b) of 0.5%Pt/g-C3N4, as well as XANES Pt L3-edge spectra of references (Pt foil and PtO). The Pt 4f core-level spectrum is normalized to the integral intensity of the C1s core-level peak, which corresponded to the spectrum of g-C3N4.

Table 1.
Results of XPS study of the cocatalyst in 0.5% Pt/g-C3N4 and 0.5% PtO/g-C3N4.
As mentioned above, an X-ray absorption spectroscopy allowed us to clarify the volume chemical state of the platinum nanoparticles deposited on g-C3N4. The XANES spectra of the Pt L3-edges of the 0.5%Pt/g-C3N4 and 0.5% PtO/g-C3N4 are presented in Figure 3b. It can be seen that the XANES spectra of the Pt L3-edge of 0.5%Pt/g-C3N4 differs from that of 0.5% PtO/g-C3N4, but has features (peaks A-D) similar to those of platinum in the metallic state (Pt foil). Thus, the XPS and XAS studies establish that in the case of 0.5%Pt/g-C3N4, the platinum nanoparticles are metallic and do not have a core–shell structure. At the same time, the spectrum of the Pt L3-edge of 0.5% PtO/g-C3N4 is similar to that of platinum oxide PtO; both have the peaks E, F, and G. In the case of 0.5% PtO/g-C3N4, the XPS and XAS confirm that the platinum nanoparticles are completely oxidized and also do not have a core-level structure.
A study of the optical properties of photocatalysts showed that the deposition of both Pt and PtO leads to an increase in absorption in the visible region, which contributes to an increase in the rate of photocatalytic reactions under the visible light irradiation (λ > 380 nm). It should be noted that after the modification of g-C3N4 with cocatalysts, there is no shift in the absorption edge of the samples obtained, since the structure of g-C3N4 does not change after the deposition of Pt or PtO (Figure 2b).
The morphology of the photocatalysts was studied via TEM (Figure 4). In the case of the PtO/g-C3N4 photocatalyst, small single cocatalyst particles of size 1–2 nm are evenly distributed over the g-C3N4 surface (Figure 4a,c). When platinum is deposited in the metallic state, platinum agglomerates of about 10 nm in size, consisting of smaller particles, can be observed (Figure 4b,d). This observation agrees well with the results of the XPS study of different surface [Pt]/[C3N4] ratios.
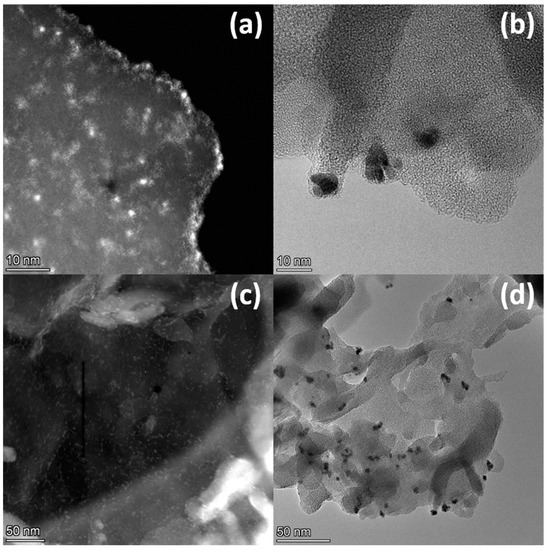
Figure 4.
(a,c) HAADF STEM images of 0.5% PtOx/g-C3N4 and (b,d) HR TEM images of 0.5% Pt/g-C3N4.
Thus, the methods used allow us to clarify the key features of the synthesized photocatalysts, which makes it possible to find the correlation between structure and photocatalytic properties. In summary, it can be concluded that photoreduction of Pt+4 under UV light results in a uniform deposition of fine platinum oxide particles onto the g-C3N4 surface. When platinum is reduced with a solution of NaBH4, Pt+4 is completely reduced to the metallic state; however, the cocatalyst particles in this case are distributed unevenly, and form aggregates on the surface of the g-C3N4.
3.2. Photocatalytic Activity
The activity of the synthesized photocatalysts was studied in the reaction of H2’s evolution from ethanol aqueous solutions under visible light irradiation. For all the photocatalysts tested, the main reaction product is H2. The highest rate of H2 evolution was achieved over the 0.5% Pt/g-C3N4 catalyst, and reached 0.6 mmol h–1 gcat–1, which exceeds the activity of unmodified g-C3N4 by 250 times. Among the photocatalysts containing platinum in the oxidized state, 0.5% PtO/g-C3N4 showed the maximum activity in the H2 evolution reaction, with rate of H2 evolution of 0.36 mmol h–1 gcat–1 (Figure 5a,b). It should be noted that an increase in the content of the cocatalyst in both the case of metallic and oxidized platinum does not lead to an increase in the rate of H2 formation. This effect may be caused by the weak adsorption of ethanol on the surface of g-C3N4, which limits the rate of hydrogen evolution in the case of composite photocatalysts.
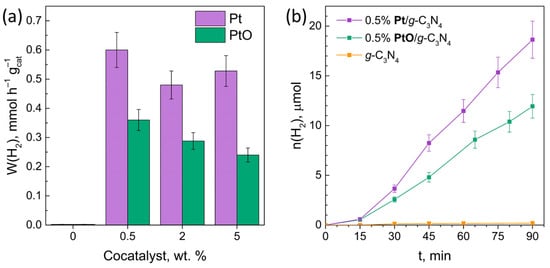
Figure 5.
(a) H2 evolution rate; (b) kinetic curves of H2 evolution. Conditions: C(cat.) = 0.5 g L–1; C0(EtOH) = 20%, V = 50 mL, t(reaction) = 90 min, λmax = 409 nm.
The higher activity of the platinum cocatalyst compared to platinum oxide is caused by differences in the energy structure of these compounds. Both during the deposition of platinum and platinum oxide on the surface of g-C3N4, heterojunctions arise at the interface between the two phases; a photogenerated electron migrates from the g-C3N4 surface to the cocatalyst particle [56,57]. However, charge transfer mechanisms are varied for different states of platinum [58].
While using metallic platinum as a cocatalyst, an electron from the conduction band of g-C3N4 falls down to the Fermi level of the metal, overcoming the Schottky barrier at the Pt—g-C3N4 contact region [59,60]. Meanwhile, the photogenerated holes remain in the valence band of g-C3N4; thus, charge carriers—electrons and holes—are spatially separated. As it has one of the highest electron work functions (5.1–5.9 eV), platinum acts as a highly efficient trap for photogenerated electrons, which allows them to significantly increase their lifetime, and, consequently, greatly contributes to increasing the rate of hydrogen evolution [61].
Platinum oxide is a semiconductor with a narrow band gap and a conduction band (CB) position of +1.3 eV, which also allows for heterojunctions of electrons from the CB of g-C3N4 to the CB of PtO [62] (Figure 6). However, in semiconductors with a narrow band gap, electrons and holes can readily recombine, leading to a decrease in photocatalytic activity [58]. Therefore, charge separation in PtO/g-C3N4 photocatalysts is not as efficient as in Pt/g-C3N4; thus, the lifetime of electrons and H2 evolution rate are decreased.
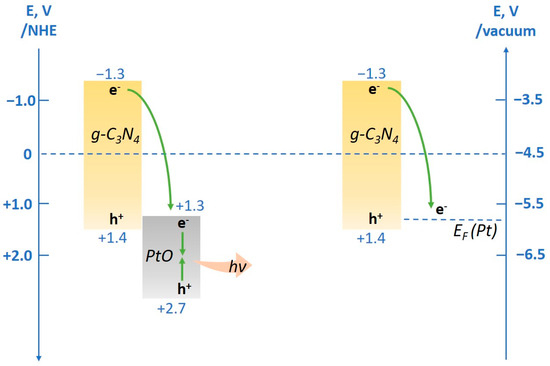
Figure 6.
Scheme of charge transfer mechanisms in PtO/g-C3N4 and Pt/g-C3N4 photocatalysts.
In addition to H2, C1-C2 products were detected in the gas phase: carbon oxides, methane, ethene, and ethane. It is worth noting that the deposition of a cocatalyst—both platinum and its oxide—significantly accelerates the rate of H2 formation, but not its byproducts, hence allowing a great increase in the selectivity of the target process (Table 2).

Table 2.
Reaction products in the gas phase after 90 minutes of the light irradiation.
Moreover, an increase in the load of the cocatalyst reduces the proportion of carbon oxides and increases the relative content of hydrocarbons (Figure 7a,b). However, in the case of photocatalysts of the PtO/g-C3N4 series, the concentration of hydrocarbons increases with the concentration of CO2, which leads to a significant decrease in the selectivity of the photocatalytic H2 production process. For both series of photocatalysts, the amount of ethane released after 90 min increases with cocatalyst loading. At the same time, the amount of ethylene is generally reduced compared to unmodified g-C3N4.
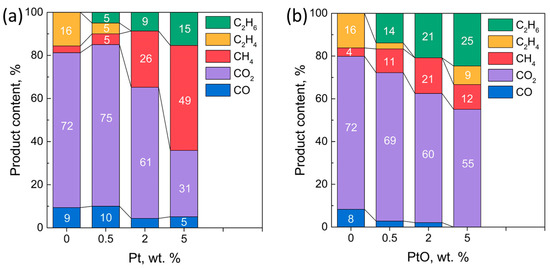
Figure 7.
Distribution of products in the gas phase without H2 over Pt/g-C3N4 (a) and PtO/g-C3N4 (b). Conditions: C(cat.) = 0.5 g L–1; C0(EtOH) = 20%, V = 50 mL, t(reaction) = 90 min, λmax = 409 nm.
During the photocatalytic process, at the first stage, ethanol is oxidized on the photocatalyst surface to acetaldehyde (Equation (2)), which then, under the action of light, decomposes into methane and CO (Equation (3)) [63,64].
C2H5OH + 2h+ = CH3CHO + 2H+
CH3CHO = CH4 + CO
Simultaneously, on the surface of the photocatalyst, the H+ reduction process occurs with the formation of H2.
Dehydration of ethanol leads to the formation of a small amount of ethylene (Equation (4)), which is then hydrogenated to form ethane (Equation (5)):
C2H5OH = C2H4 + H2O
C2H4 + H2 = C2H6
It should be noted that the rate of hydrogen formation on composite photocatalysts significantly exceeds the rate of formation of hydrocarbons. Thus, it can be concluded that the decomposition of acetaldehyde and the dehydration of water occur to a small extent.
The rate of H2 evolution as well as of selectivity in the presence of the photocatalysts considered in this work, compared to some platinized ones reported in the literature, are shown in Table 3.

Table 3.
Comparison of the activity and selectivity of the synthesized photocatalysts with literature data.
The activity of the photocatalysts synthesized in this work does not exceed the values reported in the literature for CdS and TiO2-based photocatalysts; however, it is at the level of Nb2O5 and WO3-based photocatalysts. It is also worth noting that the selectivity of the hydrogen evolution process exceeds the value presented in the literature for platinized TiO2.
4. Conclusions
In this work, photocatalysts based on g-C3N4 modified with Pt and PtO were synthesized and studied in H2 evolution from ethanol solution under visible light irradiation. Characterization of the photocatalysts using a set of research methods showed that photodeposition from H2PtCl6 solution leads to a uniform distribution of platinum oxide particles over the surface of g-C3N4. The use of NaBH4 solutions for reduction makes it possible to deposit particles of metal platinum on the surface of g-C3N4, which, despite their uneven distribution, lead to a higher rate of H2 formation than platinum oxide. An increase in the metallic platinum content leads to a gradual decrease in carbon dioxide and a significant increase in methane in the gas phase. As the PtO loading increases, the amount of carbon dioxide grows, and a large amount of ethane is produced, which on the whole results in a decrease in the relative carbon dioxide content as the platinum oxide content increases. Thus, both the state of platinum and its content affect not only the rate of hydrogen formation, but also the rate of the formation of the reaction by-products, which confirms the need to control the selectivity of the hydrogen formation process from organic electron donors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app132111739/s1, Figure S1: XRD patterns of (a) 5% Pt/g-C3N4 and (b) 5% PtO/g-C3N4; Figure S2: Tauc plot of unmodified g-C3N4.
Author Contributions
Investigation, data curation, visualization, writing—original draft preparation, A.Y.K.; investigation, data curation, visualization, writing—review and editing, funding acquisition, project administration, A.A.S.; investigation, data curation, D.D.M.; investigation, data curation, E.Y.G.; writing—review and editing, supervision, conceptualization, E.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2022-263).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The TEM study was carried out using the facilities of the shared research center “National center of investigation of catalysts” at the Boreskov Institute of Catalysis. The investigations were performed using the large-scale research facility “EXAFS spectroscopy beamline” at the Siberian Synchrotron and Terahertz Radiation Center. The authors thank Angelina Zhurenok for UV-vis spectra measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Huang, X.M.; Chen, N.; Ye, D.N.; Zhong, A.G.; Liu, H.; Li, Z.; Liu, S.Y. Structurally Complementary Star-Shaped Unfused Ring Electron Acceptors with Simultaneously Enhanced Device Parameters for Ternary Organic Solar Cells. Sol. RRL 2023, 7, 2300143. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Blanco, H.; Leaver, J.; Dodds, P.E.; Dickinson, R.; García-Gusano, D.; Iribarren, D.; Lind, A.; Wang, C.; Danebergs, J.; Baumann, M. A taxonomy of models for investigating hydrogen energy systems. Renew. Sustain. Energy Rev. 2022, 167, 112698. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef]
- Li, T.; Tsubaki, N.; Jin, Z. S-scheme heterojunction in photocatalytic hydrogen production. J. Mater. Sci. Technol. 2023, 169, 82–104. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. A new breakthrough in photocatalytic hydrogen evolution by amorphous and chalcogenide enriched cocatalysts. Chem. Eng. J. 2023, 455, 140601. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- El Ouardi, M.; El Aouni, A.; Ait Ahsaine, H.; Zbair, M.; BaQais, A.; Saadi, M. ZIF-8 metal organic framework composites as hydrogen evolution reaction photocatalyst: A review of the current state. Chemosphere 2022, 308, 136483. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti(IV) metal-organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Liu, R. Defect engineering of zeolite imidazole framework derived ZnS nanosheets towards enhanced visible light driven photocatalytic hydrogen production. Appl. Catal. B Environ. 2020, 278, 119265. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Z.; Zhang, Z.; Ao, D. Novel visible-light driven Mn0.8Cd0.2S/g-C3N4 composites: Preparation and efficient photocatalytic hydrogen production from water without noble metals. Appl. Catal. A Gen. 2016, 518, 150–157. [Google Scholar] [CrossRef]
- Jiang, X.; Gong, H.; Liu, Q.; Song, M.; Huang, C. In situ construction of NiSe/Mn0.5Cd0.5S composites for enhanced photocatalytic hydrogen production under visible light. Appl. Catal. B Environ. 2020, 268, 118439. [Google Scholar] [CrossRef]
- Li, C.Q.; Du, X.; Jiang, S.; Liu, Y.; Niu, Z.L.; Liu, Z.Y.; Yi, S.S.; Yue, X.Z. Constructing Direct Z-Scheme Heterostructure by Enwrapping ZnIn2S4 on CdS Hollow Cube for Efficient Photocatalytic H2 Generation. Adv. Sci. 2022, 9, 2201773. [Google Scholar] [CrossRef]
- Zhang, P.; Luan, D.; Lou, X.W. Fabrication of CdS Frame-in-Cage Particles for Efficient Photocatalytic Hydrogen Generation under Visible-Light Irradiation. Adv. Mater. 2020, 32, 2004561. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Sun, Y.; Dong, F.; Sheng, J. Metal-doping of halide perovskite nanocrystals for energy and environmental photocatalysis: Challenges and prospects. J. Mater. Chem. A 2022, 10, 22915–22928. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, H.G.; Lee, J.S.; Kim, J.; Li, W.; Oh, S.H. Photocatalytic hydrogen production from water over M-doped La2Ti2O7 (M = Cr, Fe) under visible light irradiation (λ > 420 nm). J. Phys. Chem. B 2005, 109, 2093–2102. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, J.; Vequizo, J.J.M.; Hisatomi, T.; Ma, Y.; Nakabayashi, M.; Takata, T.; Yamakata, A.; Shibata, N.; Domen, K. Overall Water Splitting by a SrTaO2N-Based Photocatalyst Decorated with an Ir-Promoted Ru-Based Cocatalyst. J. Am. Chem. Soc. 2023, 145, 3839–3843. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Xu, X. Ba-Modified LaTiO2N as an Efficient Visible Light Active Photocatalyst for Water Oxidation. ACS Sustain. Chem. Eng. 2020, 8, 9641–9649. [Google Scholar] [CrossRef]
- Kasahara, A.; Nukumizu, K.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. LaTiO2N as a visible-light (≤600 nm)-driven photocatalyst (2). J. Phys. Chem. B 2003, 107, 791–797. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B Environ. 2019, 257, 117855. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Akhundi, A.; Badiei, A.; Ziarani, G.M.; Habibi-Yangjeh, A.; Muñoz-Batista, M.J.; Luque, R. Graphitic carbon nitride-based photocatalysts: Toward efficient organic transformation for value-added chemicals production. Mol. Catal. 2020, 488, 110902. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Durmus, Z.; Maijenburg, A.W. A review on graphitic carbon nitride (g-C3N4)—Metal organic framework (MOF) heterostructured photocatalyst materials for photo(electro)chemical hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 36784–36813. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.; Huang, Y.; Lv, K.; Fang, S.; Wu, X.; Li, Q.; Fan, J. Sharply increasing the visible photoreactivity of g-C3N4 by breaking the intralayered hydrogen bonds. Appl. Surf. Sci. 2020, 505, 144654. [Google Scholar] [CrossRef]
- Ma, Y.; Huangfu, C.; Guo, S.; Wu, S.; Wang, Z.; Yao, L.; Huang, X.; Liu, Y.; Zhao, W. Enhanced photocatalytic nitrogen fixation on oxygen doped high specific surface area g-C3N4 under simulated sunlight. J. Photochem. Photobiol. A Chem. 2022, 433, 114208. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, X.; Wang, S.; Yan, W. Hybridization of g-C3N4 quantum dots with 1D branched TiO2 fiber for efficient visible light-driven photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 13994–14005. [Google Scholar] [CrossRef]
- Mishra, K.; Devi, N.; Siwal, S.; Gupta, V.K.; Thakur, V.K.; Mishra, K.; Siwal, S.S. Hybrid Semiconductor Photocatalyst Nanomaterials for Energy and Environmental Applications: Fundamentals, Designing, and Prospects. Adv. Sustain. Syst. 2023, 7, 2300095. [Google Scholar] [CrossRef]
- Di Liberto, G.; Cipriano, L.A.; Tosoni, S.; Pacchioni, G. Rational Design of Semiconductor Heterojunctions for Photocatalysis. Chem. A Eur. J. 2021, 27, 13306–13317. [Google Scholar] [CrossRef]
- Liang, S.; Xia, Y.; Zhu, S.; Zheng, S.; He, Y.; Bi, J.; Liu, M.; Wu, L. Au and Pt co-loaded g-C3N4 nanosheets for enhanced photocatalytic hydrogen production under visible light irradiation. Appl. Surf. Sci. 2015, 358, 304–312. [Google Scholar] [CrossRef]
- Tong, T.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Mechanistic insight into the enhanced photocatalytic activity of single-atom Pt, Pd or Au-embedded g-C3N4. Appl. Surf. Sci. 2018, 433, 1175–1183. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Tkachev, S.; Tkachenko, P.; Berdyugin, S.; Popovetskiy, P.; Gerasimov, E.; Zhurenok, A.; Kozlova, E. Platinum(IV) Carbonato Complexes: Formation via the Addition of CO2 to the [Pt(OH)6]2− Anion and Generation of Platinum(IV) Oxide Nanoparticles for the Preparation of Catalysts. Inorg. Chem. 2023, 62, 9732–9748. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Zhurenok, A.; Saraev, A.; Gerasimov, E.; Cherepanova, S.; Tkachev, S.; Plusnin, P.; Kozlova, E. Highly efficient hydrogen production under visible light over g-C3N4-based photocatalysts with low platinum content. Chem. Eng. J. 2022, 445, 136721. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, T.; Xu, T.; Li, Y.; Wang, C. Size effect of Pt co-catalyst on photocatalytic efficiency of g-C3N4 for hydrogen evolution. Appl. Surf. Sci. 2019, 464, 36–42. [Google Scholar] [CrossRef]
- Liu, M.; Xia, P.; Zhang, L.; Cheng, B.; Yu, J. Enhanced Photocatalytic H2-Production Activity of g-C3N4 Nanosheets via Optimal Photodeposition of Pt as Cocatalyst. ACS Sustain. Chem. Eng. 2018, 6, 10472–10480. [Google Scholar] [CrossRef]
- Zhang, L.; Long, R.; Zhang, Y.; Duan, D.; Xiong, Y.; Zhang, Y.; Bi, Y. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. Int. Ed. 2020, 59, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.C.; Homs, N.; Ramírez de la Piscina, P. Photocatalytic H2 production from ethanol(aq) solutions: The effect of intermediate products. Int. J. Hydrogen Energy 2016, 41, 19629–19636. [Google Scholar] [CrossRef]
- Gomes, L.E.; Nogueira, A.C.; da Silva, M.F.; Plaça, L.F.; Maia, L.J.Q.; Gonçalves, R.V.; Ullah, S.; Khan, S.; Wender, H. Enhanced photocatalytic activity of BiVO4/Pt/PtOx photocatalyst: The role of Pt oxidation state. Appl. Surf. Sci. 2021, 567, 150773. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Mädler, L.; Amal, R. Inter-relationship between Pt oxidation states on TiO2 and the photocatalytic mineralisation of organic matters. J. Catal. 2007, 251, 271–280. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Alwin, E.; Nowicki, W.; Wojcieszak, R.; Zieliński, M.; Pietrowski, M. Elucidating the structure of the graphitic carbon nitride nanomaterials via X-ray photoelectron spectroscopy and X-ray powder diffraction techniques. Dalt. Trans. 2020, 49, 12805–12813. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Tkachenko, P.; Volchek, V.; Kurenkova, A.; Gerasimov, E.; Popovetskiy, P.; Asanov, I.; Yushina, I.; Kozlova, E.; Vasilchenko, D. Photocatalytic H2 generation from ethanol and glucose aqueous solutions by PtOx/TiO2 composites. Int. J. Hydrogen Energy 2023, 48, 22366–22378. [Google Scholar] [CrossRef]
- Němec, T.; Šonský, J.; Gruber, J.; de Prado, E.; Kupčík, J.; Klementová, M. Platinum and platinum oxide nanoparticles generated by unipolar spark discharge. J. Aerosol Sci. 2020, 141, 105502. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Lisowski, W.; Jarek, M.; Nowaczyk, G.; Zielińska-Jurek, A.; Zaleska, A. Visible light photoactivity of TiO2 loaded with monometallic (Au or Pt) and bimetallic (Au/Pt) nanoparticles. Appl. Surf. Sci. 2014, 317, 1131–1142. [Google Scholar] [CrossRef]
- Bernsmeier, D.; Sachse, R.; Bernicke, M.; Schmack, R.; Kettemann, F.; Polte, J.; Kraehnert, R. Outstanding hydrogen evolution performance of supported Pt nanoparticles: Incorporation of preformed colloids into mesoporous carbon films. J. Catal. 2019, 369, 181–189. [Google Scholar] [CrossRef]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Photocatalytic Reactivity of Surface Platinized TiO2: Substrate Specificity and the Effect of Pt Oxidation State. J. Phys. Chem. B 2005, 109, 7399–7406. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Irfan, F.; Tanveer, M.U.; Moiz, M.A.; Husain, S.W.; Ramzan, M. TiO2 as an effective photocatalyst mechanisms, applications, and dopants: A review. Eur. Phys. J. B 2022, 95, 184. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Parmon, V.N. Heterogeneous semiconductor photocatalysts for hydrogen production from aqueous solutions of electron donors. Russ. Chem. Rev. 2017, 86, 870–906. [Google Scholar] [CrossRef]
- Alanazi, H.M.; AlHaddad, M.; Shawky, A.; Mohamed, R.M. Platinum oxide-supported sol-gel prepared CeO2 nanocubes for promoted photodestruction of atrazine under visible light irradiation. Catal. Commun. 2023, 177, 106646. [Google Scholar] [CrossRef]
- Halasi, G.; Ugrai, I.; Solymosi, F. Photocatalytic decomposition of ethanol on TiO2 modified by N and promoted by metals. J. Catal. 2011, 281, 309–317. [Google Scholar] [CrossRef]
- Muggli, D.S.; McCue, J.T.; Falconer, J.L. Mechanism of the Photocatalytic Oxidation of Ethanol on TiO2. J. Catal. 1998, 173, 470–483. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Zheng, W.; Xu, H.; Hou, S.; Zheng, L.; Zheng, H.; Mao, L.; Shi, X. Atomically Dispersed Pt on CdS Nanosheets for Photocatalytic Evolution of H2 and 1,1-Diethoxyethane from Ethanol. ACS Appl. Nano Mater. 2023, 6, 17161–17170. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Sarkar, M.; Patra, A.; Kailasam, K. An insight into the photo-generation of H2 and a carbon fuel additive from biomass-derived ethanol: Boosting the bio-chemical economy. J. Mater. Chem. A 2022, 10, 22289–22300. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Malik, M.A.; Narasimharao, K. Pt-Ag/Ag3PO4-WO3 nanocomposites for photocatalytic H2 production from bioethanol. Fuel 2023, 344, 127998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).