Abstract
Pentacyclic triterpenoids are one of the main functional components in Dracocephalum heterophyllum. In this study the optimal process, the fairly simple and accessible extraction and purification of triterpenoids of D. heterophyllum, was developed by a remaceration method. Remaceration is characterized by minimal loss of biologically active compounds on diffusion, which contributes to the largest depletion of raw materials. The triterpenoid yield was 2.4% under optimal conditions which was enhanced to 98.03% after purification. The triterpenoid profiles and their anticancer and antidiabetic activities were further analyzed. GC-MS analysis of triterpenoidal extract of D. heterophyllum resulted ursolic acid (71.9%) and oleanolic acid (18.1%) as the major components. Additionally, total purified triterpenoid contents of D. heterophyllum and its main components were shown to possess significant cytotoxic activity against three human breast cancer cell lines (SK-Br-3, T47D, and MCF-7). The purification of triterpenoids influenced their biological activity. The antidiabetic effect, as measured by inhibition of protein-tyrosine phosphatase (PTP-1B), of the purified fraction of triterpenoids of D. heterophyllum increased by five-fold against the enzyme. The results provide important guidance for the industrial application of D. heterophyllum confirming the prospect of developing plant extracts into effective drugs and health foods for human applications.
1. Introduction
Triterpenoids are compounds with a C30 carbon skeleton made up of six isoprene units and derived biosynthetically from the acyclic hydrocarbon, squalene [1]. The pentacyclic triterpenes can be divided into three main classes: Oleanane, ursane, and lupane. They have relatively complex cyclic structures, bearing either alcohol, aldehyde, or carboxylic acid functionalities, and represent the largest phytochemical group with an estimated more than 20,000 naturally occurring compounds [1,2]. They are common components of various plants and medicinal herbs such as mistletoe, lavender, oregano, and rosemary, as well as fruits such as apples, cranberries, figs, and olives [3,4]. Recent pharmacological studies have shown the triterpenoids oleanolic and ursolic acids to have several beneficial bioactivities, including anti-inflammatory, antitumor, hepatoprotection, and antihyperlipidemia [5,6]. Triterpenoids are used for medicinal purposes in numerous Asian cultures for their analgesic, anti-inflammatory, antipyretic, hepatoprotective, cardiotonic, and sedative effects [7]. As an important group of phytochemicals that induce numerous biological effects and display different pharmacological activities, triterpenoids are being evaluated for use in new functional foods, drugs, cosmetics, and healthcare products. The multifunctionality of triterpenoids makes them promising multi-targeting agents in the treatment of various diseases [8]. Furthermore, numerous triterpenoids have been shown to exhibit in vitro cytotoxic activity against a variety of tumor-derived cells with much lower toxicity to normal cells [4,9]. Recent investigations have shown that triterpenes and triterpenic acids, derivatives of pentacyclic triterpenes, have a multiplicity of biological effects, such as antioxidant, anti-inflammatory, anticancer, hepatoprotective, and anti-microbial activities, combined with low toxicity [10]. Due to their ability to act at various stages in the process of carcinogenesis, such as to block NF-κB activation, induce apoptosis, and inhibit proliferation, invasion, metastasis, and angiogenesis, these compounds may be considered for use in both chemoprevention and chemotherapy of cancer [11,12].
Dracocephalum heterophyllum Benth. (Lamiaceae) is one of thirty-two species of the genus Dracocephalum distributed in China [13]. It is traditionally used as an herb in Xinjiang and Tibet regions of China. Infusion of the plant is used for heart palpitations, neuralgia, migraine, headaches, and aches of catarrhal diseases. Fresh crushed leaves accelerate the healing of purulent wounds. The plant used has been widely utilized in the Amchi medical system in the Ladakh region of the Himalaya for centuries. The decoction of dried flowers and leaves is used to treat colds, coughs, and headaches [14]; the plant enhances digestion and stimulates the appetite. Anti-inflammatory, antibacterial, anti-hepatitis, antioxidant, analgesic, and anticonvulsant effects of D. heterophyllum have been documented in previous scientific reports [15,16].
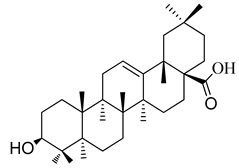
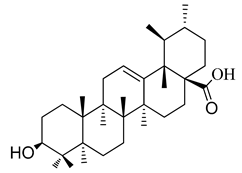
A systematic study of D. heterophyllum growing in Atush, Kashgar (Xinjiang) has not yet been carried out. Thus, the systematic evaluation of the chemical constituents was undertaken as an effort to discover bioactive compounds from this plant. In addition, the methods for selectively extracting a class of naturally bioactive compounds were developed for discovery of new medicines and herbal remedies for further pharmacological studies. Screening plant material in the search for triterpenoid-rich plant tissues has identified D. heterophyllum as a promising and highly evaluable resource. In our investigation we determined the oleanane and ursane types (oleanolic and ursolic acids) from the above-ground parts of D. heterophyllum. Their chemical structure is presented in Figure 1.
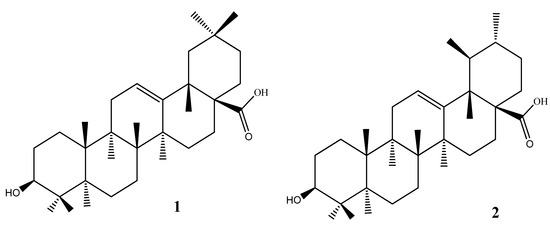
Figure 1.
The chemical structure of pentacyclic triterpenes oleanolic (1) and ursolic (2) acids.
Previously, the investigation of the ethanol extract of the aerial parts of D. heterophyllum resulted in the isolation of fourteen compounds including flavonoids, triterpenoids (ursolic and oleanolic acids), and steroids, and the quantification of four free flavonoids in the dry plant material was performed [13,17].
The present work is focused on the development of a method of extraction for total triterpenoids in the plant understudy, GC-MS evaluation of the chemical composition of total triterpenoids, determining antidiabetic and anticancer activities of crude triterpenoidal extract, total purified triterpenoids and of two previously isolated individual pure triterpenic acids (ursolic and oleanolic) from this plant species.
2. Materials and Methods
2.1. Chemicals and Plant Material
Ethanol, methanol, and chloroform were of analytical grade purchased from Tianjin Baishi Chemicals Company, Tianjin China. Vanillin, glacial acetic acid solution, perchloric acid, ursolic acid, tetra-n-butylammonium bromide, NaOH, Na2SO4, human breast cancer cell lines (SK-Br-3, T47D, and MCF-7), dimethyl sulfoxide, DPPH, pNPP, NaCl, MTT, PTP-1B inhibitor, and doxorubicin were obtained from Sigma-Aldrich chemicals GmbH Steinheim, Germany. All the chemical and reagents used were of analytical grade and double distilled water obtained through Millipore water system was used throughout the experiments.
The above-ground parts of D. heterophyllum were collected from Atush, Kashgar of Xinjiang province of China in July 2018, China, 2800 m above sea level. A voucher specimen of the plant has been deposited in the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences.
2.2. Extraction and Purification Procedures of Total Triterpenoids
Fresh plants were dried to a residual moisture content of 15% in a dark and ventilated place. The dried plant (4 kg) was crushed to size 3–5 mm mesh size. The crushed plant material was soaked with 3-folds of distilled water (relative to the mass of the plant material) for 30 min in a percolator.
An additional amount of water in a ratio of 1:5 was added to the soaked raw materials and left for 12 h. The wet raw material was dried immediately after soaking with water until the residual moisture content of 15% in a ventilated place protected from direct sunlight. Dried plant materials (3.8 kg) were powdered to 1–2 mm mesh size. The powdered plant material was loaded into a percolator and extracted with 96% ethanol (in a ratio of 1:1) for 4 h at ambient temperature.
The wet raw material was divided into two equal parts. The first part of the soaked raw material (6.8 kg) was loaded into percolator No. 1, and then 96% ethanol was poured in a raw material-extractant ratio of 1:1.5. Infusion was continued for 12 h. Then, the ethanol extract was drained from the first percolator and it was added to the second part of soaked raw material (6.8 kg) in percolator No. 2 with ratio of 1:1.5. The extract from the second percolator was used for the purification of total triterpenoids. To obtain the required product, an equal volume of water was added to the alcoholic extract from the second percolator, where triterpenoid compounds are precipitated, mainly ursolic acid. The water-diluted extract was pumped into the batch clarifier and the precipitation was allowed to proceed for 24 h at a temperature not exceeding +15 °C. After complete precipitation, the supernatant was decanted. The purification process of crude total triterpenoids was carrying out with 35% ethyl alcohol and small amount of activated carbon and filtered through filter paper. The obtained product was spread as a thin layer (up to 5 mm) on clean surface of the steel tray and dried in an oven at a temperature not above 40 °C. Residual moisture of the triterpenoids was 3.5%.
2.3. Quantitative Analysis of Triterpenoids in the Plant and the Resulting Total Triterpenoids
Quantification of total triterpenes was carried out by UV-Visible spectrophotometry at a wavelength of 547 nm after reacting triterpenes with 5% vanillin glacial acetic acid solution and perchloric acid using the following procedure. Ursolic acid was used as a reference standard. Ursolic acid (10 mg) was dissolved in 50 mL ethanol to make a stock solution having 0.2 mg/mL concentration. Working standard solutions were prepared by transferring 0.1, 0.2, 0.4, 0.6, and 0.8 mL ursolic acid standard stock solution (0.2 mg/mL) in stoppered test tubes. Solvent was then evaporated in an oven at 40 °C and 0.4 mL of the newly prepared 5% vanillin glacial acetic acid solution (0.552 g vanillin in 10 mL glacial acetic acid) and 1.6 mL perchloric acid were added. Weighed amounts of dried plant material and resulting total triterpenoids were also reacted in test tubes with 5% vanillin glacial acetic acid solution and perchloric acid in the same way. All the solutions were mixed thoroughly and heated on a water bath at 60 °C for 15 min. Solutions were cooled to room temperature and 8 mL glacial acetic acid was added to each test tube. They were mixed thoroughly and their absorbances were recorded at 547 nm against the prepared blank, having no ursolic acid, using UV-Visible spectrophotometer.
2.4. Methylation and GC-MS Analysis of Triterpenoids
Identification and analysis of chemical compounds in total triterpenoids were carried by gas-chromatographic-mass spectral (GC-MS) methods. An aliquot of total triterpenoids (0.5 mmol) was dissolved in 15 mL CHCl3, then 0.5 mL 5% NaOH and 0.5 mmol tetra-n-butylammonium bromide (powder) were added. When the solid was dissolved, a solution of 2 mmol CH3I in 15 mL CHCl3 was added, the system was heated to reflux. After the consumption of the starting materials, the mixture was washed with 15 mL 5% NaOH for several times until it was neutral. The neutral organic layer was dried with Na2SO4 [18].
Derivatized total triterpenoids were subjected to GC-MS analysis with the Agilent 7890A-5975C GC-MS, using a column HP-5MS (30 × 0.25 mm × 0.25 μm). The sample was applied (1 µL) and the GC operating parameters were as follows: Initial oven temperature was 150 °C for 2 min, then increased by 8 °C/min to 300 °C and held for 5 min. The injector temperature was 230 °C [19].
2.5. Cytotoxicity Test: MTT Assay
The cytotoxicity was assessed using the MTT assay method [20]. The total triterpenoids (as above) and two previously isolated compounds (oleanolic and ursolic acids) were tested using in vitro MTT assay for their cytotoxic activities against the SK-Br-3, T47D, and MCF-7 human breast cancer cell lines. The cells were treated with various concentrations of each compound for 48 h. All the data are represented as the mean ± S.D. of triplicate analysis. The median inhibitory concentrations (IC50, μg/mL) were determined by constructing a dose–response curve. The potent anticancer drug doxorubicin (DOX) was used as the positive control.
The test samples were dissolved in dimethyl sulfoxide (DMSO). The tumor cells were seeded into 96-well micro plates at a density of 2 × 104 cells per well in 100 μL of medium. The cells were incubated at 37 °C with various concentrations of each test sample in serum-free medium for 48 h. After 48 h, 20 μL of MTT (5 mg/mL in PBS) was added to each well of the plate. The cells were incubated at 37 °C for an additional 4 h. The medium was removed and 200 μL DMSO was added to each of the wells. After 5 min, the optical density was measured using a micro plate reader (BIO-TEK Inc., Seoul, Korea) at 550 nm. Cell viability was calculated as a percentage of viable cells in the compound-treated group versus the control group by the following equation: The inhibition rate (%) = [Optical Density (OD) (compound) − OD (Blank)/OD (Control) − OD (Blank)] × 100 [20].
2.6. Antidiaibetic Activity: PTP-1B Enzymatic Assay
Protein tyrosine phosphatase 1B (PTP-1B) activity was measured using the PTP-1B enzyme inhibition assay according to the literature [21,22]. The reagent pNPP (p-nitrophenyl phosphate disodium salt) was used as a substrate for the measurement of PTP-1B activity. Hydrolysis of the pNPP substrate produces a color reaction which measures the activity of PTP-1B. The PTP-1B inhibitor, 3-(3,5-dibromo-4-hydroxybenzoyl)-2-ethyl-N-[4-(2-thiazolylsulfamoyl)phenyl]-benzofuran-6-sulfonamide was used as the positive control. Compounds were pre-incubated with the enzyme at ambient temperature for 5 min. Then, 178 µL of buffer solution (20 mM HEPES, 150 mM NaCl, 1 mM EDTA) was added in 96-well plates. One microliter of PTP-1B protein solution (0.115 mg/mL) was added to the buffer solution. After that, 1 µL of test and positive control sample were added. Thereafter, 20 µL of the substrate pNPP (35 mM) was added and mixed for 10 min. The plate was incubated in the dark for 30 min and the reaction was terminated by adding 10 µL of 3 M NaOH. The system without the enzyme solution was used as a blank, using a micro plate reader (Spectra Max MD5, Molecular Devices, USA) absorption was measured at 405 nm. Inhibition (%) = [(OD405 − OD405 blank)/OD405 blank] × 100. IC50 was calculated from the percentage inhibition values.
3. Results and Discussions
At the preparatory stage, raw material (D. heterophyllum), after extraction with water, was dried to a residual moisture content of 15%, milled, and sifted through a sieve of the aforementioned mesh size. For extraction of triterpenoids, a fairly simple and accessible method called remaceration was chosen because it is characterized by minimal loss of biologically active compounds by diffusion, which contributes to the greatest extraction of phytochemicals. The nature of extracting, crushing the raw material, the duration and frequency of extraction have the greatest influence on the extent of extraction of biologically active substances from plant material. On this basis, we studied the impact of these factors on the extraction of triterpenoids.
3.1. Effects of Extracting Solvent
To find an effective solvent for the extraction of total triterpenoids, various solvents were tested. The use of 96% ethanol produced the highest yield of total triterpenoids (2.2 g) from 100 g of dry D. heterophyllum plant, while yield of total triterpenoids from 90%, 80%, 70%, 65%, 55% ethanol and chloroform was 2.0 g, 1.62 g, 1.10 g, 0.62 g, 0.15 g, and 2.1 g, respectively, from 100 g of dry D. heterophyllum. In our experiments, 96% ethanol was used due to high yield of extract and the lower toxicity of ethanol compared to the other solvents. Pentacyclic triterpenoids are practically insoluble in water and ethanol in a low concentration but they are soluble in chloroform, and sparingly soluble in 96% ethanol. Nevertheless, we used 96% ethanol as extracting solvent, which is the more affordable solvent as well as lower toxicity compared to chloroform.
3.2. Extraction and Purification
For the extraction and purification of triterpenoids we used the available method of solvent exchange, i.e., purified water at a ratio of 1:1 is added to the ethanol extract from the last percolator, where the substance is released into the precipitate containing triterpenoids and then allowed to stand for 24 h for complete precipitation at a temperature of not more than 15 °C. An amorphous precipitate is separated by filtration or centrifugation, which is then washed with 35% ethanol, dried and milled. Thus, after obtaining the triterpenoids, it is most efficient to carry out the cleaning of the intermediate product by way of solvent replacement. On the basis of our investigation, we found that the optimal parameters for extraction of triterpenoids are as follows: Raw materials with a particle size of 1–2 mm, the ratio of raw material:extractant in the process of infusion = 1:1.5, the number of percolators = 2 and the duration of infusion in each percolator = 12 h. This achieves maximum extraction of phytochemicals and the process proceeds with minimal loss of extractant. Figure 2 shows the total triterpenoids obtained before and after the purification with 35% ethyl alcohol and a low content of activated carbon (1 g per 100 g total triterpenoids extract). The authors studied extraction efficiency of the extraction method such as infusion, extraction under reflux and sonication using ultrasonic waves employing solvents as methanol and ethanol for the purification of ursolic acid. The results identified ultrasonic extraction using ethanol as the best among other extraction methods with high content of ursolic acid (1.89%) [23]. Published research reported highest yield of ursolic and oleanolic acids by using extraction with ethanol under refluxing and macroporous resins used for purification, which increased the content of triterpenic acids up to 92.9% [24]. The method we used, yielded 2.4% target product before purification and purification enhanced the yield to 98.03%, which is more than simple extraction with aqueous-ethanol, chloroform and purification using macroporous resins by published paper [24]. These results are reported for the first time. Total triterpenoids (before purification) is an amorphous powder, light green in color with a distinct smell and with residual moisture content of not more than 3.5%.
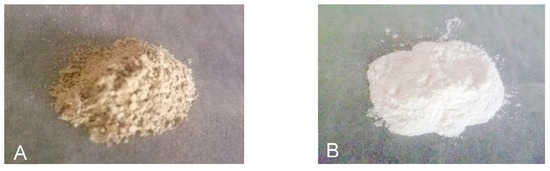
Figure 2.
The total triterpenoids before purification (A) and after purification (B).
3.3. Total Triterpene Content
A calibration curve with an R2 value 0.9994 was obtained after plotting the recorded absorbancies at wavelength maximum 547 nm against the concentrations of respective ursolic acid standard solutions (Figure 3). The amount of ursolic acid was determined using the regression equation obtained from the calibration curve. MS Excel sheet was used for constructing the calibration curve and quantification of total triterpenoids as ursolic acid equivalents. Figure 4 shows the UV-Visible spectrophotometer absorption spectrum of the triterpenoidal extracts from D. heterophyllum (A), and the total triterpenoids solution from D. heterophyllum (B) authenticating the maximum absorption at 547 nm. All the absorbances of the working standard solutions and test solutions were recorded at this wavelength.
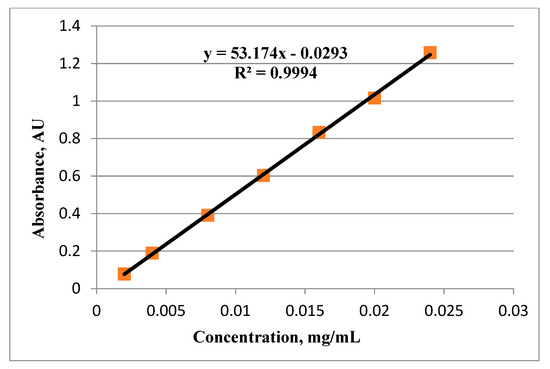
Figure 3.
Standard calibration curve of ursolic acid standard solutions.
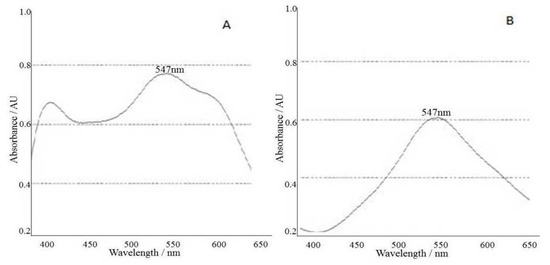
Figure 4.
UV-Visible spectrophotometer absorption spectrum of triterpenoids, extract from D. heterophyllum (A), total triterpenoids solution from D. heterophyllum (B), λ, 547 nm.
The contents of triterpenoids from the plant accumulated are 24 mg ursolic acid equivalents (UA)/g or (2.4%) of dried above-ground parts of D. heterophyllum and after purification was 980.03 mg ursolic acid equivalents (UA)/g or (98.03%), of the crude total triterpenic fraction.
3.4. Determinations of Triterpenoids by GC-MS
The pentacyclic triterpenoids ursolic and oleanolic acids, which were previously isolated from ethyl acetate and chloroform fraction [13,17], and identified by 1D NMR spectroscopy [25] were used as standards. After methylation, the sample was subjected to GC-MS producing three peaks at 16.734, 32.093 and 33.185 min corresponding to the molecular-ion peaks at m/z 74.0 (100%), 203.1 (100%) and 203.1 (100%), respectively. Structural assignments were based on analysis of fragmentation pattern of mass spectra, direct comparison of mass spectral data with profiles in the National Institute of Standards and Technology (NIST2) library, and comparisons of mass spectra with data published in the literature (Table 1).

Table 1.
The chemical composition of the total triterpenoids of D. heterophullum.
Two triterpenic acids, including ursolic acid (79.9%) and oleanolic acid (18.1%), and one fatty acid, palmitic acid (1.9%), were the major components of the purified total triterpenoids of the aboveground part of D. heterophyllum.
3.5. MTT Assay
The effects of total triterpenoids and isolated pure compounds on the proliferative response of the SK-Br-3, MCF-7, and T47D cell lines have been determined by treatment with different concentrations of the extracts. Significant reductions in cell proliferation were observed, and median inhibitory concentration values indicated that proliferation and growth of T47D cells were highly affected by ursolic acid and oleanolic acid.
The MTT analysis results showed that total triterpenoids, oleanolic and ursolic acids exhibited remarkable inhibitory activity on proliferation of SK-Br-3, T47D and MCF-7 cells in vitro compared with the positive control. With increasing concentration, the inhibitory effect was enhanced. The IC50 values (μM) of total triterpenoids and the previously isolated two triterpenoids on SK-Br-3, T47D, and MCF-7 cells have been tabulated in Table 2. The results confirmed that ursolic acid exhibited significant antiproliferative activity against T47D and MCF-7 cells with IC50 values of 1.63 ± 0.62 and 6.62 ± 1.09 μM respectively, while the IC50 values of doxorubicin was 9.0 ± 1.2 and 6.7 ± 0.9 μM, respectively. Triterpenoids, after purification, showed IC50 values of 5.91 ± 0.98, while oleanolic acid showed cytotoxic activity against SK-Br-3 cancer cells with an IC50 value of 3.08 ± 0.83 μM and an IC50 value of 2.63 ± 0.78 μM against T47D cancer cells. From these results, it appeared that total triterpenoids showed best inhibition activity against the SK-Br-3 cancer cells in comparison to pure ursolic acid, oleanolic acid, and the positive control. This might be due to some synergistic effect among the components of the total triterpenoids against the SK-Br-3 cancer cells while such type of synergism has not been observed in the case of other two cell lines. The dose–response curves for inhibition of three human breast cancer cells of the screened compounds are shown in Figure 5. Ursolic acid is widely distributed in fruits and vegetables, and has shown the ability to inhibit proliferation of breast tumor cells, induce apoptosis, scavenge free radicals, as well as regulate several anti-apoptotic proteins. Ursolic acid is known for its anti-inflammatory and antioxidant activities as well as cytotoxic activities on several human breast cancer cell lines [26]. Ursolic acid administration was found to induce autophagy and apoptosis via the GSK and Bcl-2/Caspase-3 signaling pathways, and the inhibitory effect of ursolic acid on the inflammatory response was also involved in this mechanism. The authors mentioned that these results support the promise of ursolic acid as a potential chemotherapeutic agent for breast cancer [27].

Table 2.
The IC50 values (μM) of total triterpenoids after purification, ursolic and oleanolic acids from the D. heterophyllum against SK-Br-3, T47D, and MCF-7 cells.
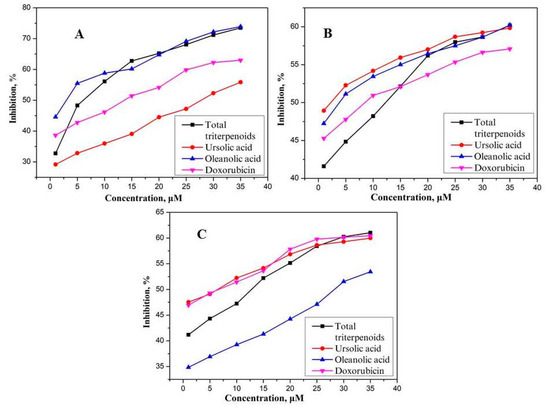
Figure 5.
Dose response curves for inhibition of three human breast cancer cells; SK-Br-3 (A), T47D (B) and MCF-7 (C).
3.6. PTP-1B Activity Assay
The total triterpenoids were tested, using different concentrations, for PTP-1B activity before and after purification. Table 3 shows that the degree of purification of triterpenoids influences the final result. Both the fractions have shown activity against the PTP-1B, but the purified fraction of triterpenoids showed significant inhibitory activities with IC50 values of 0.47 μg/mL. Among the two isolated triterpenoids, oleanolic acid showed potent activity against the PTP-1B with an IC50 value of 0.97 and ursolic acid with IC50 = 1.13 μg/mL. These results confirmed total triterpenoids (after purification) as the best PTP-1B inhibitor in comparison to other tested compounds. The dose–response curves for inhibition of PTP-1B enzyme of the screened compounds are shown in Figure 6.

Table 3.
Results of protein-tyrosine phosphatase (PTP-1B) inhibition of the total triterpenoids fractions and isolated two individual triterpenoids of D. heterophyllum.
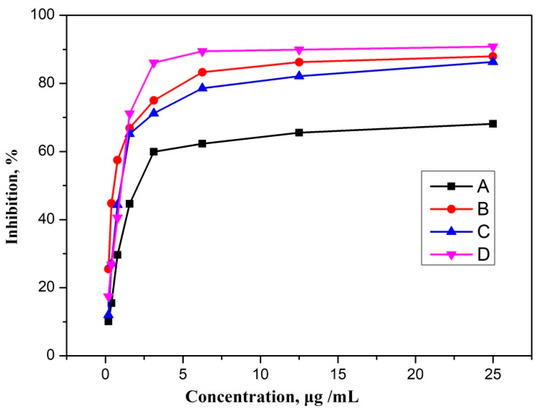
Figure 6.
Dose response curve of inhibition of PTP-1B enzyme for total triterpenoids fraction before purification (A), total triterpenoids fraction after purification (B), ursolic acid (C), and oleanolic acid (D).
4. Conclusions
The developed remaceration method is very effective for extraction, separation, and purification of total triterpenoids. This study confirmed the presence of bioactive compounds like ursolic and oleanolic acids in the aerial part of D. heterophyllum in sufficient amounts by using the remaceration method. The investigations demonstrated that the purified total triterpenoids and two previously isolated individual triterpenoids of D. heterophyllum exhibited significant cytotoxic activity against three human breast tumor cell lines as well as inhibition of the enzyme PTP-1B. The total triterpenoids extract demonstration significant antidiabetic potential, with PTB-1B IC50 values of 0.47 ± 0.18 μg/mL after purification, while the oleanolic and ursolic acids IC50 values were 0.97 ± 0.08 and 1.13 ± 0.21 μg/mL, more effective than the positive control (1.97 μg/mL), respectively. The results showed that ursolic acid exhibited significant antiproliferative activity against T47D and MCF-7 with IC50 values of 1.63 ± 0.62 and 6.62 ± 1.09 μM compared to doxorubicin. Purified total triterpenoids showed an IC50 value of 5.91 ± 0.98, while the oleanolic acid IC50 value was 3.08 ± 0.83 μM against the SK-Br-3 cancer cell and IC50 value of 2.63 ± 0.78 μM on T47D tumor cells. In summary, these results confirm the prospect of developing plant extracts into effective drugs and health foods for human applications.
Author Contributions
S.N., F.S., and L.G., performed the phytochemical investigation, designed and wrote the manuscript; S.N., W.N.S., F.S., M.N.Q., I.G., and Q.K., analyzed data; L.G. and M.H. studied the biological activities; H.A.A. and W.N.S. made a critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for financial support to the Chinese Academy of Sciences President’s International Fellowship Initiative (Grant No. 2019PB0043), Central Asian Drug Discovery & Development Center of Chinese Academy of Sciences (Grant No. CAM 201808), National Natural Science Foundation of China (Grant No. U1703235), Foreign young scholar (Grant No. 2018FYB0004) and CAS “Light of West China” Program 2018-YDYLTD-001.
Acknowledgments
All authors would like to thank the “Key Laboratory of Xinjiang Indigenous Medicinal Plants Resource Utilization and Key Laboratory of Plant Resources and Chemistry in Arid Regions” of Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences for all the facilities provided in the use of laboratories, equipment, and installations to develop this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Bishayee, A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res. Treat. 2009, 115, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, O.A.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Ruiz-Gutierrez, V. Chapter 159—Functional properties of pentacyclic triterpenes contained in pomace olive oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 1431–1438. [Google Scholar]
- Angel, R. The wealth of India. Raw materials. Kew Bull. 1978, 32, 802. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Setzer, W.; Setzer, M. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003, 3, 540–556. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.; Xu, L.; Ma, Y.; Lian, W.; Liu, Y.; Wang, Y. Optimized extraction of total triterpenoids from jujube (Ziziphus jujuba Mill.) and comprehensive analysis of triterpenic acids in different cultivars. Plants 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-κb activation induced by carcinogenic agents through suppression of IκBα kinase and p65 phosphorylation. Cancer Res. 2003, 63, 4375. [Google Scholar]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [PubMed]
- Numonov, S.R.; Usmanova, S.K.; Aisa, H.A. A triterpenoid and flavonoids from Dracocephalum heterophyllum. Chem. Nat. Compd. 2013, 48, 1109–1110. [Google Scholar]
- Ballabh, B.; Chaurasia, O.P. Traditional medicinal plants of cold desert Ladakh—Used in treatment of cold, cough and fever. J. Ethnopharmacol. 2007, 112, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-Q.; Dang, J.; Wen, H.-X.; Yuan, X.; Tao, Y.-D.; Wang, Q.-L. Anti-hepatitis, antioxidant activities and bioactive compounds of Dracocephalum heterophyllum extracts. Bot Stud 2016, 57, 16. [Google Scholar]
- Zhang, C.; Li, H.; Yun, T.; Fu, Y.; Liu, C.; Gong, B.; Neng, B. Chemical composition, antimicrobial and antioxidant activities of the essential oil of tibetan herbal medicine Dracocephalum heterophyllum Benth. Nat. Prod. Res. 2008, 22, 1–11. [Google Scholar] [CrossRef]
- Numonov, S.R.; Usmanova, S.K.; Aisa, H.A. Chemical composition of Dracocephalum heterophyllum. Chem. Nat. Compd. 2013, 49, 511–513. [Google Scholar] [CrossRef]
- Jemmali, Z.; Chartier, A.; Dufresne, C.; Elfakir, C. Optimization of the derivatization protocol of pentacyclic triterpenes prior to their gas chromatography–mass spectrometry analysis in plant extracts. Talanta 2016, 147, 35–43. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Numonov, S.R.; Safomuddin, A.; Gulmurodov, I.S.; Valiev, A.K.; Bakri, M.; Sukhrobov, P.; Habasi, M.; Setzer, W.N.; Aisa, H.A. Chemical composition of essential oil from Artemisia vachanica growing in Tajikistan. Chem. Nat. Compd. 2019, 55, 965–967. [Google Scholar]
- Bozorov, K.; Zhao, J.-Y.; Elmuradov, B.; Pataer, A.; Aisa, H.A. Recent developments regarding the use of thieno[2,3-d]pyrimidin-4-one derivatives in medicinal chemistry, with a focus on their synthesis and anticancer properties. Eur. J. Med. Chem. 2015, 102, 552–573. [Google Scholar] [CrossRef]
- Numonov, S.; Edirs, S.; Bobakulov, K.; Qureshi, N.M.; Bozorov, K.; Sharopov, F.; Setzer, N.W.; Zhao, H.; Habasi, M.; Sharofova, M.; et al. Evaluation of the antidiabetic activity and chemical composition of Geranium collinum root extracts—Computational and experimental investigations. Molecules 2017, 22, 983. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, S.F.; Atolikhshoeva, S.; Safomuddin, A.; Bakri, M.; Setzer, N.W.; Musoev, A.; Sharofova, M.; Habasi, M.; Aisa, A.H. Volatile secondary metabolites with potent antidiabetic activity from the roots of Prangos pabularia Lindl.—Computational and experimental investigations. Appl. Sci. 2019, 9, 2362. [Google Scholar] [CrossRef]
- Gao, D.; Li, N.; Li, Q.; Li, J.; Han, Z.; Fan, Y.; Liu, Z. Study of the extraction, purification and antidiabetic potential of ursolic acid from Cornus officinalis Sieb. et Zucc. Therapy 2008, 5, 697. [Google Scholar] [CrossRef]
- Pei-Jiang, C.; Shan, L.; Zi-Long, W.; Ya-Nan, W.; Xue-Wen, Z. Study on extraction and purification of ursolic acid and oleanolic acid from hawthorn fruits. Food Sci. 2007, 28, 141–144. [Google Scholar]
- Palu, D.; Bighelli, A.; Casanova, J.; Paoli, M. Identification and quantitation of ursolic and oleanolic acids in Ilex aquifolium L. Leaf extracts using 13C and 1H-NMR spectroscopy. Molecules 2019, 24, 4413. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, Y.-L.; Wang, H. Ursolic acid inhibits breast cancer growth by inhibiting proliferation, inducing autophagy and apoptosis, and suppressing inflammatory responses via the PI3K /Akt and NF-κB signaling pathways in vitro. Exp. Ther. Med. 2017, 14, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).