Abstract
This research seeks to model a packed bed with cocoa waste on an industrial scale using computer software and parametric evaluation to remove Pb(II) in solution. To achieve this, they developed multiple simulations of a packed bed using Aspen Adsorption software using different configurations of inlet flow rate and bed height through a parametric sensitivity study to evaluate the system performance using Langmuir as an isothermal model and the Linear Driving Force (LDF) model as a kinetic model. It was found that the efficiency of the adsorption process reached 99.7% for Pb(II) removal. On the other hand, the best simulation conditions considering efficiency, breakthrough time and saturation were a flow rate of 200 m3/day, a head of 5 m and an initial concentration of 1000 mg/L. This research exhibits a novel engineering perspective to anticipate the potential performance of packed bed with agro-industrial biomasses in the multiscale removal of Pb(II).
1. Introduction
Water is a vital natural resource essential for the survival of living organisms, economic growth, and human well-being. However, this resource has been in decline due to excessive use in various anthropogenic activities, which generate large volumes of wastewater loaded with multiple contaminants [1]. Water sources are affected by the presence of these pollutants originating from domestic sources, agricultural runoff, and industrial discharges, posing a risk to the health and integrity of living beings [2]. Among the various contaminants present in wastewater are heavy metals, also known as trace elements, which are classified as some of the most hazardous environmental toxins. Even at low concentrations, they represent a significant threat due to their toxicity, persistence, and bioaccumulative potential [3]. Lead (Pb(II)) metal ions are highly toxic to aquatic ecosystems and human health due to their non-biodegradable nature and their high persistence and accumulation in the environment. This heavy metal enters water bodies through various anthropogenic sources such as smelting processes, electroplating, and the discharge of industrial waste, among other activities. Pb(II) causes a range of health complications, including cardiovascular diseases, kidney failure, gastrointestinal disorders, and cognitive impairment, among others [4].
To remove heavy metals from wastewater discharged into aquatic ecosystems, various treatment techniques have been developed with the aim of eliminating these contaminants from water bodies. Among these are coagulation/flocculation processes, which use coagulants or flocculants that destabilize the contaminant particles in the water, thus facilitating their removal [5]. On the other hand, electrocoagulation is an innovative technique for water treatment, based on the use of coagulants in combination with the application of a direct current through sacrificial metal electrodes, usually made of iron or aluminum. This technique destabilizes colloidal particles and contaminants present in bodies of water [6]. One of these techniques is adsorption, a method that uses a surface phenomenon where the adsorbent is transferred to the adsorbate. It is also widely used because it is adaptable, low-cost, and easy to apply [7]. The adsorption mechanism first transports the molecules of the contaminants to be removed from the solution into the adsorbent boundary layer. Then, the diffusion of these species from the adsorbent boundary layer to the external surface of the adsorbent material occurs. Finally, the pollutants move to the interior of the porous matrix, where they attach to the available active sites, resulting in the effective retention of unwanted ions and molecules in the aqueous medium [8]. The adsorption can be divided between two main categories: chemical (chemisorption) and physical (physisorption). Chemisorption is defined by the chemical links formed by the adsorbed substance and the sorbent, which results in strong, specific, and generally irreversible interactions, as well as requiring a significant energy input [9].
The development of adsorbents based on lignocellulosic materials such as yam, cassava, corn, oil palm bagasse [10], coconut shells [11], and yam residues [12], among others, has been studied. The use of agro-industrial waste from cocoa (Theobroma cacao L.), such as husks, has been established as a sustainable alternative for the adsorption of contaminants in aqueous media. This material generates multiple by-products that stand out for their abundance, low cost, and availability in producing regions. Likewise, this material has a lignocellulosic matrix and a composition consisting of 19.82% cellulose, 9.45% hemicellulose, and 12.66% lignin, with functional groups capable of interacting with heavy metals and organic compounds [13].
These studies have highlighted the need to focus more attention on the research and development of biomass-based packed columns for potential industrial applications. Nevertheless, most studies related to adsorption are developed on a laboratory scale, which limits their direct application in real conditions. In response to this limitation, strategies have been implemented to predict the performance of adsorption systems on a larger scale, notably the use of computational tools that enable the simulation of experimental processes and thus facilitate their extrapolation to industrial contexts. Aspen Adsorption, a tool designed by AspenTech, is a simulation software focused on modelling adsorption processes in multiphase systems. This tool allows to virtually simulate the performance of adsorption columns under different operating conditions, enabling the prediction of efficiency in removing contaminants present in aqueous solutions. The use of this software not only contributes to understanding the physicochemical mechanisms that govern mass transfer and adsorbate-adsorbent interaction, but also provides fundamental support for the design, optimization, and scaling of adsorption-based water treatment technologies.
In this study, the main objective is to model an industrial-scale packed adsorption column using computational tools and parametric analysis to evaluate the removal of Pb(II) in aqueous solution using cocoa waste (Theobroma cacao L.) as an adsorbent material. This research is novel in that it uses widely available agro-industrial waste as a sustainable alternative for water decontamination, as well as applying computational simulations to predict the performance of large-scale adsorption systems. In this way, the objective is to provide quantitative information and technical criteria to facilitate the design and expansion of packed columns for the treatment of effluents contaminated with heavy metals. The selection of Pb(II) is based on its high toxicity and widespread presence in industrial process effluents, making it one of the priority heavy metals for removal studies. Various authors have pointed out that lignocellulosic residues such as cocoa shells have a notable affinity for different contaminants such as Pb(II) due to the presence of functional groups capable of forming stable complexes [14,15,16].
2. Materials and Methods
2.1. Parametric Sensitivity Study
To evaluate system performance in removing Pb(II) ions, Aspen Adsorption V10 software was used. In order to analyze the influence of operating conditions on process efficiency, a parametric sensitivity study was developed, in that the effect of varying critical system parameters was examined. Specifically, changes in bed height and feed flow rate were considered, while maintaining the initial Pb(II) concentration constant at 1000 mg/L [17,18], under operating conditions of 1 atm pressure and 30 °C temperature. Altering these variables allows the system’s performance to be analyzed under various operating conditions, making it possible to identify which factors have the greatest impact on adsorption efficiency. To establish the range of values for the parameters considered for the sensitivity study, a detailed review of multiple studies reported in the literature was conducted. This allowed us to identify different operating ranges and characteristic behaviours related to the variables studied, which were previously studied and adjusted [19,20]. Table 1 shows the range of values for the parameters considered in the sensitivity study.

Table 1.
Adsorption column analysis parameters.
2.2. Parameters and Conditions Required for the Scale-Up of the Packed Bed Column to Industrial Scale
Aspen Adsorption requires specific parameters to model and simulate the adsorption column for the removal of Pb(II) from solution. These variables are essential to define the operating conditions during the simulation of the packed adsorption column using cocoa waste as the adsorbent for the removal of heavy metals from aqueous solutions. In order to establish the parameters required for the construction of the packed adsorption column for the removal of Pb(II), various studies reported in the literature were reviewed to identify the parameters required by the Aspen Adsorption software, including the apparent density of the biomaterial, the diameter of the column, the mass transfer coefficient, the void porosity, the total porosity, and the constant values for the isothermal model. Therefore, a study, analysis, and adjustments of these parameters were carried out for this simulation model [21,22]. Table 2 presents the values for each of these parameters.

Table 2.
Values of the Factors Evaluated in the Parametric Sensitivity Analysis Study.
In order to simulate the packed column for Pb(II) adsorption, it is necessary to establish the appropriate thermodynamic package for the calculation of the physicochemical properties of the liquid phase. The precise definition of the physical properties of the process is essential for determining the characterization of the solution that interacts with the adsorbent material inside the adsorption column. These properties were calculated in this study using the ELECNRTL (Electrolyte Non-Random Two Liquid) thermodynamic model, which allows for an accurate representation of systems involving electrolytic solutions and thus ensures a more realistic description of the behaviour of the liquid phase during the adsorption process. This model accounts for molecular interactions between ionic and neutral species in aqueous solutions, enabling the estimation of properties such as fluid density, effective contaminant concentration (activity), viscosity, among others. These properties have a direct impact on key variables of the adsorption model, such as the mass transfer coefficient, and on the material balances within the column, as they allow the calculation of hydrodynamic parameters and the influence of certain factors on adsorbent performance and the breakthrough profile.
It is also necessary to configure various settings in Aspen Adsorption, such as the discretization method, the selected isotherm and kinetic models, among other conditions. The software includes several essential sections that must be properly defined to ensure accurate simulation settings. Table 3 presents the different sections to be configured along with the conditions established for each.

Table 3.
Configuration Sections for the Adsorption Column Simulation.
3. Results and Discussion
3.1. Data Obtained from Adsorption Bed Simulations for the Removal of Pb(II)
Multiple simulations of the Pb(II) adsorption bed were performed on an industrial scale using Aspen Adsorption software and different inlet flow rates and bed heights. The results obtained include the breakthrough time (B.T), which indicates the point at which the adsorbent biomaterial begins to lose its contaminant retention capacity; the saturation time (S.T), corresponding to when the adsorbent reaches its maximum adsorption capacity; and the process efficiency, which reflects how effective the material used is in carrying out the adsorption process. The simulations were conducted using the Langmuir isothermal model combined with the LDF kinetic model. Table 4 shows the results obtained from the simulations.

Table 4.
Results of the Langmuir-LDF Model Evaluation.
3.2. Parametric Sensitivity Analysis
3.2.1. Influence of Inlet Flow Rate on the Adsorption Process
Simulations were performed with flow rates of 100, 150, and 200 m3/day, considering the initial concentration (1000 mg/L) and the fixed bed height (5 m) as constants. Figure 1 shows the breakthrough curves generated from the Langmuir model coupled with LDF kinetic model. These curves indicate the evolution of the adsorption process over time, allowing fundamental parameters of bed performance to be identified: B.T, S.T, and efficiency.
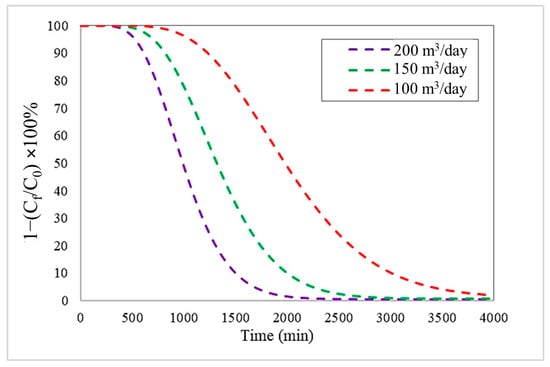
Figure 1.
Breakthrough curves at different inlet flow rates with an initial Pb(II) concentration of 1000 mg/L and a bed height of 5 m, using the Langmuir–LDF model.
These results show a significant increase in both breakthrough time (B.T.) and saturation time (S.T.) as flow rate decreases. This performance is attributable to the fact that, at lower flow rates, fluid stays in contact with the adsorbent bed longer, favouring greater diffusion of Pb(II) ions toward the active surface of the material and, consequently, more efficient use of the adsorption sites. On the contrary, at high flow rates, the reduction in residence time limits the equilibrium between the liquid and solid phases, causing faster saturation of the column. These results confirm that controlling the inlet flow rate is a determining factor in optimizing the removal capacity and useful life of the adsorption column [27].
This behaviour can be seen in the data presented in Table 3. At an inlet flow rate of 200 m3/day, the B.T. and S.T. values were 735 min and 2642 min, respectively. When the flow rate was reduced to 150 m3/day, an increase in B.T. and S.T. was recorded, reaching 981 min and 2883 min, respectively. Similarly, further reducing the flow rate to 100 m3/day led to an additional increase in these times, yielding 1474 min for B.T. and 3783 min for S.T. These values show the operational performance and useful life of the adsorbent column: high B.T. values mean a delay in the advance of Pb(II), which shows better performance. On the other hand, high S.T. values mean a higher adsorption capacity of the bioadsorbents before it needs to be regenerated or replaced. Therefore, as the flow rate decreases, better B.T. and S.T. values are obtained.
3.2.2. Influence of Bed Height on the Adsorption Process
In this study, we looked at how the height of the adsorption bed affected the process, testing heights of 3, 4, and 5 m, while maintaining a constant initial concentration of Pb(II) (1000 mg/L) and feed flow rate (200 m3/day). Figure 2 shows the breakthrough curves generated using the Langmuir isothermal model, coupled with the LDF kinetic model. These curves indicate the evolution of the adsorption process over time, allowing fundamental parameters of bed performance to be identified: B.T, S.T, and efficiency.
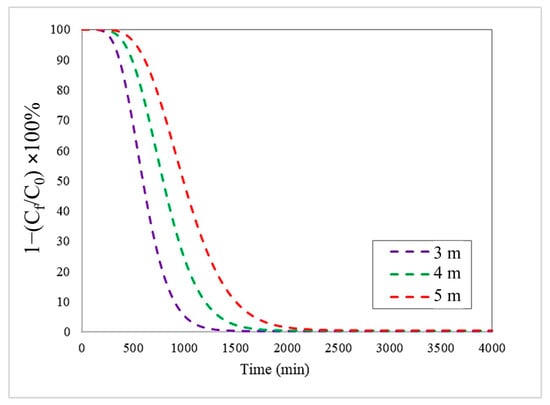
Figure 2.
Breakthrough curves at different bed heights with an inlet flow rate and initial Pb(II) concentration of 200 m3/day and 1000 mg/L, respectively, using the Langmuir–LDF model.
The results show a clear trend: as the bed height increases, there is a prolongation in both the breakthrough time (B.T.) and the saturation time (S.T.). This is due to the greater amount of biomass available in contact with the liquid phase, the higher number of active sites for adsorption, and, consequently, the improved total contaminant removal capacity. Practically speaking, this means that a higher bed favours a longer service time before the effluent reaches critical concentrations of Pb(II) [28,29].
The simulation data confirmed this trend for Pb(II) removal. As shown in Table 3, with a bed height of 3 m, B.T. and S.T. were 440 min and 1379 min, respectively. Increasing the bed height to 4 m resulted in values of 587 min for B.T. and 1913 min for S.T. Similarly, raising the bed height to 5 m further increased these times to 735 min for B.T. and 2642 min for S.T. These values show the operational performance and useful life of the adsorbent column: high B.T. values mean a delay in the advance of Pb(II), which shows better performance. On the other hand, high S.T. values mean a higher adsorption capacity of the bioadsorbent before it needs to be regenerated or replaced. Therefore, as the height of the bed increases, there is an increase in the values of B.T. and S.T.
3.3. Report on the Use of Different Simulators to Scale Packed Adsorption Columns for the Removal of Pb(II)
The results obtained from the simulation of the packed column with cocoa residues on an industrial scale demonstrated the promising performance of this adsorption system for the removal of Pb(II) in solution. In this study, the Langmuir isothermal model was combined with LDF kinetic model. Operating conditions for the bed were established, and different values were used for the inlet flow rate and bed height. Different authors have implemented simulators to predict the behaviour of packed adsorption columns for Pb(II) removal using different types of adsorbent materials such as activated carbons [30], olive tree pruning [31], among others. These studies demonstrate the advantages of implementing computational tools such as Aspen Adsorption to analyze the dynamic behaviour of systems, adjust parameters, reduce costs and time associated with experimentation, among other factors, facilitating the design, validation, and subsequent scaling of adsorption processes. Likewise, the potential of agro-industrial materials as adsorbents for the removal of Pb(II) is observed, such as Theobroma cacao L., which presented high removal values and high breakage and saturation times under industrial conditions, highlighting its potential as a sustainable adsorbent.
3.4. Limitations of the Established Assumptions
It was assumed that the adsorption process took place under isothermal conditions and without pressure drop in the column. This simplified the process, facilitating numerical resolution and allowing focus on analyzing the behaviour of the adsorbent. Likewise, the mass transfer coefficient was used as a fixed value, established by values reported in the literature. This parameter was kept constant to focus the sensitivity analysis on the variables of bed height and operating flow rate, allowing for a clearer evaluation of their influence on the adsorption process. Although these assumptions cannot express certain situations on an industrial scale, they allow for a solid and controlled understanding of the system’s performance.
4. Conclusions
The study offers a novel approach to modelling and simulating adsorption beds at an industrial scale, utilizing agro-industrial residues from Theobroma cacao L. as an adsorbent for the removal of Pb(II) in aqueous solutions. The results derived from computational simulations provide quantitative information of great relevance for the progression of adsorption processes in industry applications. Parametric sensitivity analysis showed the decisive role of operational variables, such as bed height and inlet flow rate, in system efficiency. It was observed that a larger bed increases breakthrough and saturation times, although with a decrease in adsorption efficiency, while increasing the inlet flow rate improves this efficiency but reduces the operating times of the biomaterial. Such findings are providing a solid technical basis for the design and optimization of industrial effluent treatment systems, enabling the prediction of their performance and the anticipation of process behaviour prior to implementation. Finally, it is recommended that future studies integrate economic assessments and techno-economic resilience analyses to determine the viability and sustainability of optimal adsorption column configurations in real-world application scenarios. Similarly, there are plans to develop a pilot-scale laboratory test to experimentally validate the reliability of the results obtained with the Aspen Adsorption software, thus reinforcing the robustness and applicability of this tool for modelling packed adsorption columns.
Author Contributions
Á.G.-D.: Conceptualization, Investigation, Resources, Methodology, Software, Data curation, Writing—original draft, Validation, Project administration. Á.V.-O.: Investigation, Supervision, Resources, Data curation, Validation, Writing—original draft, Project administration. R.O.-T.: Supervision, Writing—review and editing, Funding acquisition, Resources, Visualization. C.T.-T.: Conceptualization, Investigation, Resources, Data curation, Project administration, Writing—original draft, Funding acquisition, Formal analysis. J.B.-S.: Investigation, Visualization, Data curation, Writing—original draft, Validation, Software. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the results of this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank the Universidad de Cartagena for providing the equipment, reagents, and technical support for the project approved by Resolution No. 01385 and Act No. 093 of 2021. During the preparation of this manuscript, the authors used Grammarly Pro to review the English writing and ChatGPT (GPT-5.2) as a support tool for the development of the graphic abstract. The authors have reviewed and edited the result and assume full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Langmuir constant, is Langmuir’s constant and can be correlated with the variation in the adsorption area and the porosity of the adsorbent (-); | |
| Equilibrium concentration of the contaminant in solution (mg/L); | |
| Contaminant concentration at the column outlet (mg/L); | |
| Inlet contaminant concentration (mg/L); | |
| Indicates the adsorption process efficiency (-, %); | |
| Location within the adsorption bed where the equation is being evaluated (-); | |
| Mass transfer coefficient (1/s); | |
| Adsorption capacity of the contaminant at equilibrium (mg/g); | |
| Maximum loading capacity of the adsorbent (mg/g); | |
| (mg/g); | |
| Quantity of adsorption if the system were in instantaneous equilibrium in the fluid phase (mg/g); | |
| Distance between two nodes in the discretized bed (m); | |
| Previous node in the direction of flow (-); | |
| (mol/m3). |
References
- Noor, R.; Maqsood, A.; Baig, A.; Pande, C.B.; Zahra, S.M.; Saad, A.; Anwar, M.; Singh, S.K. A comprehensive review on water pollution, South Asia Region: Pakistan. Urban Clim. 2023, 48, 101413. [Google Scholar] [CrossRef]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shah, H.-U.; Khan, M.S.; Iqbal, A.; Potrich, E.; Amaral, L.S.; Rasheed, S.; Nawaz, H.; Ayub, A.; Naseem, K.; et al. Lead In drinking water: Adsorption method and role of zeolitic imidazolate frameworks for its remediation: A review. J. Clean. Prod. 2022, 368, 133010. [Google Scholar] [CrossRef]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: A review of recent developments. RSC Adv. 2023, 13, 19335–19355. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Ravindiran, G.; Sundaram, H.; Rajendran, E.M.; Ramasamy, S.; Nabil, A.Z.; Ahmed, B. Removal of azo dyes from synthetic wastewater using biochar derived from sewage sludge to prevent groundwater contamination. Urban Clim. 2023, 49, 101502. [Google Scholar] [CrossRef]
- Sukmana, H.; Bellahsen, N.; Pantoja, F.; Hodur, C. Adsorption and coagulation in wastewater treatment—Review. Prog. Agric. Eng. Sci. 2021, 17, 49–68. [Google Scholar] [CrossRef]
- Taiwo, A.F.; Chinyere, N.J. Sorption Characteristics for Multiple Adsorption of Heavy Metal Ions Using Activated Carbon from Nigerian Bamboo. J. Mater. Sci. Chem. Eng. 2016, 4, 39–48. [Google Scholar] [CrossRef]
- Asuquo, E.D.; Martin, A.D.; Nzerem, P. Evaluation of Cd(II) Ion Removal from Aqueous Solution by a Low-Cost Adsorbent Prepared from White Yam (Dioscorea rotundata) Waste Using Batch Sorption. ChemEngineering 2018, 2, 35. [Google Scholar] [CrossRef]
- Sewwandi, B.G.N.; Vithanage, M.; Wijesekara, S.S.R.M.D.H.R.; Mowjood, M.I.M.; Hamamoto, S.; Kawamoto, K. Adsorption of Cd(II) and Pb(II) onto Humic Acid–Treated Coconut (Cocos nucifera) Husk. J. Hazard. Toxic Radioact. Waste 2014, 18, 04014001. [Google Scholar] [CrossRef]
- Villabona-Ortíz, Á.; Tejada-Tovar, C.; Gonzalez-Delgado, Á.D. Adsorption of Cd2+ Ions from Aqueous Solution Using Biomasses of Theobroma cacao, Zea mays, Manihot esculenta, Dioscorea rotundata and Elaeis guineensis. Appl. Sci. 2021, 11, 2657. [Google Scholar] [CrossRef]
- Tovar, C.T.; Ortiz, Á.V.; Villadiego, M.J. Remoción de cromo hexavalente sobre residuos de cacao pretratados químicamente. Rev. UDCA Actual. Divulg. Cient. 2017, 20, 139–147. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Baligar, V.C.; He, Z.L. Heavy metal accumulation in leaves and beans of cacao (Theobroma cacao L.) in major cacao growing regions in Peru. Sci. Total Environ. 2017, 605–606, 792–800. [Google Scholar] [CrossRef]
- Obike, A.I.; Igwe, J.C.; Emeruwa, C.N.; Uwakwe, K.J. Equilibrium and kinetic studies of Cu (II), Cd (II), Pb (II) and Fe (II) adsorption from aqueous solution using cocoa (Theobroma cacao) pod husk. J. Appl. Sci. Environ. Manag. 2018, 22, 182–190. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, Á. Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks. Materials 2022, 15, 6937. [Google Scholar] [CrossRef]
- Opeolu, B.O.; Bamgbose, O.; Arowolo, T.A.; Adetunji, M.T. Utilization of maize (Zea mays) cob as an adsorbent for lead (II) removal from aqueous solutions and industrial effluents. Afr. J. Biotechnol. 2009, 8, 1567–1573. [Google Scholar]
- Elkhatib, E.A.; Moharem, M.L.; Saad, A.F.; Abdelhamed, S. Effective elimination of lead from polluted wastewater utilizing a novel nanocomposite derived from byproducts of drinking water industry. BMC Chem. 2025, 19, 188. [Google Scholar] [CrossRef]
- Upadhyay, U.; Gupta, S.; Agarwal, A.; Sreedhar, I.; Anitha, K.L. Adsorptive removal of Cd2+ ions using dolochar at an industrial-scale process optimization by response surface methodology. Environ. Sci. Pollut. Res. 2023, 30, 8403–8415. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Tejada-Tovar, C.N.; Villabona-Ortiz, Á.; González-Delgado, Á.D.; Vergara-Villadiego, J.C. Computational prediction of packed-bed reactor performance for hexavalent chromium removal from aqueous solution. J. Water Land Dev. 2025, 66, 13–18. [Google Scholar] [CrossRef]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Anitha, K.L. Simulation studies of Cu(II) removal from aqueous solution using olive stone. Clean. Mater. 2022, 5, 100128. [Google Scholar] [CrossRef]
- Lara, J.; Tejada, C.; Villabona, Á.; Arrieta, A.; Granados Conde, C. Adsorción de plomo y cadmio en sistema continuo de lecho fijo sobre residuos de cacao. Rev. ION 2016, 29, 113–124. [Google Scholar] [CrossRef]
- Soriano, A.N.; Orfiana, O.N.; Pangon, M.B.J.; Nieva, A.D.; Adornado, A.P. Simulated biosorption of Cd(II) and Cu(II) in single and binary metal systems by water hyacinth (Eichhornia crassipes) using aspen Adsorption®. ASEAN J. Chem. Eng. 2016, 16, 21–43. [Google Scholar] [CrossRef]
- Nikam, S.; Mandal, D.; Dabhade, P. LDF based parametric optimization to model fluidized bed adsorption of trichloroethylene on activated carbon particles. Particuology 2022, 65, 72–92. [Google Scholar] [CrossRef]
- Ragadhita, R.; Bayu, A.; Nandiyanto, D. Curcumin Adsorption on Zinc Imidazole Framework-8 Particles: Isotherm Adsorption Using Langmuir, Freundlich, Temkin, And Dubinin-Radushkevich Models. J. Eng. Sci. Technol. 2022, 17, 1078–1089. [Google Scholar]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb(II) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 119585. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Adi, V.S.K.; Huang, H.L.; Lin, H.P.; Huang, Z.H. Adsorption of metal ions with biochars derived from biomass wastes in a fixed column: Adsorption isotherm and process simulation. J. Ind. Eng. Chem. 2019, 76, 240–244. [Google Scholar] [CrossRef]
- Feizi, F.; Sarmah, A.K.; Rangsivek, R. Adsorption of pharmaceuticals in a fixed-bed column using tyre-based activated carbon: Experimental investigations and numerical modelling. J. Hazard. Mater. 2021, 417, 126010. [Google Scholar] [CrossRef]
- Hameed, A.; Hameed, B.H.; Almomani, F.A.; Usman, M.; Ba-Abbad, M.M.; Khraisheh, M. Dynamic simulation of lead(II) metal adsorption from water on activated carbons in a packed-bed column. Biomass Convers. Biorefin. 2022, 14, 8283–8292. [Google Scholar] [CrossRef]
- Ronda, A.; Martín-Lara, M.A.; Osegueda, O.; Castillo, V.; Blázquez, G. Scale-up of a packed bed column for wastewater treatment. Water Sci. Technol. 2018, 77, 1386–1396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.