Abstract
Animal production generates gas emissions. It is imperative to reduce them as projections suggest that emissions will continue to increase with rising temperatures, alongside the intensification of agriculture to meet global food demand. Slurry acidification in-house can reduce these emissions. In this study, an acidification technology was installed in a pig-fattening barn to evaluate the influence of the addition of a mixture of organic acids, mainly lactic acid and glycolic acid, on NH3 and GHG emissions. A total of 384 pigs were allocated to four experimental rooms, two with additive applied to the slurry pits by a spraying system and two as a control. In high-temperature conditions, the spraying system discharged additive over the slurry which, in contrast with other systems, was stored inside the rooms during the whole trial. The concentration of NH3 and GHG, the temperature, and the air extraction rate were measured continuously. A significant reduction in the emissions of the gases evaluated was achieved. NH3 emissions were reduced by 26.8%, CH4 by 23.6%, N2O by 25.0%, and CO2 by 28.7%. The role of the dynamic spraying system is considered essential to prevent the acidification effect being reversed by the buffering effect of the slurry itself.
1. Introduction
The OECD-FAO Agricultural Outlook 2020–2029 forecasts that the global meat supply will expand to meet growing demand over the period 2022–2031, reaching 377 million tonnes in 2031. Pork accounted for 35.6% of global total meat consumption in 2017, and it is projected to continue this trend in the coming decades []. The increase in global pork production over the next decade is expected to be largely driven by the recovery from African swine fever in the Asian region, and this growth accounts for two-thirds of the additional global production []. However, pork production in the European Union is projected to decline slightly as environmental concerns are expected to limit its expansion.
Therefore, its economic impact will continue to be significant, but so will its environmental impact, as livestock activities have a significant impact on virtually all aspects of the environment, including air and climate change, land and soil, water, and biodiversity []. Livestock production is known to result in gas emissions such as ammonia (NH3), methane (CH4), nitrous oxide (N2O), and carbon dioxide (CO2). In pig farms, NH3, CH4, and N2O are products of manure decomposition, while CO2 is primarily a product of animal metabolism. There are also secondary reactions in slurry, such as ammonium (NH4+), under acidic or neutral pH conditions, or NH3 at higher pH levels, which contribute directly to fine particulate matter emission and once removed, to ecosystem fertilisation, acidification, and eutrophication []. Excessive deposition of reactive nitrogen can reduce the biodiversity of terrestrial ecosystems [].
However, reducing NH3 emissions where possible is imperative, as current projections suggest that emissions will continue to increase with rising temperatures due to the current climate crisis [,], alongside the increasing intensification of agriculture to meet global food demand []. Agriculture, mainly livestock production, is the largest contributor to NH3 emissions, accounting for over 81% of global NH3 emissions []. In Spain, 96% of NH3 emissions come from agriculture, of which 43% come from livestock manure management and 16% from the pig sector []. For this reason, NH3 is the target gas of this work, although the effect of the proposed technique on greenhouse gases (GHG) was also measured, as there is little information on the effect of manure acidification on GHG emissions in pig farms.
In terms of GHG emissions from the sector, CH4 is globally distributed due to its long residence time (~8.4 years) and contributes to global warming with a global warming potential 21-times that of CO2 []. Likewise, once emitted, N2O is globally distributed due to its long residence time (~100 years) and contributes to both tropospheric warming and stratospheric ozone depletion []. CO2 is produced during the oxidation of carbon compounds in metabolic processes. Increasing the efficiency of dietary nutrient use is expected to reduce CO2 production [].
Although pig production systems have reached high performance levels over the last few decades, there is still room for improvement in terms of environmental sustainability []. Slurry additives are natural or synthetic products with different effects: a reduction in gaseous emissions, mainly NH3; a reduction in unpleasant odours; an increase in the fertilisation value of slurry; or the inactivation of pathogenic organisms, among others. Nowadays, a wide range of additives for slurry and manure treatment are available on the market. Concentrated sulphuric acid (H2SO4) is the most widely used because it is the least expensive of the acids currently available []. However, there are some disadvantages associated with the use of this acid, such as its hazardous handling, corrosiveness to equipment, and the fact that it can produce volatile sulphur compounds which are extremely toxic at low concentrations []. For this reason, it was considered necessary to investigate the efficacy of organic acids, which have been poorly studied to date []. Organic acidifiers have advantages such as their availability in nature and that they do not affect the health and safety of animals and workers, but they are weaker acids, and higher doses are required to achieve and maintain a similar acidifying effect on slurry than using mineral acids.
However, it is difficult to demonstrate the effectiveness of most of added acids at the farm level. Although it is known that slurry acidification reduces NH3 emissions, some interactions affect its effectiveness in-house: slurry pit versus emissions from soiled surfaces []; slurry composition, ventilation rate []; type of additive, organic or chemical; pH of the slurry, the presence of microorganisms, ammonium concentration, application method or animal species [,,,]. A positive relationship between temperature and NH3 emission, which is attributed to higher indoor temperatures and high ventilation rates in summer, is usually observed [,,,]. It is therefore important to evaluate the effectiveness of acidification under conditions that represent full production and complete production cycles []. In order to study the possible effect of the additive under evaluation in the most unfavourable conditions, with the highest emissions, it was decided to carry out the trial in summer.
Another of the most common problems that can have a significant impact on the effectiveness of the treatment is the large volume of mixture that needs to be handled in the case of those additives that are added directly to the slurry in-house, making the spray system critical. This problem may have contributed to the fact that other studies [,,] incorporated more sophisticated in-pit spray systems for improving the low efficacy of some additives. However, a comprehensive assessment of an organic acid mixture utilized for in-house slurry acidification during pig fattening, alongside the deployment of an innovative spraying system that enhances the advantageous effects on emission reduction from that additive, has yet to be conducted.
Based on previous reviews, the aim of this study was to evaluate the influence of the addition of a mixture of organic acids inside the pits, using a dynamic spraying system, on the acidification of the slurry and the emissions of NH3 and GHG in a commercial pig-fattening farm under summer conditions.
2. Materials and Methods
2.1. Experimental Design
The study covered 3 months during the growth and fattening phase of a batch of pigs to cover the hot season (from May to July). During the final 70 days of the fattening period, two treatments were compared: T1, the control group (no additive applied; two rooms), and T2, the acidified group, which received a variable dose of additive using a dynamic spraying system in the pits of two additional rooms. This system allows the application of an optimal amount of additive at the right time depending on the environmental conditions, by increasing the application rates at high temperatures or high NH3 concentrations indoors, according to the manufacturer’s settings. The higher the temperature or the higher the NH3 concentration, the more acidifier was sprayed.
2.2. Experimental Facilities
The study was conducted in a commercial farm located in Segovia, Spain. The animals were housed in clean and disinfected facilities. The building had 6 rooms with total slats and independent pits. Each room was 7.13 m wide, 13.10 m long, and had a 0.49–0.70 m deep dung pit under a fully slatted concrete floor with a surface area of 81.08 m2 per room (Figure 1). There were 8 pens in each room (3.09 × 3.28 m). Each room was ventilated by a 0.30 m diameter chimney fan with variable speed depending on the temperature that operated continuously. The investigation was conducted in four of the six rooms, and in order to prevent variations in ventilation rates between treatments, the fans were synchronized with the temperature in room number 2.
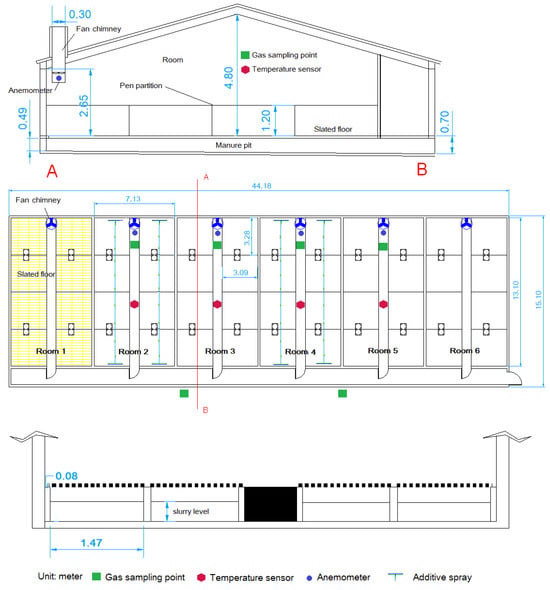
Figure 1.
Cross section (top), floor plan (middle), and pit detail (bottom) of the experimental facilities, with gas sampling points, temperature sensors, anemometers, and additive spray locations indicated. A-B: Cross-section line.
2.3. Experimental Animals and Diet
A total of 384 Pietrain boar × (Large White × Landrace) pigs, 60 days old with an average live weight of 25 kg, were allocated to four test rooms; 12 pigs were housed in each 10.13 m2 pen, i.e., 96 animals per room (48 males and 48 females). Animals were housed in pens according to their initial weight and sex. Hence, the average weights were equal among the pens, rooms, and treatments at the start of the trial. The duration of the experiment corresponded to the entire fattening period of the animals.
To analyse the productive performance of the animals, each pig was individually weighed at 60, 90, and 120 days of age. The average feed intake per pen was also monitored. The information obtained was used to calculate the production parameters (average daily gain and feed consumption and conversion).
Productive performance were compared between treatments: T1, or the control group, where no additive was applied (rooms 3 and 5), and T2 or the acidified group, where an additive was applied with the dynamic spraying system (rooms 2 and 4). The incidence of pathologies and the need for veterinary treatment (identification of animals, products, days of treatment, doses) were recorded daily. Pigs removed due to sickness or death were also recorded, indicating the cause of death or removal.
The experimental diets were formulated by Animal Data Analytics according to the raw material composition of the FEDNA tables []. The nutrient requirements of the animals were those suggested by the NRC [] according to their age. The diets were manufactured at the Proinserga factory (Fuentepelayo, Segovia).
2.4. Additive and Spraying System
The product evaluated was a biodegradable aqueous additive that was automatically applied to slurry pits using a spraying system to minimise the mixing problems commonly encountered with other systems. The components of the experimental additive were lactic acid (330 kg/t), glycolic acid (170 kg/t), polyvinyl alcohol (50 kg/t), iron sulphate (30.0 kg/t), sulphuric acid (0.600 kg/t), and water (419 kg/t).
The spray system used consisted of a 20 L/minute main pump with a 75 L storage vessel. Spray liquid was pumped through two parallel main lines to the two pit areas of each treated compartment (room 2 and room 4). A total of two branch lines were diverted from each main line to the pit area of each pen (Figure 2). These branch lines were screened from the pigs by aluminium pipes. Under the slats of each pen, the lines were connected to the centre of a spray boom fitted with 10 Lurmark HCX3 (Pentair, Cambridge, UK) nozzles on no-drip nozzle holders. This system allowed the optimum amount of additive to be applied at the right time, as it was linked to the environmental monitoring equipment in room 2 to increase the application rate at high temperatures and high NH3 concentrations, in accordance with the manufacturer’s specifications. The additive was applied throughout the experimental trial, coinciding with the final fattening period of the animals (70 days in total). Once a week, the volume of additive used was calculated by measuring the height of the storage tank.
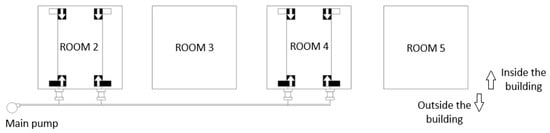
Figure 2.
Floor plan of the spraying system used.
2.5. Slurry
The manure accumulated in the indoor pits of the house throughout the test was analysed by sampling on days 21 and 42 and at the end (day 70) of the trial. PRODESTUR laboratory in Segovia (Spain) was responsible for the analyses. In each sampling, after mixing the slurry with a stick, four slurry samples were taken in each room: two in the right side of the slurry pit and two on the left side of the slurry pit. Each slurry sample was collected in a 1 L plastic cup and quickly transported to the laboratory where the contents were homogenised immediately on arrival and analysed.
The parameters analysed were pH, dry matter, organic matter, total Kjeldahl nitrogen (TKN), and ammonium nitrogen (N-NH4+). The analytical methodology used was as follows: pH, electrometric method []; dry matter, gravimetric method []; organic matter, calcination method []; total nitrogen, Kjeldahl method []; ammoniacal nitrogen, magnesium oxide method []. Ash was calculated as the difference between dry matter and organic matter.
The volume of slurry produced was estimated by measuring the pits’ height levels on a weekly basis.
2.6. Gas Emissions, Temperature, and Air Flow
During the final growth phase, the concentrations of NH3, CH4, N2O, CO2, and water vapour were measured at six sampling points, one inside each room and two in the corridor, as well as the temperature and air extraction rate (four sampling points indoors), on a semi-continuous basis (Figure 1). For measuring NH3 and GHG concentrations on the farm, a gas analyser based on photo-acoustic spectrophotometry (Innova 1412-5, SIR, S.A., Madrid, Spain) was used, with a 1309 multipoint sampler and a data acquisition system with Lumasoft 7850 software. The system required 50 s to measure the gas concentration, and 100 s for stabilisation/cleaning, allowing the measurement to be made at the six points every 15 min. This system has been used previously by other authors [,,]. Air entered the equipment through 40 m of Teflon tubing, which was found to be short enough to allow the pump to generate the necessary vacuum required for this operation. The air was filtered at the entrance of the tubing (Figure 1) using a Morgan Clean Contact filter (nSpire Health Ltd., London, UK).
A National Instruments model LM35 thermometer (Austin, TX, USA) was used to measure temperature, and the gas measurement system was also used to measure water vapour (along with the other gases, thanks to the multi-sampling technique). The water vapour concentration was only monitored to allow the gas analysers to make the appropriate internal corrections. Therefore, these values were not available in this study. Dwyer Air Velocity Transmitter, model nº 640-O (SIR, S.A., Madrid, Spain) anemometers were installed to evaluate the exhaust air flow. All measuring equipment was previously calibrated by the installation company (SIR, S.A., Madrid, Spain).
In buildings with forced ventilation, the emissions of the gases are determined by measuring the concentration of the respective gas at the air inlet and outlet and multiplying the difference by the ventilation flow [,,,,]. Therfore, the average concentration of each gas during the measurement period (15 min) was multiplied by the average ventilation flow for the same period to calculate the gas emissions.
where E is the emission in g/h, Q is the extracted air flow in m3/h, and ΔC is the difference between the concentration of each gas at the inlet and at the outlet, measured in g/m3.
E = Q × ΔC
Both the experimental design and the measurement of emissions were carried out according to the principles of the VERA Protocol 2.0 [], with some modifications. In line with this, only measurements where at least 80% of the data collected daily were correct were considered valid. To compare experimental treatments, the concentrations of gases, room temperature, and air extraction rates were monitored every 15 min and averaged hourly.
As differences in room temperatures were observed between day and night, it was considered interesting to analyse how the time of day influenced the emissions and the efficacy of the additive. Considering that at the beginning of the test, sunrise was at 6.55 a.m. and sunset was at 9.34 p.m., and that at the end of the test, sunrise was at 7.01 a.m. and sunset was at 9.45 p.m., it was considered that the measurements taken between 7.00 a.m. and 9.40 p.m. were daytime measurements and those taken between 9.41 p.m. and 6.59 a.m. were nighttime measurements.
2.7. Statistical Analysis
The statistical software used was SAS v.9.4. []. Normality was assessed (proc UNIVARIATE) using the Shapiro–Wilk test for variables related to slurry composition and performance parameters. Homoscedasticity was assessed for all variables using Levene’s test. For those variables that followed a normal distribution and were homoscedastic, the data were analysed by ANOVA (proc MIXED). For variables that followed a normal distribution but were heteroscedastic, paired t-tests with Welch correction were used. Finally, variables that were not normally distributed were analysed using the non-parametric Kruskal–Wallis test. The treatment was included as a fixed effect in the analysis of all variables. The room was considered as the experimental unit for variables related to manure composition and gas emissions. For performance measurements, the pen was considered as the experimental unit, the room was included as a random effect and body weight on day 0 as a covariate.
Regarding the analysis of gas emissions, the measurements every 15 min were grouped into hourly data. Taking into account the hourly day distribution, the hourly data were also divided into day and night; that is, we had two values (one for day and one for night) for each day. Then, the residuals of the estimated models were used to verify the assumptions of normality and homogeneity of variances. Since normality was not met, the appropriate Box–Cox transformation was applied (logarithmic transformation). A mixed linear model (PROC MIXED) was used in which the fixed effects were the treatment, week, moment (day/night), and the interaction between the treatment and moment. The random effects included the room and the week nested within the room. Finally, temperature was included as a covariate. Subsequently, the correlation of the residuals corresponding to the observations on the days of the week (day 1 to day 7) was verified by comparing the following models: without correlation, with an autoregressive model of order 1 (AR(1)), and with an autoregressive–moving average (ARMA) model with p = 1 as the autoregressive term and q = 1 as the moving average term (ARMA(1,1)). The model that best fit the data was chosen using the AIC and BIC information criteria, selecting the one with the lowest value. The selected models were the AR(1) model for NH3, CH4, and C2O and the ARMA(1,1) model for N2O. The correlation between the amount of additive applied by the dynamic spray system and the temperature and NH3 concentration in room 2 was analysed by linear regression, and, when significant, Pearson correlation coefficients were also calculated. As additive–temperature had a high R2 value, Pearson’s correlation was also performed. Differences with p ≤ 0.05 were considered statistically significant, while 0.05 < p ≤ 0.10 was considered a near significant trend. Least square means are reported.
3. Results
3.1. Ambient Conditions
Each room was ventilated with variable speed fans according to temperature. The fans in the four experimental rooms were synchronised to avoid influence between treatments, so the air extraction rate mean value in the four rooms was equal and the average temperatures in the four rooms were similar. In fact, there were no significant differences in temperature (29.8 vs. 30.6 °C; p = 0.749) and ventilation flow (8.33 vs. 8.40 m/s; p = 0.454) in the T1 and T2 rooms, respectively.
However, the daytime temperature (31.7 °C) was 9.15% higher (p < 0.001) than the nighttime temperature (28.8 °C). Similarly, as the ventilation was dependent on the indoor temperature, the daytime exhaust air velocity was 23.2% higher than the nightly flow rate (9.54 vs. 7.31 m/s; p < 0.001).
The graphs in Figure 3 show the maximum and minimum temperatures in each room, as well as the thermal oscillation during the trial. Data collected during the first 10 days of the trial were rejected because they did not reach a sufficient number of correct data, according to the VERA protocol [].
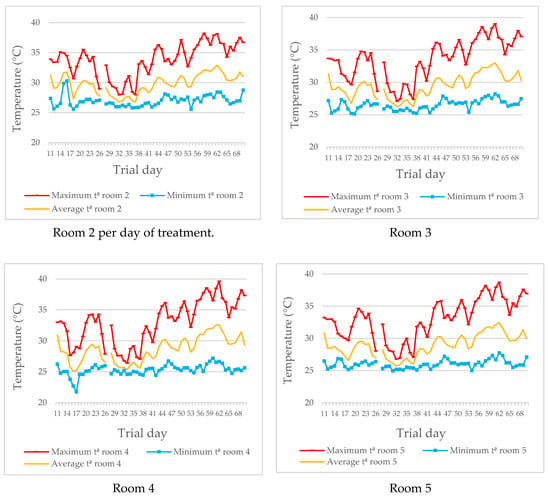
Figure 3.
Maximum, minimum and average temperature (°C) in each of the rooms during the experimental trial.
Temperatures increased as fattening progressed (0.11 °C per day on average). The lowest temperatures during the trial were recorded around day 33 of the trial (26.1 ± 0.061 °C) and the highest on day 63 of the trial (32.7 ± 0.221 °C). The average daily temperature variation was 7.15 ± 0.195 °C, with the highest temperatures in the middle hours of the day (from 15 to 18 h; 32.6 ± 0.227 °C) and the lowest in the early morning (from 4 to 6 h; 26.4 ± 0.082 °C).
3.2. Slurry Production and Composition
Slurry was allowed to accumulate in the indoor pits throughout the experimental period without being emptied. The total volume of slurry was calculated from the slurry height in the centre of the pit. In T1 (control rooms 3 and 5), the volume was 27.6 and 27.2 m3, respectively. In T2 (rooms 2 and 4), where the additive was added, the final volume was higher (30.7 and 30.8 m3, respectively). This difference was consistent with the amount of additive sprayed.
This resulted in an average slurry production per pig per day of 4.52 L, which is within the range reported in the literature (3–8 L per pig per day) according to the bibliographic review conducted in IRPP-BREF [].
The slurry composition of the different treatments is shown in Table 1. The T2 treatment, with a variable dose of additive applied with the dynamic spraying system, reduced the pH by 0.68 units. This reduction in pH could lead to an increase in the amount of nitrogen retained in the slurry, with significant differences in total Kjeldahl nitrogen (35.9% higher in T2), N-NH4+ (21% higher in T2), and organic nitrogen (2.84% higher in T2.). In agreement with other studies [], the additive significantly increased the dry matter concentration (29.7%). Significant differences between treatments were also obtained for the ash percentage (8.24% higher in T1).

Table 1.
Slurry composition in the control group (no additive applied) and acidified group (variable additive dose applied with a dynamic spraying system).
Figure 4 shows the pH evolution of the slurry in the different samplings. It is observed that throughout the entire trial, a lower pH was maintained at T2 but the reduction in pH was increased in the last stage of the fattening period.
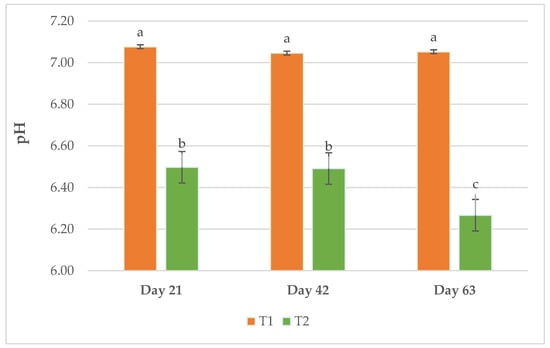
Figure 4.
pH value of slurry in the different samplings. Different letters (a, b, c) indicate p < 0.05.
3.3. Additive Use
A total of 3.35 m3 of additive was sprayed. The amount of additive applied (L) in each measurement period is shown in the Figure 5.
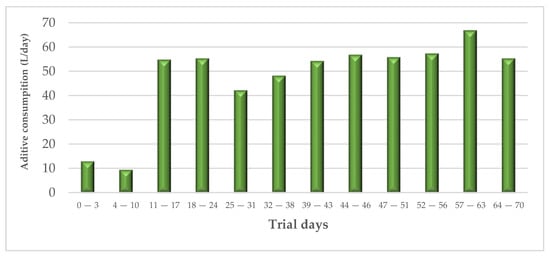
Figure 5.
Total average additive consumption during the experimental trial (in L/day).
Considering the total slurry production in rooms 2 and 4 (54.8 m3), the applied dose of acidifier was 6.12%.
The correlation between the amount of additive applied with the dynamic spraying system and the temperature and NH3 concentration in room 2 was analysed by linear regression. In the case of additive–NH3 concentration, no statistically significant relationship was observed and the R2 obtained was low (R2 = 0.146). However, as the additive temperature showed a high R2 value (R2 = 0.782), Pearson’s correlation was also performed. The Pearson’s correlation coefficient was 0.931 with statistical significance (p < 0.001).
3.4. Gas Emissions Results
The evolution of the average NH3, CH4, N2O, and CO2 concentrations per treatment during the trial is shown in Figure 6. The data collected during the first 10 days of the trial were discarded because they did not provide a sufficient number of correct data, according to the VERA protocol []. The concentrations of all gases were maintained within normal ranges in the grow-finish pig rooms throughout the experimental period.
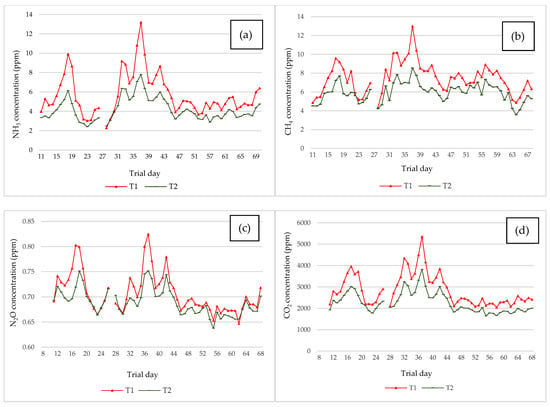
Figure 6.
Evolution of NH3 (a), CH4 (b), N2O (c), and CO2 (d) concentrations. Daily net concentration average of the rooms for each treatment during the trial (ppm). T1 = control group (no additive applied); T2 = acidified group (variable dose of additive applied with a dynamic spraying system).
The highest NH3 concentrations corresponded to T1 (control group), ranged from 2.30 to 13.2 ppm, with a mean concentration of 5.80 ± 0.285 ppm during the experimental period. T2 (acidified group) showed lower concentrations than T1 with a mean of 4.18 ± 0.158 ppm and a range of variation between 2.40 and 7.80 ppm (Figure 6a).
The highest CH4 concentration corresponded to T1 (control group), with a mean concentration of 7.49 ± 0.220 ppm, (4.3–13.0 ppm). T2 (acidified group) showed lower concentrations than T1 (5.96 ± 0.137 ppm as mean with a range 3.60–8.50 ppm, Figure 6b).
The N2O concentrations were lower in T2 (acidified group, Figure 6c) than in T1 (control group). The control group (T1) had an average N2O concentration of 0.709 ± 0.005 ppm, while T2 had a mean N2O concentration of 0.689 ± 0.003 ppm. These concentrations ranged between 0.647–0.825 and 0.637–0.751 ppm for T1 and T2, respectively (Figure 6c).
In the case of CO2 concentration (Figure 6d), the treatments showed differences. The average CO2 concentration during the first trial was 2862 ± 99.1 ppm in T1 (control group, 2041–5357 ppm) and 2268 ± 64.4 ppm in T2 (acidified group, 1642–3819 ppm).
The mean gas emissions (kg pig−1 year−1) of the different treatments are shown in Table 2.

Table 2.
Gas emissions (kg pig−1year−1) in the control group (no additive applied) and acidified group (variable dose of additive applied with a dynamic spraying system) and the effect of the time of application (day vs. night). Data collected every 15 min have been averaged for statistical analysis obtaining one day-value and one night-value per day.
The NH3 emissions obtained in T1 were 26.8% higher than in T2 (p < 0.001), and differences between daytime and nighttime emissions were observed but without statistical significance (p = 0.186).
The CH4 emissions with the control treatment were 23.6% higher than with the acidification treatment (p < 0.001) and higher emissions were observed at night than during the day (p < 0.001).
Significant reductions were also observed for N2O (25.0% less in acidified group, p < 0.001). Daytime emissions were higher than nighttime emissions, with statistical significance (p = 0.016).
Finally, the average CO2 emissions observed in our study were 28.7% higher in T1 than in T2 (p < 0.001). Emissions during the day were higher than at night but without statistical significance (p = 0.638).
The interaction between treatments and the time of day (day vs. night) did not show statistically significant differences for any of the gases, only NH3 showed a trend of a higher-efficiency effect in reducing this emission at night than during the day.
3.5. Animal Performance Results
The growth performance of the pigs housed in the different rooms is shown in Table 3. The observed production parameters were within the usual range of values observed for the category of animals evaluated. This indicates that the animals were in good health and that the treatment had no negative or positive effect on performance. In the growing period, no significant differences between treatments were observed in any of the parameters evaluated (p > 0.05). In the fattening period, the ADG was similar between treatments, but T2 (acidified group) significantly increased feed intake (p < 0.05) with a tendency to increase the feed conversion ratio (p = 0.056).

Table 3.
Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) of pigs in the control group (no additive applied) and the acidified group (variable dose of additive applied with a dynamic spraying system) during the growing (60 to 95 days of age) and fattening periods (95 to 123 days of age).
4. Discussion
4.1. Gas Emissions
Numerous studies have shown that slurry acidification is effective in reducing gas emissions []. pH is a key factor that can have a major influence on slurry characteristics and microbial activities, which can affect gas emissions, especially on NH3 and CH4 []. However, most studies have evaluated the use of inorganic acids, which are dangerous to handle and harmful to the environment, such as H2SO4, applied outside the buildings, in outdoor slurry storage. In this trial, the use of a mixture of organic acids (poorly studied so far []) combined with a novel spraying system, which applies the additive directly into the slurry pit inside the housing, was evaluated.
The NH3 concentrations in this experiment are in line with those presented by other authors and reviewed by Van Ransbeeck et al. [], in particular the work of Aarnink et al. [] and Ngwabie et al. [] whose animal husbandry conditions were very similar to those of this study. The low concentrations in these studies could be related to the high ventilation rates during the trial (summer period). More recent work [] also found low NH3 concentrations in pig-fattening facilities. NH3 emissions obtained were 2.39 and 1.75 kg pig−1 yr−1 in T1 and T2 treatments, respectively, which is in agreement with the values presented by other authors [,,,,] who obtained NH3 emissions between 1.6 and 2.8 kg pig−1 yr−1.
Low pH reduces NH3 emissions by increasing the NH4+ fraction and reducing the free NH3 fraction in the NH4+/NH3 buffer system equilibrium. Some authors have shown that slurry acidification below pH 6 can reduce NH3 emissions by 70–95% []. Fuchs et al. [] reviewed studies that reduced NH3 emissions using acetic acid and sulphuric acid, achieving efficiencies between 47 and 92%, with a pH below 6 in all studies analysed. In this trial, the application of the acidifying additive was continuous over time and homogeneous throughout the slurry pit, thanks to the dynamic spraying system installed in the pits, but the quantity and organic acids applied only reduced the pH to a value of 6.52. However, with this small reduction, it was possible to reduce NH3 emissions by 26.8%, with a long-term and sustained effect throughout the fattening period.
For NH3, differences between day and night emissions were observed, but without statistical significance (p = 0.186), being higher at night. Other authors [] found different diurnal patterns, with higher NH3 emissions during the day than at night. Higher emissions during the day have been suggested to be related to three factors: higher animal activity during the day [], higher temperatures, and higher ventilation fluxes []. However, according to Quiniou et al. [], temperature has a significant effect on the activity of feed intake between day and night. Their studies showed that at a temperature of 29 °C, only 55% of meals were consumed during the day, compared to 69% of daily meals at a temperature of 19 °C, with reduced daytime activity. Saha et al. [] observed that most animals defecated 1–2 h after feeding, with a corresponding peak in emissions. If the warmer conditions in this trial (31.7 °C) shifted feeding times to the night hours, when room temperatures were significantly lower (28.9 °C), it is possible that these emission peaks occurred at night, compensating for the higher daytime emissions due to higher temperatures and ventilation rates. This could explain why a difference in ammonia emissions was observed between day and night, with higher emissions at night but without statistical significance.
Regarding the CH4 concentration, the values obtained by Philippe et al. [] varied between 7.0 and 8.6 ppm, which is in line with our results. CH4 emissions in the control treatment were 4.06 kg CH4 pig−1 yr−1, while the acidification treatment resulted in emissions of 3.10 kg CH4 pig−1 yr−1. These emissions were similar to those found by Ni et al. [], Zifei and Powers [], or Van Ransbeeck et al. []. Other studies have demonstrated the effectiveness of acidification in reducing CH4, as microbial activity and methanogenesis are reduced in acidified slurry compared to raw slurry []. In the literature review by Fuchs et al. [], pH values below 6 achieved CH4 reductions in the range of 50 to 95%. In our study, a pH of 6.52 reduced these emissions by 23.6% throughout the experimental study. Higher CH4 emissions were observed at night than during the day (p < 0.001), which could be related to animal activities due to feeding routines at night because of the high diurnal temperatures, as explained in the previous paragraph for NH3. Other works [,] support this hypothesis since they affirmed that CH4 emissions are more affected by animal activity than by environmental conditions.
In the case of the N2O concentration, the values obtained by Philippe et al. [] were lower than ours (0.41–0.50 ppm) while the results obtained by Van Ransbeeck et al. [] were higher, varying between 0.301 and 1.304 ppm. N2O emissions were 32.0 and 24.0 g pig−1 yr−1 in T1 (control group) and T2 (acidified group), respectively; these values are in the range reported by other authors in similar studies [,,]. Acidification with the dynamic spraying system in our study had a positive effect on N2O emissions and significant reductions were observed (25.0% in the acidified group). There are inconsistencies in the reviewed literature regarding the effect of acidification on N2O emissions. Pereira et al. [] found no effect on emissions of this gas with alum-acidified slurry. However, Regueiro et al. [], in agreement with our study, found a reduction in N2O due to alum acidification, as acidification can inhibit the nitrification/denitrification process. Correspondingly, previous studies reported a decrease in nitrification in acidified manure after soil application []. Wang et al. [] achieved a 75% reduction by keeping the pH below 5.5. However, in our work, a continuous N2O reduction of 25.0% was achieved at a pH of 6.52. Due to higher daytime temperatures and ventilation rates, N2O emissions were higher during the day than at night (29.0 vs. 26.0 g pig−1 year−1; p = 0.016). Dietary patterns did not influence the emission of this gas as seems to have occurred in the case of NH3 and CH4 because feeding patterns would not play a role since the N2O is product of the pit manure’s decomposition.
Finally, with regard to the CO2 concentration, Van Ransbeeck et al. [] and Chantziaras et al. [] obtained similar values in the farms they studied, whereas the CO2 concentration was significantly lower (around 800 ppm) in the studies by Philippe et al. [] and Calvet et al. []. The average CO2 emissions observed in our study were 1321 kg CO2 pig−1 yr−1 in the control group and 942 kg CO2 pig−1 yr−1 in the acidified group. The emission values are in agreement with other authors [,,,,]. A 28.7% reduction in CO2 was found and CO2 emissions decreased with the acidification treatment because acidification can change the dry matter content, and several authors have shown that this effect is more significant in slurries with a low dry matter content []. In the case of long-term acidification, the degradation of organic matter can be slowed down (71.7 vs. 73.9% of d.w. in this work), reducing the production of carbonate and CO2 emissions. As in the case of N2O, the increase in daytime CO2 emissions due to higher temperatures and ventilation flow was not compensated by the increased nocturnal activity of the animals, and CO2 emissions were higher during the day than at night (1122 vs. 1090 kg pig−1 year−1) but without statistical significance (p = 0.638).
For all evaluated gases, interaction between treatments and the time of day (day vs. night) did not show statistically significant differences; only NH3 presented a trend of a higher efficiency at night than during the day. That is, the treatment had the same effect on emissions regardless of the time of day (day or night), which shows that its effectiveness did not vary with changes in room temperature during this test (31.7 vs. 28.8 °C; p < 0.001). It would be advisable to evaluate the efficiency of this acidifier under lower-temperature conditions (winter period) to assess its effects under these conditions.
From our results, it can be concluded that it is possible to reduce NH3 and GHG emissions with a lower pH reduction than usually used in other scientific studies and with organic acidifiers that are less dangerous and harmful to the environment. In order to prevent the acidification effect from being reversed by the buffering effect of the slurry itself, it is considered essential to have a dynamic spraying system that allows minimum and continuous doses of acid to be applied.
4.2. Spraying System
There is no extensive literature on the use of organic acidifiers in pig facilities to reduce NH3 emissions; therefore, it is difficult to assess whether the dosage is within the range of other experiments. In a previous study [], alternative acids were tested to reduce the pH under storage conditions to a value of 5.5, and the amount of lactic acid used was 380 mL per kg of slurry. In our experiment, the volume of acid used was 19.15 mL of lactic acid, 7.98 mL of glycolic acid, and 0.023 mL of sulphuric acid per kg of slurry, which is less than that used by Overmeyer et al. [], but we achieved a lower pH reduction (5.5 vs. 6.41, respectively); thus, the spraying system seems to have optimised the use of the additive, perhaps because a higher pH reduction could be achieved in the slurry surface.
Regueiro et al. [] demonstrated the effectiveness of different organic and inorganic acidifiers (sulphuric acid, lactic acid, acetic acid, citric acid, and alum) in reducing NH3 emissions during storage. Similarly, Overmeyer et al. [] compared the effectiveness of lactic acid and sulphuric acid in acidifying slurry and concluded that, in principle, any acid is suitable for acidifying slurry to a pH of 5.5. However, the organic acids are degraded and the pH rises more quickly than with mineral acids. This demonstrates the importance of implementing an additive application system when using organic acids, which allows the acidifier to be applied throughout the fattening period in the slurry pits, as in the case in our study.
The dynamic additive application system was theoretically linked to the environmental condition of measurement equipment in room 2, linking the acidifier discharge to high temperatures and a high NH3 concentration. Based on the results obtained, the relationship with high temperature was demonstrated, while the influence of the NH3 concentration could not be confirmed. This is probably due to the fact that during the trial, which was carried out in hot weather and with a high ventilation rate, the NH3 concentration did not reach the minimum programmed by the spraying system to activate the discharge. This was not the case for the temperature, which clearly influenced the application of the additive. For example, in the period between 57 and 63 days of the trial, when the maximum temperature was reached, the additive discharge was the highest (66.6 L/day), and the pH reduction was the most evident at that time. Reducing the NH3 concentration thresholds at which the sprayer is activated, as well as testing the system under winter conditions (lower ventilation and higher ammonia concentration in the rooms), would be of great interest to complement this work.
The advantage of this spraying system is twofold. On the one hand, the small, continuous, fine droplet application of the additive into the slurry pit would have kept the first layer of slurry at a sufficiently low pH to effectively reduce emissions and prevent the buffering effect or degradation of the organic acids used from reducing their effectiveness. On the other hand, applying the additive only under conditions where peak emissions could occur (high temperatures) would have achieved a reduction in the amount of additive used, with consequent economic benefits. It would be interesting to test the system’s performance over time and evaluate maintenance needs and potential environmental constraints, such as high ambient temperatures and a long-term corrosive environment in the pit.
4.3. Animal Performance
The current standard for safe NH3 levels is 25 ppm, but recent research reports indicate that maintaining a level of no more than 10 ppm may help prevent health risks to both pigs and humans []. Due to the low gas concentrations achieved during the experimental period, no difference in production performance between treatments was expected. Although the gas concentrations in the control treatment were significantly higher, they did not reach levels that could affect the animals in the short or long term (only for 4 days did the NH3 level exceed 10 ppm in the control treatment). However, there was a slight increase in feed consumption (5%) during the last phase of the fattening period, which resulted in a tendency to increase the feed conversion ratio. This could be due to the fact that the better environmental conditions in the T2 rooms (acidified slurry) resulted in higher animal activity, as poorer environmental conditions can reduce social interaction [].
Since the animal’s productive performance was not improved by the reduction in the concentration of the evaluated gases, the cost of the installation of the spraying system and of the additive used was not compensated by this possible benefit. Future research is therefore advised to assess the economic benefits of applying this technique while accounting for real market prices.
5. Conclusions
The aim of this study was to evaluate the influence of the addition of a mixture of organic acids on the acidification of pig slurry and on the emissions of NH3 and GHGs (CO2, CH4, and N2O), using a dynamic spraying system and under commercial production conditions. The following findings can be concluded based on the results:
- The mixture of organic acidifying compounds applied resulted in a significant reduction in gas emissions.
- It was possible to reduce NH3 and GHG emissions with a lower pH reduction than usually used in other scientific studies and with organic acidifiers.
- In order to prevent the acidification effect from being reversed by the buffering effect of the slurry itself, it was essential to have a dynamic spraying system that allowed minimum and continuous doses of acid to be applied.
- Applying the additive under conditions where peak emissions could occur (high temperatures) would have resulted in a reduction in the amount of additive used.
- Animal performance was not improved using the additive because, although the gas concentrations in rooms were significantly higher in the control treatment, they did not reach levels that could affect animal health.
- It would be advisable to evaluate the efficiency of this acidifier system under lower-temperature conditions (winter period), as well as the economic impact of the implementation of this technique.
- Other combinations of organic acids could be tested with this spraying system.
Author Contributions
Conceptualisation, G.M., C.P. and M.J.S.; methodology, G.M., M.R. and P.G.-R.; validation, G.M., P.G.-R., C.P. and M.J.S.; formal analysis, G.M. and M.R.; investigation, G.M.; resources, C.P.; data curation, G.M.; writing—original draft preparation, G.M.; writing—review and editing, M.R., P.G.-R., C.P. and M.J.S.; visualisation, G.M.; supervision, P.G.-R. and M.J.S.; project administration, G.M.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Miguel Ángel Ibañez, working in Statistics at the Universidad Politécnica de Madrid, for his advice on the statistical analysis of the results.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| CH4 | Methane |
| CO2 | Carbon dioxide |
| D.W. | Dry weight |
| F.W. | Fresh weight |
| FAO | Food and Agricultural Organization of the United Nations |
| FCR | Feed conversion ratio |
| FEDNA | Fundación Española para el Desarrollo de la Nutrición Animal |
| GHG | Greenhouse gases |
| H2SO4 | Sulfuric acid |
| IPCC | Intergovernmental Panel on Climate Change |
| IRPP-BREF | Best Available Techniques Reference Document for Intensive Rearing of Poultry/Pigs |
| MITECO | Ministerio para la Transición Ecológica y el Reto Demográfico |
| N2O | Nitrous oxide |
| NH3 | Ammonia |
| NH4+ | Ammonium |
| NRC | Nutrient Requirements of Swine |
| OECD | Organisation for Economic Co-operation Development |
| PM | Particulate matter |
| SE | Standard error |
| TKN | Total Kjeldahl nitrogen |
| VERA | Verification of Environmental Technologies for Agricultural Production |
References
- OECD. Market Examinations in Mexico. Case Study of the Pork Meat Market; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2019. [Google Scholar] [CrossRef]
- Anghelescu, I. EU Agricultural Outlook 2024-2035: Projected Trends and Challenges. Ew Nutrition. Available online: https://ew-nutrition.com/eu-agricultural-outlook-2024-2035-projected-trends-and-challenges/ (accessed on 21 April 2025).
- FAO. Livestock’s Long Shadow; FAO: Rome, Italy, 2006; Available online: http://www.fao.org/docrep/010/a0701e/a0701e00.HTM (accessed on 20 April 2025).
- National Research Council (NRC). Air Emissions from Animal Feeding Operations: Current Knowledge, Future Needs; National Academies Press: Washington, DC, USA, 2003. [Google Scholar] [CrossRef]
- Reaka-Kudla, M.L.; Wilson, D.E. Biodiversity II. Understanding and Protecting Our Biological Resources; Joseph Henry Press: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- Skjøth, C.A.; Geels, C. The effect of climate and climate change on ammonia emissions in Europe. Atmos. Chem. Phys. 2013, 13, 117–128. [Google Scholar] [CrossRef]
- Sutton, M.A.; Reis, S.; Riddick, S.N.; Dragosits, U.; Nemitz, E.; Theobald, M.R.; Tang, Y.S.; Braban, C.F.; Vieno, M.; Dore, A.J.; et al. Towards a climate-dependent paradigm of ammonia emission and deposition. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130166. [Google Scholar] [CrossRef]
- Malley, C.S.; Hicks, W.K.; Kulyenstierna, I.J.C.; Michalopoulou, E.; Molotoks, A.; Slater, J.; Heaps, C.G.; Ulloa, S.; Veysey, J.; Shindell, D.T.; et al. Integrated assessment of global climate, air pollution, and dietary, malnutrition and obesity health impacts of food production and consumption between 2014 and 2018. Environ. Res. Commun. 2021, 3, 075001. [Google Scholar] [CrossRef]
- Van Damme, M.; Clarisse, L.; Franco, B.; Sutton, A.M.; Erisman, J.W.; Kruit, R.W.; van Zanten, M.; Whitburn, S.; Hadji-Lazaro, J.; Hurtmans, D.; et al. Global, regional and national trends of atmospheric ammonia derived from a decadal (2008–2018) satellite record. Environ. Res. Lett. 2021, 16, 055017. [Google Scholar] [CrossRef]
- MITECO. Inventario Nacional de Emisiones a la Atmósfera. Emisiones de Contaminantes Atmosféricos. Serie 1990–2023. Informe Resumen. November 2024. Available online: https://www.miteco.gob.es/content/dam/miteco/es/calidad-y-evaluacion-ambiental/temas/sistema-espanol-de-inventario-sei-/resumen-Inventario-CA-2025.pdf (accessed on 20 April 2025).
- De Klein, C.; Novoa, R.S.A.; Ogle, S.; Smith, K.A.; Rochette, P.; Wirth, T.C. Chapter 11. N2O emissions from managed soils, and CO2 emissions from lime and urea application. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; National Greenhouse Gas Inventories Programme: Hayama, Japan, 2006; Chapter 11; pp. 11.0–11.54. Available online: http://www.ipcc-nggip.iges.or.jp/ (accessed on 20 April 2025).
- Moehn, S.; Price, J.; Zijlstra, R.; Sauer, W.; Clark, O.; Ball, R.; Atakora, J.; Feddes, J.; Zhang, Y.; Leonard, J.; et al. Diet Manipulation to Control Odor and Gas Emissions from Swine Production. In Climate Change and Managed Ecosystems; CRC Press: Boca Raton, FL, USA, 2005; pp. 295–316. [Google Scholar] [CrossRef]
- Andretta, I.; Pomar, C.; Rivest, J.; Pomar, J.; Lovatto, P.A.; Neto, J.R. The impact of feeding growing-finishing pigs with daily tailored diets using precision feeding techniques on animal performance, nutrient utilization, and body and carcass composition. J. Anim. Sci. 2014, 92, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.; Sørensen, P.; Elsgaard, L. The Fate of Sulfate in Acidified Pig Slurry during Storage and Following Application to Cropped Soil. J. Environ. Qual. 2008, 37, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Borst, G.H. Acute poisoning of pigs with hydrogen sulfide as a result of acidification of slurry on a pig farm. Tijdschr Diergeneeskd 2001, 126, 104–105. [Google Scholar]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to sulfuric acid for slurry acidification: Impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Sommer, S.; Zhang, G.; Bannink, A.; Chadwick, D.; Misselbrook, T.; Harrison, R.; Hutchings, N.; Menzi, H.; Monteny, G.; Ni, J.; et al. Algorithms Determining Ammonia Emission from Buildings Housing Cattle and Pigs and from Manure Stores. Adv. Agron. 2006, 89, 261–335. [Google Scholar] [CrossRef]
- Kavolelis, B. Influence Ventilation V Rate on Ammonia Concentration and Emission in Animal House. Pol. J. Environ. Stud. 2003, 12, 709–712. [Google Scholar]
- Cao, Y.; Bai, Z.; Misselbrook, T.; Wang, X.; Ma, L. Ammonia emissions from different pig production scales and their temporal variations in the North China Plain. J. Air Waste Manag. Assoc. 2021, 71, 23–33. [Google Scholar] [CrossRef]
- Babot, D.; Blanco, G.; Sancho, V.; Ferrer, N.; Cartanyà, J. Efecto de dos niveles de ventilación sobre las emisiones de amoniaco y los rendimientos productivos en cerdos de engorde en otoño-invierno. SUIS 2021, 178, 20–23. [Google Scholar]
- Cartanyà, J.; Blanco, G.; Sancho, V.; Ferrer, N.; Babot, D. Efecto de dos niveles de ventilación sobre las emisiones de amoniaco y los rendimientos productivos en cerdos de engorde en primavera-verano. SUIS 2021, 177, 10–15. [Google Scholar]
- Petersen, S.O.; Hutchings, N.J.; Hafner, S.D.; Sommer, S.G.; Hjorth, M.; Jonassen, K.E. Ammonia abatement by slurry acidification: A pilot-scale study of three finishing pig production periods. Agric. Ecosyst. Environ. 2016, 216, 258–268. [Google Scholar] [CrossRef]
- Tengman, C.L.; Gralapp, A.K.; Goodwin, R.N. Laboratory Testing of Commercial Manure Additives for Swine Odor Control; USDA-ARS-National Swine Research and Information Center: Ames, IA, USA, 2001. [Google Scholar]
- Stinson, R.; Lemay, S.P.; Barber, E.M.; Fonstad, T. Effectiveness of Three Manure Pit Additives; Annual Resarch Report 1999; Prairie Swine Centre INC: Saskatoon, SK, Canada, 1999; pp. 30–31. [Google Scholar]
- Andersson, M. Performance of Additives in Reducing Ammonia Emissions from Cow Slurry. Allied Nutrition. 1994. Available online: https://alliednutrition.com/wp-content/uploads/2015/09/Performance-af-Additives-in-Reducing-Ammonia-sept-2004.pdf (accessed on 14 July 2025).
- De Blas, C.y.G.G.M.; García-Rebollar, P.; Gorrachategui, M.; Mateos, G.G. Tablas FEDNA de Composición y Valor Nutritivo de Alimentos para la Fabricación de Piensos Compuestos, 4th ed.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2019. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Swine, 10th ed.; National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- BOE. Real Decreto 2257/1994, de 25 de Noviembre, por el que se Aprueba los Métodos Oficiales de Análisis de Piensos o Alimentos para Animales y sus Primeras Materias; BOE-A-1995-5541; Ministerio de la Presidencia: Madrid, Spain, 1995; Available online: https://www.boe.es/eli/es/rd/1994/11/25/2257 (accessed on 10 May 2025).[Green Version]
- BOE. Orden de 30 de Noviembre de 1976 Sobre Métodos de Análisis de Productos Fitosanitarios y Fertilizantes; BOE-A-1977-91; Presidencia del Gobierno: Madrid, Spain, 1977; Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-1977-91 (accessed on 10 May 2025).[Green Version]
- Andersson, M. Performance of Bedding Materials in Affecting Ammonia Emissions from Pig Manure. J. Agric. Eng. Res. 1996, 65, 213–222. [Google Scholar] [CrossRef]
- Amon, B.; Sryvoruchko, V.; Amon, T.; Moitzi, G. Can the additive ‘Effective Micro-Organisms (EM)’ reduce ammonia and greenhouse gas emissions from slurry stores? In Proceedings of the 11th International Conference of the FAO ESCO-RENA Network on Recycling of Agricultural, Municipal and Industrial Residues in Agriculture (RAMIRAN), Murcia, Spain, 6–9 October 2004; pp. 3181–3327. [Google Scholar][Green Version]
- Overmeyer, V.; Trimborn, M.; Clemens, J.; Hölscher, R.; Büscher, W. Acidification of slurry to reduce ammonia and methane emissions: Deployment of a retrofittable system in fattening pig barns. J. Environ. Manag. 2023, 331, 117263. [Google Scholar] [CrossRef] [PubMed]
- Kroodsma, W.; Huis in ’t Veld, J.W.H.; Scholtens, R. Ammonia emission and its reduction from cubicle houses by flushing. Livest. Prod. Sci. 1993, 35, 293–302. [Google Scholar] [CrossRef]
- Swierstra, D.; Smits, M.; Kroodsma, W. Ammonia Emission from Cubicle Houses for Cattle with Slatted and Solid Floors. J. Agric. Eng. Res. 1995, 62, 127–132. [Google Scholar] [CrossRef]
- Ogink, N.; Kroodsma, W. Reduction of Ammonia Emission from a Cow Cubicle House by Flushing with Water or a Formalin Solution. J. Agric. Eng. Res. 1996, 63, 197–204. [Google Scholar] [CrossRef]
- Braam, C.; Ketelaars, J.; Smits, M. Effects of floor design and floor cleaning on ammonia emission from cubicle houses for dairy cows. Neth. J. Agric. Sci. 1997, 45, 49–64. [Google Scholar] [CrossRef]
- Ni, J.; Heber, A.J.; Lim, T.T.; Diehl, C.A.; Duggirala, R.K.; Haymore, B.L.; Sutton, A.L. Ammonia Emission from a Large Mechanically-Ventilated Swine Building during Warm Weather. J. Environ. Qual. 2000, 29, 751–758. [Google Scholar] [CrossRef]
- VERA: Verification of Environmental Technologies Agricultural Production. Vera Test Protocol for Livestock Housing and Management Systems. September 2018. Available online: https://www.vera-verification.eu/ (accessed on 21 April 2025).[Green Version]
- SAS Institute Inc. SAS/STAT ® 9.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004; Volume 1. [Google Scholar][Green Version]
- Santonja, G.G.; Georgitzikis, K.; Scalet, B.M.; Montobbio, P.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs—Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office of the European Union: Luxembourg, 2017. [Google Scholar][Green Version]
- Van Ransbeeck, N.; Van Langenhove, H.; Demeyer, P. Indoor concentrations and emissions factors of particulate matter, ammonia and greenhouse gases for pig fattening facilities. Biosyst. Eng. 2013, 116, 518–528. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Cong, Q.; Wang, Y.; Wang, W.; Zhang, W.; Zhu, Z.; Dong, H. Responses of CH4, N2O, and NH3 emissions to different slurry pH values of 5.5–10.0: Characteristics and mechanisms. Environ. Res. 2023, 234, 116613. [Google Scholar] [CrossRef]
- Aarnink, A.; Keen, A.; Metz, J.; Speelman, L.; Verstegen, M. Ammonia Emission Patterns during the Growing Periods of Pigs Housed on Partially Slatted Floors. J. Agric. Eng. Res. 1995, 62, 105–116. [Google Scholar] [CrossRef]
- Ngwabie, N.; Jeppsson, K.-H.; Gustafsson, G.; Nimmermark, S. Effects of animal activity and air temperature on methane and ammonia emissions from a naturally ventilated building for dairy cows. Atmos. Environ. 2011, 45, 6760–6768. [Google Scholar] [CrossRef]
- Calvet, S.; Arrufat, B.; Salaet, I.; Atares, S.; Sobreviela, A.; Herrero, C.; Romero, J.; Estellés, F. A urease inhibitor reduces ammonia emission in fattening pigs reared on slatted floor in summer conditions. Biosyst. Eng. 2022, 221, 43–53. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Quantification of ammonia and hydrogen sulfide emitted from pig buildings in Korea. J. Environ. Manag. 2008, 88, 195–202. [Google Scholar] [CrossRef]
- Groot Koerkamp, P.W.G.; Metz, J.H.M.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.P.; Hartung, J.; Seedorf, J.; et al. Concentrations and Emissions of Ammonia in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Philippe, F.-X.; Laitat, M.; Canart, B.; Vandenheede, M.; Nicks, B. Gaseous emissions during the fattening of pigs kept either on fully slatted floors or on straw flow. Animal 2007, 1, 1515–1523. [Google Scholar] [CrossRef]
- Kavanagh, I.; Burchill, W.; Healy, M.; Fenton, O.; Krol, D.; Lanigan, G. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Fuchs, A.; Dalby, F.R.; Liu, D.; Kai, P.; Feilberg, A. Improved effect of manure acidification technology for gas emission mitigation by substituting sulfuric acid with acetic acid. Clean. Eng. Technol. 2021, 4, 100263. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Hansen, M.; Pedersen, S.; Rom, H. Emissions of ammonia, methane and nitrous oxide from pig houses and slurry: Effects of rooting material, animal activity and ventilation flow. Agric. Ecosyst. Environ. 2008, 124, 237–244. [Google Scholar] [CrossRef]
- Quiniou, N.; Dubois, S.; Noblet, J. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 2000, 63, 245–253. [Google Scholar] [CrossRef]
- Saha, C.; Ammon, C.; Berg, W.; Fiedler, M.; Loebsin, C.; Sanftleben, P.; Brunsch, R.; Amon, T. Seasonal and diel variations of ammonia and methane emissions from a naturally ventilated dairy building and the associated factors influencing emissions. Sci. Total. Environ. 2014, 468–469, 53–62. [Google Scholar] [CrossRef]
- Philippe, F.-X.; Laitat, M.; Wavreille, J.; Nicks, B.; Cabaraux, J.-F. Effects of a high-fibre diet on ammonia and greenhouse gas emissions from gestating sows and fattening pigs. Atmos. Environ. 2015, 109, 197–204. [Google Scholar] [CrossRef]
- Ni, J.; Heber, A.J.; Lim, T.T.; Tao, P.C.; Schmidt, A.M. Methane and Carbon Dioxide Emission from Two Pig Finishing Barns. J. Environ. Qual. 2008, 37, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Powers, W. Meta-Analysis of Greenhouse Gas Emissions from Swine Operations; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011. [Google Scholar] [CrossRef]
- Ottosen, L.D.; Poulsen, H.V.; Nielsen, D.A.; Finster, K.; Nielsen, L.P.; Revsbech, N.P. Observations on microbial activity in acidified pig slurry. Biosyst. Eng. 2009, 102, 291–297. [Google Scholar] [CrossRef]
- Jungbluth, T.; Hartung, E.; Brose, G. Greenhouse gas emissions from animal houses and manure stores. Nutr. Cycl. Agroecosyst. 2001, 60, 133–145. [Google Scholar] [CrossRef]
- Mosquera, J.; Hol, J.M.G.; Winkel, A.; Lovink, E.; Ogink, N.W.M.; Aarnink, A.J.A. Dust Emission from Animal Houses: Growing and Finishing Pigs; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2010; Available online: https://edepot.wur.nl/135664 (accessed on 20 April 2025).
- Pereira, J.L.S.; Perdigão, A.; Tavares, A.; Silva, M.E.F.; Brás, I.; Wessel, D.F. Effects of the Addition of Different Additives before Mechanical Separation of Pig Slurry on Composition and Gaseous Emissions. Agronomy 2022, 12, 1618. [Google Scholar] [CrossRef]
- Fangueiro, D.; Surgy, S.; Coutinho, J.; Vasconcelos, E. Impact of cattle slurry acidification on carbon and nitrogen dynamics during storage and after soil incorporation. J. Plant Nutr. Soil Sci. 2013, 176, 540–550. [Google Scholar] [CrossRef]
- Chantziaras, I.; De Meyer, D.; Vrielinck, L.; Van Limbergen, T.; Pineiro, C.; Dewulf, J.; Kyriazakis, I.; Maes, D. Environment-, health-, performance- and welfare-related parameters in pig barns with natural and mechanical ventilation. Prev. Vet. Med. 2020, 183, 105150. [Google Scholar] [CrossRef]
- Hinz, T.; Linke, S. A Comprehensive Experimental Study of Aerial Pollutants in and Emissions from Livestock Buildings. Part 1: Methods. J. Agric. Eng. Res. 1998, 70, 111–118. [Google Scholar] [CrossRef]
- Costa, A.; Guarino, M. Definition of yearly emission factor of dust and greenhouse gases through continuous measurements in swine husbandry. Atmos. Environ. 2009, 43, 1548–1556. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Overmeyer, V.; Kube, A.; Clemens, J.; Büscher, W.; Trimborn, M. One-time acidification of slurry: What is the most effective acid and treatment strategy? Agronomy 2021, 11, 1319. [Google Scholar] [CrossRef]
- Colina, J.; Lewis, A.; Miller, P.S. A Review of the Ammonia Issue and Pork Production; Nebraska Swine Report; University of Nebraska: Lincoln, NE, USA, 2000; pp. 24–25. [Google Scholar]
- O’Connor, E.A.; Parker, M.O.; McLeman, M.A.; Demmers, T.G.; Lowe, J.C.; Cui, L.; Davey, E.L.; Owen, R.C.; Wathes, C.M.; Abeyesinghe, S.M. The impact of chronic environmental stressors on growing pigs, Sus scrofa (Part 1): Stress physiology, production and play behaviour. Animal 2010, 4, 1899–1909. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).