Abstract
A high-performance liquid chromatography with diode array detector (HPLC-DAD) analytical method was developed for the simultaneous detection of 17 phenolic compounds, including phenols, chlorophenols, alkylphenols, and nitrophenols, in two types of water matrices: wastewater and surface water. Prior to HPLC-DAD determination, a solid-phase extraction (SPE) procedure was optimized. The proposed method uses multiwavelength analysis, with the optimum detection wavelengths selected as 268 nm, 280 nm, 386 nm, 304 nm, and 316 nm. The highest resolution was achieved using a chromatographic column, Eclipse XDB-C18 (150 × 4.6 mm, 5 μm), which was kept at 20 °C. The mobile phase consisted of a gradient elution program, with mobile phase A being a 0.1% H3PO4 aqueous solution and mobile phase B being acetonitrile. The flow rate was set at 0.6 mL/min. The 17 target phenolic compounds were fully separated in less than 27 min. All compounds showed good linear regression, with correlation coefficients higher than 0.999. The method’s quantitation limits ranged from 4.38 to 89.7 ng/L for surface water and 7.83 to 167 ng/L for wastewater. The recovery rates were in the range of 86.2–95.1% for surface water and 79.1–86.3% for wastewater. The SPE-HPLC-DAD method was proven to be fast, sensitive, accurate, and reproducible. The developed method was successfully applied for the analysis of the 17 phenolic compounds in real surface water and wastewater samples, with phenol, 2,4-DNP, and 2,4-DNP being determined at levels greater than the method’s limits of quantitation (LOQs). The proposed analytical method represents an original technical resource for the simultaneous determination of 17 phenolic compounds in environmental water matrices.
1. Introduction
Phenolic compounds are a common class of contaminants found in wastewater from various industries, including petrochemical, petroleum oil refineries, pharmaceutical, resin synthesis, textile, pulp and paper, paint, coal gasification, wood, and leather [1,2,3]. These compounds are also used as intermediates in the production of agricultural chemicals, pharmaceuticals, biocides, and dyes [4,5,6]. Phenolic contaminants are difficult to biodegrade, resulting in their persistence in wastewater at high concentrations [7]. When discharged into natural water bodies through effluent discharges, phenolic compounds can pollute various aquatic ecosystems. These compounds can negatively impact biota and pose a threat to human health due to their mutagenic and carcinogenic properties [1,3,8].
Phenolic compounds typically include chloro-, bromo-, nitro-, and alkyl phenols. Due to their persistence and toxicity in the environment, phenolic substances are considered priority contaminants and have been included in the list of hazardous substances by the United States Environmental Protection Agency (US EPA) and the European Union. These regulatory bodies have established maximum allowed concentrations (MACs) for phenols in drinking water, with the total sum quantified at 0.5 µg/L and individually quantified phenols at 0.1 µg/L [9,10,11]. For chlorophenols, toxicity increases with the number of chlorine atoms in the molecule, with pentachlorophenol being the most toxic among chlorinated phenols. The World Health Organization (WHO) recommends a reference value of 1 µg/L for chlorophenols in drinking water [12]. In Japan, the maximum allowed level of total phenols is less than 5 μg/L in drinking and tap water and less than 5 mg/L in industrial wastewater, as stated in the Japanese Water Pollution Control Law. In Romania, the maximum allowed concentration for pentachlorophenol in internal surface waters is 1 µg/L, as specified in Government decision no. 570/2016. For soil, concentrations lower than 0.02 mg/kg dry weight are considered normal values according to Order no. 756 of 3 November 1997, which approves the regulation regarding the assessment of environmental pollution [13].
Currently, the commonly used methods for determining phenolic compounds in environmental and biological samples are gas chromatography with various detectors, including electron capture detection (GC-ECD) [14], flame ionization (GC-FID), and mass spectrometer detection (GC-MS) [1]. Liquid chromatography with different detection modes is also used as an alternative, such as photometric detection (HPLC-UV) [15], mass spectrometer detection (LC-MS) [16,17], electrochemical detection [18], or capillary electrophoresis [19]. Standardized methods for phenolic compound determination in water include EPA methods 604, 625, 8041 [20], or standardized method SR EN 12673:2002 [21]. These methods involve liquid–liquid extraction or solid-phase extraction of phenolic pollutants, followed by derivatization with diazomethane, methylene chloride, pentafluorobenzyl bromide, or acetic anhydride, and analysis by GC-FID, GC-ECD, or GC-MS. However, GC techniques have limitations, such as limited applicability for non-volatile or thermally labile phenolic compounds, time-consuming sample preparation, and the need for derivatization for most compounds, as well as the requirement for careful purification steps [22]. While there are chromatographic conditions and columns that can enable the analysis of these compounds without derivatization, they are less commonly encountered due to the challenges posed by the compounds’ non-volatile and thermally labile nature. Therefore, the development of robust analytical methods that are sensitive, accurate, precise, and capable of analyzing a wide range of phenolic compounds is necessary for the detection and quantification of xenobiotic phenolic compounds in the aquatic environment. Liquid chromatography is a versatile alternative to gas chromatography, as it can handle a broad range of phenolic compounds, including non-volatile and polar ones. It also offers a variety of detection options, such as atomic emission spectrometry, UV-VIS detection, fluorescence or DAD, and mass spectrometry [23].
Previous studies have reported the determination of phenolic compounds in various matrices using HPLC techniques. For example, one study identified and quantified fourteen phenolic compounds in soil using HPLC-PDA in less than 45 min with detection limits ranging between 0.03 and 0.24 mg/L [24]. Another study developed a sensitive, selective, and accurate HPLC-DAD method for the identification and quantification of six phenolic compounds in water, with recoveries ranging from 88% to 109% [25]. Chromatographic run-times of 70 min were reported for these methods [26]. Detection of phenolic compounds in 55 min using HPLC-DAD [27] and low detection limits (0.40–5.51 µg/L) for eight phenolic compounds in water samples using HPLC determination under optimal conditions have also been reported [28].
This aim of this study was to develop, validate, and optimize a new analytical method using HPLC-DAD to quantify 17 phenolic compounds in surface waters and wastewater. The newly developed method provides a rapid and simultaneous analysis of multiple phenolic compounds in water samples with high sensitivity and selectivity. By utilizing the same mobile phase, the analysis time is reduced, making the approach more efficient and practical. Rigorous validation procedures were conducted to ensure the accuracy and reliability of the method, while optimization strategies were employed to enhance its performance.
This new analytical method has significant implications for water quality assessment and management. It enables the detection and quantification of phenolic compounds in surface waters and wastewater, facilitating the identification of potential sources of contamination and supporting effective pollution control measures. Additionally, the method contributes to environmental risk assessment by providing valuable data on the presence and concentration of phenolic pollutants, which can guide decision-making and the implementation of appropriate regulations. Moreover, the method’s application in research and development efforts will further advance our understanding of the behavior and impacts of phenolic compounds in the environment.
The novelty of this paper lies in the development of an HPLC-DAD method that significantly improves upon previous methods. The key innovation is the use of a single mobile phase, which streamlines the analysis process and enhances efficiency. This breakthrough enables the rapid and simultaneous separation and quantification of 17 phenolic compounds in water samples within an unprecedented timeframe of less than 18 min. Notably, this method achieves quantitation limits comparable to those obtained with more advanced LC-MS techniques, thereby rivaling the results achieved by more sophisticated technologies.
This novel approach has far-reaching implications for environmental monitoring and pollution control. By providing a reliable and efficient means of detecting and quantifying phenolic contaminants in wastewater and surface water matrices, this method will contribute to the comprehensive monitoring and management of these pollutants. Overall, this paper presents a significant advancement in the field of phenolic compound analysis, offering a more practical and effective approach for environmental monitoring and pollution control.
2. Materials and Methods
2.1. Chemicals and Materials
A mixture of 17 phenolic compounds was used in this study, including 2-Nitrophenol, 2,2-Dinitrophenol, 2-Methylphenol, 3-Methylphenol, 4-Methylphenol, 2-Methyl-4,6-Dinitrophenol, 2,4-Dimethylphenol, 4-Chloro-3-Methylphenol, Dinoseb (6-sec-butyl-2,4-dinitrophenol), 2,4,6-Trichlorophenol, 2,4,5-Trichlorophenol, and 2,3,4,6-Tetrachlorophenol. The individual analytical standards used were Phenol, 2-Chlorophenol, 2,4-Dichlorophenol, 2,6-Dichlorophenol, and Pentachlorophenol. All reference materials were obtained from Sigma-Aldrich (Darmstadt, Germany) and were of pure quality. Aqueous solutions were prepared using Milli-Q ultra-purified water from the Millipore system. Chromatographic purity acetonitrile (ACN) and methanol (MeOH) were purchased from Merck (Darmstadt, Germany), while analytical-grade phosphoric acid (H3PO4) was acquired from Sigma-Aldrich (Darmstadt, Germany). The physical–chemical properties of the phenolic compounds are presented in Table 1, where Log Kow represents the octanol-water partition coefficient and pKa is a number that describes the acidity of a particular molecule.

Table 1.
Physical–chemical properties of phenolic compounds.
2.2. Preparation of Standard Stock Solution and Calibration Curve
Each phenolic analytical standard, whether in the mixture or as an individual standard, was accurately weighed and dissolved in methanol (MeOH). A single standard stock solution with a concentration of 2000 µg/L was prepared in ultra-purified water. From this stock solution, precise measurements were taken and diluted 40-fold with ultra-purified water. This resulted in eight separate volumetric flasks (10 mL) containing different dilutions that corresponded to gradient concentrations of 1, 5, 10, 25, 50, 100, 200, and 500 µg/L. These eight calibration standards, along with the water samples, were analyzed using the HPLC-DAD method that was optimized in this study. The peak area and mass concentration were used to determine the linear domain, linear regression equation, and correlation coefficient of the 17 phenolic compounds.
2.3. Chromatographic Conditions
An Agilent 1200 HPLC system (Agilent Technologies, Waldbronn, Germany) was used for the analysis. The system consisted of an autosampler with a capacity of 100 positions and a variable injection volume ranging from 0.1 to 100 µL. A chromatographic column temperature box, a diode array detector capable of simultaneous acquisition of 8 wavelengths and recording of the full UV-Vis spectrum (190–900 nm), and a Zorbax Eclipse XDB C18 (Agilent Technologies, Waldbronn, Germany) chromatographic column (150 × 4.6 mm, 5 μm) were also utilized. The column temperature was maintained at 20 °C, and the detection wavelengths were set at 268 nm, 280 nm, 286 nm, 304 nm, and 316 nm. The mobile phase consisted of a 0.1% H3PO4 aqueous solution as mobile phase A and acetonitrile (ACN) as mobile phase B. Gradient elution was performed according to the following program: 0–1 min, 30% B; 1–11 min, 70% B; 11–12 min, 70% B; 12.01–17 min, 90% B; 17.1–27.1 min, 30% B (column re-equilibration). The flow rate was set at 0.6 mL/min, and the injection volume was 50 µL.
2.4. Sample Collection
The HPLC-DAD method was used to determine chlorophenols in wastewater and surface water samples. The wastewater samples were collected from the municipal wastewater treatment plants in Iasi, Galati, Targoviste, and Cluj. The samples included both influents and effluents. Surface water samples were collected upstream and downstream of the mentioned wastewater treatment plants. These samples were taken from the rivers that receive the treated wastewater, namely, the Bahlui, Siret, Ialomita, and Somes rivers.
2.5. Water Phenolic Compound Extraction
The solid-phase extraction (SPE) procedure described in this study was used to extract phenolic compounds from wastewater and surface water samples. The process involved several steps to ensure efficient extraction and concentration of the compounds. Firstly, 200 mL of the water samples were transferred into polypropylene containers. To adjust the pH of the samples, H3PO4 solution was added to achieve a pH of 2.0. Acidification is commonly performed to enhance the extraction efficiency of phenolic compounds. Strata C18 cartridges from Phenomenex (Torrance, United States) were chosen for the SPE procedure. These cartridges have a bed weight of 200 mg and a volume of 6 mL. Prior to sample loading, the cartridges were conditioned using methanol (MeOH) and ultra-purified water, 10 mL each (5 mL/min). Conditioning helps to prepare the cartridges and remove any contaminants that could interfere with the analyte extraction process. Once the cartridges were conditioned, the wastewater and surface water samples were loaded onto the SPE cartridges, using a flow rate of 5 mL/min. The extraction of the phenolic compounds onto the solid phase of the cartridges occurred during this step [28]. After loading, the cartridges were allowed to dry (30 min). Drying ensures that any excess water or solvent is removed from the cartridges, preventing dilution of the analytes during elution. Elution of the phenolic compounds from the SPE cartridges was achieved using MeOH (2 × 5 mL, 5 mL/min). This solvent is effective in extracting the compounds from the solid phase, enabling their transfer into the eluent. Eluants can contain a mixture of solvents to optimize the extraction efficiency, but in this case, MeOH was used. Following elution, the eluent containing the phenolic compounds was concentrated using a nitrogen gas flow. Concentration is important since it allows for a reduction in sample volume, resulting in higher analyte concentrations. This step is especially beneficial when dealing with low analyte concentrations in the original sample. To make the eluent suitable for high-performance liquid chromatography (HPLC) analysis, it was reconstituted with ultra-purified water to a volume of 1 mL. Reconstitution helps in achieving the desired sample volume for injection into the HPLC system.
Before HPLC analysis, the reconstituted sample was further filtered through a 0.45 µm filter syringe. Filtration removes any particulates or large molecules that could potentially interfere with the HPLC analysis or cause blockage of the analytical column. The SPE procedure employed in this study provided a robust and efficient method for extracting and concentrating phenolic compounds from wastewater and surface water samples. The combination of sample preparation techniques, air-drying, eluent composition, and filtration steps ensured the suitability and reliability of the samples for subsequent HPLC analysis.
2.6. Data Processing and Statistical Analysis
The data acquisition, processing, and reporting were performed using Agilent ChemStation software (B.02.03 (341)). Statistical analysis of the data was conducted using Microsoft Excel 2019.
3. Results and Discussion
3.1. Optimization of Detection Wavelength
The determination of the detection wavelength for each compound is crucial in order to optimize the sensitivity and selectivity of the analytical method. In this study, a DAD detector was used, which allowed for scanning of the single standard solutions of the 17 phenolic compounds in the range of 200 to 400 nm. Based on the absorption spectra obtained from the scans, the detection wavelength for each compound was determined based on its highest absorption intensity. In Figure 1a, it can be observed that Ph and 4-C-3-MP exhibited the highest absorption intensity at 268 nm, while 4-NP, 2,4-DNP, 4-MP, 2-MP, 3-MP, 2-CP, 2-M-4,6-DNP, 2,4-DNP, 2,6-DCP, Dinoseb, and 2,4,6-TCP had maximum absorption at 280 nm. Additionally, 2,4-DCP showed the highest absorption intensity at 286 nm, 2,4,5-TCP and PCP at 304 nm, and 2-NP at 316 nm. The selection of these specific detection wavelengths for each compound allows for the maxima sensitivity in detecting and quantifying their presence in the sample. By utilizing the respective absorption maximums, the method can effectively differentiate between the different phenolic compounds in the mixture. Figure 1a visually depicts the absorption spectra of the compounds at their respective detection wavelengths. This informative figure provides a clear overview of the absorption characteristics of each compound in the UV–visible region.
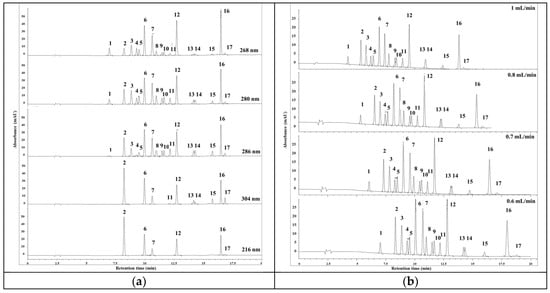
Figure 1.
Optimization data of HPLC method. (a) Chromatogram of phenolic compounds mixture solution at 268, 280, 386, 304, and 316 nm (flow rate 0.7 mL/min); (b) effect of flow rate on retention time. Note: (1) Ph, (2) 2-NP, (3) 2,4-DNP, (4) 4-MP, (5) 2-MP, (6) 3-MP, (7) 2-CP, (8) 2-M-4,6-DNP, (9) 2,4-DMP, (10) 2,6-DCP, (11) 2,4-DCP, (12) 4-C-3-MP, (13) DINOSEB, (14) 2,4,6-TCP, (15) 2,4,5-TCP, (16) 2,3,4,6-TrCP, (17) PCP (λ = 280 nm).
Overall, by carefully selecting detection wavelengths based on the highest absorption intensity of each compound, the sensitivity and accuracy of the analytical method can be enhanced, thus improving the quality of the phenolic compound analysis.
3.2. Stationary Phase
The selection of an appropriate chromatographic column is crucial for achieving efficient separation of compounds in a sample. In this study, three different columns, Poroshell 120 C18 (Agilent Technologies, Waldbronn, Germany), Luna C18 (Phenomene, Torrance, United States), and Eclipse XDB-C18 (Agilent Technologies, Waldbronn, Germany), were evaluated for their suitability in separating 17 phenolic compounds.
The Poroshell 120 C18 column showed a low retention of the phenolic compounds, regardless of whether isocratic or gradient elution modes were utilized. This lack of retention resulted in inadequate resolution between the chromatographic peaks, which is essential for accurate compound identification and quantification. Therefore, this column was deemed unsuitable for the separation of the phenolic compounds in this study.
Using the Luna C18 column, the phenolic compounds overlapped within a short run time of less than 15 min. To address this issue, the experimental conditions were modified by reducing the temperature and adjusting the mobile phase flow rate. However, these adjustments only resulted in eluting 15 chromatographic peaks, indicating the inability of the Luna C18 column to achieve satisfactory separation of all 17 phenolic compounds.
In contrast, the Eclipse XDB-C18 column met all the chromatographic requirements, including favorable resolution, acceptable retention time, and short run time, while also producing sharp and symmetric peaks. This column outperformed the other two columns in achieving efficient separation of the 17 phenolic compounds. Therefore, it was selected for further experiments. The successful selection of the Eclipse XDB-C18 column highlights the importance of carefully evaluating various column options to optimize the separation of target compounds. By utilizing a column that offers satisfactory resolution, retention, and peak symmetry, accurate and reliable analysis of the phenolic compounds can be obtained.
3.3. Mobile Phase
The selection of an appropriate mobile phase is crucial for the successful separation of compounds in HPLC analysis. In the case of phenolic compounds, most of them are susceptible to ionization. Therefore, the addition of a suitable concentration of acid in the aqueous component of the mobile phase has a significant impact on enhancing the peak shape and separation degree of the targeted compounds [29,30]. In previous studies, gradient elution has commonly been used to achieve satisfactory separation of phenolic compounds in most samples. The mobile phase typically consists of a binary system comprising an aqueous solution and an organic solvent such as acetonitrile or methanol. Organic solvents like ACN or MeOH are commonly used, while formic acid, acetic acid, and phosphoric acid are usually employed for the separation and identification of phenolic compounds [31,32,33,34].
In this study, a mobile phase consisting of a 0.1% H3PO4 aqueous solution and acetonitrile was used. This choice resulted in good peak shape symmetry for the 17 targeted phenolic compounds. It was found that achieving effective separation of the target analytes posed a challenge using isocratic elution. Therefore, the gradient elution mode was adopted to significantly enhance the separation effect. Initially, parameter settings for gradient elution were established, but only 15 out of the 17 peaks were observed. The organic-to-aqueous phase ratio was subsequently modified multiple times until a final gradient elution scheme was obtained. The final gradient elution consisted of the following time intervals and percentage ratios: 0–1 min, 30% B; 1–11 min, 70% B; 11–12 min, 70% B; 12.01–17 min, 90% B; 17.1–27.1 min, 30% B (followed by column re-equilibration). The modification of the gradient elution parameters allowed for the successful separation and determination of the 17 phenolic compounds with satisfactory retention times and peak shapes. This optimized mobile phase composition and gradient elution scheme proved to be effective in achieving the desired separation.
The discussion on the mobile phase selection and optimization highlights the importance of tailoring the chromatographic conditions to the specific compounds of interest. By carefully considering the properties of the analytes and their interactions with the mobile phase components, the overall chromatographic performance can be significantly improved. The information provided in this study on the successful application of 0.1% H3PO4 aqueous solution and acetonitrile as the mobile phase for the separation and determination of phenolic compounds can be beneficial for future research and analysis in this field. It provides insights into the challenges and considerations associated with optimizing the mobile phase composition to achieve satisfactory separation results.
3.4. Flow Rate and Column Temperature Optimization
The flow rate in HPLC analysis plays an important role in the retention and separation of phenolic compounds. In this study, it was found that the flow rate had a significant impact on the retention of the analytes, as depicted in Figure 1b. Interestingly, unlike some other studies, it was observed that decreasing the flow rate had a beneficial effect on the separation of the phenolic compounds [24].
Initially, a flow rate of 1 mL/min was used, which resulted in acceptable retention times for the compounds. However, it was noted that there was an overlap between Dinoseb and 2,4,6-Trichlorophenol, indicating insufficient separation between these two analytes. To overcome this issue, the flow rate was reduced to 0.6 mL/min. This adjustment led to a significant improvement in the resolution of peak separation, with acceptable peak widths. Therefore, the optimal flow rate for this analysis was determined to be 0.6 mL/min.
The column temperature is another parameter that can influence HPLC performance. It affects the mobile phase viscosity, retention times, and selectivity [35]. In this study, three different column temperatures (15, 20, and 25 °C) were compared to assess their effects on compound separation and peak width. It was found that modifying the column temperature between 15 and 25 °C did not generate a substantial change in the separation or peak width of the compounds, possibly due to the use of gradient elution mode (as shown in Figure 2). As a result, a column temperature of 20 °C was chosen for the experiment.
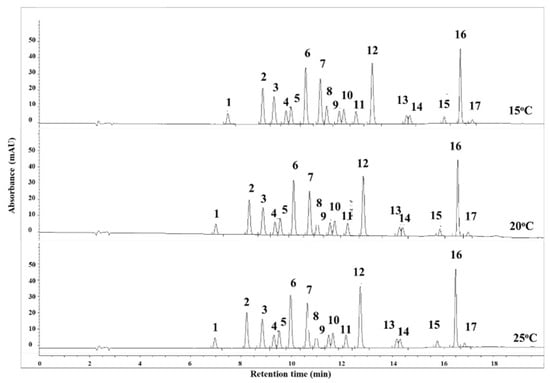
Figure 2.
The effect of chromatographic column temperatures on the separation of phenolic compounds. Note: (1) Ph, (2) 2-NP, (3) 2,4-DNP, (4) 4-MP, (5) 2-MP, (6) 3-MP, (7) 2-CP, (8) 2-M-4,6-DNP, (9) 2,4-DMP, (10) 2,6-DCP, (11) 2,4-DCP, (12) 4-C-3-MP, (13) DINOSEB, (14) 2,4,6-TCP, (15) 2,4,5-TCP, (16) 2,3,4,6-TrCP, (17) PCP (0.6 mL/min, λ = 280 nm).
The discussion on flow rate and column temperature highlights the importance of optimizing these parameters to achieve optimal separation and resolution of the phenolic compounds. The results demonstrate that a decrease in flow rate can improve the separation, while the column temperature within a certain range may not have a significant impact on the chromatographic performance. Understanding the impact of flow rate and column temperature on HPLC analysis can guide future research and analysis in this field. By carefully selecting and optimizing these parameters, researchers can achieve better separation and resolution of phenolic compounds, leading to more reliable and accurate quantification and identification of target analytes (Figure 3). The optimized parameters are given in Table 2.
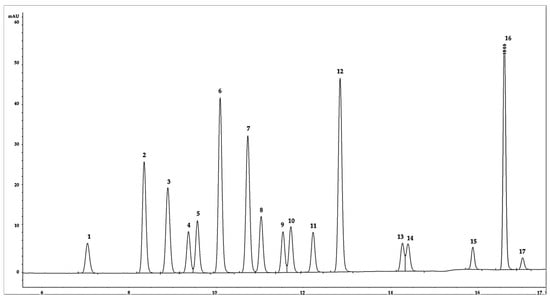
Figure 3.
Final chromatogram of the 17 phenolic compounds separation. Note: (1) Ph, (2) 2-NP, (3) 2,4-DNP, (4) 4-MP, (5) 2-MP, (6) 3-MP, (7) 2-CP, (8) 2-M-4,6-DNP, (9) 2,4-DMP, (10) 2,6-DCP, (11) 2,4-DCP, (12) 4-C-3-MP, (13) DINOSEB, (14) 2,4,6-TCP, (15) 2,4,5-TCP, (16) 2,3,4,6-TrCP, (17) PCP.

Table 2.
Optimized HPLC-DAD parameters.
It is worth noting that the specific optimal values of flow rate and column temperature may vary depending on the chromatographic system, column, and analytes of interest. Therefore, further studies and method development could explore different flow rates and column temperatures to find the most suitable conditions for specific phenolic compounds and sample matrices.
3.5. Optimization of the Solid Phase Extraction Procedure
The solid-phase extraction (SPE) procedure was developed using an Automatic SPE AutoTrace 280 instrument, from Thermo Scientific Dionex (Waltham, USA). A 200 mL sample was adjusted to pH 2 using phosphoric acid. This pH adjustment was performed to prevent partial deprotonation of the phenolic compounds, which could affect their retention on the SPE cartridges [36].
Three different types of SPE cartridges were evaluated for the recovery yield of chlorophenols: Strata C18 with a hydrophobic stationary phase, Strata X with a polymeric stationary phase, and Supelclean Coconut Charcoal cartridges (Phenomene, Torrance, United States) containing activated carbon adsorbent. The SPE procedure involved pre-conditioning the cartridges using methanol and ultra-purified water (10 mL each). Then, the sample was loaded onto the cartridges with a flow rate of 5 mL/min. The stationary phase was rinsed with 10 mL of ultra-purified water at a flow rate of 20 mL/min, and dried using nitrogen for 30 min. The phenolic compounds were subsequently eluted using methanol (2 × 5 mL) at a flow rate of 5 mL/min. The solvent was evaporated until dryness under a nitrogen current, and the samples were reconstituted with 1 mL of ultra-purified water.
The results showed that the Strata C18 cartridges provided the highest recovery values, with values greater than 88% for all compounds (as shown in Table 3). This indicates that the hydrophobic stationary phase of the Strata C18 cartridges strongly interacts with the chlorophenols, leading to efficient retention and elution. On the other hand, the Strata X cartridges with a polymeric stationary phase and the Supelclean Coconut Charcoal cartridges containing activated carbon adsorbent resulted in lower recovery yields.

Table 3.
Recovery values (%) obtained during the SPE optimization procedure.
Further analysis was conducted by repeating the SPE procedure using the Strata C18 cartridges, but with sample pH adjusted to 3 and 5, respectively. It was found that the recovery values obtained at these pH levels were lower than the initial tests at pH 2. This suggests that the pH adjustment of the sample has an impact on the retention and recovery of the phenolic compounds. The lower recovery at higher pH levels could be attributed to decreased interactions between the analytes and the hydrophobic stationary phase of the cartridges.
The discussion on the SPE procedure and the different types of cartridges used highlights the importance of selecting an appropriate cartridge for efficient retention and elution of the target analytes. The results demonstrate that the Strata C18 cartridges with a hydrophobic stationary phase were most effective for the recovery of chlorophenols in this study. The pH adjustment of the sample also plays a significant role in the extraction efficiency, with lower pH (pH 2) providing better recovery.
Understanding the optimal conditions for solid-phase extraction is crucial for the accurate and reliable determination of target analytes in samples. The insights gained from this study can guide future method development and analysis in similar applications, ultimately leading to improved extraction efficiency and analytical results.
3.6. HPLC-DAD Method Validation
The validation procedure of the HPLC-DAD method in this study was conducted following the guidelines set by the International Conference on Harmonization (ICH) [37]. The ICH guidelines recommend the use of standard solutions, blank samples, and spiked samples to evaluate the performance characteristics of a developed analytical method. This guideline provides a comprehensive framework for assessing the performance characteristics of analytical methods. The validation criteria specified in the mentioned guideline encompass parameters such as accuracy, precision, linearity, selectivity, limit of detection (LOD), and limit of quantitation (LOQ).
3.6.1. Linearity, LOD, and LOQ
Linearity refers to the ability of the method to produce a linear relationship between the concentration of the analyte and its response. A calibration curve was established using standard solutions of known concentrations, and the linearity of the method was assessed by analyzing multiple points within the calibration range. The correlation coefficient (R2) obtained from the calibration curve indicates the linearity of the method. A high R2 value close to 1 indicates a strong linear relationship between the analyte concentration and its response.
The limit of detection (LOD) and limit of quantitation (LOQ) are measures of the lowest concentration of an analyte that can be reliably detected and quantified, respectively. These parameters were determined by analyzing blank samples spiked with known low concentrations of the analyte, in descending order, and calculating the signal-to-noise ratio. The LOD represents the level at which the analyte’s signal is distinguishable from the background noise, while the LOQ represents the lowest concentration that can be quantitatively determined with acceptable accuracy and precision. Method LOQs were calculated taking into account the concentration factor and the recovery yield.
The results presented in Table 4 demonstrate the linearity of the developed HPLC-DAD method for the quantification of 17 phenolic compounds. The correlation coefficients (R2) obtained for all compounds were greater than 0.999, indicating a strong linear relationship between the analyte concentrations and their corresponding response signals. This indicates that the method has a high degree of accuracy in quantifying the phenolic compounds.

Table 4.
Items for regression equations, instrumental quantitation limits, and method LODs and LOQs.
The LOD and LOQ values obtained further highlight the sensitivity of the method for the determination of trace phenolic compounds in both surface water and wastewater samples. The instrumental quantitation limit (IOQ) ranged from 5.0 to 20 µg/L, demonstrating the capability of the method to detect and quantify phenolic compounds at low concentrations. The LOQ values, which represent the lowest concentrations that can be quantitatively determined with acceptable accuracy and precision, ranged from 4.38 to 89.7 ng/L for surface water and 7.83 to 167 ng/L for wastewater. These values indicate that the method has the sensitivity required to detect and accurately quantify phenolic compounds even at very low levels in environmental samples.
The high sensitivity of the method is crucial for environmental analysis, as phenolic compounds can occur at trace levels in water samples, and their presence may have harmful effects on ecosystems and human health. With the developed HPLC-DAD method, these compounds can be reliably detected and quantified, allowing for a comprehensive assessment of their concentrations in surface water and wastewater. The robust linear relationship, high correlation coefficients, and low LOD/LOQ values obtained in this study indicate that the developed HPLC-DAD method is reliable and sensitive for the determination of trace phenolic compounds in environmental samples. This method can be a valuable tool in assessing the levels of phenolic compounds in water bodies, helping to monitor and manage potential environmental risks associated with these compounds.
3.6.2. Precision, Accuracy, Stability, and Recovery
Precision refers to the repeatability or reproducibility of the method. It was evaluated by analyzing replicate samples and calculating the relative standard deviation (RSD) of the analyte peak areas or retention times. A low RSD indicates good precision, indicating that the method can provide consistent and reliable results.
The evaluation of instrumental repeatability, intra-day precision, inter-day precision, and stability of the standard solution provides crucial information on the reliability and robustness of the developed HPLC-DAD method for the analysis of phenolic compounds in environmental samples. The results presented in Table 4 indicate that the method exhibits excellent instrumental repeatability, with relative standard deviation (RSD) values ranging from 0.68% to 2.07% for surface water and wastewater samples. These low RSD values demonstrate the high precision and reproducibility of the method, suggesting that the instrument and analytical conditions are stable and consistently provide accurate and precise results over multiple chromatographic runs.
The intra-day precision results, obtained by analyzing six sub-samples in the same day, yielded RSD values ranging from 2.83% to 5.72% for surface water and wastewater samples. These values indicate that the method has acceptable precision for analyzing phenolic compounds in environmental samples, with relatively small variations observed within a single day. The inter-day precision results, which assessed the precision over two different days, determined by two different analysts, yielded RSD values ranging from 8.55% to 14.7% for surface water and wastewater samples. Although these values are slightly higher compared to the intra-day precision, they are still within an acceptable range for HPLC methods. These results suggest that the method can provide consistent and reliable measurements even when performed on different days and by different analysts.
The stability of the standard solution, which was evaluated over a period of 48 h, yielded RSD values ranging from 2.72% to 3.51%. These results indicate that the standard solution remains stable over the specified time period, without significant degradation or changes in the concentration of the phenolic compounds. This stability is an important characteristic for accurate and reliable quantitative analysis. The obtained RSD% values for instrumental repeatability, intra-day precision, inter-day precision, and stability of the standard solution are consistent with those reported in other phenolic compound analysis methods [24]. This demonstrates that the developed HPLC-DAD method is comparable to established methods and can provide reliable and precise measurements for the analysis of phenolic compounds in environmental samples. The results of instrumental repeatability, intra-day precision, inter-day precision, and stability evaluation indicate that the developed HPLC-DAD method is robust, accurate, and reproducible, making it suitable for routine analysis of phenolic compounds in environmental samples.
The recovery of the analytes was also assessed during the validation process. It determines the efficiency of the method in extracting and quantifying the analyte from the sample matrix. Recovery was evaluated by spiking matrix samples with known concentrations of the analyte and comparing the measured concentrations with the expected concentrations. The recovery is expressed as a percentage, and higher recovery values indicate better extraction efficiency.
The recovery experiment performed to assess the accuracy of the HPLC-DAD method provides valuable information on the reliability of the method in determining the true concentration of phenolic compounds in environmental samples. This helps determine the method’s reliability in accurately quantifying the true concentration of phenolic compounds. If the recovery experiment shows good agreement between the measured and expected concentrations, it indicates that the method is reliable and trustworthy in determining the true levels of phenolic compounds in the samples. The results presented in Table 5 indicate that the method exhibits satisfactory recovery and accuracy performance. The recoveries of the 17 phenolic compounds ranged from 86.2% to 95.1% for surface water and from 79.1% to 86.3% for wastewater samples. These recovery values suggest that the method is capable of accurately quantifying the phenolic compounds in environmental samples, with a good level of precision.

Table 5.
Instrument repeatability, method precision, stability, and accuracy (% ±RDS%).
The low RSD values, all below 5%, obtained for both surface water and wastewater samples further highlight the precision and reliability of the method. These low RSD values indicate that the method consistently provides accurate measurements and that the variation in results between replicates is minimal.
The satisfactory recovery and accuracy performance of the HPLC-DAD method for the quantification of phenolic compounds in surface water and wastewater samples is crucial for environmental analysis. Accurate determination of the true concentration of these compounds is vital for assessing their potential impact on the environment and human health.
The results obtained in this study are in line with previous studies that have reported recovery values and RSD% within similar ranges for the analysis of phenolic compounds in water matrices. This suggests that the developed HPLC-DAD method performs comparably to established methods and can provide reliable and accurate results for the analysis of phenolic compounds in environmental samples. In conclusion, the recovery experiment results demonstrate that the HPLC-DAD method has satisfactory recovery and accuracy performance for the quantification of phenolic compounds in both surface water and wastewater samples. The method shows high precision, with low RSD values, and accurately determines the true concentration of phenolic compounds in environmental samples. This confirms the reliability and suitability of the method for routine analysis of phenolic compounds in water matrices.
Accuracy refers to the closeness of the measured value to the true value or the target value. It was assessed by analyzing spiked samples with known concentrations of the analyte and comparing the recovered concentrations with the expected values. The accuracy can be expressed as percentage recovery, and the method is considered accurate if the recovery values fall within an acceptable range (typically within 80–120%).
The results obtained in this study showed that the accuracy of the method ranged between 85% and 97%. These recovery values indicate that the method generally provides measurements close to the true or target values. However, it is worth noting that some of the recovery values obtained fell below the lower limit of the acceptable range (80%). The accuracy of a method is influenced by various factors, including sample preparation, matrix effects, instrument calibration, and potential interferences. Variations in these factors can affect the recovery of the analyte and lead to deviations from the expected values.
It is important to consider factors that may have contributed to the observed recovery values below 80%. Potential causes may include incomplete recovery during the sample preparation step, matrix interferences affecting the analyte measurement, or calibration issues. Evaluating and addressing these factors can help improve the accuracy of the method. Nevertheless, it should be noted that recovery values above 100% are also encountered in some cases, which could be due to factors such as co-elution of interfering compounds or instrumental limitations.
3.7. Sample Analysis
The HPLC-DAD method was employed in this study to analyze and quantify chlorophenols in wastewater and surface water samples collected from various locations. This study focused on samples obtained from municipal wastewater treatment plants in Iasi, Galati, Targoviste, and Cluj, as well as upstream and downstream locations of the rivers that received the treated wastewater (Bahlui, Siret, Ialomita, and Somes rivers). The inclusion of both influent and effluent wastewater samples allowed for an assessment of the treatment efficiency and the potential release of chlorophenols into the receiving water bodies. By comparing the levels of chlorophenols in influent and effluent samples, it is possible to evaluate the effectiveness of the treatment processes in removing these compounds. Surface water samples collected upstream and downstream of the wastewater treatment plants provided insights into the impact of the treated wastewater on the receiving water bodies. Monitoring chlorophenol levels across these locations can help assess any potential contamination or dispersion of these compounds after wastewater discharge.
The results of the analysis showed the presence of Phenol in the influent sample of WWTP Iasi with a concentration of 267 ng/L, and 2,4-DNP in the influents of WWTPs Iasi (248 ng/L) and Galati (443 ng/L). These compounds were the only ones detected among the targeted chlorophenols. The complexity of the analyzed water matrices may have contributed to the inability to detect other targeted compounds. The presence of various organic and inorganic compounds in wastewater and surface water can interfere with the detection and quantification of specific analytes. Therefore, the absence of other chlorophenols in the samples may be attributed to their lower concentrations or their interaction with other components in the water matrices, making their detection challenging. To confirm the occurrence of the detected compounds, comparisons were made between the adsorption spectra of the phenolic compounds in the real water samples and those of individual standard solutions. This additional analysis helped to verify the presence of the detected compounds and ensure the reliability of the results. The overlaid absorption spectra presented in Figure 4 provide visual confirmation of the similarities between the spectra of the real water samples and the standards.
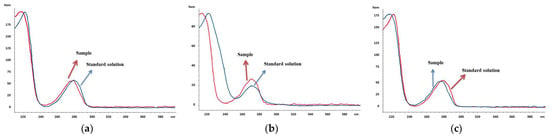
Figure 4.
Overlaid adsorption spectra for confirmation of Phenol (a) and 2,4-DNP (b) in WWTP Iasi influent and 2,4-DNP (c) WWTP Galati influent.
The values lower than LOQs in the analyzed water samples indicates that these compounds may not be major contaminants in the studied wastewater treatment plants and surrounding surface water bodies. However, it is important to note that this study focused on targeted compounds, and there may be other untargeted or unknown chlorophenols present. Further research and analysis could explore the presence and behavior of other chlorophenols or develop more sensitive methods for their detection. Evaluating the potential sources and pathways of chlorophenols in wastewater and surface water systems could also contribute to a more comprehensive understanding of their occurrence and fate in the environment.
The findings of this study provide insights into the presence and concentration of chlorophenols in the studied wastewater and surface water samples. These results can contribute to the assessment and management of water quality, as well as inform future monitoring and treatment strategies aimed at mitigating the potential risks associated with chlorophenol contamination.
4. Comparison with Previous Methods
The present study highlights the development of an HPLC-DAD method for the identification and quantification of phenolic compounds in water matrices, comparing the results with previous analytical methods reported in the literature (Table 6). The proposed method demonstrates lower quantification limits compared to most of the HPLC methods published previously. Several studies mentioned in the present study employed HPLC-UV techniques for the quantification of phenolic compounds.
For instance, a study conducted in Japan in 2006 reported the quantification of six phenolic compounds from water, using an HPLC-UV technique, through direct injection (without extraction) with derivatization with 4-Fluoro-7-nitro-2,1,3-benzoxadiazole. The LOD values were situated between 4 and 10 µg/L [15]. Another study in Poland in 2010 quantified phenolic compound using HPLC-UV without derivatization, employing SPE extraction [38]. Similarly, an HPLC-UV method developed in Africa in 2010 quantified 11 phenolic compounds from water matrices, after SPE extraction [39]. These studies reported LOD values ranging from 0.5 to 10 µg/L and LOQ values ranging from 0.01 to 0.90 mg/L [38,39]. In a more recent study conducted in Japan in 2017, an HPLC-UV analytical method was reported for the quantification of five phenolic compounds from water matrices. The method employed direct injection, after derivatization with 4-nitrobenzoyl chloride, yielding LOD values ranging from 6 to 50 µg/L [15]. In 2019, two UHPLC/PDA methods were reported in France and Brazil. The French study targeted only two compounds, and employed SPE extraction, while the Brazilian study quantified 14 phenolic compounds from wastewater using the dispersive liquid–liquid microextraction (DLLME) procedure. The studies reported LOQ values of 1 ng/L for 2-CP and 12 ng/L for 2,4-DCP [40] and from 0.5 to 10 µg/L, respectively [41].

Table 6.
Comparison with previous analytical methods.
Table 6.
Comparison with previous analytical methods.
| No. | Country | Compounds | Analytical Method | Year | Derivatization | Water Matrices | Extraction | LOQ/LOD | Values | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | China | 2-C-3-MP | ESI-LC-MS/MS | 2003 | - | Wastewater samples | SPE-C18 | LOD (ng/L) | 2 | [42] |
| 2,4-DCP | ||||||||||
| 2,4,6-TCP | ||||||||||
| 2,4,5-TCP | ||||||||||
| PCP | ||||||||||
| 2,4-DMP | ||||||||||
| 2 | China | 2-CP | LC-APCI-MS | 2006 | - | Water | SPE—oasis HLB (Polypropylene) | LOD (ng/L) | 30 | [43] |
| 3-CP | 2.5 | |||||||||
| 4-CP | 0.4 | |||||||||
| 2,4-DCP | 3.5 | |||||||||
| 2,5-DCP | 5.0 | |||||||||
| 2,6-DCP | 5.3 | |||||||||
| 3,4-DCP | 6.2 | |||||||||
| 3,5-DCP | 1.0 | |||||||||
| 2,3,4-TCP | 2.3 | |||||||||
| 2,3,5-TCP | 2.0 | |||||||||
| 2,3,6-TCP | 2.0 | |||||||||
| 2,4,5-TCP | 2.0 | |||||||||
| 2,4,6-TCP | 2.0 | |||||||||
| 3,4,5-TCP | 2.0 | |||||||||
| 2,3,4,5-TrCP | 0.7 | |||||||||
| 2,3,4,6-TrCP | 1.5 | |||||||||
| 2,3,5,6-TrCP | 1.7 | |||||||||
| PCP | 3.5 | |||||||||
| 3 | Iran | 2-CP | GC-ECD | 2007 | Acetic anhydride | Water | Liquid-liquid microextraction (LLME) | LOD (µg/L) | 1.0 | [44] |
| 3-CP | 1.0 | |||||||||
| 4-CP | 2.0 | |||||||||
| 2,4-DCP | 0.5 | |||||||||
| 2,5-DCP | 0.5 | |||||||||
| 2,6-DCP | 0.5 | |||||||||
| 3,4-DCP | 0.5 | |||||||||
| 3,5-DCP | 0.5 | |||||||||
| 2,3,4-TCP | 0.02 | |||||||||
| 2,3,5-TCP | 0.02 | |||||||||
| 2,3,6-TCP | 0.02 | |||||||||
| 2,4,5-TCP | 0.02 | |||||||||
| 2,4,6-TCP | 0.02 | |||||||||
| 3,4,5-TCP | 0.02 | |||||||||
| 2,3,4,5-TrCP | 0.01 | |||||||||
| 2,3,4,6-TrCP | 0.01 | |||||||||
| 2,3,5,6-TrCP | 0.01 | |||||||||
| PCP | 0.01 | |||||||||
| 4 | Japan | P | HPLC-UV | 2009 | 4-Fluoro-7-nitro-2,1,3-benzoxadiazole | Water | Direct injection | LOD (µg/L) | 4 | [45] |
| 2-CP | 6 | |||||||||
| 4-CP | 5 | |||||||||
| 2,6-DCP | 8 | |||||||||
| 2,4-DCP | 6 | |||||||||
| 2,4,6-TCP | 10 | |||||||||
| 5 | Poland | P | HPLC-UV | 2010 | - | Water | SPE-C18 | LOD (µg/L) | 0.7 | [38] |
| 2-NP | 0.5 | |||||||||
| 2-CP | 0.6 | |||||||||
| 2,4-DNP | 0.7 | |||||||||
| 2-M-4,6-DNP | 0.6 | |||||||||
| 4-NP | 0.8 | |||||||||
| 2,4-DMP | 0.7 | |||||||||
| 4-C-3-MP | 3.0 | |||||||||
| 2,4,6-TCP | 0.9 | |||||||||
| 2,4-DCP | 0.7 | |||||||||
| PCP | 0.7 | |||||||||
| 6 | Africa | P | HPLC-UV | 2010 | - | Water | SPE—polystyrene divinyl benzene | LOQ (mg/L) | 0.03 | [39] |
| 4-NP | 0.90 | |||||||||
| 2-NP | 0.01 | |||||||||
| 2-CP | 0.18 | |||||||||
| 2,4-DNP | 0.34 | |||||||||
| 2,4-DMP | 0.08 | |||||||||
| 4,6-DNoC | 0.06 | |||||||||
| 4-C-3-MP | 0.04 | |||||||||
| 2,4-DCP | 0.22 | |||||||||
| 2,4,6-TCP | 0.09 | |||||||||
| PCP | 0.05 | |||||||||
| 7 | Portugal | 2-amino-4-CP | LC-MS/MS | 2014 | - | Water | SPE—styrene divinylbenzene copolymer | LOQ (mg/L) | 2 | [17] |
| 4-CP | ||||||||||
| 3-CP | ||||||||||
| 2-CP | ||||||||||
| PCP | ||||||||||
| 4-C-3-MP | ||||||||||
| 2,4-DCP | ||||||||||
| 2,4,5-TCP | ||||||||||
| 2,4,6-TCP | ||||||||||
| 8 | Tunisia | 3,5-DCP | GC-ECD | 2015 | Acetylation | Water | SPE—polystyrene–divinylbenzene | LOD (µg/L) | 6.6 | [14] |
| 2,3-DCP | 6.2 | |||||||||
| 2,3,5-TCP | 4 | |||||||||
| 2,4,5-TCP | 2.6 | |||||||||
| 2,3,5,6-TrCP | 2.9 | |||||||||
| PCP | 0.5 | |||||||||
| 9 | Japan | 2-CP | HPLC-UV | 2017 | 4-Nitrobenzoyl Chloride | Water | Direct injection | LOD (µg/L) | 6 | [15] |
| 4-CP | 20 | |||||||||
| 2,4-DCP | 6 | |||||||||
| 2,6-DCP | 20 | |||||||||
| 2,4,6-TCP | 50 | |||||||||
| 10 | France | 2-CP | UHPLC/PDA | 2019 | - | Water | SPE-oasis HLB (Polypropylene) | LOQ (ng/L) | 1 | [40] |
| 2,4-DCP | 12 | |||||||||
| 11 | Brazil | P | UHPLC/PDA | 2019 | - | Wastewater | DLLME | LOQ (µg/L) | 5 | [41] |
| 4-NP | 2.5 | |||||||||
| m,p-cresol | 5 | |||||||||
| o-cresol | 5 | |||||||||
| 2-CP | 5 | |||||||||
| 2,4-DNP | 5 | |||||||||
| 2-NP | 5 | |||||||||
| 2,4-DMP | 10 | |||||||||
| 2,6-DCP | 10 | |||||||||
| 4-C-3-MP | 5 | |||||||||
| 2,4-DCP | 5 | |||||||||
| 2-M-3,5-DNP | 2.5 | |||||||||
| 2,4,6-TCP | 10 | |||||||||
| 2,4,5-TCP | 10 | |||||||||
| Dinoseb | 5 | |||||||||
| 12 | Romania | Fenol | HPLC-DAD | 2023 | - | Surface water Wastewater | SPE-C18 | LOQ (ng/L) | 34.3 | This study |
| 2-NP | 7 | |||||||||
| 4-NP, 2,4-DNP | 23.2 | |||||||||
| 4-MP | 54.4 | |||||||||
| 2-MP | 42.8 | |||||||||
| 3-MP | 52.7 | |||||||||
| 2-CP | 61.9 | |||||||||
| 2-M-4,6-DNP | 37 | |||||||||
| 2,4-DMP | 23.8 | |||||||||
| 2,6-DCP | 55.4 | |||||||||
| 2,4-DCP | 34.9 | |||||||||
| 4-C-3-MP | 38 | |||||||||
| DINOSEB | 77.4 | |||||||||
| 2,4,6-TCP | 64.5 | |||||||||
| 2,4,5-TCP | 34.3 | |||||||||
| 2,3,4,6-TrCP | 68.4 | |||||||||
| PCP | 33.7 |
The studies conducted in China in 2003 and 2006 and Portugal in 2010 utilized advanced analytical techniques, specifically ESI-LC-MS/MS (electrospray ionization–liquid chromatography–tandem mass spectrometry) and APCI-LC-MS (atmospheric pressure chemical ionization–liquid chromatography–mass spectrometry), to detect phenolic compounds in water samples. In the 2003 study, the ESI-LC-MS/MS technique was employed to detect six different phenolic compounds. The limit of detection (LOD) for these compounds was set at 2 ng/L, which means that the analytical method could reliably detect these compounds at concentrations as low as 2 ng/L in the water samples [42]. Prior to analysis, solid-phase extraction (SPE) was carried out to isolate and concentrate the phenolic compounds from the water matrix. In the 2006 study, the APCI-LC-MS technique was used to detect a larger set of 17 phenolic compounds. The LOD for these compounds ranged from 0.4 to 30 ng/L [43]. In 2014, the study conducted in Portugal quantified nine phenolic compounds, the established LOQ being 2 mg/L for each of them [17]. Similar to previous studies, SPE extraction was performed prior to analysis to concentrate the phenolic compounds from the water samples.
Gas chromatographic techniques coupled with an electron capture detector (ECD), involving derivatization, were employed in studies conducted in Iran and Tunisia. The Iranian study detected 17 compounds with LOD values ranging from 0.01 to 1.0 µg/L [44], whereas the Tunisian study detected 6 compounds with LOD values ranging from 0.5 to 6.6 µg/L [14].
The present study makes a significant contribution to the field by developing an HPLC-DAD method with lower quantification limits for the identification and quantification of phenolic compounds in water matrices compared to previous methods. The quantification limits obtained in the present study, ranging from 7.0 to 77 ng/L, are comparable to those achieved using more advanced LC-MS techniques. This indicates that the HPLC-DAD method developed in this study can provide accurate and reliable results, even at low concentrations of phenolic compounds.
One of the main advantages of the HPLC-DAD method over LC-MS techniques is its lower analysis cost. LC-MS methods often require expensive equipment and consumables, making them less accessible for many laboratories with limited resources. In contrast, HPLC-DAD is a widely available and cost-effective technique that can provide comparable results in terms of accuracy and reliability. This makes the HPLC-DAD method a valuable alternative for the analysis of phenolic compounds in water matrices, particularly for labs with budget constraints. Furthermore, the lower quantification limits achieved by the HPLC-DAD method can contribute to a more comprehensive understanding of the presence and concentration of phenolic compounds in water samples. Phenolic compounds have been associated with various health and environmental impacts, and accurate quantification is essential for risk assessment and implementing appropriate mitigation strategies. The improved sensitivity of the HPLC-DAD method may enable detection and quantification of phenolic compounds at lower concentrations, leading to a more accurate assessment of their potential effects.
However, it is important to acknowledge that each analytical method has its own strengths and limitations, and the choice of method should be based on the specific requirements of the study. While the HPLC-DAD method offers advantages in terms of cost-effectiveness and lower quantification limits, LC-MS techniques may provide additional capabilities such as structural elucidation and the ability to quantify a wider range of phenolic compounds. Therefore, researchers should carefully consider the specific objectives of their study before selecting the most appropriate analytical method.
5. Strengths, Limitations, and Future Work
The strengths of this study lie in its contributions to the field of water quality assessment and monitoring. By developing a new analytical method using HPLC-DAD, this study addresses the limitations of existing methods and provides a more efficient and practical approach for quantifying phenolic compounds in water samples. The rigorous validation procedures and optimization strategies employed ensure the accuracy and reliability of the method. Additionally, the ability to simultaneously analyze multiple compounds saves time and resources, making it a valuable tool for future research and monitoring efforts. The high sensitivity and selectivity of the method enhance its capacity to detect and quantify phenolic pollutants accurately.
The developed HPLC-DAD method demonstrates lower quantification limits compared to most HPLC methods published previously. The method’s quantification limits ranging from 7.0 to 77 ng/L are comparable to those achieved using more advanced LC-MS techniques, indicating its accuracy and reliability even at low concentrations of phenolic compounds. Additionally, the cost-effectiveness of the HPLC-DAD method makes it a valuable alternative for analyzing phenolic compounds in water matrices, particularly for laboratories with budget constraints. The method’s lower quantification limits can enhance our understanding of the presence and concentration of phenolic compounds in water samples, contributing to a more accurate assessment of their potential effects.
Although this study presents valuable contributions, there are certain limitations that should be considered. One limitation is the scope of this study, which focuses on a specific set of 17 phenolic compounds. While these compounds may be relevant, other phenolic pollutants could also be present in surface waters and wastewater. A more comprehensive analysis that includes a wider range of phenolic compounds would provide a more detailed understanding of phenolic contamination. Additionally, this study’s generalizability may be limited to the specific water matrices and analytical conditions utilized. Evaluating the method’s performance and applicability in different environmental settings and water sources is necessary to establish its wider utility.
To address the limitations mentioned above, future studies can consider various research directions. Expanding the number and variety of phenolic compounds analyzed would provide a more comprehensive understanding of phenolic contamination. Additionally, testing the developed method on a broader range of water matrices would help determine its applicability across different environmental settings. Long-term monitoring using the method can track trends in phenolic compound levels, identify potential sources of contamination, and evaluate the effectiveness of pollution control measures. Comparing the developed method with existing methods contributed to the understanding of its advantages and limitations, enabling researchers to choose the most suitable approach.
The applications of the developed method are significant for water quality assessment and management. Routine use of the method can aid in monitoring and identifying phenolic compounds as potential markers of pollution, supporting decision-making and targeted pollution control measures. The method can contribute to environmental risk assessment by evaluating the potential risks posed by phenolic compounds to aquatic ecosystems and human health. Regulatory agencies can benefit from the method’s accuracy and reliability in enforcing water quality standards and regulations. Finally, the method’s application in research and development efforts can enhance our understanding of the behavior, fate, and impacts of phenolic compounds in the environment, leading to the development of effective mitigation strategies.
Therefore, while this study has strengths in developing a new analytical method for quantifying phenolic compounds, it also has limitations related to the scope of compounds analyzed and generalizability. Future work should focus on expanding the range of compounds analyzed, testing the method in different matrices, and comparing it with existing methods. The applications of the method extend to water quality assessment, environmental risk assessment, regulatory compliance, and further research to advance our understanding of phenolic compound behavior and impacts.
6. Conclusions
This paper presents a novel multiwavelength HPLC-DAD method that allows for the simultaneous determination of 17 phenolic compounds in surface water and wastewater matrices. The developed method offers several advantages, including high sensitivity, accuracy, and short chromatographic run-time. A single extraction method using solid-phase extraction (SPE) was also developed, which simplifies the isolation and concentration of the target phenolic compounds from the water matrices. The optimized SPE-HPLC-DAD method was validated to ensure its reliability and precision. The results obtained from the validation studies, including instrumental repeatability, intra-day and inter-day precision, stability of the standard solution, and recovery experiments, demonstrate that the method performs well in terms of precision, accuracy, and sensitivity. The low RSD values obtained for various parameters indicate excellent repeatability and reproducibility of the method. The developed SPE-HPLC-DAD method offers a fast and accurate approach for the detection and quantification of a wide range of phenolic compounds. With a short chromatographic run-time of 17 min, the method provides a rapid analysis, which is beneficial for routine monitoring and assessment purposes. Furthermore, the quantification limits achieved in this study, ranging from 7.0 to 77 ng/L, are on par with those attained through more advanced LC-MS techniques. This achievement signifies the exceptional sensitivity and precision of the HPLC-DAD method developed in this research. Even at such low concentrations, the method consistently delivers accurate and reliable results for the quantification of phenolic compounds. Moreover, this method has the advantage of utilizing a single mobile phase for the simultaneous analysis of phenolic compounds in both surface water and wastewater matrices, which simplifies the analytical process and increases efficiency.
Overall, the developed SPE-HPLC-DAD method presented in this study makes a significant contribution to the field of environmental analysis by offering an effective approach for detecting and quantifying phenolic compounds in surface water and wastewater. The method’s sensitivity, accuracy, and rapidity provide a valuable tool for assessing the presence and potential environmental impact of these compounds. Moreover, the practical applications of this study’s results extend to ensuring water safety, improving wastewater treatment processes, and guiding pollution control efforts. The adoption of this developed method by various stakeholders involved in water management, environmental monitoring, and research can lead to enhanced water quality assessment and management practices.
Author Contributions
Conceptualization, I.P., T.G. and F.L.C.; methodology, F.P., V.I.I. and F.L.C.; software, F.P., T.G. and V.I.I.; validation, I.P., L.F.P. and F.L.C.; formal analysis, I.P., L.F.P. and F.P.; investigation, V.I.I., T.G. and F.L.C.; resources, F.P. and T.G.; data curation, I.P. and L.F.P.; writing—original draft preparation, I.P., L.F.P. and F.P.; writing—review and editing, V.I.I. and F.L.C.; visualization, T.G.; supervision, F.L.C., V.I.I. and T.G.; project administration, F.L.C.; funding acquisition, F.P. and T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Nucleu” Program within the National Research Development and Innovation Plan 2022–2027 with the support of Romanian Ministry of Research, Innovation, and Digitalization, contract no. 3N/2022, Project code PN 23 22 01 01. The APC was funded by National Research and Development Institute for Industrial Ecology—ECOIND.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was carried out through the “Nucleu” Program within the National Research Development and Innovation Plan 2022–2027 with the support of Romanian Ministry of Research, Innovation, and Digitalization, contract no. 3N/2022, Project code PN 23 22 01 01. We acknowledge the use of AI technology for its support in improving the English grammar in the final stage of our manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, D.H.; Choi, S.; Park, J.; Kim, K.; Oh, J.E. Phenolic compounds in the freshwater environment in South Korea: Occurrence and tissue-specific distribution. Sci. Total Environ. 2023, 905, 166914. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, A.Y.; Ozdemir, O.K. Phenol removal from synthetic solution using low pressure membranes coated with graphene oxide and carbon. Chem. Pap. 2018, 72, 327–335. [Google Scholar] [CrossRef]
- Barik, M.; Das, C.P.; Verma, A.K.; Sahoo, S.; Sahoo, N.K. Metabolic profiling of phenol biodegradation by an indigenous Rhodococcuspyridinivorans strain PDB9T N-1 isolated from paper pulp wastewater. Int. Biodeterior. Biodegrad. 2021, 158, 105168. [Google Scholar] [CrossRef]
- Czaplicka, M. Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 2004, 322, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J.; Duda, W. Phenols—Sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Jensen, J. Chlorophenols in the terrestrial environment. Rev. Environ. Contam. T 1996, 146, 25–51. [Google Scholar]
- Wei, D.; Li, M.; Meng, Q.; Yan, L.; Feng, R.; Zhang, Y.; Fan, D.; Pang, X.; Du, B.; Wei, Q. Aerobic biodegradation of 2,6-dichlorophenol in a nitrifying granular sludge reactor: System performance and microbial community evolution. J. Water Process. Eng. 2020, 37, 101524. [Google Scholar] [CrossRef]
- Acosta, C.A.; Pasquali, C.E.L.; Paniagua, G.; Garcinuño, R.M.; Hernando, P.F. Evaluation of total phenol pollution in water of San martin canal from Santiago del Estero, Argentina. Environ. Pollut. 2018, 236, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Lourido, M.; Landin, P.; Garcia-Jares, C.; Cela, R. Optimization of a derivatization-solid-phase microextraction method for the analysis of thirty phenolic pollutants in water samples. J. Chromatogr. A 2002, 963, 137–148. [Google Scholar] [CrossRef]
- Gonzalez-Toledo, E.; Prat, M.D.; Alpendurada, M.F. Solid-phase microextraction coupled to liquid chromatography for the analysis of phenolic compounds in water. J. Chromatogr. A 2001, 923, 45–52. [Google Scholar] [CrossRef]
- Asan, A.; Isildak, I. Determination of major phenolic compounds in water by reversed-phase liquid chromatography after pre-column derivatization with benzoyl chloride. J. Chromatogr. A 2003, 988, 145–149. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pentachlorophenol in Drinking-Water; WHO/SDE/WSH/03.04/62; Background document for development of WHO Guidelines for Drinking-water Quality; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- The Ministerial Order 756/1997 Establishes the Alert and Intervention Thresholds for Concentrations of Pollutants in Soils, in Correlation with the Specific Purpose of Land Use (Recreational Land Use). Available online: https://legislatie.just.ro/Public/DetaliiDocument/13572 (accessed on 1 May 2023).
- Hassine, S.B.; Hammami, B.; Touil, S.; Driss, M.R. Determination of Chlorophenols in Water Samples Using Solid-Phase Extraction Enrichment Procedure and Gas Chromatography Analysis. Bull. Environ. Contam. Toxicol. 2015, 95, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Yasuhiko, H. Simple HPLC–UV Analysis of Phenol and Its Related Compounds in Tap Water after Pre-Column Derivatization with 4-Nitrobenzoyl Chloride. J. Anal. Sci. Meth. Instrum. 2017, 7, 18–28. [Google Scholar] [CrossRef][Green Version]
- Tang, C.; Tan, J. Determination of Chlorophenols in Sewage Sludge and Soil by High-Performance Liquid Chromatography–Tandem Mass Spectrometry with Ultrasonic-assisted and Solid Phase Extraction. Anal. Lett. 2017, 50, 2959–2974. [Google Scholar] [CrossRef]
- Tavares da Fonseca Lopes Mourato, M.S. Determination of Chlorophenols in Water by LC-MS/MS. Case Study: 2-Amino-4-Chlorophenol. 2014. Available online: https://www.semanticscholar.org/paper/Determination-of-Chlorophenols-in-water-by-LC-MS-Mourato/0ea8d609e3bc6841ad8b15fa37a0879ecfc629ba (accessed on 1 May 2023).
- Alonso, M.C.; Puig, D.; Silgoner, I.; Grasserbauer, M.; Barcelo, D. Determination of priority phenolic compounds in soil samples by various extraction methods followed by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 1998, 823, 231–239. [Google Scholar] [CrossRef]
- Jauregui, O.; Puignou, L.; Galceran, M.T. New carrier electrolytes for the separation of chlorophenols by capillary electrophoresis. Electrophoresis 2000, 21, 611–618. [Google Scholar] [CrossRef]
- U.S. EPA. Method 8014A: Phenols by Gas Chromatography: Capillary Column Technique; Revision 1 in February 2007; U.S. EPA: Washington, DC, USA, 1995. [Google Scholar]
- SR EN 12673:2002; Water Quality. Determination by Gas Chromatography of Some Chlorophenols in Water. International Organization for Standardization: Geneva, Switzerland, 2002.
- Kartal, A.A.; Divrikli, U.; Elci, L. Determination of chlorophenols in wastewater with methyl chloroformate derivatization, solid phase extraction, and gas chromatography–mass spectrometry. Anal. Lett. 2015, 48, 2723–2738. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Jia, C.; Zhou, C.; Xie, Y.; Wang, M.; Zhou, C.; Li, X.; Du, Y.; Lu, F. A new method for simultaneous determination of 14 phenolic acids in agricultural soils by multiwavelength HPLC-PDA analysis. RSC Adv. 2022, 12, 14939–14944. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, R.M.; Barragan-Mendoza, L.; Peraza-Campos, A.L.; Muniz-Valencia, R.; Ceballos-Magana, S.G.; Parra-Delgado, H. Validation of an HPLC-DAD method for the determination of plant phenolics. Rev. Bras. Farm. 2019, 29, 689–693. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Zhang, Y.; Zhang, X.; Zhang, Z.; Liao, Y.; Zhang, B. A New Method for Simultaneous Determination of Phenolic Acids, Alkaloids and Limonoids in Phellodendri Amurensis Cortex. Molecules 2019, 24, 709. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xie, Q.; Di, H.; Liang, Y.; Ma, G.; Yi, Z.; Zhang, P.; Fu, P.; Li, J. Molecular complex based dispersive liquid–liquid microextraction for simultaneous HPLC determination of eight phenolic compounds in water samples. J. Mol. Liq. 2020, 309, 113115. [Google Scholar] [CrossRef]
- Liu, A.H.; Li, L.; Xu, M.; Lin, Y.H.; Guo, H.Z.; Guo, D.A. Simultaneous quantification of six major phenolic acids in the roots of Salvia miltiorrhiza and four related traditional Chinese medicinal preparations by HPLC–DAD method. J. Pharm. Biomed. Anal. 2006, 41, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yuan, Z.; Rong, L.; Zhang, Y.; Xiong, G.; Liu, Y.; Li, C. An Optimized Method for Extraction and Characterization of Phenolic Compounds in Dendranthema indicum var. aromaticum Flower. Sci. Rep. 2019, 9, 7745. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.; MagalhAes, B.E.A. Phenolic content and antioxidant capacity of infusions herbs: Optimization of phenolic extraction and HPLC-DAD method. An. Acad. Bras. Cienc. 2020, 92, e20190646. [Google Scholar] [CrossRef] [PubMed]
- Valkama, E.; Salminen, J.P.; Koricheva, J.; Pihlaja, K. Comparative Analysis of Leaf Trichome Structure and Composition of Epicuticular Flavonoids in Finnish Birch Species. Ann. Bot. 2003, 91, 643–655. [Google Scholar] [CrossRef]
- Bo, N.; Chen, L.; Yi, C.; Shi, X.; Zhao, M.J.E. A high-performance liquid chromatography method for simultaneous detection of 20 bioactive components in tea extracts. Electrophoresis 2019, 40, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Shoaib Khan, H.M.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef]
- Kalili, K.M.; de Villiers, A. Recent developments in the HPLC separation of phenolic compounds. J. Sep. Sci. 2011, 34, 854–876. [Google Scholar] [CrossRef]
- Bladek, J.; Sliwakowski, M. Phenols. Solid-phase extraction. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. In Encyclopedia of Separation Science; Academic Press: Cambridge, MA, USA, 2000; pp. 3776–3783. [Google Scholar]
- ICH Harmonised Tripartite Guideline Q2 (R1), European Medicines Agency 2005, 1, 20, 05. Available online: https://www.fda.gov/media/152208/download (accessed on 1 May 2023).
- Gilala, J. Determination of Phenols in Water by High Performance Liquid Chromatography with a Uv-Detector. Bachelor’s Thesis, Central Ostrobothnia University of Applied Sciences, Degree Programme in Chemistry and Technology, Kokkola, Finland, 2010. [Google Scholar]
- Opeolu, B.O.; Fatoki, O.S.; Odendaal, J. Development of a solid-phase extraction method followed by HPLC-UV detection for the determination of phenols in water. Int. J. Phys. Sci. 2010, 5, 576–581. [Google Scholar]
- Kadmi, Y.; Favier, L.; Yehya, T.; Soutrel, I.; Simion, A.I.; Vial, C.; Wolbert, D. Controlling contamination for determination of ultra-trace levels of priority pollutants chlorophenols in environmental water matrices. Arab. J. Chem. 2019, 12, 2905–2913. [Google Scholar] [CrossRef]
- Salcedo, G.M.; Kupski, L.; Degang, L.; Marube, L.C.; Caldas, S.S.; Primel, E.G. Determination of fifteen phenols in wastewater from petroleum refinery samples using a dispersive liquid—Liquid microextraction and liquid chromatography with a photodiode array detector. Microchem. J. 2019, 146, 722–728. [Google Scholar] [CrossRef]
- Loos, R.; Hanke, G.; Eisenreich, S.J. Multi-component analysis of polar water pollutants using sequential solid-phase extraction followed by LC-ESI-MS. J. Environ. Monit. 2003, 5, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Chen, X. Simultaneous Determination of 19 Chlorophenols in Water by Liquid Chromatography-Mass Spectrometry with Solid-Phase Extraction. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1369–1380. [Google Scholar] [CrossRef]
- Fattahi, N.; Assadi, Y.; Hosseini, M.R.M.; Jahromi, E.Z. Determination of chlorophenols in water samples using simultaneous dispersive liquid–liquid microextraction and derivatization followed by gas chromatography-electron-capture detection. J. Chromatogr. A 2007, 1157, 23–29. [Google Scholar] [CrossRef]
- Yasuhiko, H.; Youichi, F. HPLC-UV Analysis of Phenol and Chlorophenols in Water After Precolumn Derivatization with 4-Fluoro-7-nitro-2,1,3-benzoxadiazole. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2372–2383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).