Modulation of Campylobacter jejuni Motility, Adhesion to Polystyrene Surfaces, and Invasion of INT407 Cells by Quorum-Sensing Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Essential Oils and Pure Compounds
2.3. Extract Preparation
2.4. Determination of Minimal Inhibitory Concentrations
2.5. Autoinducer-2 Bioassay
2.6. Motility Assay
2.7. Adhesion to Polystyrene Surfaces
2.8. Invasion and Adhesion of INT407 Cells
2.9. Statistical Analysis
3. Results
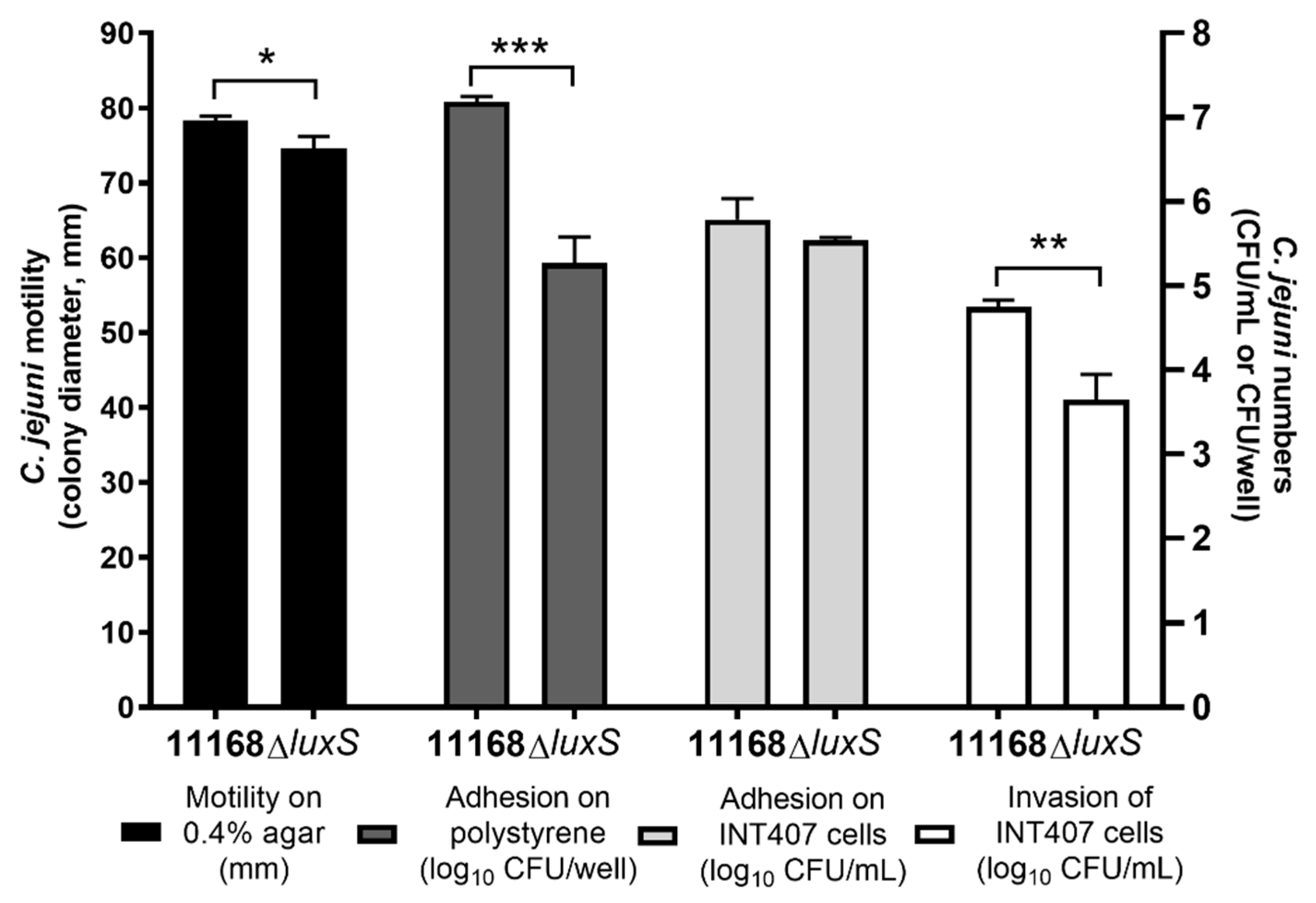
3.1. Comparison of Wild-Type C. jejuni 11168 with the luxS-Deficient Mutant
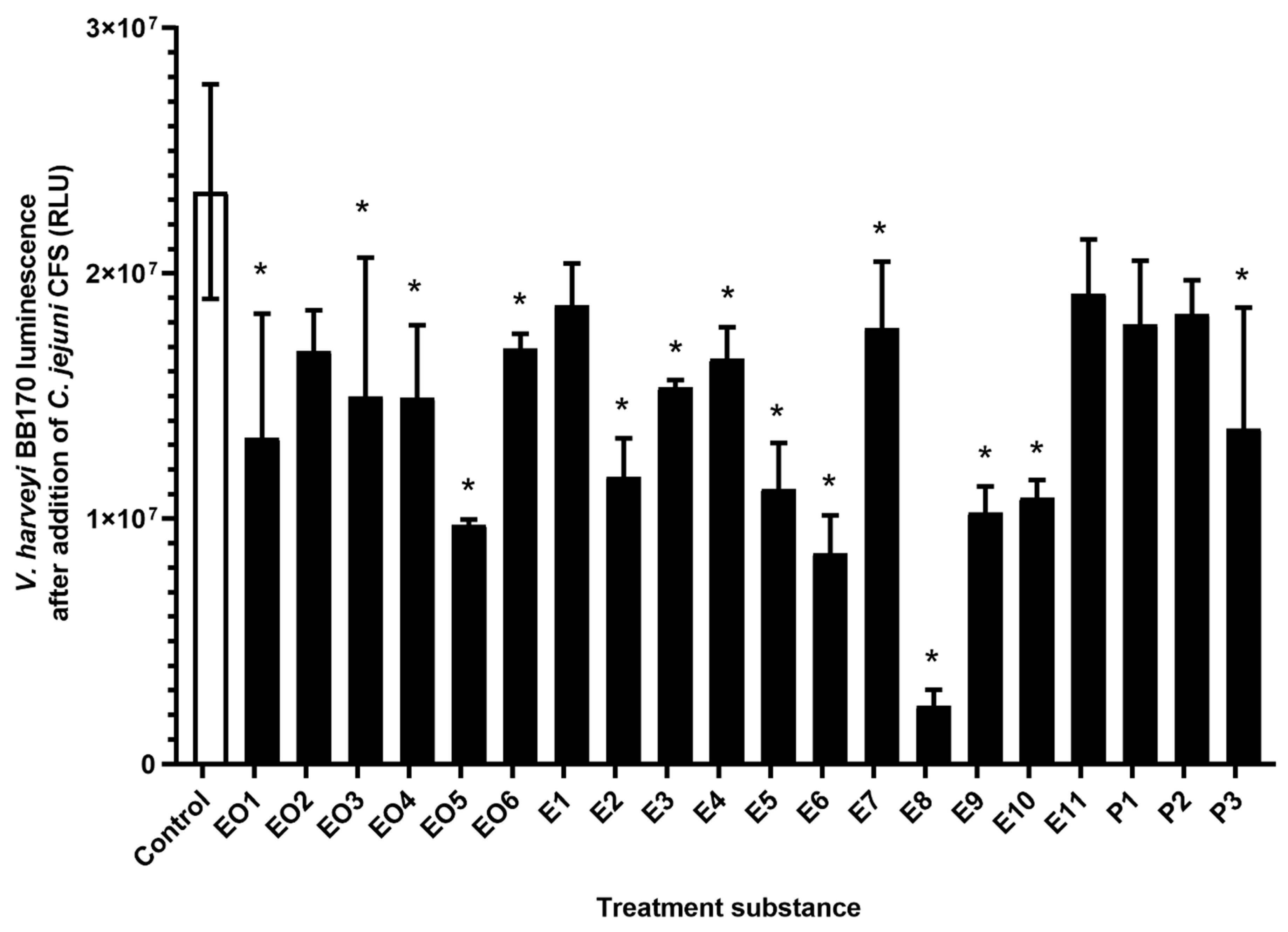
3.2. Inhibition of Quorum Sensing of C. jejuni with Essential Oils, Ethanolic Extracts, and Pure Compounds
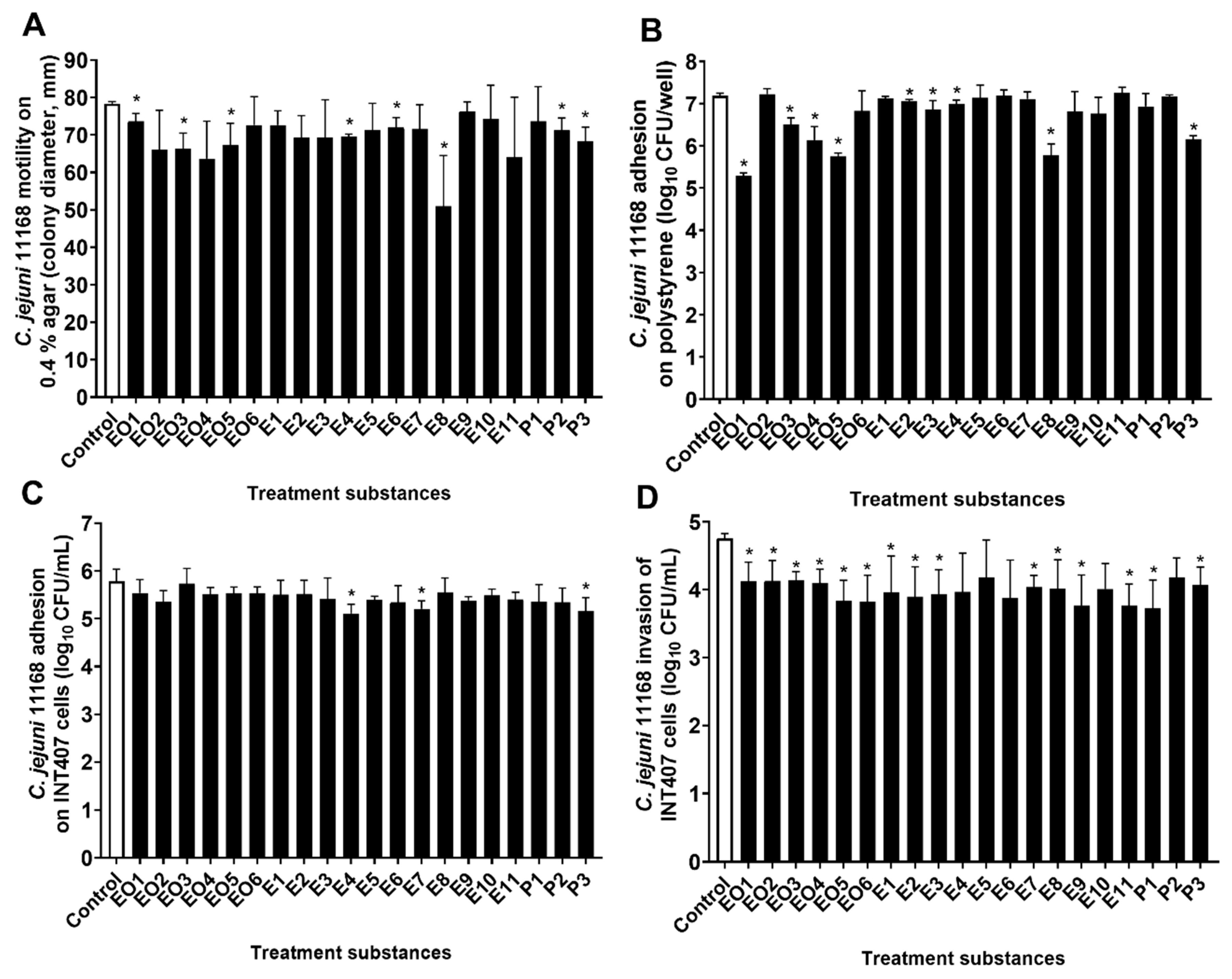
3.3. Modulation of C. jejuni Motility
3.4. Modulation of C. jejuni Adhesion to Polystyrene Surfaces
3.5. Modulation of Adhesion to and Invasion of INT407 Cells by C. jejuni 11168
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Ellyn, P.; Marder, M.P.H. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 324–328. [Google Scholar]
- EFSA and ECDC. European Food Safety Authority; European Centre for Disease Prevention and Control The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, e05598. [Google Scholar]
- Helms, M.; Simonsen, J.; Olsen, K.E.P.; Mølbak, K. Adverse health events associated with antimicrobial drug resistance in Campylobacter species: A registry-based cohort study. J. Infect. Dis. 2005, 191, 1050–1055. [Google Scholar] [CrossRef]
- BIOHAZ EFSA Panel on Biological Hazards. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar]
- Deep, A.; Chaudhary, U.; Gupta, V. Quorum sensing and bacterial pathogenicity: From molecules to disease. J. Lab. Physicians 2011, 3, 4–11. [Google Scholar] [CrossRef]
- Asfour, H.Z. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Choi, J.; Shin, D.; Ryu, S. Implication of quorum sensing in Salmonella enterica serovar typhimurium virulence: The luxS gene is necessary for expression of genes in pathogenicity island 1. Infect. Immun. 2007, 75, 4885–4890. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Deblais, L.; Kassem, I.I.; Kathayat, D.; Rajashekara, G. Novel small molecule modulators of quorum sensing in avian pathogenic Escherichia coli (APEC). Virulence 2018, 9, 1640–1657. [Google Scholar] [CrossRef]
- Gorelik, O.; Levy, N.; Shaulov, L.; Yegodayev, K.; Meijler, M.M.; Sal-Man, N. Vibrio cholerae autoinducer-1 enhances the virulence of enteropathogenic Escherichia coli. Sci. Rep. 2019, 9, 4122. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.; Fothergill, J.L. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiol. Lett. 2009, 290, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elvers, K.T.; Park, S.F. Quorum sensing in Campylobacter jejuni: Detection of a luxS encoded signaling molecule. Microbiol. Read. Engl. 2002, 148, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.; Hardie, K.R.; Burgess, N.; Doherty, N.; Kirke, D.; Holden, M.T.G.; Linforth, R.; Cornell, K.A.; Taylor, A.J.; Hill, P.J.; et al. LuxS: Its role in central metabolism and the in-vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 2002, 148, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.J.; Acheson, D.W.K. Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect. Immun. 2002, 70, 1761–1771. [Google Scholar] [CrossRef]
- Jeon, B.; Itoh, K.; Misawa, N.; Ryu, S. Effects of quorum sensing on flaA transcription and autoagglutination in Campylobacter jejuni. Microbiol. Immunol. 2003, 47, 833–839. [Google Scholar] [CrossRef]
- Holmes, K.; Tavender, T.J.; Winzer, K.; Wells, J.M.; Hardie, K.R. AI-2 does not function as a quorum sensing molecule in Campylobacter jejuni during exponential growth in vitro. BMC Microbiol. 2009, 9, 214. [Google Scholar] [CrossRef]
- Quiñones, B.; Miller, W.G.; Bates, A.H.; Mandrell, R.E. Autoinducer-2 production in Campylobacter jejuni contributes to chicken colonization. Appl. Environ. Microbiol. 2009, 75, 281–285. [Google Scholar] [CrossRef]
- Plummer, P.; Sahin, O.; Burrough, E.; Sippy, R.; Mou, K.; Rabenold, J.; Yaeger, M.; Zhang, Q. Critical role of LuxS in the virulence of Campylobacter jejuni in a guinea pig model of abortion. Infect. Immun. 2012, 80, 585–593. [Google Scholar] [CrossRef]
- Adler, L.; Alter, T.; Sharbati, S.; Gölz, G. Phenotypes of Campylobacter jejuni luxS mutants are dependent on strain background, kind of mutation and experimental conditions. PLoS ONE 2014, 9, e104399. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.Ø.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.-P.; Rygaard, J.; Moser, C.; Eberl, L.; et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiol. Read. Engl. 2005, 151, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Harjai, K.; Kumar, R.; Singh, S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2010, 58, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-Y.; Yuan, M.; Cui, Z.-Q.; Wu, Z.-M.; Yu, Z.-J.; Song, K.; Tang, B.; Fu, B.-D. Rutin inhibits quorum sensing, biofilm formation and virulence genes in avian pathogenic Escherichia coli. Microb. Pathog. 2018, 119, 54–59. [Google Scholar] [CrossRef]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Castillo, S.; Dávila-Aviña, J.; Heredia, N.; Garcia, S. Antioxidant activity and influence of citrus by-product extracts on adherence and invasion of Campylobacter jejuni and on the relative expression of cadF and ciaB. Food Sci. Biotechnol. 2017, 26, 453–459. [Google Scholar] [CrossRef]
- Bezek, K.; Kurinčič, M.; Knauder, E.; Klančnik, A.; Raspor, P.; Bucar, F.; Smole Možina, S. Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother. Res. PTR 2016, 30, 1527–1532. [Google Scholar] [CrossRef]
- Bassler, B.L.; Greenberg, E.P.; Stevens, A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997, 179, 4043–4045. [Google Scholar] [CrossRef]
- Alperth, F.; Turek, I.; Weiss, S.; Vogt, D.; Bucar, F. Qualitative and quantitative analysis of different Rhodiola rosea rhizome extracts by UHPLC-DAD-ESI-MSn. Sci. Pharm. 2019, 87, 8. [Google Scholar] [CrossRef]
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Možina, S.S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef]
- Negretti, N.M.; Konkel, M.E. Methods to Study Campylobacter jejuni Adherence to and Invasion of Host Epithelial Cells. In Campylobacter jejuni: Methods and Protocols; Butcher, J., Stintzi, A., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2017; pp. 117–127. ISBN 978-1-4939-6536-6. [Google Scholar]
- Kerekes, E.-B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Poli, J.-P.; Guinoiseau, E.; de Rocca Serra, D.; Sutour, S.; Paoli, M.; Tomi, F.; Quilichini, Y.; Berti, L.; Lorenzi, V. Anti-quorum sensing activity of 12 essential oils on Chromobacterium violaceum and specific action of cis-cis-p-menthenolide from Corsican Mentha suaveolens ssp. insularis. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Asif, M.; Tahseen, Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, R.; Tandon, S.; Pandey, R. Thyme oil reduces biofilm formation and impairs virulence of Xanthomonas oryzae. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; del R. Moreira, M.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Omwenga, E.O.; Hensel, A.; Pereira, S.; Shitandi, A.A.; Goycoolea, F.M. Antiquorum sensing, antibiofilm formation and cytotoxicity activity of commonly used medicinal plants by inhabitants of Borabu sub-county, Nyamira County, Kenya. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Tolmacheva, A.A.; Rogozhin, E.A.; Deryabin, D.G. Antibacterial and quorum sensing regulatory activities of some traditional eastern European medicinal plants. Acta Pharm. Zagreb Croat. 2014, 64, 173–186. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; van der Zeijst, B.A.; Ayling, R.; Newell, D.G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 1993, 139 Pt 6, 1171–1175. [Google Scholar] [CrossRef]
- Hermans, D.; Van Deun, K.; Martel, A.; Van Immerseel, F.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 82. [Google Scholar] [CrossRef]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.-L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef] [PubMed]
- Reeser, R.J.; Medler, R.T.; Billington, S.J.; Jost, B.H.; Joens, L.A. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 2007, 73, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Zorko, Š.; Toplak, N.; Kovač, M.; Bucar, F.; Jeršek, B.; Smole Možina, S. Antiadhesion activity of juniper (Juniperus communis L.) preparations against Campylobacter jejuni evaluated with PCR-based methods. Phytother. Res. PTR 2018, 32, 542–550. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Inhibition (%) for Treated Cultures Compared to Control Culture | |||
|---|---|---|---|---|
| Code | QS | Motility | Adhesion to Polystyrene Surfaces | Invasion of INT407 Cells |
| EO1 | 51 ± 11.6 | 6 ± 0.1 | 99 ± 0.9 | 76 ± 4.3 |
| EO2 | 37 ± 1.7 | 16 ± 2.0 | −10 ± 0.1 | 76 ± 4.5 |
| EO3 | 44 ± 9.4 | 15 ± 0.8 | 79 ± 1.6 | 76 ± 2.0 |
| EO4 | 44 ± 4.9 | 19 ± 2.4 | 91 ± 3.8 | 78 ± 3.1 |
| EO5 | 66 ± 0.7 | 14 ± 1.0 | 96 ± 1.2 | 88 ± 5.6 |
| EO6 | 36 ± 0.7 | 7 ± 0.6 | 56 ± 3.2 | 88 ± 7.3 |
| E1 | 29 ± 1.3 | 7 ± 0.3 | 13 ± 0.1 | 84 ± 9.8 |
| E2 | 58 ± 4.5 | 11 ± 0.8 | 26 ± 0.1 | 86 ± 8.5 |
| E3 | 43 ± 0.4 | 11 ± 1.4 | 54 ± 1.4 | 85 ± 7.0 |
| E4 | 38 ± 1.5 | 11 ± 0.2 | 36 ± 0.4 | 84 ± 9.8 |
| E5 | 60 ± 5.2 | 9 ± 0.7 | 11 ± 0.4 | 73 ± 7.9 |
| E6 | 71 ± 6.9 | 8 ± 0.2 | −1 ± 0.1 | 87 ± 10.2 |
| E7 | 33 ± 2.8 | 9 ± 0.6 | 15 ± 0.3 | 81 ± 2.8 |
| E8 | 96 ± 15.7 | 35 ± 7.6 | 96 ± 3.7 | 82 ± 7.1 |
| E9 | 64 ± 3.5 | 3 ± 0.1 | 57 ± 3.2 | 90 ± 8.8 |
| E10 | 61 ± 2.3 | 5 ± 0.5 | 62 ± 2.9 | 82 ± 7.1 |
| E11 | 27 ± 1.5 | 18 ± 3.8 | −18 ± 0.3 | 90 ± 6.3 |
| P1 | 32 ± 2.3 | 6 ± 0.6 | 44 ± 1.6 | 91 ± 8.2 |
| P2 | 31 ± 1.2 | 9 ± 0.3 | 2 ± 0.1 | 73 ± 4.0 |
| P3 | 50 ± 10.4 | 13 ± 0.6 | 91 ± 0.9 | 79 ± 4.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimunović, K.; Ramić, D.; Xu, C.; Smole Možina, S. Modulation of Campylobacter jejuni Motility, Adhesion to Polystyrene Surfaces, and Invasion of INT407 Cells by Quorum-Sensing Inhibition. Microorganisms 2020, 8, 104. https://doi.org/10.3390/microorganisms8010104
Šimunović K, Ramić D, Xu C, Smole Možina S. Modulation of Campylobacter jejuni Motility, Adhesion to Polystyrene Surfaces, and Invasion of INT407 Cells by Quorum-Sensing Inhibition. Microorganisms. 2020; 8(1):104. https://doi.org/10.3390/microorganisms8010104
Chicago/Turabian StyleŠimunović, Katarina, Dina Ramić, Changyun Xu, and Sonja Smole Možina. 2020. "Modulation of Campylobacter jejuni Motility, Adhesion to Polystyrene Surfaces, and Invasion of INT407 Cells by Quorum-Sensing Inhibition" Microorganisms 8, no. 1: 104. https://doi.org/10.3390/microorganisms8010104
APA StyleŠimunović, K., Ramić, D., Xu, C., & Smole Možina, S. (2020). Modulation of Campylobacter jejuni Motility, Adhesion to Polystyrene Surfaces, and Invasion of INT407 Cells by Quorum-Sensing Inhibition. Microorganisms, 8(1), 104. https://doi.org/10.3390/microorganisms8010104





