The Environmental Lifecycle of Antibiotics and Resistance Genes: Transmission Mechanisms, Challenges, and Control Strategies
Abstract
1. Introduction
2. Environmental Circular Pathways of Antibiotics and ARGs
2.1. Sources and Emission Characteristics of Antibiotics
| Source | ARGs | Relative Abundance (Copies/16S rRNA Gene Copies) | References |
|---|---|---|---|
| Sludge sampled from municipal wastewater treatment plant | tetA, tetB, tetE, tetG, tetH, tetS, tetT, tetX, sul1, sul2, qnrB, and ermC | (1.5 ± 2.3) × 109–(2.2 ± 2.8) × 1011 copies/g dry weight | [20] |
| Municipal wastewater | tetA, tetC, tetG, tetM, tetO, tetW, tetX, sul1, and sul2 | 3.6 × 101(teW) to 5.4 × 106 (tetX) copies mL−1 6.4 × 1012(tetW) to 1.7 × 1018 (sull) copies d−1 | [21] |
| Sludge sampled from hospital wastewater treatment plant | blaOXA-48, CTX-M, and blaIMP blaTEM | 5.36 × 1011–1.90 × 1012 copies/g dry weight | [22] |
| Hospital wastewater | sul1, blaSHV, catA1, aacC2, and tetA | 1.94 × 101, 4.39 × 10−3, 6.83 × 10−5, 5.67 × 10−3, 3.46 × 10−3 | [23] |
| sul1, sul2, sul3, and tetQ | 1.79 × 101~6.67 × 101, 7.33 × 10−2~3.38 × 101, 9.22 × 10−2~5.9 × 101, 2.8 × 101~7.47 × 101 | [24] | |
| Livestock wastewater | tetL, strB, sul2, tetG, ermB, sul1, tetX, and cmlA | tetL(1.36~0.39), strB (0.82~0.52), sul2 (0.96~0.64), tetG (1.81~0.67), ermB (1.17~0.71), sul1 (1.51~0.93), tetX (1.17~0.94), and cmlA(1.73~1.14) | [25] |
| tetX, ermF, ermB, mefA, tetM, and sul2 | 2.43 × 1011–5.69 × 1010 copies/mL | [26] | |
| sul1, sul2, and tetM | 3.84 × 101, 1.62 × 101, 2.33 × 101 | [27] | |
| tetC and tetO | 7.3 × 103, 1.7 × 101 | [21] | |
| Pharmaceutical industry wastewater | tetA, tetC, tetG, tetL, tetM, and tetO | 1.4 × 101, 3.2 × 102, 5.1 × 102, 6.1 × 102, 1.1 × 102, 1.0 × 100, 1.8 × 100, 1.6 × 101, 3.7 × 103 | [28] |
| sul1, sul2, tetA, qacE, and qacED1 | 101 to 102 | [29] | |
| Soil irrigated with recycled water | tetG, tetW, sulI, sulII, and intI1 | Highest abundance of sul2 and intI1; the abundances were 8.43 × 107 copies g−1 dry soil and 7.62 × 107 copies g−1 dry soil | [30] |
| Drinking water | sul1, sul2, tetC, tetG, tetX, tetA, tetB, tetO, tetM, and tetW | Total concentrations of ARGs belonging to either the sulfonamide or tetracycline resistance gene class were above 105 copies mL−1 | [31] |
| River sediments | TEM, sul1, and sul2 | 1.09 × 10−1 ~1.06 × 10−1 | [32] |
2.1.1. Medical and Domestic Wastewater
2.1.2. Livestock and Agricultural Activities
2.1.3. Pharmaceutical Industry Wastewater
2.1.4. Major Antibiotic Families and ARG Subtypes in the Environment
2.2. Migration Pathways in Environmental Media
2.2.1. The Aquatic Environment
2.2.2. Soil
2.2.3. Atmosphere
2.3. Ultimate Exposure Pathways of Drug Resistance to Human Health
2.3.1. Contamination of Drinking Water and the Food Chain
2.3.2. Airborne Transmission and Lung Colonization
2.3.3. Ecological Traceability of Multidrug-Resistant Bacteria
2.4. Detection and Quantification of Resistance Genes in the Environment
3. Molecular Mechanisms for the Emergence and Spread of Drug Resistance
3.1. The Molecular Basis of Bacterial Drug Resistance
3.1.1. Intrinsic Resistance
- (1)
- Bacteria alter extracellular membrane permeability
- (2)
- Active transport of bacteria via efflux pumps
- (3)
- Bacterial modification of antibiotic target molecules
- (4)
- Bacteria inactivate antibiotics through enzymes
3.1.2. Acquired Drug Resistance: Gene Mutations and Horizontal Transfer (HGT)
3.2. Environmental Pressures Driving and Synergistically Influencing the Evolution of Drug Resistance
3.2.1. Environmental Pressure as a Driving Force
- (1)
- Antibiotics
- (2)
- Heavy metals
- (3)
- Disinfectant
3.2.2. Synergistic Effects of Environmental Stresses
Co-Selection Resistance Driven by Multiple Pollutants
- (1)
- Co-resistance
- (2)
- Cross-resistance
- (3)
- Co-regulation
Regulatory Network of Quorum Sensing (QS) System-Regulated Biofilm Resistance
3.3. Critical Pathways for Horizontal Gene Transfer (HGT)
3.3.1. Mobile Genetic Elements
- (1)
- Plasmids
- (2)
- ICEs/IMEs
- (3)
- Bacteriophages
- (4)
- Transposons
- (5)
- Integrons
3.3.2. Conjugation: The Role of Plasmids and Integrons
3.3.3. Transformation: Capture and Integration of Free DNA
3.3.4. Transduction: Phage-Mediated Gene Delivery
3.3.5. Gene Delivery Potential of Outer Membrane Vesicles (OMVs)
4. Mechanisms of Antibiotic and ARG Removal in Biological Treatment Technologies
4.1. The Dual Role of Biofilms in Antibiotic Removal
4.1.1. Adsorption and Barrier Effects of EPSs
4.1.2. Biodegradation and Enzyme-Catalysed Conversion
4.2. Efficiency and Limitations of Key Treatment Processes
| Processing Techniques | Operating Conditions | ARGs Kind | Removal Effect | References |
|---|---|---|---|---|
| Activated sludge treatment | tetO and tetW | 3 logs | [202] | |
| ermB, tetW, and sul2 | 1.29–2.45 log (ermB), 1.13–1.62 log (tetW), 0.26–0.53 log (sul2) | [64] | ||
| CASS | tetA, tetO, tetW, sulI, sulII, and blaCTX-M | >2.60 ± 0.015 log (tetO); >2.66 ± 0.023 log (tetW) | [203] | |
| A/O | tet, erm, sul, qnr, and bla | 16.90% (total ARGs), 64.50% (tet), 92.00% (erm) | [200] | |
| A/A/O | tet, erm, sul, qnr, and bla | 56.00% (tet), 70.40–87.00% (erm) | [200] | |
| sulI, sulII, tetO, tetW, and tetQ | 1.69 logs, 1.44 logs, 2.31 logs, 2.13 logs, 2.5 logs | [204] | ||
| Membrane bioreactor | sulII, tetO, and tetW | 2.57 logs, 7.06 logs, 6 logs | [205] | |
| AnMBR | blandM-1, blaCTX-M-15, and blaOXA-48 | 2.76–3.84 logs | [206] | |
| A/O-MBR | sulI, sulII, tetC, tetX, ereA, and int1 | 0.5–5.6 logs | [11] | |
| Aerobic granular sludge | tetW, sul2, sul1, intI1, and ermB | 2.02 log (tetW), 1.43 log (sul2), 0.77 log (sul1), 0.55 log (intI1), 0.08 log (ermB) | [64] | |
| Anaerobic digestion | 40 °C, 56 °C, 60 °C, and 63 °C. | tetW, tetX, qnrA, and intI1 | Decreased ARGs except qnrA by 89–96% and ~99% at 40 °C and other temperatures, and decreased qnrA by 99% at 40, 60, and 63 °C | [207] |
| MAD | 35 °C, sludge retention time (SRT) 20 d. | sulI, sulII, tetA, tetO, tetX, bla, and TEM bla | Decreased extracellular ARGs by 0.11 1.22 logs | [208] |
| TAD | 55 °C, SRT 20 d. | sulI, sulII, tetA, tetO, tetX, bla, and TEM blaSHV | Decreased extracellular ARGs by 0.33 1.46 logs | [208] |
| Composting | Kitchen waste | tetA, tetB, tetC, tetG, tetM, tetO, tetQ, tetW, tetX, sul1, sul2, sul3, and dfrA7, qnrB, qnrS, acc(6′)-Ibcr, ermB, ermF, ermQ, ermX, and mefA | Total ARGs: 99.68–99.98% (tetracyclines: >99%; sulfonamides: 5.35–8534.69%; quinolones: 837.30–99.29%; macrolides: 4425.46–98.14%) | [209] |
| Cattle manure | ermB, ermF, ermQ, ermX, sul1, sul2, sulA, tetA, tetB, tetC, tetE, tetG, tetK, tetM, tetO, tetQ, tetW, tetX | Total ARGs: 52.69% | [210] | |
| Sewage sludge | ermB, ermC, sul1, sul2, tetC, tetG, and tetO | ermB, ermC, sul1, and tetC: 25.7%, 42.4%, 69.4%, and 44.6%, respectively | [211] | |
| CW-surface flow | Capacity: 600m/d HLR: 350–450mm/d HRT: 6h | sulI, sulII, sulIII, tetA, tetB, tetC, tetE, tetH, tetM, tetO, tetW, qnrB, qnrS, and qepA | 77.8% in summer, 59.5% in winter | [212] |
| CW-horizontal subsurface flow | Capacity: 500 m/d | intl1, sulI, sulII, dfrA, aac6, tetO, qnrA, blaNMD1, blaKPC, blaCTX, and ermB | 145.6–98.9% | [213] |
| CW-vertical subsurface flow | HLR: 5.1 cm/d | tet genes and intI1 | 33.2–99.1% | [214] |
| Constructed wetland | sulI, sulII, tetO, tetW, and tetQ | 1.5 logs, 0.48 log, 2.1 logs, 1.5 logs, 2.1 logs | [204] | |
| Microalgae | bla-Tem and ermB | 0.56logs, 1.75 logs | [215] | |
| sul1, tetQ, blaKPC, and intl1 | 1.2–4.9 logs, 2.7–6.3 logs, 0–1.5 logs, 1.2–4.8 logs | [216] |
4.2.1. Conventional Activated Sludge (CAS) Versus Membrane Bioreactor (MBR)
4.2.2. Aerobic Granular Sludge and Anaerobic Digestion Technology
4.2.3. Ecological Restoration Potential of Artificial Wetlands and Microalgal Systems
4.2.4. Emerging Technologies
- CRISPR-Cas gene editing technology
- 2.
- Nano-material technology
4.3. Risk of Secondary Contamination of Treatment Byproducts
4.3.1. Enrichment and Release of ARGs in Sludge
4.3.2. Environmental Toxicity of Intermediate Degradation Products
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moser, C.; Lerche, C.J.; Thomsen, K.; Hartvig, T.; Schierbeck, J.; Jensen, P.Ø.; Ciofu, O.; Høiby, N. Antibiotic therapy as personalized medicine—General considerations and complicating factors. APMIS 2019, 127, 361–371. [Google Scholar] [CrossRef]
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global trends in antibiotic consumption during 2016–2023 and future projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Patrolecco, L.; Rauseo, J.; Ademollo, N.; Grenni, P.; Cardoni, M.; Levantesi, C.; Luprano, M.L.; Caracciolo, A.B. Persistence of the antibiotic sulfamethoxazole in river water alone or in the co-presence of ciprofloxacin. Sci. Total Environ. 2018, 640–641, 1438–1446. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Ding, C.; Jin, M.; Ma, J.; Chen, Z.; Shen, Z.; Yang, D.; Shi, D.; Liu, W.; Kang, M.; Wang, J.; et al. Nano-Al2O3 can mediate transduction-like transformation of antibiotic resistance genes in water. J. Hazard. Mater. 2021, 405, 124224. [Google Scholar] [CrossRef]
- Church, N.A.; McKillip, J.L. Antibiotic resistance crisis: Challenges and imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, Z.; Sun, L.; Dong, C.; Jin, Y.; Hu, B.; Cheng, D. Mobile genetic elements mediate the cross-media transmission of antibiotic resistance genes from pig farms and their risks. Sci. Total Environ. 2024, 926, 172115. [Google Scholar] [CrossRef]
- Yin, F.; Hao, W.; Zhang, H.; Miao, J.; Shi, H. Pollution status and spatial distribution of antibiotic resistance genes in urban surface water and surrounding soil media. J. Environ. Chem. Eng. 2024, 12, 113856. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhou, S.; Jiang, X.; Ma, X.; Liu, C. Robust performance of a membrane bioreactor for removing antibiotic resistance genes exposed to antibiotics: Role of membrane foulants. Water Res. 2018, 130, 139–150. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of antibiotic resistance genes (ARGs) in various wastewater treatment processes: An overview. Crit. Rev. Environ. Sci. Technol. 2020, 52, 571–630. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, L.; Li, Z.-H.; Zhang, X.; Sheng, G.-P. Quantifying the occurrence and transformation potential of extracellular polymeric substances (EPS)-associated antibiotic resistance genes in activated sludge. J. Hazard. Mater. 2021, 408, 124428. [Google Scholar] [CrossRef]
- Wang, M.; Lian, Y.; Wang, Y.; Zhu, L. The role and mechanism of quorum sensing on environmental antimicrobial resistance. Environ. Pollut. 2023, 322, 121238. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dai, R.; Chen, M.; Khan, S.J.; Wang, Z. Applications of membrane bioreactors for water reclamation: Micropollutant removal, mechanisms and perspectives. Bioresour. Technol. 2018, 269, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Kaur Sodhi, K.; Singh, C.K. Recent development in the sustainable remediation of antibiotics: A review. Total Environ. Res. Themes 2022, 3–4, 100008. [Google Scholar] [CrossRef]
- Godijk, N.G.; Bootsma, M.C.J.; Bonten, M.J.M. Transmission routes of antibiotic resistant bacteria: A systematic review. BMC Infect. Dis. 2022, 22, 482. [Google Scholar] [CrossRef]
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef]
- Ben, W.; Wang, J.; Pan, X.; Qiang, Z. Dissemination of antibiotic resistance genes and their potential removal by on-farm treatment processes in nine swine feedlots in Shandong Province, China. Chemosphere 2017, 167, 262–268. [Google Scholar] [CrossRef]
- Al Salah, D.M.M.; Ngweme, G.N.; Laffite, A.; Otamonga, J.-P.; Mulaji, C.; Poté, J. Hospital wastewaters: A reservoir and source of clinically relevant bacteria and antibiotic resistant genes dissemination in urban river under tropical conditions. Ecotoxicol. Environ. Saf. 2020, 200, 110767. [Google Scholar] [CrossRef]
- Szekeres, E.; Baricz, A.; Chiriac, C.M.; Farkas, A.; Opris, O.; Soran, M.-L.; Andrei, A.-S.; Rudi, K.; Balcázar, J.L.; Dragos, N.; et al. Abundance of antibiotics, antibiotic resistance genes and bacterial community composition in wastewater effluents from different Romanian hospitals. Environ. Pollut. 2017, 225, 304–315. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Liu, J.; Zhang, G.; Tong, Y.; Ma, N. Exploring the correlations between antibiotics and antibiotic resistance genes in the wastewater treatment plants of hospitals in Xinjiang, China. Environ. Sci. Pollut. Res. 2016, 23, 15111–15121. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Cai, Y.; Xing, S.; Mi, J.; Liao, X. The relationship between culturable doxycycline-resistant bacterial communities and antibiotic resistance gene hosts in pig farm wastewater treatment plants. Ecotoxicol. Environ. Saf. 2020, 206, 111164. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Zhang, J.; Tong, J.; Chen, M.; Wei, Y. Seasonal variation and removal efficiency of antibiotic resistance genes during wastewater treatment of swine farms. Environ. Sci. Pollut. Res. 2015, 24, 9048–9057. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hao, L.; Guo, X.; Wang, N.; Ye, B. Prevalence of antibiotic resistance genes of wastewater and surface water in livestock farms of Jiangsu Province, China. Environ. Sci. Pollut. Res. 2015, 22, 13950–13959. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Yang, M.; Tian, Z.; Ren, L.; Zhang, S. Abundance and Distribution of Tetracycline Resistance Genes and Mobile Elements in an Oxytetracycline Production Wastewater Treatment System. Environ. Sci. Technol. 2012, 46, 7551–7557. [Google Scholar] [CrossRef]
- González-Plaza, J.J.; Blau, K.; Milaković, M.; Jurina, T.; Smalla, K.; Udiković-Kolić, N. Antibiotic-manufacturing sites are hot-spots for the release and spread of antibiotic resistance genes and mobile genetic elements in receiving aquatic environments. Environ. Int. 2019, 130, 104735. [Google Scholar] [CrossRef]
- Wang, F.-H.; Qiao, M.; Lv, Z.-E.; Guo, G.-X.; Jia, Y.; Su, Y.-H.; Zhu, Y.-G. Impact of reclaimed water irrigation on antibiotic resistance in public parks, Beijing, China. Environ. Pollut. 2014, 184, 247–253. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Yang, F.; Yang, J.; Yin, D. Prevalence of sulfonamide and tetracycline resistance genes in drinking water treatment plants in the Yangtze River Delta, China. Sci. Total Environ. 2014, 493, 626–631. [Google Scholar] [CrossRef]
- Chen, H.; Bai, X.; Jing, L.; Chen, R.; Teng, Y. Characterization of antibiotic resistance genes in the sediments of an urban river revealed by comparative metagenomics analysis. Sci. Total Environ. 2019, 653, 1513–1521. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Xu, L.; Strong, P.J.; Chen, H. Antibiotic-resistant genes and antibiotic-resistant bacteria in the effluent of urban residential areas, hospitals, and a municipal wastewater treatment plant system. Environ. Sci. Pollut. Res. 2014, 22, 4587–4596. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Dinh, Q.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.-J.; Blanchard, M.; Eurin, J.; Chevreuil, M. Fate of antibiotics from hospital and domestic sources in a sewage network. Sci. Total Environ. 2017, 575, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Yang, Z.; Yang, Y.; Huang, N.; Arku, J.E.; Mao, G.; Wang, Y. Global trends and prospects in the removal of pharmaceuticals and personal care products: A bibliometric analysis. J. Water Process Eng. 2021, 41, 102004. [Google Scholar] [CrossRef]
- Pempek, J.A.; Holder, E.; Proudfoot, K.L.; Masterson, M.; Habing, G. Short communication: Investigation of antibiotic alternatives to improve health and growth of veal calves. J. Dairy Sci. 2018, 101, 4473–4478. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.-P.; Sun, Y.-B.; Zhao, Z.-H. Degradation of sulfadiazine antibiotics by water falling film dielectric barrier discharge. Chin. Chem. Lett. 2014, 25, 187–192. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Cha, C.-J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I.; Baquero, F. Selection of a Multidrug Resistance Plasmid by Sublethal Levels of Antibiotics and Heavy Metals. mBio 2014, 5, e01918-14. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Lin, H.; Sun, W.; Zhang, Z.; Chapman, S.J.; Freitag, T.E.; Fu, J.; Zhang, X.; Ma, J. Effects of manure and mineral fertilization strategies on soil antibiotic resistance gene levels and microbial community in a paddy–upland rotation system. Environ. Pollut. 2016, 211, 332–337. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Y.; Zheng, L.; Ji, L.; Kong, X.; Du, J.; Wang, H.; Duan, L.; Niu, T.; Liu, J.; et al. Identification and Distribution of Antibiotic Resistance Genes and Antibiotic Resistance Bacteria in the Feces Treatment Process: A Case Study in a Dairy Farm, China. Water 2024, 16, 1575. [Google Scholar] [CrossRef]
- Milaković, M.; Vestergaard, G.; González-Plaza, J.J.; Petrić, I.; Kosić-Vukšić, J.; Senta, I.; Kublik, S.; Schloter, M.; Udiković-Kolić, N. Effects of industrial effluents containing moderate levels of antibiotic mixtures on the abundance of antibiotic resistance genes and bacterial community composition in exposed creek sediments. Sci. Total Environ. 2020, 706, 136001. [Google Scholar] [CrossRef]
- Tong, J.; Lu, X.; Zhang, J.; Sui, Q.; Wang, R.; Chen, M.; Wei, Y. Occurrence of antibiotic resistance genes and mobile genetic elements in enterococci and genomic DNA during anaerobic digestion of pharmaceutical waste sludge with different pretreatments. Bioresour. Technol. 2017, 235, 316–324. [Google Scholar] [CrossRef]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Knapp, C.W.; McCluskey, S.M.; Singh, B.K.; Campbell, C.D.; Hudson, G.; Graham, D.W. Antibiotic Resistance Gene Abundances Correlate with Metal and Geochemical Conditions in Archived Scottish Soils. PLoS ONE 2011, 6, e27300. [Google Scholar] [CrossRef]
- Novo, A.; André, S.; Viana, P.; Nunes, O.C.; Manaia, C.M. Antibiotic resistance, antimicrobial residues and bacterial community composition in urban wastewater. Water Res. 2013, 47, 1875–1887. [Google Scholar] [CrossRef]
- Wright, M.S.; Peltier, G.L.; Stepanauskas, R.; McArthur, J.V. Bacterial tolerances to metals and antibiotics in metal-contaminated and reference streams. FEMS Microbiol. Ecol. 2006, 58, 293–302. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- dos Santos, D.F.K.; Istvan, P.; Quirino, B.F.; Kruger, R.H. Functional Metagenomics as a Tool for Identification of New Antibiotic Resistance Genes from Natural Environments. Microb. Ecol. 2017, 73, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Salzman, E.; Gesierich, C.; Gardner, S.; Ammerman, M.; Eisenberg, M.; Wigginton, K. Wastewater surveillance of antibiotic-resistant bacteria for public health action: Potential and challenges. Am. J. Epidemiol. 2025, 194, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Męcik, M.; Stefaniak, K.; Harnisz, M.; Korzeniewska, E. Hospital and municipal wastewater as a source of carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa in the environment: A review. Environ. Sci. Pollut. Res. 2024, 31, 48813–48838. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zeng, Z.; Wu, Y.; Wang, Y.; Shen, L.; Huang, X.; Wang, X.; Sun, Y. Characteristics of microplastics in typical poultry farms and the association of environment microplastics colonized-microbiota, waterfowl gut microbiota, and antibiotic resistance genes. J. Hazard. Mater. 2025, 490, 137808. [Google Scholar] [CrossRef]
- Pavelquesi, S.L.; de Oliveira Ferreira, A.C.; Rodrigues, A.R.; de Souza Silva, C.M.; Orsi, D.C.; da Silva, I.C. Presence of Tetracycline and Sulfonamide Resistance Genes in Salmonella spp.: Literature Review. Antibiotics 2021, 10, 1314. [Google Scholar] [CrossRef]
- Saha, S.; Xiong, J.-Q.; Patil, S.M.; Ha, G.-S.; Hoh, J.-K.; Park, H.-K.; Chung, W.; Chang, S.W.; Khan, M.A.; Park, H.B.; et al. Dissemination of sulfonamide resistance genes in digester microbiome during anaerobic digestion of food waste leachate. J. Hazard. Mater. 2023, 452, 131200. [Google Scholar] [CrossRef] [PubMed]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestępski, S.; Konopka, I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ji, M.; Zhai, H.; Guo, Y.; Liu, Y. Occurrence of antibiotics and antibiotic resistance genes in WWTP effluent-receiving water bodies and reclaimed wastewater treatment plants. Sci. Total Environ. 2021, 796, 148919. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.A.; van Holst, S.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate of antibiotics and antibiotic resistance genes during conventional and additional treatment technologies in wastewater treatment plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Xie, J.; Jin, L.; Wu, D.; Pruden, A.; Li, X. Inhalable Antibiotic Resistome from Wastewater Treatment Plants to Urban Areas: Bacterial Hosts, Dissemination Risks, and Source Contributions. Environ. Sci. Technol. 2022, 56, 7040–7051. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Zhu, Y.-g.; Chen, Q.-l.; Shen, F.; Wu, Y.; Xu, S.; Fan, H.; Da, G.; Huang, R.-j.; et al. Global Survey of Antibiotic Resistance Genes in Air. Environ. Sci. Technol. 2018, 52, 10975–10984. [Google Scholar] [CrossRef]
- Xu, L.; Ouyang, W.; Qian, Y.; Su, C.; Su, J.; Chen, H. High-throughput profiling of antibiotic resistance genes in drinking water treatment plants and distribution systems. Environ. Pollut. 2016, 213, 119–126. [Google Scholar] [CrossRef]
- Bai, X.; Ma, X.; Xu, F.; Li, J.; Zhang, H.; Xiao, X. The drinking water treatment process as a potential source of affecting the bacterial antibiotic resistance. Sci. Total Environ. 2015, 533, 24–31. [Google Scholar] [CrossRef]
- Hao, H.; Shi, D.-y.; Yang, D.; Yang, Z.-w.; Qiu, Z.-g.; Liu, W.-l.; Shen, Z.-q.; Yin, J.; Wang, H.-r.; Li, J.-w.; et al. Profiling of intracellular and extracellular antibiotic resistance genes in tap water. J. Hazard. Mater. 2019, 365, 340–345. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. mBio 2012, 3, e00305-11. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.; Corcoran, C.; Prentice, E.; Moodley, M.; Mendelson, M.; Poirel, L.; Nordmann, P.; Brink, A.J. Emergence of plasmid-mediated colistin resistance (MCR-1) among Escherichia coli isolated from South African patients. S. Afr. Med. J. 2016, 106, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Butaye, P.; Catry, B.; Dewulf, J.; Maes, D.; Pardon, B.; Callens, B.; Vanrobaeys, M.; Opsomer, G.; de Kruif, A.; Haesebrouck, F. Effect of Antimicrobial Consumption and Production Type on Antibacterial Resistance in the Bovine Respiratory and Digestive Tract. PLoS ONE 2016, 11, e0146488. [Google Scholar] [CrossRef]
- Xie, J.; Jin, L.; Luo, X.; Zhao, Z.; Li, X. Seasonal Disparities in Airborne Bacteria and Associated Antibiotic Resistance Genes in PM2.5 between Urban and Rural Sites. Environ. Sci. Technol. Lett. 2018, 5, 74–79. [Google Scholar] [CrossRef]
- Gunathilaka, G.U.; Tahlan, V.; Mafiz, A.I.; Polur, M.; Zhang, Y. Phages in urban wastewater have the potential to disseminate antibiotic resistance. Int. J. Antimicrob. Agents 2017, 50, 678–683. [Google Scholar] [CrossRef]
- Liu, X.; Song, D.; He, X.; Wang, Z.; Zeng, M.; Deng, K. Nanopore structure of deep-burial coals explored by AFM. Fuel 2019, 246, 9–17. [Google Scholar] [CrossRef]
- Zhou, Z.-C.; Feng, W.-Q.; Han, Y.; Zheng, J.; Chen, T.; Wei, Y.-Y.; Gillings, M.; Zhu, Y.-G.; Chen, H. Prevalence and transmission of antibiotic resistance and microbiota between humans and water environments. Environ. Int. 2018, 121, 1155–1161. [Google Scholar] [CrossRef]
- Loftie-Eaton, W.; Tucker, A.; Norton, A.; Top, E.M. Flow cytometry and real-time quantitative PCR as tools for assessing plasmid persistence. Appl. Environ. Microbiol. 2014, 80, 5439–5446. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Guo, F.; Wu, W.; Zhang, T. Characterization of tetracycline resistant bacterial community in saline activated sludge using batch stress incubation with high-throughput sequencing analysis. Water Res. 2013, 47, 4207–4216. [Google Scholar] [CrossRef]
- Zheng, H.-S.; Guo, W.-Q.; Wu, Q.-L.; Ren, N.-Q.; Chang, J.-S. Electro-peroxone pretreatment for enhanced simulated hospital wastewater treatment and antibiotic resistance genes reduction. Environ. Int. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- An, D.; Chung-Wah-Cheong, J.; Yu, D.-Y.; Balaratnasingam, C. Alpha-Smooth Muscle Actin Expression and Parafoveal Blood Flow Pathways Are Altered in Preclinical Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2022, 63, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Y.; Che, Y.; Xia, Y.; Li, L.; Xiong, W.; Zhang, T. Bioprospecting for β-lactam resistance genes using a metagenomics-guided strategy. Appl. Microbiol. Biotechnol. 2017, 101, 6253–6260. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-G.; Huang, Q.; Yin, X.; Zhang, T. Source tracking of antibiotic resistance genes in the environment—Challenges, progress, and prospects. Water Res. 2020, 185, 116127. [Google Scholar] [CrossRef] [PubMed]
- Loh John, T.; Beckett Amber, C.; Scholz Matthew, B.; Cover Timothy, L. High-Salt Conditions Alter Transcription of Helicobacter pylori Genes Encoding Outer Membrane Proteins. Infect. Immun. 2018, 86, 10-1128. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Ma, X.; Wang, H.; Shi, B. Metabolic response of bacterial community to sodium hypochlorite and ammonia nitrogen affected the antibiotic resistance genes in pipelines biofilm. Water Res. 2024, 252, 121179. [Google Scholar] [CrossRef]
- Korry, B.J.; Cabral, D.J.; Belenky, P. Metatranscriptomics Reveals Antibiotic-Induced Resistance Gene Expression in the Murine Gut Microbiota. Front. Microbiol. 2020, 11, 322. [Google Scholar] [CrossRef]
- Amirmozafari, N.; Fallah Mehrabadi, J.; Habibi, A. Association of the exotoxin A and exoenzyme S with antimicrobial resistance. Arch. Iran. Med. 2016, 19, 353–358. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Ju, F. Using Culture-Enriched Phenotypic Metagenomics for Targeted High-Throughput Monitoring of the Clinically Important Fraction of the β-Lactam Resistome. Environ. Sci. Technol. 2022, 56, 11429–11439. [Google Scholar] [CrossRef]
- Jia, S.; Li, T.; Zhang, X.-X. Integrated metagenomic and metatranscriptomic analyses of ultraviolet disinfection effects on antibiotic resistance genes and bacterial communities during wastewater treatment. Ecotoxicology 2021, 30, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Shi, Q.-S.; Huang, X.-M.; Xie, X.-B. The Three Bacterial Lines of Defense against Antimicrobial Agents. Int. J. Mol. Sci. 2015, 16, 21711–21733. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.; Astani, A.; Eslami, G.; Khaledi, M.; Afkhami, H.; Rostami, S.; Zarei, M.; Rezaei Khozani, N.; Zandi, H. Upstream region of OprD mutations in imipenem-resistant and imipenem-sensitive Pseudomonas isolates. AMB Express 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2014, 13, 42–51. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Van Bambeke, F. Antibiotic efflux pumps in prokaryotic cells: Occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother. 2003, 51, 1055–1065. [Google Scholar] [CrossRef]
- Van Bambeke, F. Antibiotic efflux pumps in eukaryotic cells: Occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J. Antimicrob. Chemother. 2003, 51, 1067–1077. [Google Scholar] [CrossRef]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef]
- Lin, X.; Poeta, P.; Peng, B. Editorial: The Molecular Mechanisms of Antibiotic Resistance in Aquatic Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 586460. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Kumar, V.; Ding, Y.; Ero, R.; Serra, A.; Lee, B.S.T.; Wong, A.S.W.; Shi, J.; Sze, S.K.; Yang, L.; et al. Ribosome protection by antibiotic resistance ATP-binding cassette protein. Proc. Natl. Acad. Sci. USA 2018, 115, 5157–5162. [Google Scholar] [CrossRef]
- Marshall, S.H.; Donskey, C.J.; Hutton-Thomas, R.; Salata, R.A.; Rice, L.B. Gene Dosage and Linezolid Resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 2002, 46, 3334–3336. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Singh, C.K.; Kumar, M.; Singh, D.K. Whole-genome sequencing of Alcaligenes sp. strain MMA: Insight into the antibiotic and heavy metal resistant genes. Front. Pharmacol. 2023, 14, 1144561. [Google Scholar] [CrossRef] [PubMed]
- Pelchovich, G.; Schreiber, R.; Zhuravlev, A.; Gophna, U. The contribution of common rpsL mutations in Escherichia coli to sensitivity to ribosome targeting antibiotics. Int. J. Med. Microbiol. 2013, 303, 558–562. [Google Scholar] [CrossRef]
- Khosravi, A.D.; Etemad, N.; Hashemzadeh, M.; Khandan Dezfuli, S.; Goodarzi, H. Frequency of rrs and rpsL mutations in streptomycin-resistant Mycobacterium tuberculosis isolates from Iranian patients. J. Glob. Antimicrob. Resist. 2017, 9, 51–56. [Google Scholar] [CrossRef]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef]
- Bujnakova, D.; Strakova, E.; Kmet, V. In vitro evaluation of the safety and probiotic properties of Lactobacilli isolated from chicken and calves. Anaerobe 2014, 29, 118–127. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Aggarwal, A.; Bhalla, M.; Fatima, K.H. Detection of New Delhi metallo-beta-lactamase enzyme gene blaNDM-1 associated with the Int-1 gene in Gram-negative bacteria collected from the effluent treatment plant of a tuberculosis care hospital in Delhi, India. Access Microbiology 2020, 2, e000125. [Google Scholar] [CrossRef]
- Nadeem, S.F.; Gohar, U.F.; Tahir, S.F.; Mukhtar, H.; Pornpukdeewattana, S.; Nukthamna, P.; Moula Ali, A.M.; Bavisetty, S.C.B.; Massa, S. Antimicrobial resistance: More than 70 years of war between humans and bacteria. Crit. Rev. Microbiol. 2020, 46, 578–599. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Guo, J.; de la Fuente-Nunez, C.; Wang, J.; Han, B.; Tao, H.; Liu, J.; Wang, X. Bacterial resistance to antibacterial agents: Mechanisms, control strategies, and implications for global health. Sci. Total Environ. 2023, 860, 160461. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.; Yin, H.; Nghiem, L.D. Monitoring antibiotic resistance genes in wastewater treatment: Current strategies and future challenges. Sci. Total Environ. 2021, 783, 146964. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Soler, N.; Forterre, P. Vesiduction: The fourth way of HGT. Environ. Microbiol. 2020, 22, 2457–2460. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, M.; Shintani, M. Microbial evolution through horizontal gene transfer by mobile genetic elements. Microb. Biotechnol. 2024, 17, e14408. [Google Scholar] [CrossRef]
- Huo, M.; Xu, X.; Mi, K.; Ma, W.; Zhou, Q.; Lin, X.; Cheng, G.; Huang, L. Co-selection mechanism for bacterial resistance to major chemical pollutants in the environment. Sci. Total Environ. 2024, 912, 169223. [Google Scholar] [CrossRef]
- Lu, J.; Jin, M.; Nguyen, S.H.; Mao, L.; Li, J.; Coin, L.J.M.; Yuan, Z.; Guo, J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018, 118, 257–265. [Google Scholar] [CrossRef]
- Argudín, M.A.; Hoefer, A.; Butaye, P. Heavy metal resistance in bacteria from animals. Res. Vet. Sci. 2019, 122, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Yang, B.; Fei, Y.; Yu, J.; An, R.; Duan, L. Heavy metal contamination in surface sediments from lakes and their surrounding topsoils of China. Environ. Sci. Pollut. Res. 2021, 28, 29118–29130. [Google Scholar] [CrossRef] [PubMed]
- Merchel Piovesan Pereira, B.; Wang, X.; Tagkopoulos, I. Biocide-Induced Emergence of Antibiotic Resistance in Escherichia coli. Front. Microbiol. 2021, 12, 640923. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Weigand Michael, R.; Oh, S.; Hatt Janet, K.; Krishnan, R.; Tezel, U.; Pavlostathis Spyros, G.; Konstantinidis Konstantinos, T. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201–e01218. [Google Scholar] [CrossRef]
- Hasman, H.; Aarestrup Frank, M. tcrB, a Gene Conferring Transferable Copper Resistance in Enterococcus faecium: Occurrence, Transferability, and Linkage to Macrolide and Glycopeptide Resistance. Antimicrob. Agents Chemother. 2002, 46, 1410–1416. [Google Scholar] [CrossRef]
- Di Cesare, A.; Eckert, E.M.; D’Urso, S.; Bertoni, R.; Gillan, D.C.; Wattiez, R.; Corno, G. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 2016, 94, 208–214. [Google Scholar] [CrossRef]
- Fang, L.; Li, X.; Li, L.; Li, S.; Liao, X.; Sun, J.; Liu, Y. Co-spread of metal and antibiotic resistance within ST3-IncHI2 plasmids from E. coli isolates of food-producing animals. Sci. Rep. 2016, 6, 25312. [Google Scholar] [CrossRef]
- Slipski, C.J.; Zhanel, G.G.; Bay, D.C. Biocide Selective TolC-Independent Efflux Pumps in Enterobacteriaceae. J. Membr. Biol. 2018, 251, 15–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Su, B.; Chen, R.; Lin, J.; Lin, Z.; Wang, D.; Yu, Y. A study on the role that quorum sensing play in antibiotic-resistant plasmid conjugative transfer in Escherichia coli. Ecotoxicology 2018, 27, 209–216. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Wang, L.; Zhu, M.; Zhu, X.; Qian, C.; Li, W. Responses of biofilm microorganisms from moving bed biofilm reactor to antibiotics exposure: Protective role of extracellular polymeric substances. Bioresour. Technol. 2018, 254, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sheng, G.-P. Microbial extracellular polymeric substances (EPS) acted as a potential reservoir in responding to high concentrations of sulfonamides shocks during biological wastewater treatment. Bioresour. Technol. 2020, 313, 123654. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, C.; Diao, X.; Meng, L.; Lu, H. Interactions between tetracycline and extracellular polymeric substances in anammox granular sludge. Bioresour. Technol. 2019, 293, 122069. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Yang, Q.; Li, X.; Yuan, W.; Chen, Y.; Wang, R. Impacts of long-term exposure to tetracycline and sulfamethoxazole on the sludge granules in an anoxic-aerobic wastewater treatment system. Sci. Total Environ. 2019, 684, 67–77. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Sun, Z. Degradation mechanism of montmorillonite-enhanced antibiotic wastewater: Performance, antibiotic resistance genes, microbial communities, and functional metabolism. Bioresour. Technol. 2022, 352, 127098. [Google Scholar] [CrossRef]
- He, Q.; Xie, Z.; Fu, Z.; Wang, M.; Xu, P.; Yu, J.; Ma, J.; Gao, S.; Chen, L.; Zhang, W.; et al. Interaction and removal of oxytetracycline with aerobic granular sludge. Bioresour. Technol. 2021, 320, 124358. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, X.; Kang, J.; Pei, F.; Du, R.; Ye, Z.; Ding, H.; Ping, W.; Ge, J. Intraspecific and interspecific quorum sensing of bacterial community affects the fate of antibiotic resistance genes during chicken manure composting under penicillin G stress. Bioresour. Technol. 2022, 347, 126372. [Google Scholar] [CrossRef]
- McKinney, C.W.; Loftin, K.A.; Meyer, M.T.; Davis, J.G.; Pruden, A. tet and sul Antibiotic Resistance Genes in Livestock Lagoons of Various Operation Type, Configuration, and Antibiotic Occurrence. Environ. Sci. Technol. 2010, 44, 6102–6109. [Google Scholar] [CrossRef]
- Smillie, C.; Garcillán-Barcia, M.P.; Francia, M.V.; Rocha Eduardo, P.C.; de la Cruz, F. Mobility of Plasmids. Microbiol. Mol. Biol. Rev. 2010, 74, 434–452. [Google Scholar] [CrossRef]
- Yano, H.; Shintani, M.; Tomita, M.; Suzuki, H.; Oshima, T. Reconsidering plasmid maintenance factors for computational plasmid design. Comput. Struct. Biotechnol. J. 2019, 17, 70–81. [Google Scholar] [CrossRef]
- Loftie-Eaton, W.; Rawlings, D.E. Diversity, biology and evolution of IncQ-family plasmids. Plasmid 2012, 67, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Delavat, F.; Miyazaki, R.; Carraro, N.; Pradervand, N.; van der Meer, J.R. The hidden life of integrative and conjugative elements. FEMS Microbiol. Rev. 2017, 41, 512–537. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.; Oliveira, P.H.; de la Cruz, F.; Rocha, E.P.C. Host Range and Genetic Plasticity Explain the Coexistence of Integrative and Extrachromosomal Mobile Genetic Elements. Mol. Biol. Evol. 2018, 35, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J. Defense systems are pervasive across chromosomally integrated mobile genetic elements and are inversely correlated to virulence and antimicrobial resistance. Nucleic Acids Res. 2023, 51, 4385–4397. [Google Scholar] [CrossRef]
- Brown-Jaque, M.; Calero-Cáceres, W.; Muniesa, M. Transfer of antibiotic-resistance genes via phage-related mobile elements. Plasmid 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Ross, A.; Ward, S.; Hyman, P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef]
- Koskella, B.; Hernandez, C.A.; Wheatley, R.M. Understanding the Impacts of Bacteriophage Viruses: From Laboratory Evolution to Natural Ecosystems. Annu. Rev. Virol. 2022, 9, 57–78. [Google Scholar] [CrossRef]
- Frost, L.S.; Leplae, R.; Summers, A.O.; Toussaint, A. Mobile genetic elements: The agents of open source evolution. Nat. Rev. Microbiol. 2005, 3, 722–732. [Google Scholar] [CrossRef]
- Razavi, M.; Kristiansson, E.; Flach, C.-F.; Larsson, D.G.J. The Association between Insertion Sequences and Antibiotic Resistance Genes. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Partridge Sally, R.; Kwong Stephen, M.; Firth, N.; Jensen Slade, O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Escudero José, A.; Loot, C.; Nivina, A.; Mazel, D. The Integron: Adaptation On Demand. Microbiol. Spectr. 2015, 3, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Jové, T.; Da Re, S.; Denis, F.; Mazel, D.; Ploy, M.-C. Inverse Correlation between Promoter Strength and Excision Activity in Class 1 Integrons. PLOS Genet. 2010, 6, e1000793. [Google Scholar] [CrossRef] [PubMed]
- Lopatkin, A.J.; Meredith, H.R.; Srimani, J.K.; Pfeiffer, C.; Durrett, R.; You, L. Persistence and reversal of plasmid-mediated antibiotic resistance. Nat. Commun. 2017, 8, 1689. [Google Scholar] [CrossRef]
- Qiu, Z.; Yu, Y.; Chen, Z.; Jin, M.; Yang, D.; Zhao, Z.; Wang, J.; Shen, Z.; Wang, X.; Qian, D.; et al. Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc. Natl. Acad. Sci. USA 2012, 109, 4944–4949. [Google Scholar] [CrossRef]
- Molin, S.; Tolker-Nielsen, T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr. Opin. Biotechnol. 2003, 14, 255–261. [Google Scholar] [CrossRef]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus Biofilms Promote Horizontal Transfer of Antibiotic Resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef]
- Grohmann, E.; Christie, P.J.; Waksman, G.; Backert, S. Type IV secretion in Gram-negative and Gram-positive bacteria. Mol. Microbiol. 2018, 107, 455–471. [Google Scholar] [CrossRef]
- Kohler, V.; Vaishampayan, A.; Grohmann, E. Broad-host-range Inc18 plasmids: Occurrence, spread and transfer mechanisms. Plasmid 2018, 99, 11–21. [Google Scholar] [CrossRef]
- Fischer, W.; Tegtmeyer, N.; Stingl, K.; Backert, S. Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function. Front. Microbiol. 2020, 11, 1592. [Google Scholar] [CrossRef]
- Shen, Z.; Tang, C.M.; Liu, G.-Y. Towards a better understanding of antimicrobial resistance dissemination: What can be learnt from studying model conjugative plasmids? Mil. Med. Res. 2022, 9, 3. [Google Scholar] [CrossRef]
- Virolle, C.; Goldlust, K.; Djermoun, S.; Bigot, S.; Lesterlin, C. Plasmid Transfer by Conjugation in Gram-Negative Bacteria: From the Cellular to the Community Level. Genes. 2020, 11, 1239. [Google Scholar] [CrossRef]
- Nazir, A.; Nazir, A.; Zuhair, V.; Aman, S.; Sadiq, S.U.R.; Hasan, A.H.; Tariq, M.; Rehman, L.U.; Mustapha, M.J.; Bulimbe, D.B. The Global Challenge of Antimicrobial Resistance: Mechanisms, Case Studies, and Mitigation Approaches. Health Sci. Rep. 2025, 8, e71077. [Google Scholar] [CrossRef]
- Granato, E.T.; Palmer, J.D.; Kirk, C.; Sharp, C.; Shillcock, G.; Foster, K.R. Horizontal gene transfer of molecular weapons can reshape bacterial competition. PLOS Biol. 2025, 23, e3003095. [Google Scholar] [CrossRef] [PubMed]
- Griffith, F. The Significance of Pneumococcal Types. J. Hyg. 2009, 27, 113–159. [Google Scholar] [CrossRef] [PubMed]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef] [PubMed]
- Johnsborg, O.; Eldholm, V.; Håvarstein, L.S. Natural genetic transformation: Prevalence, mechanisms and function. Res. Microbiol. 2007, 158, 767–778. [Google Scholar] [CrossRef]
- Blokesch, M. Natural competence for transformation. Curr. Biol. 2016, 26, R1126–R1130. [Google Scholar] [CrossRef]
- Attaiech, L.; Charpentier, X. Silently transformable: The many ways bacteria conceal their built-in capacity of genetic exchange. Curr. Genet. 2016, 63, 451–455. [Google Scholar] [CrossRef]
- García-Curiel, L.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N. Toward understanding the signals of bacteriocin production by Streptococcus spp. and their importance in current applications. World J. Microbiol. Biotechnol. 2021, 37, 15. [Google Scholar] [CrossRef]
- Johnston, C.; Martin, B.; Fichant, G.; Polard, P.; Claverys, J.-P. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014, 12, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Huang, L.; Biville, F.; Zhu, D.; Wang, M.; Jia, R.; Chen, S.; Sun, K.; Yang, Q.; et al. Use of Natural Transformation To Establish an Easy Knockout Method in Riemerella anatipestifer. Appl. Environ. Microbiol. 2017, 83, e00127-17. [Google Scholar] [CrossRef] [PubMed]
- Dunning Hotopp, J.C. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011, 27, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Ryan, M.G.; Li, J.; Burger, A.; Eppley, J.M.; Hackl, T.; DeLong, E.F. Distinct horizontal gene transfer potential of extracellular vesicles versus viral-like particles in marine habitats. Nat. Commun. 2025, 16, 2126. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Bøhn, T.; Townsend, J.P. Detecting rare gene transfer events in bacterial populations. Front. Microbiol. 2014, 4, 415. [Google Scholar] [CrossRef]
- Gabashvili, E.; Osepashvili, M.; Koulouris, S.; Ujmajuridze, L.; Tskhitishvili, Z.; Kotetishvili, M. Phage Transduction is Involved in the Intergeneric Spread of Antibiotic Resistance-Associated blaCTX-M, mel, and tetM Loci in Natural Populations of Some Human and Animal Bacterial Pathogens. Curr. Microbiol. 2019, 77, 185–193. [Google Scholar] [CrossRef]
- Kline, K.A.; Chiang, Y.N.; Penadés, J.R.; Chen, J. Genetic transduction by phages and chromosomal islands: The new and noncanonical. PLOS Pathog. 2019, 15, e1007878. [Google Scholar] [CrossRef]
- Balcázar, J.L. How do bacteriophages promote antibiotic resistance in the environment? Clin. Microbiol. Infect. 2018, 24, 447–449. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, B.; Zheng, S.; Yan, W.; Yu, X.; Ye, C. High temperatures promote antibiotic resistance genes conjugative transfer under residual chlorine: Mechanisms and risks. J. Hazard. Mater. 2025, 483, 136675. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2018, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nomura, N.; Abe, K. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecology 2020, 96, fiaa031. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Yang, K.; Wang, Z. Correlation between Exogenous Compounds and the Horizontal Transfer of Plasmid-Borne Antibiotic Resistance Genes. Microorganisms 2020, 8, 1211. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.S.; Domingues, S.; Harms, K.; Fricke, W.F.; Johnsen, P.J.; da Silva, G.J.; Nielsen, K.M. Natural Transformation Facilitates Transfer of Transposons, Integrons and Gene Cassettes between Bacterial Species. PLoS Pathog. 2012, 8, e1002837. [Google Scholar] [CrossRef]
- Fulsundar, S.; Harms, K.; Flaten, G.E.; Johnsen, P.J.; Chopade, B.A.; Nielsen, K.M.; Kivisaar, M. Gene Transfer Potential of Outer Membrane Vesicles of Acinetobacter baylyi and Effects of Stress on Vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483. [Google Scholar] [CrossRef]
- Kelly, B.G.; Vespermann, A.; Bolton, D.J. Horizontal gene transfer of virulence determinants in selected bacterial foodborne pathogens. Food Chem. Toxicol. 2009, 47, 969–977. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Philip, L. Variation in cell surface characteristics and extracellular polymeric substances during the biodegradation of monocyclic and heterocyclic aromatic hydrocarbons in single and multi-substrate systems. Environ. Technol. 2017, 39, 3115–3126. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.-P.; Ma, Y.; Wang, L.-F.; Yu, H.-Q. Roles of extracellular polymeric substances (EPS) in the migration and removal of sulfamethazine in activated sludge system. Water Res. 2013, 47, 5298–5306. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Y.; Khanal, S.K.; Lu, H.; Fang, H.; Zhao, Q. Understanding the Role of Extracellular Polymeric Substances on Ciprofloxacin Adsorption in Aerobic Sludge, Anaerobic Sludge, and Sulfate-Reducing Bacteria Sludge Systems. Environ. Sci. Technol. 2018, 52, 6476–6486. [Google Scholar] [CrossRef]
- Wunder, D.B.; Bosscher, V.A.; Cok, R.C.; Hozalski, R.M. Sorption of antibiotics to biofilm. Water Res. 2011, 45, 2270–2280. [Google Scholar] [CrossRef]
- Xu, Q.; Han, B.; Wang, H.; Wang, Q.; Zhang, W.; Wang, D. Effect of extracellular polymer substances on the tetracycline removal during coagulation process. Bioresour. Technol. 2020, 309, 123316. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Müller, E.; Schüssler, W.; Horn, H.; Lemmer, H. Aerobic biodegradation of the sulfonamide antibiotic sulfamethoxazole by activated sludge applied as co-substrate and sole carbon and nitrogen source. Chemosphere 2013, 92, 969–978. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Nguyen, Q.A.; Zhang, J.; Liang, S. Improving sulfonamide antibiotics removal from swine wastewater by supplying a new pomelo peel derived biochar in an anaerobic membrane bioreactor. Bioresour. Technol. 2021, 319, 124160. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhang, L.; Zhang, W.; Jia, J.; Dai, H.; Wang, Z. Responses of nitrification performance, triclosan resistome and diversity of microbes to continuous triclosan stress in activated sludge system. J. Environ. Sci. 2020, 92, 211–223. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef]
- Schug, H.; Isaacson, C.W.; Sigg, L.; Ammann, A.A.; Schirmer, K. Effect of TiO2 Nanoparticles and UV Radiation on Extracellular Enzyme Activity of Intact Heterotrophic Biofilms. Environ. Sci. Technol. 2014, 48, 11620–11628. [Google Scholar] [CrossRef]
- Zhou, S.; Liao, Z.; Zhang, B.; Hou, R.; Wang, Y.; Zhou, S.; Zhang, Y.; Ren, Z.J.; Yuan, Y. Photochemical Behavior of Microbial Extracellular Polymeric Substances in the Aquatic Environment. Environ. Sci. Technol. 2021, 55, 15090–15099. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, J.H.; Shin, J.; Jang, H.M.; Kim, S.; Song, M.S.; Kim, Y.M. Quantitative and qualitative changes in antibiotic resistance genes after passing through treatment processes in municipal wastewater treatment plants. Sci. Total Environ. 2017, 605–606, 906–914. [Google Scholar] [CrossRef]
- Xue, G.; Jiang, M.; Chen, H.; Sun, M.; Liu, Y.; Li, X.; Gao, P. Critical review of ARGs reduction behavior in various sludge and sewage treatment processes in wastewater treatment plants. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1623–1674. [Google Scholar] [CrossRef]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012, 421–422, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yang, L.; Duan, R.; Chen, Z. Monitoring and evaluation of antibiotic resistance genes in four municipal wastewater treatment plants in Harbin, Northeast China. Environ. Pollut. 2016, 212, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M. Effects of Advanced Treatment Systems on the Removal of Antibiotic Resistance Genes in Wastewater Treatment Plants from Hangzhou, China. Environ. Sci. Technol. 2013, 47, 8157–8163. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef]
- Cheng, H.; Hong, P.-Y. Removal of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes Affected by Varying Degrees of Fouling on Anaerobic Microfiltration Membranes. Environ. Sci. Technol. 2017, 51, 12200–12209. [Google Scholar] [CrossRef]
- Burch, T.R.; Sadowsky, M.J.; LaPara, T.M. Modeling the fate of antibiotic resistance genes and class 1 integrons during thermophilic anaerobic digestion of municipal wastewater solids. Appl. Microbiol. Biotechnol. 2016, 100, 1437–1444. [Google Scholar] [CrossRef]
- Zou, Y.; Tu, W.; Wang, H.; Fang, T. Anaerobic digestion reduces extracellular antibiotic resistance genes in waste activated sludge: The effects of temperature and degradation mechanisms. Environ. Int. 2020, 143, 105980. [Google Scholar] [CrossRef]
- Zhao, C.; Xin, L.; Xu, X.; Qin, Y.; Wu, W. Dynamics of antibiotics and antibiotic resistance genes in four types of kitchen waste composting processes. J. Hazard. Mater. 2022, 424, 127526. [Google Scholar] [CrossRef]
- Gou, C.; Wang, Y.; Zhang, X.; Zhong, R.; Gao, Y. Effects of chlorotetracycline on antibiotic resistance genes and the bacterial community during cattle manure composting. Bioresour. Technol. 2021, 323, 124517. [Google Scholar] [CrossRef]
- Qiu, X.; Zhou, G.; Wang, H.; Wu, X. The behavior of antibiotic-resistance genes and their relationships with the bacterial community and heavy metals during sewage sludge composting. Ecotoxicol. Environ. Saf. 2021, 216, 112190. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Q.; Nie, X.; Chen, B.; Xiao, Y.; Zhou, Q.; Liao, W.; Liang, X. Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017, 173, 99–106. [Google Scholar] [CrossRef]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.-H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, J.; Liu, C.; Liu, L.; Liu, Y.; Fan, H. Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chem. Eng. J. 2017, 308, 692–699. [Google Scholar] [CrossRef]
- da Silva Rodrigues, D.A.; da Cunha, C.C.R.F.; do Espirito Santo, D.R.; de Barros, A.L.C.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ. Sci. Pollut. Res. 2021, 28, 67822–67832. [Google Scholar] [CrossRef]
- Ovis-Sánchez, J.O.; Perera-Pérez, V.D.; Buitrón, G.; Quintela-Baluja, M.; Graham, D.W.; Morales-Espinosa, R.; Carrillo-Reyes, J. Exploring resistomes and microbiomes in pilot-scale microalgae-bacteria wastewater treatment systems for use in low-resource settings. Sci. Total Environ. 2023, 882, 163545. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-B.; Guo, M.-T.; Wei, W.-J.; Yang, J. Reductions of bacterial antibiotic resistance through five biological treatment processes treated municipal wastewater. Environ. Sci. Pollut. Res. 2016, 23, 19495–19503. [Google Scholar] [CrossRef] [PubMed]
- Bisognin, R.P.; Wolff, D.B.; Carissimi, E.; Prestes, O.D.; Zanella, R. Occurrence and fate of pharmaceuticals in effluent and sludge from a wastewater treatment plant in Brazil. Environ. Technol. 2019, 42, 2292–2303. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Tong, J.; Tang, A.; Wang, H.; Liu, X.; Huang, Z.; Wang, Z.; Zhang, J.; Wei, Y.; Su, Y.; Zhang, Y. Microbial community evolution and fate of antibiotic resistance genes along six different full-scale municipal wastewater treatment processes. Bioresour. Technol. 2019, 272, 489–500. [Google Scholar] [CrossRef]
- Xiao, Y.; Yaohari, H.; De Araujo, C.; Sze, C.C.; Stuckey, D.C. Removal of selected pharmaceuticals in an anaerobic membrane bioreactor (AnMBR) with/without powdered activated carbon (PAC). Chem. Eng. J. 2017, 321, 335–345. [Google Scholar] [CrossRef]
- Wen, Q.; Yang, L.; Zhao, Y.; Huang, L.; Chen, Z. Insight into effects of antibiotics on reactor performance and evolutions of antibiotic resistance genes and microbial community in a membrane reactor. Chemosphere 2018, 197, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ding, R.; Zhang, Y.; Gao, Y.; Tian, Z.; Zhang, T.; Yang, M. Abundance and distribution of Macrolide-Lincosamide-Streptogramin resistance genes in an anaerobic-aerobic system treating spiramycin production wastewater. Water Res. 2014, 63, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, X.; Wang, Y.; Du, G.; Tay, J.-H.; Li, J. Piggery wastewater treatment by aerobic granular sludge: Granulation process and antibiotics and antibiotic-resistant bacteria removal and transport. Bioresour. Technol. 2019, 273, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, S.; Wang, S.; Su, H. Analysis of aerobic granules under the toxic effect of ampicillin in sequencing batch reactors: Performance and microbial community. J. Environ. Manag. 2017, 204, 152–159. [Google Scholar] [CrossRef]
- Yang, D.; Wang, J.; Qiu, Z.; Jin, M.; Shen, Z.; Chen, Z.; Wang, X.; Zhang, B.; Li, J.-W. Horizontal transfer of antibiotic resistance genes in a membrane bioreactor. J. Biotechnol. 2013, 167, 441–447. [Google Scholar] [CrossRef]
- Liao, J.; Liu, C.; Liu, L.; Li, J.; Fan, H.; Ye, J.; Zeng, Z. Influence of hydraulic retention time on behavior of antibiotics and antibiotic resistance genes in aerobic granular reactor treating biogas slurry. Front. Environ. Sci. Eng. 2019, 13, 31. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Pruden, A. Effect of temperature on removal of antibiotic resistance genes by anaerobic digestion of activated sludge revealed by metagenomic approach. Appl. Microbiol. Biotechnol. 2015, 99, 7771–7779. [Google Scholar] [CrossRef]
- Yin, F.; Dong, H.; Zhang, W.; Zhu, Z.; Shang, B. Antibiotic degradation and microbial community structures during acidification and methanogenesis of swine manure containing chlortetracycline or oxytetracycline. Bioresour. Technol. 2018, 250, 247–255. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Q.; Wu, Y.; Wang, D.; Yang, Q.; Chen, F.; Wu, Y.; Pi, Z.; Chen, Z.; Li, X.; et al. Effect of clarithromycin on the production of volatile fatty acids from waste activated sludge anaerobic fermentation. Bioresour. Technol. 2019, 288, 121598. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Ince, B.; Ince, O.; Orhon, D. Acute effect of erythromycin on metabolic transformations of volatile fatty acid mixture under anaerobic conditions. Chemosphere 2015, 124, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, R.L.R.; Gressler, V. Impact of Antibiotics on Biogas Production. In Improving Biogas Production; Springer: Cham, Switzerland, 2019; pp. 181–198. [Google Scholar]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Montemurro, N.; Pérez, S.; Rodelas, B.; Osorio, F.; Pozo, C. Insights into the removal of pharmaceutically active compounds from sewage sludge by two-stage mesophilic anaerobic digestion. Sci. Total Environ. 2021, 789, 147869. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Li, B.; Ma, L.; Wang, Y.; Huang, D.; Zhang, T. Antibiotic resistance genes and human bacterial pathogens: Co-occurrence, removal, and enrichment in municipal sewage sludge digesters. Water Res. 2016, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Yoo, H.; Lee, J.; Choi, E.J.; Park, J. Exploring the antibiotic resistome in activated sludge and anaerobic digestion sludge in an urban wastewater treatment plant via metagenomic analysis. J. Microbiol. 2019, 58, 123–130. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.-D.; Liu, Y.-S.; Ying, G.-G.; Liu, S.-S.; He, L.-Y.; Su, H.-C.; Hu, L.-X.; Chen, F.-R.; Yang, Y.-Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Zheng, J.; Huang, X.; Wang, Z.; Liu, Y.; Zhu, G. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 2013, 91, 1088–1093. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.-J.; Liu, Y.-S.; Hu, L.-X.; He, L.-Y.; Zhao, J.-L.; Wang, T.-T.; Ying, G.-G. Fate and removal of antibiotics and antibiotic resistance genes in hybrid constructed wetlands. Environ. Pollut. 2019, 249, 894–903. [Google Scholar] [CrossRef]
- Cardinal, P.; Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Beattie, S.A.; Bartel, C.N.; Elliott, A.D.; Montero, O.F.; Lokesh, S.; et al. Macrophytes may not contribute significantly to removal of nutrients, pharmaceuticals, and antibiotic resistance in model surface constructed wetlands. Sci. Total Environ. 2014, 482–483, 294–304. [Google Scholar] [CrossRef]
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.; Li, H.; Mao, S.; Wang, D.; Xiang, Z.; Guo, R.; Chen, J. The dual function of the algal treatment: Antibiotic elimination combined with CO2 fixation. Chemosphere 2018, 211, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Liang, Y.; Du, X.; Yang, C.; Yang, L.; Xie, J.; Zhao, R.; Tong, Y.; Qiu, S.; et al. Phage-delivered sensitisation with subsequent antibiotic treatment reveals sustained effect against antimicrobial resistant bacteria. Theranostics 2020, 10, 6310–6321. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Z.; Kuang, X.; Long, T.-F.; Li, G.; Ren, H.; He, B.; Yan, J.-R.; Liao, X.-P.; Liu, Y.-H.; Chen, L.; et al. Re-engineering a mobile-CRISPR/Cas9 system for antimicrobial resistance gene curing and immunization in Escherichia coli. J. Antimicrob. Chemother. 2022, 77, 74–82. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, J.; Jiang, Q.; Qiao, B.; He, B.; Yin, W.; Qiao, J.; Liu, Y. Split crRNA with CRISPR-Cas12a enabling highly sensitive and multiplexed detection of RNA and DNA. Nat. Commun. 2024, 15, 8342. [Google Scholar] [CrossRef]
- Song, Z.; Yu, Y.; Bai, X.; Jia, Y.; Tian, J.; Gu, K.; Zhao, M.; Zhou, C.; Zhang, X.; Wang, H.; et al. Pathogen-Specific Bactericidal Method Mediated by Conjugative Delivery of CRISPR-Cas13a Targeting Bacterial Endogenous Transcripts. Microbiol. Spectr. 2022, 10, e01300-22. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e00829-22. [Google Scholar] [CrossRef]
- Chen, F.; Du, H.; Tao, M.; Xu, L.; Wang, C.; White, J.C.; Wang, Z.; Xing, B. Nitrogen-Doped Carbon Dots Facilitate CRISPR/Cas for Reducing Antibiotic Resistance Genes in the Environment. J. Agric. Food Chem. 2024, 72, 3397–3405. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Q.; Huang, S.; Zhang, N.; Sun, D. Advancements in CRISPR-Cas-based strategies for combating antimicrobial resistance. Microbiol. Res. 2025, 298, 128232. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.Ø.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C.; et al. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ying, Q.; Chen, W.; Diao, Y. Study on the effect of partial filling of foam metal on the solidification performance of ice storage spheres. Int. J. Refrig. 2024, 158, 365–377. [Google Scholar] [CrossRef]
- Lam, P.L.; Wong, R.S.M.; Lam, K.H.; Hung, L.K.; Wong, M.M.; Yung, L.H.; Ho, Y.W.; Wong, W.Y.; Hau, D.K.P.; Gambari, R.; et al. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem.-Biol. Interact. 2020, 320, 109023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-d.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.-q.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Senasu, T.; Ruengchai, N.; Khamdon, S.; Lorwanishpaisarn, N.; Nanan, S. Hydrothermal Synthesis of Cadmium Sulfide Photocatalyst for Detoxification of Azo Dyes and Ofloxacin Antibiotic in Wastewater. Molecules 2022, 27, 7944. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Huang, W.; He, L.; Lin, Z.; Zhou, J.; He, Q. Toxic effects of terpinolene on Microcystis aeruginosa: Physiological, metabolism, gene transcription, and growth effects. Sci. Total Environ. 2020, 719, 137376. [Google Scholar] [CrossRef]
- Adamczyk, S.; Latvala, S.; Poimala, A.; Adamczyk, B.; Hytönen, T.; Pennanen, T. Diterpenes and triterpenes show potential as biocides against pathogenic fungi and oomycetes: A screening study. Biotechnol. Lett. 2023, 45, 1555–1563. [Google Scholar] [CrossRef]
- Shalabayev, Z.; Baláž, M.; Khan, N.; Nurlan, Y.; Augustyniak, A.; Daneu, N.; Tatykayev, B.; Dutková, E.; Burashev, G.; Casas-Luna, M.; et al. Sustainable Synthesis of Cadmium Sulfide, with Applicability in Photocatalysis, Hydrogen Production, and as an Antibacterial Agent, Using Two Mechanochemical Protocols. Nanomaterials 2022, 12, 1250. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Shen, C.; Xu, Y. Inactivation of antibiotic-resistant bacteria and antibiotic resistance genes by electrochemical oxidation/electro-Fenton process. Water Sci. Technol. 2020, 81, 2221–2231. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Guo, X.; Wang, W.; Wang, L.; Zhang, H.; Deng, L.; Yang, H.; He, T.; Wu, P.; et al. Mechanistic insights for efficient removal of intracellular and extracellular antibiotic resistance genes by iron-based nanocopper: Intracellular oxidative stress and internalization of nanocopper. J. Hazard. Mater. 2025, 484, 136745. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Teng, X.; Liang, Z.; Zhu, L.; Xu, G.; Chen, C.; Ma, K.; Liu, R.; Zhou, L.; et al. A TbPO4-based capturer for environmental extracellular antibiotic genes by interrogating lanthanide phosphates nanoneedles. J. Hazard. Mater. 2022, 423, 127139. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, F.; Nie, C.; Ma, L.; Cheng, C.; Haag, R. Biocatalytic Nanomaterials: A New Pathway for Bacterial Disinfection. Adv. Mater. 2021, 33, 2100637. [Google Scholar] [CrossRef] [PubMed]
- Nõlvak, H.; Truu, M.; Tiirik, K.; Devarajan, A.K.; Peeb, A.; Truu, J. The effect of synthetic silver nanoparticles on the antibiotic resistome and the removal efficiency of antibiotic resistance genes in a hybrid filter system treating municipal wastewater. Water Res. 2023, 237, 119986. [Google Scholar] [CrossRef]
- Wei, H.; Ma, J.; Su, Y.; Xie, B. Effect of nutritional energy regulation on the fate of antibiotic resistance genes during composting of sewage sludge. Bioresour. Technol. 2020, 297, 122513. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal Variability in Bacterial and Fungal Diversity of the Near-Surface Atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Li, D.; Li, B.; Sun, X.; Ma, L.; Qi, H. A new insight into the ARG association with antibiotics and non-antibiotic agents—Antibiotic resistance and toxicity. Environ. Pollut. 2022, 293, 118524. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, B.; Jia, Y.; Qi, Y.; Li, H.; Zhang, Q.; Wang, H. Fate of antibiotic resistance genes during sludge anaerobic fermentation: Roles of different sludge pretreatment. Environ. Res. 2024, 263, 120139. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Sui, Q.; Tong, J.; Jiang, C.; Lu, X.; Zhang, Y.; Wei, Y. Impacts of addition of natural zeolite or a nitrification inhibitor on antibiotic resistance genes during sludge composting. Water Res. 2016, 91, 339–349. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R. Role of anaerobic sludge digestion in handling antibiotic resistant bacteria and antibiotic resistance genes—A review. Bioresour. Technol. 2021, 330, 124970. [Google Scholar] [CrossRef]
- Yu, K.; Qiu, Y.; Shi, Y.; Yu, X.; Dong, T.; Wu, Y.; Li, H.; Huang, L. Association of long-term effects of low-level sulfamethoxazole with ovarian lipid and amino acid metabolism, sex hormone levels, and oocyte maturity in zebrafish. Ecotoxicol. Environ. Saf. 2022, 247, 114234. [Google Scholar] [CrossRef]
- Timraz, K.; Xiong, Y.; Al Qarni, H.; Hong, P.-Y. Removal of bacterial cells, antibiotic resistance genes and integrase genes by on-site hospital wastewater treatment plants: Surveillance of treated hospital effluent quality. Environ. Sci. Water Res. Technol. 2017, 3, 293–303. [Google Scholar] [CrossRef]
- Yu, C.; Pang, H.; Wang, J.-H.; Chi, Z.-Y.; Zhang, Q.; Kong, F.-T.; Xu, Y.-P.; Li, S.-Y.; Che, J. Occurrence of antibiotics in waters, removal by microalgae-based systems, and their toxicological effects: A review. Sci. Total Environ. 2022, 813, 151891. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Li, X.-Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Wijaya, A.J.; Anžel, A.; Richard, H.; Hattab, G. Current state and future prospects of Horizontal Gene Transfer detection. NAR Genom. Bioinform. 2025, 7, lqaf005. [Google Scholar] [CrossRef]
- Douglas, G.M.; Langille, M.G.I. Current and Promising Approaches to Identify Horizontal Gene Transfer Events in Metagenomes. Genome Biol. Evol. 2019, 11, 2750–2766. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and Barriers to, Horizontal Gene Transfer between Bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Liu, F.; Luo, Y.; Xu, T.; Lin, H.; Qiu, Y.; Li, B. Current examining methods and mathematical models of horizontal transfer of antibiotic resistance genes in the environment. Front. Microbiol. 2024, 15, 1371388. [Google Scholar] [CrossRef]
- Zhu, S.; Hong, J.; Wang, T. Horizontal gene transfer is predicted to overcome the diversity limit of competing microbial species. Nat. Commun. 2024, 15, 800. [Google Scholar] [CrossRef]
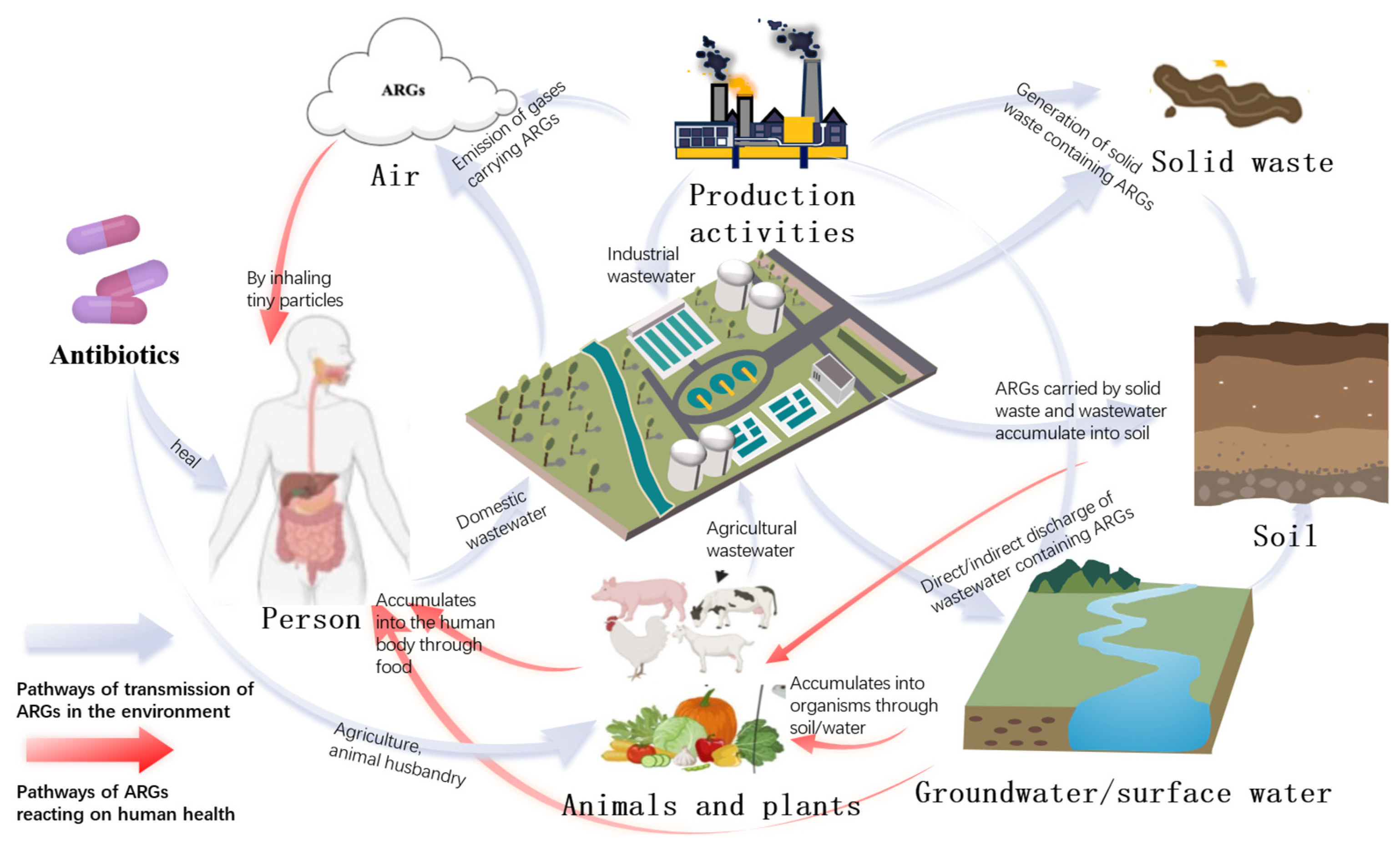


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tang, J.; Wang, X.; Ma, X.; Yuan, H.; Gao, C.; Guo, Q.; Guo, X.; Wan, J.; Dagot, C. The Environmental Lifecycle of Antibiotics and Resistance Genes: Transmission Mechanisms, Challenges, and Control Strategies. Microorganisms 2025, 13, 2113. https://doi.org/10.3390/microorganisms13092113
Li Z, Tang J, Wang X, Ma X, Yuan H, Gao C, Guo Q, Guo X, Wan J, Dagot C. The Environmental Lifecycle of Antibiotics and Resistance Genes: Transmission Mechanisms, Challenges, and Control Strategies. Microorganisms. 2025; 13(9):2113. https://doi.org/10.3390/microorganisms13092113
Chicago/Turabian StyleLi, Zhiguo, Jialu Tang, Xueting Wang, Xiaoling Ma, Heng Yuan, Congyong Gao, Qiong Guo, Xiaoying Guo, Junfeng Wan, and Christophe Dagot. 2025. "The Environmental Lifecycle of Antibiotics and Resistance Genes: Transmission Mechanisms, Challenges, and Control Strategies" Microorganisms 13, no. 9: 2113. https://doi.org/10.3390/microorganisms13092113
APA StyleLi, Z., Tang, J., Wang, X., Ma, X., Yuan, H., Gao, C., Guo, Q., Guo, X., Wan, J., & Dagot, C. (2025). The Environmental Lifecycle of Antibiotics and Resistance Genes: Transmission Mechanisms, Challenges, and Control Strategies. Microorganisms, 13(9), 2113. https://doi.org/10.3390/microorganisms13092113








