Seed-Derived Synthetic Microbial Communities (SynComs) from Medicago Wild Relatives Modulate Early Plant Microbiome Assembly and Phenotypic Traits in Lucerne (Medicago sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Bacterial Strains and SynCom Inoculum Preparation
Cultivar Selection and Germination
2.2. Overview of the Conventional Phenotyping and HTP Imaging of the SynCom Effects Across Developmental Stages
2.2.1. Early Seedling Growth Under Glasshouse Conditions
2.2.2. Controlled Environments and Phenotyping Setup at PPV Facility, Horsham
2.2.3. Early-Stage Phenotyping at Glasshouse, Bundoora (24 DAP)
2.2.4. Mid-Stage Phenotyping at PPV, Horsham (63 DAP)
2.2.5. Late-Stage Phenotyping at Glasshouse, Bundoora (76 DAP)
2.3. HTP Image Analysis
2.4. DNA Extraction, 16S rRNA Amplicon Library Preparation and Sequencing
2.5. Data Analysis
2.5.1. Statistical Analysis for Conventional Phenotyping Measurements
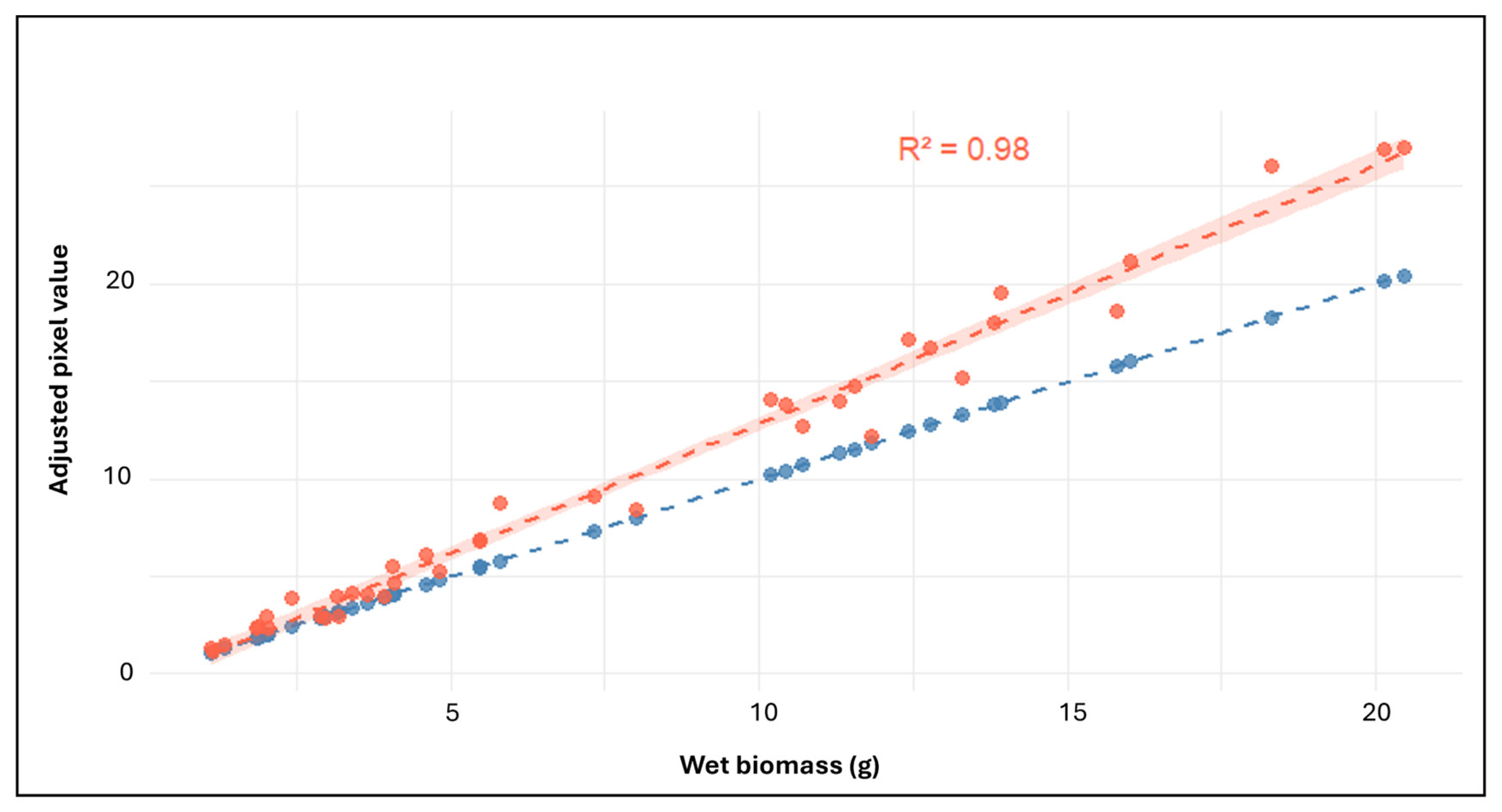
2.5.2. Validation of HTP-Derived Biomass Estimates
2.5.3. Microbiome Data Processing and Statistical Analysis
3. Results
3.1. Microbiome Dynamics at 24 DAP
3.1.1. Microbiome Sequencing and Data Processing
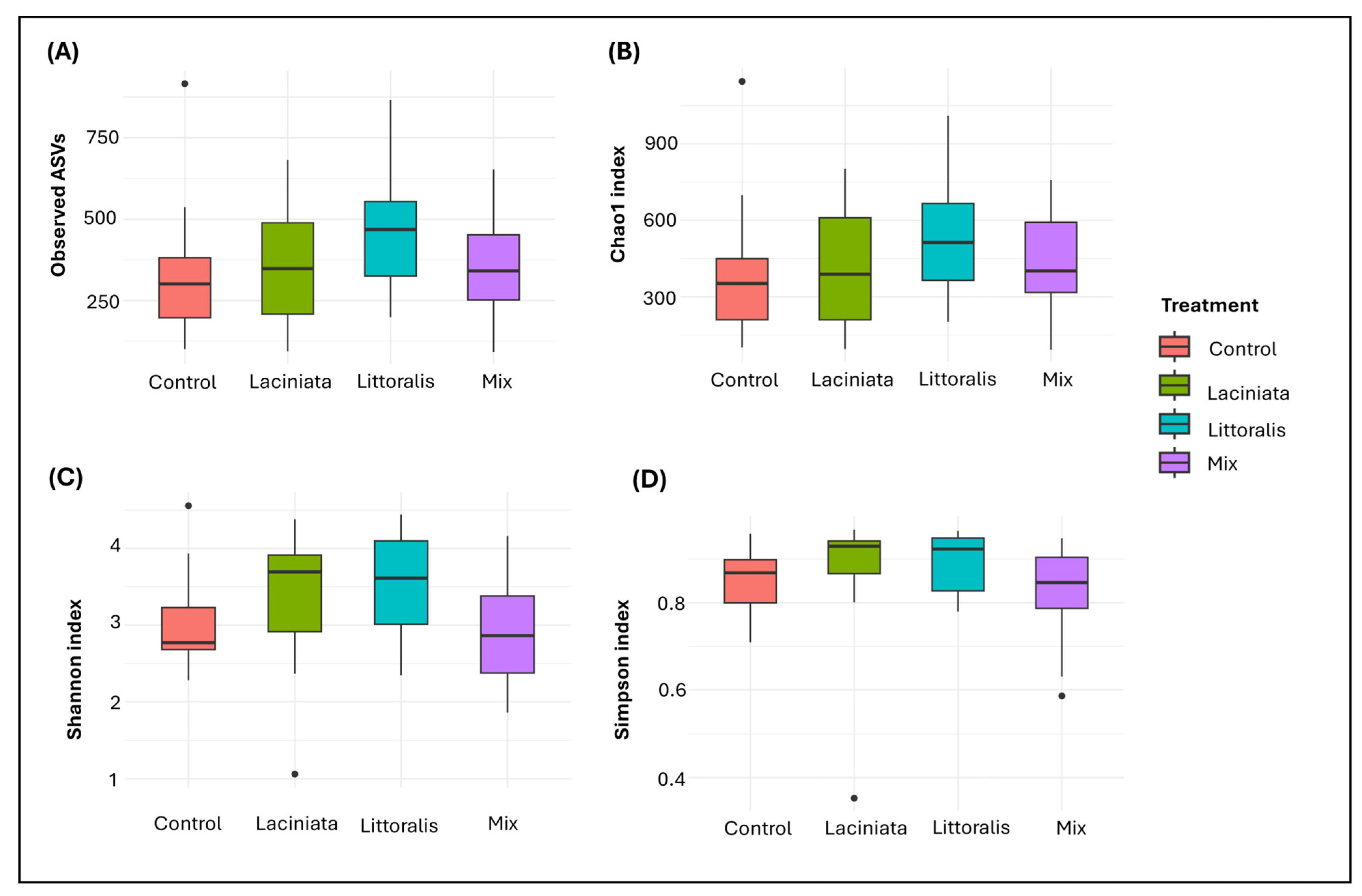
3.1.2. Microbial Diversity Analysis
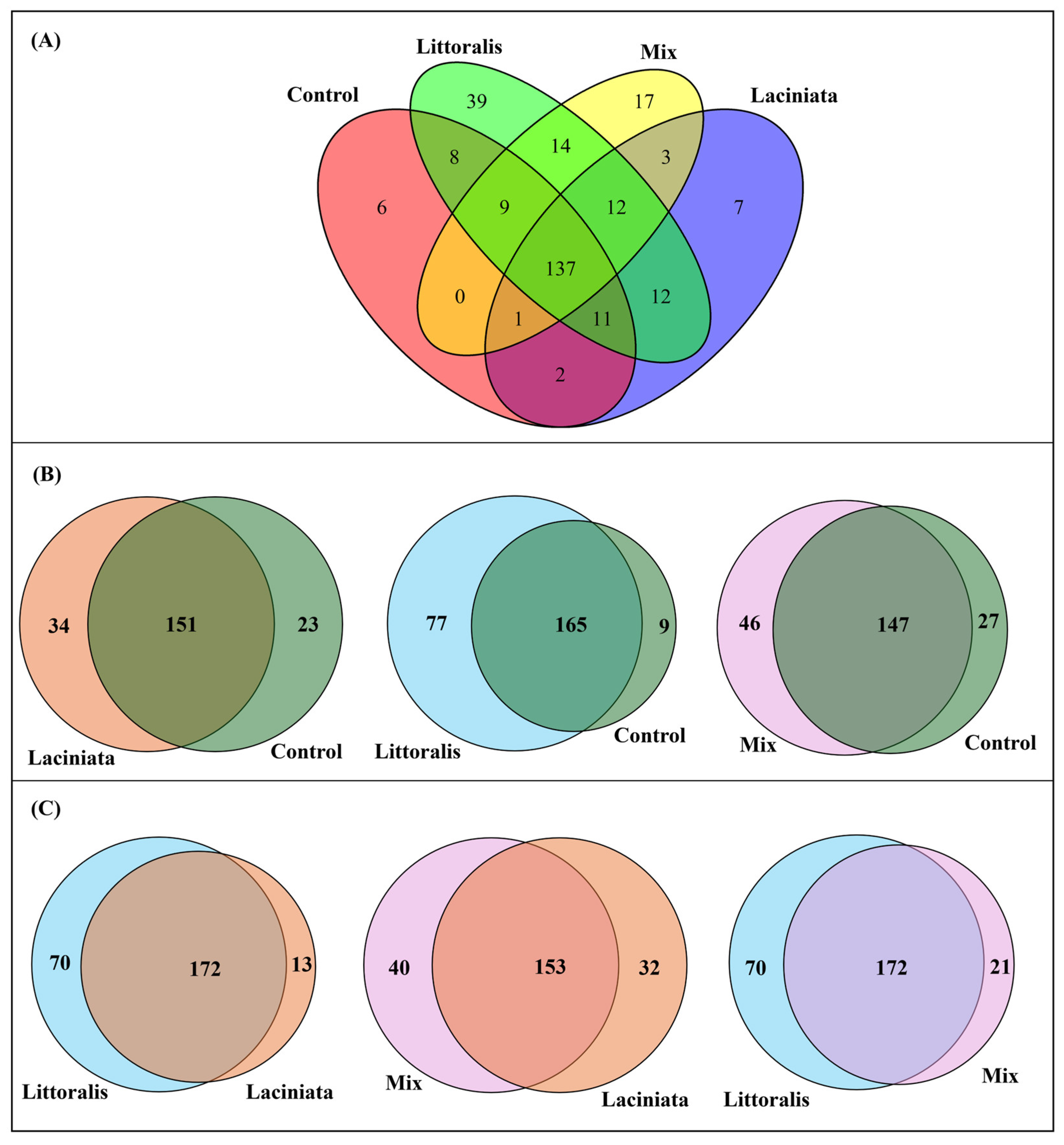
3.1.3. Core Microbiome Analysis
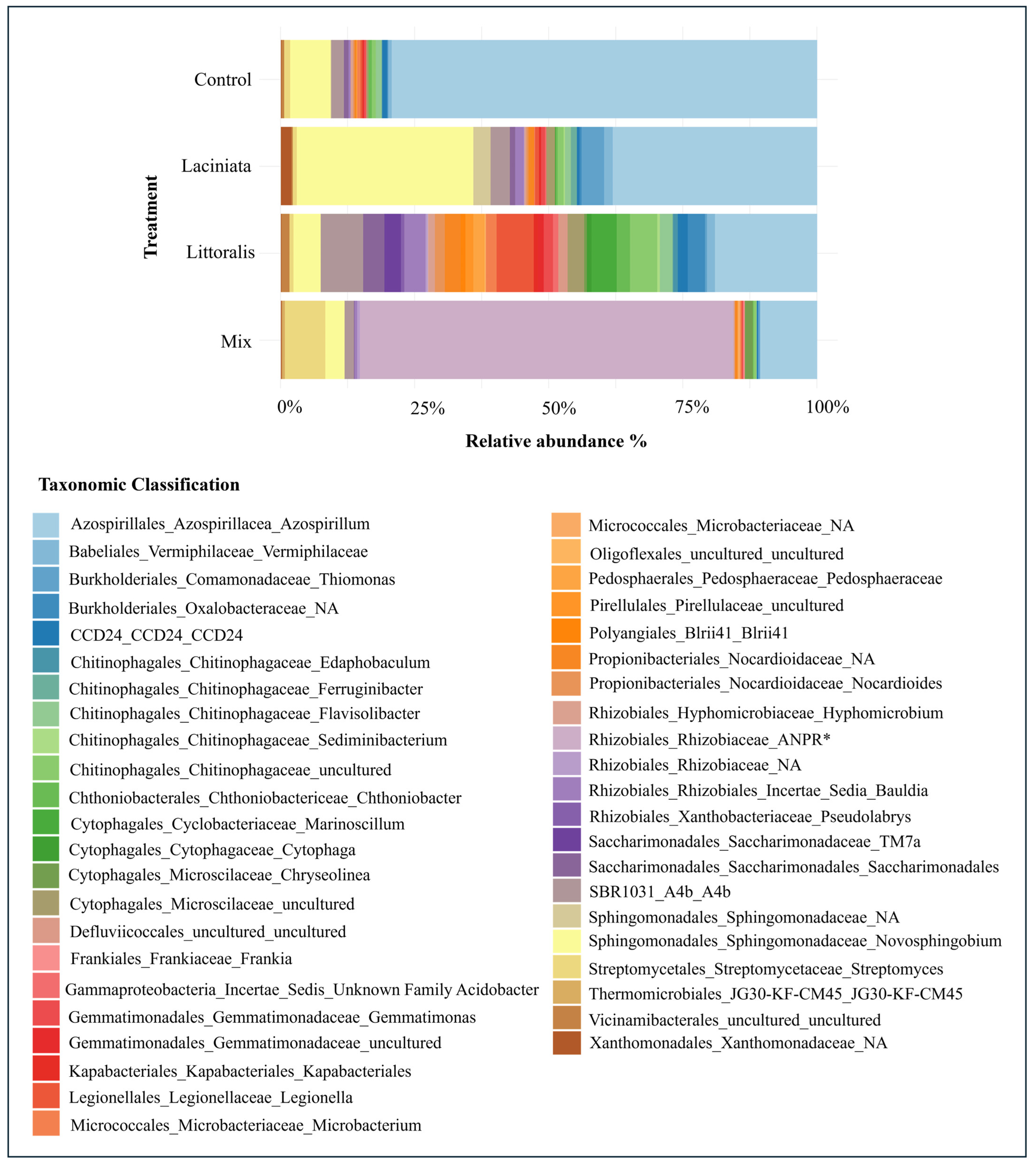
3.1.4. Indicator Taxa Analysis
3.2. Overview of Conventional Phenotyping Measurements
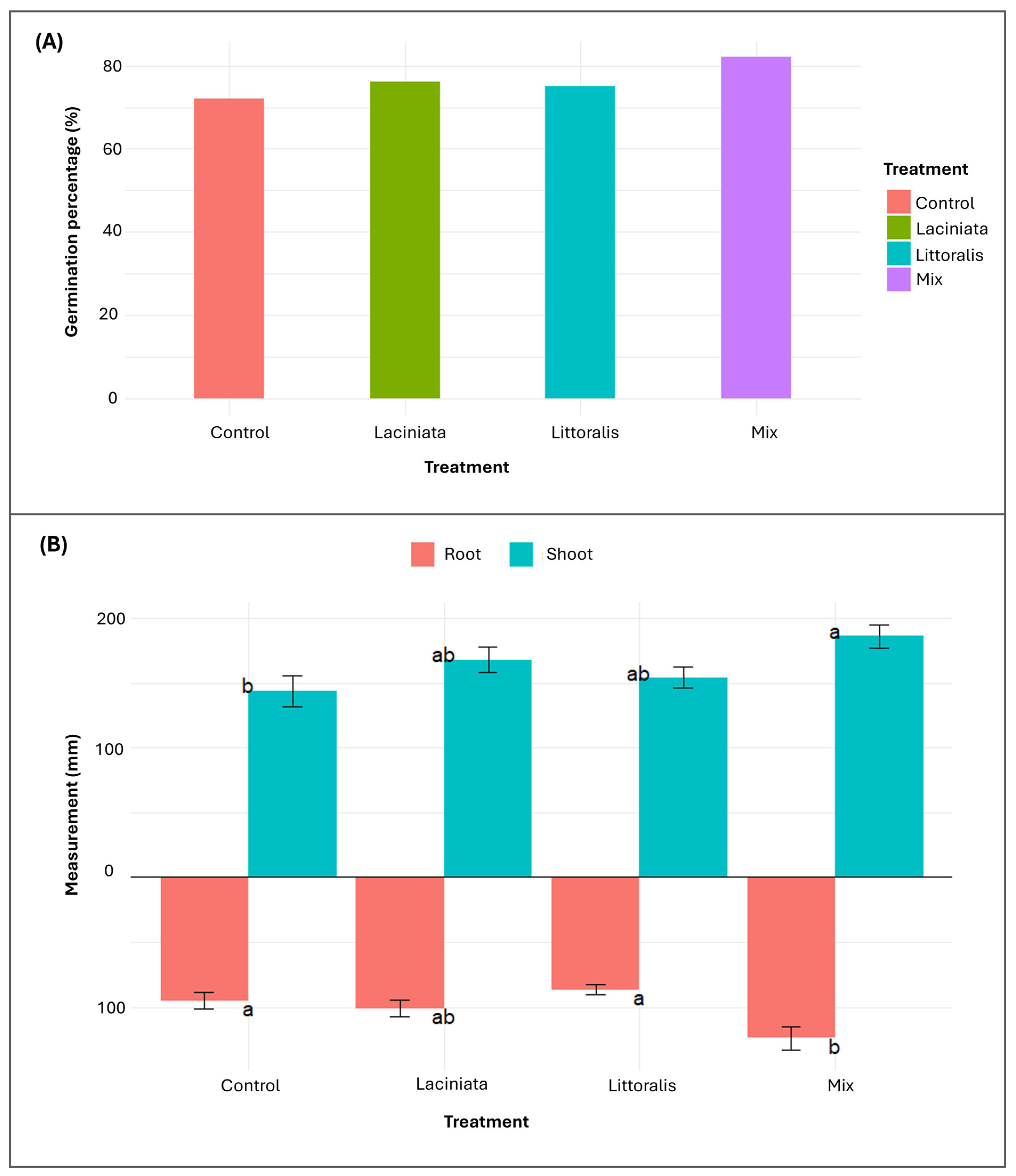
3.2.1. Evaluating the Impact of SynCom Treatments on Germination and Early-Stage Plant Growth
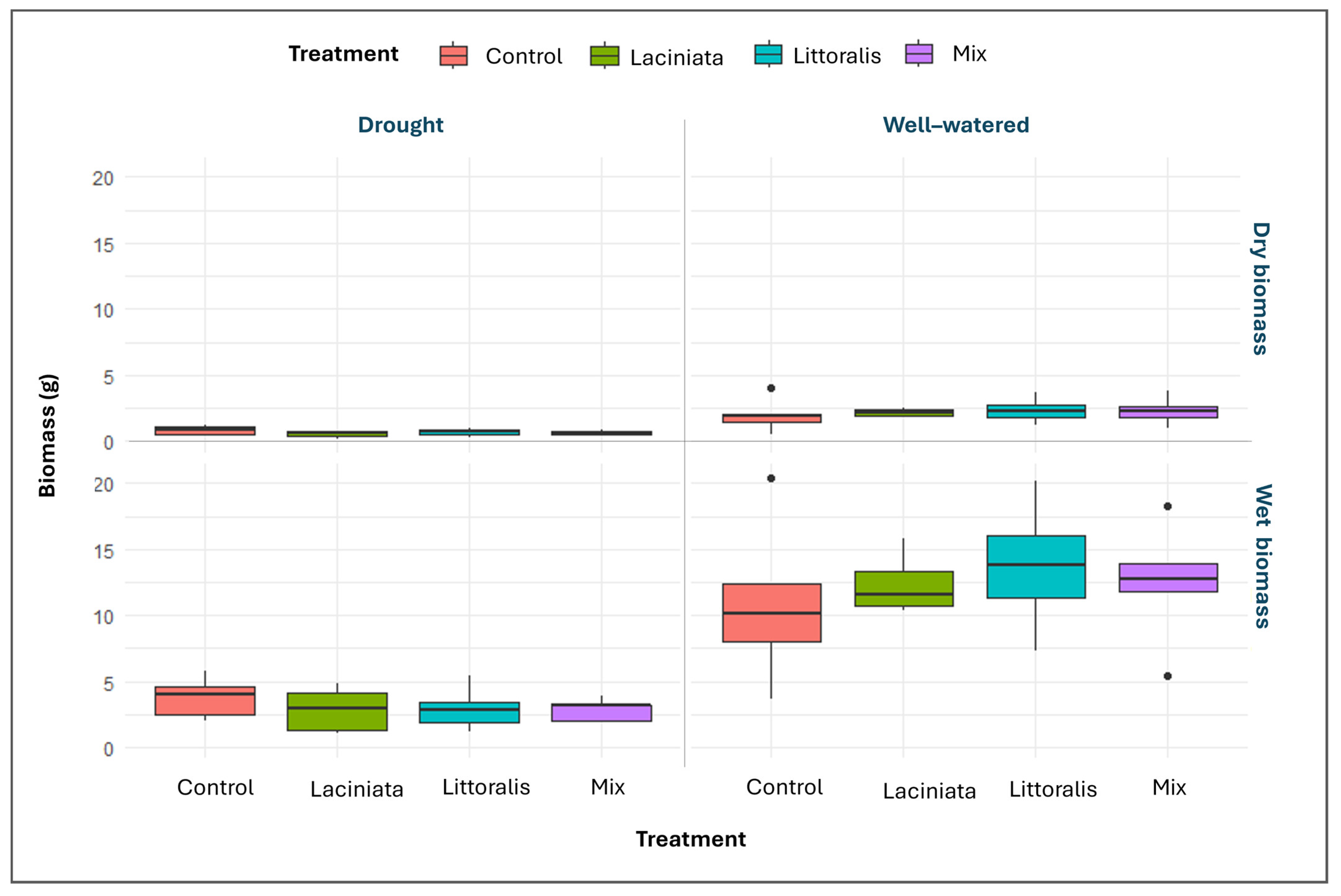
3.2.2. Evaluating Plant Aerial Biomass at 63 DAP Across SynCom Treatments and Watering Conditions
3.2.3. Post-Stress Recovery and Growth Progression Across Timepoints
3.3. Overview of HTP Phenotyping Using Image-Based Analysis
3.3.1. Validation of HTP Imaging Against Conventional Biomass Measurements
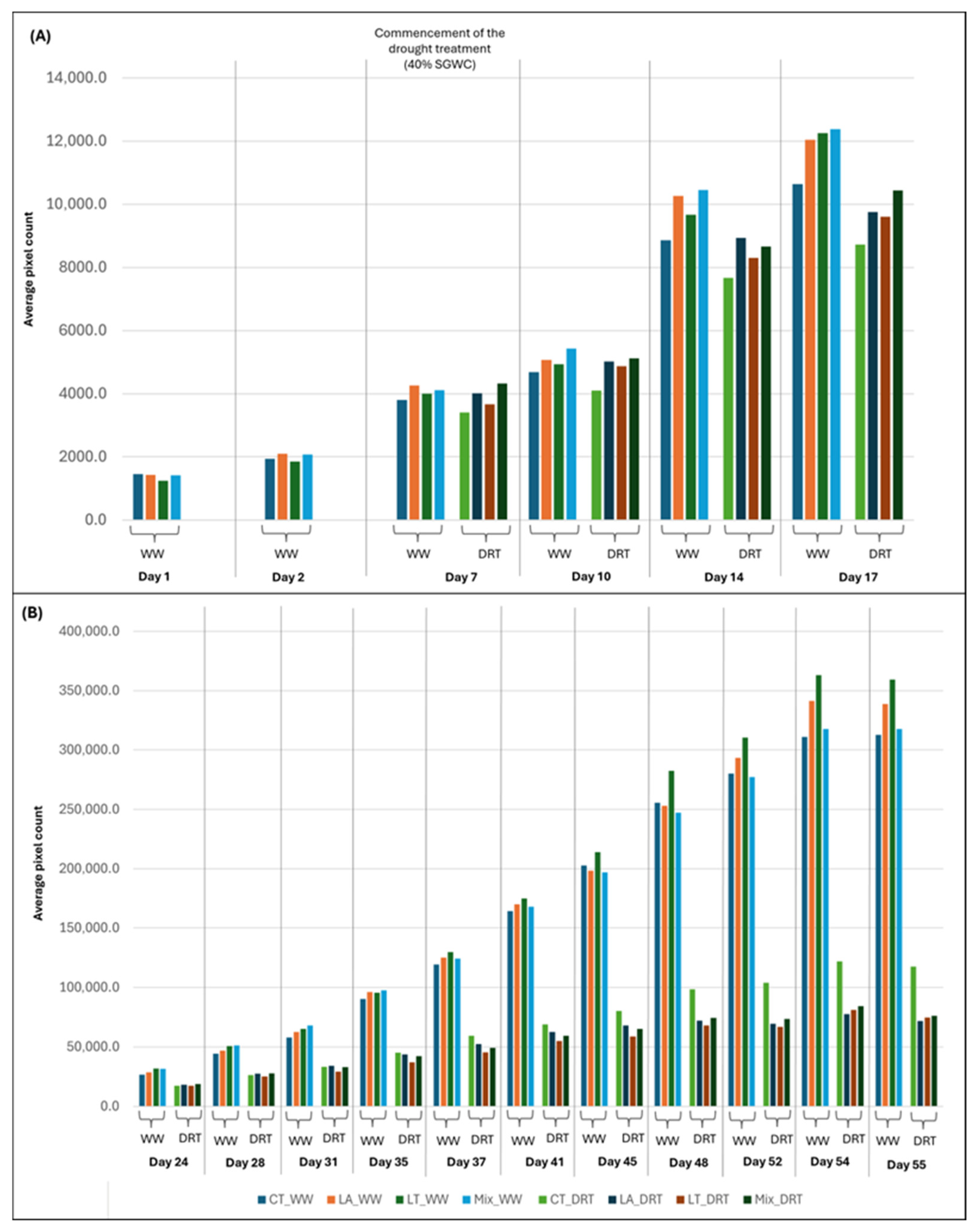
3.3.2. Early-Phase Imaging Analysis (Day 1–17)
3.3.3. Late-Phase Imaging Analysis (Day 24 to Day 55)
4. Discussion
4.1. A Holistic/Multifaceted Approach to Evaluating SynCom Efficacy on Lucerne Growth
4.2. Functional Microbiome Restructuring Underpins Early Growth Responses
4.3. Temporal Dynamics of SynCom-Mediated Growth Under Contrasting Water Regimes
4.4. Insights from Phenotyping and Microbiome Approaches in Evaluating SynCom Efficacy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 16S rRNA | 16S ribosomal ribonucleic acid |
| ANI | Average nucleotide identity |
| ANOVA | Analysis of variance |
| ANPR | Allorhizobium–Neorhizobium–Pararhizobium–Rhizobium |
| ASV | Amplicon sequence variant |
| CWRs | Crop wild relatives |
| DAP | Days after planting |
| DNA | Deoxyribonucleic acid |
| GA | Gibberellins |
| HTP | High-throughput phenotyping |
| IAA | Indole-3-acetic acid |
| LA | Laciniata SynCom (three-strain SynCom from M. laciniata) |
| LT | Littoralis SynCom (three-strain SynCom from M. littoralis) |
| Mix | Mix SynCom (six-strain SynCom combining LA and LT) |
| NB | Nutrient broth |
| NCBI | National Centre for Biotechnology Information |
| PPV | Plant Phenomics Victoria |
| QIIME2 | Quantitative Insights Into Microbial Ecology 2 |
| SGWC | Soil gravimetric water content |
| SynComs | Synthetic communities |
References
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.-T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Buée, M.; De Boer, W.; Martin, F.; van Overbeek, L.; Jurkevitch, E. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 2009, 321, 189–212. [Google Scholar] [CrossRef]
- Berg, G.; Dorador, C.; Egamberdieva, D.; Kostka, J.E.; Ryu, C.-M.; Wassermann, B. Shared governance in the plant holobiont and implications for one health. FEMS Microbiol. Ecol. 2024, 100, fiae004. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.E.; Antonielli, L.; Mitter, B.; Trognitz, F.; Sessitsch, A. Heritability and functional importance of the Setaria viridis bacterial seed microbiome. Phytobiomes J. 2020, 4, 40–52. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, K.; Verma, A.; Verma, S.K. Seed inhabiting bacterial endophytes of maize promote seedling establishment and provide protection against fungal disease. Microbiol. Res. 2022, 255, 126926. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Jacques, M.-A.; Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Raizada, M.N. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef]
- Tariq, A.; Guo, S.; Farhat, F.; Shen, X. Engineering synthetic microbial communities: Diversity and applications in soil for plant resilience. Agronomy 2025, 15, 513. [Google Scholar] [CrossRef]
- Khan, S.T. Consortia-based microbial inoculants for sustaining agricultural activities. Appl. Soil Ecol. 2022, 176, 104503. [Google Scholar] [CrossRef]
- de Souza, R.S.C.; Armanhi, J.S.L.; Arruda, P. From microbiome to traits: Designing synthetic microbial communities for improved crop resiliency. Front. Plant Sci. 2020, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Egidi, E.; Qiu, Z.; Macdonald, C.A.; Verma, J.P.; Trivedi, P.; Wang, J.; Liu, H.; Singh, B.K. Synthetic community improves crop performance and alters rhizosphere microbial communities. J. Sustain. Agric. Environ. 2022, 1, 118–131. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, S.; Ning, X.; Qiao, M.; Chen, W.; Zhang, P.; Liu, K.; Ding, Y. A synergistic indole-3-acetic acid-producing synthetic bacterial consortium benefits walnut seedling growth. Agronomy 2024, 14, 1657. [Google Scholar] [CrossRef]
- Qi, M.; Berry, J.C.; Veley, K.W.; O’Connor, L.; Finkel, O.M.; Salas-González, I.; Kuhs, M.; Jupe, J.; Holcomb, E.; Glavina del Rio, T.; et al. Identification of beneficial and detrimental bacteria impacting sorghum responses to drought using multi-scale and multi-system microbiome comparisons. ISME J. 2022, 16, 1957–1969. [Google Scholar] [CrossRef]
- Simonin, M.; Préveaux, A.; Marais, C.; Garin, T.; Arnault, G.; Sarniguet, A.; Barret, M. Transmission of synthetic seed bacterial communities to radish seedlings: Impact on microbiota assembly and plant phenotype. Peer Community J. 2023, 3, 329. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Humm, E.A.; Still, D.W.; Shi, B.; Pellegrini, M.; de la Roca, G.; Veliz, E.; Maymon, M.; Bru, P.; Huntemann, M.; et al. Medicago root nodule microbiomes: Insights into a complex ecosystem with potential candidates for plant growth promotion. Plant Soil 2022, 471, 507–526. [Google Scholar] [CrossRef]
- Flores-Duarte, N.J.; Navarro-Torre, S.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D. Nodule synthetic bacterial community as legume biofertilizer under abiotic stress in estuarine soils. Plants 2023, 12, 2083. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, J.T.; Shannon, J.G.; Lee, J.-D. Harnessing the potential of forage legumes, alfalfa, soybean, and cowpea for sustainable agriculture and global food security. Front. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef]
- Lei, Y.; Hannoufa, A.; Yu, P. The use of gene modification and advanced molecular structure analyses towards improving alfalfa forage. Int. J. Mol. Sci. 2017, 18, 298. [Google Scholar] [CrossRef] [PubMed]
- Wigley, K.; Moot, D.; Wakelin, S.A.; Laugraud, A.; Blond, C.; Seth, K.; Ridgway, H. Diverse bacterial taxa inhabit root nodules of lucerne (Medicago sativa L.) in New Zealand pastoral soils. Plant Soil 2017, 420, 253–262. [Google Scholar] [CrossRef]
- Le, X.H.; Franco, C.M.M.; Ballard, R.A.; Drew, E.A. Isolation and characterisation of endophytic actinobacteria and their effect on the early growth and nodulation of lucerne (Medicago sativa L.). Plant Soil 2016, 405, 13–24. [Google Scholar] [CrossRef]
- Chandel, A.; Mann, R.; Kaur, J.; Tannenbaum, I.; Norton, S.; Edwards, J.; Spangenberg, G.; Sawbridge, T. Australian native Glycine clandestina seed microbiota hosts a more diverse bacterial community than the domesticated soybean Glycine max. Environ. Microbiome 2022, 17, 56. [Google Scholar] [CrossRef]
- Herath Dissanayakalage, S.S.; Kaur, J.; Achari, S.R.; Sawbridge, T.I. Identification of in planta bioprotectants against Fusarium wilt in Medicago sativa L. (lucerne) from a collection of bacterial isolates derived from Medicago seeds. Front. Microbiol. 2025, 16, 1544521. [Google Scholar] [CrossRef]
- Herath Dissanayakalage, S.S.; Kaur, J.; Sawbridge, T.I. Genotype-driven shifts in the Medicago seed microbiome reveal diversity loss during domestication from wild relatives to lucerne (Medicago sativa). Preprints 2025, 2025071964. [Google Scholar]
- Yousfi, N.; Sihem, N.; Ramzi, A.; Abdelly, C. Growth, photosynthesis and water relations as affected by different drought regimes and subsequent recovery in Medicago laciniata (L.) populations. J. Plant Biol. 2016, 59, 33–43. [Google Scholar] [CrossRef]
- Badri, M.; Arraouadi, S.; Huguet, T.; Aouani, M. Comparative effects of water deficit on Medicago laciniata and Medicago truncatula lines sampled from sympatric populations. J. Plant Breed. Crop Sci. 2010, 2, 259–266. [Google Scholar]
- Friesen, M.L.; von Wettberg, E.J.B.; Badri, M.; Moriuchi, K.S.; Barhoumi, F.; Chang, P.L.; Cuellar-Ortiz, S.; Cordeiro, M.A.; Vu, W.T.; Arraouadi, S.; et al. The ecological genomic basis of salinity adaptation in Tunisian Medicago truncatula. BMC Genom. 2014, 15, 1160. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Bakker, P.A.H.M. Interactions between plants and beneficial Pseudomonas spp.: Exploiting bacterial traits for crop protection. Antonie Van Leeuwenhoek 2007, 92, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Mackiewicz, B.; Marta Kinga, L.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann. Agric. Environ. Med. 2016, 23, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Suhaimi, N.S.M.; Irawan, B.; Mohamed, Z.; Mispan, M.S. Associations of Pantoea with rice plants: As friends or foes? Agriculture 2021, 11, 1278. [Google Scholar] [CrossRef]
- Gehan, M.A.; Fahlgren, N.; Abbasi, A.; Berry, J.C.; Callen, S.T.; Chavez, L.; Doust, A.N.; Feldman, M.J.; Gilbert, K.B.; Hodge, J.G.; et al. PlantCV v2: Image analysis software for high-throughput plant phenotyping. PeerJ 2017, 5, e4088. [Google Scholar] [CrossRef]
- Klukas, C.; Chen, D.; Pape, J.-M. Integrated analysis platform: An open-source information system for high-throughput plant phenotyping. Plant Physiol. 2014, 165, 506–518. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina paired-end reAd mergeR. Bioinformatics 2013, 30, 614–620. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, alpha diversity, and statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Foster, Z.S.L.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef]
- Figueiredo dos Santos, L.; Fernandes Souta, J.; de Paula Soares, C.; Oliveira da Rocha, L.; Luiza Carvalho Santos, M.; Grativol, C.; Fernando Wurdig Roesch, L.; Lopes Olivares, F. Insights into the structure and role of seed-borne bacteriome during maize germination. FEMS Microbiol. Ecol. 2021, 97, fiab024. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Li, M.; Zhang, K.; Ma, W.; Zheng, L.; Xu, H.; Cui, B.; Liu, R.; Yang, Y.; et al. Functional assembly of root-associated microbial consortia improves nutrient efficiency and yield in soybean. J. Integr. Plant Biol. 2021, 63, 1021–1035. [Google Scholar] [CrossRef]
- Chai, Y.N.; Ge, Y.; Stoerger, V.; Schachtman, D.P. High-resolution phenotyping of sorghum genotypic and phenotypic responses to low nitrogen and synthetic microbial communities. Plant Cell Environ. 2021, 44, 1611–1626. [Google Scholar] [CrossRef]
- Yassue, R.M.; Galli, G.; Borsato, R., Jr.; Cheng, H.; Morota, G.; Fritsche-Neto, R. A low-cost greenhouse-based high-throughput phenotyping platform for genetic studies: A case study in maize under inoculation with plant growth-promoting bacteria. Plant Phenom. J. 2022, 5, e20043. [Google Scholar] [CrossRef]
- Rostamikia, Y.; Kouchaksaraei, M.T.; Asgharzadeh, A.; Rahmani, A. Effect of plant growth promoting rhizobacteria (PGPR) and cold stratification on seed germination and early growth of Corylus avellana L. Austrian J. For. Sci. 2016, 4, 337–352. [Google Scholar]
- Garcia-Lemos, A.M.; Großkinsky, D.K.; Saleem Akhtar, S.; Nicolaisen, M.H.; Roitsch, T.; Nybroe, O.; Veierskov, B. Identification of root-associated bacteria that influence plant physiology, increase seed germination, or promote growth of the christmas tree species Abies nordmanniana. Front. Microbiol. 2020, 11, 566613. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Kumar, P.P. Regulation of seed germination: The involvement of multiple forces exerted via gibberellic acid signaling. Mol. Plant 2019, 12, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Y.; Jiang, G.; Sun, H.; Liu, X.; Han, L. Seed color represents salt resistance of alfalfa seeds (Medicago sativa L.): Based on the analysis of germination characteristics, seedling growth and seed traits. Front. Plant Sci. 2023, 14, 1104948. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, Z.; Ye, J.; Verma, J.P.; Li, J.; Singh, B.K. Effective colonisation by a bacterial synthetic community promotes plant growth and alters soil microbial community. J. Sustain. Agric. Environ. 2022, 1, 30–42. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2013, 8, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Arnault, G.; Marais, C.; Préveaux, A.; Briand, M.; Poisson, A.-S.; Sarniguet, A.; Barret, M.; Simonin, M. Seedling microbiota engineering using bacterial synthetic community inoculation on seeds. FEMS Microbiol. Ecol. 2024, 100, fiae027. [Google Scholar] [CrossRef] [PubMed]
- Marín, O.; González, B.; Poupin, M.J. From microbial dynamics to functionality in the rhizosphere: A systematic review of the opportunities with synthetic microbial communities. Front. Plant Sci. 2021, 12, 650609. [Google Scholar] [CrossRef]
- Bakker, J.D. Increasing the utility of indicator species analysis. J. Appl. Ecol. 2008, 45, 1829–1835. [Google Scholar] [CrossRef]
- Tran, V.C.; Nguyen, T.H.; Hoang, T.L.A.; Pham, T.T.M.; Nguyen, T.M.; Tran, T.D.; Nguyen, T.H.G.; Han, V.-C.; Tran, A.T.; La, V.H. Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth. Open Agric. 2024, 9, 20220392. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Liu, R.; Shi, J.; Qiu, X.; Lei, J.; Zhao, X.; Wang, C.; Ge, M.; Xu, H.; et al. A simplified SynCom based on core–helper strain interactions enhances symbiotic nitrogen fixation in soybean. J. Integr. Plant Biol. 2025, 67, 1582–1598. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Roussely-Provent, V.; Walser, J.-C.; Schlaeppi, K. Deciphering composition and function of the root microbiome of a legume plant. Microbiome 2017, 5, 2. [Google Scholar] [CrossRef]
- Zgadzaj, R.; Garrido-Oter, R.; Jensen, D.B.; Koprivova, A.; Schulze-Lefert, P.; Radutoiu, S. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. USA 2016, 113, E7996–E8005. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Mitter, B.; Oswald, A.; Schloter-Hai, B.; Schloter, M.; Declerck, S.; Sessitsch, A. Rhizosphere microbiomes of potato cultivated in the High Andes show stable and dynamic core microbiomes with different responses to plant development. FEMS Microbiol. Ecol. 2016, 93, fiw242. [Google Scholar] [CrossRef]
- Abbasi, S.; Spor, A.; Sadeghi, A.; Safaie, N. Streptomyces strains modulate dynamics of soil bacterial communities and their efficacy in disease suppression caused by Phytophthora capsici. Sci. Rep. 2021, 11, 9317. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Yin, C.; Hulbert, S.; Paulitz, T.C. Core rhizosphere microbiomes of dryland wheat are influenced by location and land use history. Appl. Environ. Microbiol. 2020, 86, e02135-19. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, W.; Zhang, Y.; Yang, Q.; Yang, B.; Liang, T.; Ling, J.; Dong, J. Keystone PGPR ecological effect: An inoculation case study of diazotrophic Novosphingobium sp. N034 on mangrove plant Kandelia obovate. Appl. Soil Ecol. 2024, 202, 105567. [Google Scholar] [CrossRef]
- Kolton, M.; Erlacher, A.; Berg, G.; Cytryn, E. The Flavobacterium genus in the plant holobiont: Ecological, physiological, and applicative insights. In Microbial Models: From Environmental to Industrial Sustainability; Castro-Sowinski, S., Ed.; Springer Singapore: Singapore, 2016; pp. 189–207. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E. Chapter two—How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 2010; Volume 108, pp. 77–136. [Google Scholar]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Sahu, P.K.; Pandey, A.; White, J.F.; Verma, S.K. Endophytic Burkholderia: Multifunctional roles in plant growth promotion and stress tolerance. Microbiol. Res. 2022, 265, 127201. [Google Scholar] [CrossRef]
- Hao, X.; Wang, X.; Chen, C.; Liu, R.; Yin, Y.; Yao, J.; Xiao, Z.; Liu, X.; Shen, X.; Liu, X. Synthetic bacterial communities reshape microbial communities and enhance nutrient supply in desertified land of Northwest China. Appl. Soil Ecol. 2023, 189, 104972. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Udaondo, Z.; Montero-Curiel, M.; Wittich, R.-M.; García-Puente, A.; Segura, A. Clover root exudates favor Novosphingobium sp. HR1a establishment in the rhizosphere and promote phenanthrene rhizoremediation. mSphere 2021, 6, 00412–00421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Bai, D.-S.; Yang, X.; Zhang, Y.; Luo, X.-G. Soil sulfur cycle bacteria and metabolites affected by soil depth and afforestation conditions in high-sulfur coal mining areas. Appl. Soil Ecol. 2023, 185, 104802. [Google Scholar] [CrossRef]
- Jurkevitch, E.; Minz, D.; Ramati, B.; Barel, G. Prey range characterization, ribotyping, and diversity of soil and rhizosphere Bdellovibrio spp. isolated on phytopathogenic bacteria. Appl. Environ. Microbiol. 2000, 66, 2365–2371. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Sun, J.; Ma, Y. A degeneration gradient of poplar trees contributes to the taxonomic, functional, and resistome diversity of bacterial communities in rhizosphere soils. Int. J. Mol. Sci. 2021, 22, 3438. [Google Scholar] [CrossRef]
- Ikeda, S.; Okazaki, K.; Takahashi, H.; Tsurumaru, H.; Minamisawa, K. Seasonal shifts in bacterial community structures in the lateral root of sugar beet grown in an andosol field in Japan. Microbes Environ. 2023, 38, ME22071. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.-S.; Suh, M.K.; Eom, M.K.; Lee, J.; Lee, J.-S. A novel plant growth-promoting rhizobacterium, Rhizosphaericola mali gen. nov., sp. nov., isolated from healthy apple tree soil. Sci. Rep. 2024, 14, 1038. [Google Scholar] [CrossRef]
- Sun, C.; Xiao, J.; Bai, L.; Bai, J.; Liu, J.; Geng, L.; Zhang, Y. Defined and natural PAH contaminations shift PAH-degrading bacterial community in rhizosphere of ornamental plant species Echinacea purpurea L. Environ. Technol. Innov. 2023, 31, 103189. [Google Scholar] [CrossRef]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Mažylytė, R.; Kailiuvienė, J.; Mažonienė, E.; Orola, L.; Kaziūnienė, J.; Mažylytė, K.; Lastauskienė, E.; Gegeckas, A. The co-inoculation effect on Triticum aestivum growth with synthetic microbial communities (SynComs) and their potential in agrobiotechnology. Plants 2024, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Bano, Q.; Ilyas, N.; Bano, A.; Zafar, N.; Akram, A.; Hassan, F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013, 45, 13–20. [Google Scholar]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.H.; Omar, M.N.; Salama, S. Enhancement of drought tolerance in Triticum aestivum L. seedlings using Azospirillum brasilense NO40 and Stenotrophomonas maltophilia B11. Bull. Natl. Res. Cent. 2021, 45, 95. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. Plant microbiome: Stress response. In Interaction to Gene Induction: An Ecofriendly Mechanism of PGPR-mediated Stress Management in the Plant; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; pp. 217–232. [Google Scholar]
- Liu, Q.; Zhao, X.; Liu, Y.; Xie, S.; Xing, Y.; Dao, J.; Wei, B.; Peng, Y.; Duan, W.; Wang, Z. Response of sugarcane rhizosphere bacterial community to drought stress. Front. Microbiol. 2021, 12, 716196. [Google Scholar] [CrossRef]
- Tardieu, F.; Cabrera-Bosquet, L.; Pridmore, T.; Bennett, M. Plant phenomics, from sensors to knowledge. Curr. Biol. 2017, 27, R770–R783. [Google Scholar] [CrossRef]
- Okada, M.; Barras, C.; Toda, Y.; Hamazaki, K.; Ohmori, Y.; Yamasaki, Y.; Takahashi, H.; Takanashi, H.; Tsuda, M.; Hirai, M.Y.; et al. High-throughput phenotyping of soybean biomass: Conventional trait estimation and novel latent feature extraction using UAV remote sensing and deep learning models. Plant Phenom. 2024, 6, 0244. [Google Scholar] [CrossRef]
- Yang, W.; Duan, L.; Chen, G.; Xiong, L.; Liu, Q. Plant phenomics and high-throughput phenotyping: Accelerating rice functional genomics using multidisciplinary technologies. Curr. Opin. Plant Biol. 2013, 16, 180–187. [Google Scholar] [CrossRef]
- Elangovan, A.; Duc, N.T.; Raju, D.; Kumar, S.; Singh, B.; Vishwakarma, C.; Gopala Krishnan, S.; Ellur, R.K.; Dalal, M.; Swain, P.; et al. Imaging sensor-based high-throughput measurement of biomass using machine learning models in rice. Agriculture 2023, 13, 852. [Google Scholar] [CrossRef]
- Golzarian, M.R.; Frick, R.A.; Rajendran, K.; Berger, B.; Roy, S.; Tester, M.; Lun, D.S. Accurate inference of shoot biomass from high-throughput images of cereal plants. Plant Methods 2011, 7, 2. [Google Scholar] [CrossRef]
- LemnaTec. Advanced Plant Phenotyping. Available online: https://www.lemnatec.com/applications/advanced-plant-phenotyping/ (accessed on 18 March 2025).
- Delgado-Baquerizo, M.; Trivedi, P.; Trivedi, C.; Eldridge, D.J.; Reich, P.B.; Jeffries, T.C.; Singh, B.K. Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef]
- Dimech, A.M.; Kaur, S.; Breen, E.J. Mapping and quantifying unique branching structures in lentil (Lens culinaris Medik.). Plant Methods 2024, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Beiko, R.G. 16S rRNA gene analysis with QIIME2. In Microbiome Analysis: Methods and Protocols; Beiko, R.G., Hsiao, W., Parkinson, J., Eds.; Springer: New York, NY, USA, 2018; pp. 113–129. [Google Scholar]
- Lundberg, D.S.; Yourstone, S.; Mieczkowski, P.; Jones, C.D.; Dangl, J.L. Practical innovations for high-throughput amplicon sequencing. Nat. Methods 2013, 10, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Šic Žlabur, J.; Radman, S.; Fabek Uher, S.; Opačić, N.; Benko, B.; Galić, A.; Samirić, P.; Voća, S. Plant response to mechanically-induced stress: A case study on specialized metabolites of leafy vegetables. Plants 2021, 10, 2650. [Google Scholar] [CrossRef]
- Yadav, A.; Chen, M.; Acharya, S.M.; Yang, Y.; Zhao, T.Z.; Chakraborty, R. A stable 15-member bacterial SynCom promotes Brachypodium growth under drought stress. bioRxiv 2024. [Google Scholar] [CrossRef]
- Pereg, L.; McMillan, M. Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 2015, 80, 349–358. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2015, 67, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, T.; Zhang, F.; Yang, X.; Yang, C.; He, F.; Long, R.; Gao, T.; Jiang, Y.; Yang, Q.; et al. RAD-seq-based high-density linkage maps construction and quantitative trait loci mapping of flowering time trait in alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 899681. [Google Scholar] [CrossRef] [PubMed]


| Bacterial Isolate Lab ID and NCBI Accession Number | Isolation Source (Host Plant Species) | Taxonomic Classification (Kraken Database) | ANI % | NCBI Reference Genome ID |
|---|---|---|---|---|
| Lu_LA164_003 (PQ756890) | M. laciniata | Pantoea agglomerans pv. betae | 97.41% | Pantoea agglomerans (GCF_019048385.1) |
| Lu_LA841_009 (PQ756893) | Pantoea allii | 99.80% | Pantoea allii (GCF_003148935.1) | |
| Lu_LA164_012 (PQ756889) | Pseudomonas graminis | 85.93% | Pseudomonas graminis DSM 11,363 (GCF_900111735.1) | |
| Lu_LT198_003 (PQ756897) | M. littoralis | Pantoea agglomerans pv. betae | 97.43% | Pantoea agglomerans (GCF_019048385.1) |
| Lu_LT198_002 (PQ756896) | Pantoea allii | 80.43% | Pantoea allii (GCF_003148935.1) | |
| Lu_LT198_W003 (PQ756901) | Pseudomonas graminis | 99.85% | Pseudomonas graminis DSM 11,363 (GCF_900111735.1) |
| Control vs. SynCom-Treated Groups | |||||
|---|---|---|---|---|---|
| Comparison | Shared Taxa | Unique to Control | Unique to Treatment | Fisher’s Exact Test | Chi-Square Test |
| CT vs. LA | 72.60% | 11.10% | 16.30% | 0.1958 | 0.2331 |
| CT vs. LT | 65.70% | 3.60% | 30.70% | 3.02 × 10−12 | 8.18 × 10−11 |
| CT vs. Mix | 66.80% | 12.30% | 20.90% | 0.0502 | 0.0626 |
| Among SynCom-Treated Groups | |||||
| LT vs. LA | 67.45% | 27.50% (LT) | 5.10% (LA) | 6.14 × 10−9 | 2.97 × 10−8 |
| LT vs. MIX | 65.40% | 26.60% (LT) | 8.00% (Mix) | 3.09 × 10−6 | 7.52 × 10−6 |
| LA vs. MIX | 68.00% | 14.22% (LA) | 17.78% (Mix) | 0.4331 | 0.4731 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herath Dissanayakalage, S.S.; Kaur, J.; Li, T.; Dimech, A.M.; Sawbridge, T.I. Seed-Derived Synthetic Microbial Communities (SynComs) from Medicago Wild Relatives Modulate Early Plant Microbiome Assembly and Phenotypic Traits in Lucerne (Medicago sativa L.). Microorganisms 2025, 13, 2114. https://doi.org/10.3390/microorganisms13092114
Herath Dissanayakalage SS, Kaur J, Li T, Dimech AM, Sawbridge TI. Seed-Derived Synthetic Microbial Communities (SynComs) from Medicago Wild Relatives Modulate Early Plant Microbiome Assembly and Phenotypic Traits in Lucerne (Medicago sativa L.). Microorganisms. 2025; 13(9):2114. https://doi.org/10.3390/microorganisms13092114
Chicago/Turabian StyleHerath Dissanayakalage, Shenali Subodha, Jatinder Kaur, Tongda Li, Adam M. Dimech, and Timothy I. Sawbridge. 2025. "Seed-Derived Synthetic Microbial Communities (SynComs) from Medicago Wild Relatives Modulate Early Plant Microbiome Assembly and Phenotypic Traits in Lucerne (Medicago sativa L.)" Microorganisms 13, no. 9: 2114. https://doi.org/10.3390/microorganisms13092114
APA StyleHerath Dissanayakalage, S. S., Kaur, J., Li, T., Dimech, A. M., & Sawbridge, T. I. (2025). Seed-Derived Synthetic Microbial Communities (SynComs) from Medicago Wild Relatives Modulate Early Plant Microbiome Assembly and Phenotypic Traits in Lucerne (Medicago sativa L.). Microorganisms, 13(9), 2114. https://doi.org/10.3390/microorganisms13092114








