Effects of Italian Mediterranean Organic Diet on the Gut Microbiota: A Pilot Comparative Study with Conventional Products and Free Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention Diets
2.3. Data Collection
2.4. Anthropometry
2.5. Bioimpedance Analysis (BIA)
2.6. Eating Habits Evaluation
2.7. Gut Microbiota
2.8. Statistical Analysis
2.8.1. Taxonomic Profiling and Multivariate Analyses
2.8.2. Sex-Based Microbiota Variations
3. Results
3.1. Study Participants and Baseline Characteristics
3.2. Nutritional Composition of the Dietary Interventions
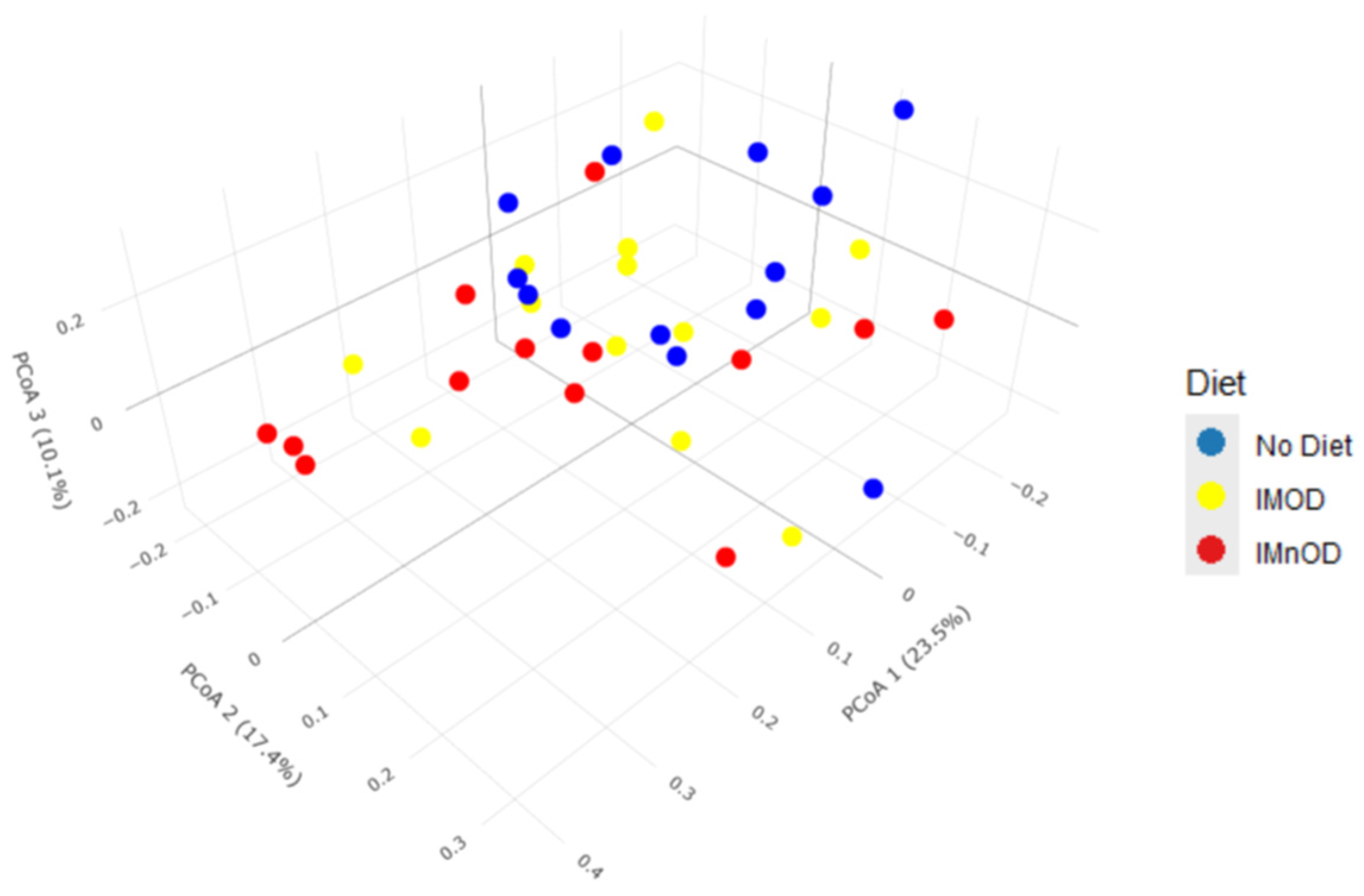
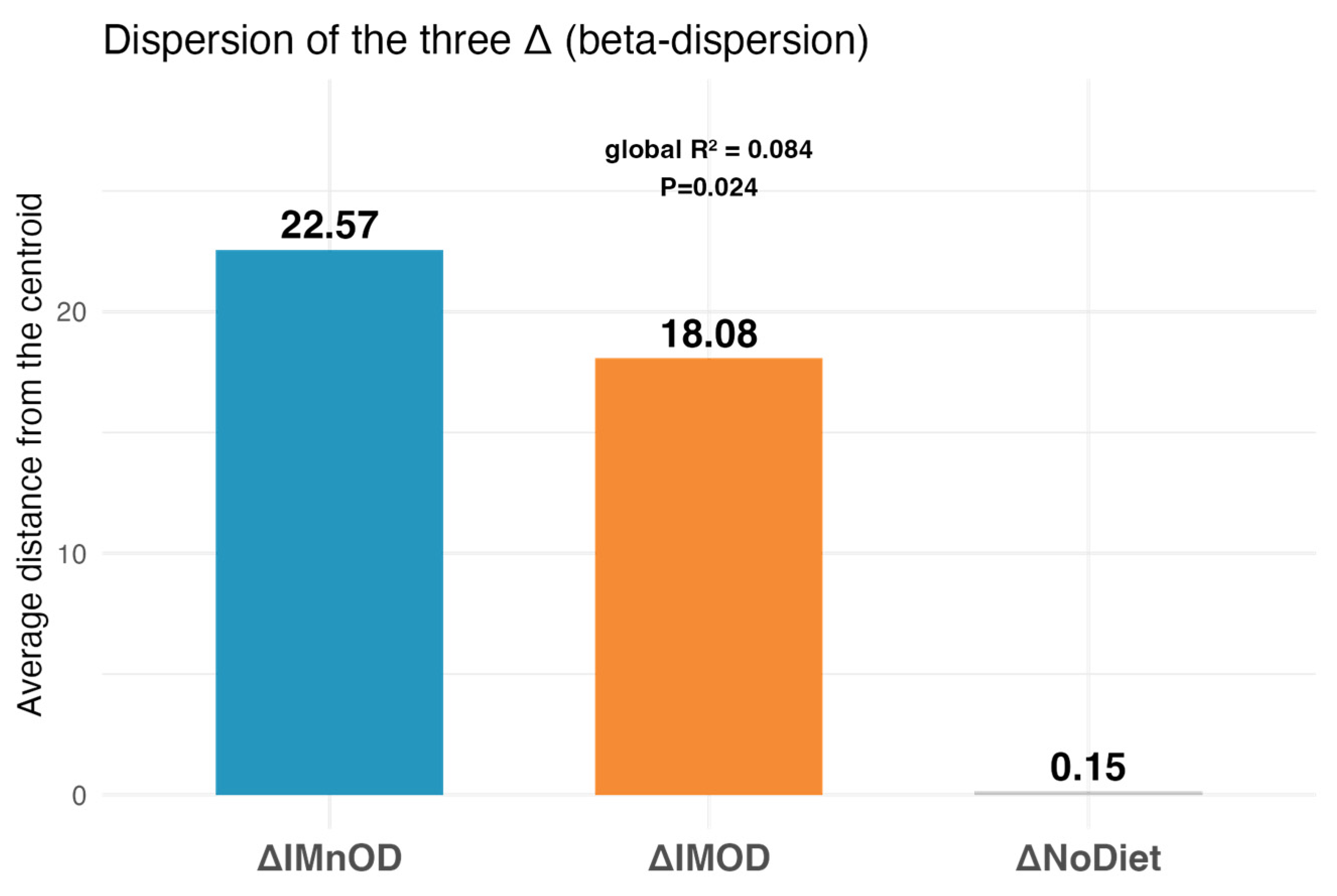
3.3. Gut Microbiota Diversity and Composition Across Dietary Interventions
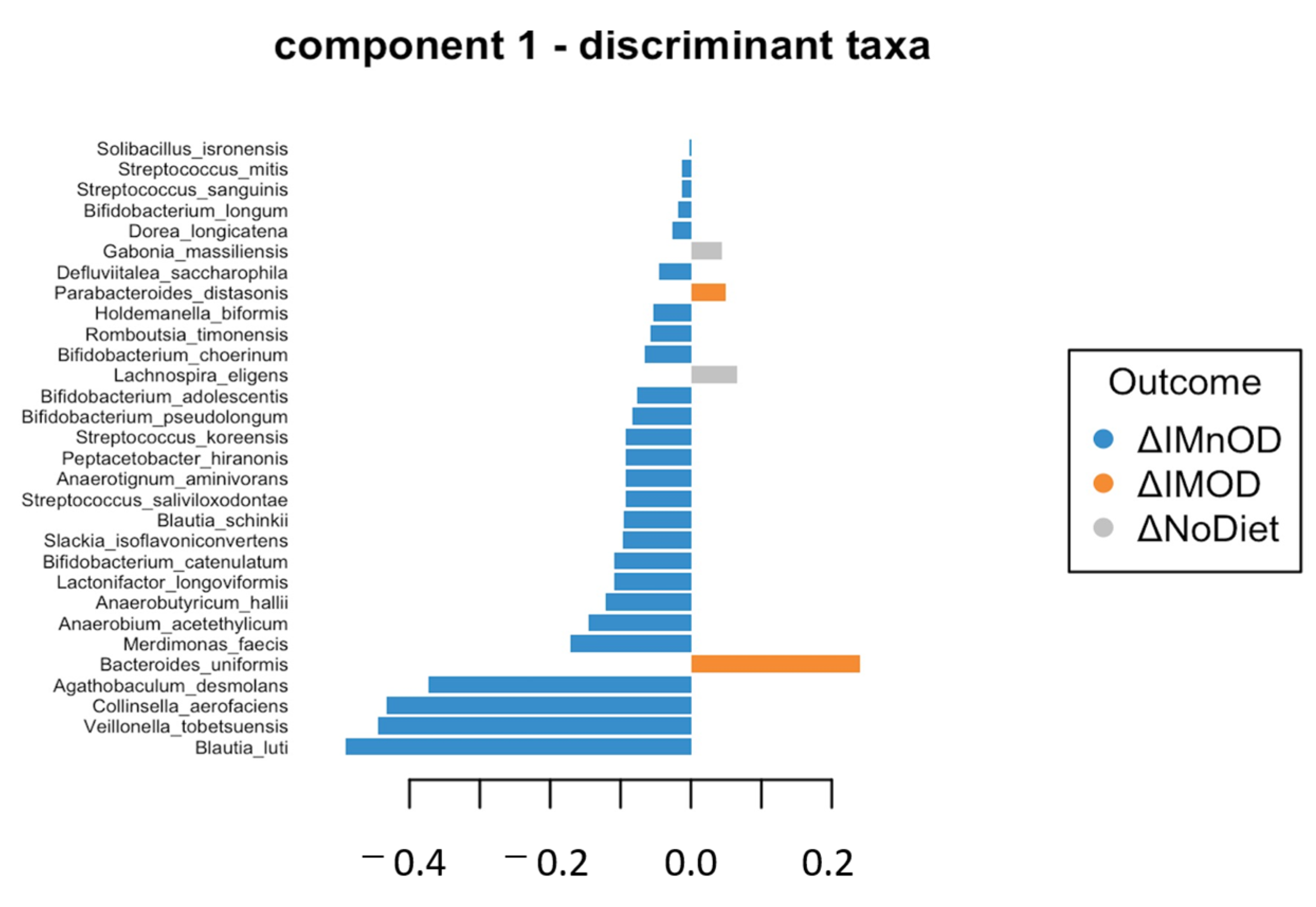
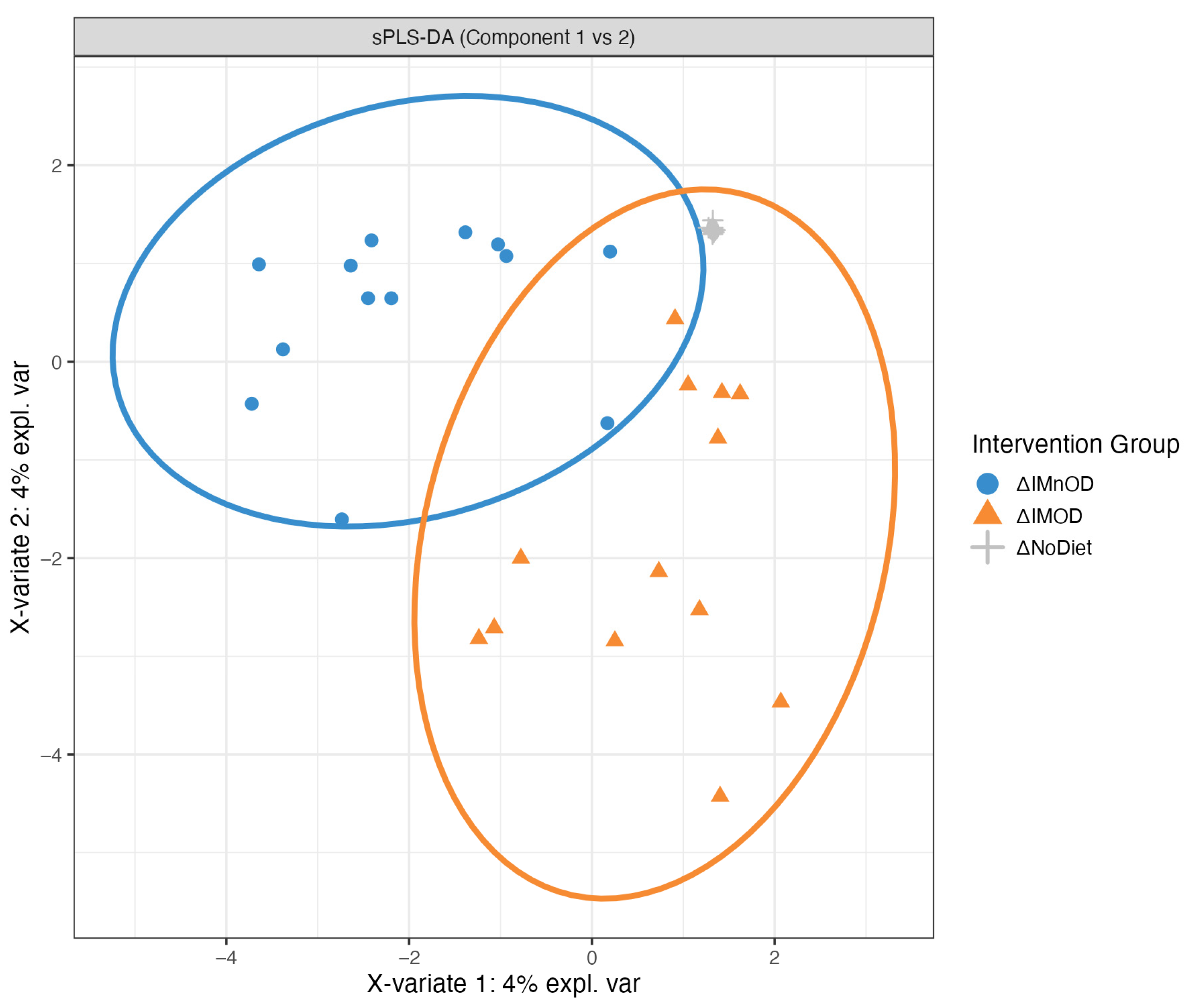
3.4. sPLS-DA and Microbial Discriminant Signatures
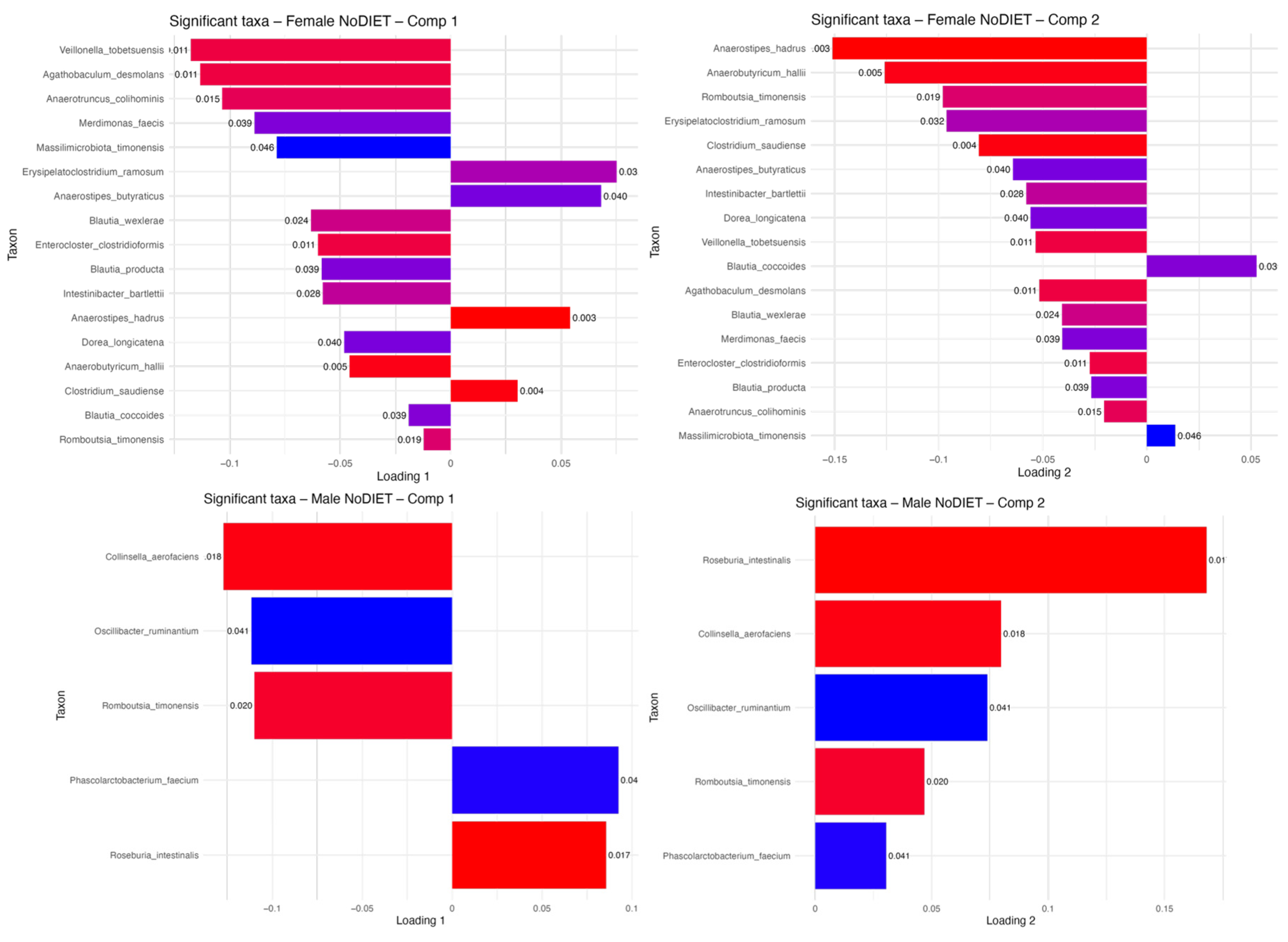
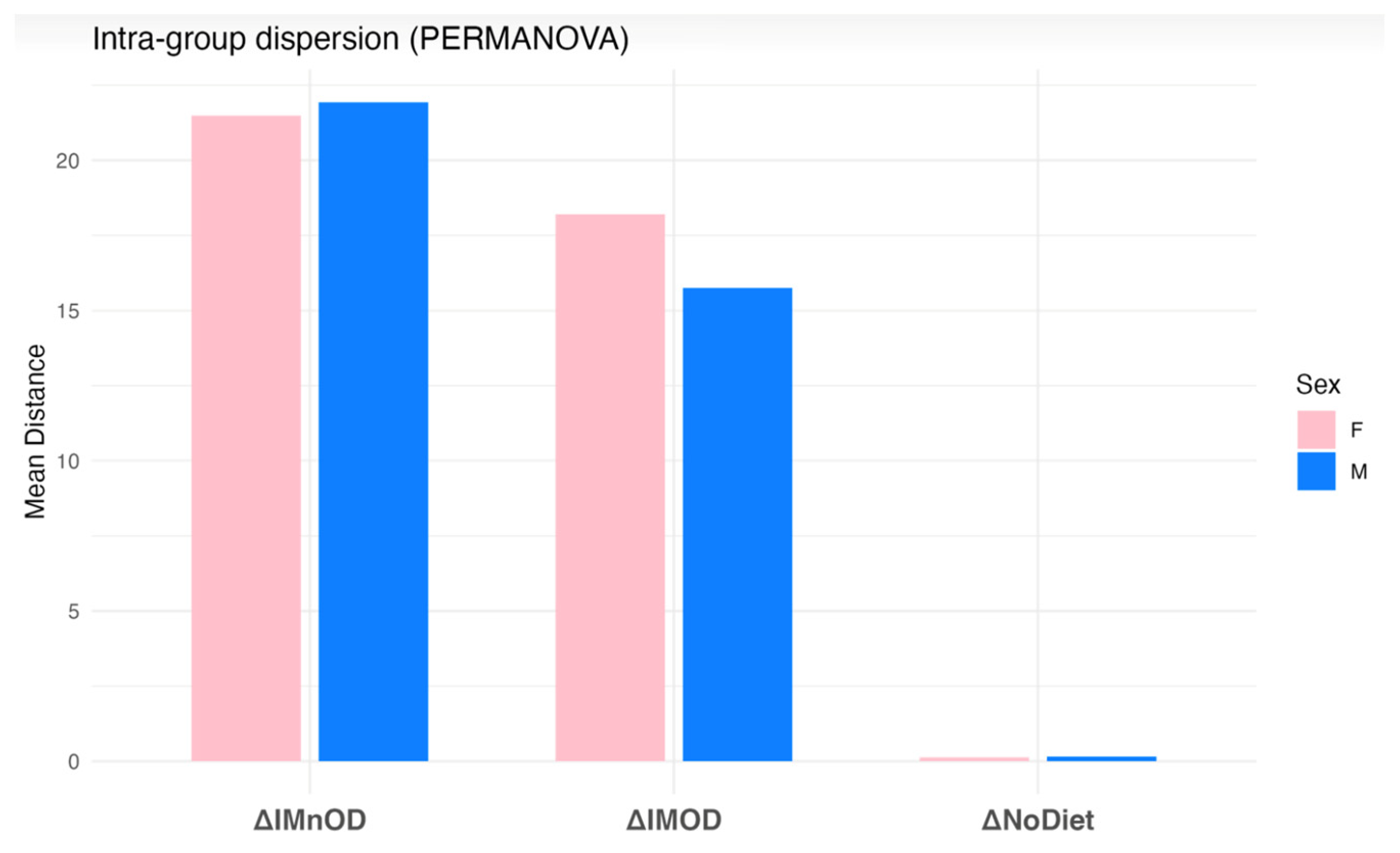
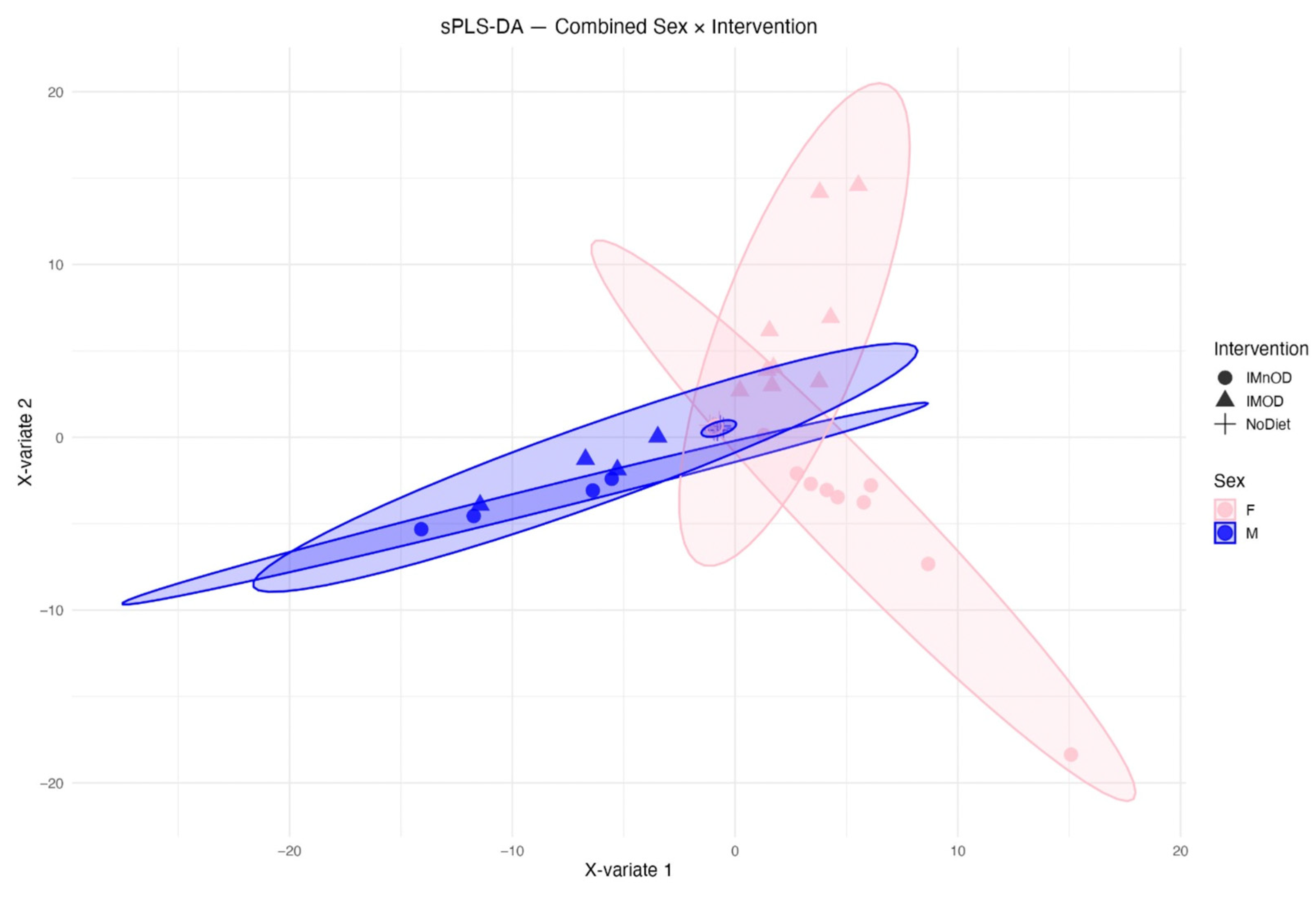
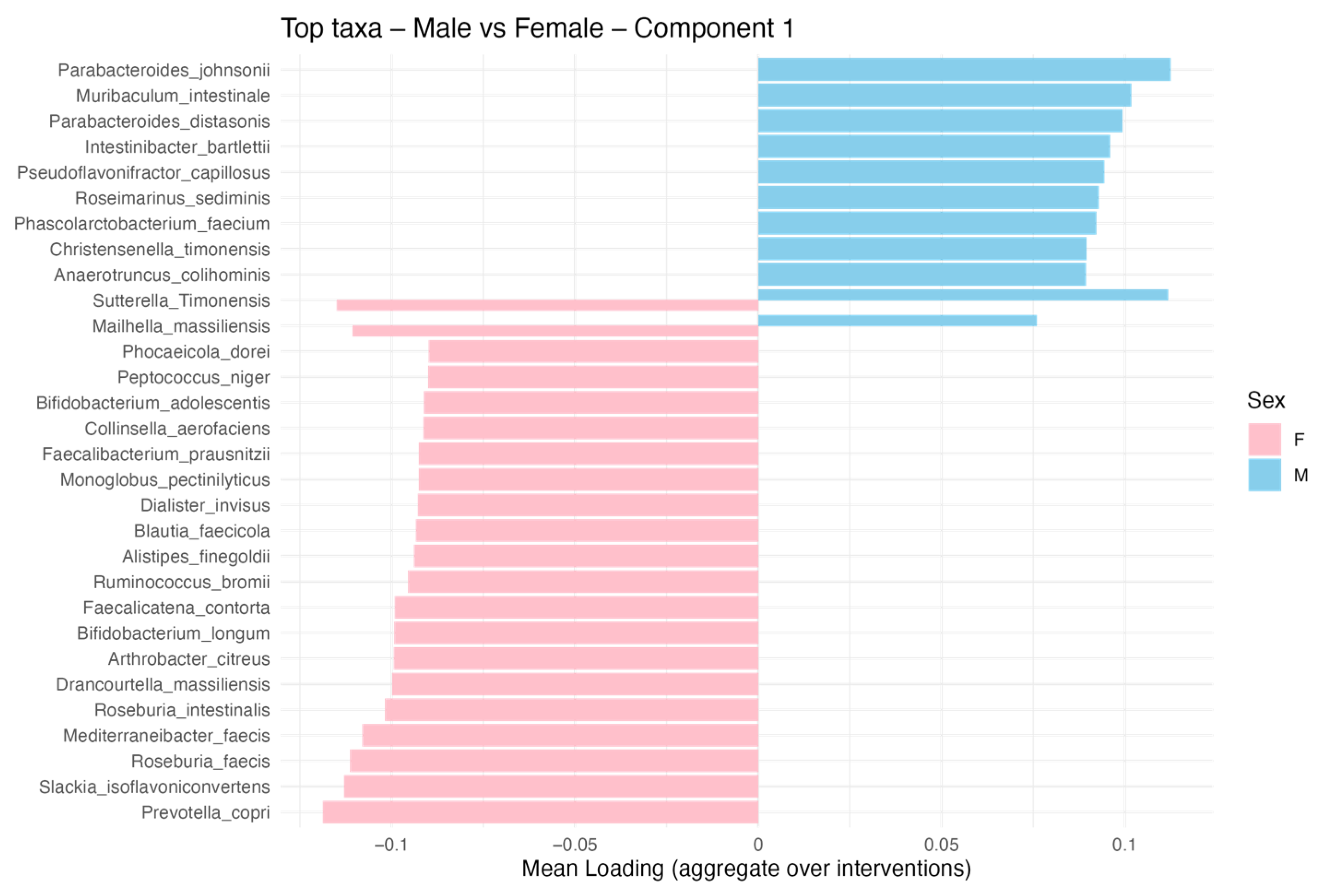
3.5. Sex-Specific Effects on Gut Microbiota Composition
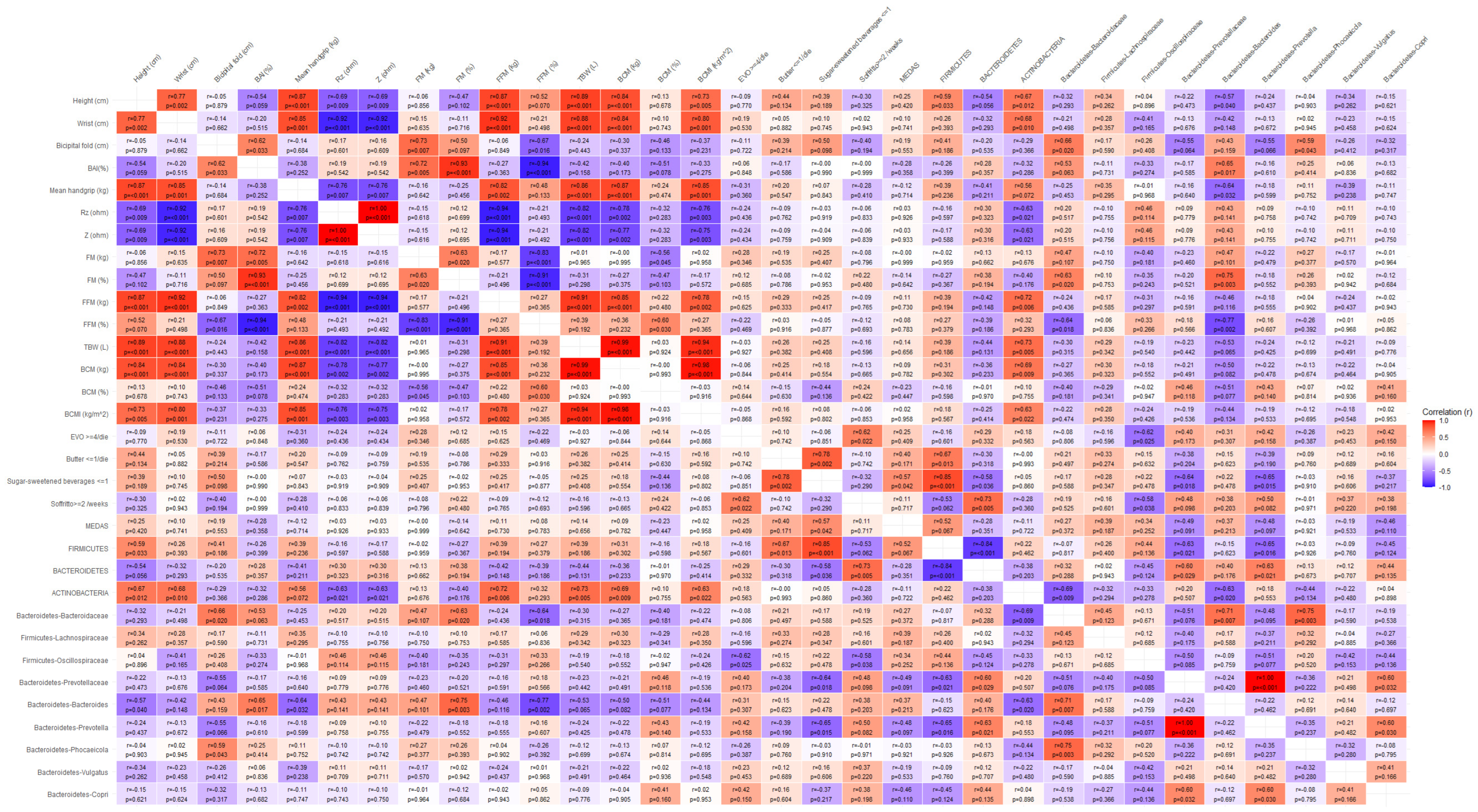
3.6. Correlation Analysis Between Microbiota and Body Composition Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-PBA | 3-Phenoxybenzoic Acid |
| ANOVA | Approximate One-Way Analysis Of Variance |
| BAI | Body Adiposity Index |
| BCM | Body Cell Mass |
| BCMI | Body Cell Mass Index |
| BIA | Bioimpedance Analysis |
| BMI | Body Mass Index |
| BMR | Basal Metabolic Rate |
| DAPs | Dialkylphosphates |
| ECM | Extracellular Mass |
| ECW | Extracellular Water |
| FFM | Fat Free Mass |
| FFQ | Food Frequency Questionnaire |
| FM | Fat Mass |
| GM | Gut Microbiota |
| ICW | Intracellular Water |
| IMnOD | Italian Mediterranean Non-Organic Diet |
| IMOD | Italian Mediterranean Organic Diet |
| MEDAS | Mediterranean Diet Adherence Screener |
| No Diet | Free Diet |
| OTUs | Operational Taxonomic Units |
| PCoA | Principal Coordinates Analysis |
| PERMANOVA | Permutational Multivariate Analysis Of Variance |
| PhA | Phase Angle |
| REML | Restricted Maximum Likelihood |
| Rz | Resistance |
| SCFAs | Short Chain Fatty Acids |
| SD | Standard Deviation |
| sPLS-DA | Partial Least Squares Discriminant Analysis |
| TBW | Total Body Water |
| WHO | World Health Organization |
| WHR | Waist-To-Height Ratio |
| Xc | Reactance |
| Z | Impedance |
Appendix A






References
- Di Renzo, L.; Gualtieri, P.; Frank, G.; Cianci, R.; Caldarelli, M.; Leggeri, G.; Raffaelli, G.; Pizzocaro, E.; Cirillo, M.; De Lorenzo, A. Exploring the Exposome Spectrum: Unveiling Endogenous and Exogenous Factors in Non-Communicable Chronic Diseases. Diseases 2024, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Gualtieri, P.; La Placa, G.; Frank, G.; Della Morte, D.; De Lorenzo, A.; Di Renzo, L. The Relationship between Exposome and Microbiome. Microorganisms 2024, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, H.; Di, S.; Guo, C.; Zhang, H.; Zhang, S.; Gold, A.; Wang, Y.; Hu, M.; Wu, D.; et al. Mapping Pesticide-Induced Metabolic Alterations in Human Gut Bacteria. Nat. Commun. 2025, 16, 4355. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, R.; Gunnigle, E.; Geissen, V.; Clarke, G.; Nagpal, J.; Cryan, J.F. Pesticide Exposure and the Microbiota-Gut-Brain Axis. ISME J. 2023, 17, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Hayashi, M.; Hirayama, M.; Nishiwaki, H.; Ito, M.; Saito, I.; Tsuboi, Y.; Isobe, T.; Ohno, K. Effects of Pesticide Intake on Gut Microbiota and Metabolites in Healthy Adults. Int. J. Environ. Res. Public Health 2022, 20, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhao, J. A Review on the Health Effects of Pesticides Based on Host Gut Microbiome and Metabolomics. Front. Mol. Biosci. 2021, 8, 632955. [Google Scholar] [CrossRef] [PubMed]
- Popli, S.; Badgujar, P.C.; Agarwal, T.; Bhushan, B.; Mishra, V. Persistent Organic Pollutants in Foods, Their Interplay with Gut Microbiota and Resultant Toxicity. Sci. Total Environ. 2022, 832, 155084. [Google Scholar] [CrossRef] [PubMed]
- Poulia, K.-A.; Bakaloudi, D.R.; Alevizou, M.; Papakonstantinou, E.; Zampelas, A.; Chourdakis, M. Impact of Organic Foods on Chronic Diseases and Health Perception: A Systematic Review of the Evidence. Eur. J. Clin. Nutr. 2025, 79, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, A.E.; Lee, S.G.; Ranadheera, C.S. Potential Associations between Organic Dairy Products, Gut Microbiome, and Gut Health: A Review. Food Res. Int. 2023, 172, 113195. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Rodriguez, J. Nutrition and Microbiome. Handb. Exp. Pharmacol. 2022, 274, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; De Lorenzo, A.; Merra, G.; Gualtieri, P. Comment on: “A Systematic Review of Organic Versus Conventional Food Consumption: Is There a Measurable Benefit on Human Health? Nutrients 2020, 12, 7”. Nutrients 2020, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.M.; Frederiksen, K.; Raaschou-Nielsen, O.; Hansen, J.; Kyrø, C.; Tjønneland, A.; Olsen, A. Organic Food Consumption Is Associated with a Healthy Lifestyle, Socio-Demographics and Dietary Habits: A Cross-Sectional Study Based on the Danish Diet, Cancer and Health Cohort. Public Health Nutr. 2022, 25, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Barański, M.; Średnicka-Tober, D.; Rempelos, L.; Hasanaliyeva, G.; Gromadzka-Ostrowska, J.; Skwarło-Sońta, K.; Królikowski, T.; Rembiałkowska, E.; Hajslova, J.; Schulzova, V.; et al. Feed Composition Differences Resulting from Organic and Conventional Farming Practices Affect Physiological Parameters in Wistar Rats-Results from a Factorial, Two-Generation Dietary Intervention Trial. Nutrients 2021, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Kasti, A.N.; Katsas, K.; Petsis, K.; Lambrinou, S.; Synodinou, K.D.; Kapetani, A.; Smart, K.L.; Nikolaki, M.D.; Halvatsiotis, P.; Triantafyllou, K.; et al. Is the Mediterranean Low Fodmap Diet Effective in Managing Irritable Bowel Syndrome Symptoms and Gut Microbiota? An Innovative Research Protocol. Nutrients 2024, 16, 1592. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Rio, P.; Massaro, M.G.; Caldarelli, M.; Frank, G.; Della-Morte, D.; Gasbarrini, A.; Gambassi, G.; De Lorenzo, A.; et al. Role of Nutrients in Modulating Microbiota and Immunity in COVID-19 Disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5927–5945. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Noce, A.; Bigioni, M.; Calabrese, V.; Della Rocca, D.G.; Di Daniele, N.; Tozzo, C.; Di Renzo, L. The Effects of Italian Mediterranean Organic Diet (IMOD) on Health Status. Curr. Pharm. Des. 2010, 16, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; Cianci, R.; Raffaelli, G.; Peluso, D.; Bigioni, G.; De Lorenzo, A. Sex-Specific Adherence to the Mediterranean Diet in Obese Individuals. Nutrients 2024, 16, 3076. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a Food Frequency Questionnaire for Use in Italian Adults Living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Itani, L.; El Ghoch, M.; Gualtieri, P.; Frank, G.; Raffaelli, G.; Pellegrini, M.; Di Renzo, L. Difference in Body Composition Patterns between Age Groups in Italian Individuals with Overweight and Obesity: When BMI Becomes a Misleading Tool in Nutritional Settings. Nutrients 2024, 16, 2415. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 3 May 2025).
- Pala, B.; Tocci, G.; Nardoianni, G.; Barbato, E.; Cianci, R.; Gualtieri, P.; Frank, G.; Di Renzo, L. Hormonal Balance and Cardiovascular Health: Exploring the Interconnection between Menopause, Body Composition, and Thyroid Function in a Cohort of Hypertensive Women. Appl. Sci. 2024, 14, 7772. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A Better Index of Body Adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Marchetti, M.; Cioccoloni, G.; Gratteri, S.; Capria, G.; Romano, L.; Soldati, L.; Mele, M.C.; Merra, G.; Cintoni, M.; et al. Role of Phase Angle in the Evaluation of Effect of an Immuno-Enhanced Formula in Post-Surgical Cancer Patients: A Randomized Clinical Trial. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.; Pala, B.; Gualtieri, P.; Tocci, G.; La Placa, G.; Di Renzo, L. Adherence to Mediterranean Diet and Implications for Cardiovascular Risk Prevention. Nutrients 2025, 17, 1991. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Sasso, G.F.; Andreoli, A.; Sorge, R.; Candeloro, N.; Cairella, M. Improved Prediction Formula for Total Body Water Assessment in Obese Women. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 535–538. [Google Scholar] [PubMed]
- Analisi Microbiota. Wellmicro. Available online: https://wellmicro.com (accessed on 3 May 2025).
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R Package for ’omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Rio, P.; Marrone, A.; Giambra, V.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Inflammaging: The Next Challenge-Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences. Biomedicines 2024, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “The Sentinel of Gut”: Their Intestinal Significance with and beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Cardelle-Cobas, A.; Mondragon, A.D.C.; Cepeda, A.; Rodriguez, J.A.; Miranda, J.M. Modulation of Gut Microbiota by Whole Encapsulated Brown Seaweed (Himanthalia elongata) in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2025, 17, 2047. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia Intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, C.; Jiang, L.; Zhong, X.; Huang, G.; Mo, G.; Cao, D.; Peng, X. Ruminococcus bromii-Generated Acetate Alleviated Clonorchis Sinensis-Induced Liver Fibrosis in Mice. Front. Microbiol. 2025, 16, 1532599. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Azinheiro, S.; Pereira, S.G. Modulation of Gut Microbiota by Diet and Probiotics: Potential Approaches to Prevent Gestational Diabetes Mellitus. Gut Microbiome 2023, 4, e17. [Google Scholar] [CrossRef] [PubMed]
- Tataru, C.; Martin, A.; Dunlap, K.; Peras, M.; Chrisman, B.S.; Rutherford, E.; Deitzler, G.E.; Phillips, A.; Yin, X.; Sabino, K.; et al. Longitudinal Study of Stool-Associated Microbial Taxa in Sibling Pairs with and without Autism Spectrum Disorder. ISME Commun. 2021, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Garcia, A.; Greppi, A.; Constancias, F.; Ruscheweyh, H.-J.; Gasser, J.; Hurley, K.; Sturla, S.J.; Schwab, C.; Lacroix, C. Anaerobutyricum hallii Promotes the Functional Depletion of a Food Carcinogen in Diverse Healthy Fecal Microbiota. Front. Microbiomes 2023, 2, 1194516. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut Microbes from the Phylogenetically Diverse Genus Eubacterium and Their Various Contributions to Gut Health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Baharlouei, P.; Koh, E.H.Y.; Pirvu, D.G.; Rehmani, R.; Arcos, M.; Puri, S. A Comprehensive Analysis of Organic Food: Evaluating Nutritional Value and Impact on Human Health. Foods 2024, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; AlHussaini, K.I. Pesticides: Unintended Impact on the Hidden World of Gut Microbiota. Metabolites 2024, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, P.; Cui, Y.; Hu, X.; Chen, F.; Ma, C. Alleviation Effects of Microbial Metabolites from Resveratrol on Non-Alcoholic Fatty Liver Disease. Foods 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Pang, J.; Li, J.; Mi, L.; Ru, D.; Feng, J.; Li, X.; Zhao, A.; Cai, L. The Effects of Organic Food on Human Health: A Systematic Review and Meta-Analysis of Population-Based Studies. Nutr. Rev. 2024, 82, 1151–1175. [Google Scholar] [CrossRef] [PubMed]
- Attaye, I.; Bird, J.K.; Nieuwdorp, M.; Gül, S.; Seegers, J.F.M.L.; Morrison, S.; Hofkens, S.; Herrema, H.; Bui, N.; Puhlmann, M.-L.; et al. Anaerobutyricum soehngenii Improves Glycemic Control and Other Markers of Cardio-Metabolic Health in Adults at Risk of Type 2 Diabetes. Gut Microbes 2025, 17, 2504115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dai, M.-Y.; Huang, R.-Y.; Duan, J.-Y.; Zhang, T.; Bao, W.-M.; Zhang, J.-Y.; Gui, S.-Q.; Xia, S.-M.; Dai, C.-T.; et al. Parabacteroides Distasonis Ameliorates Hepatic Fibrosis Potentially via Modulating Intestinal Bile Acid Metabolism and Hepatocyte Pyroptosis in Male Mice. Nat. Commun. 2023, 14, 1829. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Xu, L.; Gu, G.; Dong, Z. Parabacteroides johnsonii Inhibits the Onset and Progression of Colorectal Cancer by Modulating the Gut Microbiota. J. Transl. Med. 2025, 23, 734. [Google Scholar] [CrossRef] [PubMed]
- Chénard, T.; Malick, M.; Dubé, J.; Massé, E. The Influence of Blood on the Human Gut Microbiome. BMC Microbiol. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Bae, M.; Szamosvári, D.; Cassilly, C.D.; Bolze, A.S.; Jackson, D.R.; Xavier, R.J.; Clardy, J. Collinsella aerofaciens Produces a pH-Responsive Lipid Immunogen. J. Am. Chem. Soc. 2023, 145, 7071–7074. [Google Scholar] [CrossRef] [PubMed]
- Hata, S.; Nakajima, H.; Hashimoto, Y.; Miyoshi, T.; Hosomi, Y.; Okamura, T.; Majima, S.; Nakanishi, N.; Senmaru, T.; Osaka, T.; et al. Effects of Probiotic Bifidobacterium bifidum G9-1 on the Gastrointestinal Symptoms of Patients with Type 2 Diabetes Mellitus Treated with Metformin: An Open-Label, Single-Arm, Exploratory Research Trial. J. Diabetes Investig. 2022, 13, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A Bacterial Genus with Promising Human Health Applications. FEMS Microbiol. Rev. 2023, 47. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Venkatesh, S.; Ke, K.; Barratt, M.J.; Gordon, J.I. A Human Gut Faecalibacterium prausnitzii Fatty Acid Amide Hydrolase. Science 2024, 386, eado6828. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.M.; Reimer, R.A. Unlocking a Novel Determinant of Athletic Performance: The Role of the Gut Microbiota, Short-Chain Fatty Acids, and “Biotics” in Exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Zheng, H.-M.; Zhang, G.-X.; Chen, F.-L.; Chen, L.-D.; Yang, Z.-C. High Oscillospira Abundance Indicates Constipation and Low BMI in the Guangdong Gut Microbiome Project. Sci. Rep. 2020, 10, 9364. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Pillai, S.; Gagnon, C.A.; Foster, C.; Ashraf, A.P. Exploring the Gut Microbiota: Key Insights Into Its Role in Obesity, Metabolic Syndrome, and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2024, 109, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.C.; Silverman, J.D.; Dressman, H.K.; Wei, Z.; Dallow, E.P.; Armstrong, S.C.; Seed, P.C.; Rawls, J.F.; David, L.A. Short-Chain Fatty Acid Production by Gut Microbiota from Children with Obesity Differs According to Prebiotic Choice and Bacterial Community Composition. mBio 2020, 11, e00914–e00920. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.-K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium, Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | |
|---|---|
| Sample size | 39 (100) |
| Females | 27 (69.23) |
| Males | 12 (30.77) |
| Age (years) | 38.46 ± 10.35 |
| Height (cm) | 166.56 ± 7.10 |
| Weight (kg) | 67.12 ± 14.37 |
| BMI (kg/m2) | 24.03 ± 3.93 |
| IMOD | IMnOD | p-Value | |
|---|---|---|---|
| Kcal | 2087.00 ± 12.72 | 2069.00 ± 12.72 | 0.31 |
| Proteins (%) | 20.65 ± 0.07 | 20.55 ± 0.07 | 0.31 |
| Lipids (%) | 34.85 ± 0.21 | 34.55 ± 0.21 | 10.00 |
| Carbohydrates (%) | 44.72 ± 0.10 | 44.57 ± 0.10 | 0.31 |
| Proteins (g) | 103.32 ± 0.38 | 102.77 ± 0.38 | 0.31 |
| Lipids (g) | 77.55 ± 0.62 | 76.66 ± 0.62 | 0.31 |
| Carbohydrates (g) | 239.29 ± 2.26 | 236.09 ± 2.26 | 0.31 |
| Total Fibers (g) | 42.77 ± 1.30 | 40.92 ± 1.30 | 0.31 |
| Cholesterol (mg) | 209.50 ± 2.12 | 206.50 ± 2.12 | 0.31 |
| Saturated Fatty Acids (g) | 18.415 ± 0.01 | 18.40 ± 0.01 | 0.31 |
| Saturated Fatty Acids (%/kcal tot) | 8.31 ± 0.10 | 8.17 ± 0.10 | 0.31 |
| Salt (g) | 3.85 ± 0.07 | 3.75 ± 0.07 | 0.31 |
| Sodium (mg) | 1561.38 ± 25.48 | 1525.34 ± 25.48 | 0.31 |
| Glycemic Index | 57.98 ± 0.18 | 57.72 ± 0.18 | 0.31 |
| ω3 | 3.65 ± 0.21 | 3.35 ± 0.21 | 0.31 |
| ω6 | 10.22 ± 0.10 | 10.07 ± 0.10 | 0.31 |
| ω6/ω3 | 3.02 ± 0.14 | 2.81 ± 0.14 | 0.31 |
| ORAC | 19,882.50 ± 976.51 | 18,501.50 ± 976.51 | 0.31 |
| PRAL | −1.93 ± 4.22 | −7.91 ± 4.22 | 0.31 |
| MAI | 15.05 ± 0.07 | 14.95 ± 0.07 | 10.00 |
| AI | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.31 |
| TI | 0.22 ± 0.01 | 0.20 ± 0.01 | 0.31 |
| Variables | No Diet (n = 13) | IMOD (n = 13) | IMnOD (n = 13) | p-Value |
|---|---|---|---|---|
| Females | 9 | 9 | 9 | - |
| Males | 4 | 4 | 4 | - |
| Weight (kg) | 67.12 ± 15.21 | 66.03 ± 15.20 | 65.54 ± 15.22 | 0.88 |
| BMI (kg/m2) | 24.03 ± 4.04 | 23.53 ± 3.99 | 23.68 ± 4.02 | 0.79 |
| Neck circumference (cm) | 33.62 ± 3.70 | 32.82 ± 3.12 | 32.96 ± 3.51 | 0.78 |
| Waist circumference (cm) | 75.62 ± 11.12 | 74.74 ± 11.48 | 74.11 ± 9.92 | 0.84 |
| Abdomen circumference (cm) | 84.97 ± 10.46 | 82.08 ± 10.62 | 84.09 ± 10.69 | 0.49 |
| Hip circumference (cm) | 97.26 ± 6.96 | 95.96 ± 6.66 | 96.83 ± 6.92 | 0.63 |
| WHR | 0.78 ± 0.09 | 0.78 ± 0.09 | 0.76 ± 0.07 | 0.99 |
| Wrist circumference (cm) | 15.37 ± 1.16 | 15.14 ± 1.05 | 15.17 ± 1.17 | 0.61 |
| Bicipital Fold (mm) | 9.00 ± 4.82 | 6.21 ± 3.84 | 8.63 ± 4.44 | 0.13 |
| Tricipital Fold (mm) | 18.67 ± 8.80 | 14.42 ± 7.38 | 12.92 ± 5.68 | 0.21 |
| Subscapular fold (mm) | 16.08 ± 9.35 | 9.55 ± 4.93 | 12.13 ± 5.69 | 0.05 |
| Suprailiac fold (mm) | 14.67 ± 11.14 | 11.38 ± 5.75 | 10.29 ± 4.81 | 0.38 |
| Mean arms circumference (cm) | 27.97 ± 3.81 | 27.30 ± 3.47 | 27.71 ± 3.39 | 0.65 |
| Mean mid thighs circumference (cm) | 50.21 ± 4.67 | 50.63 ± 3.83 | 50.73 ± 4.06 | 0.81 |
| Mean root thighs circumference (cm) | 57.00 ± 4.82 | 57.13 ± 3.80 | 57.53 ± 4.39 | 0.94 |
| BAI (%) | 27.33 ± 3.48 | 26.55 ± 3.01 | 27.43 ± 3.20 | 0.54 |
| Variables | No Diet (n = 13) | IMOD (n = 13) | IMnOD (n = 13) | p-Value |
|---|---|---|---|---|
| Rz (ohm) | 577.00 ± 78.50 | 581.67 ± 83.15 | 585.91 ± 84.75 | 0.92 |
| Xc (ohm) | 60.62 ± 8.33 | 60.81 ± 10.56 | 61.12 ± 10.05 | 0.98 |
| PhA (°) | 6.05 ± 0.60 | 6.00 ± 0.74 | 6.01 ± 0.78 | 0.96 |
| Z (ohm) | 580.20 ± 78.71 | 584.88 ± 83.48 | 589.14 ± 84.97 | 0.91 |
| FM (kg) | 18.98 ± 8.26 | 17.72 ± 7.83 | 17.87 ± 8.25 | 0.81 |
| FM (%) | 25.72 ± 7.89 | 26.08 ± 8.22 | 26.79 ± 8.56 | 0.88 |
| FFM (kg) | 48.96 ± 11.64 | 48.55 ± 11.31 | 47.55 ± 10.87 | 0.88 |
| FFM (%) | 73.38 ± 7.84 | 73.92 ± 8.22 | 73.21 ± 8.56 | 0.94 |
| TBW (L) | 34.92 ± 10.34 | 35.08 ± 9.86 | 33.58 ± 10.78 | 0.83 |
| TBW (%) | 48.92 ± 6.07 | 48.83 ± 8.00 | 49.04 ± 6.40 | 0.99 |
| ECW (L) | 17.65 ± 8.15 | 15.50 ± 3.47 | 15.33 ± 3.29 | 0.21 |
| ECW (%) | 44.90 ± 9.00 | 46.87 ± 3.06 | 46.98 ± 2.90 | 0.31 |
| ICW (L) | 20.67 ± 12.20 | 17.77 ± 4.82 | 17.46 ± 4.59 | 0.26 |
| ICW (%) | 50.37 ± 8.79 | 53.13 ± 3.06 | 53.04 ± 2.90 | 0.12 |
| BCM (kg) | 28.48 ± 9.27 | 26.85 ± 6.48 | 27.78 ± 8.13 | 0.74 |
| BCM (%) | 39.54 ± 4.39 | 40.86 ± 5.58 | 40.37 ± 7.91 | 0.71 |
| BCMI (kg/m2) | 10.10 ± 2.36 | 9.53 ± 1.56 | 9.96 ± 2.09 | 0.54 |
| Discriminative Microbial Features | Loading 1 | Loading 2 | ΔIMOD | ΔIMnOD | ΔNoDiet | p-Value | FDR |
|---|---|---|---|---|---|---|---|
| Blautia luti | −0.49 | - | 3.08 | 6.86 | 0.01 | 0.06 | 0.21 |
| Veillonella tobetsuensis | −0.44 | - | 0.00 | 0.01 | 0.00 | 0.01 | 0.08 * |
| Collinsella aerofaciens | −0.43 | - | 0.89 | 2.41 | 0.01 | 0.01 | 0.08 * |
| Agathobaculum desmolans | −0.37 | - | 0.01 | 0.01 | 0.00 | 0.01 | 0.08 * |
| Bacteroides uniformis | 0.24 | - | 0.46 | −0.83 | −0.01 | 0.02 | 0.12 |
| Merdimonas faecis | −0.17 | - | 0.00 | 0.01 | 0.00 | 0.04 | 0.18 |
| Anaerobium acetethylicum | −0.14 | - | 0.00 | 0.01 | 0.00 | 0.04 | 0.18 |
| Anaerobutyricum hallii | −0.12 | −0.02 | 1.16 | 1.47 | 0.01 | 0.01 | 0.08 * |
| Lactonifactor longoviformis | −0.10 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Bifidobacterium catenulatum | −0.10 | - | 0.00 | 0.06 | 0.00 | 0.12 | 0.21 |
| Slackia isoflavoniconvertens | −0.09 | - | −0.01 | 0.04 | −0.01 | 0.73 | 0.75 |
| Blautia schinkii | −0.09 | - | −0.01 | 0.11 | −0.01 | 0.11 | 0.21 |
| Streptococcus saliviloxodontae | −0.09 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Anaerotignum aminivorans | −0.09 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Peptacetobacter hiranonis | −0.09 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Streptococcus koreensis | −0.09 | - | 0.00 | 0.04 | 0.00 | 0.12 | 0.21 |
| Bifidobacterium pseudolongum | −0.08 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Bifidobacterium adolescentis | −0.07 | - | 0.22 | 1.01 | 0.00 | 0.61 | 0.65 |
| Lachnospira eligens | 0.06 | - | −0.26 | −0.64 | −0.01 | 0.85 | 0.86 |
| Bifidobacterium choerinum | −0.06 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Romboutsia timonensis | −0.05 | - | 0.95 | 1.18 | −0.01 | 0.01 | 0.01 * |
| Holdemanella biformis | −0.05 | - | 0.08 | 0.61 | −0.01 | 0.65 | 0.69 |
| Parabacteroides distasonis | 0.04 | - | 0.15 | −0.60 | 0.01 | 0.01 | 0.08 * |
| Defluviitalea saccharophila | −0.04 | - | −0.01 | 0.01 | 0.01 | 0.07 | 0.21 |
| Gabonia massiliensis | 0.04 | - | −0.02 | −0.04 | 0.01 | 0.08 | 0.21 |
| Dorea longicatena | −0.02 | - | 0.97 | 1.61 | −0.01 | 0.01 | 0.08 * |
| Bifidobacterium longum | −0.01 | - | 0.49 | 1.86 | −0.01 | 0.48 | 0.56 |
| Streptococcus sanguinis | −0.01 | - | 0.00 | 0.01 | 0.00 | 0.12 | 0.21 |
| Streptococcus mitis | −0.01 | - | 0.01 | 0.04 | −0.01 | 0.49 | 0.56 |
| Solibacillus isronensis | −0.01 | - | 0.01 | 0.03 | −0.01 | 0.16 | 0.24 |
| Anaerostipes hadrus | - | −0.63 | 2.69 | 1.18 | −0.01 | 0.01 | 0.01 * |
| Erysipelatoclostridium ramosum | - | −0.40 | 0.31 | 0.01 | −0.01 | 0.02 | 0.12 |
| Arthrobacter citreus | - | −0.35 | 0.44 | 0.05 | 0.01 | 0.16 | 0.24 |
| Bifidobacterium pseudocatenulatum | - | −0.30 | 0.91 | 0.33 | 0.01 | 0.26 | 0.36 |
| Escherichia coli | - | −0.21 | 0.42 | −0.05 | 0.01 | 0.21 | 0.31 |
| Lacrimispora saccharolytica | - | −0.15 | 0.16 | 0.03 | 0.00 | 0.06 | 0.21 |
| Flavonifractor plautii | - | −0.15 | 0.68 | 0.11 | 0.01 | 0.17 | 0.24 |
| Megasphaera indica | - | −0.13 | 0.46 | 0.03 | −0.01 | 0.07 | 0.21 |
| Prevotella disiens | - | −0.13 | 0.02 | 0.01 | 0.00 | 0.32 | 0.41 |
| Phocaeicola coprocola | - | −0.13 | 0.84 | −0.01 | −0.01 | 0.61 | 0.65 |
| Desulfovibrio simplex | - | −0.09 | 0.23 | −0.01 | −0.01 | 0.49 | 0.56 |
| Clostridium saudiense | - | −0.08 | 0.85 | 0.46 | −0.01 | 0.01 | 0.08 * |
| Flintibacter butyricus | - | −0.08 | 0.25 | −0.03 | 0.01 | 0.16 | 0.24 |
| Peptoniphilus asaccharolyticus | - | −0.07 | 0.06 | 0.01 | 0.00 | 0.32 | 0.41 |
| Granulicatella adiacens | - | −0.07 | 0.01 | 0.01 | −0.01 | 0.28 | 0.37 |
| Anaerotruncus rubiinfantis | - | −0.07 | 0.14 | 0.04 | 0.01 | 0.94 | 0.94 |
| Enterococcus hermanniensis | - | −0.07 | 0.65 | 0.00 | 0.00 | 0.12 | 0.21 |
| Anaerostipes butyraticus | - | −0.07 | 0.51 | −0.04 | −0.01 | 0.04 | 0.18 |
| Streptococcus lactarius | - | −0.05 | 0.12 | 0.00 | 0.00 | 0.12 | 0.21 |
| Clostridium saccharogumia | - | −0.05 | 0.12 | −0.05 | −0.01 | 0.11 | 0.21 |
| Clostridium Disporicum | - | −0.05 | 0.26 | 0.00 | 0.00 | 0.12 | 0.21 |
| Dialister succinatiphilus | - | 0.04 | −0.59 | −0.21 | −0.01 | 0.41 | 0.51 |
| Eubacterium eligens | - | −0.04 | 0.85 | −0.03 | −0.01 | 0.49 | 0.56 |
| Actinomyces odontolyticus | - | −0.03 | 0.03 | 0.00 | 0.00 | 0.12 | 0.21 |
| Ruminococcus lactaris | - | −0.03 | 0.30 | −0.03 | −0.01 | 0.54 | 0.61 |
| Anaerovibrio lipolyticus | - | −0.02 | 0.12 | 0.00 | 0.00 | 0.12 | 0.21 |
| Butyricimonas synergistica | - | −0.01 | 0.06 | 0.00 | 0.00 | 0.12 | 0.21 |
| Clostridium jeddahense | - | −0.01 | 0.01 | −0.01 | −0.01 | 0.27 | 0.36 |
| Duodenibacillus massiliensis | - | 0.01 | −0.04 | −0.01 | 0.01 | 0.08 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Renzo, L.; Frank, G.; Pala, B.; Cianci, R.; La Placa, G.; Raffaelli, G.; Palma, R.; Peluso, D.; De Lorenzo, A.; Gualtieri, P.; et al. Effects of Italian Mediterranean Organic Diet on the Gut Microbiota: A Pilot Comparative Study with Conventional Products and Free Diet. Microorganisms 2025, 13, 1694. https://doi.org/10.3390/microorganisms13071694
Di Renzo L, Frank G, Pala B, Cianci R, La Placa G, Raffaelli G, Palma R, Peluso D, De Lorenzo A, Gualtieri P, et al. Effects of Italian Mediterranean Organic Diet on the Gut Microbiota: A Pilot Comparative Study with Conventional Products and Free Diet. Microorganisms. 2025; 13(7):1694. https://doi.org/10.3390/microorganisms13071694
Chicago/Turabian StyleDi Renzo, Laura, Giulia Frank, Barbara Pala, Rossella Cianci, Giada La Placa, Glauco Raffaelli, Roselisa Palma, Daniele Peluso, Antonino De Lorenzo, Paola Gualtieri, and et al. 2025. "Effects of Italian Mediterranean Organic Diet on the Gut Microbiota: A Pilot Comparative Study with Conventional Products and Free Diet" Microorganisms 13, no. 7: 1694. https://doi.org/10.3390/microorganisms13071694
APA StyleDi Renzo, L., Frank, G., Pala, B., Cianci, R., La Placa, G., Raffaelli, G., Palma, R., Peluso, D., De Lorenzo, A., Gualtieri, P., & on behalf of Clinical Nutrition and Nutrigenomics Project Group. (2025). Effects of Italian Mediterranean Organic Diet on the Gut Microbiota: A Pilot Comparative Study with Conventional Products and Free Diet. Microorganisms, 13(7), 1694. https://doi.org/10.3390/microorganisms13071694











