Diversity and Metabolic Potential of Gut Bacteria in Dorcus hopei (Coleoptera: Lucanidae): Influence of Fungus and Rotten Wood Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Rearing Conditions
2.2. Sample Dissection and Microbial DNA Extraction
2.3. PCR Amplification and 16S rRNA Sequencing
2.4. Processing of Sequence Data
2.5. Statistical and Bioinformatics Analysis
3. Results
3.1. Larval Gut Bacterial Alpha Diversity
3.2. Intestinal Bacterial Community Structure
3.3. Prediction of Gut Bacterial Function of D. hopei Larvae on Different Diets
3.4. Network Analysis of Gut Bacteria in D. hopei Larvae on Different Diets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Che, L.; Li, Y.; Dan, L.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Kudo, R.; Masuya, H.; Endoh, R.; Kikuchi, T.; Ikeda, H. Gut bacterial and fungal communities in ground-dwelling beetles are associated with host food habit and habitat. ISME J. 2019, 13, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Shaikh, A.F.; Pawar, K.D.; Xie, R.; Sun, J.; Kandasamy, S.; Pandit, R.S. Evaluation of cellulose degrading bacteria isolated from the gut-system of cotton bollworm, Helicoverpa armigera and their potential values in biomass conversion. PeerJ 2021, 9, e11254. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R. Beneficial interactions between insects and gut bacteria. Indian J. Microbiol. 2009, 49, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Nishimiya, Y.; Sasaki, Y.C.; Tsuda, S. Discovery of hyperactive antifreeze protein from phylogenetically distant beetles questions its evolutionary origin. Int. J. Mol. Sci. 2021, 22, 3637. [Google Scholar] [CrossRef] [PubMed]
- Songvorawit, N.; Butcher, B.A.; Chaisuekul, C. Decaying wood preference of stag beetles (Coleoptera: Lucanidae) in a tropical dry-evergreen forest. Environ. Entomol. 2017, 46, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic beetles as indicator species for dead-wood amount and temperature in European beech forests. Ecol. Indic. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- Tanahashi, M.; Hawes, C.J. The presence of a mycangium in European Sinodendron cylindricum (Coleoptera: Lucanidae) and the associated yeast symbionts. J. Insect Sci. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, M.; Matsushita, N.; Togashi, K. Are stag beetles fungivorous? J. Insect Physiol. 2009, 55, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Barcoto, M.O.; Carlos-Shanley, C.; Fan, H.; Ferro, M.; Nagamoto, N.S.; Bacci, M.; Currie, C.R.; Rodrigues, A. Fungus-growing insects host a distinctive microbiota apparently adapted to the fungiculture environment. Sci. Rep. 2020, 10, 12384. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Prem Anand, A.A.; Vennison, S.J.; Sankar, S.G.; Gilwax Prabhu, D.I.; Vasan, P.T.; Raghuraman, T.; Jerome Geoffrey, C.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef]
- Kaczmarczyk-Ziemba, A.; Wagner, G.K.; Grzywnowicz, K.; Kucharczyk, M.; Zielińska, S. The microbiome profiling of fungivorous black tinder fungus beetle Bolitophagus reticulatus reveals the insight into bacterial communities associated with larvae and adults. PeerJ 2019, 7, e6852. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Qi, F.; Wang, Z. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kikuchi, Y. Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect Sci. 2020, 41, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Kaltenpoth, M. Beetle–bacterial symbioses: Endless forms most functional. Annu. Rev. Entomol. 2022, 67, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Choi, M.Y.; Kim, J.W.; Lee, S.; Ahn, J.; Song, J.; Kim, S.; Weon, H. Effects of diet type, developmental stage, and gut compartment in the gut bacterial communities of two Cerambycidae species (Coleoptera). J. Microbiol. 2017, 55, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.B.; Hsiao, E.Y. Microbiomes as sources of emergent host phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, S.; Peres, H.; Martins, N.; Serra, C.; Santos, R.A.; Monroig, Ó.; Oliva-Teles, A. Use of black soldier fly (Hermetia illucens) larvae meal in diets for gilthead seabream juveniles: Effects on growth-related gene expression, intermediary metabolism, digestive enzymes, and gut microbiota modulation. Aquaculture 2024, 580, 740357. [Google Scholar] [CrossRef]
- Xin, L.; Chen, Y.; Rong, W.; Qin, Y.; Li, X.; Guan, D. Gut Microbiota Analysis in Silkworms (Bombyx mori) Provides Insights into Identifying Key Bacterials for Inclusion in Artificial Diet Formulations. Animals 2024, 14, 1261. [Google Scholar] [CrossRef] [PubMed]
- Kuranouchi, T.; Nakamura, T.; Shimamura, S.; Kojima, H.; Goka, K.; Okabe, K.; Mochizuki, A. Nitrogen fixation in the stag beetle, Dorcus (Macrodorcus) rectus (Motschulsky) (Col., Lucanidae). J. Appl. Entomol. 2006, 130, 471–472. [Google Scholar] [CrossRef]
- Mohammed, W.S.; Ziganshina, E.E.; Shagimardanova, E.I.; Gogoleva, N.E.; Ziganshin, A.M. Comparison of intestinal bacterial and fungal communities across various xylophagous beetle larvae (Coleoptera: Cerambycidae). Sci. Rep. 2018, 8, 10073. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xiang, X.; Wan, X. Divergence in gut bacterial community among life stages of the rainbow stag beetle Phalacrognathus muelleri (Coleptera: Lucanidae). Insects 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Bin, X.; Wang, P.; Shen, Y.; Xiang, X.; Jafir, M.; Wan, X. Investigation of fungal community structure in the gut of the stag beetle Dorcus hopei (Coleoptera; Lucanidae): Comparisons among developmental stages. Microb. Ecol. 2024, 87, 70. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chu, S.; Shi, Z.; You, R.; Chen, H. Marked variations in diversity and functions of gut microbiota between wild and domestic stag beetle Dorcus hopei hopei. BMC Microbiol. 2024, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Han, S.H.; Park, S.J. Development of Seven Microsatellite Markers Using Next Generation Sequencing for the Conservation on the Korean Population of Dorcus hopei (E. Saunders, 1854) (Coleoptera, Lucanidae). Int. J. Mol. Sci. 2015, 16, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Tang, M. Community structure of gut bacteria of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) larvae during overwintering stage. Sci. Rep. 2017, 7, 14242. [Google Scholar] [CrossRef] [PubMed]
- Morales-Jiménez, J.; Zúñiga, G.; Ramírez-Saad, H.C.; Hernández-Rodríguez, C. Gut-associated bacteria throughout the life cycle of the bark beetle Dendroctonus rhizophagus Thomas and Bright (Curculionidae: Scolytinae) and their cellulolytic activities. Microb. Ecol. 2012, 64, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Delalibera, I., Jr.; Handelsman, J.; Raffa, K.F. Contrasts in cellulolytic activities of gut microorganisms between the wood borer, Saperda vestita (Coleoptera: Cerambycidae), and the bark beetles, Ips pini and Dendroctonus frontalis (Coleoptera: Curculionidae). Environ. Entomol. 2005, 34, 541–547. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Xu, Z.Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wu, Y.; Zhang, F.; Kuang, Y.; Li, C.; Sun, R.; Hui, C. Evidence for cross transmission of pathogens between wild hooded cranes and domestic geese. J. Avian Biol. 2023, 2023, e03083. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamada, K.D.; Tomii, K.; Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 2018, 34, 2490–2492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, T.; Xu, M.; Zhao, Y.; Shen, P.; Wang, Y. 16S rRNA sequencing-based evaluation of the protective effects of Hua-Zhuo-Jie-Du on rats with chronic atrophic gastritis. BMC Complement. Med. Ther. 2022, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Gibert, C.; Escarguel, G. PER-SIMPER—A new tool for inferring community assembly processes from taxon occurrences. Glob. Ecol. Biogeogr. 2019, 28, 374–385. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, J.; Zhang, J.; Li, D.; Xu, C.; Kuzyakov, Y. Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Tillage Res. 2020, 196, 104491. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Shi, Y.; Bai, Y.; Chu, H. Soil pH correlates with the co-occurrence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil Biol. Biochem. 2018, 121, 185–192. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Maiques, E.; Angelova, A.; Carrasco, P.; Moya, A.; Latorre, A. Diet shapes the gut microbiota of the omnivorous cockroach Blattella germanica. FEMS Microbiol. Ecol. 2015, 91, fiv022. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiao, S.; Li, X.; Li, M. Bacterial and fungal gut communities of Agrilus mali at different developmental stages and fed different diets. Sci. Rep. 2018, 8, 15634. [Google Scholar] [CrossRef] [PubMed]
- Chouaia, B.; Goda, N.; Mazza, G.; Alali, S.; Florian, F.; Gionechetti, F.; Callegari, M.; Gonella, E.; Magoga, G.; Fusi, M.; et al. Developmental stages and gut microenvironments influence gut microbiota dynamics in the invasive beetle Popillia japonica Newman (Coleoptera: Scarabaeidae). Environ. Microbiol. 2019, 21, 4343–4359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhang, S.; Zhang, Y.; Cao, Y.; Wang, L. The effect of pine wood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle on intestinal bacterial community of insect vector Monochamus saltuarius (Coleoptera: Cerambycidae). Forests 2022, 13, 1673. [Google Scholar] [CrossRef]
- Berasategui, A.; Axelsson, K.; Nordlander, G.; Schmidt, A.; Borg-Karlson, A.-K.; Gershenzon, J.; Terenius, O.; Kaltenpoth, M. The gut microbiota of the pine weevil is similar across Europe and resembles that of other conifer-feeding beetles. Mol. Ecol. 2016, 25, 4014–4031. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zeng, J.; Cui, Z.; Geng, S.; Song, X.; Zhang, F.; Su, X.; Li, H. Distinct gut bacterial composition in Anoplophora glabripennis reared on two host plants. Front. Microbiol. 2023, 14, 1199994. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Ashraf, M.Z.; Modlinger, R.; Synek, J.; Schlyter, F.; Roy, A. Unravelling the gut bacteriome of Ips (Coleoptera: Curculionidae: Scolytinae): Identifying core bacterial assemblage and their ecological relevance. Sci. Rep. 2020, 10, 18572. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Hofman, C.A.; Netshifhefhe, S.R.; Duncan, F.D.; Honap, T.P.; Lesnik, J.; Lewis, C.M. Taxonomic features and comparisons of the gut microbiome from two edible fungus-farming termites (Macrotermes falciger; M. natalensis) harvested in the Vhembe district of Limpopo, South Africa. BMC Microbiol. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Beza-Beza, C.F.; Mikaelyan, A. Wood fibers are a crucial microhabitat for cellulose- and xylan- degrading bacteria in the hindgut of the wood-feeding beetle Odontotaenius disjunctus. Front. Microbiol. 2023, 14, 1173696. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, C.; Luo, X.; Wulamu, D.; Zhan, S.; Huang, Y.; Yang, H. Dynamics of the intestinal bacterial community in black soldier fly larval guts and its influence on insect growth and development. Insect Sci. 2023, 30, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Wang, X.; Wang, G.; Shang, S.; Dou, Z.; Luo, Y. Gut lignocellulose activity and microbiota in Asian longhorned beetle and their predicted contribution to larval nutrition. Front. Microbiol. 2022, 13, 899865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional Molecular Ecological Networks. mBio 2010, 1, e00169-00110. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.M.; Relman, D.A. The application of ecological theory toward an understanding of the human microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, P.; Qin, Y.; Tu, Q.; Yang, Y.; He, Z.; Schadt, C.W.; Zhou, J. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol. 2016, 18, 205–218. [Google Scholar] [CrossRef] [PubMed]
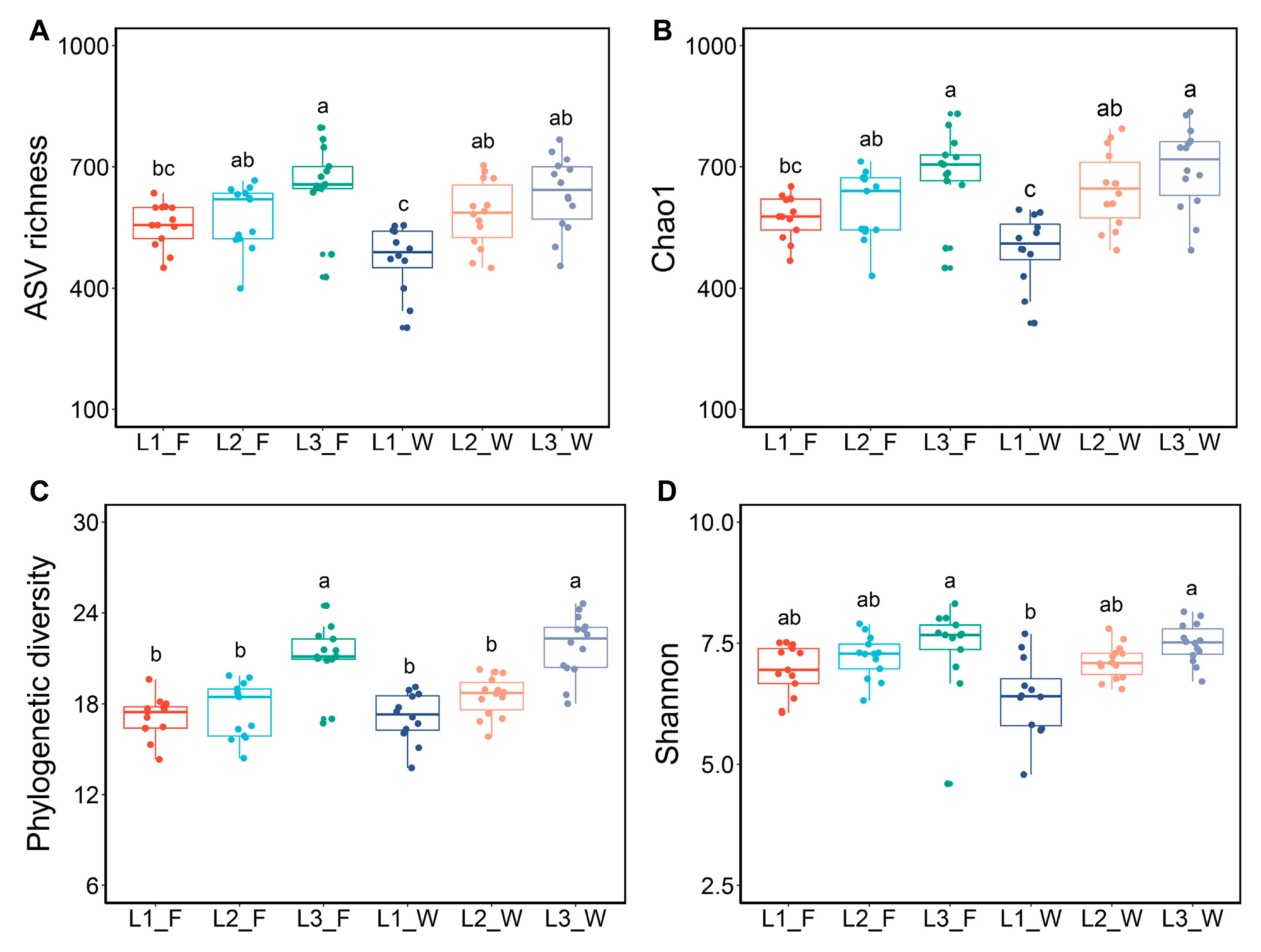
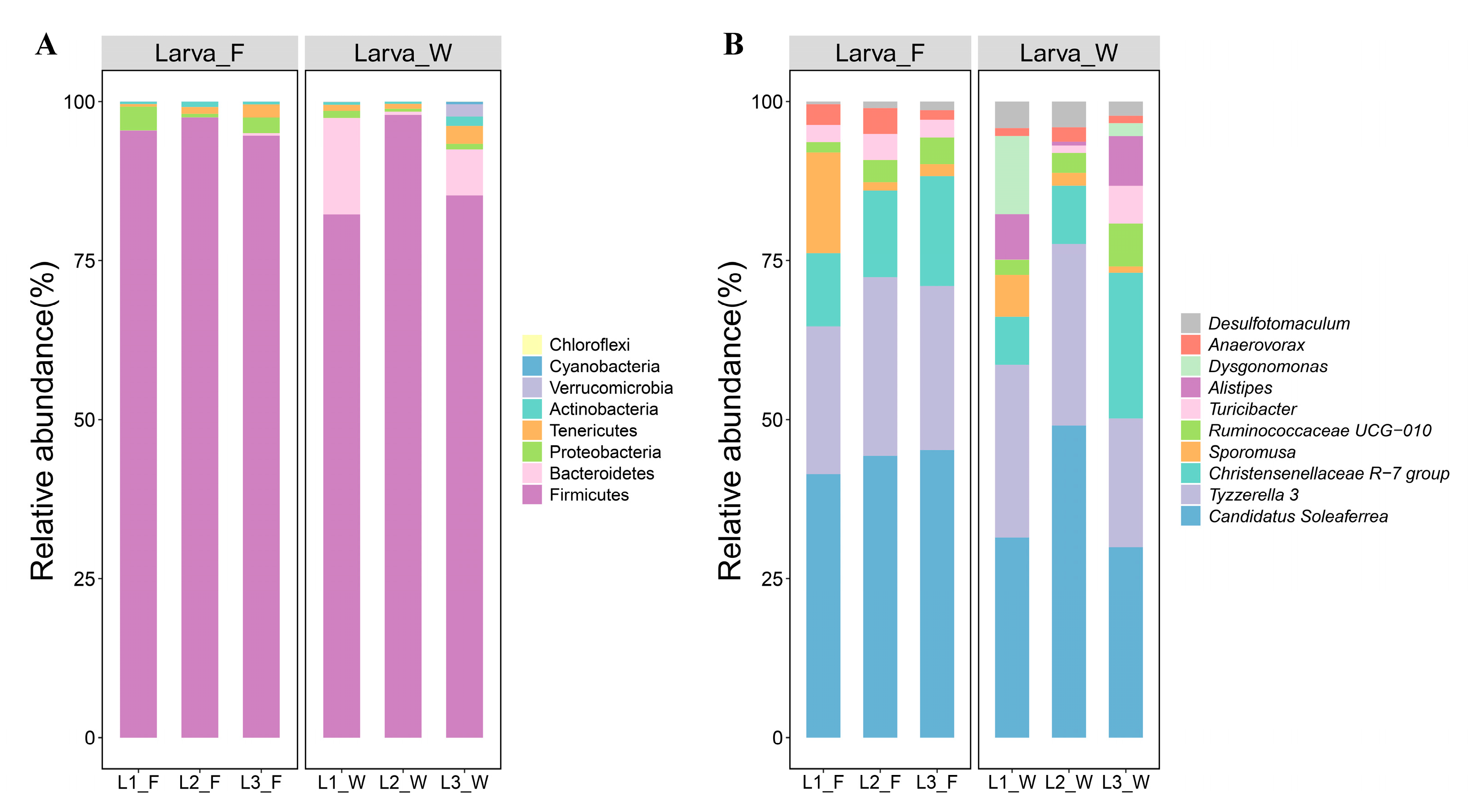

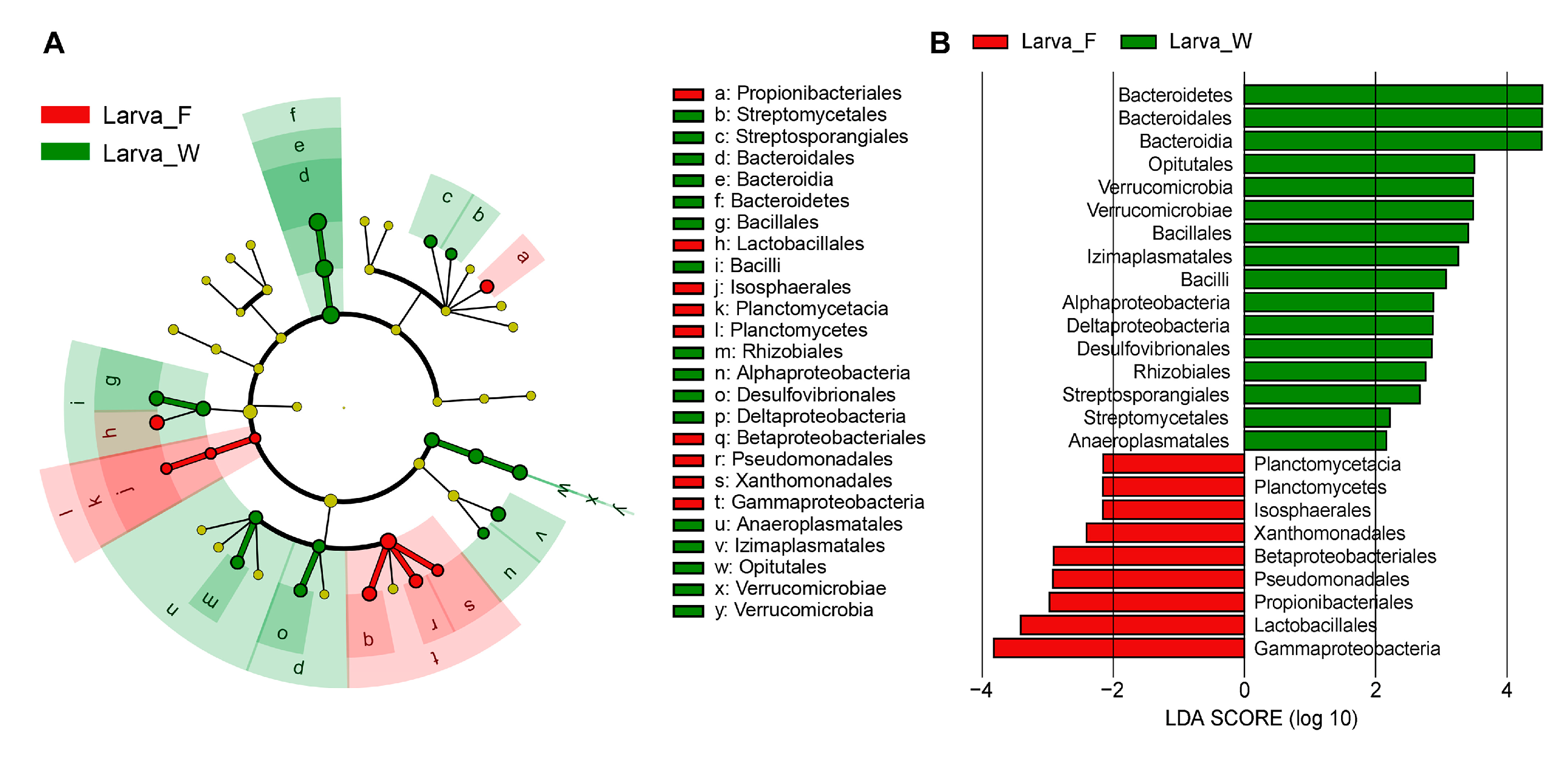
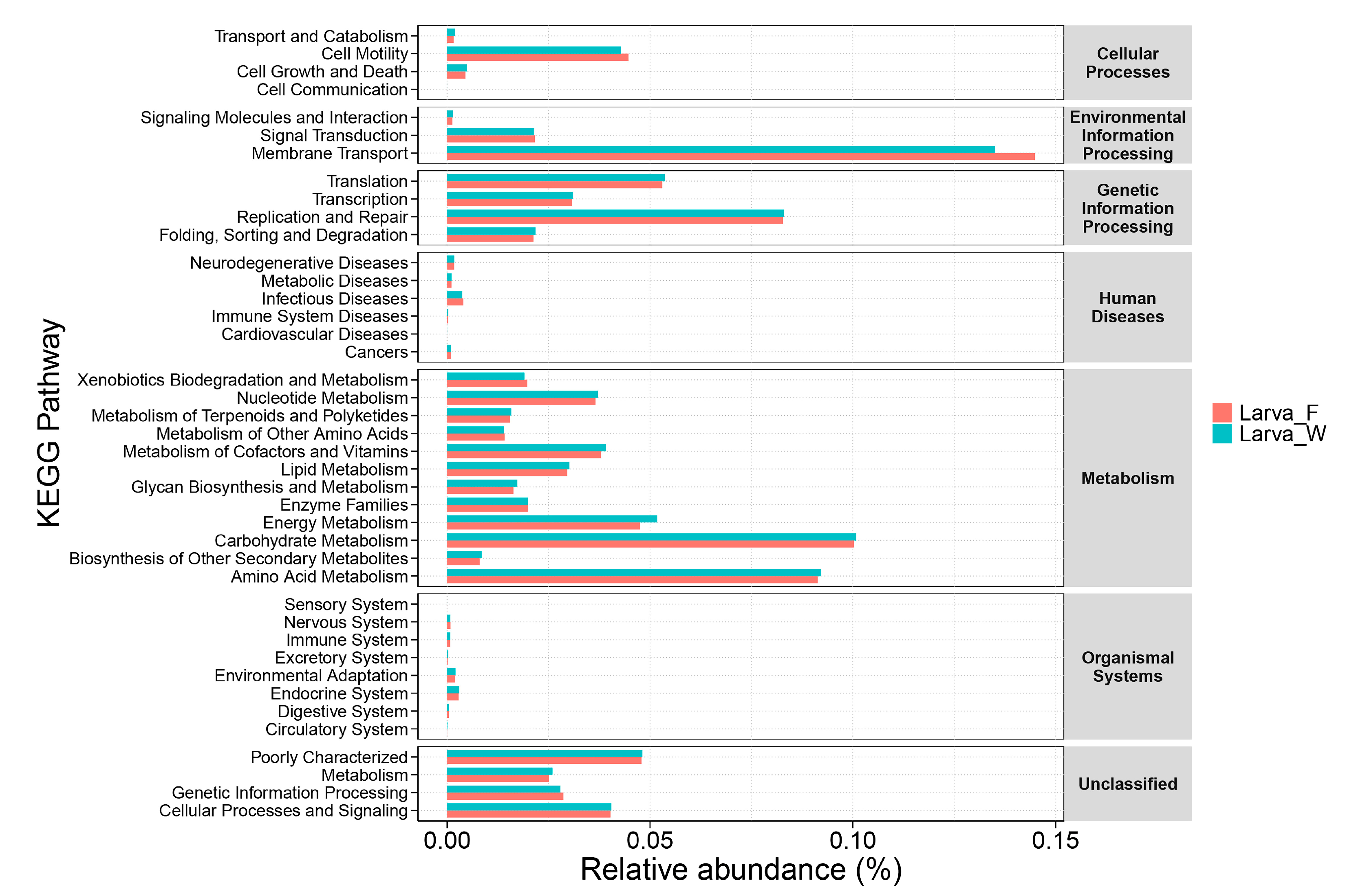
| Stages | ANOSIM | |
|---|---|---|
| r | p | |
| L1_F vs. L2_F | 0.129 | 0.023 |
| L1_F vs. L3_F | 0.272 | 0.001 |
| L2_F vs. L3_F | 0.350 | 0.001 |
| L1_W vs. L2_W | 0.324 | 0.001 |
| L1_W vs. L3_W | 0.251 | 0.003 |
| L2_W vs. L3_W | 0.356 | 0.001 |
| Larva_F vs. Larva_W | 0.211 | 0.001 |
| Taxonomy | Contribution (%) |
|---|---|
| Larva_F vs. Larva_W | |
| g__Candidatus Soleaferrea | 19.8 |
| g__Tyzzerella 3 | 11.3 |
| g__Christensenellaceae R-7 group | 9.8 |
| g__Sporomusa | 9.2 |
| g__Alistipes | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Bin, X.; Xiang, X.; Wan, X. Diversity and Metabolic Potential of Gut Bacteria in Dorcus hopei (Coleoptera: Lucanidae): Influence of Fungus and Rotten Wood Diets. Microorganisms 2025, 13, 1692. https://doi.org/10.3390/microorganisms13071692
Wang P, Bin X, Xiang X, Wan X. Diversity and Metabolic Potential of Gut Bacteria in Dorcus hopei (Coleoptera: Lucanidae): Influence of Fungus and Rotten Wood Diets. Microorganisms. 2025; 13(7):1692. https://doi.org/10.3390/microorganisms13071692
Chicago/Turabian StyleWang, Pan, Xiaoyan Bin, Xingjia Xiang, and Xia Wan. 2025. "Diversity and Metabolic Potential of Gut Bacteria in Dorcus hopei (Coleoptera: Lucanidae): Influence of Fungus and Rotten Wood Diets" Microorganisms 13, no. 7: 1692. https://doi.org/10.3390/microorganisms13071692
APA StyleWang, P., Bin, X., Xiang, X., & Wan, X. (2025). Diversity and Metabolic Potential of Gut Bacteria in Dorcus hopei (Coleoptera: Lucanidae): Influence of Fungus and Rotten Wood Diets. Microorganisms, 13(7), 1692. https://doi.org/10.3390/microorganisms13071692







