Improving the Properties of Laccase Through Heterologous Expression and Protein Engineering
Abstract
1. Introduction
2. Hosts for Heterogeneous Expression
2.1. Heterogeneous Expression with Bacteria as Hosts
2.1.1. Cloning and Heterologous Expression
2.1.2. Enzyme Properties from Bacterial Hosts
2.1.3. Advantages and Disadvantages of Laccase Expression in Bacteria
2.2. Heterologous Expression in Fungi as Hosts
2.2.1. Cloning and Heterologous Expression in Fungi
2.2.2. Enzyme Properties from Fungal Hosts
2.2.3. Advantages and Disadvantages of Laccase Expression in Fungi
3. Comparison of Laccase Expression in Bacterial and Fungal
4. Modification of Catalytic Properties of Laccase
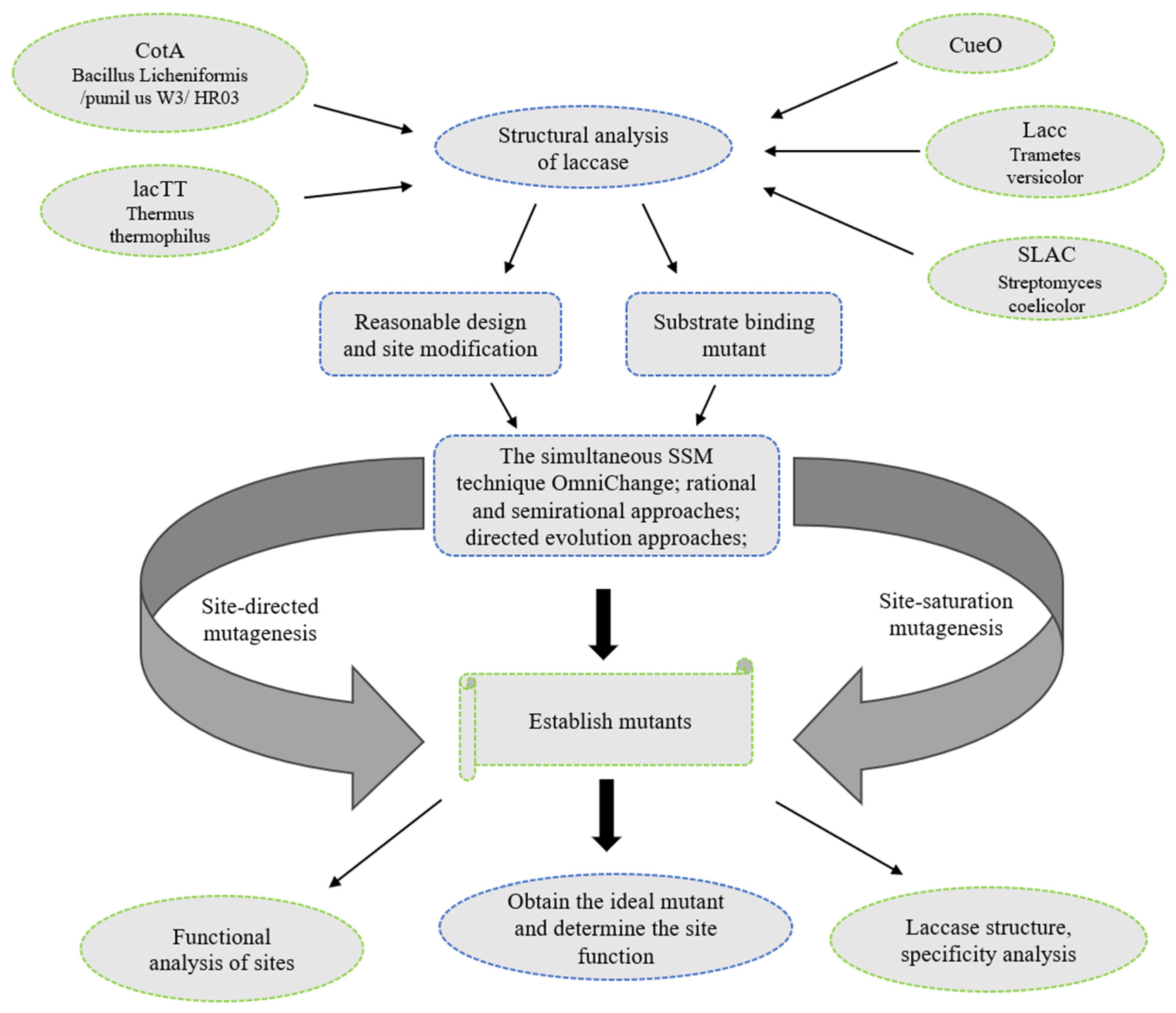
4.1. Laccase Modification Design
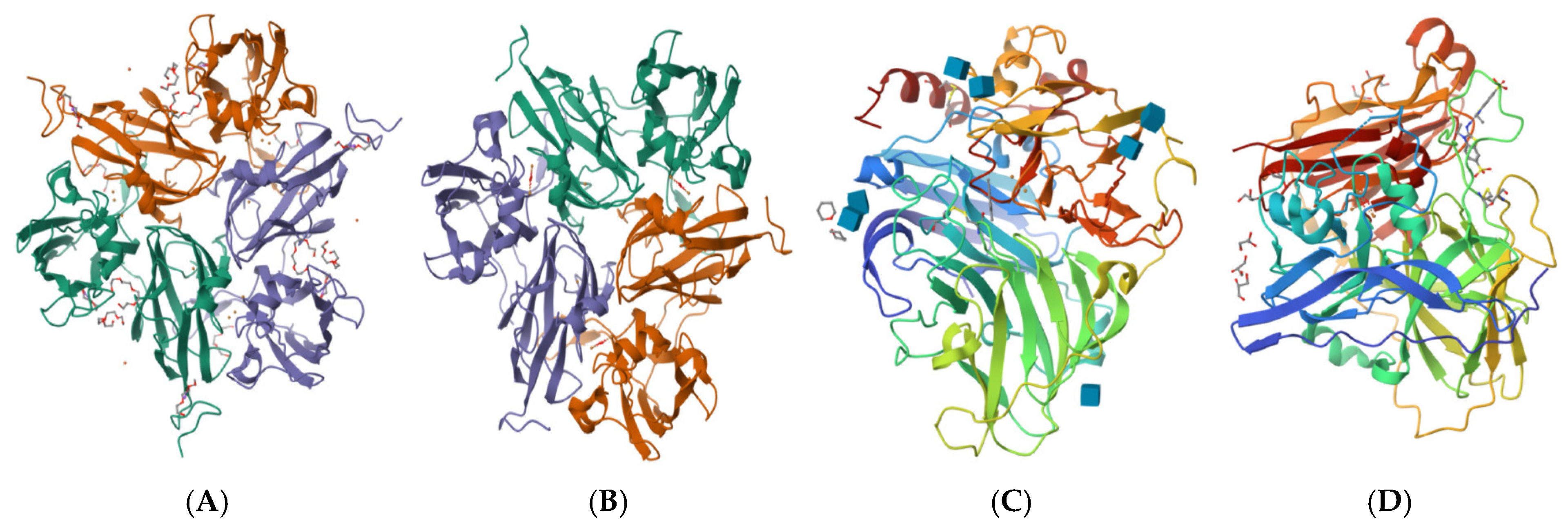
4.1.1. Laccase Structure
Structure of Laccase Protein
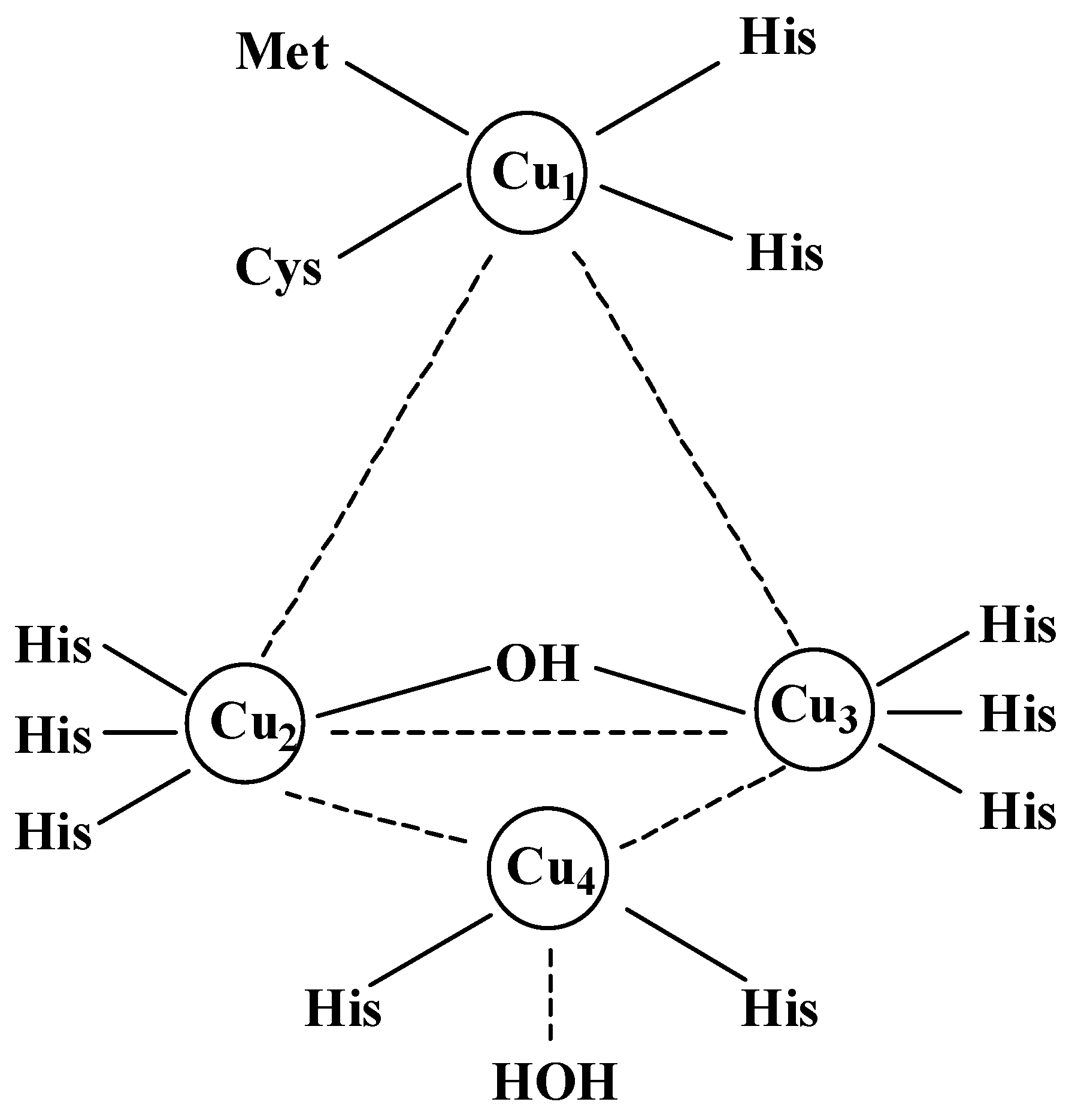
Copper Structure in Laccase
Laccase Gene and Function
4.1.2. Design Method
4.2. Laccase Modification
4.2.1. Protein Site Modification
Active Site
Hydrophobic Site
Glycosylation Site
4.3. Changes in Biochemical Properties of Laccase
4.3.1. Laccase pH Adaptability
4.3.2. Laccase Temperature Change
4.3.3. Substrate Specificity
5. Conclusions
6. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Fu, Y.; Shen, D.; Lindahl, K.; Wei, S. Characterization and identification of an archaeological “lacquer” pipe. Herit. Sci. 2024, 12, 142. [Google Scholar] [CrossRef]
- Dong, C.-D.; Tiwari, A.; Anisha, G.S.; Chen, C.-W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation*. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, F.; Berhe, M.; Zhou, R.; Li, D.; Li, H.; Yang, L.; Zhou, T.; Zhang, Y.; Wang, L.; et al. Genome-wide identification, classification, and expression profiling of LAC gene family in sesame. BMC Plant Biol. 2024, 24, 1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Owusu-Fordjour, M.; Xu, L.; Ding, Z.; Gu, Z. Immobilization of Laccase on Magnetic Chelator Nanoparticles for Apple Juice Clarification in Magnetically Stabilized Fluidized Bed. Front. Bioeng. Biotechnol. 2020, 8, 589. [Google Scholar] [CrossRef]
- Huang, W.; He, X.; Wu, J.; Ma, X.; Han, J.; Wang, L.; Wang, Y. The evaluation of deep eutectic solvents and ionic liquids as cosolvents system for improving cellulase properties. Ind. Crops Prod. 2023, 197, 116555. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.; Wang, M.; Xu, L.; Qian, J.; Guan, G.; Xu, B. Clarification of Sugarcane Juice Catalyzed by Magnetic Immobilized Laccase Intensified by Alternating Magnetic Field. Foods 2025, 14, 444. [Google Scholar] [CrossRef]
- Tulek, A.; Karatas, E.; Cakar, M.M.; Aydin, D.; Yilmazcan, O.; Binay, B. Optimisation of the Production and Bleaching Process for a New Laccase from Madurella mycetomatis, Expressed in Pichia pastoris: From Secretion to Yielding Prominent. Mol. Biotechnol. 2021, 63, 24–39. [Google Scholar] [CrossRef]
- Mekureyaw, M.F.; Junker, A.L.; Bai, L.; Zhang, Y.; Wei, Z.; Guo, Z. Laccase based per- and polyfluoroalkyl substances degradation: Status and future perspectives. Water Res. 2025, 271, 122888. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.; Ngo, H.H.; Guo, W.; Zhang, S. Advances in thermostable laccase and its current application in lignin-first biorefinery: A review. Bioresour. Technol. 2020, 298, 122511. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Bhatia, S.; Batra, N. Laccase: Sustainable production strategies, heterologous expression and potential biotechnological applications. Int. J. Biol. Macromol. 2024, 280, 135745. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Sun, X.; Wei, X.; Lin, Z.; Zhang, X.; Shi, J.; Battino, M.; Gong, Y.; Shi, B.; et al. SERS determination of hydroxy-α-sanshool in spicy hotpot seasoning: The strategy to restrain the interference of capsaicin and its mechanism. Food Chem. 2023, 413, 135644. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, H.; Wang, M.; Yu, X.; Cui, Y.; Xu, L.; Ma, A.; Ding, Z.; Huo, S.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; Wang, Y.; Cai, C.; Ayaz, A.; Alabbosh, K.F.; Khan, K.A.; Han, S.; Zhu, D. The Architecture of Adaptive Lignin Biosynthesis Navigating Environmental Stresses in Plants. J. Agron. Crop Sci. 2025, 211, e70012. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Tian, Y.; Li, Z.; Gao, J.; Yan, Y.; Peng, R.; Yao, Q. Heterologous expression and characterization of a laccase from Laccaria bicolorin in Pichia pastoris. Biotechnol. Biotechnol. Equip. 2015, 30, 63–68. [Google Scholar] [CrossRef]
- Singh, A.K.; Abellanas-Perez, P.; de Andrades, D.; Cornet, I.; Fernandez-Lafuente, R.; Bilal, M. Laccase-based biocatalytic systems application in sustainable degradation of pharmaceutically active contaminants. J. Hazard. Mater. 2025, 485, 136803. [Google Scholar] [CrossRef]
- Liu, J.; Hu, C.; Meng, X.; Sun, Y.; Zhao, B.; Lin, Z. Metal covalent organic frameworks-based laccase-like nanozyme for oxidative degradation and identification of phenolic pollutants. J. Hazard. Mater. 2025, 487, 137142. [Google Scholar] [CrossRef]
- Nazar, M.; Xu, Q.; Zahoor; Ullah, M.W.; Khan, N.A.; Iqbal, B.; Zhu, D. Integrated laccase delignification with improved lignocellulose recalcitrance for enhancing enzymatic saccharification of ensiled rice straw. Ind. Crops Prod. 2023, 202, 116987. [Google Scholar] [CrossRef]
- Thathola, P.; Melchor-Martínez, E.M.; Adhikari, P.; Hernández Martínez, S.A.; Pandey, A.; Parra-Saldívar, R. Laccase-mediated degradation of emerging contaminants: Unveiling a sustainable solution. Environ. Sci. Adv. 2024, 3, 1500–1512. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.-X.; Ye, Y.-F.; Ma, J.-X.; Li, X.; Si, J.; Cui, B.-K. Laccase immobilization and its degradation of emerging pollutants: A comprehensive review. J. Environ. Manag. 2024, 359, 120984. [Google Scholar] [CrossRef]
- Aghaee, M.; Salehipour, M.; Rezaei, S.; Mogharabi-Manzari, M. Bioremediation of organic pollutants by laccase-metal–organic framework composites: A review of current knowledge and future perspective. Bioresour. Technol. 2024, 406, 131072. [Google Scholar] [CrossRef]
- Dai, C.; Hou, Y.; Xu, H.; Umego, E.C.; Huang, L.; He, R.; Ma, H. Identification of a thermophilic protease-producing strain and its application in solid-state fermentation of soybean meal. J. Sci. Food Agric. 2022, 102, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-J.; Park, J.-H. Refolding, characterization, and dye decolorization ability of a highly thermostable laccase from Geobacillus sp. JS12. Protein Expr. Purif. 2020, 173, 105646. [Google Scholar] [CrossRef] [PubMed]
- Ece, S.; Lambertz, C.; Fischer, R.; Commandeur, U. Heterologous expression of a Streptomyces cyaneus laccase for biomass modification applications. Amb. Express 2017, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Fernandez, B.J.; Risso, V.A.; Rueda, A.; Sanchez-Ruiz, J.M.; Alcalde, M. Ancestral Resurrection and Directed Evolution of Fungal Mesozoic Laccases. Appl. Environ. Microbiol. 2020, 86, e00778-20. [Google Scholar] [CrossRef]
- He, R.; Xing, H.; Wang, Z.; Ding, W.; Zhu, P.; Liu, B.; Ma, H. Establishment of an Enzymatic Membrane Reactor for Angiotensin-Converting Enzyme Inhibitory Peptides Preparation from Wheat Germ Protein Isolates. J. Food Process Eng. 2016, 39, 296–305. [Google Scholar] [CrossRef]
- Yan, J.-K.; Pei, J.-J.; Ma, H.-L.; Wang, Z.-B.; Liu, Y.-S. Advances in antitumor polysaccharides from phellinus sensu lato: Production, isolation, structure, antitumor activity, and mechanisms. Crit. Rev. Food Sci. Nutr. 2017, 57, 1256–1269. [Google Scholar] [CrossRef]
- Shang, L.; Bai, X.; Chen, C.; Liu, L.; Li, M.; Xia, X.; Wang, Y. Isolation and identification of a Bacillus megaterium strain with ochratoxin A removal ability and antifungal activity. Food Control 2019, 106, 106743. [Google Scholar] [CrossRef]
- Ngea, G.L.N.; Yang, Q.; Tchabo, W.; Castoria, R.; Zhang, X.; Zhang, H. Leuconostoc mesenteroides subsp. mesenteroides LB7 isolated from apple surface inhibits P. expansum in vitro and reduces patulin in fruit juices. Int. J. Food Microbiol. 2021, 339, 109025. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Hu, J.; Chen, X.; Qiu, Y.; Shi, J.; Wang, G.; Xu, J. Isolation and characterization of Bacillus amyloliquefaciens MQ01, a bifunctional biocontrol bacterium with antagonistic activity against Fusarium graminearum and biodegradation capacity of zearalenone. Food Control 2021, 130, 108259. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Q.; Zheng, Z.; Zhou, J.; Cui, Y.; Jin, W.; Gao, R. Isolation of Protease-Producing Bacteria from Shrimp Paste and the Characteristics of Fermenting Catfish Paste. J. Aquat. Food Product Technol. 2022, 31, 332–343. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.; Yolandani; Ma, H.; Ashokkumar, M. Dynamic changes of microbial communities during natural solid-state fermentation of soybean meal and isolation of dominant bacteria for peptide production. Food Biosci. 2023, 56, 103154. [Google Scholar] [CrossRef]
- Mei, S.; Chen, X. Investigation into the anti-inflammatory mechanism of coffee leaf extract in LPS-induced Caco-2/U937 co-culture model through cytokines and NMR-based untargeted metabolomics analyses. Food Chem. 2023, 404, 134592. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Zhang, J.; Cui, L.; Lu, H.; Zhu, Y.; Zhao, Y.; Fan, S.; Xiao, X. Barley β-glucan inhibits digestion of soybean oil in vitro and lipid-lowering effects of digested products in cell co-culture model. Food Res. Int. 2023, 164, 112378. [Google Scholar] [CrossRef] [PubMed]
- Boasiako, T.A.; Ekumah, J.-N.; Yaqoob, S.; Aregbe, A.Y.; Li, Y.; Ashiagbor, K.; Lu, W.; Boateng, I.D.; Ma, Y. Synergistic effects of lactobacillus strains and Acetobacter pasteurianus on jujube puree’s product functionality and quality. Heliyon 2024, 10, e24447. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.; Imtiaz, A.; Awan, K.A.; Murtaza, M.S.; Mubeen, B.; Yinka, A.A.; Boasiako, T.A.; Alsulami, T.; Rehman, A.; Khalifa, I.; et al. Impact of fermentation through synergistic effect of different lactic acid bacteria (mono and co-cultures) on metabolic and sensorial profile of mulberry juice. J. Food Meas. Charact. 2024, 18, 9364–9384. [Google Scholar] [CrossRef]
- Mei, S.; Kitts, D.D.; Chen, X. Coffee leaf polyphenol-rich extracts alleviated lipopolysaccharide-induced intestinal barrier dysfunction: Insights from a Caco-2/U937 co-culture model. Food Biosci. 2024, 61, 104639. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, S.; Jain, K.K.; Sharma, K.K. Metabolic coupling in the co-cultured fungal-yeast suite of Trametes ljubarskyi and Rhodotorula mucilaginosa leads to hypersecretion of laccase isozymes. Fungal Biol. 2019, 123, 913–926. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Li, G.; Liu, K.; Liu, Y.; He, J.; Lei, J. Preparation and application of a chemically modified laccase and copper phosphate hybrid flower-like biocatalyst. Biochem. Eng. J. 2019, 144, 235–243. [Google Scholar] [CrossRef]
- Tanaka, T.; Kondo, A. Cell-surface display of enzymes by the yeast Saccharomyces cerevisiae for synthetic biology. FEMS Yeast Res. 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Chen, Q. Lanthanide ion (Ln3+)-based upconversion sensor for quantification of food contaminants: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3531–3578. [Google Scholar] [CrossRef]
- Gao, X.; Ye, C.; Ma, H.; Zhang, Z.; Wang, J.; Zhang, Z.-H.; Zhao, X.; Ho, C.-T. Research Advances in Preparation, Stability, Application, and Possible Risks of Nanoselenium: Focus on Food and Food-Related Fields. J. Agric. Food Chem. 2023, 71, 8731–8745. [Google Scholar] [CrossRef]
- Dai, C.; Shu, Z.; Ma, C.; Yan, P.; Huang, L.; He, R.; Ma, H. Isolation of a surfactin-producing strain of Bacillus subtilis and evaluation of the probiotic potential and antioxidant activity of surfactin from fermented soybean meal. J. Sci. Food Agric. 2024, 104, 8469–8479. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Q.; Dhanasekaran, S.; Godana, E.A.; Zhang, X.; Yang, Q.; Zhao, L.; Zhang, H. The necrosis-inducing protein (NIP) gene contributes to Penicillium expansum virulence during postharvest pear infection. Food Res. Int. 2022, 158, 111562. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Dhanasekaran, S.; Yang, Q.; Ngea, G.L.N.; Godana, E.A.; Zhang, H. Degradation and stress response mechanism of Cryptococcus podzolicus Y3 on ochratoxin A at the transcriptional level. Lwt-Food Sci. Technol. 2022, 157, 113061. [Google Scholar] [CrossRef]
- Hailei, W.; Chaozhi, T.; Guangli, Y.; Ping, L. A novel membrane-surface liquid co-culture to improve the production of laccase from Ganoderma lucidum. Biochem. Eng. J. 2013, 80, 27–36. [Google Scholar] [CrossRef]
- Zhou, C.; Li, B.; Yang, W.; Liu, T.; Yu, H.; Liu, S.; Yang, Z. A Comprehensive Study on the Influence of Superheated Steam Treatment on Lipolytic Enzymes, Physicochemical Characteristics, and Volatile Composition of Lightly Milled Rice. Foods 2024, 13, 240. [Google Scholar] [CrossRef]
- Pullmann, P.; Weissenborn, M.J. Improving the Heterologous Production of Fungal Peroxygenases through an Episomal Pichia pastoris Promoter and Signal Peptide Shuffling System. ACS Synth. Biol. 2021, 10, 1360–1372. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Abdullatypov, A.V.; Larionova, A.P.; Trubitsin, I.V.; Alferov, S.V.; Ponamoreva, O.N.; Leontievsky, A.A. Expression of thermophilic two-domain laccase from Catenuloplanes japonicus in Escherichia coli and its activity against triarylmethane and azo dyes. PeerJ 2021, 9, e11646. [Google Scholar] [CrossRef]
- Xu, C.-L.; Zhu, C.-Y.; Li, Y.-N.; Gao, J.; Zhang, Y.-W. Heparinase III with High Activity and Stability: Heterologous Expression, Biochemical Characterization, and Application in Depolymerization of Heparin. J. Agric. Food Chem. 2024, 72, 3045–3054. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, B.; Bai, J.; Fan, S.; Daglia, M.; Li, J.; Zhao, Y.; He, Y.; Zhu, L.; Xiao, X. Heterologous expression and enzymatic characteristics of sulfatase from Lactobacillus plantarum dy-1. Food Funct. 2024, 15, 5439–5449. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Zhang, Y.; Xu, Y.; Chen, X.; Huang, W.; Li, P. The MYB transcriptional factor BrMYB108 regulates Auxin-mediated delayed leaf senescence in postharvest Pak Choi. Postharvest Biol. Technol. 2025, 219, 113181. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Bai, J.; Huang, M.; He, Y.; Zhao, Y.; Xiao, X. Enzymatic Mechanism of a β-Glucosidase from Lactiplantibacillus plantarum Dy-1 with Potential Applications in the Release of Bound Phenolics in Fermentation Barley. J. Agric. Food Chem. 2025, 73, 4164–4173. [Google Scholar] [CrossRef]
- He, L.; Yan, M.; Naeem, M.; Chen, M.; Chen, Y.; Ni, Z.; Chen, H. Enhancing Manganese Peroxidase: Innovations in Genetic Modification, Screening Processes, and Sustainable Agricultural Applications. J. Agric. Food Chem. 2024, 72, 26040–26056. [Google Scholar] [CrossRef]
- Zhang, Y.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, H. Overexpression of the SDR gene improves the ability of Meyerozyma guilliermondii to degrade patulin in pears and juices. Food Chem. 2023, 417, 135785. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, J.; Saeed, M.; Hussain, N.; Chen, X.; Guo, Z.; Yong, Y.; Chen, H. Molecular Modification Strategies of Nitrilase for Its Potential Application in Agriculture. J. Agric. Food Chem. 2024, 72, 15106–15121. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, S.; He, L.; Feng, Y.; Saeed, M.; Ma, Y.; Ni, Z.; Zhu, D.; Chen, H. High-throughput screening and identification of lignin peroxidase based on spore surface display of Bacillus subtilis. J. Sci. Food Agric. 2025, 105, 2179–2189. [Google Scholar] [CrossRef]
- Khodakarami, A.; Goodarzi, N.; Hoseinzadehdehkordi, M.; Amani, F.; Khodaverdian, S.; Khajeh, K.; Ghazi, F.; Ranjbar, B.; Amanlou, M.; Dabirmanesh, B. Rational design toward developing a more efficient laccase: Catalytic efficiency and selectivity. Int. J. Biol. Macromol. 2018, 112, 775–779. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, T.; Zhong, Q.; Wang, G. Crystal structure of CotA laccase complexed with 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) at a novel binding site. Acta Crystallogr. F Struct. Biol. Commun. 2016, 72, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Zúñiga, L.D.; Millán-Pacheco, C.; Viniegra-González, G.; Villegas, E.; Arregui, L.; Rojo-Domínguez, A. Molecular dynamics on laccase from Trametes versicolor to examine thermal stability induced by salt bridges. Chem. Phys. 2019, 517, 253–264. [Google Scholar] [CrossRef]
- Yang, M.; Yun, J.; Zhang, H.; Magocha, T.A.; Zabed, H.; Xue, Y.; Fokum, E.; Sun, W.; Qi, X. Genetically Engineered Strains: Application and Advances for 1,3-Propanediol Production from Glycerol. Food Technol. Biotechnol. 2018, 56, 3–15. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, S.; Capocasa, F.; Battino, M.; Mazzoni, L.; Mezzetti, B. Improved nutritional quality in fruit tree species through traditional and biotechnological approaches. Trends Food Sci. Technol. 2021, 117, 125–138. [Google Scholar] [CrossRef]
- Ekumah, J.-N.; Ma, Y.; Akpabli-Tsigbe, N.D.K.; Kwaw, E.; Ma, S.; Hu, J. Global soil distribution, dietary access routes, bioconversion mechanisms and the human health significance of selenium: A review. Food Biosci. 2021, 41, 100960. [Google Scholar] [CrossRef]
- Movahedi, A.; Aghaei-Dargiri, S.; Barati, B.; Kadkhodaei, S.; Wei, H.; Sangari, S.; Yang, L.; Xu, C. Plant Immunity Is Regulated by Biological, Genetic, and Epigenetic Factors. Agronomy 2022, 12, 2790. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Razack, S.A.; Ponpandian, L.N.; Zhang, G.; Yun, J.; Huang, J.; Lee, D.; Li, X.; Dou, Y.; Qi, X. Microbial hosts for production of D-arabitol: Current state-of-art and future prospects. Trends Food Sci. Technol. 2022, 120, 100–110. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.S.S.; Xue, S.; Islam, F.; Ikram, A.U.; Abdullah, M.; Liu, S.; Tappiban, P.; Chen, J. A comprehensive overview of omics-based approaches to enhance biotic and abiotic stress tolerance in sweet potato. Hortic. Res. 2024, 11, uhae014. [Google Scholar] [CrossRef]
- Gupta, V.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Functional substitution of domain 3 (T1 copper center) of a novel laccase with Cu ions. Int. J. Biol. Macromol. 2019, 123, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase Production from Lignocellulosic Agricultural Wastes by Solid-State Fermentation: A Review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef]
- Aza, P.; de Salas, F.; Molpeceres, G.; Rodriguez-Escribano, D.; de la Fuente, I.; Camarero, S. Protein Engineering Approaches to Enhance Fungal Laccase Production in S. cerevisiae. Int. J. Mol. Sci. 2021, 22, 1157. [Google Scholar] [CrossRef]
- Gupta, N.; Farinas, E.T. Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores. Protein Eng. Des. Sel. 2010, 23, 679–682. [Google Scholar] [CrossRef]
- Bu, T.; Yang, R.; Zhang, Y.; Cai, Y.; Tang, Z.; Li, C.; Wu, Q.; Chen, H. Improving decolorization of dyes by laccase from Bacillus licheniformis by random and site-directed mutagenesis. PeerJ 2020, 8, e10267. [Google Scholar] [CrossRef] [PubMed]
- Kwiatos, N.; Jedrzejczak-Krzepkowska, M.; Krzeminska, A.; Delavari, A.; Paneth, P.; Bielecki, S. Evolved Fusarium oxysporum laccase expressed in Saccharomyces cerevisiae. Sci. Rep. 2020, 10, 3244. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Li, Y.; Wang, F.; Li, S.; Shi, G.; Ding, Z. Comparative transcriptomics and transcriptional regulation analysis of enhanced laccase production induced by co-culture of Pleurotus eryngii var. ferulae with Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2020, 104, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, X.; Liu, B.; Battino, M.; Meng, X.; Zhang, F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biol. Technol. 2024, 215, 113006. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Feng, F. Combined strategies for improving production of a thermo-alkali stable laccase in Pichia pastoris. Electron. J. Biotechnol. 2017, 28, 7–13. [Google Scholar] [CrossRef]
- Hiraishi, T.; Tachibana, K.; Asakura, N.; Abe, H.; Maeda, M. Enhanced expression of a recombinant multicopper oxidase, CueO, from Escherichia coli and its laccase activity towards aromatic substrates. Polym. Degrad. Stab. 2019, 164, 1–8. [Google Scholar] [CrossRef]
- Caloglu, B.; Binay, B. Utilization potential of agro-industrial by-products and waste sources: Laccase production in bioreactor with Pichia pastoris. Biochem. Eng. J. 2023, 193, 108854. [Google Scholar] [CrossRef]
- Nasoohi, N.; Khajeh, K.; Mohammadian, M.; Ranjbar, B. Enhancement of catalysis and functional expression of a bacterial laccase by single amino acid replacement. Int. J. Biol. Macromol. 2013, 60, 56–61. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Ponpandian, L.N.; Zhang, G.; Yun, J.; Qi, X. Harnessing L-arabinose isomerase for biological production of D-tagatose: Recent advances and its applications. Trends Food Sci. Technol. 2021, 107, 16–30. [Google Scholar] [CrossRef]
- Tang, X.; Ravikumar, Y.; Zhang, G.; Yun, J.; Zhao, M.; Qi, X. D-allose, a typical rare sugar: Properties, applications, and biosynthetic advances and challenges. Crit. Rev. Food Sci. Nutr. 2024, 65, 2785–2812. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Adeyanju, A.A.; Onyeaka, H.; Nwonuma, C.O.; Olaniran, A.F.; Alejolowo, O.O.; Inyinbor, A.A.; Oluyori, A.P.; Zhou, C. A review on rebaudioside M: The next generation steviol glycoside and noncaloric sweetener. J. Food Sci. 2024, 89, 6946–6965. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Liaqat, F.; Khazi, M.I.; Liaqat, N.; Nawaz, M.Z.; Zhu, D. Lignin biotransformation: Advances in enzymatic valorization and bioproduction strategies. Ind. Crops Prod. 2024, 216, 118759. [Google Scholar] [CrossRef]
- Wu, Z.; Li, P.; Chen, Y.; Chen, X.; Feng, Y.; Guo, Z.; Zhu, D.; Yong, Y.; Chen, H. Rational Design for Enhancing Cellobiose Dehydrogenase Activity and Its Synergistic Role in Straw Degradation. J. Agric. Food Chem. 2024, 72, 24620–24631. [Google Scholar] [CrossRef]
- Yang, Y.; Ghatge, S.; Hur, H.-G. Improvement of thermoalkaliphilic laccase (CtLac) by a directed evolution and application to lignin degradation. Appl. Microbiol. Biotechnol. 2023, 107, 273–286. [Google Scholar] [CrossRef]
- Pardo, I.; Camarero, S. Exploring the Oxidation of Lignin-Derived Phenols by a Library of Laccase Mutants. Molecules 2015, 20, 15929–15943. [Google Scholar] [CrossRef]
- Mateljak, I.; Alcalde, M. Engineering a Highly Thermostable High-Redox Potential Laccase. ACS Sustain. Chem. Eng. 2021, 9, 9632–9637. [Google Scholar] [CrossRef]
- Popovic, M.C.; Stanisic, M.; Prodanovic, R. State of the Art Technologies for High Yield Heterologous Expression and Production of Oxidoreductase Enzymes: Glucose Oxidase, Cellobiose Dehydrogenase, Horseradish Peroxidase, and Laccases in Yeasts P. pastoris and S. cerevisiae. Fermentation 2024, 10, 93. [Google Scholar] [CrossRef]
- Stanzione, I.; Pezzella, C.; Giardina, P.; Sannia, G.; Piscitelli, A. Beyond natural laccases: Extension of their potential applications by protein engineering. Appl. Microbiol. Biotechnol. 2020, 104, 915–924. [Google Scholar] [CrossRef]
- Antosova, Z.; Sychrova, H. Yeast Hosts for the Production of Recombinant Laccases: A Review. Mol. Biotechnol. 2016, 58, 93–116. [Google Scholar] [CrossRef]
- Martinkova, L.; Kotik, M.; Markova, E.; Homolka, L. Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: A review. Chemosphere 2016, 149, 373–382. [Google Scholar] [CrossRef]
- Bertrand, B.; Martinez-Morales, F.; Trejo-Hernandez, M.R. Upgrading Laccase Production and Biochemical Properties: Strategies and Challenges. Biotechnol. Prog. 2017, 33, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Estrada, C.; de Jesus Rostro-Alanis, M.; Munoz-Gutierrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldivar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation—A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Arya, S.K. Utility of laccase in pulp and paper industry: A progressive step towards the green technology. Int. J. Biol. Macromol. 2019, 134, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-Y. Discoloration of indigo dyes by eco-friendly biocatalysts. Dye. Pigment. 2021, 184, 108749. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. Biotechnol. 2019, 95, 481–494. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Hever, E.; Santhanam, V.; Alberi, S.; Dhara, A.; Bols, M.; Nasheuer, H.-P.; Murphy, P.V. Synthesis of C-glycoside analogues of isopropyl β-D-1-thiogalactopyranoside (IPTG) and 1-β-D-galactopyranosyl-2-methylpropane. Conformational analysis and evaluation as inhibitors of the lac repressor in E. coli and as galactosidase inhibitors. Org. Biomol. Chem. 2024, 22, 7460–7477. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, M.; Lu, Z.; Lu, F.; Zhang, C. Heterologous production of a temperature and pH-stable laccase from Bacillus vallismortis fmb-103 in Escherichia coli and its application. Process Biochem. 2017, 55, 77–84. [Google Scholar] [CrossRef]
- Wang, J.; Yu, S.; Li, X.; Feng, F.; Lu, L. High-level expression of Bacillus amyloliquefaciens laccase and construction of its chimeric variant with improved stability by domain substitution. Bioprocess Biosyst. Eng. 2020, 43, 403–411. [Google Scholar] [CrossRef]
- Zhang, T.; Han, M.; Yu, H.; Meng, S.; Zhang, Z.; An, Q. Effects of single and mixed metal ions on laccase activities of Pleurotus ostreatus CY 568 in submerged fermentation. Mycosystema 2024, 43, 230201. [Google Scholar]
- Trubitsina, L.I.; Tishchenko, S.V.; Gabdulkhakov, A.G.; Lisov, A.V.; Zakharova, M.V.; Leontievsky, A.A. Structural and functional characterization of two-domain laccase from Streptomyces viridochromogenes. Biochimie 2015, 112, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, A.; Golestani, A.; Lakzaei, M.; Rasi Varaei, S.S.; Aminian, M. Expression optimization, purification, and functional characterization of cholesterol oxidase from Chromobacterium sp. DS1. PLoS ONE 2019, 14, e0212217. [Google Scholar] [CrossRef] [PubMed]
- Trubitsina, L.I.; Lisov, A.V.; Belova, O.V.; Trubitsin, I.V.; Demin, V.V.; Konstantinov, A.I.; Zavarzina, A.G.; Leontievsky, A.A. Transformation of low molecular compounds and soil humic acid by two domain laccase of Streptomyces puniceus in the presence of ferulic and caffeic acids. PLoS ONE 2020, 15, e0239005. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Bibra, M.; Chadha, B.S.; Sani, R.K. Enhanced hydrolysis of lignocellulosic biomass with doping of a highly thermostable recombinant laccase. Int. J. Biol. Macromol. 2019, 137, 232–237. [Google Scholar] [CrossRef]
- Yang, X.; Gu, C.; Lin, Y. A novel fungal laccase from Sordaria macrospora k-hell: Expression, characterization, and application for lignin degradation. Bioprocess Biosyst. Eng. 2020, 43, 1133–1139. [Google Scholar] [CrossRef]
- Ahlawat, S.; Singh, D.; Yadav, A.; Singh, A.K.; Virdi, J.S.; Sharma, K.K. Proteomic analysis reveals the damaging role of low redox laccase from Yersinia enterocolitica strain 8081 in the midgut of Helicoverpa armigera. Biotechnol. Lett. 2020, 42, 2189–2210. [Google Scholar] [CrossRef]
- Mandic, M.; Djokic, L.; Nikolaivits, E.; Prodanovic, R.; O’Connor, K.; Jeremic, S.; Topakas, E.; Nikodinovic-Runic, J. Identification and Characterization of New Laccase Biocatalysts from Pseudomonas Species Suitable for Degradation of Synthetic Textile Dyes. Catalysts 2019, 9, 629. [Google Scholar] [CrossRef]
- Mohtashami, M.; Fooladi, J.; Haddad-Mashadrizeh, A.; Housaindokht, M. Molecular cloning, expression and characterization of poxa1b gene from Pleurotus ostreatus. Mol. Biol. Rep. 2019, 46, 981–990. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, K.K.; Bhardwaj, K.N.; Chakraborty, S.; Kuhad, R.C. Multiple Genes in a Single Host: Cost-Effective Production of Bacterial Laccase (cotA), Pectate Lyase (pel), and Endoxylanase (xyl) by Simultaneous Expression and Cloning in Single Vector in E. coli. PLoS ONE 2015, 10, e0144379. [Google Scholar] [CrossRef]
- Yang, L.-H.; Qiao, B.; Xu, Q.-M.; Liu, S.; Yuan, Y.; Cheng, J.-S. Biodegradation of sulfonamide antibiotics through the heterologous expression of laccases from bacteria and investigation of their potential degradation pathways. J. Hazard. Mater. 2021, 416, 125815. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Zhu, G.; Wang, L.; Bai, H.; Qian, Y.; Zhou, X.; Yin, Q.; Zhang, Y. Expression of a deep-sea bacterial laccase from Halomonas alkaliantartica and its application in dyes decolorization. Ann. Microbiol. 2023, 73, 19. [Google Scholar] [CrossRef]
- Adiguzel, A.O.; Konen-Adiguzel, S.; Cilmeli, S.; Mazmanci, B.; Yabalak, E.; Ustun-Odabasi, S.; Kaya, N.G.; Mazmanci, M.A. Heterologous expression, purification, and characterization of thermo- and alkali-tolerant laccase-like multicopper oxidase from Bacillus mojavensis TH309 and determination of its antibiotic removal potential. Arch. Microbiol. 2023, 205, 287. [Google Scholar] [CrossRef] [PubMed]
- Vaelimets, S.; Pedetti, P.; Virginia, L.J.; Hoang, M.N.; Sauer, M.; Peterbauer, C. Secretory expression of recombinant small laccase genes in Gram-positive bacteria. Microb. Cell Factories 2023, 22, 72. [Google Scholar] [CrossRef]
- Bian, L.; Zheng, M.; Chang, T.; Zhou, J.; Zhang, C. Degradation of Aflatoxin B1 by recombinant laccase extracellular produced from Escherichia coli. Ecotoxicol. Environ. Saf. 2022, 244, 114062. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Wang, Z.; Xie, B.; Xiong, Z.; Li, H.; Ahmed, M.; Fang, F.; Li, J.; Li, X. Cloning, expression and application of a novel laccase derived from water buffalo ruminal lignin-degrading bacteria. Int. J. Biol. Macromol. 2024, 266, 131109. [Google Scholar] [CrossRef]
- Carla Martini, M.; Berini, F.; Ausec, L.; Casciello, C.; Vacca, C.; Pistorio, M.; Lagares, A.; Mandic-Mulec, I.; Marinelli, F.; Florencia Del Papa, M. Identification and Characterization of a Novel Plasmid-Encoded Laccase-Like Multicopper Oxidase from Ochrobactrum sp. BF15 Isolated from an On-Farm Bio-Purification System. Food Technol. Biotechnol. 2021, 59, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, L.; Li, Y.; Xu, Z.; Ge, X.; Zhang, Y.; Wang, N.; Wang, S.; Yang, W.; Lu, F.; et al. The heterologous expression, characterization, and application of a novel laccase from Bacillus velezensis. Sci. Total Environ. 2020, 713, 136713. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Yin, Q.; Fang, W.; Xiao, Y.; Fang, Z. Expression of a thermo- and alkali-philic fungal laccase in Pichia pastoris and its application. Protein Expr. Purif. 2019, 154, 16–24. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, S.Y.; Park, H.; Jeon, S.J. Expression, refolding, and characterization of a small laccase from Thermus thermophilus HJ6. Protein Expr. Purif. 2015, 114, 37–43. [Google Scholar] [CrossRef]
- Balcazar-Lopez, E.; Helena Mendez-Lorenzo, L.; Alberto Batista-Garcia, R.; Esquivel-Naranjo, U.; Ayala, M.; Kumar, V.V.; Savary, O.; Cabana, H.; Herrera-Estrella, A.; Luis Folch-Mallol, J. Xenobiotic Compounds Degradation by Heterologous Expression of a Trametes sanguineus Laccase in Trichoderma atroviride. PLoS ONE 2016, 11, e0147997. [Google Scholar] [CrossRef]
- Endo, K.; Hayashi, Y.; Hibi, T.; Hosono, K.; Beppu, T.; Ueda, K. Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J. Biochem. 2003, 133, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Stanišić, M.; Ilić Đurđić, K.; Prodanović, O.; Polović, N.; Prodanović, R. Dopamine-modified pectin for a Streptomyces cyaneus laccase induced microbeads formation, immobilization, and textile dyes decolorization. Environ. Technol. Innov. 2021, 22, 101399. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Qu, R.; Zhang, G.; Rajoka, M.S.R.; Shao, D.; Jiang, C.; Shi, J. Heterologous expression of Oenococcus oeni sHSP20 confers temperature stress tolerance in Escherichia coli. Cell Stress Chaperones 2018, 23, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, I.; Zahoor; Sethupathy, S.; Xu, Q.; Zhu, B.; Shah, S.W.A.; Zhuang, Z.; Zhu, D. Optimization of heterologous production of Bacillus ligniniphilus L1 laccase in Escherichia coli through statistical design of experiments. Microbiol. Res. 2023, 274, 127416. [Google Scholar] [CrossRef]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Molina, M.A.; Winnik, D.L.; Busi, M.V.; Farina, J.I.; Villalba, L.L.; Zapata, P.D. Isolation of a laccase-coding gene from the lignin-degrading fungus Phlebia brevispora BAFC 633 and heterologous expression in Pichia pastoris. J. Appl. Microbiol. 2018, 124, 1454–1468. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Albarracin-Pardo, D.A.; Rivera-Hoyos, C.M.; Morales-Alvarez, E.D.; Poutou-Pinales, R.A.; Cardozo-Bernal, A.M.; Quevedo-Hidalgo, B.E.; Pedroza-Rodriguez, A.M.; Diaz-Rincon, D.J.; Rodriguez-Lopez, A.; et al. Media improvement for 10 L bioreactor production of rPOXA 1B laccase by P. pastoris. 3 Biotech 2019, 9, 447. [Google Scholar] [CrossRef]
- Antosova, Z.; Herkommerova, K.; Pichova, I.; Sychrova, H. Efficient secretion of three fungal laccases from Saccharomyces cerevisiae and their potential for decolorization of textile industry effluentA comparative study. Biotechnol. Progress 2018, 34, 69–80. [Google Scholar] [CrossRef]
- Iimura, Y.; Sonoki, T.; Habe, H. Heterologous expression of Trametes versicolor laccase in Saccharomyces cerevisiae. Protein Expr. Purif. 2018, 141, 39–43. [Google Scholar] [CrossRef]
- Darvishi, F.; Moradi, M.; Jolivalt, C.; Madzak, C. Laccase production from sucrose by recombinant Yarrowia lipolytica and its application to decolorization of environmental pollutant dyes. Ecotoxicol. Environ. Saf. 2018, 165, 278–283. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, S.; Xia, Y.; Xia, L. Expression of a thermotolerant laccase from Pycnoporus sanguineus in Trichoderma reesei and its application in the degradation of bisphenol A. J. Biosci. Bioeng. 2018, 125, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X.; Ng, T.B.; Lin, J.; Ye, X. Laccase Gene Family in Cerrena sp. HYB07: Sequences, Heterologous Expression and Transcriptional Analysis. Molecules 2016, 21, 1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, X.; Kang, Y.; Xiao, Y.; Liu, H. Degradation and detoxification of azo dyes with recombinant ligninolytic enzymes from Aspergillus sp. with secretory overexpression in Pichia pastoris. R. Soc. Open Sci. 2020, 7, 200688. [Google Scholar] [CrossRef]
- Wang, B.; Yan, Y.; Xu, J.; Fu, X.; Han, H.; Gao, J.; Li, Z.; Wang, L.; Tian, Y.; Peng, R.; et al. Heterologous Expression and Characterization of a Laccase from Laccaria bicolor in Pichia pastoris and Arabidopsis thaliana. J. Microbiol. Biotechnol. 2018, 28, 2057–2063. [Google Scholar] [CrossRef]
- Nitheranont, T.; Watanabe, A.; Asada, Y. Heterologous expression of two minor laccase isozyme cDNAs from the edible mushroom Grifola frondosa. Biosci. Biotechnol. Biochem. 2017, 81, 2367–2369. [Google Scholar] [CrossRef]
- Zhuo, R.; Yu, H.; Yuan, P.; Fan, J.; Chen, L.; Li, Y.; Ma, F.; Zhang, X. Heterologous expression and characterization of three laccases obtained from Pleurotus ostreatus HAUCC 162 for removal of environmental pollutants. J. Hazard. Mater. 2018, 344, 499–510. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Tron, T.; Keshavarz, T. Laccase from Aspergillus niger: A novel tool to graft multifunctional materials of interests and their characterization. Saudi J. Biol. Sci. 2018, 25, 545–550. [Google Scholar] [CrossRef]
- Aza, P.; Molpeceres, G.; Ruiz-Duenas, F.J.; Camarero, S. Heterologous Expression, Engineering and Characterization of a Novel Laccase of Agrocybe pediades with Promising Properties as Biocatalyst. J Fungi 2021, 7, 359. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Li, Z.; Yin, W.B. Rational design for fungal laccase production in the model host Aspergillus nidulans. Sci. China Life Sci. 2019, 62, 84–94. [Google Scholar] [CrossRef]
- Nguyen Duc, H.; Nguyen Thi My, L.; Chew, K.W.; Park, S.-M.; Show, P.L. Characterization of a recombinant laccase from Fusarium oxysporum HUIB02 for biochemical application on dyes removal. Biochem. Eng. J. 2021, 168, 107958. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Ahmed, M.; Wadhwa, R.; Aggarwal, S.; Mustafiz, A.; Rajagopalan, G. High Production of Trametes cinnabarina Laccase (lac1) by Suspended and Immobilized Cells of Recombinant Pichia pastoris from Crude Glycerol. Waste Biomass Valorization 2022, 13, 2149–2168. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, Q.; Zhu, L.; Pan, C. Characterization of a Recombinant Laccase B from Trametes hirsuta MX2 and Its Application for Decolorization of Dyes. Molecules 2022, 27, 1581. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Pei, Z.; Wang, B.; Peng, R.; Yao, Q. Characterization and Degradation of Triphenylmethane Dyes and Their Leuco-Derivatives by Heterologously Expressed Laccase from Coprinus cinerea. Cell Biochem. Funct. 2024, 42, e4127. [Google Scholar] [CrossRef]
- Liu, N.; Li, B.; Zhao, X. Improving heterologous expression of laccase by Pichia pastoris via vanillin-induced stress response and its application for removing inhibitors of lignocellulose hydrolysate. Sustain. Energy Fuels 2024, 8, 5254–5270. [Google Scholar] [CrossRef]
- Magalhaes, F.F.; Neves, M.C.; Pedro, A.Q.; Freire, M.G.; Santos-Ebinuma, V.C.; Tavares, A.P.M. Recombinant laccase biosynthesis for efficient polydopamine coating. Biochem. Eng. J. 2024, 212, 109483. [Google Scholar] [CrossRef]
- Sevillano, L.; Vijgenboom, E.; van Wezel, G.P.; Diaz, M.; Santamaria, R.I. New approaches to achieve high level enzyme production in Streptomyces lividans. Microb. Cell Fact. 2016, 15, 28. [Google Scholar] [CrossRef]
- Wang, B.; Yan, Y.; Tian, Y.; Zhao, W.; Li, Z.; Gao, J.; Peng, R.; Yao, Q. Heterologous expression and characterisation of a laccase from Colletotrichum lagenarium and decolourisation of different synthetic dyes. World J. Microbiol. Biotechnol. 2016, 32, 40. [Google Scholar] [CrossRef]
- Zhuo, R.; Zhang, J.; Yu, H.; Ma, F.; Zhang, X. The roles of Pleurotus ostreatus HAUCC 162 laccase isoenzymes in decolorization of synthetic dyes and the transformation pathways. Chemosphere 2019, 234, 733–745. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, G.; Lian, L.; Guo, L.; Wang, W.; Yang, Z.; Miao, J.; Chen, B.; Xie, B. Cloning and Expression Analysis of Vvlcc3, a Novel and Functional Laccase Gene Possibly Involved in Stipe Elongation. Int. J. Mol. Sci. 2015, 16, 28498–28509. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Liang, S.; Zhao, L.; Liu, Z. Heterologous expression of a laccase from Pycnoporus sanguineus in Pichia pastoris. J. Biotechnol. 2008, 136, S315. [Google Scholar] [CrossRef]
- Litwinska, K.; Bischoff, F.; Matthes, F.; Bode, R.; Rutten, T.; Kunze, G. Characterization of recombinant laccase from Trametes versicolor synthesized by Arxula adeninivorans and its application in the degradation of pharmaceuticals. AMB Express 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Vijayakumar, V.E.; Venkataraman, K. A Systematic Review of the Potential of Pichia pastoris (Komagataella phaffii) as an Alternative Host for Biologics Production. Mol. Biotechnol. 2024, 66, 1621–1639. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, H.; Hu, C.; Tron, T.; Lin, J.; Wang, J.; Sun, B. Molecular docking studies and in vitro degradation of four aflatoxins (AFB1, AFB2, AFG1, and AFG2) by a recombinant laccase from Saccharomyces cerevisiae. J. Food Sci. 2020, 85, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, Z.; Zhang, S.; Yang, X. The catalytic properties of Thermus thermophilus SG0.5JP17-16 laccase were regulated by the conformational dynamics of pocket loop 6. Biochim. Et Biophys. Acta-Gen. Subj. 2021, 1865, 129872. [Google Scholar] [CrossRef]
- Dabirmanesh, B.; Khajeh, K.; Ghazi, F.; Ranjbar, B.; Etezad, S.M. A semi-rational approach to obtain an ionic liquid tolerant bacterial laccase through pi-type interactions. Int. J. Biol. Macromol. 2015, 79, 822–829. [Google Scholar] [CrossRef]
- Van Wieren, A.; Colen, P.; Majumdar, S. A project-oriented biochemistry laboratory for protein engineering and structure-function using small laccase enzyme from Streptomyces coelicolor. Biochem. Mol. Biol. Educ. 2023, 51, 708–718. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Galli, C.; Gentili, P.; Jolivalt, C.; Madzak, C.; Vadala, R. How is the reactivity of laccase affected by single-point mutations? Engineering laccase for improved activity towards sterically demanding substrates. Appl. Microbiol. Biotechnol. 2011, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Machczynski, M.C.; Vijgenboom, E.; Samyn, B.; Canters, G.W. Characterization of SLAC: A small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci. 2004, 13, 2388–2397. [Google Scholar] [CrossRef]
- Xie, T.; Liu, Z.; Wang, G. Structural basis for monolignol oxidation by a maize laccase. Nat. Plants 2020, 6, 231–237. [Google Scholar] [CrossRef]
- de Salas, F.; Canadas, R.; Santiago, G.; Virseda-Jerez, A.; Vind, J.; Gentili, P.; Martinez, A.T.; Guallar, V.; Munoz, I.G.; Camarero, S. Structural and biochemical insights into an engineered high-redox potential laccase overproduced in Aspergillus. Int. J. Biol. Macromol. 2019, 141, 855–867. [Google Scholar] [CrossRef]
- Lopes, P.; Koschorreck, K.; Pedersen, J.N.; Ferapontov, A.; Lorcher, S.; Pedersen, J.S.; Urlacher, V.B.; Ferapontova, E.E. Bacillus Licheniformis CotA Laccase Mutant: ElectrocatalyticReduction of O2 from 0.6 V (SHE) at pH 8 and in Seawater. Chemelectrochem 2019, 6, 2043–2049. [Google Scholar] [CrossRef]
- Prins, A.; Kleinsmidt, L.; Khan, N.; Kirby, B.; Kudanga, T.; Vollmer, J.; Pleiss, J.; Burton, S.; Le Roes-Hill, M. The effect of mutations near the T1 copper site on the biochemical characteristics of the small laccase from Streptomyces coelicolor A3(2). Enzym. Microb. Technol. 2015, 68, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Verma, P. Multicopper oxidase laccases with distinguished spectral properties: A new outlook. Heliyon 2020, 6, e03972. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Guo, Y.; Qiao, Y.; Ma, Q.; Ji, C.; Zhao, L. Degradation of zearalenone and aflatoxin B1 by Lac2 from Pleurotuspulmonarius in the presence of mediators. Toxicon 2021, 201, 1–8. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Y.; Zhang, Y.; Fang, W.; Xiao, Y.; Fang, Z. Role of N-glycosylation on the specific activity of a Coprinopsis cinerea laccase Lcc9 expressed in Pichia pastoris. J. Biosci. Bioeng. 2019, 128, 518–524. [Google Scholar] [CrossRef]
- Salony; Garg, N.; Baranwal, R.; Chhabra, M.; Mishra, S.; Chaudhuri, T.K.; Bisaria, V.S. Laccase of Cyathus bulleri: Structural, catalytic characterization and expression in Escherichia coli. Biochim. Biophys. Acta 2008, 1784, 259–268. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Guo, W.; Jia, L.; Fu, Y.; Gui, S.; Lu, F. Cloning, expression, and characterization of a thermostable and pH-stable laccase from Klebsiella pneumoniae and its application to dye decolorization. Process Biochem. 2017, 53, 125–134. [Google Scholar] [CrossRef]
- Pardo, I.; Rodriguez-Escribano, D.; Aza, P.; de Salas, F.; Martinez, A.T.; Camarero, S. A highly stable laccase obtained by swapping the second cupredoxin domain. Sci. Rep. 2018, 8, 15669. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, K.Z.; Wang, Y.J.; Yan, N.; Liao, X.R.; Guan, Z.B. Enhancing the decolorization activity of Bacillus pumilus W3 CotA-laccase to Reactive Black 5 by site-saturation mutagenesis. Appl. Microbiol. Biotechnol. 2020, 104, 9193–9204. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, P.; Portaccio, M.; Lepore, M.; Delfino, I. Optical Properties of Laccases and Their Use for Phenolic Compound Detection and Quantification: A Brief Review. Appl. Sci. 2023, 13, 12929. [Google Scholar] [CrossRef]
- Alcalde, M. Laccases: Biological Functions, Molecular Structure and Industrial Applications. In Industrial Enzymes; Springer: Berlin/Heidelberg, Germany, 2007; pp. 461–476. [Google Scholar]
- Yu, L.; Liu, A.; Kuang, J.; Wei, R.; Wang, Z.; Tian, C. Analysis of the electron transfer pathway in small laccase by EPR and UV–vis spectroscopy coupled with redox titration. Magn. Reson. Lett. 2024, 4, 200116. [Google Scholar] [CrossRef]
- Wang, J.-X.; Vilbert, A.C.; Cui, C.; Mirts, E.N.; Williams, L.H.; Kim, W.; Zhang, Y.J.; Lu, Y. Increasing Reduction Potentials of Type 1 Copper Center and Catalytic Efficiency of Small Laccase from Streptomyces coelicolor through Secondary Coordination Sphere Mutations. Angew. Chem. Int. Ed. 2023, 62, e202314019. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Zhan, J.; Lin, Y.; Yang, X. Axial bonds at the T1 Cu site of Thermus thermophilus SG0.5JP17-16 laccase influence enzymatic properties. FEBS Open Bio 2019, 9, 986–995. [Google Scholar] [CrossRef]
- Fujihiro, S.; Higuchi, R.; Hisamatsu, S.; Sonoki, S. Metabolism of hydroxylated PCB congeners by cloned laccase isoforms. Appl. Microbiol. Biotechnol. 2009, 82, 853–860. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Olbrich, A.C.; Schild, J.N.; Urlacher, V.B. Correlation between the T1 copper reduction potential and catalytic activity of a small laccase. J. Inorg. Biochem. 2019, 201, 110843. [Google Scholar] [CrossRef]
- Rodgers, C.J.; Blanford, C.F.; Giddens, S.R.; Skamnioti, P.; Armstrong, F.A.; Gurr, S.J. Designer laccases: A vogue for high-potential fungal enzymes? Trends Biotechnol. 2010, 28, 63–72. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Zhao, J.; Yue, Q.; Yan, Y.; Gao, Z.; Dong, Y.; Zhang, Z.; Fan, Y.; Tian, J.; et al. Crystal structures of multicopper oxidase CueO G304K mutant: Structural basis of the increased laccase activity. Sci. Rep. 2018, 8, 14252. [Google Scholar] [CrossRef]
- Enguita, F.J.; Martins, L.O.; Henriques, A.O.; Carrondo, M.A. Crystal structure of a bacterial endospore coat component—A laccase with enhanced thermostability properties. J. Biol. Chem. 2003, 278, 19416–19425. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhang, S.; Chang, T.; Zhang, J.; Zhu, X.; Zhang, C. Enhanced catalytic performance and pH stability of Streptomyces Laccase Y230R and its degradation of malachite green. Int. J. Biol. Macromol. 2024, 277, 134108. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yao, Q.; Yu, G.; Liu, S.; Yun, J.; Xiao, X.; Deng, Z.; Li, H. Biochemical Characterization of a Novel Bacterial Laccase and Improvement of Its Efficiency by Directed Evolution on Dye Degradation. Front. Microbiol. 2021, 12, 633004. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Jiang, H.; Chen, C.C.; Liu, G.L.; Hu, Z.; Chi, Z.M.; Chi, Z. Production, Gene Cloning, and Overexpression of a Laccase in the Marine-Derived Yeast Aureobasidium melanogenum Strain 11-1 and Characterization of the Recombinant Laccase. Mar. Biotechnol. 2019, 21, 76–87. [Google Scholar] [CrossRef]
- Xie, T.; Li, J.; Wang, G. Tailoring CotA Laccase Substrate Specificity by Rationally Reshaping Pocket Edge. Chembiochem 2024, 25, e202400660. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, X.; Shi, Y.; Lin, J.; Irfan, M.; Tao, Y. Essential role of the N- and C-terminals of laccase from Pleurotus florida on the laccase activity and stability. Appl. Biochem. Biotechnol. 2014, 174, 2007–2017. [Google Scholar] [CrossRef]
- Theerachat, M.; Emond, S.; Cambon, E.; Bordes, F.; Marty, A.; Nicaud, J.M.; Chulalaksananukul, W.; Guieysse, D.; Remaud-Simeon, M.; Morel, S. Engineering and production of laccase from Trametes versicolor in the yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 125, 267–274. [Google Scholar] [CrossRef]
- Moore, J.C.; Rodriguez-Granillo, A.; Crespo, A.; Govindarajan, S.; Welch, M.; Hiraga, K.; Lexa, K.; Marshall, N.; Truppo, M.D. “Site and Mutation”-Specific Predictions Enable Minimal Directed Evolution Libraries. ACS Synth. Biol. 2018, 7, 1730–1741. [Google Scholar] [CrossRef]
- Santiago, G.; de Salas, F.; Lucas, M.F.; Monza, E.; Acebes, S.; Martinez, Á.T.; Camarero, S.; Guallar, V. Computer-Aided Laccase Engineering: Toward Biological Oxidation of Arylamines. ACS Catal. 2016, 6, 5415–5423. [Google Scholar] [CrossRef]
- Sheng, S.; Jia, H.; Topiol, S.; Farinas, E.T. Engineering CotA Laccase for Acidic pH Stability Using Bacillus subtilis Spore Display. J. Microbiol. Biotechnol. 2017, 27, 507–513. [Google Scholar] [CrossRef]
- Fasan, R.; Meharenna, Y.T.; Snow, C.D.; Poulos, T.L.; Arnold, F.H. Evolutionary history of a specialized p450 propane monooxygenase. J. Mol. Biol. 2008, 383, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Mate, D.M.; Gonzalez-Perez, D.; Falk, M.; Kittl, R.; Pita, M.; De Lacey, A.L.; Ludwig, R.; Shleev, S.; Alcalde, M. Blood tolerant laccase by directed evolution. Chem. Biol. 2013, 20, 223–231. [Google Scholar] [CrossRef]
- Bao, C.; Zhang, Q. Modulation of protein activity and assembled structure by polymer conjugation: PEGylation vs glycosylation. Eur. Polym. J. 2019, 112, 263–272. [Google Scholar] [CrossRef]
- Khalid, N.; Asgher, M.; Qamar, S.A. Evolving trend of Boletus versicolor IBL-04 by chemical mutagenesis to overproduce laccase: Process optimization, 3-step purification, and characterization. Ind. Crops Prod. 2020, 155, 112771. [Google Scholar] [CrossRef]
- Kolyadenko, I.; Scherbakova, A.; Kovalev, K.; Gabdulkhakov, A.; Tishchenko, S. Engineering the Catalytic Properties of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. Int. J. Mol. Sci. 2022, 23, 65. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Yang, X.; Lin, Y. Four second-sphere residues of Thermus thermophilus SG0.5JP17-16 laccase tune the catalysis by hydrogen-bonding networks. Appl. Microbiol. Biotechnol. 2018, 102, 4049–4061. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, S.; Xie, Y.; Fang, Z.; Xiao, Y.; Fang, W.; Zhang, X. Mechanism of the salt activation of laccase Lac15. Biochem. Biophys. Res. Commun. 2020, 521, 997–1002. [Google Scholar] [CrossRef]
- Durao, P.; Bento, I.; Fernandes, A.T.; Melo, E.P.; Lindley, P.F.; Martins, L.O. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: Structural, biochemical, enzymatic and stability studies. J. Biol. Inorg. Chem. 2006, 11, 514–526. [Google Scholar] [CrossRef]
- Deshpande, N.; Wilkins, M.R.; Packer, N.; Nevalainen, H. Protein glycosylation pathways in filamentous fungi. Glycobiology 2008, 18, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Reyna, M.; Liu, W.C.; Jeng, W.Y.; Lee, C.C.; Hsu, C.A.; Wen, T.N.; Wang, A.H.; Shyur, L.F. Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS ONE 2015, 10, e0120601. PLoS ONE 2015, 10, e0120601. [Google Scholar] [CrossRef]
- Mao, G.; Wang, K.; Wang, F.; Li, H.; Zhang, H.; Xie, H.; Wang, Z.; Wang, F.; Song, A. An Engineered Thermostable Laccase with Great Ability to Decolorize and Detoxify Malachite Green. Int. J. Mol. Sci. 2021, 22, 11755. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yang, K.; Wang, X.; Wang, Y.; Zhang, H.; Huang, H.; Su, X.; Yao, B.; Luo, H.; et al. Development of an efficient protein expression system in the thermophilic fungus Myceliophthora thermophila. Microb. Cell Factories 2023, 22, 236. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Santiago, G.; Gentili, P.; Lucas, F.; Monza, E.; Medrano, F.J.; Galli, C.; Martínez, A.T.; Guallar, V.; Camarero, S. Re-designing the substrate binding pocket of laccase for enhanced oxidation of sinapic acid. Catal. Sci. Technol. 2016, 6, 3900–3910. [Google Scholar] [CrossRef]
- Hu, J.; Wang, F.; Ma, A.; Zhuang, G.; Liu, Y.; Lu, J.; Guo, C.; Liu, C. Farnesol stimulates laccase production in Trametes versicolor. Eng. Life Sci. 2016, 16, 364–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Zheng, Z.; Liu, S.; Yang, K. Extraction, enzymatic properties and application of laccase from Pleurotus citrinopileatus residue. Food Mach. 2024, 40, 188–194. [Google Scholar]
- Altinel, B.; Cetintas, B.; Tuluk, K.; Akgun, I.H.; Sargin, S. Production of laccase enzyme via solid-state fermentation and its application in white and whole meal wheat bread. Cereal Res. Commun. 2025, 53, 979–992. [Google Scholar] [CrossRef]
- Dong, Y.; Chidar, E.; Karboune, S. Investigation of in situ and ex situ mode of lactic acid bacteria incorporation and the effect on dough extensibility, bread texture and flavor quality during shelf-life. Food Chem. X 2024, 24, 101857. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Gao, Y.; Wang, F.; Zhou, P.; Zhang, Y.; Li, Y.; Wang, K.; Rui, T.; Zhu, G.; et al. Study on the improvement of Rye bread quality by bacterial Laccase derived from Streptomyces coelicolor. J. Cereal Sci. 2025, 123. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, R.; Gao, Y.; Guan, J.; Chen, Z.; Zhang, Y.; Li, Y.; Zhu, G.; Wang, W.; Zhou, L.; et al. Comparison of the effects of three different fungal laccases on the quality of rye bread. Food Chem. 2025, 482, 144035. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Priyadarshi, R.; Zhang, W.L.; Khezerlou, A.; Rhim, J.W. Innovative application of laccase enzyme in food packaging. Trends Food Sci. Technol. 2024, 151, 104623. [Google Scholar] [CrossRef]
- Tang, P.; Zheng, T.; Ran, R.; Xiong, Y.; Li, G. Collagen films functionalized with gallic acid in the presence of laccase for beef preservation. Food Packag. Shelf Life 2023, 38, 101100. [Google Scholar] [CrossRef]
- Tang, P.; Zheng, T.; Yang, C.; Li, G. Enhanced physicochemical and functional properties of collagen films cross-linked with laccase oxidized phenolic acids for active edible food packaging. Food Chem. 2022, 393, 133353. [Google Scholar] [CrossRef]
- Xu, L.; Li, B.; Liu, T.; Ma, A.; Zhuang, G.; Qian, J.; Cui, Y.; Huo, S.; Xia, J.; Wang, F. Highly Efficient Degradation of 2-Methylisoborneol by Laccase Assisted by a Micro-Electric Field. Catalysts 2024, 14, 649. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Dong, W.; Hu, X. Laccase immobilized on amino modified magnetic biochar as a recyclable biocatalyst for efficient degradation of trichloroethylene. Int. J. Biol. Macromol. 2024, 282, 136709. [Google Scholar] [CrossRef]
- Han, W.; Zhao, Y.; Chen, Q.; Xie, Y.; Zhang, M.; Yao, H.; Wang, L.; Zhang, Y. Laccase surface-display for environmental tetracycline removal: From structure to function. Chemosphere 2024, 365, 143286. [Google Scholar] [CrossRef]
- Li, Z.; Ning, K.; Guo, N.; Shao, Y.; Li, S.; Wu, D.; Ren, X.; Wang, L. Application of activated carbon-immobilized laccase for tetracycline degradation. Environ. Technol. Innov. 2024, 36, 103873. [Google Scholar] [CrossRef]
- Wang, X.; Meng, F. Construction of a continuous packed bed laccase reactor for the elimination of tetracyclines in seawater. J. Environ. Chem. Eng. 2024, 12, 111939. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Kaur, I.; Sodhi, H.S.; Choudhary, R.; Ercisli, S.; Fidan, H.; Dasci, E.; Ullah, R.; Bari, A. Purification, kinetic characterization of thermostable multicopper oxidase from the oyster mushroom and its versatility for greener agro-pulp bio bleaching in the paper industry. Cell. Mol. Biol. 2024, 70, 1–9. [Google Scholar] [CrossRef]
- Ni, H.; Yang, F.; Wang, L.; Li, B.; Li, H.; Liu, J.; Jiang, Z.; Cheng, W. Directed evolution improves the catalytic activity of laccase in papermaking. Chin. J. Biotechnol. 2025, 41, 308–320. [Google Scholar] [CrossRef]
- Patel, K.; Vaghamshi, N.; Shah, K.; Duggirala, S.M.; Ghelani, A.; Dudhagara, P.; Shyu, D.J.H. Synergistic Use of Thermostable Laccase and Xylanase in Optimizing the Pre-Bleaching of Kraft Pulp. Catalysts 2024, 14, 1. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Gentili, P.; Vitali, A.; Cerra, S.; Palmeri, F.; Fratoddi, I.; Polentarutti, M.; Bais, G.; Gullo, L.; Cartoni, A. Electrospray deposition of starch-containing laccase: A green technique for low-cost and eco-friendly biosensors. Biosens. Bioelectron. 2025, 267, 116758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, M.; Tang, H.; Zhang, M. A novel laccase/titanium carbide modified nickel foam electrode for amperometric detection of dopamine. Microchem. J. 2024, 199, 109944. [Google Scholar] [CrossRef]
| Host | Laccase Source | Expression Strategy | Vector | Inducer | Reaction Substrate | Activity (U/L, ABTS) | Ref. |
|---|---|---|---|---|---|---|---|
| E. cloni 10G | Geobacillus sp. | Extracellular | pRham N-His SUMO Kan | -- | ABTS | 37 | [104] |
| E. coli Top10 | Sordaria macrospora | Extracellular | pET-30a-LacSM | Cu2+ | SGZ, ABTS, 2,6-DMP, and guaiacol | 239 | [105] |
| Escherichia coli BL21 (DE3) | Y. enterocolitica | Intracellular | pTZ57R/T | IPTG (1 m M) | ABTS | 3671 | [106] |
| Escherichia coli BL21 (DE3) | Geobacillus sp. | Intracellular | Topo blunt vector | IPTG (0.3 mM) | SGZ, ABTS, 2,6-DMP and guaiacol | -- | [22] |
| E. coli Rosetta (DE3) | Pseudomonas Species | Extracellular | pRSETB | IPTG (1 m M) | ABTS and guaiacol | -- | [107] |
| Escherichia coli BL21 | Pleurotus ostreatus | Extracellular | Pet-22b (+) | IPTG (1 m M) and CuSO4 (0.25 mM) | ABTS | 1539 | [108] |
| E. coli BL21(DE3) | -- | Extracellular /Intracellular | pET28a | IPTG (0.6 mM) and CuCl2 (0.25 mM) | ABTS | -- | [109] |
| E. coli M15 (pREP4) | Streptomyces puniceus | Extracellular | pQE-30 | IPTG | -- | -- | [103] |
| E. coli BL21(DE3) | Bacillus amyloliquefaciens | Extracellular /Intracellular | pET-20 (b) + /lac and pPICZαB/lac | IPTG (0.03 mmol/L) | ABTS and SDZ | 20255 | [99] |
| E. coli M15 (pREP4) | Catenuloplanes japonicus | Intracellular | pQE-30 | IPTG (0.2 mM) and CuSO4 (1 mM) | ABTS and 2,6-DMP | -- | [48] |
| E. coli DH5α | seven bacteria laccase genes | Extracellular | pET-Ompa and pET-Lpp | IPTG (1 mM) | SDZ, SMZ and SMX | -- | [110] |
| Escherichiacoli DH10B | Streptomyces viridochromogenes | Intracellular | pAL-TA | IPTG (0.1 mM) and CuSO4 (0.25 mM) | ABTS | -- | [101] |
| E. coli BL21 (DE3) | Bacillus vallismortis | Extracellular | pET-23a | Cu2+ (0.25 mM) | ABTS | 1580 | [98] |
| E. coli DH5α | Geothermobacter hydrogeniphilus | Intracellular | pET-22b | IPTG (0.2 mM) | -- | -- | [111] |
| E. coli DH5α and E. coli BL21 (DE3) | Bacillus mojavensis | Intracellular | pET-14b | IPTG (0.3 mM) | ABTS, 2,6-DMP and SDZ | -- | [112] |
| Streptomyces lividans and Bacillus subtilis | Streptomyces coelicolor, Streptomyces viridosporus and Amycolatopsis | Intracellular | pBE-S | CuSO4 (100 µM) | ABTS | 1950 | [113] |
| E. coli BL21 (DE3) | Bacillus vallismortis | Extracellular /Intracellular | pET-28a | Methanol (6%, v/v) | ABTS | 1545.6 | [114] |
| E. coli BL21 (DE3) | Bacillus cereus and Ochrobactrum pseudintermedium | Intracellular | pET28a (+) | IPTG (0.2 mM) | ABTS | 7.54 | [115] |
| E. coli BL21 (DE3) | Ochrobactrum sp. | Intracellular | pUC59 and pET22b (+) | IPTG (0.4-1 mM) | ABTS, 2,6-DMP and SDZ | -- | [116] |
| Host | Laccase Source | Expression Strategy | Vector | Inducer | Reaction Substrate | Activity (U/L, ABTS) | Ref. |
|---|---|---|---|---|---|---|---|
| Pichia pastoris | Cerrena sp. | Extracellular | pMD18-T | Cu2+ (0.25 mM) | ABTS | -- | [133] |
| Pichia pastoris | Aspergillus sp. | Extracellular | pPIC9 K-Lac, pPIC9 K-MnP and pPIC9 K-LiP | -- | ABTS, and veratryl alcohol | -- | [134] |
| Pichia pastoris | Madurella mycetomatis | Extracellular | pPICZA and pPICZαA | Methanol (1%) | ABTS, SGZ and 2,6-DMP | -- | [7] |
| Pichia pastoris | Laccaria bicolor | Extracellular | pMD18-T | -- | ABTS | -- | [135] |
| Pichia pastoris | Coprinopsis cinerea | Extracellular | pPIC9K | Methanol | ABTS | 3138 | [118] |
| Pichia pastoris | Grifola frondosa | Extracellular | pPICZA | CuSO4 | ABTS and 2,6-DMP | -- | [136] |
| Pichia pastoris | Phlebia brevispora | Extracellular | pGEM-T Easy | Methanol (0.5%) | ABTS | -- | [127] |
| Pichia pastoris | Pleurotus ostreatus | Extracellular | pPIC3.5K | Methanol | ABTS | 500 | [137] |
| Pichia pastoris | Rigidoporus sp. | Extracellular | -- | CuSO4 (0.3 M) | ABTS | -- | [138] |
| Saccharomyces cerevisiae | Trametes versicolor | Extracellular | pYES2 | Cu2+ | ABTS | 45 | [130] |
| Saccharomyces cerevisiae | Aspergillus niger | Extracellular | -- | -- | ABTS | -- | [138] |
| Saccharomyces cerevisiae | Agrocybe pediades | Extracellular | pJMP9.1 | CuSO4 (2 mM) | ABTS | 778 | [139] |
| A. nidulans | Pycnoporus sanguineus | Extracellular | pMD18-t | Cu2+ (0.1 mmol/L) | -- | -- | [140] |
| Trichoderma atroviride | Trametes(Pycnoporus) sanguineus | Extracellular | pGEM-T Easy | CuSO4 (100 μM) | ABTS, guaiacol, syringaldazine and o-dianisidine | -- | [120] |
| Trichoderma reesei | Pycnoporus sanguineus | Extracellular | pD915 | Lactose (2% w/v) | ABTS | -- | [132] |
| Pichia pastoris | Fusarium oxysporum | Extracellular | pPIC9K | Cu2+ (0.08 Mm) | ABTS | 21966 | [141] |
| Pichia pastoris | Trametes cinnabarina | Extracellular | -- | -- | ABTS | 2851 | [142] |
| Pichia pastoris | Trametes hirsuta | Extracellular | -- | Methanol | ABTS | 2590 | [143] |
| Pichia pastoris | Coprius cinerea | Extracellular | pPICZB | -- | ABTS | 2760 | [144] |
| Pichia pastoris | Pleurotus ostreatus | Extracellular | Vector | ABTS | 285.7 | [145] | |
| Komagataella phaffii | Trametes versicolor | Extracellular | pMD18-T | CuSO4 (0.1 mM) and Methanol | ABTS | -- | [146] |
| Host | Culture Cycle (h) | Range of Enzyme Activity (U/L, ABTS) | Sources of Laccase Genes | Expression Strategies |
|---|---|---|---|---|
| Fungi | 24–48 | 200–3000 | Bacteria and fungi | Primarily extracellular |
| Bacteria | 16–36 | 200–2000 | Bacteria and fungi (primarily bacteria) | Intracellular and extracellular expression |
| Source Strain | Mutant Protein | Control | Mutant Position | WT Km | Km | WT Specific Activity | Mutant kcat/Km | Substrate | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | R178V, K433T | Lac15 | R178 and K433 | 2031.22, 125.51 | 1736.25, 182.98 | [200] | |||
| Thermus thermophilus | M460L | lacTT | Axial residue | 378.49 | 72.40 | 15.85 | 3.69 | guaiacol | [178] |
| Streptomyces coelicolor A3 (2) | SLAC-V290N | SLAC | T1 copper site | 5.088 | 1.999 | 1.615 | 2.226 | 2,6-DMP | [166] |
| Pleurotus ostreatus | POXA1cΔ13-R5V | Wild-type | N- and C-terminals | 0.97 | 1.13 | 2.67 | 25.98 | Guaiacol | [189] |
| Pichia pastoris | D500G | 04lac | - | 44.0 | 58.2 | - | - | ABTS | [75] |
| Bacillus HR03 | T415G, T415I, T418I, T415G/T418I | Native | T415 and T418 | 6.7 | 1.1, 4.1, 3.4, 2.3 | 2.34 | 9.54, 0.81, 0.13, 0.15 | SGZ | [57] |
| Fusarium oxysporum | 4C1, 4A9 | Gr2 | - | 739.0 | 504.4, 271.7 | 0.02 | 0.03, 0.22 | DMP | [72] |
| Coprinopsis cinerea | N313Q/N454Q | nLcc9 | N-glycosylation sites | 1.10 × 10−5 | 1.96 × 10−5 | 1.95 × 107 | 3.93 × 107 | ABTS | [169] |
| basidiomycetes | C14F12, CA32F1 | 3A4 | Residues at substrate-binding pocket (six in total) | 7.0 | 14.2, 9.9 | 22 | 17.7, 26 | SA | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, G.; Li, B.; Xu, L.; Qian, J.; Zou, B.; Huo, S.; Ding, Z.; Cui, K.; Wang, F. Improving the Properties of Laccase Through Heterologous Expression and Protein Engineering. Microorganisms 2025, 13, 1422. https://doi.org/10.3390/microorganisms13061422
Guan G, Li B, Xu L, Qian J, Zou B, Huo S, Ding Z, Cui K, Wang F. Improving the Properties of Laccase Through Heterologous Expression and Protein Engineering. Microorganisms. 2025; 13(6):1422. https://doi.org/10.3390/microorganisms13061422
Chicago/Turabian StyleGuan, Guoqiang, Beidian Li, Ling Xu, Jingya Qian, Bin Zou, Shuhao Huo, Zhongyang Ding, Kai Cui, and Feng Wang. 2025. "Improving the Properties of Laccase Through Heterologous Expression and Protein Engineering" Microorganisms 13, no. 6: 1422. https://doi.org/10.3390/microorganisms13061422
APA StyleGuan, G., Li, B., Xu, L., Qian, J., Zou, B., Huo, S., Ding, Z., Cui, K., & Wang, F. (2025). Improving the Properties of Laccase Through Heterologous Expression and Protein Engineering. Microorganisms, 13(6), 1422. https://doi.org/10.3390/microorganisms13061422







