Abstract
In Colombia, tropical febrile illnesses represent one of the most important causes of clinical attention. Febrile illnesses in the tropics are mainly zoonotic and have a broad etiology. The Colombian surveillance system monitors some notifiable diseases. However, several etiologies are not monitored by this system. In the present review, we describe eleven different etiologies of zoonotic tropical febrile illnesses that are not monitored by the Colombian surveillance system but have scientific, historical, and contemporary data that confirm or suggest their presence in different regions of the country: Anaplasma, Arenavirus, Bartonella, relapsing fever group Borrelia, Coxiella burnetii, Ehrlichia, Hantavirus, Mayaro virus, Orientia, Oropouche virus, and Rickettsia. These could generate a risk for the local population, travelers, and immigrants, due to which they should be included in the mandatory notification system, considering their importance for Colombian public health.
Keywords:
Alphavirus; Anaplasma; arbovirus; arenavirus; Bartonella; Borrelia; Colombia; Coxiella burnetii; Ehrlichia; hantavirus; Orientia; Orthobunyavirus; Rickettsia 1. Introduction
Colombia is a Latin American country whose climate is isothermal; it has a constant temperature with only rainy and dry seasons with slight variation throughout the year. It has five natural geographic regions: the Amazon Rainforest, the Andes Mountain Range region, the Caribbean coast, the Grassland Plains, and the Pacific coast. At least 85% of the national territory is composed of tropical and subtropical regions; thus, tropical diseases represent one of the most critical affections for Colombians and travelers who visit the country [1,2,3].
Febrile illnesses represent the leading cause of clinical attention in tropical and subtropical regions [4]. The etiology of tropical fevers is broad and includes a significant number of pathogens, most of them zoonotic, which may have arisen from wild animal species favored by different wildlife intervention activities, such as intensified agriculture, accelerated urbanization, and eco-tourism, among others [5,6,7].
Some etiologies of zoonotic tropical febrile illnesses (e.g., leptospirosis, yellow fever, and equine encephalitis) are notifiable diseases for the Colombian national surveillance system (Instituto Nacional de Salud (INS)—https://www.ins.gov.co accessed on 1 May 2023). However, several others, whose importance has been demonstrated in other countries (e.g., spotted fever group rickettsioses in Brazil and scrub typhus in Chile) [8,9], are not monitored by local authorities despite the scientific, historical, or contemporary evidence that confirms or suggests their circulation in Colombia.
Although for some of these non-notifiable zoonotic pathogens (e.g., Rickettsia), the national surveillance center (INS) can perform a confirmatory diagnosis in case of high clinical suspicion, diagnostic methods for others are only available in some higher education research groups’ laboratories that work with these zoonotic infectious agents, using commercial kits or in-house methods. Reference laboratories from international centers such as the CDC (Centers for Disease Control and Prevention) are an alternative way to reach a confirmatory diagnosis, mainly in case of outbreaks of an unknown disease [10]. In addition, research groups have contributed to knowledge about these non-notifiable zoonotic febrile illnesses and their repercussions on the local population’s health.
This review aims to summarize and describe the evidence of other etiologies of zoonotic tropical fevers that are, or probably are, circulating in the country and are not part of the notifiable diseases in Colombia.
3. Hantavirus
Orthohantaviruses (Hantaviridae family) are emerging viruses whose reservoirs are small rodent species and affect approximately 150,000 to 200,000 people worldwide annually [38]. Hantavirus infection occurs after exposure to contaminated aerosols or excreta from infected rodents [38]. A total of 40 hantavirus species have been described, 22 of which are pathogenic for humans. Those limited to Europe and Asia are known as Old World hantaviruses (e.g., Puumala virus and Hantaan virus), and those reported in America are known as New World hantaviruses (e.g., Sin Nombre virus and Andes virus) [38]. Clinically, two forms of hantavirus disease have been described: hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS) [39,40].
HFRS is caused by Old World hantaviruses. It is a non-specific febrile illness with signs of renal damage such as oliguria, proteinuria, and hematuria in severe cases. Low platelet counts and lymphocytosis are the main laboratory abnormalities. The mortality rate ranges from less than 1% to 15% and depends on the type of virus implicated, with Hantaan virus being the most pathogenic [39,41].
On the other hand, HCPS is caused by New World hantaviruses. It is a severe pulmonary acute illness that clinically develops as a non-specific febrile illness that evolves as classic acute respiratory distress syndrome. It has a mortality rate of more than 40% due to pulmonary edema, respiratory insufficiency, or cardiogenic shock, and is fatal than HFRS [39,42].
Laboratory diagnosis of hantavirus infection is usually made using serological methods, which can include the detection of IgM antibodies with ELISA or evidence of seroconversion of IgG antibodies between acute and convalescent serum samples. PCR can detect the presence of the viral RNA, which can be considered a confirmatory diagnosis. In addition, hantavirus antigens in formalin-fixed tissues can be evidenced with immunohistochemistry (IHC), which can be used in post-mortem cases to establish the cause of death [43]. Although the virus can be isolated, this process must be only performed in reference laboratories [43].
In the Americas, since 1993, when the first case of HCPS was identified in the United States, countries such as Brazil, Argentina, and Chile, among others, have continuously been describing novel HCPS human cases [44]. However, some confirmed cases with mild clinical courses, incompatible with the classic HCPS, have also been reported in these countries [45,46]. In Colombia, the first record of human exposure to hantavirus was reported in 2004 among rural male workers from 12 towns of Córdoba and Sucre departments [26]. Ten years later, in Monteria (Córdoba department), the first confirmed case of hantavirus disease, not clinically compatible with HCPS, was identified and diagnosed using seroconversion [47]. Further studies performed in the Caribbean (Córdoba department) and Orinoquia regions (Meta department) identified novel cases due to hantavirus infection among febrile patients, finding 6% (6/100) and 3% (3/100) of cases, respectively, all of them confirmed using seroconversion with IgG ELISA [27,48]. In neither study, cases were clinically compatible HCPS, raising the possibility that other hantaviruses related with mild cases of HFRS (e.g., Seoul virus) might be circulating in the country [49].
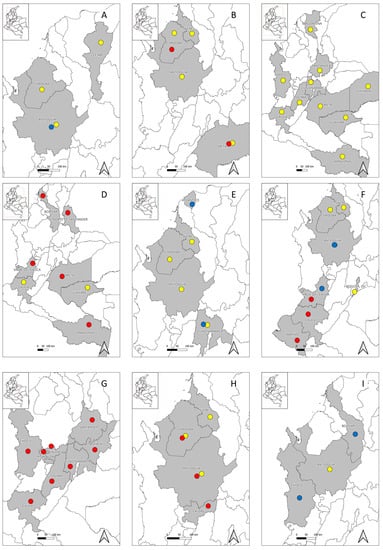
Furthermore, several serological studies performed in different Colombian regions have evidenced human exposure to hantaviruses among different population groups, such as healthy inhabitants from six urban municipalities of Córdoba department [24], febrile patients in urban settlements of the Urabá region (Antioquia department) in whom no seroconversion was evidenced [21], and indigenous populations from rural communities of Córdoba (Embera Katío and Tuchín communities) and Cesar departments (Kankuamos community) [22,23,25], suggesting that at least one hantavirus native to Colombia is circulating among rural inhabitants and that, probably, another hantavirus might be present in urban regions (Table 1) (Figure 1B). Exposure to hantaviruses has also been evidenced in several rodent species in the departments of Córdoba, Sucre, and Antioquia [50,51,52], as well as the description of a novel hantavirus designated as “Necoclí” virus [53], whose importance for human and animal health is still unknown. This evidence suggests that at least one human pathogenic hantavirus is present in Colombia, about which little is known to date. Further studies among wild rodent populations are thus needed because some species may be acting as reservoirs and shedders of these viruses.
4. Mayaro Virus
Mayaro virus (MAYV) is an arbovirus that belongs to the Alphavirus genus (Togaviridae family). It is part of the Semliki complex along with other viruses, such as Chikungunya, Semliki Forest, Ross River, and O’nyong-nyong [54]. In its natural wild cycle, MAYV is transmitted by female mosquitoes of the genus Haemagogus, mainly Haemagogus janthinomys; however, its introduction into urban environments also involves other mosquito species, such as Aedes and Culex spp., which may be acting as vectors of MAYV [55,56].
Cases due to MAYV have been limited to several regions of Central and South America, mainly those surrounding the Amazon basin. Over the last few years, MAYV cases have continuously increased, suggesting that it could be imperative to identify it as a novel epidemic arbovirus [57]. Due to the clinical, epidemiological, and biological similarities with the Chikungunya virus (CHIKV), which is also present in the same regions, it may be probable that cases due to MAYV are misdiagnosed as CHIKV [58].
MAYV usually causes a mild and self-limited AUFI with unspecific clinical manifestations, of which arthralgia and maculopapular rash are the most important during its acute phase; fever during MAYV infection is usually biphasic, reappearing after an afebrile period, which may differentiate it from other arboviral etiologies [54,59]. During the convalescent phase, more than half of the patients suffer from long-term arthralgia, persisting for several months, similar to CHIKV infection [60]. Although most cases are mild and self-limited, MAYV infection can also produce several complications, such as neurological compromise, heart damage, chronic intermittent fever, and hemorrhages, which can be so severe that death can occur [54].
Laboratory diagnosis can be performed using virological methods to detect viral RNA with PCR or cell culture isolation. The latter is rarely used as a first-line diagnostic tool and is only performed in reference laboratories [61,62]. Serological methods can also be used; ELISA or other immunoassay methods can detect anti-MAYV IgM antibodies. However, results must be interpreted carefully, as cross-reactivity can occur with other alphaviruses. Thus, its detection is only presumptive, and confirmation requires demonstrating IgG seroconversion in paired serum samples without evidence of seroconversion to other alphaviruses [63,64].
In Colombia, although no clinical case due to MAYV has been reported to date, the circulation of this virus in several regions of the country has already been evidenced. The first report came in 1961, when MAYV was successfully isolated from anthropophilic Psorophora mosquitoes collected in San Vicente de Chucurí, Santander department [65], raising the possibility that these arthropods may be involved in MAYV local eco-epidemiology. In 1964, seropositivity was found among the rural populations from several regions of Chocó, Tolima, Cundinamarca, Santander, Meta, and Vichada departments, finding different percentages of exposure, with the highest ones being 60% (3/5) and 24.5% (38/155), and being found in Cumaribo municipality (Vichada department) and Barrancabermeja municipality (Santander department), respectively [30]. In 1969, seropositivity was also found among military recruits from different regions of the country [32]. In 1970, seropositivity was found among indigenous populations in the region of Araracuara, Puerto Santander, Amazonas department [28]. Since then, an epidemiological silence took place until 2007, when during a febrile illness sentinel program, seropositivity was detected in Guaviare and Magdalena departments [31]. Furthermore, more recently, in 2022, during an arboviral surveillance study performed in the department of Cauca, seropositivity was found among healthy individuals (Table 1) (Figure 1C) [29]. This evidence suggests that MAYV is actively circulating in rural regions of the country, apparently generating more asymptomatic cases.
Although its importance may be underestimated to date, a study showed, using mathematical analysis, that several regions are at greater risk of a possible MAYV epidemic once the mosquitoes adapt to an urban environment or MAYV adapts to urban or urban–wild transition mosquitoes such as Aedes aegypti or Aedes albopictus, respectively [66]. Thus, MAYV might be one of the most critical threats in the future due to accelerated urbanization and deforestation, adapting to other anthropophilic mosquitoes such as Aedes spp., whose competence as a MAYV vector has already been demonstrated and are circulating in an urban cycle [67].
5. Oropouche Virus
Oropouche virus (OROV) is a member of the Simbu serogroup of the Orthobunyavirus genus (Bunyaviridae family); it is the etiological agent of Oropouche fever, one of the etiologies of AUFIs, mainly in the Amazon basin, and probably a candidate for the next great arboviral epidemic across the Americas, along with Venezuelan equine encephalitis virus and MAYV [68,69]. OROV is a vector-borne infectious agent mainly transmitted by the bite of Culicoides paraensis [70]. However, other mosquito species, such as Culex quinquefasciatus, Coquilletidia venezuelensis, and Aedes serratus, might also be involved, with C. paraensis and Cx. quinquefasciatus being the vectors related to the urban cycle and the remaining two species being in their natural wild cycle [69]. Some mammals and wild bird species might act as natural reservoirs in the natural wild cycle [71,72].
The first OROV human infection was notified in the village of Vega de Oropouche, Trinidad and Tobago [73]. Since then, isolated cases have been reported in many South American countries (e.g., Argentina, Bolivia, and Ecuador), and disease outbreaks have been reported in Brazil, Peru, and Panama [69].
Clinically, Oropouche fever develops as an AUFI with non-specific symptomatology, such as headache, myalgia, arthralgia, chills, and dizziness [72,74]. In more than half of cases, clinical manifestations recur a few days after the initial febrile episode. Even though severe cases are unusual, complications can occur, of which aseptic meningitis is the most significant [75]. Recovery is complete without any apparent sequel, even in severe cases; to date, no fatal cases have been reported [74].
Laboratory diagnosis can be made using serological procedures in order to detect specific IgG and IgM antibodies with ELISA, IFA, PRNT, or Hemagglutination Inhibition (HAI) tests [72]. A single positive serum result in a clinically compatible patient can highly suggest OROV infection; however, confirmatory diagnosis requires the isolation of the virus, which is only performed in reference laboratories, or the detection of viral RNA with PCR [72].
In Colombia, the first record of the presence of OROV was reported in 1961, in which seropositivity was found among 27.3% (6/22) of primates sampled in the municipality of Lizama, Santander department, using Neutralization Test 50 LD50 [30]. No human cases were reported until 2021, when a case of Oropouche fever was reported in the municipality of Turbaco (Bolivar department) and was confirmed with viral culture, IFA, and PCR [76], with this being the first clinical case of Oropouche fever confirmed in Colombia.
Only two additional studies have been performed in which seropositivity was found among different populations from Colombia, including patients from Guaviare department enrolled in a febrile illness sentinel program [31] and healthy rural villagers from four municipalities of Cauca department [29]. In addition, one study performed among febrile patients in four urban municipalities of Amazonas, Meta, Norte de Santander, and Valle Del Cauca departments detected the presence of OROV using molecular methods (Table 1) (Figure 1D) [33]. These findings suggest that local transmission of OROV infection is occurring in Colombia, probably more frequently in rainy seasons, and that Oropouche fever is one of the causes of AUFIs in the country. However, its importance remains underestimated due to the absence of studies and previous reports in many regions throughout the country.
6. Anaplasma
Anaplasma is a genus of obligate intracellular Gram-negative bacteria of veterinary and human health importance transmitted via tick bite [77]. Two Anaplasma species, Anaplasma phagocytophilum and Anaplasma capra, have been implicated as human pathogens. However, it has been suggested that anaplasmosis may be a significant emerging infectious disease in the future, as several novel and yet unrecognized Anaplasma species are continuously being detected in a wide range of hosts [78,79].
The main human pathogenic species is A. phagocytophilum, the etiological agent of human granulocytic anaplasmosis (HGA), a tick-borne infectious disease transmitted by several Ixodes species [80]. Clinically, it can range from asymptomatic infection to fatal disease. It usually develops one or two weeks after tick exposure as a non-specific febrile illness accompanied by severe headache, myalgia, arthralgia, sweating, and stiff neck as the most common symptoms [80]. The disease can be severe in 35% of cases; however, the fatality rate is less than 1%, and life-threatening complications include acute respiratory distress syndrome, acute renal failure, and hemodynamic collapse [81,82].
Laboratory confirmatory diagnosis includes PCR on DNA extracted from blood samples, IgG seroconversion with IFA, and direct observation of morulae (microcolonies of Anaplasma) in the cytoplasm of granulocytes of peripheral blood smears with microscopy. Confirmatory diagnosis should be performed in clinically compatible patients with risk factors such as living or having traveled to endemic regions or those bitten by a tick [83].
Although the tick species recognized as competent vectors of A. phagocytophilum are not present within Colombia up to now, one probable case of anaplasmosis has been reported in a febrile livestock farmer in the city of Cartagena (Atlántico department), whose diagnosis was only performed using microscopy with Wright–Giemsa staining and who fully recovered after treatment with doxycycline [84]. Another probable case of co-infection of human anaplasmosis/dengue fever was reported in the municipality of Villeta (Cundinamarca department) [85]. Although no recognized Anaplasma vectors have been found in Colombia, studies performed in Argentina have reported the detection of Anaplasma spp. of unknown pathogenicity in anthropophilic ticks of Amblyomma spp. [86,87], suggesting another competent vector may be present in South America.
Exposure to Anaplasma spp. has also been evidenced in different Colombian regions, such as livestock farming workers in Córdoba and Sucre departments [88], febrile patients from Villeta municipality (Cundinamarca department) [85], livestock farming workers from San Pedro de Los Milagros (Antioquia department) [89], and febrile patients from the Magdalena Medio region (Table 2) (Figure 1E) [90]. These findings suggest and reinforce the possibility that at least one human pathogenic Anaplasma sp. may be present in the country.

Table 2.
Serological and molecular studies on Anaplasma, Bartonella, Borrelia, Coxiella burnetii, Ehrlichia, and Orientia in Colombia.
7. Bartonella
Bartonella is a group of fastidious intra-erythrocytic Gram-negative bacteria. They have many animal reservoirs, including humans, and are transmitted by a wide range of vectors, including sand flies, lice, fleas, and probably ticks [100,101]. Bartonella bacilliformis, Bartonella quintana, and Bartonella henselae are classically known as the pathogenic species of this genus. Infections caused by Bartonella spp. can be mild and sometimes asymptomatic. However, there are also clinically symptomatic cases, with some of them being severe and even fatal depending on the host (e.g., immune status and underlying diseases) and microorganism factors (e.g., species involved and bacterial load) [100,102].
The most known diseases due to Bartonella spp. include Carrion’s disease, trench fever, and cat-scratch disease. However, other diseases include chronic lymphadenopathy, bacteremia, culture-negative endocarditis, bacillary angiomatosis, and bacillary peliosis [102]. Carrion’s disease is a biphasic illness caused by B. bacilliformis and transmitted by sand flies of Lutzomya spp. During its acute phase, the disease is known as Oroya fever. This acute febrile hemolytic illness causes anemia and non-specific symptomatology, with a fatality rate as high as 90% if patients are not opportunely treated. The chronic phase of the disease is known as “verruga peruana” (Peruvian wart), which is a blood-filled nodular skin lesion similar to a hemangioma that can persist for several months [103]. Trench fever is caused by B. quintana and is transmitted by the clothing louse Pediculus humanus. It can develop as a mild self-limited febrile illness or a prolonged recurrent debilitating febrile illness with asymptomatic periods in between [104]. B. henselae mainly causes cat-scratch disease, although other species, such as Bartonella clarridgeiae, can also be implicated, and cat scratches or bites transmit it. Usually, it is characterized by the development of a papular lesion at the site of inoculation accompanied by proximal regional lymphadenopathy, and patients usually develop a fever with non-specific symptomatology. Atypical clinical presentations of cat-scratch disease include Parinaud’s oculoglandular syndrome, encephalitis, endocarditis, and fever of unknown origin [105].
Diagnosis of bartonellosis is usually clinical. However, some laboratory methodologies can be performed to confirm the etiological diagnosis. Although Bartonella is a fastidious bacterium, it can be isolated by blood culture, and it can also be observed in peripheral blood smears during the acute phase of B. bacilliformis infection (Oroya fever). Serological methods such as ELISA and IFA can detect IgM or IgG antibodies and evidence seroconversion if acute and convalescent samples are available. The bacterial DNA can be detected by performing PCR on blood and lymph node aspirates, mainly in cases with high suspicion but negative culture attempts [106,107].
In Colombia, Carrion’s disease was unknown until 1936, when a high-mortality outbreak of febrile illness associated with severe hemolytic anemia took place among the indigenous population in the southwest of Nariño department and lasted until 1941, with more than 6000 cases [108,109]. Although similar cases have been diagnosed in other parts of the country, such as Bogotá, D.C. [110], these patients had a history of travel to the endemic area in Nariño. Late in 1941, novel cases occurred in villages on the border of the department of Cauca [111], which were close to the endemic area in the Nariño department. No confirmed cases out of the endemic area were reported until 1988, when an anemic febrile patient from Pradera municipality, Valle del Cauca department, was diagnosed with bartonellosis using microscopy [112]. Although the vector of Carrion’s disease in Colombia is unknown, historical data suggest that Lutzomya colombiana could be one of the vectors [112]. Despite the historical data on the presence of Carrión’s disease in Colombia, no novel cases have been reported. Several hypotheses have been raised in order to explain the origin and extinction of Carrion’s disease in Nariño. Apparently, the disease may have been imported from Peru with the entry of Peruvian soldiers into the Nariño region during the Colombia–Peru postwar period, and the extinction of the epidemic might have been due to the probable exhaustion of human reservoirs, considering the high lethality of the disease [113]. According to historical data, Carrion’s disease was prevalent in Colombia’s South Pacific subregion in the department of Nariño and surrounding areas in Cauca department. Thus, it is necessary to include Oroya fever among the differential diagnoses of malaria and other febrile illnesses, and the “verruga peruana” among the dermatological lesions in this region.
Since 2000, only a few studies have been performed to determine the exposure to Bartonella in Colombia. Studies performed among rural workers from several municipalities of Córdoba and Sucre departments [88], inhabitants of Monteria and Cereté municipalities (Córdoba department) [91], and homeless population from Bogotá, D.C. [92] evidenced different Bartonella spp. seropositivity rates (Table 2). Furthermore, the pathogen B. quintana was confirmed in clothing lice collected from homeless people from Bogotá, D.C. [92]. Although no confirmed cases of B. henselae infection have been reported to date, clinical cases compatible with cat-scratch disease in young children have been reported in Medellin, Antioquia department [114], and in Cali, Valle del Cauca department [115], as well as a Parinaud’s oculoglandular syndrome in a hospital in Medellin, Antioquia (Figure 1F) [116]. These bacteria could be circulating in domestic animals; however, these data remain unknown, as only one study has evidenced exposure to Bartonella spp. in 10.1% (26/258) of dogs from Bogotá, D.C., using antigens of B. henselae, B. clarridgeiae, and Bartonella vinsonii subsp. berkhoffii [117].
8. Relapsing Fever Group Borrelia
Borrelia is a bacterial genus of Gram-negative spirochetes. They can be classified into two groups: the Borrelia burgdorferi sensu lato complex, which includes at least 20 different genospecies, and the relapsing fever group, which includes many species [118]. Both of them are implicated in human infectious diseases, causing two well-described illnesses: Lyme borreliosis, caused by Borrelia burgdorferi sensu stricto, Borrelia afzelii, and Borrelia garinii [119], and relapsing fever, which is caused by several relapsing fever group Borrelia spp. [120].
Relapsing fever is an episodic AUFI that can be transmitted by the human clothing lice or ticks of the Argasidae and Ixodidae families, with the genus Ornithodoros spp. being the most important in Latin America [120,121]. The disease develops abruptly as a febrile illness with chills and other non-specific symptomatology, lasting for approximately three to five days, followed by an afebrile period that ends with further febrile episodes. Usually, three to thirteen recurrences can occur; the highest number of febrile recurrences occurs when the disease is left untreated; however, the severity of the febrile episodes usually decreases with every recurrence. Significant organ involvement can also occur, which is most frequent in louse-borne relapsing fever, resulting in a poor prognosis with a high mortality risk when organ damage is severe [120]. Tick-borne relapsing fever is a zoonotic disease for which many animal species might act as reservoirs. The principal risk factors for acquiring the disease are poverty and overcrowding for louse-borne relapsing fever and performing activities that increase the risk of tick exposure for tick-borne relapsing fever [120].
For the relapsing fever group Borrelia, diagnosis can be made by visualizing the bacteria in blood samples using dark-field microscopy or stains such as Wright–Giemsa during the febrile paroxysms; further, the detection of the bacterial DNA using amplification with PCR can be performed [122].
In the Americas, cases of relapsing fever have been reported in many countries from North, Central, and South America. The distribution of the disease and the implicated Borrelia species may depend on the geographical distribution of its tick vector [121,123].
The first documented case of tick-borne relapsing fever in Colombia was reported in 1906 in Villeta municipality (Cundinamarca department) [124]. During the same year, a febrile illness due to yellow fever and tick-borne relapsing fever occurred at Muzo mines in Boyacá department and lasted until early 1907, with a fatality rate of 20% [125,126]. One year later, in 1907, novel cases of tick-borne relapsing fever were reported in Manizales municipality (Caldas department) [127]. In 1923, new cases of relapsing fever were reported in Bucaramanga city (Santander department), and due to these findings, a tick collection across different regions of Colombia was made, detecting Borrelia spp. from Ornithodoros rudis collected in Buenaventura, Barranquilla, Bucaramanga, Tumaco, Quibdó, Lloró, and Itsmina [128]. In 1928, 91 cases of relapsing fever diagnosed using microscopy, of which 29 were foreigners and 62 were Colombians, were reported in San Juan (Chocó department) [129]. In 1934, novel cases were reported in Villeta and Puerto Lievano municipalities (Cundinamarca department) [124]. Subsequently, cases have increasingly been reported in other regions in Caldas, Cauca, Tolima, and Risaralda (Figure 1G) [130].
Seropositivity against Borrelia spp. using B. burgdorferi antigens has been reported in patients in a hospital in Cali (Valle Del Cauca) [94]; rural workers from the municipalities of Monteria, Cereté, Lorica, and Cotorra (Córdoba department) [93]; and febrile patients from Puerto Berrio, Puerto Nare, and Cimitarra municipalities (Antioquia and Santander departments) [90]. However, these serological data must be interpreted carefully due to cross-reactivity among Borrelia spp. [122,131]. Thus, it is likely that these results were due to exposure to other Borrelia species of the relapsing fever group or other infectious agents, such as Treponema species.
Attempts to detect Borrelia spp. in animals have been made, finding positivity using molecular methods in bats from Macaregua cave, Santander department [132,133], and bats captured in different municipalities (Montelíbano, Tierralta, San Antero, Montería, Lorica, and Moñitos) in Córdoba department [134] forming a new taxon. Furthermore, in Córdoba department, the presence of the anthropophilic tick Ornithodoros puertoricensis was described [135], in which in Panama, a novel species of the relapsing fever group borreliae (Borrelia puertoricensis), of unknown pathogenicity, was detected [136]. All these findings highlight the need for more research to understand Borrelia’s local epidemiology. However, there are enough historical data to state that relapsing fever must be included in the differential diagnosis of AUFIs in Colombia.
9. Coxiella burnetii
Coxiella is a bacterial genus composed of intracellular Gram-negative rods [137]. To date, only Coxiella burnetii has been associated with human disease. It has two antigenic phase variants: the phase I variant, which is the virulent form of the bacteria that can be found in nature and predominates in chronic forms of the disease, and the phase II variant, which is an attenuated form of the bacteria and is associated with acute forms of disease [137,138]. C. burnetii is an aerosol-borne pathogen; its natural form is highly resistant to chemical agents and extreme environmental conditions. Thus, it is considered extraordinarily infectious and one of the most feared potential bioterrorism agents [139].
The disease caused by C. burnetii is called Q fever (query fever). It mainly affects people living in rural areas in contact with cattle [138]. Q fever has an incubation period of two to three weeks, and it does not have a typical form, being clinically variable between patients and making its diagnosis challenging [140]. Q fever is a self-limited acute febrile illness accompanied by headaches, myalgias, arthralgias, and cough as the principal non-specific clinical manifestations [138,141]. Although the disease can be mild in most cases, some patients develop severe and chronic forms of the disease, which include prolonged fever, neurologic signs, hepatitis, atypical pneumonia, and myocarditis associated with pericarditis, with the latter two being the leading causes of death [138,141].
For laboratory confirmation of Q fever, paired serum samples must demonstrate seroconversion with IgG IFA using C. burnetii phase I and II antigens. IgM antibodies can also be requested but are less specific; thus, they only provide limited diagnostic values without IgG results. PCR can also test blood or serum samples during the disease’s acute phase to detect the DNA of C. burnetii. Isolation of the pathogen can be performed in cell cultures; however, it is challenging and requires a Biosafety Level 3 laboratory, since its manipulation can be hazardous and laboratory transmission has already been reported [142]. Chronic Q fever can be confirmed with the detection of anti-phase I IgG antibodies ≥ 1:1024; tissue biopsies can also be tested using PCR or IHC [142].
In Colombia, four cases of Q fever have been reported to date. The first one occurred in 2012 and developed as culture-negative infectious endocarditis in a farmer from the municipality of Nariño, Antioquia department; the disease was accompanied by splenic infarction, retinal emboli, and renal failure; the diagnosis of Q fever was made using serology, finding positive titers of phase I antibodies [143]. Another case, which developed as acute atypical pneumonia in a patient who may have acquired the infection in a rural area in the municipality of Manizales, Caldas department, was reported in the same year. The diagnosis was made with serology, ruling out other atypical microorganisms [144]. A third case was reported in 2014 in a patient with a background of livestock contact while working as a farmer in a tropical rural area of Monteria municipality, Córdoba department. The patient was enrolled during a surveillance epidemiological study and was clinically asymptomatic but with mild signs and symptoms of heart damage. The diagnosis was made with serology, which evidenced elevated titers of phase I and phase II antibodies [145]. Finally, in 2021, a case of acute Q fever with generalized maculopapular and purpuric rash was reported by a zootechnician who may have acquired the infection while he was working with goats that had recently given birth in a tropical rural area in the Magdalena Medio region, Antioquia department; it was diagnosed using PCR [146].
Several seroprevalence studies have been performed in different regions of Colombia, evidencing different seropositivity rates among slaughterhouse personnel from the city of Medellin [96] and livestock farmers from the North and Magdalena Medio regions [95] and San Pedro de Los Milagros municipality [89] in Antioquia department. Seropositivity was also found among livestock farmers from rural areas of Monteria (Córdoba department) [98] and inhabitants of San Marcos, Cotorra, Lorica, Monteria, and Cienaga de Oro municipalities (Córdoba and Sucre departments) [88]. Additionally, molecular detection of C. burnetii was evidenced among asymptomatic farmers from Puerto Berrío, Puerto Nare, and Puerto Triunfo (Antioquia department) (Table 2) (Figure 1H) [97]. These data suggest that C. burnetii is actively circulating in Colombia.
Contact with domestic livestock animals, including cattle, goats, and sheep, may represent the main risk for acquiring the infection, as evidence of C. burnetii has been detected in these animals from Colombia [95,147] and their products [98]. Furthermore, C. burnetii may also be circulating in a wild cycle, as evidence of the bacteria was found among bats that inhabit Macaregua cave, Santander department, Colombia [148]; thus, cavers and people who perform eco-touristic activities may be aware of the risk that Q fever may represent for them. Q fever remains a minor suspected etiology of febrile illness in Colombia; its symptomatology is not characteristic; and diagnostic methods for its confirmation are not offered routinely, which is probably why few reported cases and high human seropositivity rates exist.
10. Ehrlichia
Ehrlichia genus is composed of obligate intracellular Gram-negative pleomorphic tick-borne bacteria, which infect a wide range of host cells, including endothelial and hemopoietic cells (e.g., macrophages and neutrophils), in which they form clusters known as morulae [149]. To date, Ehrlichia chaffeensis, Ehrlichia ewingii, and Ehrlichia muris subsp. eauclairensis have been implicated as human pathogens and are restricted to specific areas of the United States due to the distribution of their tick vectors. Nevertheless, novel species are continuously being detected from several animal hosts. It is likely that some of these may have a zoonotic potential [149,150,151]. On the other hand, several authors have suggested that Ehrlichia canis, a pathogen of dogs, may also be linked to human disease [152]; however, to date, this is still controversial, and more studies are needed to clarify it.
E. chaffeensis is the primary human pathogen; it causes human monocytic ehrlichiosis (HME), which is a mild non-specific febrile illness [153]. However, in 15% of patients, a severe form of the disease can be developed with meningoencephalitis, acute renal failure, myocarditis, lung damage, gastrointestinal hemorrhage, and coagulopathies [153,154]. Little is known about the disease caused by the other two pathogenic species, but reports from case series suggest that they may cause a milder disease similar to HME [155,156].
Laboratory diagnosis of ehrlichiosis is similar to anaplasmosis and should be performed in clinically compatible cases with high-risk factors. The reference method is IFA, with which IgG seroconversion must be evidenced. Amplification with PCR of the bacterial DNA extracted from blood samples can also be used for diagnosis in the early stages of the disease; culture isolation is also possible, but it is only performed in specialized laboratories; and direct visualization of morulae in peripheral blood samples using microscopy can be highly suggestive mainly in risk populations [79,157].
In Colombia, three presumed cases of human ehrlichiosis have been reported to date, but two lack reliable diagnosis methods to be classified as confirmed cases [158,159]. The only convincing reported case is a febrile pediatric patient from a rural area of central-western Colombia who had come into contact with dog ticks and was diagnosed using qPCR [160].
Furthermore, several serological studies have evidenced exposure to Ehrlichia spp. in Antioquia department among livestock farmers from the North and Magdalena Medio regions [95] and San Pedro de Los Milagros municipality [89], and among febrile patients from the Magdalena Medio region (Table 2) (Figure 1I) [90]. In addition, E. canis has been confirmed from dogs samples in several regions, including the Cauca department [161], where a comprehensive study was performed, revealing that humans are not usually infected by this species [162]. Thus, these findings should reinforce surveillance studies to understand if Ehrlichia spp. could be an emerging human pathogen in Colombia.
11. Orientia
Orientia spp. are a bacterial genus formed by small intracellular Gram-negative rods transmitted by chiggers, the larval stage of trombiculid mites [163]. Classically, Orientia tsutsugamushi was the only recognized species for a long time; however, recent studies have proposed two novel species, Candidatus Orientia chuto and Candidatus Orientia chiloensis, both pathogenic for humans [164].
The disease caused by these species is known as scrub typhus. This re-emerging vector-borne infectious disease develops as an AUFI associated with rash and a necrotic eschar at the site of inoculation [165]. The disease can be mild or severe; in the latter, respiratory, neurological, renal, gastrointestinal, and cardiovascular damage can occur, compromising the patient’s life [166].
For confirmatory diagnosis of scrub typhus, a four-fold rise in seroconversion of IgG antibodies between acute and convalescent serum samples using IFA is required. However, ELISA detection of IgG or IgM antibodies can also be performed [167]. Molecular methods such as PCR can also be performed to detect bacterial DNA. In some cases, bacterial DNA can be detected in whole-blood samples. Using an eschar sample or a swab of the uncrusted lesion is preferable, as it is more likely to detect bacterial DNA due to the high load of bacteria in them [168]. Isolation can also be performed; however, it can be only performed in reference laboratories [167].
Classically, scrub typhus was restricted to the Asia-Pacific region in a geographic zone known as the “tsutsugamushi triangle,” which has been known as the only endemic region worldwide [169]. However, several studies have evidenced autochthonous cases from the United Arab Emirates and exposition to Orientia spp. in several countries of Africa [170,171]. In Latin America, a newly endemic region has been found in Chile, where seropositivity and autochthonous scrub typhus cases have been evidenced, as well as the vector involved in transmitting the disease [9,172,173,174]. Since then, serological evidence has been found in Peru and Honduras, suggesting that scrub typhus might be more widespread than initially thought and could be one of the etiologies of AUFIs in Latin America [164,175,176].
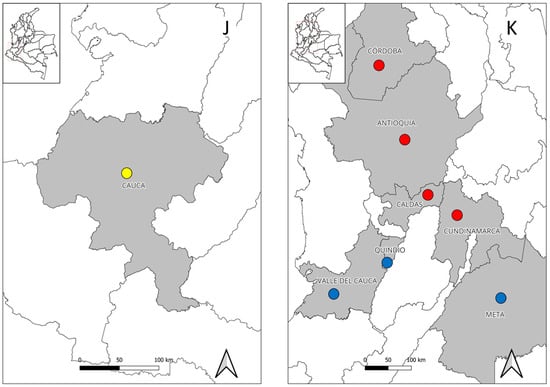
In Colombia, no attempt to detect the bacterium or the exposition to it was made until 2022, when a study found seropositivity to Orientia spp., confirmed with Western blot, among inhabitants of four municipalities of Cauca department (Table 2) (Figure 1J) [99], which should encourage to perform more studies to establish if Orientia is part of febrile illnesses in the region, and if so, determine which species are involved, as well as its possible implications for Colombian public health or whether further surveillance by local health systems is warranted.
12. Rickettsia
Rickettsia spp. is a bacterial genus of the order Rickettsiales and family Rickettsiaceae, which includes small obligate intracellular Gram-negative short rods [177]. These can be classified into four groups: the spotted fever group (SFG), the typhus group (TG), the transitional group, and the ancestral group, of which the first three include several human pathogenic species [178]. A total of 27 Rickettsia spp. have been described, and at least seventeen of them are recognized as human pathogenic species [179], of which, to date, Rickettsia rickettsii, Rickettsia typhi, Rickettsia prowazekii, and Rickettsia akari are the most important. However, emerging species, such as Rickettsia parkeri, and geographically restricted species, such as Rickettsia conorii and Rickettsia africae, are also highly important pathogenic species [180].
Clinically, rickettsioses are among the most important and underrated causes of AUFIs in several regions worldwide [181]. SFG rickettsioses, transmitted by ticks, develop as febrile illnesses accompanied by non-specific symptoms with the development of rash, usually macular or maculopapular, which is highly variable and depends on the pathogen. Some species can induce the formation of an eschar at the site of inoculation, which can be single or multiple; fatality is variable and depends on the species, with R. rickettsii being the deadliest one and generating, in severe cases, pulmonary, renal, and neurologic damage [180,182]. TG rickettsioses, caused by R. prowazekii (epidemic typhus transmitted by lice) and R. typhi (murine typhus transmitted by fleas), are also febrile illnesses with non-specific symptoms, among which rash and gastrointestinal symptomatology such as nausea and vomiting can be present in about half of the cases. Murine typhus is generally mild, but epidemic typhus can be severe, with a case fatality rate as high as 50% due to complications that include endocarditis, pneumonia, renal failure, meningitis, and septic shock, among others [180,183,184].
Laboratory diagnosis of rickettsioses is complex and only sometimes available in endemic areas. The gold standard method for confirmatory diagnosis is IFA for IgG antibodies, which need to be performed on paired serum samples to evidence a four-fold seroconversion. Only single-sample results cannot be used for laboratory confirmation, as antibody titers can be observed in persons even four years after the acute illness, mainly in endemic areas. However, a single titer of at least 1:256 obtained during a clinically epidemiologically compatible illness can also suggest rickettsiosis [185,186]. PCR amplification can also be a helpful tool. Detection of bacterial DNA in whole-blood samples cannot always be performed, as Rickettsia spp. infect endothelial cells and do not circulate in large numbers in the bloodstream until the disease has progressed severely. However, samples such as a skin biopsy of a rash lesion, the inoculation eschar, or a swab sample of the uncrusted lesion can be used for PCR amplification, as these samples have a high bacterial load [186,187]. Rickettsia can be isolated; however, isolation can only be performed in specialized laboratories [186].
In Colombia, for the period from 1902 to 1986, there are many historical data regarding the presence of different rickettsioses, which include cases of SFG, TG, and unspecific rickettsioses in several regions of the departments of Antioquia, Atlántico, Bolivar, Boyacá, Caldas, Cauca, Cundinamarca, Córdoba, Huila, Magdalena, Meta, Nariño, Norte de Santander, Santander, Tolima, and Valle Del Cauca [188]. Since then, a significant number of studies have been performed in several regions in the last twenty years, establishing at least four endemic regions for rickettsioses: Tobia valley in Cundinamarca department, the north region of Caldas department, the Urabá region in Antioquia department, and the Caribbean region in Córdoba department [189]; however, other regions, such as the Orinoquia region, may also be considered crucial, as it is probably endemic [190,191].
In Cundinamarca department, the first cases of SFG rickettsiosis due to R. rickettsii were described in 1935 by Dr. Luis Patiño Camargo, when an epidemic of the disease occurred in the town of Tobia, in which 65 cases were reported, of which only 3 recovered, giving a high fatality rate of 95% [192]. Years later, in 1941, two novel fatal cases from areas close to village of Tobia were reported [193]. Since then, an epidemiological silence occurred until 2007, when two novel fatal cases of R. rickettsii rickettsiosis were reported in Villeta municipality, near the same region where the disease was initially discovered [194]. Several years later, fifteen non-fatal cases of SFG rickettsioses were detected in the same municipality during a febrile illness surveillance study, of which twelve had co-infections with dengue or leptospirosis [85]. Due to the high fatality rate of R. rickettsii rickettsiosis, febrile cases detected in Tobia valley and adjacent areas should be carefully managed, as this region is highly endemic for this pathogenic species.
In the department of Caldas, the first reports of confirmed cases were performed in 1942, 1943, and 1946, all reported as TG rickettsioses [188]. Since then, novel confirmed cases have been reported in 2008, when a study detected fourteen murine typhus cases in the department’s north part [195]. Several years later, a prospective study performed in the same region, specifically in Aguadas, Filadelfia, and Salamina municipalities, evidenced several SFG and TG rickettsioses [196]. Finally, in another surveillance study, eight febrile patients enrolled in the municipalities of Belalcázar, Salamina, Filadelfia, Noracasia, and San José were confirmed as SFG and TG rickettsioses [197]. These studies confirm that murine typhus and at least one pathogenic SFG Rickettsia sp. are circulating in Caldas department.
In the Urabá region, department of Antioquia, the first reports of rickettsioses cases occurred in 2006, when an outbreak of R. rickettsii infection was reported in the municipality of Necoclí, which had a fatality rate of 35% [10]. In addition, during a non-malarial febrile illness surveillance study, nine cases of rickettsioses, where three of them were co-infections with other febrile illness etiologies, were detected among inhabitants of three municipalities: Apartado, Necoclí, and Turbo [21]. Years later, another study also found six confirmed cases of SFG rickettsioses among febrile patients from the same region [198]. The first case of rickettsiosis due to the emerging pathogen R. parkeri strain Atlantic Rainforest was also confirmed in the municipality of Turbo [199]. Fatal cases have also been reported in the region, with the first one being a fatal pediatric case due to R. rickettsii in the municipality of Chigorodó [200], and the second one being a fatal case due to co-infection between R. rickettsii and Leptospira interrogans serovar Copenhageni in a patient from Carepa municipality [201]. These studies clearly show that the Urabá region is an endemic region for at least two different SFG rickettsioses. Furthermore, not so far away from the Urabá Antioquia region, in the municipality of Uramita, four cases of R. rickettsii infection confirmed with laboratory and epidemiological association have also been reported, suggesting that it may also be considered an important region in which rickettsioses may be circulating [202,203].
Finally, in the department of Córdoba, the first report of an unspecific rickettsiosis took place in 1947 [188]. Since then, novel cases occurred in 2011, when twenty cases of R. rickettsii infection were detected in the municipality of Los Córdobas; four of these died, giving a fatality rate of 20% [204]. Years later, two SFG rickettsioses cases were identified in Monteria municipality during an AUFI surveillance study [48]. Although only two studies have evidence of the presence of cases of rickettsioses in this region, the fact of having detected cases due to R. rickettsii and evidence of seropositivity to SFG Rickettsia spp. in several municipalities of the north of the department [205] should alert local authorities to consider this region as highly endemic for this pathogen.
Besides these regions, several cases of rickettsioses have been detected in other regions. In the department of Quindío, fifteen cases of rickettsioses, with five of them being co-infections with dengue, were detected among febrile patients in several health institutions [206]. In the department of Meta, a probable case of SFG rickettsiosis with seroconversion was reported in a patient who had recently traveled to the municipality of Puerto Gaitán [190]. Finally, in the department of Valle Del Cauca, a probable case of murine typhus was reported in a febrile patient from an urban area of the city of Cali (Figure 1K) [207]. All these data reinforce the need for a better monitoring of rickettsioses in the country and further studies to establish if other regions are endemic.
13. Conclusions
Although the Colombian healthcare surveillance system monitors a significant number of zoonotic infectious diseases, several are still underrated and neglected, and some even remain undiscovered. With this review, which collected information until early 2023, we found evidence that some etiologies of non-notifiable zoonotic tropical febrile illnesses are present in several regions of Colombia, generating a risk for the local population, travelers who visit the country, and immigrants. Due to the close relationship among humans, domestic animals, and wildlife in several scenarios related to habitat destruction (e.g., intensive agriculture, accelerate urbanization, and eco-tourism) and climate change, zoonotic febrile illnesses represent a significant public health problem. These diseases negatively affect the population’s well-being, since their clinical suspicion and confirmatory diagnosis are not always available. Thus, their actual contribution to the disease burden remains unknown. It is thus necessary to carry out more studies using the One Health approach, to understand the importance of these diseases for local eco-epidemiology and public health. Different research groups, with different national and international funding, contributed to the knowledge of these infectious diseases on the national territory. However, despite this, the local surveillance system remains unaware of the importance of these illnesses for local public health, making it necessary to keep promoting and suggesting their inclusion into the mandatory notification system.
Author Contributions
Conceptualization, C.R.S.-R. and Á.A.F.-M.; writing—original draft preparation, C.R.S.-R.; writing—review and editing, C.R.S.-R., Á.A.F.-M., C.C.S.-R., M.H. and S.M.; visualization, C.C.S.-R.; supervision Á.A.F.-M.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Vicerrectoría de Investigación of Pontificia Universidad Javeriana funded the APC.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Vicerrectoría de Investigación of Pontificia Universidad Javeriana for the financial support to publish the present manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rangel-Churio, J.O. La biodiversidad de Colombia: Significado y distribución regional. Acad. Colomb. Cienc. Exactas Físicas Y Nat. 2015, 39, 176–200. [Google Scholar] [CrossRef]
- Pabón, J.D. El cambio climático global y su manifestación en Colombia. Cuad. Geogr. Rev. Colomb. Geogr. 2003, 12, 111–119. [Google Scholar]
- Villegas Vélez, Á.A.; Castrillón Gallego, C. Territorio, enfermedad y población en la producción de la geografía tropical colombiana, 1872–1934. Hist. Crit. 2006, 32, 94–117. [Google Scholar] [CrossRef]
- Bottieau, E.; Yansouni, C.P. Fever in the tropics: The ultimate clinical challenge? Clin. Microbiol. Infect. 2018, 24, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Maze, M.J.; Bassat, Q.; Feasey, N.A.; Mandomando, I.; Musicha, P.; Crump, J.A. The epidemiology of febrile illness in sub-Saharan Africa: Implications for diagnosis and management. Clin. Microbiol. Infect. 2018, 24, 808–814. [Google Scholar] [CrossRef]
- Moreira, J.; Bressan, C.S.; Brasil, P.; Siqueira, A.M. Epidemiology of acute febrile illness in Latin America. Clin. Microbiol. Infect. 2018, 24, 827–835. [Google Scholar] [CrossRef]
- Wangdi, K.; Kasturiaratchi, K.; Nery, S.V.; Lau, C.L.; Gray, D.J.; Clements, A.C.A. Diversity of infectious aetiologies of acute undifferentiated febrile illnesses in south and Southeast Asia: A systematic review. BMC Infect. Dis. 2019, 19, 577. [Google Scholar] [CrossRef]
- Martiniano, N.O.M.; Sato, T.P.; Vizzoni, V.F.; Ventura, S.F.; Oliveira, S.V.; Amorim, M.; Gazêta, G.S. A new focus of spotted fever caused by Rickettsia parkeri in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e222022. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Martínez-Valdebenito, C.; Acosta-Jamett, G.; Jiang, J.; Richards, A.L.; Abarca, K. Scrub Typhus in Continental Chile, 2016–2018. Emerg. Infect. Dis. 2019, 25, 1214–1217. [Google Scholar] [CrossRef]
- Acosta, J.; Urquijo, L.; Díaz, A.; Sepúlveda, M.; Mantilla, G.; Heredia, D.; González, M.; Parra, E.; Rey, G.; Múnera, G.; et al. Brote de rickettsiosis en Necoclí, Antioquia, febrero-marzo de 2006. Inf. Quinc. Epidemiol. Nac. 2006, 11, 177–192. [Google Scholar]
- Hallam, S.J.; Koma, T.; Maruyama, J.; Paessler, S. Review of Mammarenavirus Biology and Replication. Front. Microbiol. 2018, 9, 1751. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; de la Torre, J.C. Human Pathogenic Arenaviruses (Arenaviridae). In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 2, pp. 507–517. [Google Scholar] [CrossRef]
- Petersen, L.R.; Ksiazek, T.G. Zoonotic Viruses. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 1493–1508. [Google Scholar]
- Bonthius, D.J. Lymphocytic choriomeningitis virus: An underrecognized cause of neurologic disease in the fetus, child, and adult. Semin. Pediatr. Neurol. 2012, 19, 89–95. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.B. Lassa, Junin, Machupo and Guanarito Viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.M.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 203–212. [Google Scholar]
- Fukushi, S.; Tani, H.; Yoshikawa, T.; Saijo, M.; Morikawa, S. Serological assays based on recombinant viral proteins for the diagnosis of arenavirus hemorrhagic fevers. Viruses 2012, 4, 2097–2114. [Google Scholar] [CrossRef] [PubMed]
- Happi, A.N.; Happi, C.T.; Schoepp, R.J. Lassa fever diagnostics: Past, present, and future. Curr. Opin. Virol. 2019, 37, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Faccini-Martínez, Á.A.; Calixto, O.J.; Hidalgo, M. Bolivian hemorrhagic fever: A narrative review. Travel. Med. Infect. Dis. 2021, 40, 102001. [Google Scholar] [CrossRef]
- Trapido, H.; Sanmartín, C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am. J. Trop. Med. Hyg. 1971, 20, 631–641. [Google Scholar] [CrossRef]
- Ruelas, D.; Pacheco, V.; Inche, B.; Tinoco, N. A preliminary review of Nephelomys albigularis (Tomes, 1860) (Rodentia: Cricetidae), with the description of a new species from the Peruvian montane forests. Zootaxa 2021, 5027, 175–210. [Google Scholar] [CrossRef]
- Arroyave, E.; Londoño, A.F.; Quintero, J.C.; Agudelo-Flórez, P.; Arboleda, M.; Díaz, F.J.; Rodas, J.D. Etiología y caracterización epidemiológica del síndrome febril no palúdico en tres municipios del Urabá antioqueño, Colombia. Biomedica 2013, 33, 99–107. [Google Scholar]
- Restrepo, B.; Rodas, J.D.; Montoya-Ruiz, C.; Zuluaga, A.M.; Parra-Henao, G.; Agudelo-Flórez, P. Evidencia serológica retrospectiva de infecciones por Leptospira spp., dengue, hantavirus y arenavirus en indígenas Emberá-Katío, Colombia. Rev. Chil. Infectol. 2016, 33, 472–473. [Google Scholar] [CrossRef]
- Bolaños, A.; Montoya-Ruiz, C.; Perez-Peréz, J.C.; Rodas, J.D.; Mattar, S. Seroprevalence of arenavirus and hantavirus in indigenous populations from the Caribbean, Colombia. Rev. Soc. Bras. Med. Trop. 2019, 53, e201901322019. [Google Scholar] [CrossRef]
- Guzmán, C.; Mattar, S.; Levis, S.; Pini, N.; Figueiredo, T.; Mills, J.; Salazar-Bravo, J. Prevalence of antibody to hantaviruses in humans and rodents in the Caribbean region of Colombia determined using Araraquara and Maciel virus antigens. Mem. Inst. Oswaldo Cruz. 2013, 108, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Barrera, S.; Martínez, S.; Tique-Salleg, V.; Miranda, J.; Guzmán, C.; Mattar, S. Seroprevalence of Hantavirus, Rickettsia y Chikungunya in the indigenous population of Tuchín, Córdoba. Infectio 2015, 19, 75–82. [Google Scholar] [CrossRef]
- Máttar, S.; Parra, M. Serologic evidence of hantavirus infection in humans, Colombia. Emerg. Infect. Dis. 2004, 10, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; Mattar, S.; Rodriguez, D.; Tique, V.; Rodríguez, I. First serological evidence of hantavirus infection in humans from the Orinoquia region of Colombia. Braz. J. Infect. Dis. 2016, 20, 507–508. [Google Scholar] [CrossRef]
- Prias Landinez, E.; Bernal Cubides, C.; Vargas de Torres, S.; Romero Leon, M. Encuesta serológica de virus transmitidos por artrópodos. Boletín Oficina Sanit. Panamericana 1970, 68, 134–141. [Google Scholar]
- Gil-Mora, J.; Acevedo-Gutiérrez, L.Y.; Betancourt-Ruiz, P.L.; Martínez-Diaz, H.C.; Fernández, D.; Bopp, N.E.; Olaya-Másmela, L.A.; Bolaños, E.; Benavides, E.; Villasante-Tezanos, A.; et al. Arbovirus Antibody Seroprevalence in the Human Population from Cauca, Colombia. Am. J. Trop. Med. Hyg. 2022, 107, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Groot, H. Estudios sobre virus transmitidos por artrópodos en Colombia. Rev. Acad. Colomb. Cien Exac Fis. Nat. 1964, 12, 3–23. [Google Scholar]
- Hidalgo, M.; Castañeda, E.; Méndez, J.; Travassos da Rosa, A.; Valbuena, G. Detección de anticuerpos contra arbovirus y rickettsias en sueros provenientes del programa centinela de entidades febriles, 2000–2004. Inf. Quinc. Epidemiol. Nac. 2007, 12, 81–96. [Google Scholar]
- Evans, A.S.; Casals, J.; Opton, E.M.; Borman, E.K.; Levine, L.; Cuadrado, R.R. A nationwide serum survey of Colombian military recruits, 1966. I. Description of sample and antibody patterns with arboviruses, polioviruses, respiratory viruses tetanus, and treponematosis. Am. J. Epidemiol. 1969, 90, 292–303. [Google Scholar] [CrossRef]
- Ciuoderis, K.A.; Berg, M.G.; Perez, L.J.; Hadji, A.; Perez-Restrepo, L.S.; Aristizabal, L.C.; Forberg, K.; Yamaguchi, J.; Cardona, A.; Weiss, S.; et al. Oropouche virus as an emerging cause of acute febrile illness in Colombia. Emerg. Microbes Infect. 2022, 11, 2645–2657. [Google Scholar] [CrossRef]
- Mattar, S.; Guzman, C.; Arrazola, J.; Soto, E.; Barrios, J.; Pini, N.; Levis, S.; Salazar-Bravo, J.; Mills, J.N. Antibody to arenaviruses in rodents, Caribbean Colombia. Emerg. Infect. Dis. 2011, 17, 1315–1317. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Bonilla-Aldana, D.K.; Risquez, A.; Paniz-Mondolfi, A.; Suárez, J.A. Should we be concerned about Venezuelan hemorrhagic fever?—A reflection on its current situation in Venezuela and potential impact in Latin America amid the migration crisis. New Microbes New Infect. 2021, 44, 100945. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Montoya-Ruíz, C.; Faccini-Martínez, Á.A.; Rodas, J.D. An updated review and current challenges of Guanarito virus infection, Venezuelan hemorrhagic fever. Arch. Virol. 2022, 167, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Castellar, A.; Guevara, M.; Rodas, J.D.; Londoño, A.F.; Arroyave, E.; Díaz, F.J.; Levis, S.; Blanco, P.J. Primera evidencia de infección por el virus de la coriomeningitis linfocítica (arenavirus) en roedores Mus musculus capturados en la zona urbana del municipio de Sincelejo, Sucre, Colombia. Biomedica 2017, 37, 75–85. [Google Scholar]
- Munir, N.; Jahangeer, M.; Hussain, S.; Mahmood, Z.; Ashiq, M.; Ehsan, F.; Akram, M.; Ali Shah, S.M.; Riaz, M.; Sana, A. Hantavirus diseases pathophysiology, their diagnostic strategies and therapeutic approaches: A review. Clin. Exp. Pharm. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Londoño, A.F.; Levis, S.; Rodas, J.D. Hantavirus como agentes emergentes de importancia en Suramérica. Biomedica 2011, 31, 451–464. [Google Scholar] [CrossRef]
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus infections. Clin. Microbiol. Infect. 2019, 21S, e6–e16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Du, H.; Wang, L.M.; Wang, P.Z.; Bai, X.F. Hemorrhagic Fever with Renal Syndrome: Pathogenesis and Clinical Picture. Front. Cell. Infect. Microbiol. 2016, 6, 1. [Google Scholar] [CrossRef]
- Llah, S.T.; Mir, S.; Sharif, S.; Khan, S.; Mir, M.A. Hantavirus induced cardiopulmonary syndrome: A public health concern. J. Med. Virol. 2018, 90, 1003–1009. [Google Scholar] [CrossRef]
- Mattar, S.; Guzmán, C.; Figueiredo, L.T. Diagnosis of hantavirus infection in humans. Expert. Rev. Anti Infect. Ther. 2015, 13, 939–946. [Google Scholar] [CrossRef]
- Pini, N. Hantavirus pulmonary syndrome in Latin America. Curr. Opin. Infect. Dis. 2004, 17, 427–431. [Google Scholar] [CrossRef]
- Puerta, H.; Cantillo, C.; Mills, J.; Hjelle, B.; Salazar-Bravo, J.; Mattar, S. Hantavirus del Nuevo Mundo. Ecología y epidemiología de un virus emergente en Latinoamérica. Medicina 2006, 66, 343–356. [Google Scholar]
- Figueiredo, L.T.; Souza, W.M.; Ferrés, M.; Enria, D.A. Hantaviruses and cardiopulmonary syndrome in South America. Virus Res. 2014, 187, 43–54. [Google Scholar] [CrossRef]
- Mattar, S.; Garzon, D.; Tadeu, L.; Faccini-Martínez, A.A.; Mills, J.N. Serological diagnosis of hantavirus pulmonary syndrome in a febrile patient in Colombia. Int. J. Infect. Dis. 2014, 25, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Mattar, S.; Tique, V.; Miranda, J.; Montes, E.; Garzon, D. Undifferentiated tropical febrile illness in Cordoba, Colombia: Not everything is dengue. J. Infect. Public. Health 2017, 10, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Faccini-Martinez, Á.A. Seoul hantavirus could be an etiology of acute undifferentiated febrile illness in Colombia? Rev. MVZ Cordoba 2021, 26, 1–3. [Google Scholar]
- Alemán, A.; Iguarán, H.; Puerta, H.; Cantillo, C.; Mills, J.; Ariz, W.; Mattar, S. Primera evidencia serológica de infección por Hantavirus en roedores, en Colombia. Rev. Salud Publica 2006, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Londoño, A.F.; Díaz, F.J.; Agudelo-Flórez, P.; Levis, S.; Rodas, J.D. Genetic evidence of hantavirus infections in wild rodents from northwestern Colombia. Vector Borne Zoonotic Dis. 2011, 11, 701–708. [Google Scholar] [CrossRef]
- Blanco, P.; Arroyo, S.; Corrales, H.; Pérez, J.; Álvarez, L.; Castellar, A. Evidencia serológica de infección por hantavirus (Bunyaviridae: Hantavirus) en roedores del Departamento de Sucre, Colombia. Rev. Salud Publica 2012, 14, 755–764. [Google Scholar]
- Montoya-Ruiz, C.; Cajimat, M.N.; Milazzo, M.L.; Diaz, F.J.; Rodas, J.D.; Valbuena, G.; Fulhorst, C.F. Phylogenetic Relationship of Necoclí Virus to Other South American Hantaviruses (Bunyaviridae: Hantavirus). Vector Borne Zoonotic Dis. 2015, 15, 438–445. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.M.; Ramírez-Santana, C. Mayaro: An emerging viral threat? Emerg. Microbes Infect. 2018, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Mohammed, A.; Jayaraman, J.; Nandram, N.; Feng, R.S.; Lezcano, R.D.; Seeramsingh, R.; Daniel, B.; Lovin, D.D.; Severson, D.W.; et al. Changing patterns in the distribution of the Mayaro virus vector Haemagogus species in Trinidad, West Indies. Acta Trop. 2019, 199, 105108. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.N.; Carvalho, F.D.; De Mendonça, S.F.; Rocha, M.N.; Moreira, L.A. Vector competence of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes for Mayaro virus. PLoS Negl. Trop. Dis. 2020, 14, e00075182020. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.L.A.; Fonseca, B.A.L.D. Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Braz. J. Infect. Dis. 2017, 21, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Murray, K.O. Dengue, West Nile virus, chikungunya, Zika-and now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e00054622017. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Freitas, R.B.; Travassos da Rosa, J.F.; Gabbay, Y.B.; Mello, W.A.; LeDuc, J.W. An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am. J. Trop. Med. Hyg. 1981, 30, 674–681. [Google Scholar] [CrossRef]
- Santiago, F.W.; Halsey, E.S.; Siles, C.; Vilcarromero, S.; Guevara, C.; Silvas, J.A.; Ramal, C.; Ampuero, J.S.; Aguilar, P.V. Long-Term Arthralgia after Mayaro Virus Infection Correlates with Sustained Pro-inflammatory Cytokine Response. PLoS Negl. Trop. Dis. 2015, 9, e00041042015. [Google Scholar] [CrossRef]
- Terzian, A.C.; Auguste, A.J.; Vedovello, D.; Ferreira, M.U.; da Silva-Nunes, M.; Sperança, M.A.; Suzuki, R.B.; Juncansen, C.; Araújo, J.P., Jr.; Weaver, S.C.; et al. Isolation and characterization of Mayaro virus from a human in Acre, Brazil. Am. J. Trop. Med. Hyg. 2015, 92, 401–404. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Rojas, A.; Mohamed-Hadley, A.; de Guillén, Y.A.; Pinsky, B.A. Real-time RT-PCR for Mayaro virus detection in plasma and urine. J. Clin. Virol. 2018, 98, 1–4. [Google Scholar] [CrossRef]
- Figueiredo, L.T.; Nogueira, R.M.; Cavalcanti, S.M.; Schatzmayr, H.; da Rosa, A.T. Study of two different enzyme immunoassays for the detection of Mayaro virus antibodies. Mem. Inst. Oswaldo Cruz. 1989, 84, 303–307. [Google Scholar] [CrossRef]
- Fumagalli, M.J.; de Souza, W.M.; Romeiro, M.F.; de Souza Costa, M.C.; Slhessarenko, R.D.; Figueiredo, L.T.M. Development of an Enzyme-Linked Immunosorbent Assay to Detect Antibodies Targeting Recombinant Envelope Protein 2 of Mayaro Virus. J. Clin. Microbiol. 2019, 57, e01892-18. [Google Scholar] [CrossRef] [PubMed]
- Groot, H.; Morales, A.; Vidales, H. Virus isolations from forest mosquitoes in San Vicente de Chucuri, Colombia. Am. J. Trop. Med. Hyg. 1961, 10, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Marín, B.S.; Gandica, I.D.; Aguirre-Obando, O.A. The Mayaro virus and its potential epidemiological consequences in Colombia: An exploratory biomathematics analysis. Parasit. Vectors 2020, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Kantor, A.M.; Lin, J.; Wang, A.; Thompson, D.C.; Franz, A.W.E. Infection Pattern of Mayaro Virus in Aedes aegypti (Diptera: Culicidae) and Transmission Potential of the Virus in Mixed Infections with Chikungunya Virus. J. Med. Entomol. 2019, 56, 832–843. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Paniz-Mondolfi, A.E.; Villamil-Gómez, W.E.; Navarro, J.C. Mayaro, Oropouche and Venezuelan Equine Encephalitis viruses: Following in the footsteps of Zika? Travel. Med. Infect. Dis. 2017, 15, 72–73. [Google Scholar] [CrossRef]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Gomes, M.L.; LeDuc, J.W.; Hoch, A.L. Transmission of Oropouche virus from man to hamster by the midge Culicoides paraensis. Science 1982, 215, 1251–1253. [Google Scholar] [CrossRef]
- Gibrail, M.M.; Fiaccadori, F.S.; Souza, M.; Almeida, T.N.; Chiang, J.O.; Martins, L.C.; Ferreira, M.S.; Cardoso, D.D. Detection of antibodies to Oropouche virus in non-human primates in Goiânia City, Goiás. Rev. Soc. Bras. Med. Trop. 2016, 49, 357–360. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche virus: A new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–578. [Google Scholar] [CrossRef]
- Travassos da Rosa, J.F.; de Souza, W.M.; Pinheiro, F.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Rocha, A.G.; Freitas, R.B.; Ohana, B.A.; Travassos da Rosa, A.P.; Rogério, J.S.; Linhares, A.C. Meningite associada às infecções por vírus Oropouche. Rev. Inst. Med. Trop. Sao Paulo 1982, 24, 246–251. [Google Scholar]
- Gómez-Camargo, D.E.; Egurrola-Pedraza, J.A.; Cruz, C.D.; Popuche, D.; Ochoa-Díaz, M.M.; Guevara, C.; Silva, M.; Abente, E.J.; Ampuero, J.S. Evidence of Oropouche Orthobunyavirus Infection, Colombia, 2017. Emerg. Infect. Dis. 2021, 27, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Cabezas-Cruz, A. The genus Anaplasma: New challenges after reclassification. Rev. Sci. Tech. 2015, 34, 577–586. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Ismail, N.; McBride, J.W. Tick-Borne Emerging Infections: Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. North. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Dahlgren, F.S.; Mandel, E.J.; Krebs, J.W.; Massung, R.F.; McQuiston, J.H. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am. J. Trop. Med. Hyg. 2011, 85, 124–131. [Google Scholar] [CrossRef]
- Hansmann, Y.; Jaulhac, B.; Kieffer, P.; Martinot, M.; Wurtz, E.; Dukic, R.; Boess, G.; Michel, A.; Strady, C.; Sagez, J.F.; et al. Value of PCR, Serology, and Blood Smears for Human Granulocytic Anaplasmosis Diagnosis, France. Emerg. Infect. Dis. 2019, 25, 996–998. [Google Scholar] [CrossRef]
- Gómez Arcila, V.; Arroyo Salgado, B.J.; Bello Espinosa, A.A.; Rodríguez Escobar, Z.; Polo Andrade, E.R. Diagnóstico microbiológico compatible con Anaplasma sp. en un paciente con síndrome febril. Rev. Argent. Microbiol. 2015, 47, 78–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faccini-Martínez, Á.A.; Ramírez-Hernández, A.; Barreto, C.; Forero-Becerra, E.; Millán, D.; Valbuena, E.; Sánchez-Alfonso, A.C.; Imbacuán-Pantoja, W.O.; Cortés-Vecino, J.A.; Polo-Terán, L.J.; et al. Epidemiology of Spotted Fever Group Rickettsioses and Acute Undifferentiated Febrile Illness in Villeta, Colombia. Am. J. Trop. Med. Hyg. 2017, 97, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, A.T.; Manzoli, D.E.; Fernandez, C.; Zurvera, D.; Monje, L.D. Anaplasma species infecting questing ticks in the Iberá wetlands ecoregion, Argentina. Exp. Appl. Acarol. 2023, 89, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Tarragona, E.L.; Sebastian, P.S.; Félix, M.L.; Venzal, J.M. Novel Anaplasma (Rickettsiales: Anaplasmataceae) strain and Hepatozoon sp. cf. H. procyonis (Apicomplexa, Hepatozoidae) detected in Procyon cancrivorus (Carnivora, Procyonidae) from Argentina, with note of tick-host association. Vet. Res. Commun. 2023. [Google Scholar] [CrossRef] [PubMed]
- Máttar, S.; Parra, M. Detection of antibodies to Anaplasma, Bartonella and Coxiella in rural inhabitants of the Caribbean area of Colombia. Rev. MVZ Cordoba 2006, 11, 781–789. [Google Scholar]
- Molina-Guzmán, L.P.; Ríos-Tobón, S.; Cardona-Lopera, X.; Lopera, J.A.; Ríos-Osorio, L.A.; Gutiérrez-Builes, L.A. Occupational history of exposure to zoonotic agents in people dedicated to livestock in San Pedro de los Milagros, Antioquia, Colombia. Rev. Fac. Med. 2019, 67, 399–405. [Google Scholar] [CrossRef]
- Cabrera, R.; Mendoza, W.; López-Mosquera, L.; Cano, M.A.; Ortiz, N.; Campo, V.; Keynan, Y.; López, L.; Rueda, Z.V.; Gutiérrez, L.A. Tick-Borne-Agents Detection in Patients with Acute Febrile Syndrome and Ticks from Magdalena Medio, Colombia. Pathogens 2022, 11, 1090. [Google Scholar] [CrossRef]
- Buelvas, F.; Alvis, N.; Buelvas, I.; Miranda, J.; Mattar, S. Alta Prevalencia de Anticuerpos contra Bartonella y Babesia microti en Poblaciones Rurales y Urbanas en dos Provincias de Córdoba, Colombia. Rev. Salud Publica 2008, 10, 168–177. [Google Scholar] [CrossRef]
- Faccini-Martínez, Á.A.; Márquez, A.C.; Bravo-Estupiñan, D.M.; Calixto, O.J.; López-Castillo, C.A.; Botero-García, C.A.; Hidalgo, M.; Cuervo, C. Bartonella quintana and Typhus Group Rickettsiae Exposure among Homeless Persons, Bogotá, Colombia. Emerg. Infect. Dis. 2017, 23, 1876–1879. [Google Scholar] [CrossRef]
- Miranda, J.; Mattar, S.; Perdomo, K.; Palencia, L. Seroprevalencia de borreliosis, o enfermedad de Lyme, en una población rural expuesta de Córdoba, Colombia. Rev. Salud Publica 2009, 11, 480–489. [Google Scholar] [CrossRef]
- Palacios, R.; Torres, A.; Trujillo, R. IgG antibody reactivity to Borrelia burgdorferi sensu stricto antigens in patients with morphea in Colombia. Int. J. Dermatol. 2003, 42, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Eraso-Cadena, M.P.; Molina-Guzmán, L.P.; Cardona, X.; Cardona-Arias, J.A.; Ríos-Osorio, L.A.; Gutierrez-Builes, L.A. Serological evidence of exposure to some zoonotic microorganisms in cattle and humans with occupational exposure to livestock in Antioquia, Colombia. Cad. Saude Publica 2018, 34, e001936172018. [Google Scholar] [CrossRef] [PubMed]
- de Ruiz, H.L. Q fever in Colombia, S.A. A serological survey of human and bovine populations. Zent. Vet. B. 1977, 24, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Orrego, R.; Ríos-Osorio, L.A.; Keynan, Y.; Rueda, Z.V.; Gutiérrez, L.A. Molecular detection of Coxiella burnetii in livestock farmers and cattle from Magdalena Medio in Antioquia, Colombia. PLoS ONE 2020, 15, e02343602020. [Google Scholar] [CrossRef]
- Contreras, V.; Máttar, S.; González, M.; Álvarez, J.; Oteo, J.A. Coxiella burnetii in bulk tank milk and antibodies in farm workers at Montería, Colombia. Rev. Colomb. Cienc. Pecu. 2015, 28, 181–187. [Google Scholar] [CrossRef]
- Faccini-Martínez, Á.A.; Silva-Ramos, C.R.; Blanton, L.S.; Arroyave, E.; Martínez-Diaz, H.C.; Betancourt-Ruiz, P.; Hidalgo, M.; Walker, D.H. Serologic Evidence of Orientia Infection among Rural Population, Cauca Department, Colombia. Emerg. Infect. Dis. 2023, 29, 456–459. [Google Scholar] [CrossRef]
- Welch, D.F. Bartonella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Saengsawang, P.; Kaewmongkol, G.; Phoosangwalthong, P.; Chimnoi, W.; Inpankaew, T. Detection of zoonotic Bartonella species in ticks and fleas parasitizing free-ranging cats and dogs residing in temples of Bangkok, Thailand. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100612. [Google Scholar] [CrossRef]
- Cheslock, M.A.; Embers, M.E. Human Bartonellosis: An Underappreciated Public Health Problem? Trop. Med. Infect. Dis. 2019, 4, 69. [Google Scholar] [CrossRef]
- Garcia-Quintanilla, M.; Dichter, A.A.; Guerra, H.; Kempf, V.A.J. Carrion’s disease: More than a neglected disease. Parasit. Vectors 2019, 12, 141. [Google Scholar] [CrossRef]
- Ruiz, J. Bartonella quintana, past, present, and future of the scourge of World War I. APMIS 2018, 126, 831–837. [Google Scholar] [CrossRef]
- Nelson, C.A.; Moore, A.R.; Perea, A.E.; Mead, P.S. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health 2018, 65, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Agan, B.K.; Dolan, M.J. Laboratory diagnosis of Bartonella infections. Clin. Lab. Med. 2002, 22, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.R.; Gilioli, R.; Velho, P.E. Bartonellosis diagnosis requires careful evaluation. Braz. J. Infect. Dis. 2010, 14, 217. [Google Scholar] [CrossRef]
- Patiño Camargo, L. Bartonellosis en colombia, bartonellosis de guáitara ó fiebre verrucosa del guáitara. Rev. Fac. Med. 1939, 7, 467–501. [Google Scholar]
- Mackehenie, D. La actualidad médico social. La verruga andina y su nuevo foco colombiano. Rev. Fac. Med. 1940, 8, 350–353. [Google Scholar]
- Patiño Camargo, L.; Cifuentes, P.; Herrera, M.S. El primer caso de Bartonellosis (fiebre verrucosa del guaitara o verruga) en Bogotá. Rev. Fac. Med. 1940, 9, 351–359. [Google Scholar]
- Groot, H. Anotaciones sobre la historia y origen de las bartonellosis humana, enfermedad de Carrion, en Colombia. Pasto, Colombia: Ministerio de Trabajo Higiene y Previsión Social. Pub Lab. Hig. Nariño 1942, 42, 1–7. [Google Scholar]
- Alexander, B. A review of bartonellosis in Ecuador and Colombia. Am. J. Trop. Med. Hyg. 1995, 52, 354–359. [Google Scholar] [CrossRef]
- Sotomayor Tribín, H.A.; Faccini Martínez, Á.A. Una mirada a la bartonelosis: A propósito de dos máscaras prehispánicas donadas al Museo de Historia de Medicina de la Academia Nacional de Medicina de Colombia. Medicina 2021, 43, 450–462. [Google Scholar] [CrossRef]
- Macías, A.; Aguirre, C.; Bustamante, A.; Garcés, C.; Echeverri, V.; Díaz, A. Cat scratch disease in Medellín, Colombia. Oxf. Med. Case Rep. 2014, 2014, 43–45. [Google Scholar] [CrossRef]
- Hurtado, I.C.; Laufer, M. Systemic cat scratch disease (Bartonella henselae infection): A cause of prolonged fever that should not be overlooked. Infectio 2017, 21, 69–72. [Google Scholar]
- Arango-Ferreira, C.; Castano, J. Parinaud’s Oculoglandular Syndrome in Cat Scratch Disease. N. Engl. J. Med. 2018, 379, e312018. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.C.; Chomel, B.B.; Singhasivanon, O.U.; Namekata, D.Y.; Kasten, R.W.; Kass, P.H.; Cortés-Vecino, J.A.; Gennari, S.M.; Rajapakse, R.P.; Huong, L.T.; et al. Bartonella infection in urban and rural dogs from the tropics: Brazil, Colombia, Sri Lanka and Vietnam. Epidemiol. Infect. 2013, 141, 54–61. [Google Scholar] [CrossRef]
- Margos, G.; Gofton, A.; Wibberg, D.; Dangel, A.; Marosevic, D.; Loh, S.M.; Oskam, C.; Fingerle, V. The genus Borrelia reloaded. PLoS ONE 2018, 13, e02084322018. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers. 2016, 2, 16090. [Google Scholar] [CrossRef]
- Cutler, S.J. Relapsing Fever Borreliae: A Global Review. Clin. Lab. Med. 2015, 35, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Faccini-Martínez, Á.A.; Silva-Ramos, C.R.; Santodomingo, A.M.; Ramírez-Hernández, A.; Costa, F.B.; Labruna, M.B.; Muñoz-Leal, S. Historical overview and update on relapsing fever group Borrelia in Latin America. Parasit. Vectors 2022, 15, 196. [Google Scholar] [CrossRef]
- Fotso Fotso, A.; Drancourt, M. Laboratory Diagnosis of Tick-Borne African Relapsing Fevers: Latest Developments. Front. Public. Health 2015, 3, 254. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.E.; Krishnavahjala, A.; Garcia, M.N.; Bermudez, S. Tick-Borne Relapsing Fever Spirochetes in the Americas. Vet. Sci. 2016, 3, 16. [Google Scholar] [CrossRef]
- Roca-García, M. Contribución al Estudio de la Fiebre Espiroquetal en Colombia; Universidad Nacional, Facultad de Medicina: Bogotá, Colombia, 1939. [Google Scholar]
- Franco, R. Informe presentado al sindicato de Muzo por la misión encargada de estudiar la epidemia de fiebre observada en la mina en los meses de marzo y abril de 1907. Rev. Med. Bogota. 1907, 28, 93–105. [Google Scholar]
- Franco, R.; Toro, G.; Martinez, J. Fiebre amarilla y fiebre espiroquetal. Sesiones Científicas del Centenario. Acad. Nac. Med. Bogota 1911, 1, 169–227. [Google Scholar]
- Robledo, E. Fiebre recurrente en Manizales. Bol. Med. 1907, 1, 113–118. [Google Scholar]
- Dunn, L.H. Studies on the South American tick, Ornithodoros venezuelensis Brumpt, in Colombia. Its prevalence, distribution, and importance as an intermediate host of relapsing fever. J. Parasitol. 1927, 13, 249–255. [Google Scholar] [CrossRef]
- Pampana, E.J. Notes on colombian relapsing fever. Trans. R. Soc. Trop. Med. Hyg. 1928, 21, 315–328. [Google Scholar] [CrossRef]
- Romero Garcia, A.M. La Fiebre Recurrente; Universidad Nacional, Facultad de Medicina: Bogotá, Colombia, 1940. [Google Scholar]
- Branda, J.A.; Steere, A.C. Laboratory Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 2021, 34, e00018-19. [Google Scholar] [CrossRef] [PubMed]
- Marinkelle, C.J.; Grose, E.S. Species of Borrelia from a Colombian bat (Natalus tumidirostris). Nature 1968, 218, 487. [Google Scholar] [CrossRef]
- Muñoz-Leal, S.; Faccini-Martínez, Á.A.; Pérez-Torres, J.; Chala-Quintero, S.M.; Herrera-Sepúlveda, M.T.; Cuervo, C.; Labruna, M.B. Novel Borrelia genotypes in bats from the Macaregua Cave, Colombia. Zoonoses Public Health 2021, 68, 12–18. [Google Scholar] [CrossRef]
- López, Y.; Muñoz-Leal, S.; Martínez, C.; Guzmán, C.; Calderón, A.; Martínez, J.; Galeano, K.; Muñoz, M.; Ramírez, J.D.; Faccini-Martínez, Á.A.; et al. Molecular evidence of Borrelia spp. in bats from Córdoba Department, northwest Colombia. Parasit. Vectors 2023, 16, 5. [Google Scholar] [CrossRef]
- López, Y.; Robayo-Sánchez, L.N.; Muñoz-Leal, S.; Aleman, A.; Arroyave, E.; Ramírez-Hernández, A.; Cortés-Vecino, J.A.; Mattar, S.; Faccini-Martínez, Á.A. Ornithodoros puertoricensis (Ixodida: Argasidae) Associated with Domestic Fowl in Rural Dwellings From Córdoba Department, Caribbean Colombia. Front. Vet. Sci. 2021, 8, 704399. [Google Scholar] [CrossRef]
- Bermúdez, S.E.; Armstrong, B.A.; Domínguez, L.; Krishnavajhala, A.; Kneubehl, A.R.; Gunter, S.M.; Replogle, A.; Petersen, J.M.; Lopez, J.E. Isolation and genetic characterization of a relapsing fever spirochete isolated from Ornithodoros puertoricensis collected in central Panama. PLoS Negl. Trop. Dis. 2021, 15, e00096422021. [Google Scholar] [CrossRef]
- Drancourt, M.; Raoult, D. Coxiella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q Fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef]
- Oyston, P.C.F.; Davies, C. Q fever: The neglected biothreat agent. J. Med. Microbiol. 2011, 60, 9–21. [Google Scholar] [CrossRef]
- Melenotte, C.; Million, M.; Raoult, D. New insights in Coxiella burnetii infection: Diagnosis and therapeutic update. Expert. Rev. Anti Infect. Ther. 2020, 18, 75–86. [Google Scholar] [CrossRef]
- Greiner, A.L.; Bhengsri, S.; Million, M.; Edouard, S.; Thamthitiwat, S.; Clarke, K.; Kersh, G.J.; Gregory, C.J.; Raoult, D.; Parola, P. Acute Q Fever Case Detection among Acute Febrile Illness Patients, Thailand, 2002–2005. Am. J. Trop. Med. Hyg. 2018, 98, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR. Recomm. Rep. 2013, 62, 1–30. [Google Scholar] [PubMed]
- Betancur, C.A.; Múnera, A.G. Endocarditis por Coxiella burnetii: Fiebre Q. Acta Med. Colomb. 2012, 37, 31–33. [Google Scholar] [CrossRef]
- Meza-Cardona, J.C.; Rosso-Suárez, F. Neumonía por Coxiella burnetii: Presentación de un caso y revisión de la literatura. CES Medicina 2012, 26, 201–207. [Google Scholar]
- Mattar, S.; Contreras, V.; González, M.; Camargo, F.; Álvarez, J.; Oteo, J.A. Coxiella burnetii infection in a patient from a rural area of Monteria, Colombia. Rev. Salud Publica 2014, 16, 958–961. [Google Scholar] [CrossRef]
- Uribe Pulido, N.; Escorcia García, C.; Cabrera Orrego, R.; Gutiérrez, L.A.; Agudelo, C.A. Acute Q Fever with Dermatologic Manifestations, Molecular Diagnosis, and No Seroconversion. Open. Forum Infect. Dis. 2021, 8, ofab458. [Google Scholar] [CrossRef]
- Contreras, V.; Gonzalez, M.; Alvarez, J.; Mattar, S. Coxiella burnetii infection in sheep and goats: A public risk health, Colombia. Infectio 2018, 22, 173–177. [Google Scholar] [CrossRef]
- Silva-Ramos, C.R.; Faccini-Martínez, Á.A.; Pérez-Torres, J.; Hidalgo, M.; Cuervo, C. First molecular evidence of Coxiella burnetii in bats from Colombia. Res. Vet. Sci. 2022, 150, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Rikihisa, Y.; Dasch, G.A. Ehrlichia. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Zweygarth, E.; Vancová, M.; Broniszewska, M.; Grubhoffer, L.; Passos, L.M.F.; Ribeiro, M.F.B.; Alberdi, P.; de la Fuente, J. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int. J. Syst. Evol. Microbiol. 2016, 66, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Allerdice, M.E.J.; Sloan, L.M.; Paddock, C.D.; Munderloh, U.G.; Rikihisa, Y.; Tajima, T.; Paskewitz, S.M.; Neitzel, D.F.; Hoang Johnson, D.K.; et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int. J. Syst. Evol. Microbiol. 2017, 67, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Dumler, J.S.; Carlyon, J.A. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert. Rev. Anti Infect. Ther. 2009, 7, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, D.B.; Dawson, J.E.; Robinson, L.E. Human ehrlichiosis in the United States, 1985 to 1990. Ann. Intern. Med. 1994, 120, 736–743. [Google Scholar] [CrossRef]
- Johnson, D.K.; Schiffman, E.K.; Davis, J.P.; Neitzel, D.F.; Sloan, L.M.; Nicholson, W.L.; Fritsche, T.R.; Steward, C.R.; Ray, J.A.; Miller, T.K.; et al. Human Infection with Ehrlichia muris-like Pathogen, United States, 2007–2013. Emerg. Infect. Dis. 2015, 21, 1794–1799. [Google Scholar] [CrossRef]
- Paddock, C.D.; Liddell, A.M.; Storch, G.A. Other causes of tick-borne ehrlichioses, including Ehrlichia ewingii. In Tick-Borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005. [Google Scholar]
- Franco-Zetina, M.; Adame-Gallegos, J.; Dzul-Rosado, K. Efectividad de los métodos diagnósticos para la detección de ehrlichiosis monocítica humana y canina. Rev. Chil. Infectol. 2019, 36, 650–655. [Google Scholar] [CrossRef]
- Montes Farah, J.; De la Vega del Risco, F.; Bello Espinosa, A.; Fortich Salvador, A.S. Coinfección de babesiosis y ehrlichiosis: Un caso en Cartagena de indias, Colombia. Rev. Cienc. Biomed. 2012, 3, 339–345. [Google Scholar]
- Hidrón Botero, A.; Muñoz Ramirez, F.; Vega Miranda, J. Primer caso de ehrlichiosis monocítica humana reportado en Colombia. Infectio 2014, 18, 158–161. [Google Scholar] [CrossRef]
- De la Espriella Pérez, A.; Restrepo Gouzi, A.V.; Trujillo Honeysberg, M.R.; Calle Echeverri, D.A. Ehrlichia monocítica humana: Primer reporte de caso pediátrico en Colombia. Rev. Lat. Infect. Pediatr. 2021, 34, 41–47. [Google Scholar] [CrossRef]
- Martínez Díaz, H.C.; Gil-Mora, J.; Betancourt-Ruiz, P.; Silva-Ramos, C.R.; Matiz-González, J.M.; Villalba-Perez, M.A.; Ospina-Pinto, M.C.; Ramirez-Hernández, A.; Olaya-M, L.A.; Bolaños, E.; et al. Molecular detection of tick-borne rickettsial pathogens in ticks collected from domestic animals from Cauca, Colombia. Acta Trop. 2023, 238, 106773. [Google Scholar] [CrossRef] [PubMed]
- Forero-Becerra, E.; Patel, J.; Martínez-Díaz, H.C.; Betancourt-Ruiz, P.; Benavides, E.; Durán, S.; Olaya-Másmela, L.A.; Bolaños, E.; Hidalgo, M.; McBride, J.W. Seroprevalence and Genotypic Analysis of Ehrlichia canis Infection in Dogs and Humans in Cauca, Colombia. Am. J. Trop. Med. Hyg. 2021, 104, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Walker, D.H. Orientia. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Silva-Ramos, C.R.; Jacinavicius, F.C.; Weitzel, T.; Walker, D.H.; Faccini-Martínez, Á.A. Scrub typhus: A new cause of acute undifferentiated febrile illness in Latin America? Travel. Med. Infect. Dis. 2021, 43, 102138. [Google Scholar] [CrossRef]
- Rajapakse, S.; Weeratunga, P.; Sivayoganathan, S.; Fernando, S.D. Clinical manifestations of scrub typhus. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.V.; Sudarsan, T.I.; Prakash, J.A.; Varghese, G.M. Severe scrub typhus infection: Clinical features, diagnostic challenges and management. World J. Crit. Care Med. 2015, 4, 244–250. [Google Scholar] [CrossRef]
- Kala, D.; Gupta, S.; Nagraik, R.; Verma, V.; Thakur, A.; Kaushal, A. Diagnosis of scrub typhus: Recent advancements and challenges. Biotech 2020, 10, 396. [Google Scholar] [CrossRef]
- Jiang, J.; Martínez-Valdebenito, C.; Weitzel, T.; Farris, C.M.; Acosta-Jamett, G.; Abarca, K.; Richards, A.L. Development of a New Genus-Specific Quantitative Real-Time PCR Assay for the Diagnosis of Scrub Typhus in South America. Front. Med. 2022, 9, 831045. [Google Scholar] [CrossRef]
- Jiang, J.; Richards, A.L. Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle. Trop. Med. Infect. Dis. 2018, 3, 11. [Google Scholar] [CrossRef]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.; et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef]
- Yen, T.Y.; Zhang, Z.; Chao, C.C.; Ching, W.M.; Shu, P.Y.; Tseng, L.F.; Carvalho, A.V.A.; Tsai, K.H. Serologic Evidence for Orientia Exposure in the Democratic Republic of Sao Tome and Principe. Vector Borne Zoonotic Dis. 2019, 19, 821–827. [Google Scholar] [CrossRef]
- Abarca, K.; Martínez-Valdebenito, C.; Angulo, J.; Jiang, J.; Farris, C.M.; Richards, A.L.; Acosta-Jamett, G.; Weitzel, T. Molecular Description of a Novel Orientia Species Causing Scrub Typhus in Chile. Emerg. Infect. Dis. 2020, 26, 2148–2156. [Google Scholar] [CrossRef]
- Weitzel, T.; Acosta-Jamett, G.; Jiang, J.; Martínez-Valdebenito, C.; Farris, C.M.; Richards, A.L.; Abarca, K. Human seroepidemiology of Rickettsia and Orientia species in Chile—A cross-sectional study in five regions. Ticks Tick. Borne Dis. 2020, 11, 101503. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Silva-de la Fuente, M.C.; Martínez-Valdebenito, C.; Stekolnikov, A.A.; Pérez, C.; Pérez, R.; Vial, C.; Abarca, K.; Acosta-Jamett, G. Novel Vector of Scrub Typhus in Sub-Antarctic Chile: Evidence from Human Exposure. Clin. Infect. Dis. 2022, 74, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Kocher, C.; Jiang, J.; Morrison, A.C.; Castillo, R.; Leguia, M.; Loyola, S.; Ampuero, J.S.; Cespedes, M.; Halsey, E.S.; Bausch, D.G.; et al. Serologic Evidence of Scrub Typhus in the Peruvian Amazon. Emerg. Infect. Dis. 2017, 23, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Zhang, Z.; Belinskaya, T.; Chen, H.W.; Ching, W.M. Leptospirosis and Rickettsial Diseases Sero-Conversion Surveillance Among U.S. Military Personnel in Honduras. Mil. Med. 2022, 187, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Raoult, D. Rickettsia. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shpynov, S.; Pozdnichenko, N.; Gumenuk, A. Approach for classification and taxonomy within family Rickettsiaceae based on the Formal Order Analysis. Microbes Infect. 2015, 17, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Blanton, L.S.; Walker, D.H. Rickettsiae as Emerging Infectious Agents. Clin. Lab. Med. 2017, 37, 383–400. [Google Scholar] [CrossRef]
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. North. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Premaratna, R. Rickettsial illnesses, a leading cause of acute febrile illness. Clin. Med. 2022, 22, 2–5. [Google Scholar] [CrossRef]
- Jay, R.; Armstrong, P.A. Clinical characteristics of Rocky Mountain spotted fever in the United States: A literature review. J. Vector Borne Dis. 2020, 57, 114–120. [Google Scholar] [CrossRef]
- Bechah, Y.; Capo, C.; Mege, J.L.; Raoult, D. Epidemic typhus. Lancet Infect. Dis. 2008, 8, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Tsioutis, C.; Zafeiri, M.; Avramopoulos, A.; Prousali, E.; Miligkos, M.; Karageorgos, S.A. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: A systematic review. Acta Trop. 2017, 166, 16–24. [Google Scholar] [CrossRef]
- Blanton, L.S. Rickettsiales: Laboratory Diagnosis. In Rickettsiales; Thomas, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Stewart, A.G.; Stewart, A.G.A. An Update on the Laboratory Diagnosis of Rickettsia spp. Infection. Pathogens 2021, 10, 1319. [Google Scholar] [CrossRef]
- Myers, T.; Lalani, T.; Dent, M.; Jiang, J.; Daly, P.L.; Maguire, J.D.; Richards, A.L. Detecting Rickettsia parkeri infection from eschar swab specimens. Emerg. Infect. Dis. 2013, 19, 778–780. [Google Scholar] [CrossRef]
- Cuéllar-Sáenz, J.A.; Faccini-Martínez, Á.A.; Ramírez-Hernández, A.; Cortés-Vecino, J.A. Rickettsioses in Colombia during the 20th century: A historical review. Ticks Tick. Borne Dis. 2023, 14, 102118. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Aguirre, L.M.; Martínez-Díaz, H.C.; Betancourt-Ruiz, P.; Hidalgo, M. Historia de la rickettsiosis en Colombia. In Enfermedades Rickettsiales en Latinoamérica; Posada Arias, S., Cabrera Jaramillo, A., Monsalve Buriticá, S., Eds.; Fondo Editorial Biogénesis: Medellín, Colombia, 2020; pp. 218–239. [Google Scholar]
- Gómez-Quintero, C.H.; Faccini-Martínez, Á.A.; Botero-García, C.A.; Lozano, M.; Sánchez-Lerma, L.; Miranda, J.; Mattar, S.; Hidalgo, M. Probable case of spotted fever group rickettsial infection in a new suspected endemic area, Colombia. J. Infect. Public. Health 2017, 10, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Páez, F.A.; Martins, T.F.; Ossa-López, P.A.; Sampieri, B.R.; Camargo-Mathias, M.I. Detection of Rickettsia spp. in ticks (Acari: Ixodidae) of domestic animals in Colombia. Ticks Tick. Borne Dis. 2018, 9, 819–823. [Google Scholar] [CrossRef]
- Patino, L.; Afanador, A.; Paul, J.H. A spotted fever in Tobia, Colombia. Preliminary report. Am. J. Trop. Med. Hyg. 1937, 17, 639–653. [Google Scholar] [CrossRef]
- Patiño Camargo, L. Nuevas observaciones sobre un tercer foco de fiebre petequial (Maculosa) en el Hemisferio Americano. Boletín Oficina Sanit. Panamericana 1941, 20. Available online: https://iris.paho.org/handle/10665.2/13254 (accessed on 1 May 2023).
- Hidalgo, M.; Orejuela, L.; Fuya, P.; Carrillo, P.; Hernandez, J.; Parra, E.; Keng, C.; Small, M.; Olano, J.P.; Bouyer, D.; et al. Rocky Mountain spotted fever, Colombia. Emerg. Infect. Dis. 2007, 13, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Salguero, E.; de la Ossa, A.; Sánchez, R.; Vesga, J.F.; Orejuela, L.; Valbuena, G. Murine typhus in Caldas, Colombia. Am. J. Trop. Med. Hyg. 2008, 78, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Montoya, V.; Martínez, A.; Mercado, M.; De la Ossa, A.; Vélez, C.; Estrada, G.; Pérez, J.E.; Faccini-Martínez, A.A.; Labruna, M.B.; et al. Flea-borne rickettsioses in the north of Caldas province, Colombia. Vector Borne Zoonotic Dis. 2013, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.E.; Estrada, G.I.; Zapata, Y.; Hidalgo, M.; Serna, C.C.; Castro, D.C.; González, C. Frecuencia de anticuerpos y seroconversión frente a Rickettsia spp. en pacientes atendidos en instituciones de salud del departamento de Caldas, Colombia, 2016–2019. Biomedica 2021, 41, 103–117. [Google Scholar] [CrossRef]
- Gil-Lora, E.J.; Patiño-Gallego, J.J.; Acevedo-Gutiérrez, L.Y.; Montoya-Ruiz, C.; Rodas-González, J.D. Rickettsia spp. infection of the group of spotted fevers in febrile patients of the Urabá Antioquia, Colombia. Iatreia 2019, 32, 167–176. [Google Scholar] [CrossRef]
- Arboleda, M.; Acevedo-Gutiérrez, L.Y.; Ávila, A.; Ospina, D.; Díaz, F.J.; Walker, D.H.; Rodas, J.D. Human Rickettsiosis Caused by Rickettsia parkeri Strain Atlantic Rainforest, Urabá, Colombia. Emerg. Infect. Dis. 2020, 26, 3048–3050. [Google Scholar] [CrossRef]
- Quintero Vélez, J.C.; Faccini-Martínez, Á.A.; Rodas González, J.D.; Díaz, F.J.; Ramírez García, R.; Somoyar Ordosgoitia, P.; Parra Saad, E.A.; Osorio Quintero, L.; Rojas Arbeláez, C. Fatal Rickettsia rickettsii infection in a child, Northwestern Colombia, 2017. Ticks Tick. Borne Dis. 2019, 10, 995–996. [Google Scholar] [CrossRef]
- Ramírez-García, R.; Quintero, J.C.; Rosado, A.P.; Arboleda, M.; González, V.A.; Agudelo-Flórez, P. Leptospirosis y rickettsiosis, reto diagnóstico para el síndrome febril en zonas endémicas. Biomedica 2021, 41, 208–217. [Google Scholar] [CrossRef]
- Londoño, A.F.; Arango-Ferreira, C.; Acevedo-Gutiérrez, L.Y.; Paternina, L.E.; Montes, C.; Ruiz, I.; Labruna, M.B.; Díaz, F.J.; Walker, D.H.; Rodas, J.D. A Cluster of Cases of Rocky Mountain Spotted Fever in an Area of Colombia Not Known to be Endemic for This Disease. Am. J. Trop. Med. Hyg. 2019, 101, 336–342. [Google Scholar] [CrossRef]
- Quintero-Vélez, J.C.; Cienfuegos-Gallet, A.V.; Quintero, L.O.; Úsuga, A.F.; Cifuentes, S.; Solari, S.; Rodas, J.D.; Diaz, F.J.; Rojas, C.A. Epidemiology of Rickettsial Infection in the Municipality of Uramita, Colombia. Am. J. Trop. Med. Hyg. 2021, 105, 1013–1023. [Google Scholar] [CrossRef]
- Hidalgo, M.; Miranda, J.; Heredia, D.; Zambrano, P.; Vesga, J.F.; Lizarazo, D.; Mattar, S.; Valbuena, G. Outbreak of Rocky Mountain spotted fever in Córdoba, Colombia. Mem. Inst. Oswaldo Cruz. 2011, 106, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Londoño, A.F.; Acevedo-Gutiérrez, L.Y.; Marín, D.; Contreras, V.; Díaz, F.J.; Valbuena, G.; Labruna, M.B.; Hidalgo, M.; Arboleda, M.; Mattar, S.; et al. Human prevalence of the spotted fever group (SFG) rickettsiae in endemic zones of Northwestern Colombia. Ticks Tick. Borne Dis. 2017, 8, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Salazar, C.A.; Recalde-Reyes, D.P.; González, M.M.; Padilla Sanabria, L.; Quintero-Álvarez, L.; Gallego-Gómez, J.C.; Castano-Osorio, J.C. Clinical manifestations and laboratory findings on a case series of acute febrile syndrome with a presumptive diagnosis of dengue virus infection. Quindio, Colombia. Infectio 2016, 20, 84–92. [Google Scholar] [CrossRef]
- Patino-Nino, J.A.; Pérez-Camacho, P.M.; Aguirre-Recalde, J.A.; Faccini-Martínez, Á.A.; Montenegro-Herrera, C.A.; Hidalgo, M. Probable case of murine typhus with respiratory failure in an adolescent from the urban area of Cali, Colombia. Infectio 2016, 20, 97–100. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).