Host-Driven Phosphorylation Appears to Regulate the Budding Activity of the Lassa Virus Matrix Protein
Abstract
1. Introduction
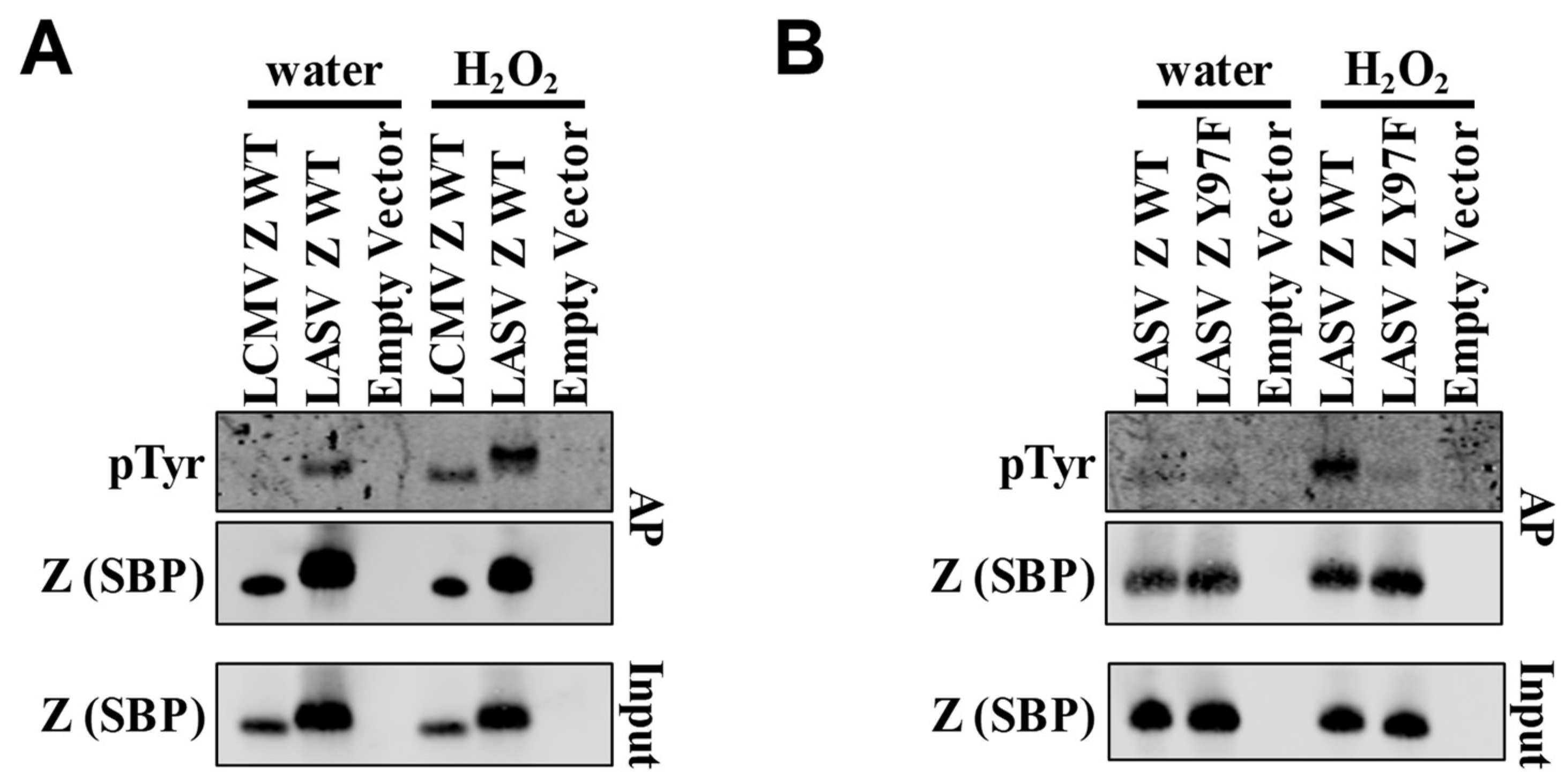
2. Results and Discussion
3. Materials and Methods
3.1. Cells and Plasmids
3.2. Identification of Phosphorylation Sites by Mass Spectrometry
3.3. Detection of Phosphoproteins by Western Blotting
3.4. Virus-Like Particle Release Assay
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Charrel, R.N.; Lamballerie, X.D. Arenaviruses other than lassa virus. Antivir. Res. 2003, 57, 89–100. [Google Scholar] [CrossRef]
- Monath, T.P.; Newhouse, V.F.; Kemp, G.E.; Setzer, H.W.; Cacciapuoti, A. Lassa virus isolation from mastomys natalensis rodents during an epidemic in sierra leone. Science 1974, 185, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulemou, K.; Sylla, O.; Kourouma, F.; Dore, A.; Soropogui, B.; Aniskin, V.; Allali, B.; et al. Mastomys natalensis and lassa fever, west africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Olayemi, A.; Cadar, D.; Magassouba, N.F.; Obadare, A.; Kourouma, F.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Rieger, T.; Bockholt, S.; et al. New hosts of the lassa virus. Sci. Rep. 2016, 6, 25280. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa fever. N. Engl. J. Med. 1986, 314, 20–26. [Google Scholar] [CrossRef]
- Bonwitt, J.; Kelly, A.H.; Ansumana, R.; Agbla, S.; Sahr, F.; Saez, A.M.; Borchert, M.; Kock, R.; Fichet-Calvet, E. Rat-atouille: A mixed method study to characterize rodent hunting and consumption in the context of lassa fever. Ecohealth 2016, 13, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Lassa fever: Review of epidemiology and epizootiology. Bull. World Health Organ. 1975, 52, 577–592. [Google Scholar]
- McCormick, J.B.; Webb, P.A.; Krebs, J.W.; Johnson, K.M.; Smith, E.S. A prospective study of the epidemiology and ecology of lassa fever. J. Infect. Dis. 1987, 155, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Frame, J.D.; Baldwin, J.M.; Gocke, D.J.; Troup, J.M. Lassa fever, a new virus disease of man from west africa: I. Clinical description and pathological findings. Am. J. Trop. Med. Hyg. 1970, 19, 670–676. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Tomori, O.; Nasidi, A.; Perez-Oronoz, G.I.; Fakile, Y.; Hutwagner, L.; McCormick, J.B. Review of cases of nosocomial lassa fever in nigeria: The high price of poor medical practice. Br. Med. J. 1995, 311, 857–859. [Google Scholar] [CrossRef]
- Ajayi, N.A.; Nwigwe, C.G.; Azuogu, B.N.; Onyire, B.N.; Nwonwu, E.U.; Ogbonnaya, L.U.; Onwe, F.I.; Ekaete, T.; Günther, S.; Ukwaja, K.N. Containing a lassa fever epidemic in a resource-limited setting: Outbreak description and lessons learned from abakaliki, nigeria (January–March 2012). Int. J. Infect. Dis. 2013, 17, e1011–e1016. [Google Scholar] [CrossRef]
- Shaffer, J.G.; Grant, D.S.; Schieffelin, J.S.; Boisen, M.L.; Goba, A.; Hartnett, J.N.; Levy, D.C.; Yenni, R.E.; Moses, L.M.; Fullah, M.; et al. Lassa fever in post-conflict sierra leone. PLOS Negl. Trop. Dis. 2014, 8, e2748. [Google Scholar] [CrossRef]
- Price, M.E.; Fisher-Hoch, S.P.; Craven, R.B.; McCormick, J.B. A prospective study of maternal and fetal outcome in acute lassa fever infection during pregnancy. Br. Med. J. 1988, 297, 584–587. [Google Scholar] [CrossRef]
- Cummins, D.; McCormick, J.B.; Bennett, D.; Samba, J.A.; Farrar, B.; Machin, S.J.; Fisher-Hoch, S.P. Acute sensorineural deafness in lassa fever. JAMA 1990, 264, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.C.S.; Wilson, S.M.; Oram, J.D. Nucleotide sequence of the s rna of lassa virus (nigerian strain) and comparative analysis of arenavirus gene products. Virus Res. 1991, 18, 151–164. [Google Scholar] [CrossRef]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B.A. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and lassa fever virus. Science 1998, 282, 2079–2081. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S. Receptor binding and cell entry of old world arenaviruses reveal novel aspects of virus-host interaction. Virology 2009, 387, 245–249. [Google Scholar] [CrossRef]
- Klewitz, C.; Klenk, H.-D.; ter Meulen, J. Amino acids from both n-terminal hydrophobic regions of the lassa virus envelope glycoprotein gp-2 are critical for ph-dependent membrane fusion and infectivity. J. Gen. Virol. 2007, 88, 2320–2328. [Google Scholar] [CrossRef]
- Lee, K.J.; Novella, I.S.; Teng, M.N.; Oldstone, M.B.A.; de la Torre, J.C. Np and l proteins of lymphocytic choriomeningitis virus (lcmv) are sufficient for efficient transcription and replication of lcmv genomic RNA analogs. J. Virol. 2000, 74, 3470–3477. [Google Scholar] [CrossRef]
- Lopez, N.; Jacamo, R.; Franze-Fernandez, M.T. Transcription and rna replication of tacaribe virus genome and antigenome analogs require n and l proteins: Z protein is an inhibitor of these processes. J. Virol. 2001, 75, 12241–12251. [Google Scholar] [CrossRef]
- Djavani, M.; Lukashevich, I.S.; Sanchez, A.; Nichol, S.T.; Salvato, M.S. Completion of the lassa fever virus sequence and identification of a ring finger open reading frame at the l rna 5′ end. Virology 1997, 235, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Lukashevich, I.S.; Djavani, M.; Shapiro, K.; Sanchez, A.; Ravkov, E.; Nichol, S.T.; Salvato, M.S. The lassa fever virus l gene: Nucleotide sequence, comparison, and precipitation of a predicted 250 kda protein with monospecific antiserum. J. Gen. Virol. 1997, 78, 547–551. [Google Scholar] [CrossRef]
- Strecker, T.; Eichler, R.; Meulen, J.t.; Weissenhorn, W.; Dieter Klenk, H.; Garten, W.; Lenz, O. Lassa virus z protein is a matrix protein sufficient for the release of virus-like particles. J. Virol. 2003, 77, 10700–10705. [Google Scholar] [CrossRef] [PubMed]
- Fehling, S.; Lennartz, F.; Strecker, T. Multifunctional nature of the arenavirus ring finger protein z. Viruses 2012, 4, 2973–3011. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Ly, H.; Liang, Y. The z proteins of pathogenic but not nonpathogenic arenaviruses inhibit rig-i-like receptor-dependent interferon production. J. Virol. 2015, 89, 2944–2955. [Google Scholar] [CrossRef] [PubMed]
- Cornu, T.I.; de la Torre, J.C. Ring finger z protein of lymphocytic choriomeningitis virus (lcmv) inhibits transcription and rna replication of an lcmv s-segment minigenome. J. Virol. 2001, 75, 9415–9426. [Google Scholar] [CrossRef] [PubMed]
- Jacamo, R.; Lopez, N.; Wilda, M.; Franze-Fernández, M.T. Tacaribe virus z protein interacts with the l polymerase protein to inhibit viral rna synthesis. J Virol 2003, 77. [Google Scholar] [CrossRef]
- Kranzusch, P.J.; Whelan, S.P.J. Arenavirus z protein controls viral rna synthesis by locking a polymerase–promoter complex. Kranzusch, P.J.; Whelan, S.P.J. Arenavirus z protein controls viral rna synthesis by locking a polymerase–promoter complex. Proc. Natl. Acad. Sci. USA 2011, 108, 19743–19748. [Google Scholar] [CrossRef]
- Schlie, K.; Maisa, A.; Freiberg, F.; Groseth, A.; Strecker, T.; Garten, W. Viral protein determinants of lassa virus entry and release from polarized epithelial cells. J. Virol. 2010, 84, 3178–3188. [Google Scholar] [CrossRef]
- Capul, A.A.; Perez, M.; Burke, E.; Kunz, S.; Buchmeier, M.J.; de la Torre, J.C. Arenavirus z-glycoprotein association requires z myristoylation but not functional ring or late domains. J. Virol. 2007, 81, 9451–9460. [Google Scholar] [CrossRef]
- Eichler, R.; Strecker, T.; Kolesnikova, L.; ter Meulen, J.; Weissenhorn, W.; Becker, S.; Klenk, H.D.; Garten, W.; Lenz, O. Characterization of the lassa virus matrix protein z: Electron microscopic study of virus-like particles and interaction with the nucleoprotein (np). Virus Res. 2004, 100, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Craven, R.C.; de la Torre, J.C. The small ring finger protein z drives arenavirus budding: Implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 2003, 100, 12978–12983. [Google Scholar] [CrossRef] [PubMed]
- Zaza, A.D.; Herbreteau, C.H.; Peyrefitte, C.N.; Emonet, S.F. Mammarenaviruses deleted from their z gene are replicative and produce an infectious progeny in bhk-21 cells. Virology 2018, 518, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Strecker, T.; Maisa, A.; Daffis, S.; Eichler, R.; Lenz, O.; Garten, W. The role of myristoylation in the membrane association of the lassa virus matrix protein z. Virol. J. 2006, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Greenwald, D.L.; de La Torre, J.C. Myristoylation of the ring finger z protein is essential for arenavirus budding. J. Virol. 2004, 78, 11443–11448. [Google Scholar] [CrossRef] [PubMed]
- Votteler, J.; Sundquist, W.I. Virus budding and the escrt pathway. Cell Host Microbe 2013, 14, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Danzy, S.; Kumar, N.; Ly, H.; Liang, Y. Biological roles and functional mechanisms of arenavirus z protein in viral replication. J. Virol. 2012, 86, 9794–9801. [Google Scholar] [CrossRef]
- Urata, S.; Noda, T.; Kawaoka, Y.; Yokosawa, H.; Yasuda, J. Cellular factors required for lassa virus budding. J. Virol. 2006, 80, 4191–4195. [Google Scholar] [CrossRef]
- Han, Z.; Lu, J.; Liu, Y.; Davis, B.; Lee, M.S.; Olson, M.A.; Ruthel, G.; Freedman, B.D.; Schnell, M.J.; Wrobel, J.E.; et al. Small-molecule probes targeting the viral ppxy-host nedd4 interface block egress of a broad range of rna viruses. J Virol 2014, 88. [Google Scholar] [CrossRef]
- Ziegler, C.M.; Eisenhauer, P.; Bruce, E.A.; Weir, M.E.; King, B.R.; Klaus, J.P.; Krementsov, D.N.; Shirley, D.J.; Ballif, B.A.; Botten, J. The lymphocytic choriomeningitis virus matrix protein ppxy late domain drives the production of defective interfering particles. PLoS Pathog 2016, 12, e1005501. [Google Scholar] [CrossRef]
- Nishi, H.; Shaytan, A.; Panchenko, A.R. Physicochemical mechanisms of protein regulation by phosphorylation. Front. Genet. 2014, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- Keck, F.; Ataey, P.; Amaya, M.; Bailey, C.; Narayanan, A. Phosphorylation of single stranded rna virus proteins and potential for novel therapeutic strategies. Viruses 2015, 7, 5257–5273. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.; Church, G.M. Collection and motif-based prediction of phosphorylation sites in human viruses. Sci. Signal. 2010, 3, rs2. [Google Scholar] [CrossRef] [PubMed]
- Bretaña, N.A.; Lu, C.-T.; Chiang, C.-Y.; Su, M.-G.; Huang, K.-Y.; Lee, T.-Y.; Weng, S.-L. Identifying protein phosphorylation sites with kinase substrate specificity on human viruses. PLoS ONE 2012, 7, e40694. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.A.; Striker, R. Phosphorylation events during viral infections provide potential therapeutic targets. Rev. Med. Virol. 2012, 22, 166–181. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Cooper, A.; Shi, W.; Bornmann, W.; Carrion, R.; Kalman, D.; Nabel, G.J. Productive replication of ebola virus is regulated by the c-abl1 tyrosine kinase. Sci. Transl. Med. 2012, 4, 123ra124. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, L.; Mittler, E.; Schudt, G.; Shams-Eldin, H.; Becker, S. Phosphorylation of marburg virus matrix protein vp40 triggers assembly of nucleocapsids with the viral envelope at the plasma membrane. Cell. Microbiol. 2012, 14, 182–197. [Google Scholar] [CrossRef]
- Bajorek, M.; Caly, L.; Tran, K.C.; Maertens, G.N.; Tripp, R.A.; Bacharach, E.; Teng, M.N.; Ghildyal, R.; Jans, D.A. The thr205 phosphorylation site within respiratory syncytial virus matrix (m) protein modulates m oligomerization and virus production. J. Virol. 2014. [Google Scholar] [CrossRef]
- Pei, Z.; Harrison, M.S.; Schmitt, A.P. Parainfluenza virus 5 m protein interaction with host protein 14-3-3 negatively affects virus particle formation. J. Virol. 2011, 85, 2050–2059. [Google Scholar] [CrossRef]
- Hemonnot, B.; Mollé, D.; Bardy, M.; Gay, B.; Laune, D.; Devaux, C.; Briant, L. Phosphorylation of the htlv-1 matrix l-domain-containing protein by virus-associated erk-2 kinase. Virology 2006, 349, 430–439. [Google Scholar] [CrossRef]
- Ziegler, C.M.; Eisenhauer, P.; Bruce, E.A.; Beganovic, V.; King, B.R.; Weir, M.E.; Ballif, B.A.; Botten, J. A novel phosphoserine motif in the lcmv matrix protein z regulates the release of infectious virus and defective interfering particles. J. Gen. Virol. 2016, 97, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Volpon, L.; Osborne, M.J.; Capul, A.A.; de la Torre, J.C.; Borden, K.L.B. Structural characterization of the z ring-eif4e complex reveals a distinct mode of control for eif4e. Proc. Natl. Acad. Sci. USA 2010, 107, 5441–5446. [Google Scholar] [CrossRef] [PubMed]
- Salvato, M.S.; Schweighofer, K.J.; Burns, J.; Shimomaye, E.M. Biochemical and immunological evidence that the 11 kda zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 1992, 22, 185–198. [Google Scholar] [CrossRef]
- Salvato, M.S.; Shimomaye, E.M. The completed sequence of lymphocytic choriomeningitis virus reveals a unique rna structure and a gene for a zinc finger protein. Virology 1989, 173, 1–10. [Google Scholar] [CrossRef]
- Hastie, K.M.; Zandonatti, M.; Liu, T.; Li, S.; Woods, V.L.; Saphire, E.O. Crystal structure of the oligomeric form of lassa virus matrix protein z. J. Virol. 2016, 90, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Volpon, L.; Osborne, M.J.; Borden, K.L.B. Nmr assignment of the arenaviral protein z from lassa fever virus. Biomol. NMR Assign. 2008, 2, 81–84. [Google Scholar] [CrossRef]
- May, E.R.; Armen, R.S.; Mannan, A.M.; Brooks, C.L. The flexible c-terminal arm of the lassa arenavirus z-protein mediates interactions with multiple binding partners. Proteins 2010, 78, 2251–2264. [Google Scholar] [CrossRef]
- Capul, A.A.; de la Torre, J.C.; Buchmeier, M.J. Conserved residues in lassa fever virus z protein modulate viral infectivity at the level of the ribonucleoprotein. J. Virol. 2011, 85, 3172–3178. [Google Scholar] [CrossRef]
- Xing, J.; Chai, Z.; Ly, H.; Liang, Y. Differential inhibition of macrophage activation by lymphocytic choriomeningitis virus and pichinde virus is mediated by the z protein n-terminal domain. J. Virol. 2015, 89, 12513–12517. [Google Scholar] [CrossRef]
- Li, W.; Cowley, A.; Uludag, M.; Gur, T.; McWilliam, H.; Squizzato, S.; Park, Y.M.; Buso, N.; Lopez, R. The embl-ebi bioinformatics web and programmatic tools framework. Nucleic Acids Res. 2015, 43, W580–W584. [Google Scholar] [CrossRef]
- Manning, J.T.; Forrester, N.; Paessler, S. Lassa virus isolates from mali and the ivory coast represent an emerging fifth lineage. Front. Microbiol. 2015, 6, 1037. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.D.; Rollin, P.E.; Ksiazek, T.G.; Hustad, H.L.; Bausch, D.G.; Demby, A.H.; Bajani, M.D.; Peters, C.J.; Nichol, S.T. Genetic diversity among lassa virus strains. J. Virol. 2000, 74, 6992–7004. [Google Scholar] [CrossRef] [PubMed]
- Shannon, L.M.W.; Thomas, S.; Daniel, C.; Hans-Peter, D.; Kelly, F.; Ketan, P.; Shelley, M.B.; William, G.D.; John, D.K.; Pierre, E.R.; et al. New lineage of lassa virus, togo, 2016. Emerg. Infect. Dis. J. 2018, 24, 599. [Google Scholar]
- Oloniniyi, O.K.; Unigwe, U.S.; Okada, S.; Kimura, M.; Koyano, S.; Miyazaki, Y.; Iroezindu, M.O.; Ajayi, N.A.; Chukwubike, C.M.; Chika-Igwenyi, N.M.; et al. Genetic characterization of lassa virus strains isolated from 2012 to 2016 in southeastern nigeria. PLOS Negl. Trop. Dis. 2018, 12, e0006971. [Google Scholar] [CrossRef] [PubMed]
- Verdecia, M.A.; Bowman, M.E.; Lu, K.P.; Hunter, T.; Noel, J.P. Structural basis for phosphoserine-proline recognition by group iv ww domains. Nat. Struct. Biol. 2000, 7, 639. [Google Scholar] [CrossRef] [PubMed]
- Smerdon, S.J.; Yaffe, M.B. Chapter 72-recognition of phospho-serine/threonine phosphorylated proteins by phospho-serine/threonine-binding domains. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 539–550. [Google Scholar]
- Liou, Y.-C.; Zhou, X.Z.; Lu, K.P. Prolyl isomerase pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem. Sci. 2011, 36, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Lee, T.K.W.; Poon, R.T.P.; Fan, S.T.; Wong, K.B.; Kwong, Y.L.; Tse, E. Pin1 interacts with a specific serine-proline motif of hepatitis b virus x-protein to enhance hepatocarcinogenesis. Gastroenterology 2007, 132, 1088–1103. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Ryo, A.; Yamamoto, N. The prolyl isomerase pin1 stabilizes the human t-cell leukemia virus type 1 (htlv-1) tax oncoprotein and promotes malignant transformation. Biochem. Biophys. Res. Commun. 2009, 381, 294–299. [Google Scholar] [CrossRef]
- Peloponese, J.-M.; Yasunaga, J.; Kinjo, T.; Watashi, K.; Jeang, K.-T. Peptidylproline cis-trans-isomerase pin1 interacts with human t-cell leukemia virus type 1 tax and modulates its activation of nf-κb. J. Virol. 2009, 83, 3238–3248. [Google Scholar] [CrossRef]
- Misumi, S.; Inoue, M.; Dochi, T.; Kishimoto, N.; Hasegawa, N.; Takamune, N.; Shoji, S. Uncoating of human immunodeficiency virus type 1 requires prolyl isomerase pin1. J. Biol. Chem. 2010, 285, 25185–25195. [Google Scholar] [CrossRef]
- Dochi, T.; Nakano, T.; Inoue, M.; Takamune, N.; Shoji, S.; Sano, K.; Misumi, S. Phosphorylation of human immunodeficiency virus type 1 capsid protein at serine 16, required for peptidyl-prolyl isomerase-dependent uncoating, is mediated by virion-incorporated extracellular signal-regulated kinase 2. J. Gen. Virol. 2014, 95, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Lusic, M.; Gutierrez, M.I.; Cereseto, A.; Del Sal, G.; Giacca, M. Concerted action of cellular jnk and pin1 restricts hiv-1 genome integration to activated cd4+ t lymphocytes. Nat. Med. 2010, 16, 329. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Murata, T.; Ryo, A.; Kawashima, D.; Sugimoto, A.; Kanda, T.; Kimura, H.; Tsurumi, T. Pin1 interacts with the epstein-barr virus DNA polymerase catalytic subunit and regulates viral DNA replication. J. Virol. 2013, 87, 2120–2127. [Google Scholar] [CrossRef]
- Guito, J.; Gavina, A.; Palmeri, D.; Lukac, D.M. The cellular peptidyl-prolyl cis/trans isomerase pin1 regulates reactivation of kaposi’s sarcoma-associated herpesvirus from latency. J. Virol. 2014, 88, 547–558. [Google Scholar] [CrossRef]
- Denu, J.M.; Tanner, K.G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 1998, 37, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
- Dephoure, N.; Gould, K.L.; Gygi, S.P.; Kellogg, D.R.; Drubin, D.G. Mapping and analysis of phosphorylation sites: A quick guide for cell biologists. Mol. Biol. Cell 2013, 24, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Baltimore, D. Defective viral particles and viral disease processes. Nature 1970, 226, 325–327. [Google Scholar] [CrossRef]
- Dutko, F.J.; Pfau, C.J. Arenavirus defective interfering particles mask the cell-killing potential of standard virus. J. Gen. Virol. 1978, 38, 195–208. [Google Scholar] [CrossRef]
- Carnec, X.; Baize, S.; Reynard, S.; Diancourt, L.; Caro, V.; Tordo, N.; Bouloy, M. Lassa virus nucleoprotein mutants generated by reverse genetics induce a robust type i interferon response in human dendritic cells and macrophages. J. Virol. 2011, 85, 12093–12097. [Google Scholar] [CrossRef]
- Popescu, M.; Schaefer, H.; Lehmann-Grube, F. Homologous interference of lymphocytic choriomeningitis virus: Detection and measurement of interference focus-forming units. J. Virol. 1976, 20, 1–8. [Google Scholar]
- Welsh, R.M.; Pfau, C.J. Determinants of lymphocytic choriomeningitis interference. J. Gen. Virol. 1972, 14, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Chesarino, N.M.; McMichael, T.M.; Hach, J.C.; Yount, J.S. Phosphorylation of the antiviral protein interferon-inducible transmembrane protein 3 (ifitm3) dually regulates its endocytosis and ubiquitination. J. Biol. Chem. 2014, 289, 11986–11992. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, F.; Lee, H.; Bedford, M.T.; Petrucci, T.; Sudol, M.; Lisanti, M.P. Tyrosine phosphorylation of β-dystroglycan at its ww domain binding motif, ppxy, recruits sh2 domain containing proteins. Biochemistry 2001, 40, 14585–14592. [Google Scholar] [CrossRef] [PubMed]
- Chesarino, N.M.; McMichael, T.M.; Yount, J.S. E3 ubiquitin ligase nedd4 promotes influenza virus infection by decreasing levels of the antiviral protein ifitm3. PLOS Pathogens 2015, 11, e1005095. [Google Scholar] [CrossRef] [PubMed]
- Cornillez-Ty, C.T.; Liao, L.; Yates, J.R.; Kuhn, P.; Buchmeier, M.J. Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J. Virol. 2009, 83, 10314–10318. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.P.; Eisenhauer, P.; Russo, J.; Mason, A.B.; Do, D.; King, B.; Taatjes, D.; Cornillez-Ty, C.; Boyson, J.E.; Thali, M.; et al. The intracellular cargo receptor ergic-53 is required for the production of infectious arenavirus, coronavirus, and filovirus particles. Cell Host Microbe 2013, 14, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.M.; Eisenhauer, P.; Kelly, J.A.; Dang, L.N.; Beganovic, V.; Bruce, E.A.; King, B.R.; Shirley, D.J.; Weir, M.E.; Ballif, B.A.; et al. A proteomics survey of junín virus interactions with human proteins reveals host factors required for arenavirus replication. J. Virol. 2018, 92, e01565-17. [Google Scholar] [CrossRef]
- Degasperi, A.; Birtwistle, M.R.; Volinsky, N.; Rauch, J.; Kolch, W.; Kholodenko, B.N. Evaluating strategies to normalise biological replicates of western blot data. PLoS ONE 2014, 9, e87293. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegler, C.M.; Eisenhauer, P.; Manuelyan, I.; Weir, M.E.; Bruce, E.A.; Ballif, B.A.; Botten, J. Host-Driven Phosphorylation Appears to Regulate the Budding Activity of the Lassa Virus Matrix Protein. Pathogens 2018, 7, 97. https://doi.org/10.3390/pathogens7040097
Ziegler CM, Eisenhauer P, Manuelyan I, Weir ME, Bruce EA, Ballif BA, Botten J. Host-Driven Phosphorylation Appears to Regulate the Budding Activity of the Lassa Virus Matrix Protein. Pathogens. 2018; 7(4):97. https://doi.org/10.3390/pathogens7040097
Chicago/Turabian StyleZiegler, Christopher M., Philip Eisenhauer, Inessa Manuelyan, Marion E. Weir, Emily A. Bruce, Bryan A. Ballif, and Jason Botten. 2018. "Host-Driven Phosphorylation Appears to Regulate the Budding Activity of the Lassa Virus Matrix Protein" Pathogens 7, no. 4: 97. https://doi.org/10.3390/pathogens7040097
APA StyleZiegler, C. M., Eisenhauer, P., Manuelyan, I., Weir, M. E., Bruce, E. A., Ballif, B. A., & Botten, J. (2018). Host-Driven Phosphorylation Appears to Regulate the Budding Activity of the Lassa Virus Matrix Protein. Pathogens, 7(4), 97. https://doi.org/10.3390/pathogens7040097




