Abstract
Background: In sub-Saharan Africa, Plasmodium falciparum is unequivocally responsible for almost all malaria cases and deaths. However, the long-neglected human P. vivax, P. ovale, and P. malariae parasites also emerge as relevant, though their prevalence and contribution to the burden of the disease are very poorly appreciated. This study aimed to bridge this gap and surveyed the circulation of non-falciparum malaria parasites among febrile patients in four regions in south Senegal. Methods: Blood samples were obtained from 1990 febrile patients during the malaria transmission seasons of 2020, 2021, and 2022 in four southern regions in Senegal (Kedougou, Kolda, Tambacounda, and Ziguinchor). Genomic DNA was isolated and tested for Plasmodium infections by using a combination of Plasmodium genus-specific qPCR and Plasmodium species-specific nested PCR. Frequencies and distribution of Plasmodium species according to region, period, and patient demographics were analyzed using R. Spatial patterns of infection were further explored and visualized with QGIS software version 3.30.2. Results: The Plasmodium positivity rate was 73.43% of which 67.92% were unique Plasmodium species infections and 32.08% were co-infections by two or three Plasmodium species. The results described the ongoing circulation of all non-falciparum species in three of the four study regions, the non-detection of P. vivax and P. malariae parasites among the samples tested in Ziguinchor, the first evidence of non-falciparum infections in Kolda and Tambacounda, as well as the first report of P. ovale in Ziguinchor. Conclusions: Our data call on clinicians to account for these species in clinical prognoses, but also on the National Malaria Control Programme to consider these species in their policy of reducing the incidence of the disease with a view to eliminating malaria in Senegal.
1. Introduction
Malaria infection rates vary widely among populations, and children younger than 5 years and primigravidae pregnant women in the WHO African region are the most affected individuals []. As the main cause of malaria in Africa, Plasmodium falciparum accounts for more than 90% of the world’s malaria mortality, thus justifying that the majority of preventive and control measures against the disease are directed towards this species.
Though there is a renewed interest in human-infecting non-falciparum species in sub-Saharan Africa, the scarcity of research on their prevalence and role in malaria disease have somewhat limited the attention paid to these species. Plasmodium vivax is well acknowledged to pose a huge threat to human health [], but the contribution of other non-falciparum species, notably P. ovale and P. malariae, to the burden of the disease remains very poorly documented. Despite being incriminated for disease severity and deaths in some studies [,,,,], many reasons contribute to explain the poor understanding of the role of non-falciparum species in malaria morbidity and mortality. These include, but are not limited to, the absence of validated sensitive and species-specific point-of-care diagnostics, their co-occurrence with P. falciparum at low parasitemia levels, and the lack of experienced microscopists to distinguish between Plasmodium species. Moreover, the ability of P. vivax and P. ovale to constitute dormant hypnozoites forms that can revive infections in the absence of new mosquito bites, along with the inability to maintain both species in long-term in vitro culture because of their specific biological features, constitute additional challenges to the assessment of their contribution to the burden of malaria.
In Senegal, the National Malaria Control Programme (NMCP) has reported, despite a substantial regional variation, a global trend of decreased malaria-related morbidity and mortality in the last two decades. In fact, the NMCP data indicate that in the southern regions, the pattern of malaria transmission varies from high in the southeast (Kedougou, Kolda, and Tambacounda), moderate in the central (Sedhiou), to low in the southwest (Ziguinchor) regions []. The NMCP routine surveillance of malaria uses the standard of care malaria Rapid Diagnostic Test (RDT) and microscopy methods for the diagnosis of malaria cases and identifies P. falciparum as the major cause. These make P. falciparum the primary target for the control and elimination of the disease, though a low-level of non-falciparum species (P. ovale, P. vivax, and P. malariae) infections is recurrently reported [,,,,,,]. Although effective and inexpensive, RDT and microscopy methods lack sufficient sensitivity to identify non-falciparum species, which further drops with the decrease in parasitemia during the disease elimination phase. Molecular methods are now widely used for the detection and identification of malaria parasites, including in mixed infections and at low parasitemia [,].
This study surveyed the circulation of the neglected non-falciparum human malaria parasites among febrile patients living in southern regions of Senegal.
2. Materials and Methods
2.1. Study Sites
This study was conducted in four of the fourteen regions of Senegal situated in the south-eastern and south-western parts of the country (Figure 1). These include Kedougou (12°32′58″ N, 12°10′58″ W), Kolda (12°53′04″ N, 14°55′11″ W), Tambacounda (13°45′12″ N, 13°40′18″ W), and Ziguinchor (12°33′29″ N, 16°16′56″ W) [].
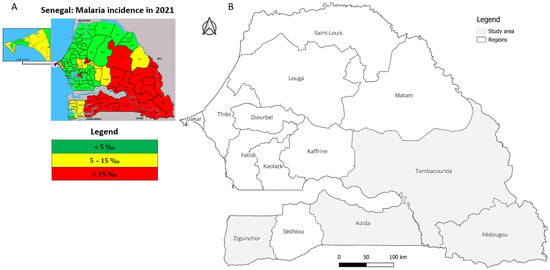
Figure 1.
Map of Senegal showing (A) the highest malaria incidence regions (red) in the south and southeast in 2021, adapted from an NMCP report [] and (B) a highlight of the four selected study regions.
A detailed description of Kedougou region (climate, rainfall, landscape, fauna, population, malaria prevalence) has been provided elsewhere [,,]. Tambacounda region, located in the southeast, is the largest region of Senegal, bordered by The Gambia, Guinea, and Mali. The rainy season runs from June to November and the majority of malaria cases occur between July and October []. The region of Kolda is located in the south, bordered by The Gambia in the north, Guinea-Bissau and Guinea in the south, Sédhiou region in the west, and Tambacounda in the east. Ziguinchor region is located in the southwest of the country, bordered by The Gambia in the north and Guinea-Bissau in the south. It is the eighth largest region of Senegal, and is geographically isolated from the north of the country by The Gambia. The region has a tropical savanna climate, with an average annual accumulated rainfall of approximately 1547 mm or 61 inches.
According to recent data from the NMCP [], Senegal recorded a total of 354,708 malaria cases. Of these, 116,983 cases (32.98%) were reported in Kolda, 101,077 (28.49%) in Tambacounda, 67,941 (19.15%) in Kédougou, and 3757 (1.06%) in Ziguinchor. Together, these regions account for approximately 81.69% of the country’s total malaria burden.
2.2. Study Design and Sample Collection
This study focused on febrile patients who consulted at the regional hospitals in Tambacounda, Kolda, and Ziguinchor, while in Kedougou patients were recruited from the primary health center and the health posts of Bandafassi, Bantako, and Ndiormi. Patients were enrolled during the malaria peak transmission seasons (from August to December) in 2020, 2021, and 2022. All patients aged 6 months and older presenting with fever (axillary temperature > 37.5 °C) and at least one symptom suggestive of malaria disease (headache, nausea, dizziness, chills, fatigue, etc.) were included in the study after obtaining their consent or assent.
A total of 1990 patients were included in the study, and venous blood samples were collected on EDTA tubes from each participant along with age and sex demographic data.
2.3. Molecular Characterization of Malaria Parasite Species
All blood samples were subjected to Plasmodium species characterization following genomic DNA (gDNA) extraction using a QIamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A Plasmodium genus-specific qPCR assay targeting the cytochrome b [] was used for the molecular detection of species-specific Plasmodium parasite DNA, followed by a nested PCR on qPCR positive DNA with primers targeting the Plasmodium spp. 18S small subunit ribosomal RNA (18S ssrRNA) genes as described previously []. Genomic DNA obtained from samples found positive by microscopy for P. falciparum, P. malariae, P. vivax, and P. ovale-infected patients were used as positive controls. Nested PCR results were scored as a categorical variable (presence versus absence of amplification).
2.4. Data Analysis
The structure of the study population was analyzed according to sex and age of patients, and their distribution according to three defined age groups: <5 years, 5–15 years, and ≥15 years. The frequency of the different Plasmodium species was determined by calculating the proportions of each species and each type of infection (single or mixed). The significance of the observed differences was assessed using Fisher’s exact test for categorical variables or the Kruskal–Wallis test for comparison of means. All statistical analyses were performed using R version 4.1 [] with a significance level of 5% and all maps were realized using QGIS version 3.30.2 [].
3. Results
3.1. Demographic Characteristics of the Study Population
A total of 1990 patients were included in the study, and their demographic characteristics are summarized in Table 1. Of these, only 7.9% originated from Ziguinchor while the majority (47.1%) were recruited in Tambacounda, the region with the lowest mean age (17.29 ± 0.56) of participants as well as the youngest population (60.9% were less than 15 years old) (Table 1). Males and females were equally represented in Tambacounda, Kedougou, and Ziguinchor, whereas male participants predominated in Kolda (Table 1). Patients aged +15 years old constitute the majority of participants irrespective of the region (Table 1).

Table 1.
Baseline characteristics of the study population.
3.2. Plasmodium Species Infections Among the Study Patients
Plasmodium genus positivity among the enrolled patients was 68.69% (1367/1990) as measured by qPCR, and all the four Plasmodium species tested in this study were detected among the patients’ samples with P. falciparum being the most dominant parasite (63.82%) (Table 2). Among the neglected non-falciparum parasites, P. ovale was detected in 41.61% of patients’ samples (Table 2), while only 2.7% (54/1990) and 2.06% (41/1990) of samples tested positive for P. vivax and P. malariae, respectively (Table 2).

Table 2.
Prevalence of Plasmodium species in relation to sex and age.
All Plasmodium species were detected in both male and female patients, but a statistically significant difference was only noted for P. falciparum (p-value = 0.04) (Table 2). With regards to age, besides P. malariae (p = 0.8), the other three Plasmodium species showed significant differences between age groups, with patients aged ≤15 years being more infected than the others (p < 0.05) (Table 2).
3.3. Single and Mixed Plasmodium Species Infections Among Patients
Patients mono-infected with Plasmodium species accounted for 42.7% (584/1367) of all detected Plasmodium infections (Figure 2). Among the 584 mono-infected samples, close to 15% were due to non-falciparum parasites at the respective proportions of 11.13%, 2.05%, and 1.54% for P. ovale, P. vivax, and P. malariae (Figure 2).
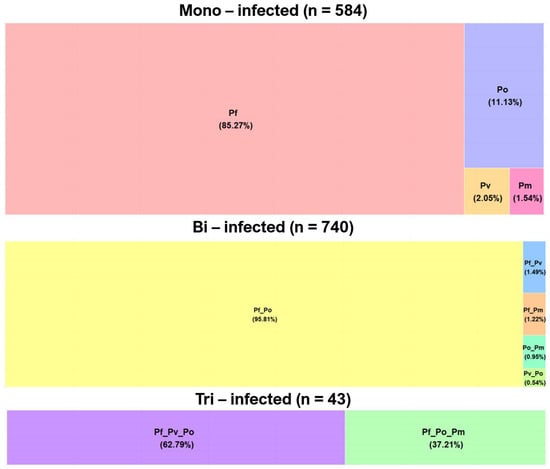
Figure 2.
Single and mixed Plasmodium species infections among the study samples (Pf: Plasmodium falciparum; Pv: P. vivax; Po: P. ovale; Pm: P. malariae).
Of the 740 bi-infected patients, 95.8% were co-infected by P. falciparum/P. ovale followed by P. falciparum/P. vivax (1.49%), P. falciparum/P. malariae (1.22%), P. ovale/P. malariae: 0.95%), and P. ovale/P.vivax (0.54%). Among the 43 samples concurrently infected by three Plasmodium species, only two combinations (P. falciparum/P. vivax/P. ovale and P. falciparum/P. ovale/P. malariae) were detected at 62.79% and 37.21%, respectively (Figure 2).
The specific frequencies of single and mixed non-Plasmodium species infections are detailed in Supplemental Material S1 for each region.
3.4. Spatio-Temporal Variation of Non-Falciparum Species Infections
The proportions of Plasmodium species varied between the study regions and sampling periods (2020, 2021, and 2022) and are illustrated in Figure 3. In Ziguinchor, P. ovale was the only non-falciparum species detected among the tested samples. This contrasts with the detection of the three non-falciparum parasites across the study periods in all the three other study regions (Kedougou, Tambacounda, and Kolda), with P. ovale being the most represented while P. vivax and P. malariae were found at lower and comparable proportions (Figure 3 and Supplemental Material S2).
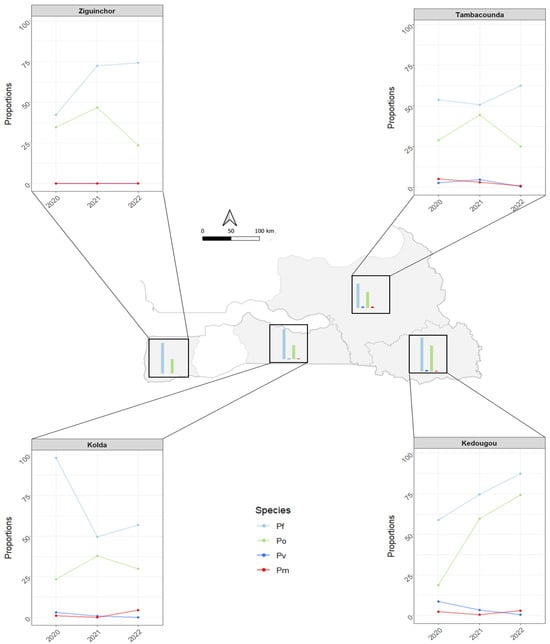
Figure 3.
Spatial and temporal distribution of Plasmodium species in the four southern regions of Senegal from 2020 to 2022 (Pf: Plasmodium falciparum; Pv: P. vivax; Po: P. ovale; Pm: P. malariae).
Between the three study periods, only P. ovale has shown substantial variation in frequency in all regions, a finding that contrasts with the low frequencies and limited variation of P. vivax and P. malariae in Tambacounda, Kedougou, and Kolda (Figure 3). The variation pattern of P. ovale was similar in Ziguinchor, Kolda, and Tambacounda, and was characterized by an important frequency increase from 20–30% in 2020 to 37.5–45% in 2021 and a subsequent drop close to 25% in 2022 (Figure 3). In Kedougou, the proportion of P. ovale increased from 18.75% in 2020 to 60% in 2021, and 74.21% in 2022 (Figure 3).
4. Discussion
In Senegal, the malaria incidence data reported by the NMCP are solely based on P. falciparum-based RDT and microscopic detection of Plasmodium species, both being indeed of great use in remote areas that lack modern laboratory capabilities. However, both methods have shown several limitations in the detection of non-falciparum species, thus hampering the estimation of the true prevalence and contribution of non-falciparum species to the global malaria burden []. To end malaria, the detection and clearance of all malaria parasites that affect humans are critical, and molecular assays are keys to this perspective, not only by confirming the high P. falciparum malaria incidences in various settings, but also by revealing instances of non-falciparum infections that have surfaced in many sub-Saharan African countries [,,,,,], including in Senegal [,,,]. However, it is worth noting that a selection bias of patient recruitment in 2020 in Kolda has resulted in the high frequency of P. falciparum infections in that area, but similar trends and infection rates were observed in 2021 and 2022 in all regions.
Besides the absence of P. vivax and P. malariae parasites in patients’ samples obtained from Ziguinchor, this study has revealed the presence of all non-falciparum parasites in samples from Kedougou, Tambacounda, and Kolda across the three periods. The results confirm early reports on the circulation of non-falciparum species in Kedougou [,,,], but, to the best of our knowledge, report the first detection of P. ovale parasite infections in Ziguinchor.
In this study, non-falciparum species were predominantly found in co-infections with P. falciparum at varying proportions across the four regions, an association that further complicates differentiation by microscopy [,] in areas where technicians are not sufficiently trained. Living in co-endemic areas with active transmission of all human non-falciparum species was shown to be predictive of exposure to infection by one malaria species with an increased risk of exposure to another malaria species []. This finding underscores the need for an improved surveillance system with quality-assured diagnosis by coupling the current standard point-of-care diagnostics with molecular surveillance to sustain Plasmodium species detection and speciation. The high proportion of younger patients (60.9% <15 years) from Tambacounda may explain the high Plasmodium infection rate among this more vulnerable population, as opposed to Ziguinchor, which has the highest proportion of older patients (81.63% ≥15 years) who are known to be less vulnerable to Plasmodium infections and malaria disease.
Though the majority of P. vivax and P. ovale infections are reported to originate from relapses of dormant hypnozoites parasites from the liver [], we cannot, at this stage, argue about whether the PCR-detected P. ovale and P. vivax parasites were new infections from recent mosquito bites or were due to recrudescence from the dormant hypnozoites parasites of old infections. The data indicate that non-falciparum Plasmodium species circulate in the study areas, and their low-level detection among patients who consulted at given health facilities suggests the potential existence of an important reservoir in the communities. Similar observations have been previously made, and have raised the question of the existence of potential “hot/cold” spots of transmission that require further investigations if the elimination objective is to be achieved in due time []. The exclusion of P. knowlesi, the fifth human-infecting Plasmodium species from our molecular panel is justified by the lack of epidemiological evidence to suspect it’s introduction in Senegal. However, we recognize the importance of proactive surveillance, especially in the context of changing ecological dynamics, increasing human–animal interactions, and mosquito vector adaptation that could facilitate zoonotic transmission.
The control of life-threatening infections due to non-falciparum species (P. ovale, P. vivax, and P. malariae) is a major obstacle to malaria elimination [], because of several factors: the diagnostic challenge posed by their low-density parasitemia; the ability of P. vivax and P. ovale to revive infections from dormant hypnozoites forms; and the inability to maintain them in long-term in vitro culture [,,] for advanced experimental studies. Future studies that integrate vector surveillance and environmental factors as well as patients’ clinical data would allow a more detailed analysis and explanation of any potential regional and spatial difference in Plasmodium species prevalence and distribution including malaria transmission dynamics and the role of specific Anopheles species in the maintenance of the observed infections. To date, maintaining the limited success gained in controlling P. falciparum malaria in the highest malaria transmission regions (Kedougou, Kolda, and Tambacounda) is likely to be challenged by the growing evidence of non-falciparum infections in the areas, thus bringing in a new paradigm for the control program that might require immediate attention. In this context, control and surveillance programs must not only adapt to address the diversity of human Plasmodium species but should also, within a proactive surveillance framework, include emerging zoonotic threats such as P. knowlesi, which is increasingly recognized for its potential for human transmission.
5. Conclusions
This study demonstrates the circulation of the neglected non-falciparum malaria parasites among febrile patients in Senegal along with the first evidence of P. ovale infections in the low transmission area of Ziguinchor. Though the primary role of P. falciparum in malaria incidence is unequivocal, the recurrence of non-falciparum malaria is increasingly becoming a major recognized and neglected public health problem that may threaten the prospect of malaria elimination in Senegal and elsewhere. To further reduce malaria and eliminate the disease, it is urgent to implement malaria molecular surveillance for improved detection of malaria parasites and proper management of malaria patients. The so-called “minor” or “neglected” malaria species deserve special attention as they can cause severe malaria and may lead to fatalities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14121201/s1.
Author Contributions
B.S.S., S.O.M.D., I.V.-W. and M.N. conceived and supervised the study; B.S.S., S.O.M.D., A.D., A.S.D., B.D. and M.N. implemented the field study and samples collection; S.O.M.D., A.D., H.A.M.D., I.S., R.S. and P.Y.B.G. performed the laboratory assays; B.S.S. and A.D. maintained the database; B.S.S., S.O.M.D., A.D. and M.N. analyzed the data and drafted the manuscript; B.S., P.M.S. and I.V.-W. substantially contributed to the review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Institut Pasteur de Dakar, and the European & Developing Countries Clinical Trials Partnership (EDCTP), which is part of the EDCTP2 program supported by the European Union (fellowship number TMA2018SF-2468). The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP and other funders. The funders had no role in the study design, data collection and analysis, writing of the manuscript, and decision to submit the article for publication.
Institutional Review Board Statement
This study was reviewed and approved by the National Ethical Committee for Research in Health Senegal under the reference 00000185MSAS/CNERS/SP, and approval date 2 November 2021.
Informed Consent Statement
Written informed consent was obtained for all study participants. For minors, consent was provided by legal guardians, and children over the age of 7 also provided assent.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article. The data supporting the conclusions of this article can be made available by the authors under reasonable request.
Acknowledgments
The authors acknowledge the support and contribution of the study participants. The recruitment teams at the health posts in Kedougou, and the regional hospitals of Kolda, Tambacounda, and Ziguinchor are thanked for their valuable support.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- WHO. World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Battle, K.; Baird, K. The global burden of Plasmodium vivax malaria is obscure and insidious. PLoS Med. 2021, 18, e1003799. [Google Scholar] [CrossRef]
- Mahgoub, H.; Gasim, G.; Musa, I.; Adam, I. Severe Plasmodium vivax malaria among Sudanese children at new Halfa hospital, eastern Sudan. Parasites Vectors 2012, 5, 154. [Google Scholar] [CrossRef]
- Nurleila, S.; Syafruddin, D.; Elyazar, I.; Baird, J. Serious and fatal illness associated with falciparum and vivax malaria among patients admitted to hospital at West Sumba in eastern Indonesia. Am. J. Trop. Med. Hyg. 2012, 87, 41. [Google Scholar] [CrossRef]
- Singh, R.; Jain, V.; Singh, P.; Bharti, P.; Thomas, T.; Basak, S.; Singh, N. First report of detection and molecular confirmation of Plasmodium ovale from severe malaria cases in central India. Trop. Med. Int. Health 2013, 18, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Wångdahl, A.; Wyss, K.; Saduddin, D.; Bottai, M.; Ydring, E.; Vikerfors, T.; Färnert, A. Severity of Plasmodium falciparum and non-falciparum malaria in travelers and migrants: A nationwide observational study over 2 decades in Sweden. J. Infect. Dis. 2019, 220, 1335–1345. [Google Scholar] [CrossRef]
- Diagne, A.; Sambe, B.; Gaba, F.; Sarr, I.; Diatta, A.; Sadio, O.; Diaw, S.; Diatta, H.; Diouf, B.; Vigan-Womas, I.; et al. Variable effects of non-falciparum species infections on malaria disease severity in high transmission regions in Senegal. Trop. Med. Health 2024, 52, 93. [Google Scholar] [CrossRef]
- PNLP. Plan stratégique national de lutte contre le paludisme au Sénégal 2021–2025. In Programme National de Lutte Contre Le Paludisme; Ministry of Health and Public Action of Senegal: Rue Aimé Césaire, Dakar, 2025. [Google Scholar]
- Trape, J.; Rogier, C.; Konate, L.; Diagne, N.; Bouganali, H.; Canque, B.; Legros, F.; Badji, A.; Ndiaye, G.; Ndiaye, P.; et al. The Dielmo project: A longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 1994, 51, 123–137. [Google Scholar] [CrossRef]
- Trape, J.; Tall, A.; Sokhna, C.; Ly, A.; Diagne, N.; Ndiath, O.; Mazenot, C.; Richard, V.; Badiane, A.; Dieye-Ba, F.; et al. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: A 22 year longitudinal study. Lancet Infect. Dis. 2014, 14, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.; Deme, A.; Gomis, J.; Dieye, B.; Durfee, K.; Thwing, J.; Fall, F.; Ba, M.; Ndiop, M.; Badiane, A.; et al. Evidence of non-Plasmodium falciparum malaria infection in Kédougou, Sénégal. Malar. J. 2017, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.; Diop, F.; Niang, O.; Sadio, B.; Sow, A.; Faye, O.; Diallo, M.; Sall, A.; Perraut, R.; Toure-Balde, A. Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kédougou, southeastern Senegal. Malar. J. 2017, 16, 497. [Google Scholar] [CrossRef]
- Niang, M.; Sane, R.; Sow, A.; Sadio, B.; Chy, S.; Legrand, E.; Faye, O.; Diallo, M.; Sall, A.; Menard, D.; et al. Asymptomatic Plasmodium vivax infections among Duffy-negative population in Kedougou, Senegal. Trop. Med. Health 2018, 46, 45. [Google Scholar] [CrossRef] [PubMed]
- Badiane, A.; Ngom, B.; Ndiaye, T.; Cunningham, D.; Campbell, J.; Gaye, A.; Sène, A.; Sy, M.; Ndiaye, D.; Nwakanma, D.; et al. Evidence of Plasmodium vivax circulation in western and eastern regions of Senegal: Implications for malaria control. Malar. J. 2024, 23, 149. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G.; Singh, B. Nested PCR analysis of Plasmodium parasites. In Malaria Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2002; pp. 189–203. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.; Jarra, W.; Pinheiro, L.; do Rosaria, V.; Thaithong, S.; Brown, K. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef] [PubMed]
- ANSD. Rapport Provisoire, Recensement Général de la Population et de l’Habitat (RGPH-5), 2023; ANSD: Dakar, Senegal, 2024. [Google Scholar]
- Niang, M.; Thiam, L.; Sow, A.; Loucoubar, C.; Bob, N.; Diop, F.; Diouf, B.; Niass, O.; Mansourou, A.; Varela, M.; et al. A molecular survey of acute febrile illnesses reveals Plasmodium vivax infections in Kedougou, southeastern Senegal. Malar. J. 2015, 14, 281. [Google Scholar] [CrossRef]
- Niang, M.; Loucoubar, C.; Sow, A.; Diagne, M.; Faye, O.; Faye, O.; Diallo, M.; Toure-Balde, A.; Sall, A. Genetic diversity of Plasmodium falciparum isolates from concurrent malaria and arbovirus co-infections in Kedougou, southeastern Senegal. Malar. J. 2016, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.; Dia, A.; Faye, O.; Weaver, S.; Diallo, M.; et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef]
- Farrugia, C.; Cabaret, O.; Botterel, F.; Bories, C.; Foulet, F.; Costa, J.; Bretagne, S. Cytochrome b Gene Quantitative PCR for Diagnosing Plasmodium falciparum Infection in Travelers. J. Clin. Microbiol. 2011, 49, 2191–2195. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System; QGIS Association: Grüt (Gossau ZH), Switzerland, 2024. [Google Scholar]
- Abba, K.; Kirkham, A.; Olliaro, P.; Deeks, J.; Donegan, S.; Garner, P.; Takwoingi, Y. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database Syst. Rev. 2014, 2015, CD011431-195. [Google Scholar] [CrossRef]
- Johnston, S.P.; Pieniazek, N.J.; Xayavong, M.V.; Slemenda, S.B.; Wilkins, P.P.; Da Silva, A.J. PCR as a Confirmatory Technique for Laboratory Diagnosis of Malaria. J. Clin. Microbiol. 2006, 44, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Gupta, P.; Sharma, A.; Singh, V.; Dash, A.; Das, A. High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Trop. Med. Int. Health 2010, 15, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Dias, F.; Figueiredo, J.; Mora, V.; Cano, J.; De Sousa, B.; Do Rosário, V.; Benito, A.; Berzosa, P.; Arez, A. Duffy Negative Antigen Is No Longer a Barrier to Plasmodium vivax–Molecular Evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Neglected Trop. Dis. 2011, 5, e1192. [Google Scholar] [CrossRef]
- Rubio, J.; Benito, A.; Roche, J.; Berzosa, P.; Garcia, P.; Mico, M.; Edu, M.; Alvar, J. Semi-nested, multiplex polymerase chain reaction for detection of human malaria parasites and evidence of Plasmodium vivax infection in Equatorial Guinea. Am. J. Trop. Med. Hyg. 1999, 60, 183–187. [Google Scholar] [CrossRef]
- Bernabeu, M.; Gomez-Perez, G.; Sissoko, S.; Niambélé, M.; Haibala, A.; Sanz, A.; Théra, M.; Fernandez-Becerra, C.; Traoré, K.; Alonso, P.; et al. Plasmodium vivax malaria in Mali: A study from three different regions. Malar. J. 2012, 11, 405. [Google Scholar] [CrossRef]
- Sambe, B.; Diagne, A.; Diatta, H.; Gaba, F.; Sarr, I.; Diatta, A.; Diaw, S.; Sané, R.; Diouf, B.; Vigan-Womas, I.; et al. Molecular detection and quantification of Plasmodium vivax DNA in blood pellet and plasma samples from patients in Senegal. Front. Parasitol. 2023, 2, 1149738. [Google Scholar] [CrossRef]
- Diallo, M.; Diongue, K.; Seck, M.; Ndiaye, M.; Diallo, I.; Diedhiou, Y.; Ndiaye, T.; Ndiaye, Y.; Badiane, A.; Ndiaye, D. Quality control of malaria microscopy reveals misdiagnosed non-falciparum species and other microscopically detectable pathogens in Senegal. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, A.; Marques, R.; Regiart, M.; Bargieri, D. Diagnostic Methods for Non-Falciparum Malaria. Front. Cell. Infect. Microbiol. 2021, 11, 536. [Google Scholar] [CrossRef]
- Culleton, R.; Ndounga, M.; Zeyrek, F.; Coban, C.; Casimiro, P.; Takeo, S.; Tsuboi, T.; Yadava, A.; Carter, R.; Tanabe, K. Evidence for the Transmission of Plasmodium vivax in the Republic of the Congo, West Central Africa. J. Infect. Dis. 2009, 200, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Baird, J. African Plasmodium vivax malaria improbably rare or benign. Trends Parasitol. 2022, 38, 683–696. [Google Scholar] [CrossRef]
- WHO. A Framework for Malaria Elimination; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Quaye, I.K.; Aleksenko, L.; Oeuvray, C.; Yewhalaw, D.; Duah, N.; Gyan, B.; Haiyambo, D.H.; Dongho, G.B.D.; Torgby, R.-A.; Amoah, L. The Pan African Vivax and Ovale Network (PAVON): Refocusing on Plasmodium vivax, ovale and asymptomatic malaria in sub-Saharan Africa. Parasitol. Int. 2021, 84, 102415. [Google Scholar] [CrossRef] [PubMed]
- Benavente, E.D.; Ward, Z.; Chan, W.; Mohareb, F.R.; Sutherland, C.J.; Roper, C.; Campino, S.; Clark, T.G. Genomic variation in Plasmodium vivax malaria reveals regions under selective pressure. PLoS ONE 2017, 12, e0177134. [Google Scholar] [CrossRef] [PubMed]
- Benavente, E.D.; Manko, E.; Phelan, J.; Campos, M.; Nolder, D.; Fernandez, D.; Velez-Tobon, G.; Castaño, A.T.; Dombrowski, J.G.; Marinho, C.R. Distinctive genetic structure and selection patterns in Plasmodium vivax from South Asia and East Africa. Nat. Commun. 2021, 12, 3160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).