Immunotherapeutics Combining a Recombinant Chimeric Protein, Monophosphoryl Lipid A, and Miltefosine Against Visceral Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Parasites, and Experimental Infection
2.2. Expression and Purification of the ChimT Protein
2.3. Experimental Groups and Treatment Protocols
2.4. Spleen Cell Culture
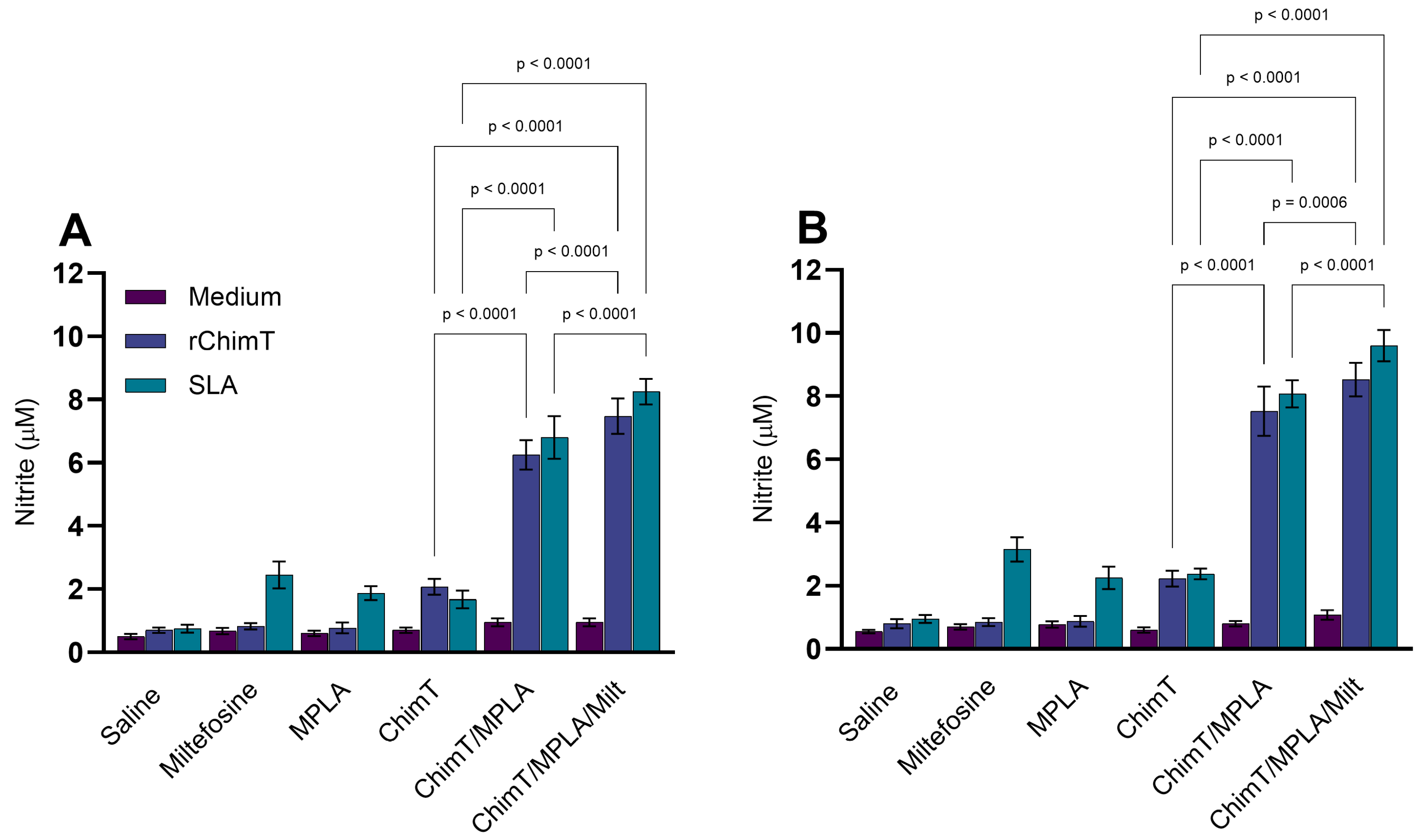
2.5. Cytokine Production and Nitrite Secretion
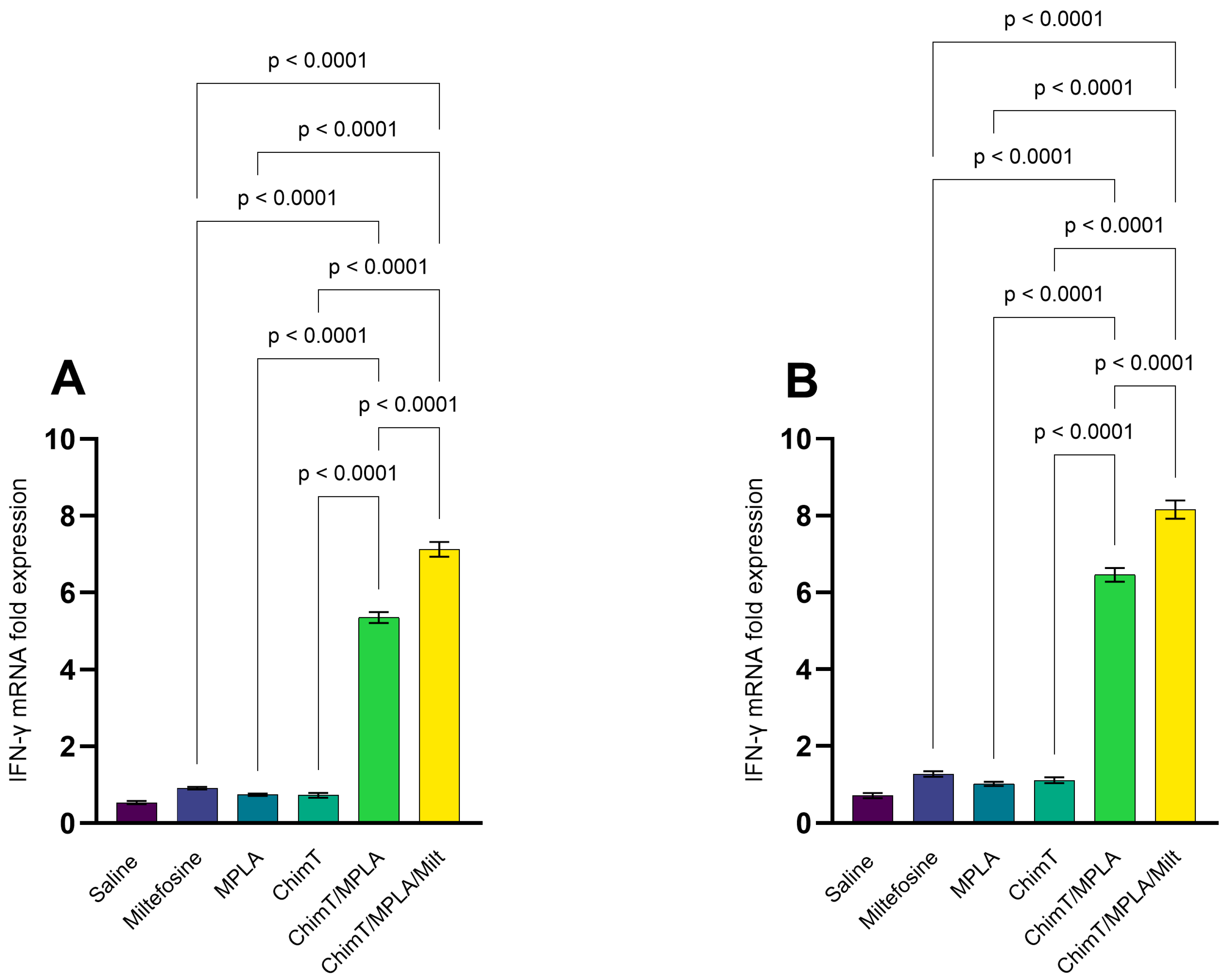
2.6. IFN-γ mRNA Expression in Stimulated Cell Cultures
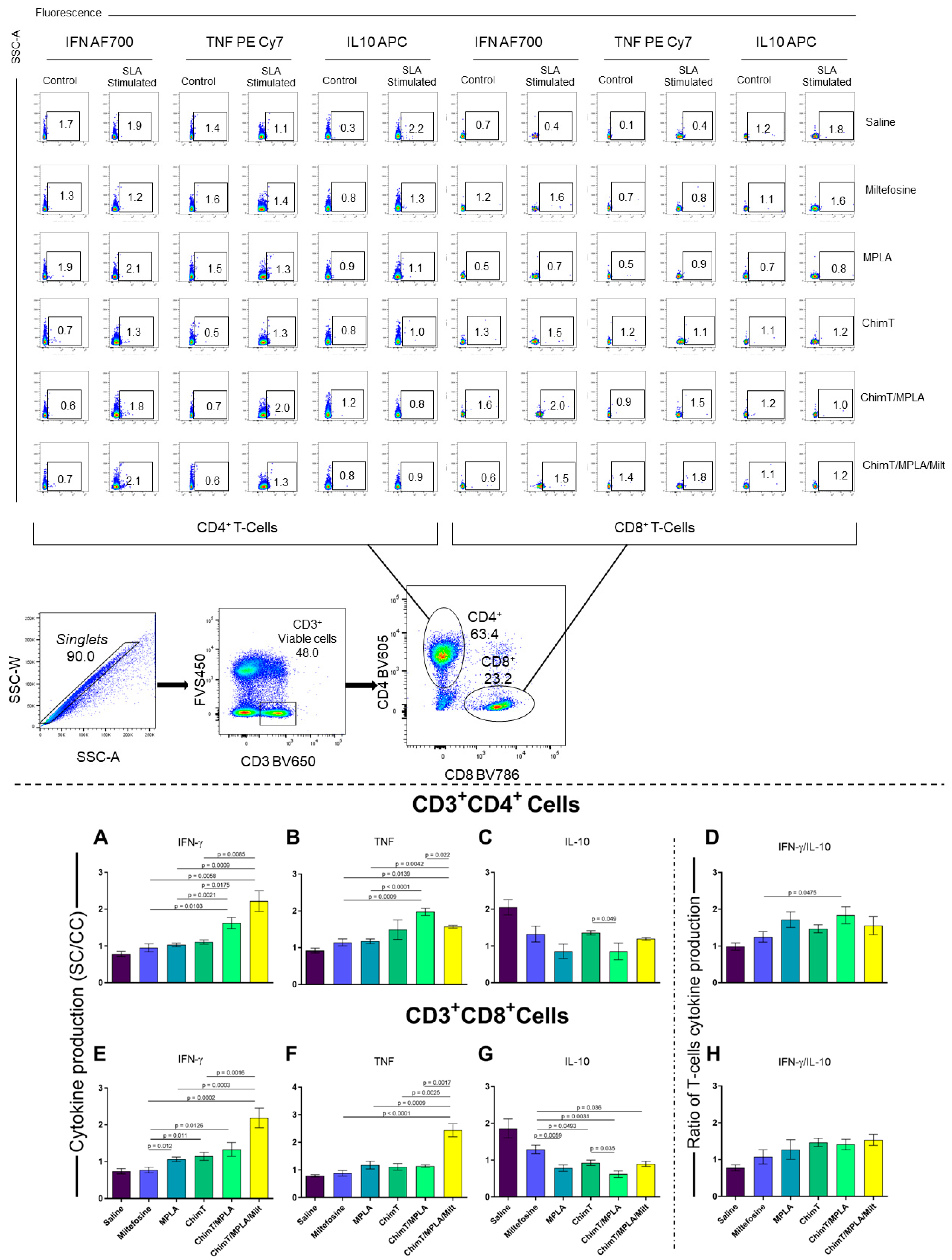
2.7. Intracytoplasmic Cytokine Profile Assessed by Flow Cytometry
2.8. Humoral Response
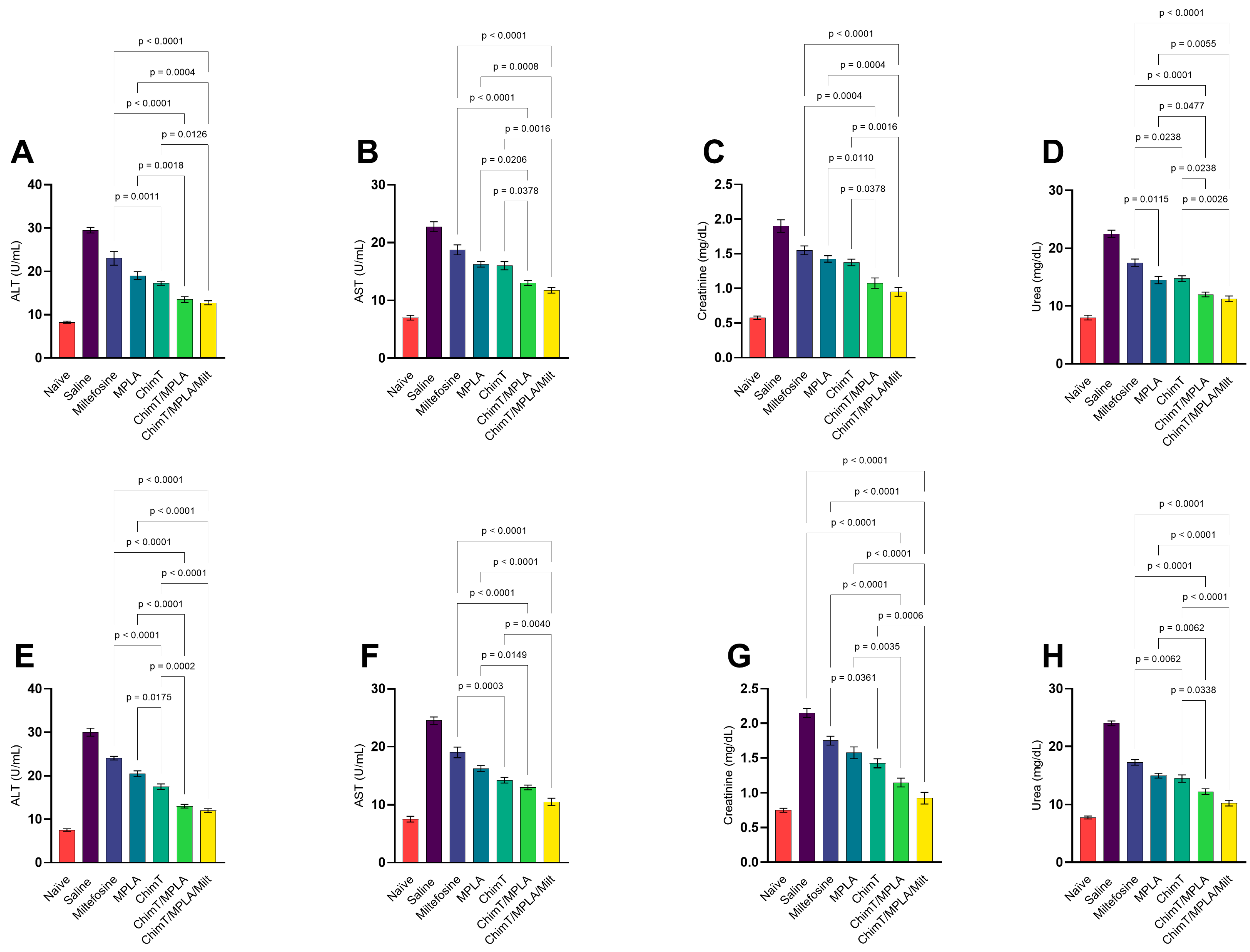
2.9. Renal and Hepatic Toxicity
2.10. Parasite Load by Limiting Dilution Technique
2.11. Parasite Load Estimated by Quantitative PCR (qPCR)
2.12. Statistical Analysis
3. Results
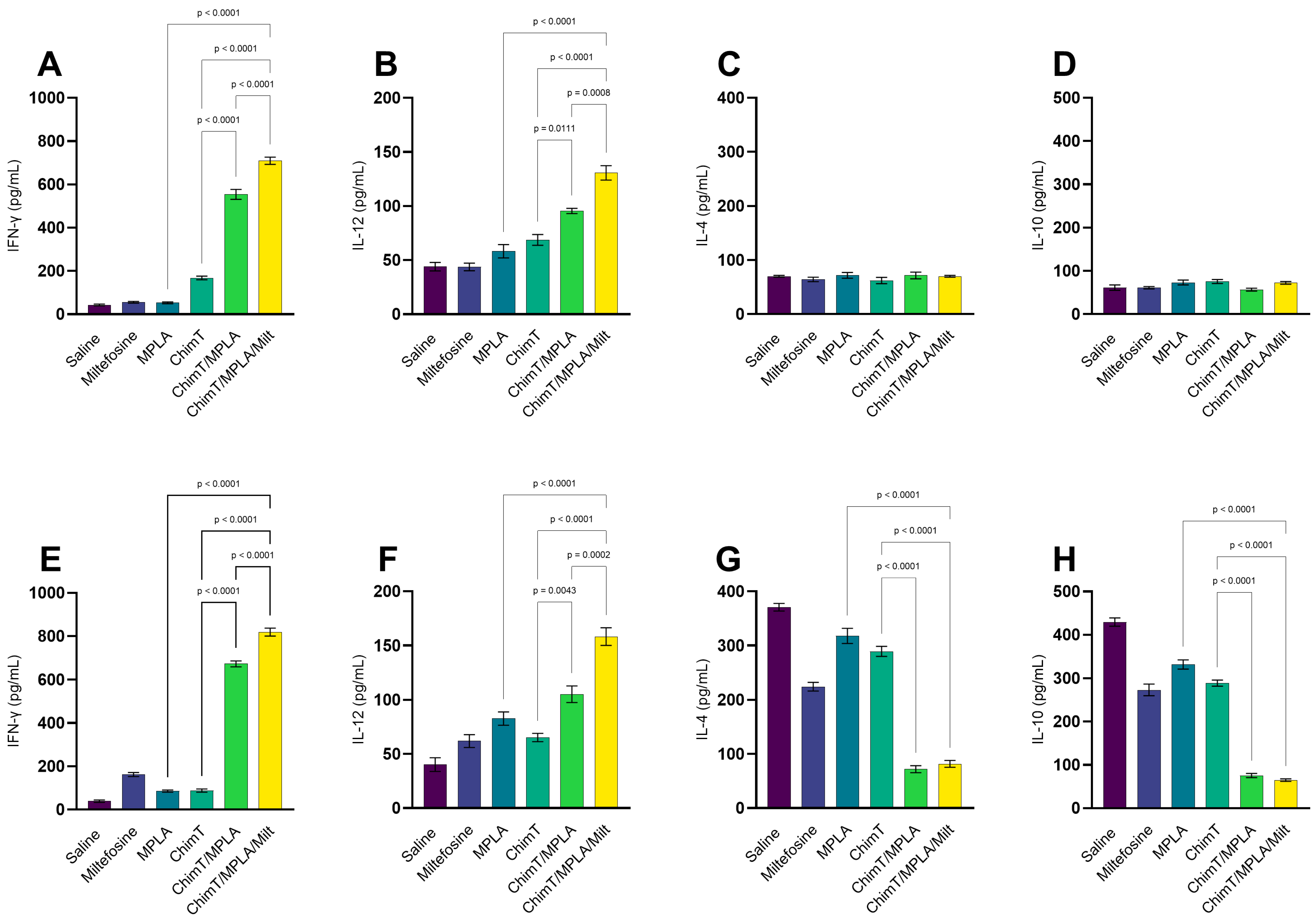
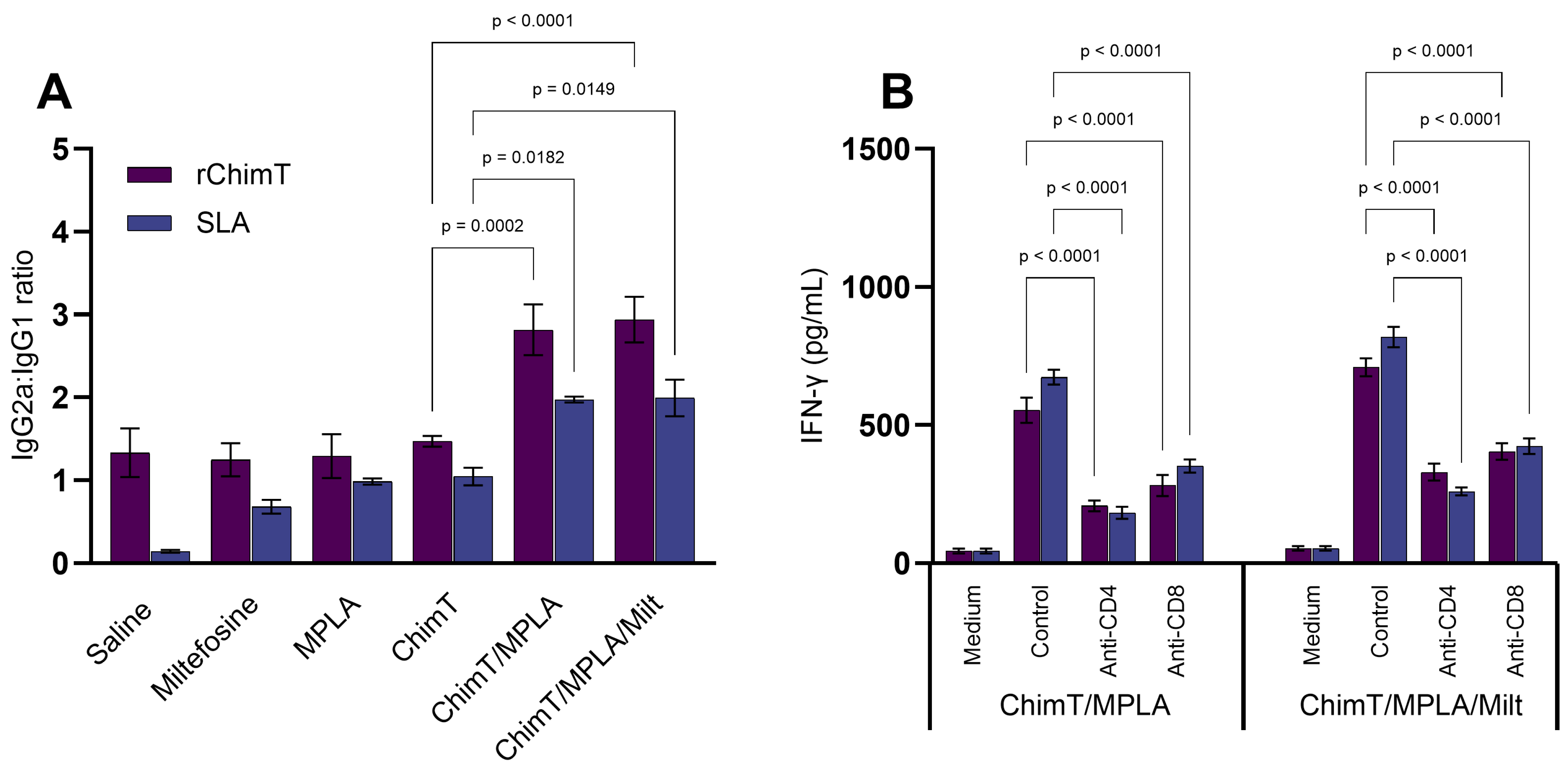
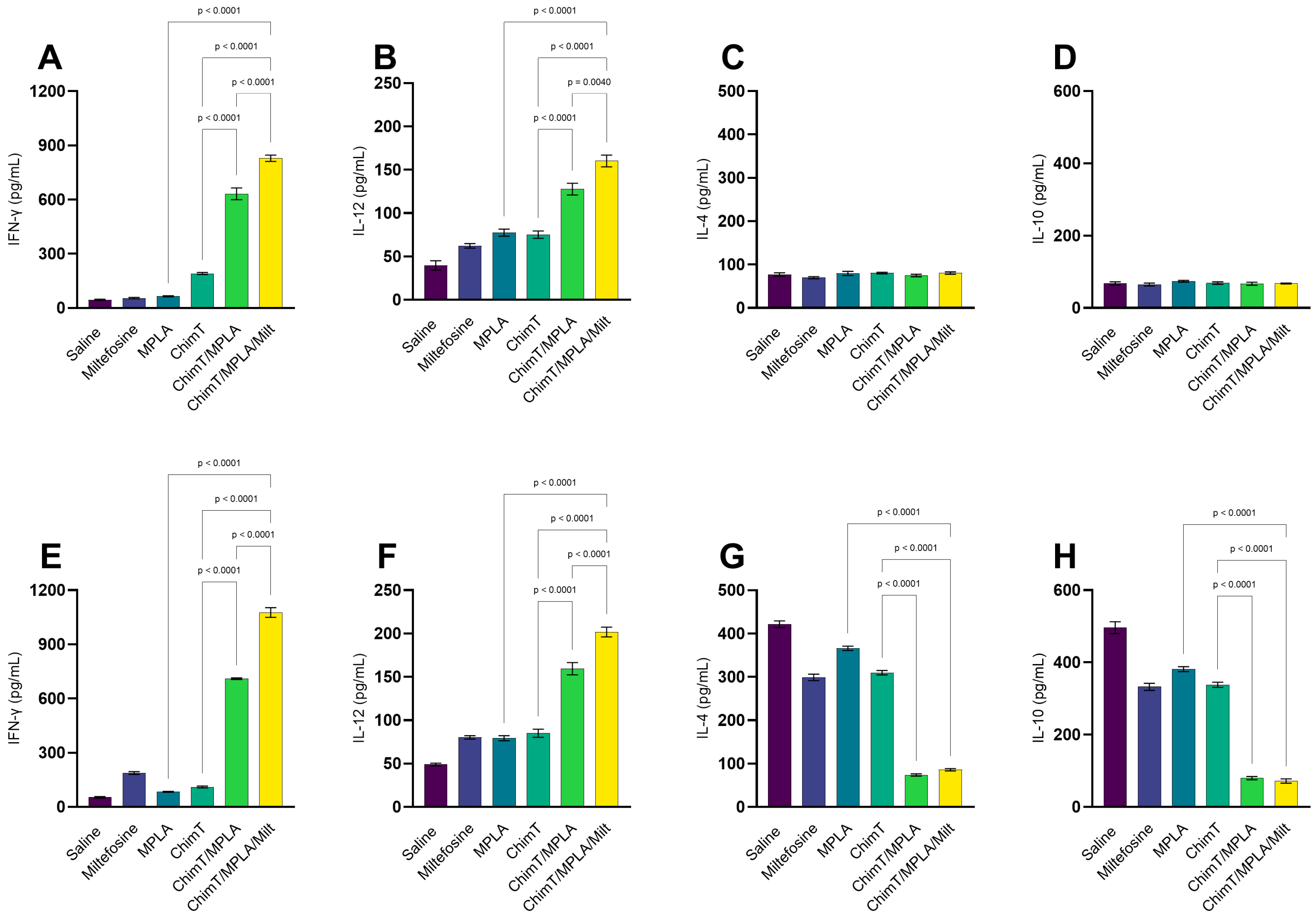
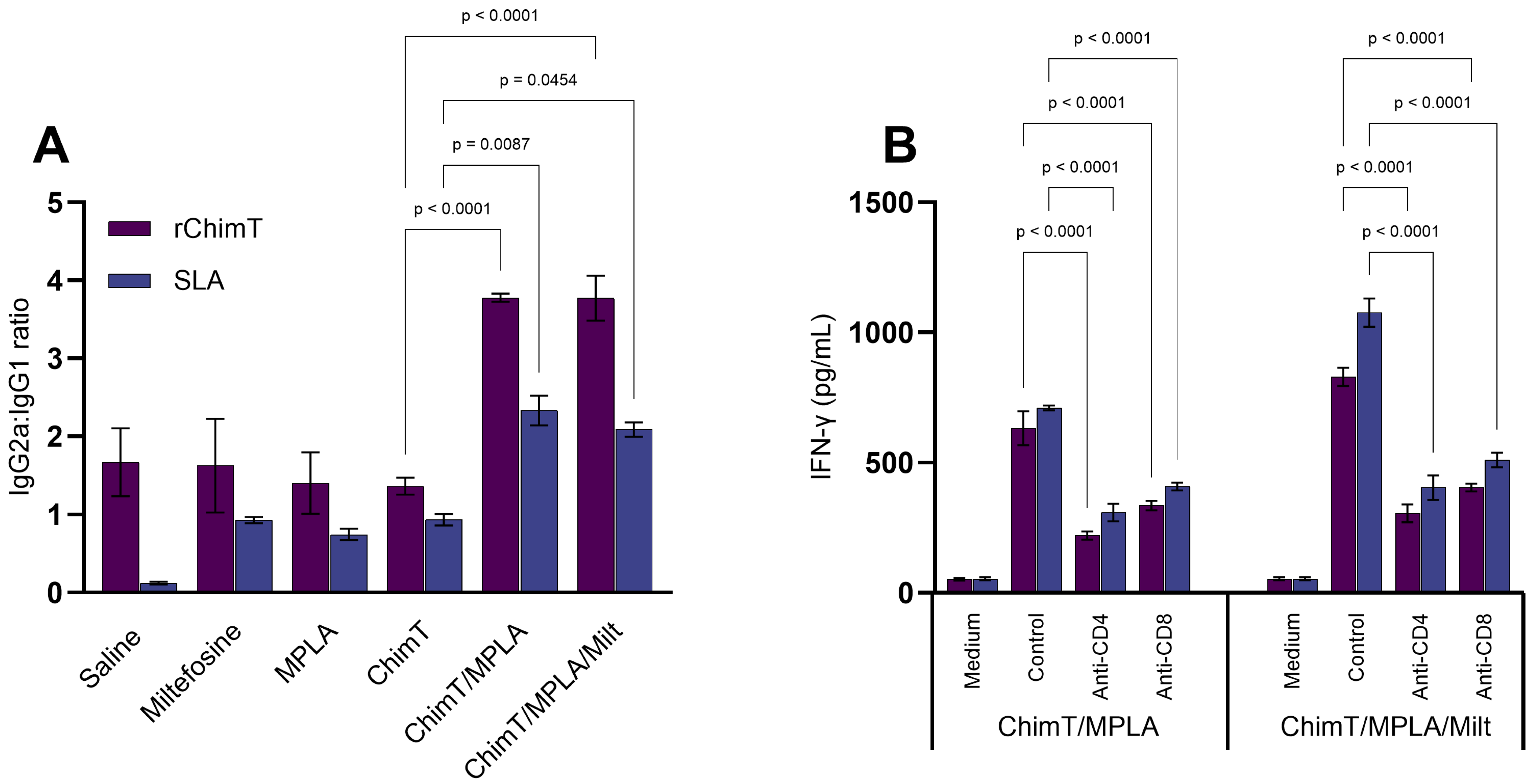
3.1. Cytokine and Antibody Responses Following Treatment
3.2. IFN-γ mRNA Expression and Nitrite Production
3.3. Toxicological Assays in Treated Mice
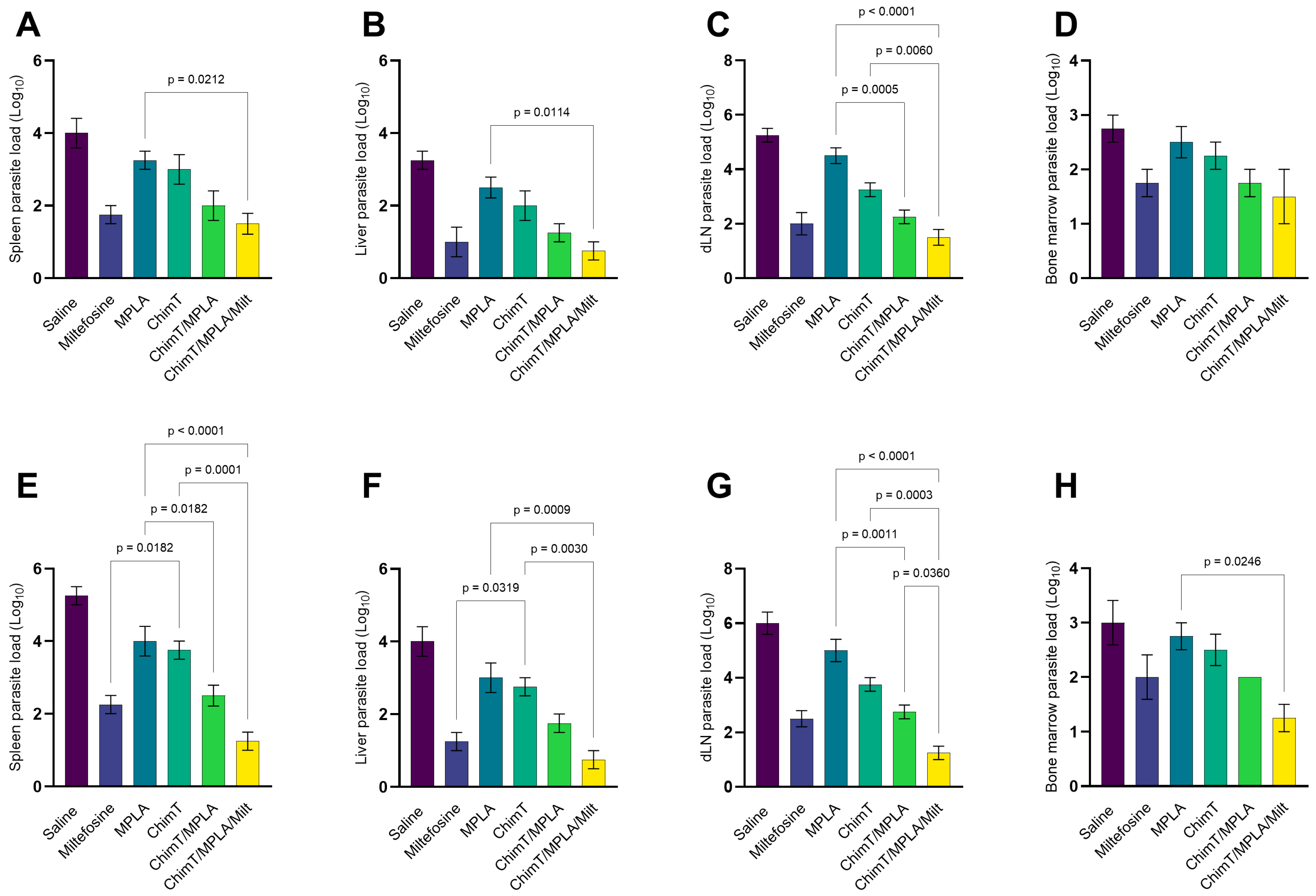
3.4. Parasite Burden Measured in Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Leishmaniasis. Available online: http://www.who.int/topics/leishmaniasis/en/2025 (accessed on 23 July 2025).
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Chang, K.P.; Costa, D.L.; Cunha, F.V.M. From infection to death: An overview of the pathogenesis of visceral leishmaniasis. Pathogens 2023, 12, 969. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, B. Emerging therapeutic targets for treatment of leishmaniasis. Expert Opin. Ther. Targets 2018, 22, 467–486. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Avery, V.M. Assay development in leishmaniasis drug discovery: A comprehensive review. Expert Opin. Drug Discov. 2022, 17, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Engel, J.; Sindermann, H.; Fischer, C.; Junge, K.; Bryceson, A.; Berman, J. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 2002, 347, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Bastola, A.; Shrestha, M.; Chalise, B.S.; Mishra, A.K.; Devkota, L. Miltefosine rescue treatment for visceral leishmaniasis relapse patient. Case Rep. Infect. Dis. 2019, 2019, 3634568. [Google Scholar] [CrossRef]
- Palić, S.; Bhairosing, P.; Beijnen, J.H.; Dorlo, T.P.C. Systematic review of host-mediated activity of miltefosine in leishmaniasis through immunomodulation. Antimicrob. Agents Chemother. 2019, 63, e02507–e02518. [Google Scholar] [CrossRef]
- Melcon-Fernandez, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and Nifuratel combination: A promising therapy for the treatment of Leishmania donovani visceral leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. [Google Scholar] [CrossRef]
- Benaim, G.; Paniz-Mondolfi, A. Unmasking the mechanism behind miltefosine: Revealing the disruption of intracellular Ca2+ homeostasis as a rational therapeutic target in leishmaniasis and Chagas disease. Biomolecules 2024, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Shankar, P.; Mishra, J.; Singh, S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasites Vectors 2016, 9, 277. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Monti-Rocha, R.; Costa, D.L.; Sesana, A.M.; Pansini, L.N.N.; Segatto, M.; Mottram, J.C.; Costa, C.H.N.; Carvalho, S.F.G.; Dietze, R. Natural resistance of Leishmania infantum to miltefosine contributes to the low efficacy in the treatment of visceral leishmaniasis in Brazil. Am. J. Trop. Med. Hyg. 2019, 101, 789–794. [Google Scholar] [CrossRef]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef]
- Rostamian, M.; Bahrami, F.; Niknam, H.M. Vaccination with whole-cell killed or recombinant leishmanial protein and toll-like receptor agonists against Leishmania tropica in BALB/c mice. PLoS ONE 2018, 13, e0204491. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Prakash, J.; Singh, O.P.; Gedda, M.R.; Chauhan, S.B.; Sundar, S.; Dubey, V.K. IFN-γ+ CD4+ T cell-driven prophylactic potential of recombinant LDBPK_252400 hypothetical protein of Leishmania donovani against visceral leishmaniasis. Cell. Immunol. 2021, 361, 104272. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Tavares, N.M.; Costa, D.; Pitombo, M.; Barbosa, L.; Fukutani, K.; Miranda, J.C.; Oliveira, C.I.; Valenzuela, J.G.; Barral, A.; et al. DNA vaccination with KMP11 and Lutzomyia longipalpis salivary protein protects hamsters against visceral leishmaniasis. Acta Trop. 2011, 120, 185–190. [Google Scholar] [CrossRef]
- Gusmão, M.R.; Ostolin, T.L.V.D.P.; Carvalho, L.M.; Costa, A.F.P.; Moreira, G.J.L.; Cardoso, J.M.O.; Aguiar-Soares, R.D.O.; Reis, A.B.; Brito, R.C.F.; Roatt, B.M. Immunoprophylaxis using polypeptide chimera vaccines plus adjuvant system promote Th1 response controlling the spleen parasitism in hamster model of visceral leishmaniasis. Vaccine 2022, 40, 5494–5503. [Google Scholar] [CrossRef]
- Campos, M.P.; Figueiredo, F.B.; Morgado, F.N.; Renzetti, A.R.D.S.; Souza, S.M.M.; Pereira, S.A.; Rodrigues-da-Silva, R.N.; Lima-Junior, J.D.C.; Luca, P.M. Leishmania infantum virulence factor A2 protein: Linear B-cell epitope mapping and identification of three main linear B-cell epitopes in vaccinated and naturally infected dogs. Front. Immunol. 2018, 9, 1690. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Alcolea, P.J.; Larraga, J.; Peris, M.P.; Esteban, A.; Cortés, A.; Ruiz-García, S.; Castillo, J.A.; Larraga, V. A non-replicative antibiotic resistance-free DNA vaccine delivered by the intranasal route protects against canine leishmaniasis. Front. Immunol. 2023, 14, 1213193. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.; Nascimento, E.; Sampaio, W.; França, J.C.; Fujiwara, R.T.; Vale, A.; Dias, E.S.; Vieira, E.; Costa, R.T.; Mayrink, W.; et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine 2008, 26, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.; Gusmão, M.R.; Costa, A.F.P.; de Brito, R.C.F.; Aguiar-Soares, R.D.O.; Cardoso, J.M.O.; Reis, A.B.; Carneiro, C.M.; Roatt, B.M. Immunochemotherapy for visceral leishmaniasis: Combinatorial action of miltefosine plus LBSapMPL vaccine improves adaptative Th1 immune response with control of splenic parasitism in experimental hamster model. Parasitology 2022, 149, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yadagiri, G.; Singh, A.; Arora, K.; Mudavath, S.L. Immunotherapy and immunochemotherapy in combating visceral leishmaniasis. Front. Med. 2023, 10, 1096458. [Google Scholar] [CrossRef]
- Ostolin, T.L.V.D.P.; Gusmão, M.R.; Mathias, F.A.S.; Cardoso, J.M.O.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Ruiz, J.C.; Resende, D.M.; de Brito, R.C.F.; Reis, A.B. A chimeric vaccine combined with adjuvant system induces immunogenicity and protection against visceral leishmaniasis in BALB/c mice. Vaccine 2021, 39, 2755–2763. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Zaldívar, M.F.; Gonçalves, A.A.M.; Resende, L.A.; Mariano, R.M.D.S.; Pereira, D.F.S.; Conrado, I.D.S.S.; Costa, M.A.F.; Lair, D.F.; Vilas-Boas, D.F.; et al. New Approaches to the Prevention of Visceral Leishmaniasis: A Review of Recent Patents of Potential Candidates for a Chimeric Protein Vaccine. Vaccines 2024, 12, 271. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, L.M.D.; Lage, D.P.; Martins, V.T.; Carvalho, A.; Roatt, B.M.; Menezes-Souza, D.; Tavares, C.A.P.; Alves, R.J.; Barichello, J.M.; et al. Treatment of murine visceral leishmaniasis using an 8-hydroxyquinoline-containing polymeric micelle system. Parasitol. Int. 2016, 65, 728–736. [Google Scholar] [CrossRef]
- Lage, D.P.; Ribeiro, P.A.F.; Dias, D.S.; Mendonça, D.V.C.; Ramos, F.F.; Carvalho, L.M.; Steiner, B.T.; Tavares, G.S.V.; Martins, V.T.; Machado, A.S.; et al. Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis. Vaccines 2020, 8, 289. [Google Scholar] [CrossRef]
- Coelho, V.T.; Oliveira, J.S.; Valadares, D.G.; Chávez-Fumagalli, M.A.; Duarte, M.C.; Lage, P.S.; Soto, M.; Santoro, M.M.; Tavares, C.A.; Fernandes, A.P.; et al. Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl. Trop. Dis. 2012, 6, e1430. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.S.; Lage, D.P.; Vale, D.L.; Freitas, C.S.; Linhares, F.P.; Cardoso, J.M.O.; Pereira, I.A.G.; Ramos, F.F.; Tavares, G.S.V.; Ludolf, F.; et al. A recombinant Leishmania amastigote-specific protein, rLiHyG, with adjuvants, protects against infection with Leishmania infantum. Acta Trop. 2022, 230, 106412. [Google Scholar] [CrossRef]
- Dias, D.S.; Ribeiro, P.A.F.; Martins, V.T.; Lage, D.P.; Ramos, F.F.; Dias, A.L.T.D.; Rodrigues, M.R.; Portela, A.S.B.; Costa, L.E.; Caligiorne, R.B.; et al. Recombinant prohibitin protein of Leishmania infantum acts as a vaccine candidate and diagnostic marker against visceral leishmaniasis. Cell. Immunol. 2018, 323, 59–69. [Google Scholar] [CrossRef]
- Lage, D.P.; Ribeiro, P.A.F.; Dias, D.S.; Mendonça, D.V.C.; Ramos, F.F.; Carvalho, L.M.; de Oliveira, D.; Steiner, B.T.; Martins, V.T.; Perin, L.; et al. A candidate vaccine for human visceral leishmaniasis based on a specific T cell epitope-containing chimeric protein protects mice against Leishmania infantum infection. NPJ Vaccines 2020, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Fumagalli, M.A.; Ribeiro, T.G.; Castilho, R.O.; Fernandes, S.O.; Cardoso, V.N.; Coelho, C.S.; Mendonça, D.V.; Soto, M.; Tavares, C.A.; Faraco, A.A.; et al. New delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Rev. Soc. Bras. Med. Trop. 2015, 48, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef]
- Van Griensven, J.; Diro, E. Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect. Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef]
- Lage, D.P.; Vale, D.L.; Linhares, F.P.; Freitas, C.S.; Machado, A.S.; Cardoso, J.M.O.; Oliveira, D.; Galvani, N.C.; Oliveira, M.P.; Oliveira-da-Silva, J.A.; et al. A recombinant chimeric protein-based vaccine containing T-cell epitopes from amastigote proteins and combined with distinct adjuvants, induces immunogenicity and protection against Leishmania infantum infection. Vaccines 2022, 10, 1146. [Google Scholar] [CrossRef]
- Vale, D.L.; Freitas, C.S.; Martins, V.T.; Moreira, G.J.L.; Machado, A.S.; Ramos, F.F.; Pereira, I.A.G.; Bandeira, R.S.; Jesus, M.M.; Tavares, G.S.V.; et al. Efficacy of an immunotherapy combining immunogenic chimeric protein plus adjuvant and amphotericin B against murine visceral leishmaniasis. Biology 2023, 12, 851. [Google Scholar] [CrossRef]
- Tavares, G.S.V.; Mendonça, D.V.C.; Pereira, I.A.G.; Oliveira-da-Silva, J.A.; Ramos, F.F.; Lage, D.P.; Machado, A.S.; Carvalho, L.M.; Reis, T.A.R.; Perin, L.; et al. A clioquinol-containing Pluronic F127 polymeric micelle system is effective in the treatment of visceral leishmaniasis in a murine model. Parasite 2020, 27, 29. [Google Scholar] [CrossRef]
- Gomes, D.C.O.; Souza, B.L.d.S.C.; Schwedersky, R.P.; Covre, L.P.; Guedes, H.L.d.M.; Lopes, U.G.; Ré, M.I.; Rossi-Bergmann, B. Intranasal immunization with chitosan microparticles enhances LACK-DNA vaccine protection and induces specific long-lasting immunity against visceral leishmaniasis. Microbes Infect. 2022, 24, 104884. [Google Scholar] [CrossRef]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef]
- Moreira, N.D.D.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.D.A.; Ker, H.G.; de Oliveira Cardoso, J.M.; Giunchetti, R.C.; Carneiro, C.M.; de Lana, M.; Reis, A.B. Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: “Leishman Donovan units” versus real-time PCR. PLoS ONE 2012, 7, e47907. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, R.; Dahal, P.; Singh-Phulgenda, S.; Brack, M.; Naylor, C.; Sundar, S.; Alves, F.; Stepniewska, K.; Guerin, P.J. Estimating the proportion of relapse following treatment of visceral leishmaniasis: Meta-analysis using Infectious Diseases Data Observatory (IDDO) systematic review. Lancet Reg. Health-Southeast Asia 2023, 22, 100317. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; Aguiar-Soares, R.D.; Coura-Vital, W.; Ker, H.G.; Moreira, N.; Vitoriano-Souza, J.; Giunchetti, R.C.; Carneiro, C.M.; Reis, A.B. Immunotherapy and immunochemotherapy in visceral leishmaniasis: Promising treatments for this neglected disease. Front. Immunol. 2014, 5, 272. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.; Ferreira, F.C.; Gusmão, M.R.; Costa, A.F.P.; de Brito, R.C.F.; Aguiar-Soares, R.D.O.; Reis, A.B.; Cardoso, J.M.O.; Carneiro, C.M.; Roatt, B.M. Heterologous vaccine therapy associated with half course of Miltefosine promote activation of the proinflammatory response with control of splenic parasitism in a hamster model of visceral leishmaniasis. Curr. Res. Immunol. 2021, 2, 194–201. [Google Scholar] [CrossRef]
- Borja-Cabrera, G.P.; Santos, F.N.; Santos, F.B.; Trivellato, F.A.; Kawasaki, J.K.; Costa, A.C.; Castro, T.; Nogueira, F.S.; Moreira, M.A.B.; Luvizotto, M.C.R.; et al. Immunotherapy with the saponin enriched-Leishmune vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine 2010, 28, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key determinants of resistance or disease progression in visceral leishmaniasis: Opportunities for novel diagnostics and immunotherapy. Front. Immunol. 2019, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Margaroni, M.; Kotsakis, S.D.; Karagouni, E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines 2020, 8, 350. [Google Scholar] [CrossRef]
- Ayari-Riabi, S.; Ben Khalaf, N.; Bouhaouala-Zahar, B.; Verrier, B.; Trimaille, T.; Benlasfar, Z.; Chenik, M.; Elayeb, M. Polylactide nanoparticles as a biodegradable vaccine adjuvant: A study on safety, protective immunity and efficacy against human leishmaniasis caused by Leishmania major. Molecules 2022, 27, 8677. [Google Scholar] [CrossRef]
- Fernández, L.; Solana, J.C.; Sánchez, C.; Jiménez, M.A.; Requena, J.M.; Coler, R.; Reed, S.G.; Valenzuela, J.G.; Kamhawi, S.; Oliveira, F.; et al. Protective efficacy in a hamster model of a multivalent vaccine for human visceral leishmaniasis (Mulevaclin) consisting of the KMP11, LEISH-F3+, and LJL143 antigens in virosomes, plus GLA-SE adjuvant. Microorganisms 2021, 9, 2253. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, T.; Tan, S.; Yu, L.; Chi, Y.; Chen, J.; Fan, X.; Lu, X. Screening helper T lymphocyte epitopes based on IFN-gamma/IL-10 ratio for developing a novel multi-epitope vaccine candidate using Wolbachia surface protein as an adjuvant against visceral leishmaniasis. Parasite Vectors 2025, 18, 116. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: From mode of action to effective vaccines. Expert Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef]
- Oliveira-Maciel, D.; Dos-Santos, J.S.; Oliveira-Silva, G.; Mello, M.F.; da Fonseca-Martins, A.M.; Carneiro, M.P.D.; Ramos, T.D.; Firmino-Cruz, L.; Gomes, D.C.O.; Rossi-Bergmann, B.; et al. MPLA and AddaVax® Adjuvants Fail to Promote Intramuscular LaAg Vaccine Protectiveness against Experimental Cutaneous Leishmaniasis. Microorganisms 2021, 9, 1272. [Google Scholar] [CrossRef]
- Costa, S.S.; Ribeiro, N.R.; Cruz, L.d.R.; Moreira, G.J.L.; Martins, V.T.; Lage, D.P.; Vale, D.L.; Costa, C.V.S.; Galvani, N.C.; Luiz, G.P.; et al. Potential immunoprophylactic use of a multi-epitope protein of CD4+ and CD8+ T cells against visceral leishmaniasis in Balb/c mice. Res. Veter-Sci. 2025, 194, 105820. [Google Scholar] [CrossRef]
- Ismail, N.; Karmakar, S.; Bhattacharya, P.; Sepahpour, T.; Takeda, K.; Hamano, S.; Matlashewski, G.; Satoskar, A.R.; Gannavaram, S.; Dey, R.; et al. Leishmania Major Centrin Gene-Deleted Parasites Generate Skin Resident Memory T-Cell Immune Response Analogous to Leishmanization. Front. Immunol. 2022, 13, 864031. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Corvo, L.; Garde, E.; Ramírez, L.; Iniesta, V.; Bonay, P.; Gómez-Nieto, C.; González, V.M.; Martín, M.E.; Alonso, C.; et al. Coadministration of the three antigenic Leishmania infantum poly (a) binding proteins as a DNA vaccine induces protection against Leishmania major infection in BALB/c mice. PLoS Negl. Trop. Dis. 2015, 9, e0003751. [Google Scholar] [CrossRef]
- Alves-Silva, M.V.; Nico, D.; Luca, P.M.; Palatnik-de-Sousa, C.B. The F1F3 recombinant chimera of Leishmania donovani-nucleoside hydrolase (NH36) and its epitopes induce cross-protection against Leishmania (V.) braziliensis infection in mice. Front. Immunol. 2019, 10, 724. [Google Scholar] [CrossRef]
- Devender, M.; Sebastian, P.; Maurya, V.K.; Kumar, K.; Anand, A.; Namdeo, M.; Maurya, R. Immunogenicity and protective efficacy of tuzin protein as a vaccine candidate in Leishmania donovani-infected BALB/c mice. Front. Immunol. 2024, 14, 1294397. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Abánades, D.R.; Parody, N.; Carrión, J.; Risueño, R.M.; Pineda, M.A.; Bonay, P.; Alonso, C.; Soto, M. The immunodominant T helper 2 (Th2) response elicited in BALB/c mice by the Leishmania LiP2a and LiP2b acidic ribosomal proteins cannot be reverted by strong Th1 inducers. Clin. Exp. Immunol. 2007, 150, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Daoudaki, M.; Diakou, A.; Frydas, S.; Fouzas, I.; Karagouni, E.; Vavatsi, N.; Haralabidis, S. Vaccination with Trichinella spirallis antigens increases CD8+ peripheral T cells and enhances the Th2 immune response in Leishmania infantum challenged mice. Int. J. Immunopathol. Pharmacol. 2009, 22, 169–174. [Google Scholar] [CrossRef]
- Martins, V.T.; Lage, D.P.; Duarte, M.C.; Costa, L.E.; Garde, E.; Rodrigues, M.R.; Chávez-Fumagalli, M.A.; Menezes-Souza, D.; Roatt, B.M.; Tavares, C.A.; et al. A new Leishmania-specific hypothetical protein, LiHyT, used as a vaccine antigen against visceral leishmaniasis. Acta Trop. 2016, 154, 73–81. [Google Scholar] [CrossRef]
- Bhowmick, S.; Ravindran, R.; Ali, N. IL-4 contributes to failure, and colludes with IL-10 to exacerbate Leishmania donovani infection following administration of a subcutaneous leishmanial antigen vaccine. BMC Microbiol. 2014, 14, 8. [Google Scholar] [CrossRef]
- Tedla, M.G.; Nahar, M.F.; Every, A.L.; Scheerlinck, J.Y. The Immune Memory Response of In Vitro-Polarised Th1, Th2, and Th17 Cells in the Face of Ovalbumin-Transgenic Leishmania major in a Mouse Model. Int. J. Mol. Sci. 2024, 25, 8753. [Google Scholar] [CrossRef]
- Nateghi-Rostami, M.; Sohrabi, Y. Memory T cells: Promising biomarkers for evaluating protection and vaccine efficacy against leishmaniasis. Front. Immunol. 2024, 15, 1304696. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.R.; Silva, D.A.M.; Katz, S.; Orikaza, C.M.; Oliveira, K.C.; Barbiéri, C.L. A new immunochemotherapy schedule for visceral leishmaniasis in a hamster model. Parasitol. Res. 2022, 121, 2849–2860. [Google Scholar] [CrossRef]
- Gupta, S.; Sane, S.A.; Shakya, N.; Vishwakarma, P.; Haq, W. CpG oligodeoxynucleotide and miltefosine, a potential combination for treatment of experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 2011, 55, 3461–3464. [Google Scholar] [CrossRef]
- Van Henten, S.; Tesfaye, A.B.; Abdela, S.G.; Tilahun, F.; Fikre, H.; Buyze, J.; Kassa, M.; Cnops, L.; Pareyn, M.; Mohammed, R.; et al. Miltefosine for the treatment of cutaneous leishmaniasis—A pilot study from Ethiopia. PLoS Negl. Trop. Dis. 2021, 15, e0009460. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine. 2018, 36, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Júnior, E.F.; Martins, T.M.; Canto-Cavalheiro, M.M.; Marques, P.R.; Portari, E.A.; Coelho, M.G.; Netto, C.D.; Costa, P.R.; Sabino, K.C.; Torres-Santos, E.C. Preclinical studies evaluating subacute toxicity and therapeutic efficacy of LQB-118 in experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 2016, 60, 3794–3801. [Google Scholar] [CrossRef]
- Majoor, A.; Michel, G.; Marty, P.; Boyer, L.; Pomares, C. Leishmaniases: Strategies in treatment development. Parasite 2025, 32, 18. [Google Scholar] [CrossRef]
- Clementi, A.; Battaglia, G.; Floris, M.; Castellino, P.; Ronco, C.; Cruz, D.N. Renal involvement in leishmaniasis: A review of the literature. Nephrol. Dial. Transplant. Plus 2011, 4, 147–152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, M.M.; Lage, D.P.; Pimenta, B.L.; Moreira, G.J.L.; Pereira, I.A.G.; Câmara, R.S.B.; Silva, A.L.; Tavares, G.S.V.; Falcão, K.O.M.; Dias, S.S.G.; et al. Immunotherapeutics Combining a Recombinant Chimeric Protein, Monophosphoryl Lipid A, and Miltefosine Against Visceral Leishmaniasis. Pathogens 2025, 14, 1202. https://doi.org/10.3390/pathogens14121202
Jesus MM, Lage DP, Pimenta BL, Moreira GJL, Pereira IAG, Câmara RSB, Silva AL, Tavares GSV, Falcão KOM, Dias SSG, et al. Immunotherapeutics Combining a Recombinant Chimeric Protein, Monophosphoryl Lipid A, and Miltefosine Against Visceral Leishmaniasis. Pathogens. 2025; 14(12):1202. https://doi.org/10.3390/pathogens14121202
Chicago/Turabian StyleJesus, Marcelo M., Daniela P. Lage, Breno L. Pimenta, Gabriel J. L. Moreira, Isabela A. G. Pereira, Raquel S. B. Câmara, Ana L. Silva, Grasiele S. V. Tavares, Karolina O. M. Falcão, Saulo S. G. Dias, and et al. 2025. "Immunotherapeutics Combining a Recombinant Chimeric Protein, Monophosphoryl Lipid A, and Miltefosine Against Visceral Leishmaniasis" Pathogens 14, no. 12: 1202. https://doi.org/10.3390/pathogens14121202
APA StyleJesus, M. M., Lage, D. P., Pimenta, B. L., Moreira, G. J. L., Pereira, I. A. G., Câmara, R. S. B., Silva, A. L., Tavares, G. S. V., Falcão, K. O. M., Dias, S. S. G., Abrão, D. M., Rodrigues, M. M., Oliveira-da-Silva, J. A., Giusta, M. S., Chávez-Fumagalli, M. A., Roatt, B. M., Galdino, A. S., Christodoulides, M., Freitas, C. S., & Coelho, E. A. F. (2025). Immunotherapeutics Combining a Recombinant Chimeric Protein, Monophosphoryl Lipid A, and Miltefosine Against Visceral Leishmaniasis. Pathogens, 14(12), 1202. https://doi.org/10.3390/pathogens14121202








