The First Study of Borrelia burgdorferi Sensu Lato Persistence in Small Mammals Captured in the Ixodes persulcatus Distribution Area in Western Siberia
Abstract
1. Introduction
2. Materials and Methods
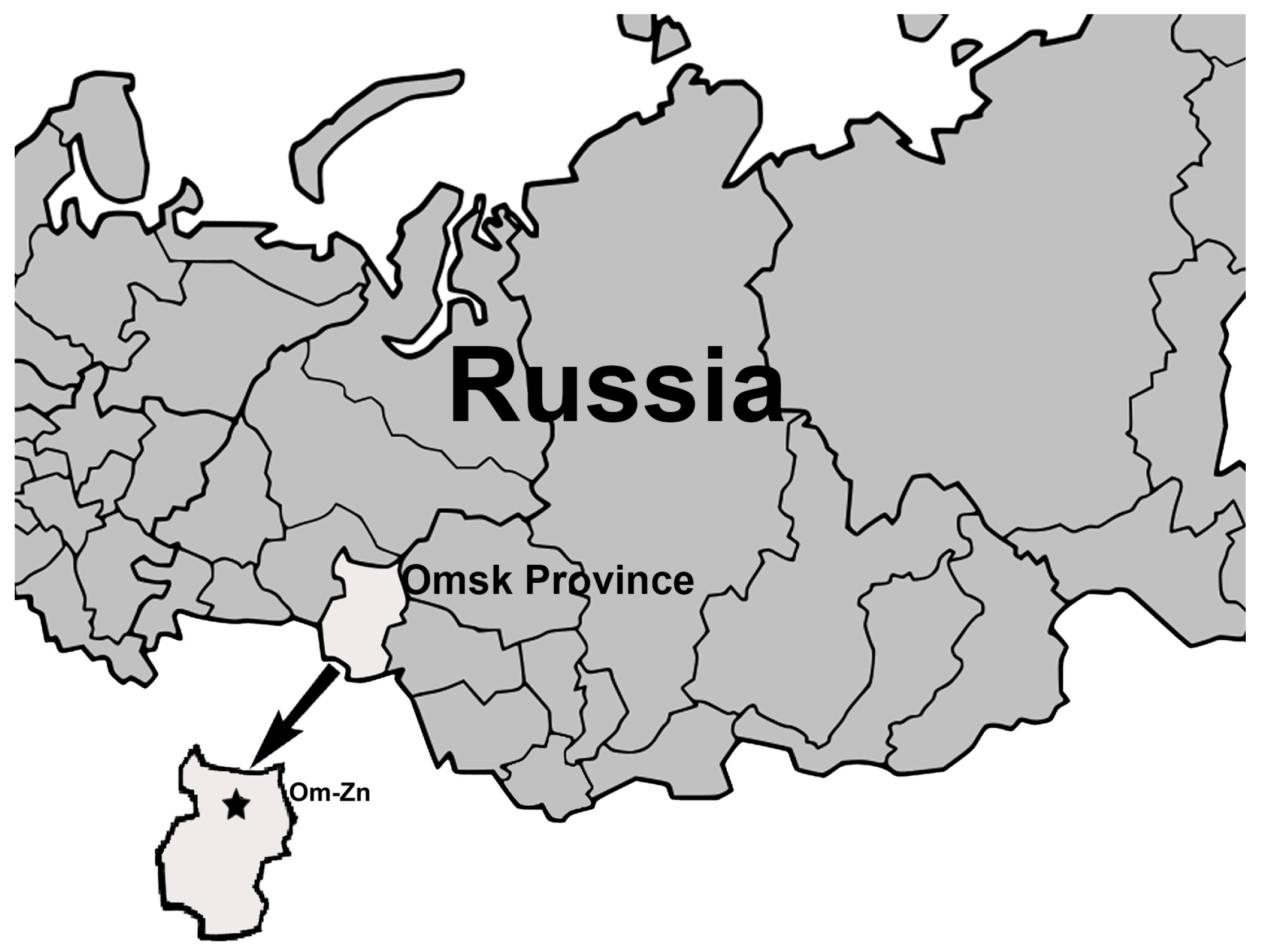
2.1. Sampling
2.2. The Study of B. burgdorferi s.l. Persistence
2.3. Laboratory Colonies of Ticks
2.4. The Study of B. burgdorferi s.l. Transmission
2.5. DNA Extraction
2.6. Detection and Genetic Characterization of B. burgdorferi s.l.
2.7. Phylogenetic Analysis
2.8. Statistical Analysis
2.9. GenBank Accession Numbers
3. Results
3.1. B. burgdorferi s.l. Prevalence in Small Mammals
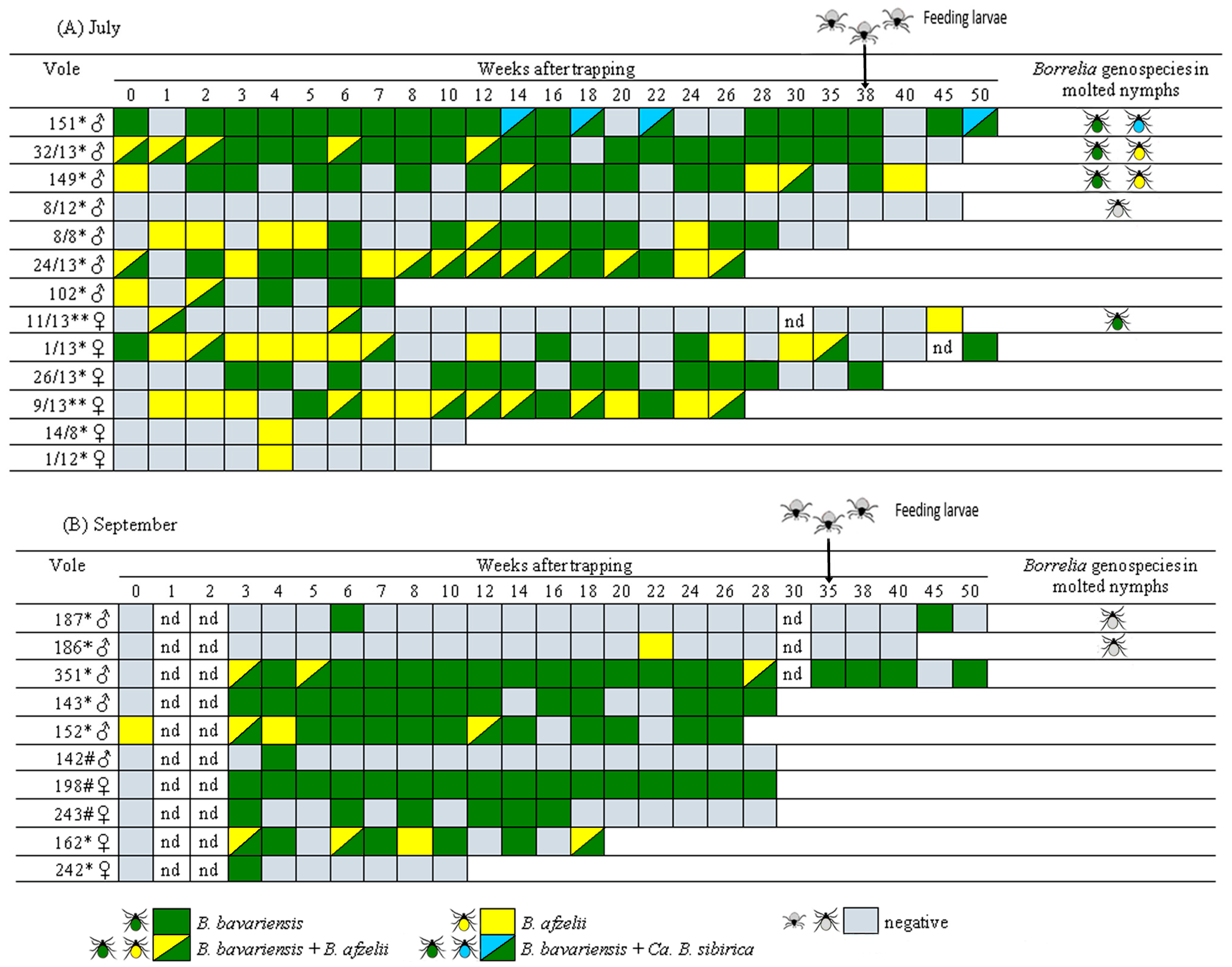
3.2. Persistence of B. burgdorferi s.l. in Naturally Infected Voles
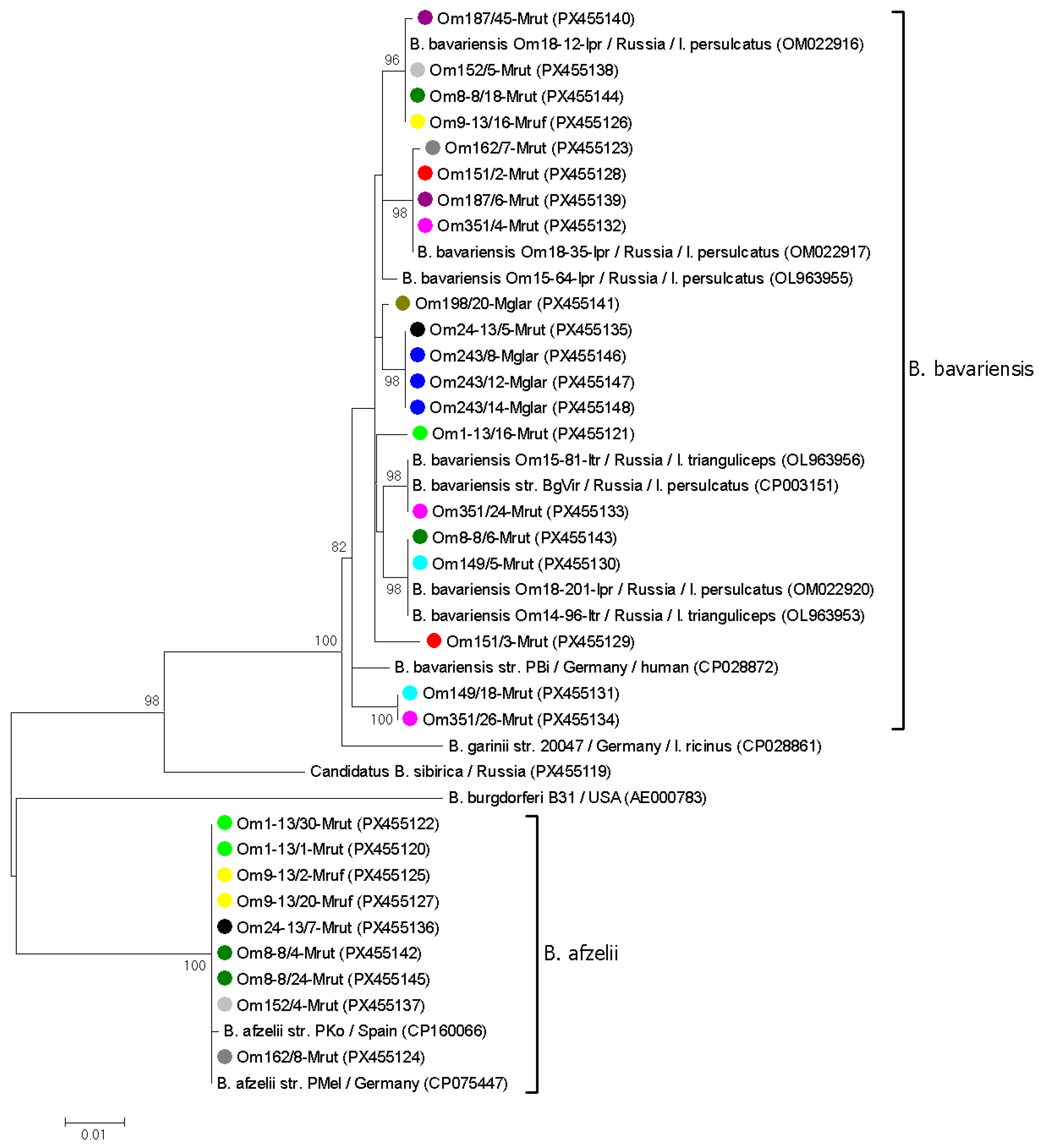
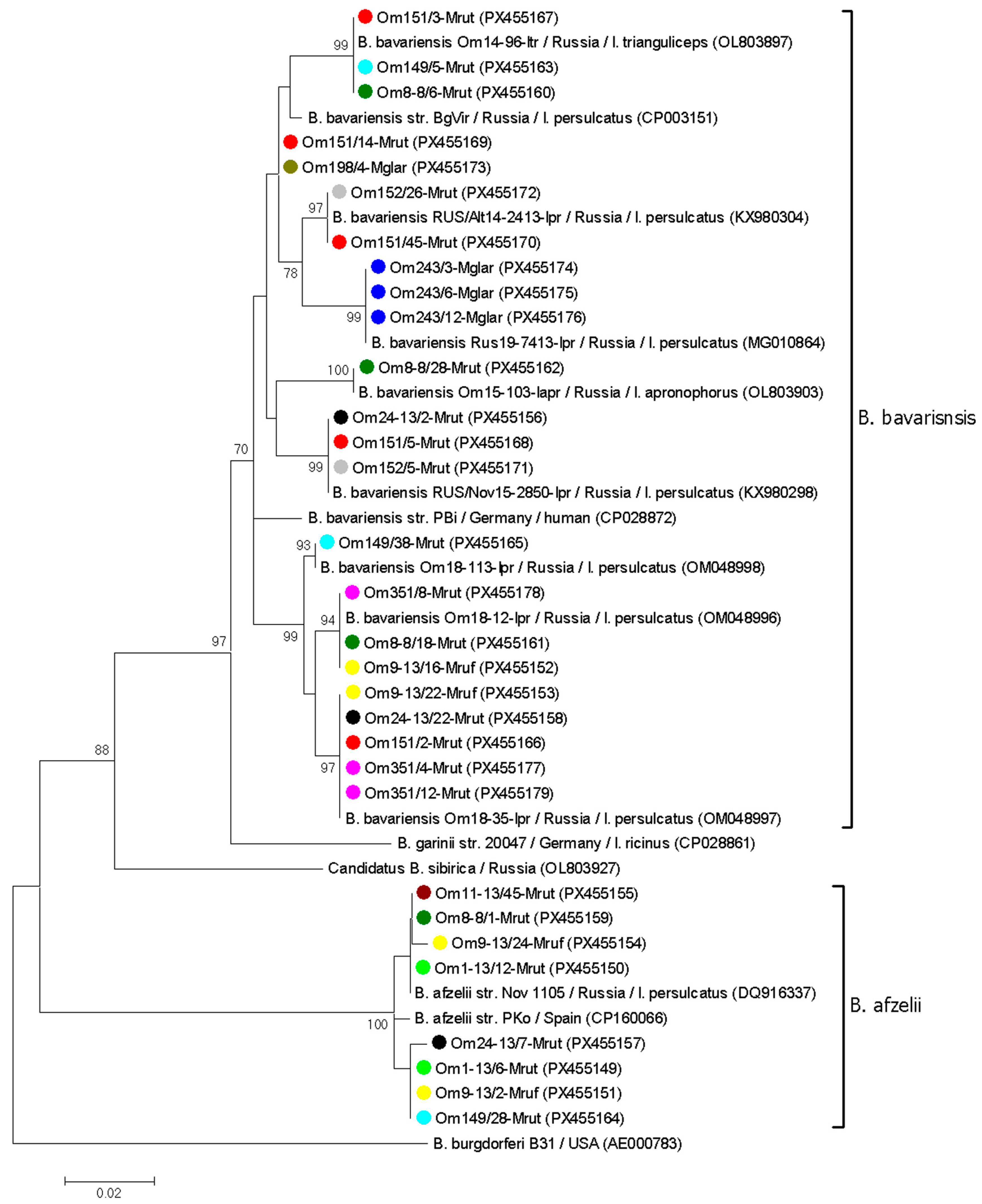
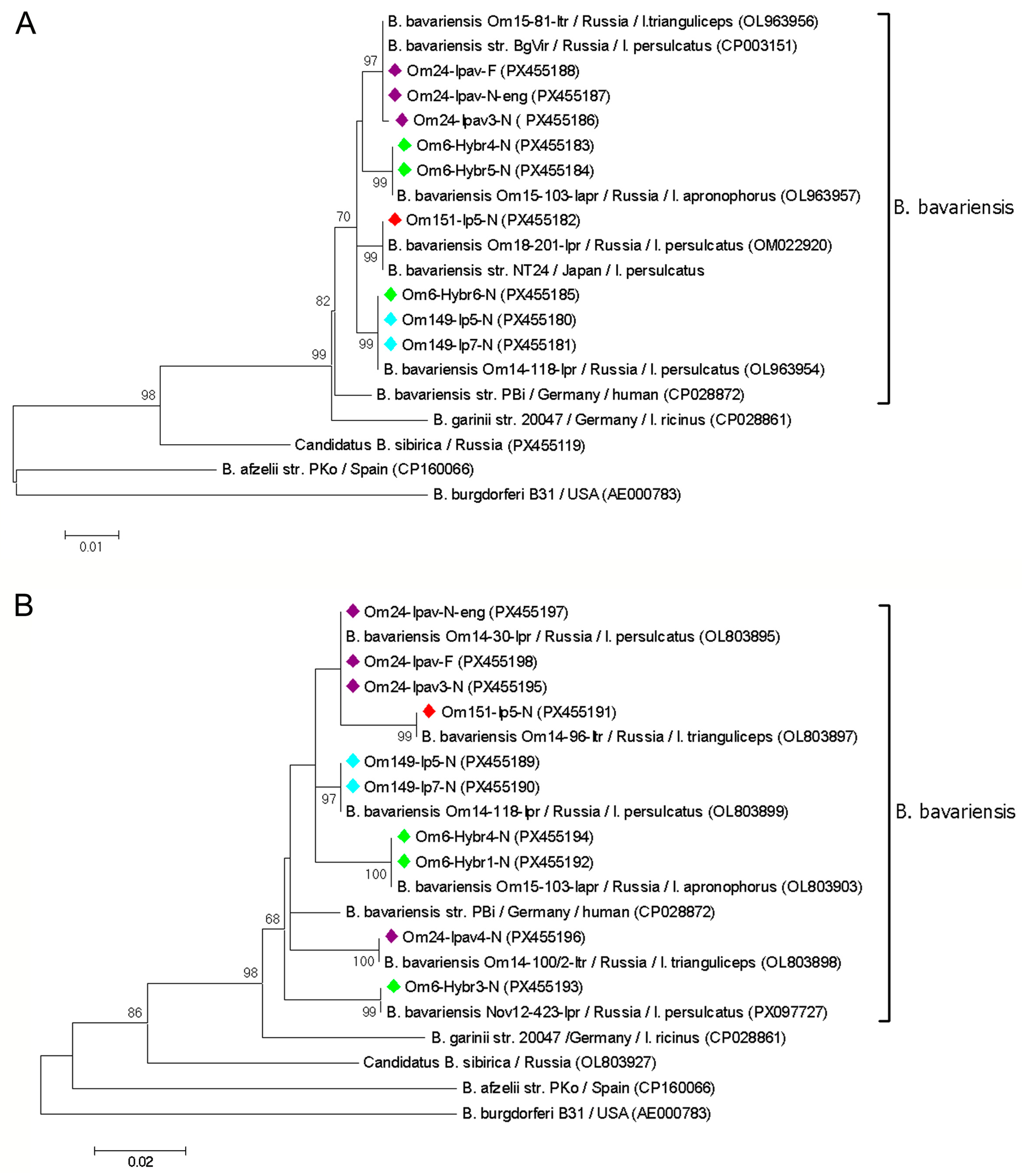
3.3. Genetic Diversity of B. burgdorferi s.l. in Voles
3.4. Transmission of B. burgdorferi s.l. by Ixodes spp.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolcott, K.A.; Margos, G.; Fingerle, V.; Becker, N.S. Host association of Borrelia burgdorferi sensu lato: A review. Ticks Tick Borne Dis. 2021, 12, 101766. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Vancova, M.; Clark, K.; Grubhoffer, L.; Oliver, J.H., Jr. Isolation of live Borrelia burgdorferi sensu lato spirochaetes from patients with undefined disorders and symptoms not typical for Lyme borreliosis. Clin. Microbiol. Infect. 2016, 22, 267.e9–15. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Mead, P.S.; Johnson, D.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef]
- Rollins, R.E.; Sato, K.; Nakao, M.; Tawfeeq, M.T.; Herrera-Mesías, F.; Pereira, R.J.; Kovalev, S.; Margos, G.; Fingerle, V.; Kawabata, H.; et al. Out of Asia? Expansion of Eurasian Lyme borreliosis causing genospecies display unique evolutionary trajectories. Mol. Ecol. 2023, 32, 786–799. [Google Scholar] [CrossRef]
- Margos, G.; Vollmer, S.A.; Cornet, M.; Garnier, M.; Fingerle, V.; Wilske, B.; Bormane, A.; Vitorino, L.; Collares-Pereira, M.; Drancourt, M.; et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 2009, 75, 5410–5416. [Google Scholar] [CrossRef]
- Rizzoli, A.; Hauffe, H.; Carpi, G.; Vourc, H.G.; Neteler, M.; Rosa, R. Lyme borreliosis in Europe. Euro. Surveill. 2011, 16, 19906. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef]
- Huegli, D.; Hu, C.M.; Humair, P.F.; Wilske, B.; Gern, L. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J. Clin. Microbiol. 2002, 40, 4735–4737. [Google Scholar] [CrossRef]
- Takano, A.; Nakao, M.; Masuzawa, T.; Takada, N.; Yano, Y.; Ishiguro, F.; Fujita, H.; Ito, T.; Ma, X.; Oikawa, Y.; et al. Multilocus sequence typing implicates rodents as the main reservoir host of human-pathogenic Borrelia garinii in Japan. J. Clin. Microbiol. 2011, 49, 2035–2039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurtenbach, K.; Hanincová, K.; Tsao, J.I.; Margos, G.; Fish, D.; Ogden, N.H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 2006, 4, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Raileanu, C.; Silaghi, C.; Fingerle, V.; Margos, G.; Thiel, C.; Pfister, K.; Overzier, E. Borrelia burgdorferi Sensu Lato in Questing and Engorged Ticks from Different Habitat Types in Southern Germany. Microorganisms 2021, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Voordouw, M.J. Co-feeding transmission in Lyme disease pathogens. Parasitology 2015, 142, 290–302. [Google Scholar] [CrossRef]
- Baum, E.; Hue, F.; Barbour, A.G. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. mBio 2012, 3, e00434-12. [Google Scholar] [CrossRef]
- Andersson, M.; Scherman, K.; Råberg, L. Multiple-strain infections of Borrelia afzelii: A role for within-host interactions in the maintenance of antigenic diversity? Am. Nat. 2013, 181, 545–554. [Google Scholar] [CrossRef]
- Jacquet, M.; Margos, G.; Fingerle, V.; Voordouw, M.J. Comparison of the Lifetime Host-to-Tick Transmission Between Two Strains of the Lyme disease pathogen Borrelia afzelii. Parasites Vectors 2016, 9, 645. [Google Scholar] [CrossRef]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef]
- Rego, R.O.; Bestor, A.; Štefka, J.; Rosa, P.A. Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PLoS ONE 2014, 9, e101009. [Google Scholar] [CrossRef]
- Derdáková, M.; Dudičák, V.; Brei, B.; Brownstein, J.S.; Schwartz, I.; Fish, D. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl. Environ. Microbiol. 2004, 70, 6783–6788. [Google Scholar] [CrossRef]
- Hanincová, K.; Ogden, N.H.; Diuk-Wasser, M.; Pappas, C.J.; Iyer, R.; Fish, D.; Schwartz, I.; Kurtenbach, K. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 2008, 74, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Gern, L.; Siegenthaler, M.; Hu, C.M.; Leuba-Garcia, S.; Humair, P.F.; Moret, J. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): Duration and enhancement of infectivity for Ixodes ricinus ticks. Eur. J. Epidemiol. 1994, 10, 75–80. [Google Scholar] [PubMed]
- States, S.L.; Huang, C.I.; Davis, S.; Tufts, D.M.; Diuk-Wasser, M.A. Co-feeding transmission facilitates strain coexistence in Borrelia burgdorferi, the Lyme disease agent. Epidemics 2017, 19, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.N.; Dykhuizen, D.E.; Qiu, W.; Dunn, J.J.; Bosler, E.M.; Luft, B.J. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 1999, 151, 15–30. [Google Scholar] [CrossRef]
- Perez, D.; Kneubühler, Y.; Rais, O.; Jouda, F.; Gern, L. Borrelia afzelii ospC Genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: Contribution of co-feeding ticks. Ticks Tick-Borne Dis. 2011, 2, 137–142. [Google Scholar] [CrossRef]
- Qiu, W.G.; Dykhuizen, D.E.; Acosta, M.S.; Luft, B.J. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics 2002, 160, 833–849. [Google Scholar] [CrossRef]
- Genne, D.; Rossel, M.; Sarr, A.; Battilotti, F.; Rais, O.; Rego, R.O.M.; Voordouw, M.J. Competition between strains of Borrelia afzelii in the host tissues and consequences for transmission to ticks. ISME J. 2021, 15, 2390–2400. [Google Scholar] [CrossRef]
- Rynkiewicz, E.C.; Brown, J.; Tufts, D.M.; Huang, C.I.; Kampen, H.; Bent, S.J.; Fish, D.; Diuk-Wasser, M.A. Closely-related Borrelia burgdorferi (sensu stricto) strains exhibit similar fitness in single infections and asymmetric competition in multiple infections. Parasites Vectors 2017, 10, 64. [Google Scholar] [CrossRef]
- Rar, V.; Livanova, N.; Tkachev, S.; Kaverina, G.; Tikunov, A.; Sabitova, Y.; Igolkina, Y.; Panov, V.; Livanov, S.; Fomenko, N.; et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit. Vectors 2017, 10, 258. [Google Scholar] [CrossRef]
- Rar, V.; Chicherina, G.; Igolkina, Y.; Fedorets, V.; Epikhina, T.; Tikunova, N. Spectrum of Ixodidae Ticks Attacking Humans in Novosibirsk Province, Russian Siberia, and Their Association with Tick-Borne Bacterial Agents. Pathogens 2025, 14, 315. [Google Scholar] [CrossRef]
- Mukhacheva, T.A.; Kovalev, S.Y. Borrelia spirochetes in Russia: Genospecies differentiation by real-time PCR. Ticks Tick Borne Dis. 2014, 5, 722–726. [Google Scholar] [CrossRef]
- Kurilshikov, A.M.; Fomenko, N.V.; Stronin, O.V.; Tikunov, A.Y.; Kabilov, M.R.; Tupikin, A.E.; Tikunova, N.V. Complete Genome Sequencing of Borrelia valaisiana and Borrelia afzelii Isolated from Ixodes persulcatus Ticks in Western Siberia. Genome Announc. 2014, 2, e01315-14. [Google Scholar] [CrossRef] [PubMed]
- Sabitova, Y.; Rar, V.; Tikunov, A.; Yakimenko, V.; Korallo-Vinarskaya, N.; Livanova, N.; Tikunova, N. Detection and genetic characterization of a putative novel Borrelia genospecies in Ixodes apronophorus/Ixodes persulcatus/Ixodes trianguliceps sympatric areas in Western Siberia. Ticks Tick Borne Dis. 2023, 14, 102075. [Google Scholar] [CrossRef]
- Kovalevskii, Y.V.; Korenberg, E.I.; Gorelova, N.B. Long-term dynamics of the epizootic process in natural foci of ixodid tick borreliosis in mountain taiga forests of the Middle Ural. Parazitologiia 2004, 38, 105–121. (In Russian) [Google Scholar] [PubMed]
- Rar, V.A.; Epikhina, T.I.; Tikunova, N.V.; Bondarenko, E.I.; Ivanov, M.K.; Iakimenko, V.V.; Mal’kova, M.G.; Tantsev, A.K. DNA detection of pathogens transmitted by Ixodid ticks in blood of small mammals inhabiting the forest biotopes in Middle Irtysh Area (Omsk Region, West Siberia). Parazitologiia 2014, 48, 37–53. (In Russian) [Google Scholar]
- Rar, V.; Yakimenko, V.; Makenov, M.; Tikunov, A.; Epikhina, T.; Tancev, A.; Bobrova, O.; Tikunova, N. High prevalence of Babesia microti ‘Munich’ type in small mammals from an Ixodes persulcatus/Ixodes trianguliceps sympatric area in the Omsk region, Russia. Parasitol. Res. 2016, 115, 3619–3629. [Google Scholar] [CrossRef] [PubMed]
- Gromov, I.M.; Polyakov, I.Y. Fauna of the USSR. Mammals, Ser. 3: Voles (Microtinae); Nauka: Leningrad, Russia, 1977; 502p. (In Russian) [Google Scholar]
- Gureev, A.A. Fauna of the USSR. Mammals, Ser. 4: Insectivores; Nauka: Leningrad, Russia, 1979; 501p. (In Russian) [Google Scholar]
- Yakimenko, V.V.; Malkova, M.G.; Shpynov, S.N. Ixodid Ticks of the Western Siberia; Omsk Scientific Vestnik: Omsk, Russia, 2013; p. 276. (In Russian) [Google Scholar]
- Rar, V.; Livanova, N.; Sabitova, Y.; Igolkina, Y.; Tkachev, S.; Tikunov, A.; Babkin, I.; Golovljova, I.; Panov, V.; Tikunova, N. Ixodes persulcatus/pavlovskyi natural hybrids in Siberia: Occurrence in sympatric areas and infection by a wide range of tick-transmitted agents. Ticks Tick Borne Dis. 2019, 10, 101254. [Google Scholar] [CrossRef]
- Margos, G.; Gatewood, A.G.; Aanensen, D.M.; Hanincová, K.; Terekhova, D.; Vollmer, S.A.; Cornet, M.; Piesman, J.; Donaghy, M.; Bormane, A.; et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2008, 105, 8730–8735. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Korenberg, E.I.; Pomelova, V.G.; Osin, N.S. Infections with Natural Focality Transmitted by Ixodid Ticks; Ginzburg, A., Zlobin, V., Eds.; Kommentariy: Moscow, Russia, 2013; 463p. (In Russian) [Google Scholar]
- Korenberg, E.I.; Kovalevskii, Y.V.; Gorelova, N.B.; Nefedova, V.V. Comparative analysis of the roles of Ixodes persulcatus and I. trianguliceps ticks in natural foci of ixodid tick-borne borrelioses in the Middle Urals, Russia. Ticks Tick Borne Dis. 2015, 6, 316–321. [Google Scholar] [CrossRef]
- Gylfe, A.; Bergström, S.; Lundström, J.; Olsen, B. Reactivation of Borrelia infection in birds. Nature 2000, 403, 724–725. [Google Scholar] [CrossRef]
- Scholz, H.C.; Margos, G.; Derschum, H.; Speck, S.; Tserennorov, D.; Erdenebat, N.; Undraa, B.; Enkhtuja, M.; Battsetseg, J.; Otgonchimeg, C.; et al. High prevalence of genetically diverse Borrelia bavariensis-like strains in Ixodes persulcatus from Selenge Aimag, Mongolia. Ticks Tick Borne Dis. 2013, 4, 89–92. [Google Scholar] [CrossRef]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Brorson, O.; Brorson, S.H. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 1997, 25, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.L.; Jurkovich, P.; Kodner, C.; Johnson, R.C. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J. Clin. Microbiol. 1991, 29, 894–896. [Google Scholar] [CrossRef]
- Sonnesyn, S.W.; Manivel, J.C.; Johnson, R.C.; Goodman, J.L. A guinea pig model for Lyme disease. Infect. Immun. 1993, 61, 4777–4784. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.Y.; Sabitova, Y.V.; Rar, V.A.; Morozov, I.M.; Gordeiko, N.S.; Allenov, A.V.; Kaverina, G.B.; Babkin, I.V.; Tikunova, N.V.; Andaev, E.I. Role of Ixodes pavlovskyi (Acari, Ixodidae) in Borreliosis Epizootic Process at the Island Russky. Probl. Osobo Opasnykh Infektsii 2021, 1, 116–121. (In Russian) [Google Scholar] [CrossRef]
- Piesman, J.; Oliver, J.R.; Sinsky, R.J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 1990, 42, 352–357. [Google Scholar] [CrossRef]
| Locus | Organism | Reaction | Primer Name | Primer Sequences 5′-3′ | T * (°C) | References |
|---|---|---|---|---|---|---|
| ITS2 | Ixodidae | conventional | F-ITS2 | cacactgagcacttactctttg | 57 | [29] |
| R1-ITS2 | actggatggctccagtattc | |||||
| cox1 | I. persulcatus | conventional | Ixodes-F | acctgatatagctttccctcg | 55 | [29] |
| Ipers-R | ttgattcctgttggaacagc | |||||
| I. pavlovskyi | conventional | Ixodes-F | acctgatatagctttccctcg | 55 | [29] | |
| Ipav-R | taatccccgtggggacg | |||||
| IGS | B. burgdorferi s.l. | Primary | NC1 | cctgttatcattccgaacacag | 50 | [29] |
| NC2 | tactccattcggtaatcttggg | |||||
| Nested | NC3 | tactgcgagttcgcgggag | 50 | |||
| NC4 | cctaggcattcaccatagac | |||||
| “Ca. B. sibirica” | Nested | Bsib | ataaaacattctaaaaaaatgaaca | 50 | This study | |
| NC4 | cctaggcattcaccatagac | [29] | ||||
| clpA | B. burgdorferi s.l. | Primary | clpAF1237 | aaagatagatttcttccagac | 50 | [41] |
| clpAR2218 | gaatttcatctattaaaagctttc | |||||
| Nested | clpAF1255 | gacaaagcttttgatattttag | 50 | |||
| clpAR2104 | caaaaaaaacatcaaattttctatctc | |||||
| p83/100 | B. burgdorferi s.l. | Primary | F7 | ttcaaagggatactgttagagag | 50 | [29] |
| F10 | aagaaggcttatctaatggtgatg | |||||
| Nested | F5 | acctggtgatgtaagttctcc | 54 | |||
| F12 | ctaacctcattgttgttagactt |
| Sampling Periods | No. of Tested Mammals | No (%/95% CI) of Samples Containing DNA of B. burgdorferi s.l. | No of Genotyped Samples | No (%/95% CI *) of Samples Containing DNA | |||
|---|---|---|---|---|---|---|---|
| Ba | Bbav | Bs | Ba + Bbav | ||||
| June 2013 | 38 | 3 (7.9/2.7–20.8) | 3 | 2 | 1 | 0 | 0 |
| September 2013 | 59 | 7 (11.9/5.9–22.5) | 7 | 2 | 5 | 0 | 0 |
| July 2014 | 124 | 14 (11.3/6.9–18.1) | 12 | 5 | 5 | 0 | 2 |
| September 2014 | 119 | 5 (4.2/1.8–9.5) | 5 | 3 | 1 | 0 | 1 |
| July 2015 | 79 | 10 (12.7/7.0–21.8) | 10 | 5 | 2 | 0 | 3 |
| September 2015 | 87 | 4 (4.6/1.8–11.2) | 4 | 1 | 2 | 0 | 1 |
| September 2016 | 95 | 6 (6.3/2.9–13.1) | 2 | 1 | 1 | 0 | 0 |
| June 2017 | 33 | 6 (18.2/8.6–34.4) | 6 | 2 | 4 | 0 | 0 |
| October 2017 | 43 | 8 (18.6/9.7–32.6) | 8 | 2 | 6 | 0 | 0 |
| September 2018 | 28 | 7 (25.0/12.7–43.4) | 7 | 4 | 3 | 0 | 0 |
| August 2024 | 32 | 13 (40.6/25.5–57.7) | 13 | 6 | 5 | 1 | 1 |
| Total | 737 | 83 (11.3/9.2–13.8) | 77 | 33 (42.9/32.4–54.0) | 35 (45.5/34.8–56.5) | 1 (1.3/0.2–7.0) | 8 (10.4/5.4–19.2) |
| Both Sampling Periods | July 2015 | September 2015 | Statistical Difference Between Sampling Periods | |
|---|---|---|---|---|
| All examined voles | ||||
| Total number | 47 | 24 | 23 | |
| No. of males (%/95% CI) | 26 (55.3/41.3–68.6) | 13 (54.2/35.1–72.1) | 13 (56.5/36.8–74.3) | |
| No. of females (%/95% CI) | 21 (44.7/31.4–58.8) | 11 (45.8/27.9–64.9) | 10 (43.5/25.6–63.2) | |
| PCR-positive voles | ||||
| Total number | 22 | 12 | 10 | |
| No. of males (%/95% CI) | 12 (54.6/34.7–73.1) | 6 (50.0/25.4–74.6) | 6 (60.0/31.3–83.2) | |
| No. of females (%/95% CI) | 10 (45.4/26.9–65.3) | 6 (50.0/25.4–74.6) | 4 (40.0/16.8–68.7) | |
| Number (%/95% CI) of voles with a portion of PCR-positive blood samples: | ||||
| 50–100% | 14 (63.6/43.0–80.3) | 9 (75/46.8–91.1) | 5 (50.0/23.7–76.3) | |
| 15–50% | 1 (4.6/0.8–21.8) | 0 | 1 (10.0/1.8–40.4) | |
| 0–15% | 7 (31.8/16.3–52.7) | 3 (25/8.9–53.2) | 4 (40.0/16.8–68.7) | |
| Number (%/95% CI) of voles with a persistence of: | ||||
| B. bavariensis | 7 (31.8/16.3–52.7) | 1 (8.3/1.5–35.4) | 6 (60.0/31.3–83.2) | |
| B. afzelii | 3 (13.6/4.8–33.3) | 2 (16.7/4.7–44.8) | 1 (10.0/1.8–40.4) | |
| B. bavariensis + B. afzelii | 11 (50.0/30.7–69.3) | 8 (66.7/39.1–86.2) | 3 (30.0/10.8–60.3) | |
| B. bavariensis + Ca. B. sibirica | 1 (4.6/0.8–21.8) | 1 (8.3/1.5–35.4) | 0 | |
| PCR-positive samples | ||||
| Total number | 224 | 142 | 82 | |
| Number (%/95% CI) of blood samples containing DNA: | ||||
| B. bavariensis | 150 (67.0/60.6–72.8) | 80 (56.3/48.1–64.2) | 70 (85.4/76.1–91.4) | χ2 = 19.8, p < 0.001 |
| B. afzelii | 34 (15.2/11.1–20.5) | 30 (21.1/15.2–28.6) | 4 (4.9/1.9–11.9) | χ2 = 10.7, p = 0.001 |
| B. bavariensis + B. afzelii | 36 (16.1/11.8–21.5) | 28 (19.7/14.0–27.0) | 8 (9.8/5.0–18.1) | χ2 = 3.8, p = 0.05 |
| B. bavariensis + Ca. B. sibirica | 4 (1.8/0.7–4.5) | 4 (2.9/1.1–7.6) | 0 | |
| Voles | Feeding Larvae | Molted Nymphs | MIR */Prevalence # (%/95% CI) of Bbsl, Ba, Bbav, and Bs in Molted Nymphs | |||||
|---|---|---|---|---|---|---|---|---|
| ID | Bbsl Species in Voles | Tick Species | Time After Vole Capture (Weeks) | ID | No of Ticks in a Pool | Presence of Bbsl DNA | Bbsl Species in Molted Ticks | |
| 151 | Bbav, | Ip | 38 | 151-Ip1 | 5 | + | Bbav, Bs | Bbsl: 21.4/7.6–47.6 * |
| Bs | Ip | 38 | 151-Ip3 | 7 | + | Bbav | Bbav: 21.4/7.6–47.6 * | |
| Ip | 38 | 151-Ip4 | 1 | - | -- | Bs: 7.1/1.3–31.5 * | ||
| Ip | 38 | 151-Ip5 | 1 | + | Bbav | |||
| 149 | Bbav | Ip | 38 | 149-Ip1 | 5 | + | Bbav, Ba | Bbsl: 22.2/10.6–40.8 * |
| Ba | Ip | 38 | 149-Ip2 | 5 | + | Bbav, Ba | Bbav: 22.2/10.6–40.8 * | |
| Ip | 38 | 149-Ip3 | 7 | + | Bbav, Ba | Ba: 11.1/3.9–28.1 * | ||
| Ip | 38 | 149-Ip4 | 7 | + | Bbav | |||
| Ip | 38 | 149-Ip5 | 1 | + | Bbav | |||
| Ip | 38 | 149-Ip6 | 1 | - | - | |||
| Ip | 38 | 149-Ip7 | 1 | + | Bbav | |||
| 32-13 | Bbav | Ip | 38 | 32-13-Ip1 | 7 | + | Bbav | Bbsl: 18.8/8.9–35.3 * |
| Ba | Ip | 38 | 32-13-Ip2 | 7 | + | Bbav | Bbav: 18.8/8.9–35.3 * | |
| Ip | 38 | 32-13-Ip3 | 5 | + | Bbav | Ba: 6.3/1.7–20.2 * | ||
| Ip | 38 | 32-13-Ip4 | 5 | + | Bbav | |||
| Ip | 42 | 32-13-Ip5 | 4 | + | Bbav, Ba | |||
| Ip | 42 | 32-13-Ip6 | 4 | + | Bbav, Ba | |||
| 11-13 | Bbav | Ip | 42 | 11-13-Ip1 | 5 | - | - | Bbsl: 10.0/1.8–40.4 * |
| Ba | Ip | 42 | 11-13-Ip2 | 5 | + | Bbav | Bbav: 10.0/1.8–40.4 * | |
| 186 | Ba | Ip | 35 | 186-Ip1 | 5 | - | - | NA |
| Ip | 35 | 186-Ip2 | 5 | - | - | |||
| Ip | 35 | 186-Ip3 | 5 | - | - | |||
| 187 | Bbav | Ip | 35 | 187-Ip1 | 4 | - | - | NA |
| Ip | 35 | 187-Ip2 | 4 | - | - | |||
| 8-12 | - | Ip | 42 | 8-12-Ip1 | 5 | - | - | NA |
| Ip | 42 | 8-12-Ip1 | 5 | - | - | |||
| 24 | Bbav | Ipav | 23 | 24-Ipav1 | 1 | - | - | Bbsl: 50.0/18.8–81.2 # |
| Ipav | 23 | 24-Ipav2 | 1 | + | Bbav | Bbav: 50.0/18.8–81.2 # | ||
| Ipav | 23 | 24-Ipav3 | 1 | + | Bbav | |||
| Ipav | 23 | 24-Ipav4 | 1 | + | Bbav | |||
| Ipav | 23 | 24-Ipav5 | 1 | - | - | |||
| Ipav | 23 | 24-Ipav6 | 1 | - | - | |||
| 6 | Bbav | Hybr | 23 | 6-Hybr1 | 1 | + | Bbav | Bbsl: 71.4/35.9–91.8 # |
| Bbav | Hybr | 23 | 6-Hybr2 | 1 | - | - | Bbav: 71.4/35.9–91.8 # | |
| Bbav | Hybr | 23 | 6-Hybr3 | 1 | + | Bbav | ||
| Bbav | Hybr | 23 | 6-Hybr4 | 1 | + | Bbav | ||
| Bbav | Hybr | 23 | 6-Hybr5 | 1 | + | Bbav | ||
| Bbav | Hybr | 23 | 6-Hybr6 | 1 | + | Bbav | ||
| Bbav | Hybr | 23 | 6-Hybr7 | 1 | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rar, V.; Yakimenko, V.; Igolkina, Y.; Sabitova, Y.; Fedorets, V.; Karimov, A.; Rubtsov, G.; Epikhina, T.; Tikunova, N. The First Study of Borrelia burgdorferi Sensu Lato Persistence in Small Mammals Captured in the Ixodes persulcatus Distribution Area in Western Siberia. Pathogens 2025, 14, 1200. https://doi.org/10.3390/pathogens14121200
Rar V, Yakimenko V, Igolkina Y, Sabitova Y, Fedorets V, Karimov A, Rubtsov G, Epikhina T, Tikunova N. The First Study of Borrelia burgdorferi Sensu Lato Persistence in Small Mammals Captured in the Ixodes persulcatus Distribution Area in Western Siberia. Pathogens. 2025; 14(12):1200. https://doi.org/10.3390/pathogens14121200
Chicago/Turabian StyleRar, Vera, Valeriy Yakimenko, Yana Igolkina, Yuliya Sabitova, Valeria Fedorets, Alfrid Karimov, Gavril Rubtsov, Tamara Epikhina, and Nina Tikunova. 2025. "The First Study of Borrelia burgdorferi Sensu Lato Persistence in Small Mammals Captured in the Ixodes persulcatus Distribution Area in Western Siberia" Pathogens 14, no. 12: 1200. https://doi.org/10.3390/pathogens14121200
APA StyleRar, V., Yakimenko, V., Igolkina, Y., Sabitova, Y., Fedorets, V., Karimov, A., Rubtsov, G., Epikhina, T., & Tikunova, N. (2025). The First Study of Borrelia burgdorferi Sensu Lato Persistence in Small Mammals Captured in the Ixodes persulcatus Distribution Area in Western Siberia. Pathogens, 14(12), 1200. https://doi.org/10.3390/pathogens14121200





