Abstract
Bluetongue is a vector-borne disease with epidemic potential. Recently, outbreaks of Bluetongue were reported across Greece, caused by the Bluetongue virus (BTV) serotype 4. Regarding its pathogenesis, BTV infection involves various target organs with limited data referring to the kidneys. The objective of this study was to identify the possible impact of BTV infection on kidneys using common renal biomarkers. Urine and blood samples collected from 30 sheep with clinical signs of bluetongue (BTV sheep) and 30 clinically healthy sheep (normal sheep) from the same farms were finally selected and included in the study from an initial population of 47 sheep per group, based on the absence of active urine sediment. Complete urinalysis was performed and urine protein to creatinine ratio (UPC) and urine gamma-glutamyl transferase to creatinine (UGGTC) ratio were determined. Blood urea nitrogen (BUN), creatinine, total proteins, albumin (ALB), and inorganic phosphate (P) were determined in serum samples. UPC and UGGTC were significantly higher (p < 0.05) in BTV sheep compared to normal, whereas urine specific gravity (USG) was significantly lower (p < 0.05). Cylindruria was also detected in BTV sheep, and absence of azotemia in BTV and normal sheep. All these findings are indicative of renal tubular injury and/or dysfunction and suggestive of an association between BTV infection and acute damage of renal tissue.
Keywords:
bluetongue; BTV4; casts; creatinine; renal injury; renal tubules; sheep; UGGTC ratio; UPC ratio; USG 1. Introduction
Bluetongue (BT) is a vector-borne disease of domestic and wild ruminants caused by Bluetongue virus (BTV), a member of the Reoviridae family (genus Orbivirus), which is mainly transmitted by Culicoides biting midges [1], but there is also possibility that trans-placental transmission may occur [2,3]. There are at least 28 serotypes of the BTV [4,5].
The virus spreads worldwide and it is responsible for economic losses. In Greece, the first incidence of BTV was in 1979, caused by the serotype 4. Subsequently, serotypes 1, 4, 9, and 16 were isolated in the epidemic of 1998–2001 [6]. The next incidence was caused by the BTV serotype 1 in 2008–2009. An outbreak with a huge number of cases took place in 2014 caused by the serotype 4 [7,8]. The latest bluetongue incursion in Greece started at the end of July 2020 from Western Macedonia and by the end of November 2020, 339 outbreaks were detected across the whole country. In all cases, Bluetongue virus serotype 4 was identified as the causative agent [6].
BTV infection can be acute, chronic, or subclinical. The clinical signs vary among ruminants, with sheep being the most susceptible [9,10]. The main symptoms are fever, reduced appetite, hyperemia of the buccal, labial, and nasal mucosa and facial edema, while more rare symptoms are halitosis, cyanotic tongues, conjunctivitis, nasal discharge, dyspnea, lameness caused by coronitis, hemorrhagic diarrhea or vomiting, and abortions [7,9,11,12]. Moreover, the severity of clinical signs seems to depend on the serotype of the virus involved [2,13].
The pathogenesis of BTV includes injury to small blood vessels in target tissues caused by BTV replication, injury and necrosis of endothelial cells, leading to vascular thrombosis, tissue infarction, and haemorrhage [14]. The main target tissues of the virus are skin, vascular system, mucous membranes of upper respiratory and gastrointestinal tract, lymph nodes, lungs, and less frequently spleen, bone marrow, liver, and heart [13,15,16]. There are limited data referring to kidneys as a target organ of BTV infection in sheep. Post-mortem examination revealed petechiae haemorrhages in the kidneys of naturally and experimentally infected sheep with BTV serotype 1 and 8 [12,17] and subcapsular haemorrhages in Poll Dorset sheep infected with BTV serotype 1 and 3 [13].
The purpose of the study was to identify possible impact of BTV infection on kidneys using common widely available renal biomarkers.
2. Results
No abnormalities were observed in the urine physical examination, as well as in the dipstick method, except for the positive protein reaction in 22 out of 30 BTV confirmed ovine cases with compatible clinical signs (BTV sheep). In 7 out of 30 clinically healthy sheep (normal sheep), traces of protein were detected by dipstick. Urine pH was also measured by the dipstick method [18] and ranged from 7.5 to 8.5 in all animals.
Mean urine specific gravity (USG) was significantly lower in BTV sheep (mean ± SE: 1.028 ± 0.001 and 1.032 ± 0.001 for BTV and normal sheep, respectively; p < 0.05). On the contrary, urine biochemical analysis revealed that average urine protein to creatinine (UPC) ratio and urine gamma-glutamyl transferase (GGT) to creatinine (UGGTC) ratio were significantly higher (p < 0.05) in BTV sheep compared to normal ones (Figure 1).
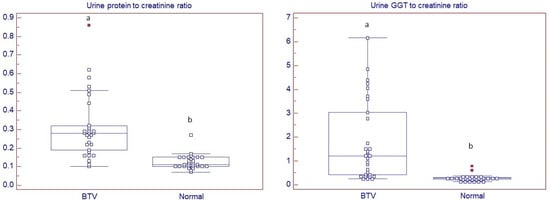
Figure 1.
Dot and box and whisker plots of urine protein to creatinine ratio and urine gamma-glutamyl transpeptidase (GGT) to creatinine ratio determined in sheep with (Bluetongue virus (BTV); n = 30) and without (normal; n = 30) clinical signs of bluetongue and without active sediment. a,b Different superscripts are indicative of statistically significant difference. (p < 0.05).
Microscopical examination of urine sediment revealed the presence of 0-3/LPF cellular casts in 7 out of 30 BTV sheep with clinical symptoms and absence of any other type of cast. There was suspicion of 0-2/LPF renal tubular epithelial cells rather than a confident distinction of transitional epithelial cells in 2 out of 30 BTV sheep. Finally, 0-5/LPF squamous epithelial cells were detected in 4 animals of each group. Only few magnesium ammonium phosphate crystals were sporadically found in animals of both groups.
As it is shown in Table 1, mean serum total proteins were significantly higher in BTV sheep than normal ones (p < 0.05). Moreover, average serum albumins tended to be significantly lower and mean serum globulins were significantly higher in BTV sheep compared to normal (p < 0.05; Table 1). Average BUN tended to be significantly higher in BTV sheep than normal ones, but within reference interval in 29 out of 30 BTV cases. Serum creatinine was not significantly different among BTV and normal sheep (p > 0.05), but the average creatinine concentration in BTV sheep was higher than the reference interval (Table 1). Mean serum inorganic phosphate was within reference interval in all animals and significantly lower in BTV sheep compared to normal (p < 0.05; Table 1).

Table 1.
Mean ± SE values and laboratory reference range of selected serum biochemical variables determined in sheep with (BTV; n = 30) and without (normal; n = 30) clinical signs of bluetongue.
3. Discussion
In the present study, the main findings of urinalysis included reduced USG, proteinuria, enzymuria, and cylindruria. Since samples with active sediment were excluded from the analysis, all these findings are indicative of acute kidney injury (AKI). USG is measured for the assessment of tubular function, reflecting the ability of the renal tubules to dilute or concentrate the glomerular filtrate. The reference interval of USG is wide among healthy adequately hydrated animals. In contrast to normal sheep, USG was lower in BTV sheep despite the presence of proteinuria that increases USG value [19]. Therefore, the concentrating ability of these animals might have been overestimated. The ability of the kidney to concentrate was progressively decreased in ewes after aminoglycoside induced toxicosis and it was considered as a consequence of acute renal injury [20].
Proteinuria was assessed by the reagent strip method and the UPC ratio. Dipstick has been extensively used for the assessment of proteinuria as it is claimed to be more convenient in clinical practice. However, the alkaline pH of urine, which is the case of ovine urine, is reported to cause false positive results in urine protein detected by the dipstick method. Moreover, UPC reflects a 24-h urine protein excretion and accounts for variances in USG and urine volume. Creatinine is excreted in urine at an approximately constant rate, which means it can be used as a standard for comparison for other substances that are also excreted in the urine. Thus, urine creatinine levels are used to normalize urine protein levels for fluctuations in urine concentration driven by the patient’s level of hydration. UPC is a quantitative method and it is used for the estimation of proteinuria by IRIS [21].
The origin of proteinuria can be prerenal, renal, or postrenal. Proteinuria can be caused due to structural or functional damage of the glomerulus, renal tubular cells, or the interstitial space of the kidneys [22]. Renal tubular proteinuria is induced by lesions that impair tubular reabsorption of low molecular weight plasma proteins that are normally filtered by glomerular capillary permselective walls [22]. In the present study, UPC ratio was significantly higher in sheep with BTV. Prerenal proteinuria is unlikely to occur, since hemoglobinuria and myoglobinuria were neither observed nor detected. Postrenal proteinuria is also unlikely as sheep with active urine sediment were excluded from the study. Hence, proteinuria in the present study seems to be of renal origin.
Febrile conditions are known to cause the so-called functional proteinuria, which is usually transient and resolves when the causative agent remits [23]. The underlying mechanism was initially thought to be stress induced due to adrenaline release [24]. However, this was not evidenced in a recent study in dogs [25]. In humans, the magnitude of fever was associated with tubular proteinuria that was caused by fever per se. It has been attributed to a transient impairment of renal tubules to reabsorb low molecular weight proteins [26]. The pathophysiology of febrile proteinuria is not entirely elucidated, while new biomarkers are under evaluation to identify the origin of proteinuria and the possible distinction between bacterial and viral etiology [27]. In the present study, we cannot exclude that proteinuria is functional due to the febrile state. Actually, the main evidence of acute renal injury in BTV sheep was cylindruria, enzymuria indicators of renal tubular damage, while USG and proteinuria are indicators of renal tubular dysfunction, i.e., impairment of concentrating ability and tubular reabsorption respectively.
Urine enzymes have been used as biomarkers for the early detection of acute renal tubular injury in humans and animals [28]. Their usefulness is attributed to the ability to differentiate ongoing from previous renal damage. GGT is a transferase enzyme present in the cell membranes of various tissues [29]. In kidneys, GGT releases in the renal tubular lumen after injury of the microvilli of the luminal surface of renal tubular epithelial cells [30].
Urine GGT has been proposed as a sensitive indicator of acute renal tubular damage in drug induced nephrotoxicosis in dogs [31,32]. Urine GGT to creatinine ratio has been significantly correlated to the 24h urinary GGT excretion in dogs with gentamicin induced nephrotoxicosis [33]. Increased urine GGT activity has been reported in sheep with induced mercuric chloride nephrotoxicity [34], as well as in sheep after sodium fluoride poisoning associated with kidney degeneration and necrosis predominantly in proximal tubules [35]. Relatedly in experimentally intoxicated sheep after gentamicin administration, urine GGT was proven a suitable early indicator of renal tubular damage [36]. The results of the same study demonstrated a significant correlation between urine GGT and its ratio to urine creatine (UGGTC), which may stabilize the variation in values result from changes in urine concentration [36]. Increased UGGTC ratio and UPC ratio were observed in sheep with systemic AA amyloidosis indicated reliably renal injury, while serum creatinine showed no alterations [37]. Amyloidosis in sheep has been highly associated with proximal convoluted tubulonephrosis along with the glomerulus lesions [38]. In the present study, UGGTC was significantly increased in BTV confirmed sheep compared to normal ones, indicating that BTV infection is possibly associated with renal tubular damage.
The presence of abnormal cellular constitutes or casts in urine sediment is indicative of renal tubular cell damage [39]. In a study that included hospitalized patients with suspicion of AKI, a scoring system based on casts and renal tubular epithelial cell was evaluated for differentiating acute tubular damage from prerenal AKI [40]. Urine sediment evaluation of the BTV sheep revealed the presence of few cellular casts and a low number of renal tubular epithelial cells. The identification of renal tubular epithelial cells is however doubtful, as their distinction from the transitional epithelial cells is challenging [41]. The presence of a low number of squamous epithelial cells is not considered significant as they derive from the distal third of the urethra, the vagina, and the prepuce and may normally exfoliate during micturition [41]. Likewise, although the presence of magnesium ammonium phosphate crystals in the urine has been associated with urinary tract infection by urease producing bacteria, the absence of bacteriuria, pyuria, and hematuria in all sheep included in the present study renders this possibility highly unlikely. Moreover, the pH of the urine of sheep was alkaline and this type of crystals is common in the alkaline pH [41].
Studies in sheep after experimentally induced nephrotoxicosis showed diffuse necrosis of tubular epithelial cells and distinct tubular protein accumulation accompanied with abnormal urinalysis findings, mainly proteinuria and glycosuria, as well as renal tubular epithelial cells, and cellular and granular casts [20]. These findings are suggestive of acute kidney injury and are in accordance with our results reflecting BTV induced renal tubular cell damage in ovine urine.
In previous studies, multifocal tubular necrosis was present in kidney biopsies of BTV serotype 17 infected sheep in Brazil [42], proximal tubular necrosis and haemorrhage was found in the kidneys of 70% experimentally infected white-tailed deer [43]. Similarly, glomerular congestion, erythrocytes and clumped protein in Bowman’s space and tubules, tubulonephrosis, interstitial mononuclear nephritis, and interstitial haemorrhages were evident in naturally infected goats with BTV serotype 1 in Spain [44]. The virus replicates into endothelial cell cytoplasm, triggering the expression of vasoactive and inflammatory mediators result in increased permeability, cell necrosis, and subsequently edema, congestion, vascular thrombosis, and haemorrhage [14].
The lower concentration of serum albumin detected in BTV sheep is mainly attributed to a mild acute phase reaction rather than to albumin loss. It is well documented that albuminuria is mainly associated with glomerular damage, while lower molecular weight proteins are detected in the urine as a result of renal tubular injury [21]. Serum total proteins were also higher in BTV sheep as a consequence of higher serum globulins, accounting for the humoral immune response to viral infection.
The glomerular filtration rate was investigated employing the traditional biomarkers BUN, creatinine, and phosphate. Both urea and phosphate are not specific indicators of renal dysfunction, as they strongly depend on food intake and are excreted in gastrointestinal tract and saliva [19]. Therefore, their values might have been affected in BTV animals by the loss of appetite. Moreover, both BUN and inorganic phosphate concentrations were within reference interval, although average BUN was higher, and serum inorganic phosphate was lower in BTV sheep compared to normal.
Creatinine is considered as a more specific biomarker of renal dysfunction compared to BUN and phosphate in ruminants. Serum creatinine was significantly higher in ewes with experimentally reduced renal mass compared to intact ones [45], as well as in gentamicin [20] or sodium fluoride intoxicated sheep [35]. However, serum creatinine concentration is not an early biomarker of reduced renal function in sheep as in other species, since creatinine concentration increases after extensive loss of nephron function [19,46]. On the contrary, renal proteinuria is an earlier biomarker of renal dysfunction before the onset of azotemia [21]. This could be the reason why, although renal proteinuria was detected, the average serum creatinine concentration was not significantly different between BTV and normal sheep. Nevertheless, the fact that the average creatinine concentration was higher than the reference range in BTV group further supports the urinalysis evidence that Bluetongue is associated with acute kidney injury.
From a clinical point of view in the light of the finding of this study, despite the absence of evidence of impaired glomerular filtration, a recommendation to avoid nephrotoxic agents seems to be reasonable.
4. Materials and Methods
4.1. Sample Size
The minimum required sample size was determined using the statistical program G*Power 3.0. The analysis was run using Means: Difference between two dependent means (matched pair) of t-tests family with desired Power 0.8 and type I error 0.05. The effect size f was calculated with the desired detectable difference for UPC values 0.1 and standard deviation 0.1. The analysis revealed that, at least 10 pairs of animals are required (Power 0.803).
4.2. Inclusion Criteria
Blood and urine samples of 47 ovine (indigenous Greek breeds) BTV clinical cases, that had been confirmed by the State Reference Laboratory by real-time reverse transcriptase/polymerase chain reaction (RRT-PCR) [6] and reported to the World Animal Health Information System of OIE were collected for further investigation of health status and supportive treatment administration. The clinical signs compatible with BTV infection included fever, facial edema, decreased appetite, halitosis, lameness, and nasal discharge [7]. None of these animals had received any medication for at least one month before sampling. A proportional number of matched samples were collected from 47 clinically healthy sheep of the same age in each flock as well. Urine sediment was evaluated in all 47 pairs of samples. In any case of active urine sediment, we excluded both the individual animal with the active sediment and the other animal of the pair. In this way, 17 pairs of samples were excluded, and finally 30 were included in the study.
4.3. Urine and Blood Sampling
Urine samples were obtained directly into sterile containers during natural voiding which was induced by holding the nostrils closed for some seconds, and by rubbing the area of the vulva or the prepuce. Urine was centrifuged at 250× g for 5 min and one vial of the supernatant was used immediately, while another was stored at −20 °C for further proteinuria and enzymuria detection. Urine sediment was immediately microscopically examined.
Blood was collected by jugular venipuncture using plein vacuum tubes (Venoject®, Terumo Europe, Leuven, Belgium) without anticoagulant. After blood clotting, the tube was centrifuged at 300× g for 10 min and the supernatant serum was transferred into plastic vials (Eppendorf Tubes®, Eppendorf AG, Hamburg, Germany). The serum vials were stored at −20 °C and were analyzed within 48 h.
4.4. Urinalysis
USG was measured immediately after centrifugation in the supernatant using a temperature compensated refractometer (Reichert TS Meter refractometer, Model 1310400A, Reichert Scientific Instruments), while dipstick method was used for the measurement of pH, hemoglobin, ketones, bilirubin, protein, and glucose concentrations, using the Multistix 10 SG (Siemens Healthcare GmbH, Erlangen, Germany) urine reagent strips.
A small volume of urine was used to resuspend the urine sediment. A drop of the resuspended sediment was microscopically examined as previously described [41]. Briefly, the number of erythrocytes and leukocytes per 40× high power field (HPF) in 10 HPFs was recorded as a range per HPF. Epithelial cells and casts were recorded by each type per 10× low power field (LPF) in 10 LPFs as a range per LPF. The presence and types of microorganisms and crystals were determined using a semiquantitative scale (none, few, moderate, many).
The samples were evaluated both on an unstained wet sediment and on air dried Giemsa stained sediment preparation to exclude bacteriuria [47] and to distinguish renal tubular epithelial cells from transitional epithelial cells [41]. Sediments were classified as active based on the presence of the following findings: >5 RBCs, > 8 WBCs per HPF, bacteriuria, or epithelial cells per LPF. However, increased number of transitional epithelial cells were interpreted as indicative of inflammation taking into consideration the concurrent increased number of WBCs [41]. Urinalysis was completed within 1 h after collection.
4.5. Biochemical Analysis
Urine protein and urine creatinine concentrations were determined with the pyrogallol red and Jaffé methods, respectively. Urine GGT activity was assessed using the Szasz’s modified method.
Urine protein to creatinine ratio (UPC) and urine GGT to creatine ratio (UGGTC) were calculated dividing the values of the urine protein concentration and the urine GGT activity by urine creatinine concentration.
Serum total protein concentration was immediately measured using a temperature compensated refractometer (Reichert TS Meter refractometer, Model 1310400A, Reichert Scientific Instruments Buffalo, NY, USA) [48]. Albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), and phosphate (P) concentrations were determined in serum. All biochemical analysis in urine and serum, except for total protein concentration, were determined using an automated biochemical analyzer (Biosystems Analyzer A25).
4.6. Data Analysis
The obtained data was analyzed with MedCalc® 9.2 software (Mariakerke, Belgium). The normality of the data was tested with Kolmogorov–Smirnov test; paired samples t-test was run to evaluate the significance of differences between normal sheep and those with clinical signs of bluetongue for each one for the urine and serum parameters evaluated. A significance level of p ≤ 0.05 was used in all comparisons.
5. Conclusions
These findings indicate the impact of BTV-4 on renal tubular structure (enzymuria, cylindruria) and function (decreased USG, proteinuria) without evidence of alteration in glomerular filtration rate (absence of severe azotemia and hyperphosphatemia). There are no histopathological studies on ovine kidneys infected by the BTV-4 serotype and further investigation such as experimental infection using the same strain of virus is required to verify urinalysis results with any anatomical derangements in the kidneys [42,43,44]. Based on this evidence, veterinary practitioners should be cautious to avoid the use of potential nephrotoxic medication at the supportive treatment protocol of bluetongue.
Author Contributions
Conceptualization, L.V.A. and P.D.K.; methodology, A.D.; software, V.M.S.; validation, A.D., L.V.A.; formal analysis, E.G.K.; investigation, E.G.K.; resources, L.V.A.; data curation, P.D.K.; writing—original draft preparation, E.G.K. and V.M.S.; writing—review and editing, L.V.A., E.G.K., V.M.S., A.D. and P.D.K.; visualization, E.G.K. and V.M.S.; supervision, L.V.A. and P.D.K.; project administration, P.D.K.; funding acquisition, L.V.A. and P.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because sampling was performed only once for the evaluation of animal health and the optimization of supportive treatment in farms with confirmed and reported cases of BTV. Moreover, all procedures were done according to the ethical standards in the Helsinki Declaration of 1975, as revised in 2000, as well as the national law inducing minimal stress in animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to further processing for other studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dijkstra, E.; van der Ven, I.J.; Meiswinkel, R.; Holzel, D.R.; Van Rijn, P.A.; Meiswinkel, R. Culicoides chiopterus as a potential vector of bluetongue virus in Europe. Vet. Rec. 2008, 162, 422. [Google Scholar] [CrossRef]
- van der Sluijs, M.T.; Schroer-Joosten, D.P.; Fid-Fourkour, A.; Vrijenhoek, M.P.; Debyser, I.; Moulin, V.; Moormann, R.J.; de Smit, A.J. Transplacental transmission of Bluetongue virus serotype 1 and serotype 8 in sheep: Virological and pathological findings. PLoS ONE 2013, 8, e81429. [Google Scholar] [CrossRef]
- Spedicato, M.; Carmine, I.; Teodori, L.; Leone, A.; Casaccia, C.; Di Gennaro, A.; Di Francesco, G.; Marruchella, G.; Portanti, O.; Marini, V.; et al. Transplacental transmission of the Italian Bluetongue virus serotype 2 in sheep. Vet. Ital. 2019, 55, 131–141. [Google Scholar] [CrossRef]
- Belbis, G.; Zientara, S.; Breard, E.; Sailleau, C.; Caignard, G.; Vitour, D.; Attoui, H. Bluetongue Virus: From BTV-1 to BTV-27. Adv. Virus Res. 2017, 99, 161–197. [Google Scholar] [CrossRef]
- Bumbarov, V.; Golender, N.; Jenckel, M.; Wernike, K.; Beer, M.; Khinich, E.; Zalesky, O.; Erster, O. Characterization of bluetongue virus serotype 28. Transbound. Emerg. Dis. 2020, 67, 171–182. [Google Scholar] [CrossRef]
- OIE. Event Summary: Bluetongue, Greece. 2020. Available online: https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=35352 (accessed on 26 December 2020).
- Katsoulos, P.D.; Giadinis, N.D.; Chaintoutis, S.C.; Dovas, C.I.; Kiossis, E.; Tsousis, G.; Psychas, V.; Vlemmas, I.; Papadopoulos, T.; Papadopoulos, O.; et al. Epidemiological characteristics and clinicopathological features of bluetongue in sheep and cattle, during the 2014 BTV serotype 4 incursion in Greece. Trop. Anim. Health Prod. 2016, 48, 469–477. [Google Scholar] [CrossRef]
- Kyriakis, C.S.; Billinis, C.; Papadopoulos, E.; Vasileiou, N.G.; Athanasiou, L.V.; Fthenakis, G.C. Bluetongue in small ruminants: An opinionated review, with a brief appraisal of the 2014 outbreak of the disease in Greece and the south-east Europe. Vet. Microbiol. 2015, 181, 66–74. [Google Scholar] [CrossRef]
- Elbers, A.R.; Backx, A.; Meroc, E.; Gerbier, G.; Staubach, C.; Hendrickx, G.; van der Spek, A.; Mintiens, K. Field observations during the bluetongue serotype 8 epidemic in 2006. I. Detection of first outbreaks and clinical signs in sheep and cattle in Belgium, France and the Netherlands. Prev. Vet. Med. 2008, 87, 21–30. [Google Scholar] [CrossRef]
- Backx, A.; Heutink, C.G.; van Rooij, E.M.; van Rijn, P.A. Clinical signs of bluetongue virus serotype 8 infection in sheep and goats. Vet. Rec. 2007, 161, 591–592. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Fthenakis, G.C.; Amiridis, G.S.; Athanasiou, L.V.; Birtsas, P.; Chatzopoulos, D.C.; Chouzouris, T.M.; Giannakopoulos, A.; Ioannidi, K.S.; Kalonaki, S.N.; et al. Experiences from the 2014 outbreak of bluetongue in Greece. Small Rumin. Res. 2016, 142, 61–68. [Google Scholar] [CrossRef]
- Darpel, K.E.; Batten, C.A.; Veronesi, E.; Shaw, A.E.; Anthony, S.; Bachanek-Bankowska, K.; Kgosana, L.; bin-Tarif, A.; Carpenter, S.; Muller-Doblies, U.U.; et al. Clinical signs and pathology shown by British sheep and cattle infected with bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Vet. Rec. 2007, 161, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, C.; Salt, J.S.; Graham, S.D.; Hopwood, K.; Wade-Evans, A.M. Bluetongue virus serotypes 1 and 3 infection in Poll Dorset sheep. Aust. Vet. J. 1998, 76, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.J.; Jagels, G.; Rossitto, P.V.; Moore, P.F.; Heidner, H.W. The pathogenesis of experimental bluetongue virus infection of calves. Vet. Pathol. 1990, 27, 223–229. [Google Scholar] [CrossRef]
- Khorajiya, J.H.; Singh, K.P.; Bhatt, P.; Saminathan, M.; Tiwari, S.; Bhat, S.A.; Vineetha, S.; Maity, M.; Panda, S.; Gupta, V.K. Haematological and biochemical alterations in native sheep experimentally infected with bluetongue virus serotype-2. Indian J. Anim. Sci. 2019, 89, 3–8. [Google Scholar]
- Sanchez-Cordon, P.J.; Pleguezuelos, F.J.; Perez de Diego, A.C.; Gomez-Villamandos, J.C.; Sanchez-Vizcaino, J.M.; Ceron, J.J.; Tecles, F.; Garfia, B.; Pedrera, M. Comparative study of clinical courses, gross lesions, acute phase response and coagulation disorders in sheep inoculated with bluetongue virus serotype 1 and 8. Vet. Microbiol. 2013, 166, 184–194. [Google Scholar] [CrossRef]
- Athanasiou, L.V.; Katsoulos, P.D.; Katsogiannou, E.G.; Polizopoulou, Z.S.; Diamantaki, M.; Kamatsos, C.; Christodoulopoulos, G. Comparison between the urine dipstick and the pH-meter to assess urine pH in sheep and dogs. Vet. Clin. Pathol. 2018, 47, 284–288. [Google Scholar] [CrossRef]
- Stockham, S.L.; Scott, M.A. Urinary System. In Fundamentals of Veterinary Clinical Pathology, 2nd ed.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 415–494. [Google Scholar]
- Garry, F.; Chew, D.J.; Hoffsis, G.F. Urinary indices of renal function in sheep with induced aminoglycoside nephrotoxicosis. Am. J. Vet. Res. 1990, 51, 420–427. [Google Scholar]
- Grauer, G.F. Measurement and Interpretation of Proteinuria and Albuminuria (Revised 2016). Available online: http://www.iris-kidney.com/education/proteinuria.html (accessed on 26 December 2020).
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. (Eds.) Diseases of the Urinary System. In Veterinary Medicine, 11th ed.; W. B. Saunders: St. Louis, MO, USA, 2017; pp. 1095–1154. [Google Scholar] [CrossRef]
- Harley, L.; Langston, C. Proteinuria in dogs and cats. Can. Vet. J. La Revue Vet. Can. 2012, 53, 631–638. [Google Scholar]
- Marks, M.I.; McLaine, P.N.; Drummond, K.N. Proteinuria in children with febrile illnesses. Arch. Dis. Child 1970, 45, 250–253. [Google Scholar] [CrossRef]
- Citron, L.E.; Weinstein, N.M.; Littman, M.P.; Foster, J.D. Urine cortisol-creatinine and protein-creatinine ratios in urine samples from healthy dogs collected at home and in hospital. J. Vet. Intern. Med. 2020, 34, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.; Skaarup, P. Urinary excretion of ten plasma proteins in patients with febrile diseases. Acta Med. Scand. 1977, 201, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.; Israel, E.; Segev, Y.; Landau, D. Febrile Proteinuria in Hospitalized Children: Characterization of Urinary Proteins. Front. Pediatr. 2018, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, D. Renal markers for assessment of renal tubular and glomerular dysfunction. J. Nephropharmacol. 2013, 2, 23–25. [Google Scholar] [PubMed]
- Goldberg, D.M. The expanding role of microsomal enzyme induction, and its implications for clinical chemistry. Clin. Chem. 1980, 26, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ilchyshyn, N.P.; Villiers, E.; Monti, P. Validation of a spectrophotometric method for GGT measurement in canine urine and determination of the urine GGT-to-creatinine ratio reference interval and biological variation in 41 healthy dogs. J. Vet. Diagn. Investig. 2019, 31, 33–39. [Google Scholar] [CrossRef]
- Clemo, F.A. Urinary enzyme evaluation of nephrotoxicity in the dog. Toxicol. Pathol. 1998, 26, 29–32. [Google Scholar] [CrossRef]
- Greco, D.S.; Turnwald, G.H.; Adams, R.; Gossett, K.A.; Kearney, M.; Casey, H. Urinary gamma-glutamyl transpeptidase activity in dogs with gentamicin-induced nephrotoxicity. Am. J. Vet. Res. 1985, 46, 2332–2335. [Google Scholar]
- Grauer, G.F.; Greco, D.S.; Behrend, E.N.; Mani, I.; Fettman, M.J.; Allen, T.A. Estimation of quantitative enzymuria in dogs with gentamicin-induced nephrotoxicosis using urine enzyme/creatinine ratios from spot urine samples. J. Vet. Intern. Med. 1995, 9, 324–327. [Google Scholar] [CrossRef]
- Robinson, M.; Trafford, J. A study of early urinary enzyme changes in mercuric chloride nephropathy in sheep. J. Comp. Pathol. 1977, 87, 275–280. [Google Scholar] [CrossRef]
- Kessabi, M.; Hamliri, A.; Braun, J.P.; Rico, A.G. Experimental acute sodium fluoride poisoning in sheep: Renal, hepatic, and metabolic effects. Fundam. Appl. Toxicol. 1985, 5, 1025–1033. [Google Scholar] [CrossRef]
- Garry, F.; Chew, D.J.; Hoffsis, G.F. Enzymuria as an index of renal damage in sheep with induced aminoglycoside nephrotoxicosis. Am. J. Vet. Res. 1990, 51, 428–432. [Google Scholar] [PubMed]
- Fernandez, A.; Mensua, C.; Biescas, E.; Lujan, L. Clinicopathological features in ovine AA amyloidosis. Res. Vet. Sci. 2003, 75, 203–208. [Google Scholar] [CrossRef]
- Mensua, C.; Carrasco, L.; Bautista, M.J.; Biescas, E.; Fernandez, A.; Murphy, C.L.; Weiss, D.T.; Solomon, A.; Lujan, L. Pathology of AA amyloidosis in domestic sheep and goats. Vet. Pathol. 2003, 40, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Schentag, J.J.; Gengo, F.M.; Plaut, M.E.; Danner, D.; Mangione, A.; Jusko, W.J. Urinary casts as an indicator of renal tubular damage in patients receiving aminoglycosides. Antimicrob. Agents Chemother. 1979, 16, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A.; Coca, S.G.; Kanbay, M.; Brewster, U.C.; Parikh, C.R. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 1615–1619. [Google Scholar] [CrossRef]
- Wamsley, H.L. Examination of the Urine Sediment. In Cowell and Tyler’s Diagnostic Cytology and Haematology of the Dog and Cat, 5th ed.; Valenciano, A.C., Cowell, R.L., Eds.; Elsevier Inc.: St. Louis, MO, USA, 2020; pp. 379–405. [Google Scholar]
- Bianchi, R.M.; Panziera, W.; Faccin, T.C.; de Almeida, G.L.; Cargnelutti, J.F.; Flores, E.F.; Kommers, G.D.; Fighera, R.A. Clinical, pathological and epidemiological aspects of outbreaks of bluetongue disease in sheep in the central region of Rio Grande do Sul. Pesq. Vet. Bras. 2017, 37, 1443–1452. [Google Scholar] [CrossRef]
- Karstad, L.; Trainer, D.O. Histopathology of experimental bluetongue disease of white-tailed deer. Can. Vet. J. 1967, 8, 247–254. [Google Scholar]
- Sanchez-Cordon, P.J.; Pedrera, M.; Risalde, M.A.; Molina, V.; Rodriguez-Sanchez, B.; Nunez, A.; Sanchez-Vizcaino, J.M.; Gomez-Villamandos, J.C. Potential role of proinflammatory cytokines in the pathogenetic mechanisms of vascular lesions in goats naturally infected with bluetongue virus serotype 1. Transbound. Emerg. Dis. 2013, 60, 252–262. [Google Scholar] [CrossRef]
- Gibson, K.J.; Thomson, C.L.; Boyce, A.C.; Karime, B.M.; Lumbers, E.R. Effects of a reduction in maternal renal mass on pregnancy and cardiovascular and renal function of the pregnant ewe. Am. J. Physiol. Renal. Physiol. 2006, 290, F1153–F1162. [Google Scholar] [CrossRef]
- Lefebvre, H.P. Renal function testing. In Nephrology and Urology of Small Animals; Bartges, J., Polzin, D., Eds.; Wiley-Blackwell: Ames, IA, USA, 2011; pp. 91–96. [Google Scholar]
- Swenson, C.L.; Boisvert, A.M.; Gibbons-Burgener, S.N.; Kruger, J.M. Evaluation of modified Wright-staining of dried urinary sediment as a method for accurate detection of bacteriuria in cats. Vet. Clin. Pathol. 2011, 40, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Katsoulos, P.D.; Athanasiou, L.V.; Karatzia, M.A.; Giadinis, N.; Karatzias, H.; Boscos, C.; Polizopoulou, Z.S. Comparison of biuret and refractometry methods for the serum total proteins measurement in ruminants. Vet. Clin. Pathol. 2017, 46, 620–624. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/Licenses/by/4.0/).