Abstract
This paper examined the effect of marble powder (MP) on air-cured cement-based materials when subjected to sulfuric acid (H2SO4) attack. Four MP replacement levels were tested: 0%, 5%, 10%, and 15% by weight of cement. The prepared samples were cured for 90 days prior to being exposed to H2SO4. Macroscopic tests for apparent density and compressive strength along with microstructural characterization using X-ray diffraction (XRD) and scanning electron microscopy (SEM) were performed to determine the effect of MP on the properties of the materials. The Rietveld method was used to analyze the amounts of different crystalline phases and amorphous calcium silicate hydrate (C-S-H). The obtained results indicate that 5% MP in air-cured cement -based materials exhibited the best behavior with acceptable resistance to acid attacks. This level of MP replacement was found to optimize the filler effect, improve the hydration process, and enhance the matrix density, which in turn reduces the permeability of the material and increases acid resistance. This is attributed to the balanced contribution of MP to phase formation, particularly calcite, which helps to counteract acid-induced dissolution, while also preserving the stability of C-S-H phases. This study provides a new perspective of the role of MP in influencing phase content (crystalline and amorphous phases) and their possible impacts on macroscopic properties such as apparent density and compressive strength. MP behaved as a filler, to improve hydration and resistance to acid attacks. Additionally, using MP as a replacement for ordinary Portland cement (OPC) offers a sustainable alternative by reducing waste and promoting the recycling of marble industry by-products, thereby contributing to environmental sustainability. It is recommended that, 5% MP is the optimal replacement content to enhance durability and mechanical properties in air-cured cement-based materials in aggressive environments, as it is both practical and achievable for infrastructure to be subjected to the aggressive environment.
1. Introduction
Cement-based materials are regarded as integral materials of the construction industry around the world, as they possess strength and can withstand considerable loading [1]. Nevertheless, the sector is increasingly challenged by long-term durability issues associated with cement materials that can be degraded by various environmental and chemical stresses. A key determinant of concrete’s longevity lies in its resistance to aggressive conditions, including exposure to carbon dioxide, sulfates, chlorides, and acids [2,3,4,5]. These detrimental agents compromise structural integrity, reduce service life, and significantly raise maintenance costs [6].
Among the most critical environmental threats to construction materials are acid attacks, commonly arising from acid rain in industrial regions, chemical discharges, and marine environments. Sulfuric acid (H2SO4) is highly aggressive towards cement-based material infrastructure. It is found in groundwater, industrial discharges, and sewage [7]. H2SO4 reacts with both hydrated and unhydrated cement, creating a significant risk to the performance and durability of concrete. Sulfuric acid leads to leaching of portlandite (CH), destabilization of calcium-silicate-hydrate (C-S-H) gel, and the formation of expansive products such as ettringite and gypsum, leading to softening, cracking, and mass loss in cement-based materials, threatening the structure of essential infrastructure such as pavements, bridges, and sewage pipelines [8,9,10]. Cement is especially susceptible to sulfuric acid due to a series of degradation reactions triggered by the dissolution of calcium phases. The first step includes the quick reaction between sulfuric acid and calcium hydroxide (CH), resulting in the formation of calcium sulfate (gypsum): CH + H2SO4 → CaSO4·2H2O.
This process consumes CH, which is usually responsible for buffering acidity, promoting acid penetration. In addition, gypsum reacts with the aluminate phases in hydrated cement to form ettringite (AFt), an expansive mineral known to generate internal tensions, cracking and disrupting the cement matrix. As acid infiltration progresses, the decalcification of C-S-H takes place, which decreases its structural integrity and eventually converts it to a silica-rich gel with a low binding capacity. Combined, these events also lead to increased porosity, decreased cohesion of the matrix, and significant loss of mechanical strength, which partially explains why sulfuric acid is regarded as one of the most destructive chemical agents for cementitious materials.
As noted, the degradation is related to the concentration of acid and the contact duration; the longer the contact, the more pore structures are enlarged, leading to increased porosity that decreases the mechanical performance of the cement-based materials [11,12,13]. Further, the production of cement-based materials can contribute to environmental degradation. Cement production in 2018 produced an estimated 1.50 ± 0.12 gigatons of CO2 emissions [14].
In relation to the degradation mechanisms stated above, it will also be necessary to consider the use of additives that may decrease acid susceptibility through microstructural changes and decreased concentrations of soluble hydration products that are part of the paste such as calcium hydroxide. Marble powder (MP) has the potential to improve the densification of the matrix due to high levels of calcite and may decrease the concentrations of CH due to filler and nucleation effects, thereby partially mitigating the detrimental impact of sulfuric acid attack.
Given these concerns associated with durability and sustainability, several researchers have concentrated on developing alternative building materials that are eco-friendly and also derived from industrial and/or by-product reuse. Industrial waste has been a major environmental liability; however, it also has great potential as a sustainable construction resource. Over recent decades, a considerable body of research has examined the incorporation of waste materials such as fly ash, blast furnace slag, silica fume, and rice husk ash as partial replacements for Portland cement [15,16,17]. Not only does this remedy waste pile up, but it also helps to significantly decrease CO2 emissions that are caused by cement production [18].
Among the industrial sectors being evaluated for their environmental impact, the marble sector is notable for producing tons of both solid and liquid waste, which are often released without treatment. This challenge has led to the identification of alternative materials and, more specifically, marble waste in the form of finely ground powder. Marble powder (MP) is a good candidate as an additive or partial substitution for cement due to its calcite content. Marble powder, as well as other limestone-based fillers, has been evaluated for its ability to improve the physical and chemical stability of cement-based materials, reduce cracking, and mitigate mass loss by improving porosity and reaction mechanism [19]. Importantly, using MP in cement formulations resulted in reduced CO2 emissions by as much as 12% and in reduced concrete costs from $40 to $33 per cubic meter. These substitutions not only improve the environmental sustainability of cement-based materials but are also cost-effective [20].
Several studies have examined the characteristics of cement-based systems with incorporated MP or other carbonate-rich fillers as a replacement for ordinary Portland cement (OPC) [21,22,23]. Previous studies have indicated beneficial contributions of MP, which can include enhanced packing of particles, increased early-age hydration through nucleation effects, lower permeability and improved density at the microstructure scale. Several studies on limestone fillers [1,9,24], which share similar chemical constituents as MP, have identified improvements in strength evolution, sulfate resistance, and lower acid ingress due to the formation of carboaluminate phases and refined pore structures. Nevertheless, while these studies provide a deeper understanding of the MP effects under standard curing conditions, the literature remains void of MP performance under continuous acid exposure when cured in air, which warrants attention regarding the utility of MP to improve chemical durability and longer-term stability in an acidic environment.
In addition to benefits in terms of performance, the use of MP provides significant environmental benefits. As a result of quarrying and processing activities, worldwide volumes of marble waste are produced. This waste is typically disposed of in landfills or in the open environment, resulting in dust pollution, contamination of soil, and disturbance of ecosystems. By incorporating MP into cement-based materials, we can divert this waste from disposal streams, serving a sustainable purpose and minimizing the environmental burden of anti-waste consequence. Additionally, displacing some portion of Portland cement with MP helps lower the emissions of CO2 because MP does not require the input of more deeply energy-intensive clinker production. This aligns with global sustainability goals to lower the carbon footprint of industrial activities and promote circularity in construction activities.
Besides its physical filler capacity, MP is also involved in chemical interactions that can affect durability in acidic environments. The calcite (CaCO3) in MP can react with the calcium hydroxide (CH) released during cement hydration, and potentially form more calcium carbonate through carbonation-like reactions. This decreases the amount of CH, which is one of the most susceptible phases to sulfuric acid dissolution, and thus reduces the leaching rate in acidic media. The presence of calcite can also influence the early hydration of aluminate phases, through the formation of carboaluminate compounds, which develop a denser and more stable microstructure. Collectively, these chemical reactions can improve resistance to acid ingress by increasing cohesion within the matrix and reducing capillary porosity, ultimately slowing degradation rates of cement structures in the presence of sulfuric acid.
Furthermore, a previous study reported that the inclusion of limestone or marble powder at replacement levels between 5% and 10% can significantly enhance resistance to acid attack. This improvement is attributed to filler and nucleation effects that contribute to a denser microstructure and lower capillary porosity [25]. For instance, Liu et al. [24] stated that limestone powder decreased acid ingress, as it contributed to forming a gypsum layer, which created a protective layer and provided a physical barrier.
This is consistent with the findings of Atabey et al. [26], who reported that mortar samples with a partial cement substitution of no greater than 10% MP showed noticeably less damage from hydrochloric acid and sodium and magnesium sulfate exposures when compared to a control mixture. But the performance improvements diminished when the substitution amount exceeded 15%, perhaps due to the dilution of reactive cementitious constituents. In fact, a partial cement substitution of no greater than 15% with MP improved both mechanical properties and chemical resistance in mortars that included andesite waste and by-products from the marble industry. The findings suggested the utility and effectiveness of such a modification for improving the overall performance of cement-based materials.
Durability studies for mortar that included marble sand indicated that the mortar degraded faster than the control mortar in acidic environments over testing periods of 14 days and 21 days [27]. In another study, which used self-compacting concrete, researchers found that MP had better performance in terms of resistance to chemical agents (sulfuric acid and sodium sulfate) compared to conventional cement-based mixtures [28].
The improved performance is reportedly linked to the chemical reactivity of calcite, which can also facilitate the transformation of CH to calcium carbonate (CaCO3), thus reducing the amount of calcium hydroxide available for acid-induced degradation reactions [9]. To be clear, the transformation is to benefit cementitious matrices by improved performance against acidic attack and other durability properties (e.g., compressive strength and mass loss). X-Ray Diffraction (XRD) analysis by Messaoudene et al. [29] has shown that MP decreases the amount of CH, which may improve the swelling of cement-based materials.
While the behavior of cement-based materials containing MP as a partial cement substitution cured in air has been examined [30,31,32,33], studies on materials cured in air and then subsequently immersed in an acidic environment do not exist. In fact, the studies on the performance of acid-attacked cement-based materials in these conditions are notably lacking. Such curing conditions influence hydration kinetics and microstructural developments of cement-based materials concerning the chemical degradation behavior.
Despite the existing literature on MP as a cement replacement, studies have concentrated on ordinary curing conditions, or just the overall mechanical properties of cement-based materials. To the best of our knowledge, no studies have specifically evaluated the chemical degradation of MP-modified cement-based materials when subjected to aggressive sulfuric acid after air curing. While this may seem insignificant, it is an important gap given air curing can cause reduced moisture availability, reduced reaction kinetics, and a different microstructure that can all contribute to acid resistance, and there has yet to be an adequate understanding of its contribution. Therefore, there is a clear need for a systematic study of the macro- and micro-scale influence of MP-modified cementitious materials conditioned under air and subjected to aggressive H2SO4 environments.
To contribute to this gap, the present study examines the effects of partially substituting an ordinary Portland cement (OPC) with marble powder (MP) at substitution levels of 0%, 5%, 10%, and 15%. It focuses on the macro-scale properties (apparent density and compressive strength) and microstructural characteristics (chemical phase identification, Si/Ca ratios) of air-cured cement-based materials. The cement mixes were stored at an air temperature equal to 22 ± 2 °C and a relative humidity of 20 ± 1% for 90 days. After this duration, the specimens were submerged in a 5% sulfuric acid (H2SO4) solution for 1, 2, 4, and 6 months. After 6 months of being immersed in sulfuric acid, X-ray diffraction (XRD) analysis based on Reitveld refinement through X’Pert HighScore Plus software (Version 2.1b) was carried out in order to quantify crystalline phase changes of cement-based materials containing MP. Additionally, microstructural analysis by scanning electron microscopy (SEM) was also performed to find out the elemental mapping and Si/Ca ratio. The study contributes a critical contribution toward understanding how to optimize OPC formulations with MP to improve the mechanical performance and the chemical resistance of cement-based materials in aggressive acidic environments.
2. Materials and Methods
2.1. Materials
The Ordinary Portland cement (OPC) utilized in this study was CEM I 42.5R, supplied by Biskria Cement Company (Biskra, Algeria). The cement is characterized by a true density of 3.15 g/cm3 and a Blaine Specific Surface Area of 357.18 m2/kg. This OPC type was selected because it has a 95% clinker and 5% gypsum composition which is commercially available and is closest to pure clinker in Algeria, given that there is no cement made from 100% clinker locally produced. Another advantage is that CEM I 42.5R has a wide usage in Algeria, making it a relevant reference material in this study.
The marble powder (MP) was collected as waste from aggregate processing at the CHATT/Fil-Fila quarry, located in the Skikda region of northeastern Algeria. This material was subjected to fine grinding in the laboratory to achieve the same fineness as the OPC. After grinding, the MP reached a true density of 2.74 g/cm3 and a specific surface area of 386.95 m2/kg, confirming its suitability for use as a partial cement substitute. The MP was selected since it is an abundant industrial by-product in Algeria, and presents a sustainable material that could enhance the durability of cement-based materials. The high content of calcite and fine particle size will enable partial replacement of cement, with its local availability demonstrating clear relevance to local construction applications.
The chemical composition and physical properties of both OPC and MP were determined via standardized testing procedures; the results are given in Table 1. These properties include major oxide contents (CaO, SiO2, Al2O3, Fe2O3, etc.), loss on ignition (L.O.I.), insoluble residue (I.R.), and particle size distribution.

Table 1.
Properties of the OPC and WMP [32].
According to the results shown in Table 1, the MP exhibits a higher loss on ignition (LOI) compared to OPC. This is mainly attributed to its elevated calcium carbonate (CaCO3) content. During the LOI test, CaCO3 decomposes upon heating and releases CO2, resulting in a significant mass loss. This behavior is typical of carbonate-based materials and is well documented in cement and mineral chemistry [34,35].
2.2. Methods
2.2.1. Sample Preparation and Curing Conditions
Cement-based material samples were prepared that involved a partial substitution of OPC with MP at four substitution levels: 0%, 5%, 10%, and 15% by mass. The water-to-binder ratio (w/b) for each of the mixes prepared was kept constant at 0.40 [30].
For each mix, six (6) cubic specimens (50 × 50 × 50 mm3) were prepared in accordance with ASTM C109/C109M [36] to perform the physical and mechanical testing. In addition to the cubic specimens, six (6) prisms were prepared (40 × 40 × 160 mm3) so that a cross-section could be extracted from the middle portion of the prism to analyze the samples using an X-Ray Diffraction (XRD) (Bruker AXS, Germany) and a scanning electron microscope (SEM) (Bruker, Germany).
After demolding, the specimens were stored at an air temperature of 22 ± 2 °C and a relative humidity of 20 ± 1%, consistent with prior studies [30,31,32,33] to ensure continuity and comparability with past studies. The samples were maintained in this curing condition for 90 days. After reaching the curing period, all specimens were immersed in a 5% sulfuric acid solution (H2SO4) prepared using distilled water to simulate acid attack.
The 5% concentration of sulfuric acid was selected in order to simulate more aggressive, real-world conditions, such as those that would be observed in a sewage system, wastewater treatment plant discharged effluent, or agricultural areas influenced by acid rain. Actual environmental concentrations are generally less than this value, but 5% H2SO4 allows for controlled accelerated degradation conditions that enables evaluation of long-term chemical resistance within a workable experimental timeframe that reflects past studies of acid attack in cementitious materials [9,37,38].
The duration of immersion was for 1, 2, 4, and 6 months together, while the 5% sulfuric acid solution was replaced weekly to maintain a constant concentration during the entire immersion period.
2.2.2. Determination of Macro Properties
Apparent Density: During each assessment period, apparent density was calculated according to Kirgiz [39] after removing specimens from the acid solution using the following equation:
where:
is the apparent density (g/cm3),
is the mass of the dry specimen (g),
is the total volume of the specimen (cm3).
Each specimen was first dried to a constant mass before measurement. The volume was calculated based on the nominal dimensions of the cubes (50 × 50 × 50 mm3 = 125 cm3). The average value from three specimens per substitute ratio and testing age was reported.
Compressive Strength: Following the determination of the apparent density, compressive strength test was performed according to ASTM C109/C109M [36] standards. At different ages (1, 2, 4 and 6 months) and for each mixture (0%, 5%, 10%, and 15% MP), compressive strength was calculated as the average of six individual specimens for each mixture to ensure the reliability of the results. To determine the compressive strength, the following equation was used:
where:
is the compressive strength (MPa),
F is the failure load (N),
A is the loading area (mm2).
2.2.3. Microstructural Characterization
In order to evaluate the changes in microstructure due to sulfuric acid exposure and MP addition, advanced analytical techniques were employed after 6 months of sulfuric acid exposure.
- X-Ray Diffraction (XRD): Powder XRD was employed for mineralogical analysis of hardened cement-based materials containing MP at different levels. Prior to analysis, a cross-section from the middle portion of each prism was extracted (40 × 40 × 8 ± 2 mm), dried in silica gel at a temperature of 20 °C, crushed, and then sieved to a particle size of <50 µm to reduce the effect of rehydration. XRD patterns were obtained from a diffractometer with a radiation source (λ = 1.54056 Å), and a to 70° (2θ) scan area, and a step of 0.002°.
The XRD analysis combined with Rietveld refinement will allow for quantification of changes in crystalline phases that may be caused by exposure to sulfuric acid and the addition of MP to the hydration products. This will help identify phase changes, for example, the formation or reduction of portlandite (CH), ettringite, gypsum, and other crystalline phases.
- Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS): SEM-EDS analysis was performed on the cross-sectional surfaces of air-cured cement paste specimens exposed to sulfuric acid attack. For the purposes of this study, the SEM and EDS were concerned solely with examining microstructural phase changes and elemental distributions (Si, Ca, Al) without concern for cracks, voids, or general morphology. To support the XRD/Rietveld results, elemental mapping and Si/Ca ratios were generated to confirm proposed changes to the C-S-H gel and other cementitious phases, as a function of MP substitution and acid attack.
The samples were cut in the middle at the prisms’ 40 × 40 × 160 mm (thickness of 8 ± 2 mm) to be examined after 6 months of exposure to sulfuric acid. After that, these samples were dried on silica gel at 20 °C until they attained a constant weight, which required 2 to 4 days. Since surface defects should be minimized, the microstructures of the samples were examined from their cross-sectional surfaces.
Following the procedure outlined in [33], a precision diamond saw was used to cut the volume of samples (8 ± 2 mm thick) from the area of interest in order to minimize the adsorption of a significant volume of gas in high-vacuum equipment (electron microscopes). The final sample size was 10 mm × 10 mm × 10 mm.
Scanning electron microscope requires a flat and highly polished surface. For this purpose, in the beginning, the samples were cold-mounted in an epoxy resin. In the second step, the sample’s surface was grinded using abrasive papers with water to make it flat by eliminating the features of the saws using the Grinder-Polisher Machine Minitech 263 from the brand “PRESI, Germany”. The following grades of abrasive papers were used: 120, 320, 600, 800, 1200. After grinding the samples were polished with diamond paste (with dimensions of 6 µm and 1 µm) using the Beta Grinder-Polisher Machine from the brand “BUEHLER, USA”. the grinding and polishing operations take about 2 min and 14 min respectively, with a rotation speed of about 300 rpm. After that, the cleaned sample surface was covered with a conductive path made of copper tape and they were then carbon coated to obtain electrical conductivity and to prevent electrical charging of the samples. A conductive layer with a thickness of 30 nm was applied in a vacuum of 1.3 × 10−4 Pa (10−6 Torr) with the use of the thermal evaporation method and by using sharpened graphite rods [30]. Finally, the samples can be analyzed using SEM to determine their microstructural properties. The SEM was used under high vacuum, at an accelerating voltage of 20 kV and a working distance of 10 mm–15 mm. The samples were viewed at a magnification of 1000×. Each frame was digitized at the resolution of 1536 × 1092 pixels. This is reasonable compromise between obtaining a sufficient sampling area and adequate enough resolution to distinguish the phases of interest.
The amorphous phase (mostly C-S-H gel) was the focus of the EDS analysis, and elemental mapping was performed to determine the distribution of the most important elements (Si, Ca, and Al). The Al/Si ratio of C-S-H was estimated by linear regression using data points representing phases without ettringite or monosulfate (AFm) by plotting the Al/Ca versus Si/Ca ratios for each specimen and taking sixty (60) measurement points. Together, these points provided enough information to evaluate the composition of the C-S-H phase. Data collection took approximately three hours per sample.
3. Results
3.1. Macro Properties
3.1.1. Apparent Density
Figure 1 displays the variations in the apparent density for the cement-based materials containing MP as a partial replacement for OPC at percentages of 0%, 5%, 10%, and 15% at various exposure ages (1, 2, 4, and 6 months), under sulfuric acid attack. The error bars represent the standard deviation (SD) of triplicate measurements.
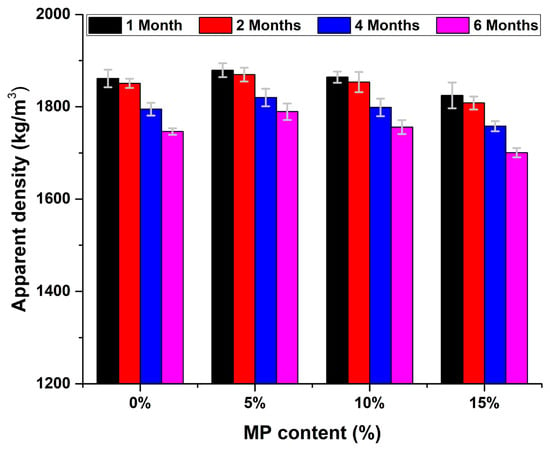
Figure 1.
Effect of sulfuric acid attack on the apparent density of air-cured cement-based materials containing marble powder (MP) at different replacement level (0% MP, 5% MP, 10% MP, 15% MP).
The apparent density results of air-cured cement pastes exposed to sulfuric acid attack showed a progressive trend that reflects the effect of replacing cement with MP at different proportions. A gradual decline in apparent density was observed over time in all mixtures, primarily due to ongoing microstructural deterioration caused by interactions with the acidic environment particularly the formation of soluble products such as calcium sulfate (gypsum) [1]. Nevertheless, the 5% MP replacement exhibited the highest average compared to the control sample and other replacement levels. It could be related to the filler effect, because the fine marble particles could reduce porosity and improve packing in the matrix and hence could reduce permeability and could improve acid resistance [40]. Moreover, although 10% replacement had improved performance over control (0%), 10% was not as effective as 5%, indicating that there may be a limit of effective replacement level. The 15% replacement showed an overall decrease in density due to excessive replacement of reactive cement with an inert material; this resulted in reduced formation of hydration products (C-S-H), increased porosity, and a higher vulnerability to chemical deterioration [41].
Once the MP level exceeds 5%, the apparent density continues to gradually fall because with the increase in inert MP, reactive cement is proportionately reduced, and there are fewer hydration products such as C-S-H. A corresponding increase in porosity, weaker matrix packing, and resistance to acid attack results. This trend demonstrates that although an optimal amount of MP exists to enhance density and acid resistance, at some point the benefits are outweighed by dilution and a reduction in reactivity.
The data is consistent with recent studies that indicate that marble powder as a partial cement replacement increases the apparent density at lower proportions (up to 10%) due to its filling effect and reduction of voids in a cementitious matrix [42]. The study refers to the acid resistance in terms of a dense and less porous material. In this study the optimized use of marble powder led to an overall lowering of acid permeability by the apparent density increase, especially with sulfuric acid. This greatly improved the durability of the chemical action with the cement-based materials matrix [1].
3.1.2. Compressive Strength
The acid resistance of cement pastes was evaluated using a 5% sulfuric acid solution. Samples from the different mixtures (0% MP, 5% MP, 10% MP, and 15% MP) were immersed in the 5% sulfuric acid for 1, 2, 4, and 6 months and the results are shown in Figure 2. The error bars correspond the standard deviation of triplicate measurements.

Figure 2.
Effect of sulfuric acid attack on the compressive strength of air-cured cement-based materials containing marble powder (MP) at different replacement level (0% MP, 5% MP, 10% MP and 15% MP).
The compressive strength of the specimens decreased over the immersed time. The reduction in compressive strength is due to the degradation of the hydrates in the hardened cement matrix due to the aggressive acidic environment, in this case sulfuric acid, acting on them and especially the calcium silicate hydrate (C-S-H) phases [43].
At 30 days of immersion, the compressive strength of the reference mix 0% MP was 45.13 MPa, while the 5% MP, 10% MP, and 15% MP mixes had a compressive strength of 48.37, 45.92, and 39.15 MPa, respectively. At 60 days the compressive strength had decreased to 39.76, 43.34, 40.42, and 32.98 MPa respectively. At 120 days the compressive strength continued to decrease to 34.23, 38.99, 35.38, and 26.29 MPa, and again decreased at 180 days to 28.47, 34.08, 29.14, and 20.11 MPa for 0% MP, 5% MP, 10% MP, and 15% MP, respectively.
All immersion periods of the 5% MP mix showed the highest compressive strength, indicating that the replacement of 5% cement with MP improves the resistance of cement pastes to acid. This improvement can be attributed to the filler effect, which increases paste density and reduces its permeability to acids. Furthermore, the reaction between calcium carbonate in marble powder and sulfuric acid consumes a portion of the acid, thereby minimizing its attack on primary hydration products and helping to preserve the paste’s strength.
Regarding the 10% MP mix, a moderate decrease is seen in compressive strength relative to the 5% MP specimen, which is presumably due to partial dilution of the reactive clinker phases in turn slightly limiting C-S-H formation, but still resulting in improved packing of the matrix compared to the 15% MP mix.
On the other hand, the 15% MP mix had the lowest compressive strength, due to the excess MP which provided an excess dilution effect from the MP which weakened the cohesion between the components that are required for effective and rapid strength development in the cement paste. This reduction may also be explained by the substantial lower quantity of C3S and C2S in the blended cement-based materials, which are mainly responsible for compressive strength. This is often referred to as pozzolanic reaction dilution [30,31,32].
Therefore, it can safely be inferred and concluded that the MP had a beneficial, positive effect although it is limited to some level of substitution.
These findings are consistent with previous studies showing enhanced acid resistance of cement-based materials with a replacement of 5% OPC with MP. However, the reduced performance in the 15% MP mix confirms that MP acts primarily as a filler with limited pozzolanic activity at higher replacement level [27].
During the testing phase, macroscopic observations indicated that samples containing 10% and 15% MP had a visibly greater number of surface defects (small cracks, and signs of localized deterioration) than the 5% MP and reference mixes (0% MP). The 5% MP samples presented a density and uniformity appearance with minimal surface defects, reinforcing the observation that 5% MP was an adequate balance of strength, density, and durability.
The reduction in compressive strength at higher MP replacement levels is governed by the combined effects of (1) reduced C-S-H formation due to dilution of clinker phases and (2) the formation of a less stable and more degraded C-S-H gel under sulfuric acid attack. When MP content exceeds the optimal level (5%), the dilution of reactive components such as C3S and C2S limits the amount of newly generated C-S-H, while the filler effect becomes insufficient to compensate for the reduction in hydration products. Moreover, sulfuric acid exposure promotes decalcification and silica leaching from the C-S-H structure, resulting in a more disordered and weaker gel. This interplay between clinker dilution, reduced C-S-H content, and acid-driven deterioration explains the significant loss of strength at 10% and especially 15% MP. Thus, although MP contributes positively through its filler effect at low levels, excessive replacement compromises hydration kinetics, durability, and long-term mechanical performance.
3.2. Microstructural Results
3.2.1. X-Ray Diffraction and Rietveld Analysis
The XRD patterns of air-cured cement-based materials with different MP contents (0%, 5%, 10%, and 15%) after immersion in sulfuric acid are presented in Figure 3. At first glance, the diffraction peaks at all MP contents appear to be of nearly the same positions, indicating same phase assemblage. However, differences in peak intensities indicate variations in phase content. In particular, the diffraction intensities decrease as the MP content increases, which indicates the decrease in concentration of the crystalline phase due to both MP substitution and acid-induced degradation.
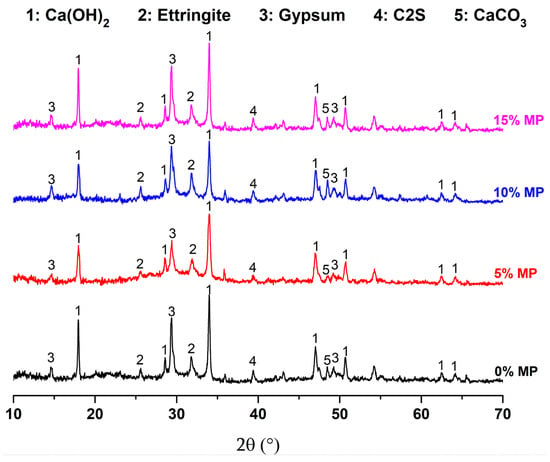
Figure 3.
The measured XRD patterns and calculated patterns by Rietveld refinement of air-cured cement-based materials containing MP at different levels at 0% MP, 5% MP, 10% MP and 15% MP, after sulfuric acid immersion.
3.2.2. Qualitative Phase Identification
The spectrograms obtained from Figure 3 were treated using X’Pert High Score software (Version 2.1b) to determine crystalline phases using ICDD “International Centre for Diffraction Data” cards. The results of XRD measurements were analyzed using the Rietveld method.
The spectrograms obtained from XRD analysis show the presence of portlandite (Ca(OH)2) and ettringite that are the result of hydration reactions. Also, the presence of anhydrous cement grains in the mixtures such as belite C2S. It was noticed, the presence of gypsum and Calcite (CaCO3). The latter is mostly ascribed to the MP addition.
The uncrystallized zones which are observed between peaks in the mixes may be attributed to the amorphous gel of C-S-H. The latter consists of many small globules having a disordered layered structure [44,45,46]. As a result, it is not detectable by XRD in the cement paste XRD pattern [47,48,49].
3.2.3. Effect of MP on Phase Intensity and Acid Resistance
The peak intensity of the alite, belite, and aluminate reduced progressively with the increase in MP substitution, primarily due to the dilution effect and the susceptibility of calcium-rich phases to acid attack [50]. The findings indicate that MP substitution reduces the content of reactive clinker phases, weakening the system’s resistance to sulfuric acid attack.
The XRD patterns of the cement pastes with different MP content show notable changes in the broad diffuse halo, which is usually connected to poorly crystalline C-S-H gel and is focused around 2θ ≈ 29.5–30.5°, with some broad elevation around 32.5° [48,49]. The intensity of this halo increased at 5% MP in comparison to the control, indicating better C-S-H preservation under acid exposure. This is attributed to MP’s filler effect, which densifies the matrix and minimizes acid penetration. This interpretation aligns with the findings by Aliabdo et al. [51], who noted enhanced durability in systems incorporating finely ground limestone. Nevertheless, C-S-H halo intensity slightly decreased when MP content increased to 10%, illustrating that higher substitution began diluting the cement content without providing noticeable additional benefits. At 15% MP, a further decrease in the amorphous halo was noted, indicating a drop in the presence of C-S-H. This can be attributed to the over-replacement of the clinker phases, which are the major precursors of C-S-H, causing restricted hydration and a poorly developed binding phase. The reduction in C-S-H content supports the conclusion that over-replacement at this level weakens acid resistance through reduced gel formation and a less protective matrix. This trend supports Singh et al. [52]. Observation that over-substitution with inert fillers may delay hydration kinetics and lower the amount of produced hydrates.
It should be mentioned that the increased diffuse halo at 5% MP does not always mean that more C-S-H is formed as a result of hydration alone. Instead, under conditions of acid exposure, there may be more preservation of the original C-S-H, as MP could act as a physical barrier or buffer against acid ingress. This protective mechanism can be blamed for the increased resistance observed in samples with 5% MP, as the key strength-contributing phase C-S-H remains less deteriorated. This behavior is consistent with the results of a study that compared acid resistance in cementitious systems with and without mineral additions [53].
Portlandite (CH), another significant hydration product, exhibited a different pattern. CH is very reactive under acidic conditions and dissolves easily, releasing Ca2+ and forming secondary products such as gypsum. In the control mix (0% MP), CH peaks were clearly observed, but their intensity declined as MP content increased to 10%, suggesting both a reduction in CH formation and progressive leaching under acid conditions. But with 15% MP, a relative increase in peak intensity of CH was observed, which should not be interpreted as an absolute increase in the production of CH. Instead, this is probably because two things occur: first, the dilution of the cement reduces the quantity of C-S-H produced, limiting the pozzolanic consumption of CH; and second, localized retention or re-precipitation of CH in less-exposed areas can still be detected in the XRD pattern. This increase may also arise from the relative, as opposed to absolute, abundance of CH due to the lower content of other hydrates. This confirms the decreased resistance to acid attack at high MP contents, where the protective effects observed at lower dosages are no longer dominant. Supporting this explanation, Refs. [30,31,32] demonstrated that 15% MP replacement increases porosity, thereby facilitating deeper acid penetration and heterogeneous degradation, which may lead to non-uniform phase distribution.
Ettringite (AFt) followed a similar behavior. Its peak intensity decreased with increasing MP substitution up to 10%, which may result from acid-induced decomposition or transformation of AFt into gypsum. For 15% MP, the ettringite peak intensity exhibited an increase once more. This phenomenon can be explained by secondary reactions with available tricalcium aluminate (C3A), sulfate ions from the acid, and the increased availability of calcite from MP. This kind of interaction can promote the formation of calcium carboaluminates [20,32,54,55], which have been found to stabilize ettringite in acidic conditions.
Gypsum formation, nevertheless, increased progressively with more MP content. This is attributed to the reaction between sulfuric acid and calcium-rich phases such as CH and C3A, which results in the formation of gypsum as a secondary product [56].
Peaks for calcite also intensified progressively with MP substitution, as would be expected based on the fact that MP mainly consists of CaCO3 [16,54,57,58]. Moreover, acid-induced carbonation of leached calcium ions may contribute to the calcite signal detected by XRD [59]. Hence, MP not only alters the phase composition as a result of cement dilution but also facilitates the formation of more acid-resistant secondary phases like gypsum and calcite, though at the cost of reducing the amount of C-S-H and other strength-contributing phases.
It is important to note that the increased ettringite content observed at higher MP levels—particularly at 15% MP—has direct microstructural consequences. Excessive ettringite formation is known to produce volumetric expansion within the cement matrix, generating internal tensile stresses that can initiate microcracks and widen existing pores [60,61]. In the present study, the slight rise in AFt content at 15% MP likely intensified microcracking, which is consistent with the reduced and for this mixture. This microcracking effect, combined with the already weakened C-S-H gel at high MP levels, provides a mechanistic explanation for the pronounced drop in compressive strength at 15% MP.
Similarly, gypsum formation plays a dual role under sulfuric acid attack. At lower MP contents (5–10%), the formation of a thin gypsum layer may temporarily act as a protective barrier, slowing acid ingress and partially preserving C-S-H [62]. However, at 15% MP, the higher CaCO3 availability and increased CH dissolution promote excessive gypsum crystallization. This leads to expansion, pore plugging followed by tensile microcracking, and progressive material weakening [63]. Thus, at high MP levels, gypsum contributes more to microstructural deterioration rather than protection, which correlates with the substantial losses in apparent density and compressive strength observed in this mixture.
3.2.4. Rietveld Refinement Applied to Quantitative Phase Analysis
The quantitative analysis of the mineralogical phases of the cement-based materials with MP, which were acid-exposed, was performed by Rietveld refinement, and the results are shown in Table 2.

Table 2.
Mass percentage of each component in cement-based materials containing MP at different levels after sulfuric acid immersion determined by XRD/Rietveld.
The information data provides an assessment of the evolution of phases, considering the increasing MP content, and corroborates the qualitative observations gleaned from the XRD patterns (Figure 3). With the increase in MP substitution from 0% to 15%, there was an observable decline in the mass percentage of the primary clinker phases. The Alite (C3S) content diminished from 1.47% in the control mix to 0.96% at 15% MP. Also, belite (β-C2S) and aluminate (C3A) showed a decline from 4.30% to 2.78% and 2.86% to 1.82%, respectively. This trend indicates the dilution of reactive cement ingredients and the dissolution susceptibility of these phases in acidic environments [64]. The absence of ferrite (C4AF) in all samples indicates that it was completely degraded under sulfuric acid attack, corroborating the findings by Tsubone et al. [65] that reported the instability of this phase in low pH environments.
Portlandite (CH) decreased from 5.73% in the control sample to 4.11% at 10% MP. This supports the hypothesis of clinker hydration reduction and CH leaching in acidic conditions, which is associated with hydration because CH is leached as Ca2+ [66]. In the presence of acid attack, the MP helps to reduce the rate of CH leaching by filling voids, which indirectly contributes to maintaining better mechanical properties. Interestingly, the CH content increased to 4.89% with 15% MP. As noted before, this does not indicate increased CH formation, which would cause an apparent increase due to decreased C-S-H (35.28% at 15% MP). The reduction in the rate of pozzolanic reaction also contributes to the formation of CH. Additionally, the increase in porosity with high MP content may also increase the CH leached in less exposed areas, increasing its concentration [32]. Consequently, this leads to a less dense matrix, resulting in reduced and at a high replacement level.
The gypsum content increased progressively with MP addition, from 8.84% for the control to 10.54% at 15% MP. This is reflective of the reaction of sulfuric acid with CH and C3A, which generates gypsum as a secondary product; this product causes ongoing microstructural deterioration and thus contributes to the decline in and .
Ettringite remained relatively stable in all the levels but showed a slight increase at 15% MP (10.97%), which may be attributed to secondary reactions between calcite and C3A, as well as the presence of sulfate [66]. These reactions lead to the formation of calcium carboaluminate that enhance the stability of ettringite in acidic environments. At lower MP replacement levels (5% and 10%), the stable ettringite formation contributes to increase both the and , as it helped improve the packing density and hydration of the cement-based materials matrix. However, at 15% MP, the slight increase in ettringite formation disturbs the hydration causing expansion and microcracking in the matrix, leading to decrease in both and .
The content of calcite rose considerably with MP substitution, increasing from 3.51% (0% MP) to 12.58% (15% MP), which agrees with the natural composition of MP and also the additional calcite formed by carbonation upon acid attack [67]. At the lower replacement levels (5% and 10%), the increased calcite content contributed positively to the ad and cd. This was likely a result of the effect of MP acting as a filler, reducing porosity, increasing packing density, and providing a more dense matrix of cement-based materials matrix, leading to better mechanical properties. Nevertheless, the increase in calcite at 15% MP substitution levels, while initially contributing to the densification, likely began to have a detrimental effect. The excess calcite content may have contributed to increased carbonation in acidic environments, which led to a weakening and microcracking of the matrix. This disruption in the microstructure of cement-based materials likely contributed to the observed decrease in and at the higher MP content.
The total crystalline content increased from 36.92% in the control to 44.48% at 15% MP, indicating that acid-resistant phases such as calcite and gypsum (the new crystalline secondary phases) become increasingly dominant with increasing MP content. This increase suggests better resistance to acidic conditions and contributes to the improved structural integrity at 5% and 10% MP, which helps increase and at lower levels. The MP is certainly plays a protective role, thereby reducing the porosity and significantly increasing the compactness of the cement-based materials matrix. In contrast, the amorphous phase, which is primarily composed of C-S-H, showed a non-linear trend. It increased to 49.23% at 5% MP, reflecting better retention of existing C-S-H possibly due to the filler effect of MP, which helped enhance packing density and reduce porosity. This improved microstructure led to higher of apparent density and compressive strength. However, the C-S-H content is dropped to 44.09% at 10% MP compared to the 5% MP sample, though it remained close to the control mix (43.57%), and further decreased to 35.28% at 15% MP. This decrease at higher MP contents is caused by cement dilution and less availability of clinker, which reduces the extent of hydration and gel formation [68].
At 15% MP, the significant decrease in the amorphous phase and the increase in crystalline phases resulted in a shift in the hydration process. While the acid-resistant crystalline phases provide durability enhancement, the reduction in the amorphous phase (C-S-H), critical for strength development, this shift leads to reduced the apparent density and compressive strength at this higher replacement level (15%).
The observations made through XRD correlate directly with the macroscopic mechanical performance mentioned earlier. In the 5% MP mix, there was sufficient C-S-H remaining; this allows for a higher apparent density and compressive strength as a result of matrix packing and filler effects. In the 10% MP sample, C-S-H was diluted enough to slightly lessen strength, consistent with intermediate mechanical values. However, in the 15% MP sample, C-S-H was diluted enough to lower density and compressive strength, mainly due to the dilution of reactive clinker phases (not a result of an acid attack). This demonstrates that excessive replacement of reactive matrix with inert MP reduced the formation of the primary binding phase (C-S-H), which is needed to develop mechanical resistance and acid durability. Overall, these results suggest that 5% MP is the best replacement level to maintain microstructural characteristics and macroscopic performance.
The unquantified loss representing unidentified amorphous material, residual water, or degradation products varied between 12.92% and 20.24%, which reflects the complexity of acid-induced phase transformations and the limitation of XRD in detecting poorly crystalline or trace phases [60].
Rietveld analysis confirms that MP significantly alters the phase composition of cement pastes subjected to sulfuric acid attack. While low MP content (5%) has the potential to enhance acid resistance via filler effects and better C-S-H retention, high content (15%) results in cement dilution, increased porosity, and reduced durability. The patterns noted in both observations (Figure 3) and calculations (Table 2) confirmed the analysis and demonstrated that the quantification technique was effective and reliable.
3.3. Microstructural and Elemental Characterization via SEM-EDS
Back-scattered electron (BSE) images and energy-dispersive X-ray spectroscopy (EDS) elemental mapping of cement-based materials containing MP are presented in Figure 4. In addition, quantitative EDS, including elemental compositions, is shown in Table 3 and Figure 5.
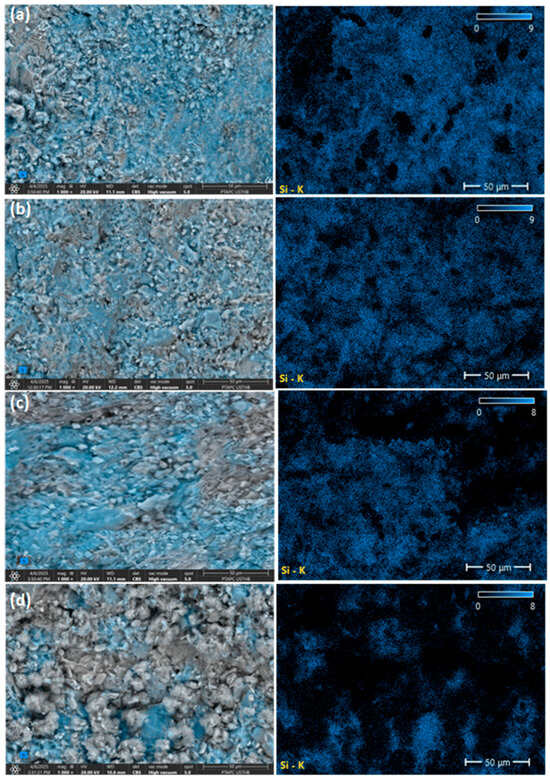
Figure 4.
Back-scattered electron (BSE) images (left) and Si EDS mappings (right) of air-cured cement-based materials containing MP at different levels: (a) 0% MP; (b) 5% MP; (c) 10% MP; (d) 15% MP, after sulfuric acid immersion. The blue color represents the Si elements in the images.

Table 3.
Chemical elements of air-cured cement-based materials containing MP at different levels (0% MP, 5% MP, 10% MP, and 15% MP) after sulfuric acid immersion—results obtained from EDS analysis.
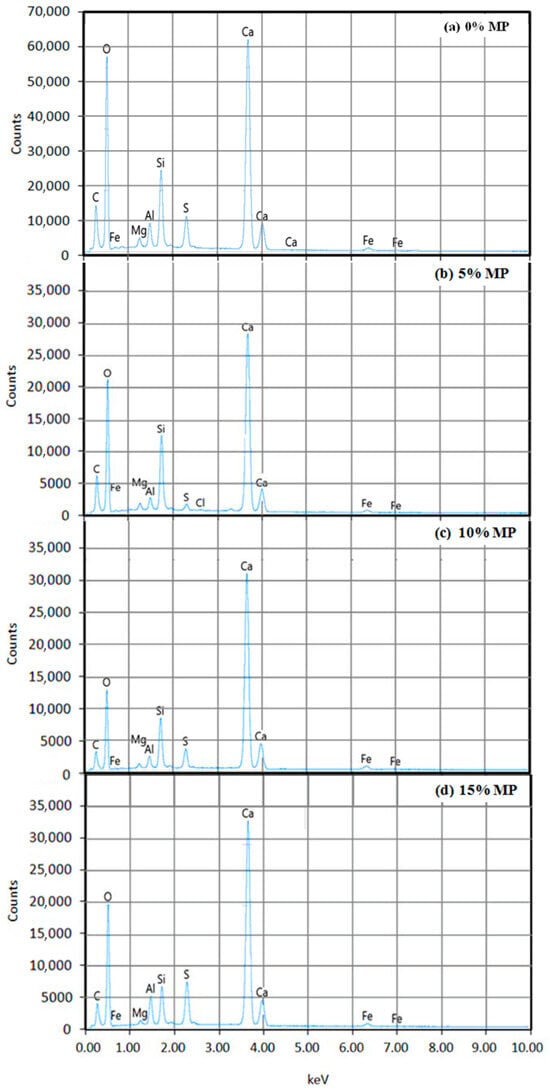
Figure 5.
Energy-dispersive X-ray spectroscopy (EDX) of cement-based materials containing MP at different levels: (a) 0% MP; (b) 5% MP; (c) 10% MP; (d) 15% MP (results obtained with a 20-kV accelerating voltage).
The BSE images and EDS elemental mappings (Figure 4) indicate that incorporating 5% MP enhances the retention of silicon (Si) as evidenced by the more intense blue color of the Si phase. This improvement can neither be due to the hydration of cement, since MP is an inert material and does not take part in hydration reactions. Instead, the observed microstructural benefits are explained by the physical filler effect of MP particles [55,69], which results in a denser microstructure and more efficient packing. The densification would definitely improve the resistance of the cement matrix against sulfuric acid attack by limiting acid penetration and reducing leaching of calcium and silicon from the C-S-H phase. However, at 10% MP, the Si content slightly decreased compared to the 5% MP sample, although it remained close to the levels observed in the control mix (0%).
When 15% MP was incorporated into the binder, a more noticeable reduction in the content of Si was observed. Such reduction in Si could be attributed to two factors: (1) the dilution effect, where additional inert MP replaces reactive clinker, and (2) the increased vulnerability to sulfuric acid, which accelerates the leaching of silicon out of the C-S-H structure [55]. This is clearly evident at 15% MP, where black areas observed in the BSE images correlate with degraded zones with lower Si presence.
Significant differences in elemental composition with respect to MP substitution levels were found by the EDS analysis (Figure 5 and Table 3).
As can be seen from Table 3, the use of up to 15% of MP as a cement substitute led the content of calcium hydrate (Ca) in the mix to increase from 28% in the control mix (0% MP) to 34.30% when using 15% MP owing to the presence of calcite (CaCO3) in the MP [30]. Moreover, the content of iron hydrate (Fe) in the cement-based materials increased from 1% in the control mix (0% MP) to 1.40% at 10% MP and decreased again to 1.20% at 15% MP, but it was still higher than the 0% MP result. This variation is explained due to the reaction of the CaCO3 with C4AF, forming calcium carbo-aluminoferrites (CCAF) [70]. Additionally, the increase in aluminum hydrate (Al) content of specimens having 15% of MP, rising from 1.70% in the control mix to 2.60% at 15% MP, is caused by the reaction between C3A and CaCO3, leading to the formation of calcium carbo-aluminates (CCA) [20,32,54].
Regarding the silicon hydrate (Si) content, it increased from 5.10% in the control mix (0% MP) to 6.50% at 5% MP, then declined to 5.00% at 10% MP and further dropped to 3.10% at 15% MP. These findings are consistent with the Si elemental mapping images (Figure 4), where the intensity of the blue color indicating Si concentration—was highest at 5% MP and consistently decreasing with increasing contents of MP. Since Si is a fundamental component of C-S-H, the primary hydration product responsible for strength development in cement-based materials, this trend suggests that the incorporation of 5% MP enhances C-S-H formation, likely due to improved particle packing and filler effects [55]. The reduction in the Si content at 10% MP compared to that in 5% MP is evident, although the results remain very close to the control mix (0% MP). Nonetheless, beyond this point, it is shown that dilution of reactive cement by increasing MP content decreases the availability of silica required for C-S-H formation, resulting in decreased durability and strength [33]. A noticeable carbon (C) peak appears in Figure 5. The C content increased with the incorporation of MP, reaching a peak at 10% MP (8.90%). This increase could be attributed to two main sources: (1) the intrinsic carbon from the CaCO3 present in MP, and (2) the formation of carbonation products as a result of acid-induced decalcification and subsequent carbonation of calcium-rich phases during the exposure period. In addition, a sulfur peak (S) is also observed in Figure 5 which confirms the formation of gypsum and ettringite, along with C-S-H and CH [33,58]. The S content at higher levels of MP exhibited a significant increase, most notably at 15% MP (4.60%), suggesting more sulfate salt deposition, such as gypsum or ettringite, that typically occurs due to sulfuric acid attack. These sulfate-containing phases are indicative of more severe chemical degradation and microstructural damage.
Overall, these findings support the conclusion that a modest MP substitution of 5% improves C-S-H retention and slows degradation under sulfuric acid attack because of enhanced particle packing and filler effect, while a 10% MP substitute yielded performance almost identical to the control mix, with C-S-H content slightly reduced. However, a high substitute level (15%) compromises the mechanical performance of the cementitious matrix as well as its chemical stability, likely due to the decrease in the silicate (Si) content and the increase in the portlandite (CH) content [33].
C-S-H Phase Characterization by Elemental Ratios
To investigate the C-S-H phase in air-cured cement-based materials containing MP under the influence of sulfuric acid immersion, SEM-EDS analyses were carried out after 6 months of acid immersion. The findings are presented in Figure 6.
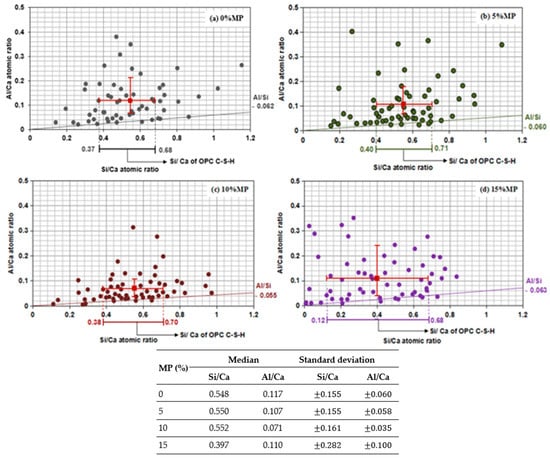
Figure 6.
Plot of Al/Ca as a function of the Si/Ca atomic ratios of the EDS measurements of cement-based materials containing MP at different levels: (a) 0% MP; (b) 5% MP; (c) 10% MP; (d) 15% MP (results obtained with a 20-kV accelerating voltage).
60 data points per sample were collected using EDS analysis, enabling the creation of a plot correlating Al/Ca with Si/Ca. This plot enabled the determination of the C-S-H phase composition. The Al/Si ratio of the C-S-H was derived from the slope of a line drawn through points representing mixed analyses of CH and C-S-H without aluminum iron monosulfate (AFm) or ettringite. Lower Si/Ca ratios data points were also represented in mixed analyses of C-S-H and CH. The majority of the data points on this line were within the Si/Ca ratio range of C-S-H [33,71].
The analysis confirmed a broad spread within the data points, which necessitated the utilization of scattering indicators. Furthermore, the resultant distributions had asymmetry. Toubal Seghir et al. [33] noted that to account for the influence of outliers in the arithmetic mean and standard deviation, the interquartile range was implemented to measure spread in the central portion of the data. The plots provided the median values of cement-based materials containing MP exposed to acid attack, as well as the lower and upper quartile values for Al/Si and Si/Ca ratios.
The plots provided in Figure 6 also confirm that incorporating MP in the cement–based materials enhance retention of the C-S-H phase when it is exposed to sulfuric acid, rather than promoting its formation, as indicated by the Si/Ca and Al/Si ratios. The ratios provide valuable insights into the composition as well as structural stability of the C-S-H phase, which determines the material’s strength and durability.
For the control mix (0% MP), the Si/Ca ratio is between 0.37 and 0.68, while having an Al/Si ratio of 0.062. The results of Si/Ca are a typical balance between silica and calcium in a hydrated cement paste, where the C-S-H gel is the main hydration product. The Al/Si ratio indicates the occurrence of aluminum-containing phases, such as AFm or ettringite, which are commonly found in OPC hydration.
When 5% MP was incorporated into the binder, the Si/Ca ratio increased slightly (ranging from 0.40 to 0.71), and the Al/Si ratio remained relatively stable (0.060). The increase in the Si/Ca ratio could be attributed to the denser matrix created by MP’s filler effect, which physically protects the C-S-H from acid penetration and degradation.
For 10% MP, the Si/Ca ratio is between 0.38 and 0.70, which is close to that of the control mix (0% MP). This suggests that an increase in MP does not affect the Si/Ca ratio. This stability may be due to the continued enhancement of packing and hydration efficiency at this lower MP content. The Si content in the samples at 10% MP did not show appreciable differences from the control mix, which can be expected because the Si/Ca ratio remained stable. The slight change in this ratio at this percentage of MP shows that the C-S-H retention is still effective, and the role of MP as a filler still improves the cement matrix without significant degradation under the influence of sulfuric acid. The Al/Si ratio decreased slightly to 0.055, showing limited AFm or ettringite phase development. This supports the rationale that MP primarily influences microstructure through physical mechanisms and not chemical reactivity.
At 15% MP, the Si/Ca ratio ranged from 0.12 to 0.68, reflecting a marked decline from both the control mix and low MP contents. This reduction in the Si/Ca ratio can be attributed to two major reasons: (1) the dilution effect caused by the higher MP content, which reduces the amount of reactive silica available in the mixture, and (2) the acid attack, which accelerates the leaching of silica and calcium from the C-S-H structure.
The BSE images show the presence of black particles, which is synonymous with the voiding of silicon (Si) leading to the dissolution of the C-S-H gel while sulfuric acid is present. Higher levels of MP lead to increased cement reactivity, thus also decreasing the overall silica content available for the formation of C-S-H. At the same time, sulfuric acid intensifies the dissolution of C-S-H phase, thus leading to increased silicon (Si) loss. Moreover, the results for the 15% MP mix also showed high amounts of data point dispersion, suggesting C-S-H composition was highly variable. This requires that with higher MP contents, the behavior of the cement matrix becomes more unpredictable, leading to inconsistent material properties and reduced durability. Variability of this type is undesirable because it reduces the reliability of the material for structural application, especially when exposed to sulfuric acid. This research highlights the fact that even though MP is beneficial for packing and hydration retention at lower contents, at higher levels it becomes harmful due to lower reactivity and greater susceptibility to acid-induced degradation.
The decrease in Si/Ca ratio observed with higher MP content suggests a lower availability of silicon, which is an essential component for the formation of the C-S-H gel structure. In cementitious systems, an optimal Si/Ca ratio leads to the formation of a well-ordered, more polymerized C-S-H gel, which contributes to higher density and compressive strength.
When the Si/Ca ratio decreases, as seen with increasing MP content, the degree of polymerization of C-S-H may be affected, leading to the formation of less polymerized, more disordered C-S-H structures. This disordering results in a less dense network, which in turn reduces the material’s overall strength and mechanical resistance. The lower density and disorder of the C-S-H network can result in fewer interlinked C-S-H chains, which are critical for load-bearing and the development of compressive strength.
Additionally, the reduced amount of reactive Si due to MP replacement can lead to the formation of less effective C-S-H gel, which contributes to the overall weakening of the cement matrix, particularly at higher MP levels (15% MP). In essence, the lower Si content results in incomplete or less stable C-S-H formation, which could explain the lower compressive strength observed at higher MP contents.
These findings indicate that the decrease in Si/Ca ratio with increased MP replacement (especially at 15% MP) is the result of both a decrease in the amount of C-S-H formed (dilution of clinker phases) as well as formation of a less polymerized, less stable, more silica-deficient C-S-H gel. This situation agrees with previous works that have shown lower Si/Ca ratios correspond to shorter silicate chains, less bridging tetrahedra and weaker C-S-H structures [34,72]. In addition, sulfuric acid further accelerates the decalcification of cement and leaching of silica, compounding the structural disorder of C-S-H [73,74]. Thus, the lower Si/Ca values obtained for the 15% MP condition reflects both reduced C-S-H formation as well as a mechanically weaker C-S-H gel which helps to explain the lower compressive strength results for this mix.
Overall, the reduced Si/Ca ratio is indicative of a diluted C-S-H network, with implications for both structural integrity and mechanical performance. This supports the notion that excessive replacement of cement with MP (especially at higher levels such as 15%) compromises the formation of the key binding phase (C-S-H), leading to the reduced strength observed in the tests.
These observations align with previous studies regarding the impact of acid attacks on cementitious materials. The C-S-H structure usually deteriorates in acidic environments, primarily due to the silica component, which is the essential component of this phase [75]. At higher MP levels, the reduced amount of reactive cement components combined with the leaching effect of the sulfuric acid results in a significant decrease in Si, thus impairing the durability and performance of the material upon acid exposure [76]. During this phase (15% MP), the Al/Si ratio increases to 0.063. This is due to the reaction of CaCO3 provided by MP with C3A, which led to the formation of calcium carboaluminates. The addition of a new chemical component is involved in the ettringite development [32].
4. Conclusions
The purpose of this study was to investigate the macro and microstructural properties of air-cured cement-based materials with MP when subjected to acid attack by sulfuric acid. Based on the results, the following conclusions could be made:
- Partial replacement of OPC with 5% MP improved both macro- and microstructural properties, including apparent density and compressive strength, due to the filler effect and reduced matrix permeability, enhancing resistance to acid attack.
- X-ray Diffraction and Rietveld analysis revealed that MP significantly altered the phase composition. MP increased calcite content, preserved C-S-H phases, and promoted gypsum formation. Ettringite remained stable, with only a minor increase at 15% MP. Low MP contents (5–10%) effectively enhanced acid durability by retaining C-S-H and improving density.
- SEM-EDS analysis confirmed that incorporating up to 5% MP increased Ca and Si content, enhanced hydration, and improved particle packing, leading to better acid resistance.
- The Al/Ca vs. Si/Ca ratio analysis showed that 10% MP maintains C-S-H preservation and reduces leaching of aluminum-containing phases under acid attack, demonstrating acceptable durability.
- At 10% MP replacement, performance was comparable to the control, indicating no detrimental effect on durability.
- At 15% MP, reductions in all performance metrics were observed, likely due to dilution effects and increased silica leaching under sulfuric acid exposure.
The study demonstrates that MP can be used as a sustainable partial replacement of OPC in cement-based materials exposed to aggressive environments. Replacement levels up to 10% provide satisfactory durability, while 5% MP showed the most notable improvements in density and compressive strength. Future research could focus on:
- Optimizing MP particle size and chemical composition to further enhance acid resistance.
- Investigating long-term performance and durability under varying environmental conditions.
- Exploring the synergistic effects of MP with other supplementary cementitious materials to improve mechanical and chemical resistance.
In summary, MP is a promising sustainable alternative to OPC for applications requiring enhanced durability against acid attack, particularly in air-cured cement-based materials.
Author Contributions
Conceptualization, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Data curation, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Formal analysis, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Funding acquisition, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Investigation A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Methodology, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Project administration, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Resources, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Software, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Supervision, A.B., N.T.S., L.S. and Y.A.; Validation, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Visualization, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T.; Writing—original draft, A.B., N.T.S., L.S., Y.A. and Y.T.; Writing—review and editing, A.B., N.T.S., L.S., Y.A., M.B., K.H., C.B. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to acknowledge the support of the Civil Engineering Laboratory at the University Yahia Fares of Medea, Algeria, for enabling the experimental tests to be performed. Additionally, the authors would especially acknowledge the support of the Platform for Physico-Chemical Analyses (PTAPC) at the University of Science and Technology Houari Boumediene, Algeria, for providing the facilities and assistance that enabled the XRD and SEM analyses to be carried out.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, S.; Wang, Z. Effect of Limestone Powder on Acid Attack Characteristics of Cement Pastes. Mater. Sci. 2014, 20, 503–508. [Google Scholar] [CrossRef]
- Jahani, F.; Devinny, J.; Mansfeld, F.; Rosen, I.G.; Sun, Z.; Wang, C. Investigations of sulfuric acid corrosion of concrete. I: Modeling and chemical observations. J. Environ. Eng. 2001, 127, 572–579. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Riyahi, A.; Jebter, A.A.; Hejazi, F. Investigation on durability of concrete exposed to chloride, sulfate attack and steel corrosion. IOP Conf. Ser. Earth Environ. Sci. 2019, 357, 12011. [Google Scholar] [CrossRef]
- Chang, H.; Wang, P.; Jin, Z.; Li, G.; Feng, P.; Ye, S.; Liu, J. Durability and Aesthetics of Architectural Concrete under Chloride Attack or Carbonation. Materials 2020, 13, 839. [Google Scholar] [CrossRef]
- Bassuoni, M.T.; Nehdi, M.L. Resistance of self-consolidating concrete to sulfuric acid attack with consecutive pH reduction. Cem. Concr. Res. 2007, 37, 1070–1084. [Google Scholar]
- Naseer, A.; Tantray, M.A. Sulfuric acid’s effects on the characteristics of regular Portland cement concrete under various environmental circumstances. Libr. Prog. Int. 2024, 44, 19096–19103. [Google Scholar]
- Irico, S.; De Meyst, L.; Qvaeschning, D.; Alonso, M.C.; Villar, K.; De Belie, N. Severe Sulfuric Acid Attack on Self-Compacting Concrete with Granulometrically Optimized Blast-Furnace Slag-Comparison of Different Test Methods. Materials 2020, 13, 1431. [Google Scholar]
- Al-Sodani, K.A.A.; Adewumi, A.A.; Mohd Ariffin, M.A.; Salami, B.A.; Yusuf, M.O.; Ibrahim, M.; AlAteah, A.H.; Al-Tholaia, M.M.H.; Shamsah, S.M.I.; Ismail, M. Acid Resistance of Alkali-Activated Natural Pozzolan and Limestone Powder Mortar. Sustainability 2022, 14, 14451. [Google Scholar] [CrossRef]
- Zivica, V.; Bajza, A. Acidic attack of cement based materials—A review: Part 1. Principle of acidic attack. Constr. Build. Mater. 2001, 15, 331–340. [Google Scholar] [CrossRef]
- Selim, F.A.; Hashem, F.S.; Amin, M.S. Mechanical, microstructural and acid resistance aspects of improved hardened Portland cement pastes incorporating marble dust and fine kaolinite sand. Constr. Build. Mater. 2020, 251, 118992. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J.A. Acid corrosion resistance of different cementing materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- Sengul, O.; Tasdemir, M.A. Compressive strength and rapid chloride permeability of concretes with ground fly ash and slag. J. Mater. Civ. Eng. 2009, 21, 494–501. [Google Scholar] [CrossRef]
- Abbas, Y.; Djebien, R.; Toubal Seghir, N.; Benaimeche, O. Enhancing mechanical behaviour and durability of high performance concrete with silica fume, ground blast furnace slag, and marble powder. J. Appl. Eng. Sci. 2023, 13, 137–146. [Google Scholar] [CrossRef]
- Madandoust, R.; Ranjbar, M.M.; Moghadam, H.A.; Mousavi, S.Y. Mechanical properties and durability assessment of rice husk ash concrete. Biosyst. Eng. 2011, 110, 144–152. [Google Scholar] [CrossRef]
- Yang, K.H.; Jung, Y.B.; Cho, M.S.; Tae, S.H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J. Clean. Prod. 2015, 103, 774–783. [Google Scholar] [CrossRef]
- Boutlikht, M.; Douadi, A.; Khitas, N.E.H.; Messai, A.; Hebbache, K.; Belebchouche, C.; Smarzewski, P.; Tawfik, T.A. Optimizing of Self-Compacting Concrete (SCC): Synergistic Impact of Marble and Limestone Powders—A Technical and Statistical Analysis. Buildings 2025, 15, 1043. [Google Scholar]
- Arel, H.Ş. Recyclability of Waste Marble in Concrete Production. J. Clean. Prod. 2016, 131, 179–188. [Google Scholar] [CrossRef]
- Chakkor, O. Durability and Microstructural Evaluation of Geopolymer Mortars Exposed to Sulphuric Acid Using Industrial By-Product Fillers. Polymers 2025, 17, 2310. [Google Scholar] [CrossRef]
- Boukhelkhal, A.; Benguit, A.; Lakhdar, A. Study of the Durability Against Acid Sulfuric Attack and Accelerated Carbonation of Eco-Efficient Self-Compacting Concrete Blended with Marble Powder. Int. J. Integr. Eng. 2025, 17, 241–255. [Google Scholar]
- Wang, Q.; Kunther, W.; Li, Y.; Visalakshi, T.; Gomasa, R.; Amroun, S.; De Souza, D.J.; Kasaniya, M.; Tole, I.; Li, X.; et al. Sulfate Attack Testing Approaches from Concrete to Cement Paste: A Review by RILEM TC 298-EBD. Mater. Struct. 2025, 58, 232. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, Z.G.; Kong, Y.N.; Li, L.H.; Rao, M.J. Acid Attack on Cement Paste Containing Limestone Powder. Adv. Mater. Res. 2013, 724, 1589–1592. [Google Scholar] [CrossRef]
- Kore, S.D.; Vyas, A.K.; Syed Ahmed Kabeer, K.I. A brief review on sustainable utilisation of marble waste in concrete. Int. J. Sustain. Eng. 2020, 13, 264–279. [Google Scholar] [CrossRef]
- Atabey, İ.İ.; Çelikten, S.; Canbaz, M. Chemical resistance of hardened mortar containing andesite and marble industry waste powder. Chall. J. Concr. Res. Lett. 2023, 14, 31–38. [Google Scholar] [CrossRef]
- Chahour, K.; Safi, B. Mechanical behavior and chemical durability of marble-based mortar: Application to panels subjected to punching. Constr. Build. Mater. 2020, 232, 117245. [Google Scholar] [CrossRef]
- Benguit, A.; Azzouz, L.; Boukhelkhal, A. Durabilité des bétons autoplaçants à base de poudre de marbre vis-à-vis des attaques sulfates, acides et carbonatation accélérée. In Proceedings of the 1st International Congress on Advances in Geotechnical Engineering and Construction Management ICAGECM’19, Skikda, Algeria, 9–10 December 2019; Volume I, pp. 66–72. [Google Scholar]
- Messaoudene, I.; Jauberthie, R. Marble Filler: Does It Retard the Dissolution of Cement Constituents? Int. Sch. Res. Not. 2011, 2011, 695123. [Google Scholar] [CrossRef]
- Toubal Seghir, N.; Mellas, M.; Sadowski, Ł.; Żak, A. Effects of marble powder on the properties of the air-cured blended cement paste. J. Clean. Prod. 2018, 183, 858–868. [Google Scholar] [CrossRef]
- Toubal Seghir, N.; Benaimeche, O.; Krzywiński, K.; Sadowski, Ł. Ultrasonic Evaluation of Cement-Based Building Materials Modified Using Marble Powder Sourced from Industrial Wastes. Buildings 2020, 10, 38. [Google Scholar] [CrossRef]
- Toubal Seghir, N.; Mellas, M.; Sadowski, Ł.; Krolicka, A.; Zak, A. The Utilization of Waste Marble Dust as a Cement Replacement in Air-Cured Mortar. Sustainability 2019, 11, 2215. [Google Scholar] [CrossRef]
- Toubal Seghir, N.; Mellas, M.; Sadowski, Ł.; Krolicka, A.; Zak, A. The Effect of Curing Conditions on the Properties of Cement- Based Composites Blended with Waste Marble Dust. JOM-J. Miner. Met. Mater. Soc. 2019, 71, 1002–1015. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Hewlett, P.; Liska, M. (Eds.) Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- ASTM C109/C109 M-99; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). Annual Book of ASTM Standards 4; ASTM International: West Conshohocken, PA, USA, 1999.
- Panchal, V.C.; Vesmawala, G.R. Durability Performance of Binary and Ternary Blend Self-Compacting Concrete Using Waste Marble Powder and Lime Powder. Int. J. Eng. Adv. Technol. 2020, 9, 3381–3387. [Google Scholar]
- Amar, B.; Boukhelkhal, A.; Lakhdar, A. Durability self-compacting mortar under external chemical attacks based on composite binder. Cem.-Wapno-Beton = Cem. Lime Concr. 2024, 29, 71–81. [Google Scholar] [CrossRef]
- Kirgiz, M.S. Advancements in mechanical and physical properties for marble powder-cement composites strengthened by nanostructured graphite particles. Mech. Mater. 2016, 92, 223–234. [Google Scholar] [CrossRef]
- Kashyap, V.S.; Sancheti, G.; Yadav, J.S. Durability and microstructural behavior of Nano silica-marble dust concrete. Clean. Mater. 2023, 7, 100165. [Google Scholar]
- Abbas, M.M.; Muntean, R. Marble Powder as a Sustainable Cement Replacement: A Review of Mechanical Properties. Sustainability 2025, 17, 736. [Google Scholar] [CrossRef]
- SAli, S.; Ahmad, S.; Ullah, I. Discover Civil Engineering Utilization of waste marble dust as cement and sand replacement in concrete. Discov. Civ. Eng. 2024, 1, 15. [Google Scholar]
- Hashem, F.S.; Amin, M.S.; El-Gamal, S.M.A. Improvement of acid resistance of Portland cement pastes using rice husk ash and cement kiln dust as additives. J. Therm. Anal. Calorim. 2013, 111, 1391–1398. [Google Scholar]
- Zhang, Q.; Ye, G. Quantitative analysis of phase transition of heated Portland cement paste. J. Therm. Anal. Calorim. 2013, 112, 629–636. [Google Scholar]
- Jennings, H.M. A model for the microstructure of calcium silicate hydrate in cement paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Jennings, H.M. Refinements to colloid model of C-S-H in cement: CM-II. Cem. Concr. Res. 2008, 38, 275–289. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Mirmoghtadaei, R.; Shen, L.; Hargraves, J. A New Method to Predict Final Products of Red Mud-Slag-Based Alkali-Activated Materials Using Complete Phase Analysis of Precursors. Sustainability 2023, 15, 3473. [Google Scholar] [CrossRef]
- Asghari-Kaljahi, E.; Mansouri, H.; Hoseinzadeh, Z. Experimental study on the stabilization of fine grained soils by steel furnace slag and lime for using as subbase material. Int. J. Pavement Res. Technol. 2024, 1–15. [Google Scholar] [CrossRef]
- Huo, G.; Jiang, X.; Sun, X.; Li, H.; Shi, H. Performance of high-belite calcium sulfoaluminate cement subjected to hydrochloric acid and sulfuric acid. Front. Mater. 2024, 10, 1282919. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd-Elmoaty, A.E.M.; Hassan, H.H. Utilization of crushed clay brick in concrete industry. Alex. Eng. J. 2014, 53, 151–168. [Google Scholar] [CrossRef]
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials—A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Berger, F.; Bogner, A.; Hirsch, A.; Ukrainczyk, N.; Dehn, F.; Koenders, E. Thermodynamic Modeling and Experimental Validation of Acetic Acid Attack on Hardened Cement Paste: Effect of Silica Fume. Materials 2022, 15, 8355. [Google Scholar] [CrossRef]
- Ergün, A. Effects of the usage of diatomite and waste marble powder as partial replacement of cement on the mechanical properties of concrete. Constr. Build. Mater. 2011, 25, 806–812. [Google Scholar] [CrossRef]
- Ulubeyli, G.C.; Artir, R. Properties of Hardened Concrete Produced by Waste Marble Powder. Procedia-Soc. Behav. Sci. 2015, 195, 2181–2190. [Google Scholar] [CrossRef]
- Makhloufi, Z.; Bederina, M.; Bouhicha, M.; Kadri, E.H. Effect of mineral admixtures on resistance to sulfuric acid solution of mortars with quaternary binders. Phys. Procedia 2014, 55, 329–335. [Google Scholar] [CrossRef]
- Ercikdi, B.; Külekci, G.; Yilmaz, T. Utilization of granulated marble wastes and waste bricks as mineral admixture in cemented paste backfill of sulphide-rich tailings. Constr. Build. Mater. 2015, 93, 573–583. [Google Scholar] [CrossRef]
- Sadek, D.M.; El-Attar, M.M.; Ali, H.A. Reusing of Marble and Granite Powders in Self-Compacting Concrete for Sustainable Development. J. Clean. Prod. 2016, 121, 19–32. [Google Scholar] [CrossRef]
- Chavez Panduro, E.A.; Torsæter, M.; Gawel, K.; Bjørge, R.; Gibaud, A.; Bonnin, A.; Schlepütz, C.M.; Breiby, D.W. Computed X-ray Tomography Study of Carbonate Precipitation in Large Portland Cement Pores. Cryst. Growth Des. 2019, 19, 5850–5857. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Attigobe, E.K.; Rizkalla, S.H. Response of Concrete to Sulfuric Acid. ACI Mater. J. 1988, 85, 481–488. [Google Scholar] [CrossRef]
- Monteny, J.; De Belie, N.; Taerwe, L. Resistance of Different Types of Concrete Mixtures to Sulfuric Acid. Mater. Struct. 2003, 36, 242–249. [Google Scholar] [CrossRef]
- Dillard, R.J.; Murray, C.D.; Deschenes, R.A. Belitic calcium sulfoaluminate cement subjected to sulfate attack and sulfuric acid. Constr. Build. Mater. 2022, 343, 128089. [Google Scholar] [CrossRef]
- Tsubone, K.; Yamaguchi, Y.; Ogawa, Y.; Kawai, K. Deterioration of Concrete Immersed in Sulfuric Acid for a Long Term. Key Eng. Mater. 2016, 711, 659–664. [Google Scholar] [CrossRef]
- Monteny, J.; Vincke, E.; Beeldens, A.; De Belie, N.; Taerwe, L.; Van Gemert, D.; Verstraete, W. Chemical, microbiological, and in situ test methods for biogenic sulfuric acid corrosion of concrete. Cem. Concr. Res. 2000, 30, 623–634. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the carbonation of concrete with supplementary cementitious materials: A critical review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Wang, T.; Yang, W.; Zhang, J. Experimental Studies on Mechanical Properties and Microscopic Mechanism of Marble Waste Powder Cement Cementitious Materials. Crystals 2022, 12, 868. [Google Scholar] [CrossRef]
- Abbas, Y.; Djebien, R.; Toubal Seghir, N. Utilization of marble waste as fine aggregate in the composition of high-performance concrete containing mineral additions. Can. J. Civ. Eng. 2024, 52, 45–59. [Google Scholar] [CrossRef]
- Ingram, K.D.; Daugherty, K.E. A Review of Limestone Additions to Portland Cement and Concrete. Cem. Concr. Compos. 1991, 13, 165–170. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Richardson, I.G. The Calcium Silicate Hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Carde, C.; François, R. Effect of the Leaching of Calcium Hydroxide from Cement Paste on Mechanical and Physical Properties. Cem. Concr. Res. 1997, 27, 539–550. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Yu, X.; Huang, J. Decalcification of Calcium Silicate Hydrate (C-S-H) under Aggressive Solution Attack. Constr. Build. Mater. 2022, 342, 127988. [Google Scholar] [CrossRef]
- Das, K.K.; Wu, X.; Noh, G.; Lee, J.H.; Jang, J.G. Effect of acid attack coupled with elevated temperatures on carbonation-cured calcium sulfoaluminate and ordinary Portland cement paste. Case Stud. Constr. Mater. 2025, 23, e05150. [Google Scholar] [CrossRef]
- Kovalčíková, M.; Eštoková, A. Leaching of calcium and silicon from cement composites in the aggressive. Pollack Period. 2014, 9, 123–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).