Abstract
Nanomaterials play a beneficial role in regulating the function of cement-based materials. The effects and mechanism of graphene oxide (GO) on foam behavior in solutions and air-entraining behavior of cement mortar were studied, and its effect on the microstructure of cement mortar was also investigated. The results show that a synergy between GO’s hydrophobicity and the air-entraining agent’s hydrophobic chains drove more agent molecules to adsorb onto the GO surface, subsequently spreading and aggregating across the bubbles. GO effectively assisted the air entraining agent to refine the bubble size, improved the bubble stability of aqueous solutions, and had excellent air entraining performance in the fresh cement mortar, as well as the optimum air-void adjustment performance of hardened cement mortars. With the addition of 0.4‰ GO, the loss rate of gas content in the GO mixed mortar was 10.3%, which was 55.8% lower than that when only using AEA. The addition of 0.4‰ of GO effectively increased the volume fraction of the cement mortar system. GO reduced the pore volume in the mortar through the filling effect and nucleation effect to reduce the total porosity and refine the microstructure of the mortar.
1. Introduction
Cement-based materials are porous structural materials composed of hardened cement slurry and aggregate, and the porosity structure directly affects the workability and durability of concrete [1,2,3,4]. The appropriate introduction of small, uniform and airtight bubbles can not only improve the workability of the mix, but also eliminate the segregation and bleeding of newly mixed concrete [5]. It is more conducive to improving the durability of hardened concrete such as freeze–thaw resistance, medium penetration resistance and structural stability [6,7]. Therefore, the stability of air bubbles introduced into the freshly mixed cement-based materials is very important.
In recent decades, with people’s in-depth understanding of the application of nanomaterials in cement-based materials, research on the use of nanomaterials to improve the performance of cement-based materials has emerged rapidly. Nanomaterials can fill some micropores in cement sand, improve the pore structure, and provide a new research idea for improving the mechanical properties and durability of cement-based materials. And some theoretical achievements have been made [8,9,10]. Among them, Graphene Oxide (GO) has undergone extensive research because of its unique large thick ring structure, rich active oxygen-containing functional groups, its own enhanced physical properties, and excellent characteristics of better dispersion in water than other nanomaterials (such as SiO2) [11]. In the existing literature, many scholars have added GO to cement-based materials, hoping to utilize the excellent mechanical properties of GO to enhance the mechanical properties and freeze–thaw resistance of cement-based materials [12,13]. Zeng et al. [14] found that 0.03 wt% GO can increase the compressive strength of mortar by 41.9%, which provided a basis for utilizing GO materials to enhance the mechanical strength of cement-based materials. Cheng, Z.J.N. et al. [15] prepared a fly ash material coated with graphene oxide for use as an additive; the coated GO could then generate nucleation effects to promote the hydration process and provide pore-infilling effects to decrease the interfacial transition zone width (about 12.5%) and the crack ratio in the interfacial transition zone (over 10%). Compared with the plain cement pastes, the workability, mechanical strength, and impermeability of the GO-coated fly ash modified cementitious slurry can be significantly reinforced up to 21.6%, 38.4% and 106.4%, respectively. M.J. Mahmoodi et al. [16] revealed that graphene oxide nanosheets were investigated to enhance the drying shrinkage strain of concrete, which provided important data for utilizing GO to enhance the volume stability and durability of cement materials. Sun et al. [17] showed that the inclusion of GO effectively reduced the thickness and volume fraction of interface transition zone (ITZ) by 11.64% and 11.77%, and reduced the porosity of slurry, thus making the overall microstructure of mortar more uniform. Indukuri et al. [18] pointed out that when GO was added at 0.04 wt%, the 28-day compressive strength was 46.6% higher than that of ordinary cement slurry, and the flexural strength was 75% higher, which provided direct evidence that GO can significantly enhance the toughness of cement materials. Graphene oxide enhanced the permeability barrier of cement mortar, greatly reduced the water permeability of cement composite, and thus improved its durability. L. Djenaoucine et al. [19,20] reduced the corrosion rate using GO as a corrosion inhibitor, and also found that the effect of GO as a nucleation site had a positive effect on the cement paste nano-properties at advanced ages. They revealed that the flexural strength of cement mortars with the combination of GO and superplasticizer (SP) after 28 days increased by 21.86%, compared with reference mortar [21]. In addition, M. Izadifar et al. [22,23] discovered that there is a close interaction between reduced graphene oxide (rGO) and the hydration product of cement, namely hydrated calcium silicate (CSH). This indicates that rGO has significant application potential in cement-based materials.
At present, the studies on GO in the field of cement-based materials mainly focus on the mechanical properties, durability properties and microstructure of cement-based materials, while the studies on bubble behavior in cement-based materials are rarely involved. Most of the existing research has focused on the effects of graphene and modified graphene materials on the microstructure, mechanical properties and durability of cement, but there is a lack of research on the influence of bubble behavior. This is mainly due to the difficulty in testing bubble behavior and the scarcity of teams with such research conditions. In this paper, GO, as a two-dimensional flexible material with high regularity and large specific surface area, assisted air entraining agent to regulate the porosity of cement mortar. The effects of GO on foam behavior, porosity content and porosity microstructure of mortar were systematically investigated from the aspects of bubble in solution, gas content of freshly mixed mortar and porosity parameters of hardened mortar. This study aims to explore the synergistic effect between GO and AEA through the following aspects: (1) the foaming properties of the mixed solution; (2) the influence on the air entraining behavior, and (3) the resulting microstructure of the cement mortar. It provides technical guidance and scientific new way for the regulation and optimization of bubble structure in the field of cement-based materials.
2. Materials and Methods
2.1. Materials
Cement: PO 42.5 was obtained from Jiangnan-xiaoyetian Cement Co., Ltd. (Nanjing, China), whose chemical composition and physical and mechanical properties are shown in Table 1 and Table 2; A Chinese standard sand with a nominal grain size of 0.08–2 mm was used as the fine aggregate.; Standard hard water: The total content of calcium ions and magnesium ions is 1500 mg/L, The mass ratio of calcium ions to magnesium ions is 2.4 to 1.0; Air entraining agent (AEA): Molecular formula: (C2H4O)nC12H26O4SNa were purchased from Aladdin Chemical Co., Ltd. (Shanghai, China); GO (purity > 99%, single layer ratio of 99%) were purchased from Nanjing XFnano (Nanjing, China), and the morphology is shown in Figure 1.

Table 1.
Chemical composition and mineral composition of cement (w%).

Table 2.
Physical and mechanical properties of cement.
Figure 1.
The topography of GO.
2.2. Methods
2.2.1. Test of Bubble Properties
The bubble size, quantity and image of the prepared solution were measured at 25 °C using DFA100 dynamic foam analyzer from Krüss (Hamburg, Germany). Parameter setting: bubbling 1.5 min, stable bubble 18.5 min, a total of 20 min, gas flow of 0.2 L/min. Test procedure: Firstly, 50 g 0.3 wt% AEA solution was configured as the control sample. By ultrasound (5 min), 0.05 g, 0.1 g and 0.25 g GO were uniformly dispersed in 50 g water and 0.1 wt%, 0.2 wt% and 0.5 wt% GO dispersions were configured. The mixed liquid of AEA and GO is prepared by mixing the aforementioned 0.3 wt% AEA solution with the corresponding GO suspension in the exact quantities as specified in the design. Before configuration, each sample was tested in parallel 3 times, and the intermediate effective value was taken as the final test result.
2.2.2. Test of Performance of Fresh Mixed Mortar
The gas content of fresh mixed mortar was tested at the beginning and after 1 h. Experimental conditions: 675 g cement; 1350 g standard sand; water-cement ratio 0.5; AEA dosage is 0.2‰ of cement mass; the dosage of GO is 0.10‰, 0.20‰ and 0.40‰ of cement mass, respectively; stirring time: slow stirring for 60 s, standing for 90 s, and then fast stirring for 60 s, a total of 210 s.
2.2.3. Test of Performance of Hardened Mortar
Hardened mortar blocks were obtained after 14 days of standard curing of the mortar specimens. The test blocks were cut into 1 cm thin slices (5 cm × 3 cm × 1 cm), and the air voids of the hardened mortar were tested by X-ray tomography (X-ray CT, ZEISS Context) (Dublin, OH, USA), including gas content of the hardened mortar, average bubble diameter and aperture distribution. Three-dimensional air void structure reconstruction images were obtained. The test parameters are set as follows: voltage: 70 KV, power: 7 W, resolution: 12 μm, exposure time: 0.14 s.
The above hardened mortar specimens doped with AEA and AEA + 0.20‰GO were cut into about 1 cm cubes. Then these small cubes were gold-plated and then bonded to the test platform using conductive glue. These small cubes were tested and observed by FEI Quanta 250 scanning electron microscope (SEM) (Waltham, MA, USA). The SEM images of the specimens and the element composition and content analysis of microregions were obtained. N2 adsorption and desorption curves, specific surface area and pore volume were measured by TriStarII 3020 automated specific surface and porosity analyzer (BET) (Noklossor, GA, USA). The samples were ground into powder and passed through a 200-mesh sieve. The portion of powder that passed through the sieve was collected and degassed at 120 °C for 2 h before analysis.
3. Results and Discussions
3.1. Effect of GO on Bubble Behavior in Solution
Firstly, the bubble behavior of the solution with GO in the air entraining agent was studied. Figure 2 shows the bubble size and number of the solution at the end of 1.5 min bubbling, at the middle and the end of 20 min test, as well as the foam height at the end of 1.5 min bubbling. As can be seen from Figure 2a, the bubble size introduced into the aqueous solution with GO is much smaller than that into the control solution without GO at 1.5 min, 10 min and 20 min, and the more GO is added, the smaller the bubble size will be introduced into the aqueous solution. As can be seen from Figure 2b, the number of bubbles introduced into the aqueous solution with GO is higher than that in the control solution without GO, and the more GO is added, the more bubbles will be introduced into the aqueous solution. As can be seen from Figure 2c, the foam height of the air entraining agent solution after adding GO is higher than that of the control solution without adding GO, and it increases with the increase in GO content, indicating that the air entraining effect of the air entraining agent solution after adding GO is improved. The smaller the size and number of bubbles in the solution, the finer the foam and the better the stability.
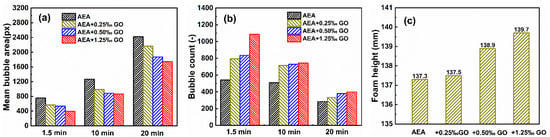
Figure 2.
Mean bubble areas (a), bubble counts (b) and foam height (c) of the air entraining agents at different times.
Figure 3 more intuitively shows the bubble shape and size distribution of the measured sample at 1.5 min, 10 min, and 20 min. The bubbles introduced by the control group and the two aqueous solutions after adding GO gradually increased in size and decreased in number with the extension of bubble stabilization time. However, the bubbles introduced into the aqueous solution after adding GO were significantly more and finer than those in the control group at 10 min and 20 min, and the bubble stability was better, and the greater the amount of GO, the more obvious the effect was. It shows that GO can effectively assist the entraining agent to refine the bubble size of the solution and improve the stability of the solution bubble.
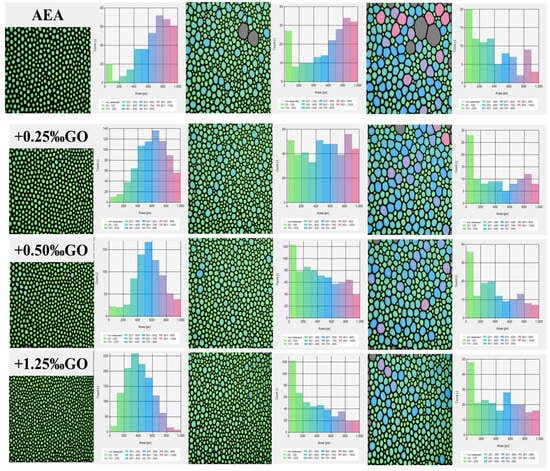
Figure 3.
Bubble photographs in a unit area of the air entraining agents at 1.5 min (left), 10 min (middle) and 20 min (right)).
3.2. Effect of GO on Gas Content of Freshly Mixed Mortar
The air entraining and bubble stabilization performance of GO assisted air entraining agent in cement mortar was further explored. The initial and 1 h gas content of mortar was shown in Figure 4. The initial gas content of the mortar in the control group with AEA only was about 13.3%, and the initial gas content of the mortar with 0.1‰, 0.2‰ and 0.4‰GO was 14.6%, 17.8% and 22.3%, respectively. After 1 h, the loss rate of gas content in the control group was 23.3%, while the loss rate of gas content in the GO mixed mortar was 21.2%, 14.1% and 10.3%, respectively. It can be clearly seen that due to the addition of GO, the loss rate of 1 h content in the cement mortar has decreased by more than half (based on the comparative calculation, the specific value of the relative decrease in the loss rate of gas content is 55.8%). It shows that GO can obviously assist the air entraining agent in mortar and stabilize the air content of mortar, and the higher the dosage, the more obvious the effect of assisting air entraining.
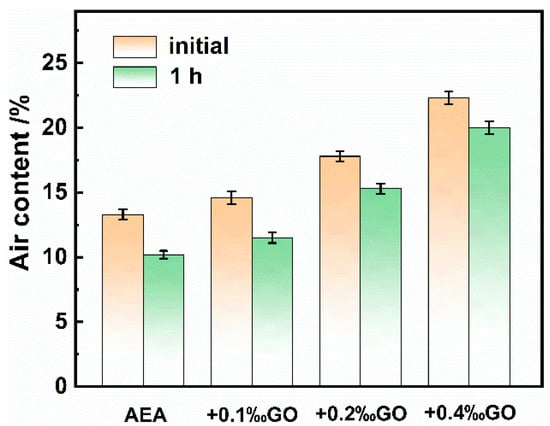
Figure 4.
Air contents of the fresh cement mortars of the air entraining agents.
3.3. Effect of GO on Air Void of Hardened Mortar
Table 3 lists the air void parameters of hardened mortar specimens tested by CT. The gas content of hardened mortar in the control group with only AEA air entraining agent is 7.9%, while the gas content of hardened mortar with GO is higher than that in the control group (10.3%, 13.7% and 18.4%, respectively), which is consistent with the law of gas content of freshly mixed mortar discussed in Section 3.2. This means that the addition of 0.4‰ of GO effectively increased the volume fraction of the cement mortar system. The volume fraction was 132.9% higher than that of the control sample without GO addition. In addition, the total number and total air void of the mortar specimens in the test group doped with GO were significantly more than those in the control group, and the mortar specimens with higher GO content had the largest number.

Table 3.
Air-void parameters of the hardened cement mortar by CT.
Figure 5 shows the number of air voids and the proportion distribution of air voids in hardened mortar specimens. As can be seen from Figure 5a, the proportion of small air voids less than 200 μm in hardened mortar specimens doped with GO is larger than that in the control group only doped with AEA; on the contrary, the proportion of large air voids greater than 200 μm is smaller, and the number of small air voids in mortar specimens with high GO content is greater. As can be seen from Figure 5b, the proportion of air voids less than 200 μm in the GO doped hardened mortar specimen is larger than that in the control group; on the contrary, the proportion of air voids greater than 200 μm is smaller than that in the control group. The above results show that the hardened mortar specimens doped with GO have more hardened air voids and smaller diameter, and the change law is consistent with the change law of bubble properties in the solution.
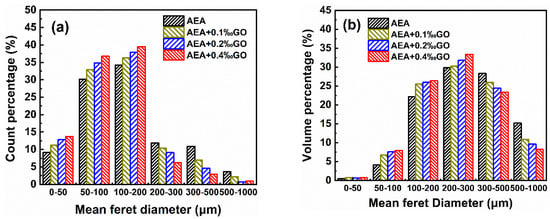
Figure 5.
Count percentage (a) and volume percentage (b) of the hardened cement mortar specimens contained air entraining agents and GO.
Figure 6 shows the image of all air voids extracted by CT from mortar specimens selected in the same cube area. It can be seen more and more directly that hardened mortar specimens doped with GO introduced more and more hardened air voids than those in the control group, and hardened mortar specimens with higher GO content had better air voids. The above results show that GO can effectively assist the air entraining agent to refine the air voids in the hardened mortar, make the air voids small and fine, and improve the stability of the bubbles in the mortar.
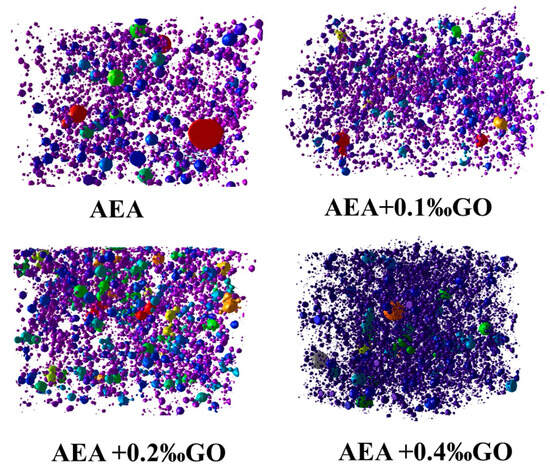
Figure 6.
Three-dimensional air-void distribution of the hardened cement mortar specimens contained air entraining agents and GO.
The influence of GO on the total porosity of hardened mortar specimens was further studied. Figure 7 shows the N2 absorption and desorption curve, specific surface area and total empty capacity of mortar specimens doped with AEA and AEA + 0.2‰GO. As can be seen from Figure 7a, the adsorption isotherms of samples in both groups are type II isotherms (S-type isotherms), and the adsorption amount of nitrogen in mortar specimens doped with AEA + 0.2‰GO is smaller than that in the AEA control group, indicating that the addition of GO reduces the internal pore channels, micropore capacity, porosity and denser structure of mortar specimens. As can be seen from Figure 7b, it further indicates that GO reduces the specific surface area and total pore capacity in the mortar, resulting in lower porosity. This is because GO, as a nanomaterial, can promote the hydration reaction rate and hydration effect of cement to generate C-S-H gel through filling effect and nucleation effect, and provides more nucleation sites for C-S-H gel to generate more C-S-H gel, so that it can be converted into a dense network structure and refine the micro-pores and micro-cracks inside the mortar. The number of harmful pores in cement mortar can be reduced, the internal microstructure of mortar can be improved, and the porosity of cement-based materials can be reduced [24,25].
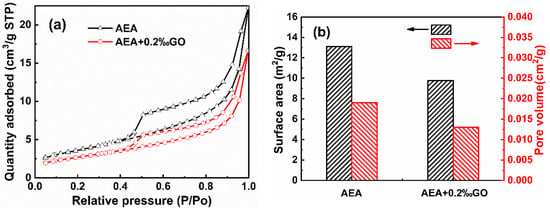
Figure 7.
N2 adsorption–desorption curves (a) and physical adsorption data (b) of the hardened cement mortar specimens.
3.4. Synergistic Mechanism of GO and Air Entraining Agent
In order to explore the mechanism of the influence of the self-assembly system of AEA and GO on bubbles, we used SEM and EDX tests to observe the air void structure in the cement mortar and the distribution of elements on the air void walls. By measuring the distribution abundance of carbon elements at different positions around the air voids, the locations where AEA and GO are enriched can be inferred. This, in turn, helps to explain why, when AEA and GO are used together, the air void structure of the material is significantly optimized. Figure 8 shows the SEM image and micro-energy spectrum of mortar specimens doped with AEA and AEA + 0.2‰GO. Quantitative analysis of carbon element was carried out on the stomatal wall and outside the stomatal area of hardened mortar test block. Table 4 lists the carbon element content in different regions of mortar specimens doped with AEA and AEA + 0.2‰GO. As can be seen from Table 4, the mass fraction of carbon element on the air void wall of the mortar specimen doped with AEA air entraining agent is 6.04%, while the mass fraction of carbon element outside the air void wall is 0.45%. The large difference in the content of carbon element on the air void wall and the outside of air void is mainly due to the fact that the AEA air entraining agent is self-assembled and directional arrangement on the gas–liquid interface of the bubbles in the newly mixed mortar through the hydrophobic association between the hydrophobic chains, so that the bubbles are easier to generate and stabilize. The molecular formula of AEA is (C2H4O)nC12H26O4SNa, which contains rich carbon element. Therefore, the difference in the content of carbon element on and outside the air void wall can indicate that the air entraining agent is mainly concentrated in the gas–liquid interface of the bubbles in the newly mixed mortar. The mass fraction of carbon element on the air void wall of the mortar specimen mixed with 0.2‰ GO is 10.05%, while the mass fraction of carbon element outside the air void wall is 1.15%. The content of carbon element on the air void wall is much higher than that outside the air void wall, which proves that the extra GO with rich carbon element is mainly distributed in the gas–liquid interface of the bubbles compared with the control group. It is used in conjunction with air entraining agents to make bubbles easier to form and stabilize.
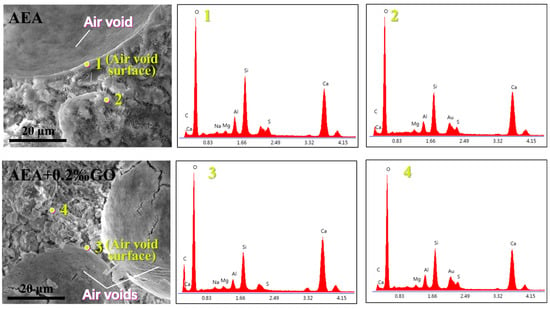
Figure 8.
The SEM images and EDS patterns of the hardened cement mortar specimens.

Table 4.
Carbon element content of energy spectrum probe position.
Air entraining agent is a surfactant molecule with hydrophobic chain segment at one end and hydrophilic group at the other end. It is amphiphilic and can be self-assembled and oriented at the gas–liquid interface or oil-water interface, thereby reducing the surface tension of aqueous solution [26] and making it easier for bubbles to form and stabilize. GO is a two-dimensional flexible material with high regularity and large specific surface area, which has the advantage of being centrally arranged at liquid–gas, liquid–liquid and liquid-solid interfaces [27]. When GO is added to the air entraining agent, due to the large thick ring structure and π-π stacking in the molecular structure of GO, GO itself has a strong hydrophobicity. Through hydrophobic self-assembly between water and the hydrophobic chain of the air entraining agent [28,29,30], more air entraining agent molecules are adsorbed on the surface of GO, and multiple “hydrophobic-hydrophilic” surfactant molecular structures are formed. This self-assembled structure spreads out and aggregates at the gas–liquid interface to form a continuous network structure, increase the thickness of the bubble liquid film, and make the surface of the bubble have strong viscoelasticity, thus improving the stability of the bubble, as shown in Figure 9. Furthermore, GO and air entraining agent play a synergic role to refine the bubble size of the solution, improve the stability of the solution bubble, and have excellent air entraining performance and porosity adjustment performance of hardened mortar in the newly mixed mortar.
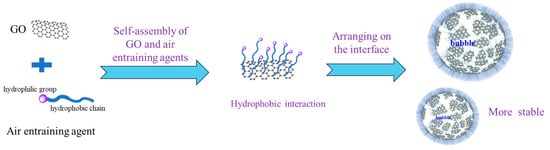
Figure 9.
The synergistic effect of GO and entraining agents.
4. Conclusions
The mechanism of synergism between GO and air entraining agent was investigated. Due to its excellent hydrophobicity and flexibility, GO is self-assembled with the hydrophobic chain of the air entraining agent through hydrophobic interaction, so that more air entraining agent molecules are adsorbed on the surface of GO, forming multiple “hydrophobic-hydrophilic” surfactant molecular structures, spreading and aggregating on the surface of the bubble, increasing the liquid film thickness of the bubble, and thus improving the stability of the bubble. GO effectively assists the air entraining agent to refine the bubble size of the solution, improve the stability of the solution bubble, and has excellent air entraining performance and stable gas content in the newly mixed mortar, so that the air voids in the hardened mortar are small and fine; And the greater the dosage of GO is, the more obvious the effect takes place. Thanks to the synergy between GO and AEA, we can expect that these materials will be widely applied to enhance the properties of cement-based materials in the future, such as mechanical properties, freeze–thaw resistance, and water permeability resistance, etc. Although the price of GO is relatively high, its content is very low. It has significant application value in some high-end cement-based material applications (such as ultra-high-performance concrete, artistic concrete, impact-resistant protective concrete, marine engineering concrete, etc.).
Author Contributions
Conceptualization, M.Q.; methodology, M.Q.; investigation, M.Q., G.C., Y.F., Y.L. and M.S.; data curation, M.Q., G.C., Y.F. and Y.L.; writing—original draft preparation, M.Q., G.C., Y.F. and Y.L.; writing—review and editing, M.S.; supervision, M.Q.; project administration, M.Q.; funding acquisition, M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 52578292, 52178213) and High-level Talent Research Start-up Project of Nanjing Xiaozhuang University.
Data Availability Statement
The original data is provided under the requested conditions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, R.; Hu, Z.; Li, Y.; Wang, K.; Zhang, H. Review on the deterioration and approaches to enhance the durability of concrete in the freeze–thaw environment. Constr. Build. Mater. 2022, 321, 126371. [Google Scholar] [CrossRef]
- Netinger Grubeša, I.; Marković, B.; Vračević, M.; Tunkiewicz, M.; Szenti, I.; Kukovecz, Á. Pore structure as a response to the freeze/thaw resistance of mortars. Materials 2019, 12, 3196. [Google Scholar] [CrossRef]
- Shuldyakov, K.; Trofimov, B.; Kramar, L. Stable microstructure of hardened cement mortar-a guarantee of the durability of concrete. Case Stud. Constr. Mater. 2020, 12, e00351. [Google Scholar]
- Li, W.; Zhou, Y.; Yin, J.; Peng, Y.; Wang, Y.; Tang, S.; Shi, Y.; Wang, Y.; Wang, L. Study on thermodynamics based hydration of low-heat Portland cement and compensation effect of magnesium oxide admixtures. J. Zhejiang Univ.-SCIENCE A 2025, 26, 305–319. [Google Scholar] [CrossRef]
- Yu, F.; Lou, Z.; Yan, N. Effect of the compounding of an antifoaming agent and a viscosity modifying agent on the frost resistance of mold bag concrete. Constr. Build. Mater. 2021, 308, 125016. [Google Scholar] [CrossRef]
- Shah, H.A.; Yuan, Q.; Zuo, S. Air entrainment in fresh concrete and its effects on hardened concrete-a review. Constr. Build. Mater. 2021, 274, 121835. [Google Scholar] [CrossRef]
- Şahin, Y.U.Ş.A.; Akkaya, Y.; Boylu, F.; Taşdemir, M.A. Characterization of air entraining admixtures in concrete using surface tension measurements. Cement Concrete Comp. 2017, 82, 95–104. [Google Scholar] [CrossRef]
- Ren, J.; Luo, X.; Bai, R.; Pan, C.; Zhang, J. Pore characteristics of different phase in nano-modified concrete and their influences on the compressive strength. J. Build. Eng. 2022, 46, 103784. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Tolkou, A.K.; Efstathiou, S.; Rahdar, A.; Favvas, E.P.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials in cementitious composites: An update. Molecules 2021, 26, 1430. [Google Scholar] [CrossRef] [PubMed]
- Makul, N. Advanced smart concrete-a review of current progress, benefits and challenges. J. Clean. Prod. 2020, 274, 122899. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, L.; Zhao, W.; Lazaro, S.A.M.; Li, X.; Tang, S. Dielectric and mechanical properties of cement pastes incorporated with magnetically aligned reduced graphene oxide. Dev. Built Environ. 2024, 18, 100471–100481. [Google Scholar] [CrossRef]
- Zeng, H.; Lai, Y.; Qu, S.; Qin, Y. Graphene oxide-enhanced cementitious materials under external sulfate attack: Implications for long structural life. ACS Appl. Nano Mater. 2020, 3, 9784–9795. [Google Scholar] [CrossRef]
- Zeng, H.; Lai, Y.; Qu, S.; Yu, F. Effect of graphene oxide on permeability of cement materials: An experimental and theoretical perspective. Constr. Build. Mater. 2021, 41, 102326. [Google Scholar] [CrossRef]
- Zeng, H.; Lai, Y.; Qu, S.; Yu, F. Exploring the effect of graphene oxide on freeze-thaw durability of air-entrained mortars. Constr. Build. Mater. 2022, 324, 126708. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, Y.; Wu, J.; Guo, X.; Chen, W.; Gao, Y. Graphene oxide-coated fly ash for high performance and low-carbon cementitious composites. J. Mater. Res. Technol. 2023, 25, 6710–6724. [Google Scholar] [CrossRef]
- Mahmoodi, M.J.; Khamehchi, M.; Safi, M. Comprehensive modelling of drying shrinkage strain of graphene oxide nanosheet concrete. Adv. Cem. Res. 2024, 36, 1–14. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Z.; Ling, L.; Memon, S.A.; Ren, J.; Liu, B.; Xing, F. Influence of graphene oxide on interfacial transition zone of mortar. J. Nanomater. 2020, 11, 8919681. [Google Scholar] [CrossRef]
- Indukuri, C.S.R.; Nerella, R.; Madduru, S.R.C. Workability, microstructure, strength properties and durability properties of graphene oxide reinforced cement paste. Aust. J. Civ. Eng. 2020, 18, 73–81. [Google Scholar] [CrossRef]
- Djenaoucine, L.; Argiz, C.; Picazo, Á.; Moragues, A.; Galvez, J.C. The corrosion-inhibitory influence of graphene oxide on steel reinforcement embedded in concrete exposed to a 3.5M NaCl solution. Cement Concrete Comp. 2025, 155, 105835. [Google Scholar] [CrossRef]
- Djenaoucine, L.; Picazo, A.; de la Rubia, M.A.; Galvez, J.C.; Moragues, A. Effect of graphene oxide on the hydration process and macro-mechanical properties of cement. Boletín Soc. Española Cerámica Vidrio 2024, 63, 294–303. [Google Scholar] [CrossRef]
- Djenaoucine, L.; Picazo, Á.; de la Rubia, M.Á.; Moragues, A.; Gálvez, J.C. Influence of Graphene Oxide on Mechanical Properties and Durability of Cement Mortar. Materials 2024, 17, 1445. [Google Scholar] [CrossRef]
- Izadifar, M.; Dolado, J.S.; Thissen, P.; Ukrainczyk, N.; Koenders, E.; Ayuela, A. Theoretical Elastic Constants of Tobermorite Enhanced with Reduced Graphene Oxide through Hydroxyl vs Epoxy Functionalization: A First-Principles Study. J. Phys. Chem. C 2023, 127, 18117–18126. [Google Scholar] [CrossRef]
- Izadifar, M.; Dolado, J.S.; Thissen, P.; Ayuela, A. Interactions between Reduced Graphene Oxide with Monomers of (Calcium) Silicate Hydrates: A First-Principles Study. Nanomaterials 2021, 11, 2248. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Ultrastable particle stabilized foams. Angew. Chem. Int. Ed. 2006, 45, 3526–3530. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Zhu, W.; Qin, W. Solubility study on the surfactants functionalized reduced graphene oxide. Colloid Surf. A 2018, 538, 79–85. [Google Scholar] [CrossRef]
- Yu, L.; Wu, R. Using graphene oxide to improve the properties of ultra-highperformance concrete with fine recycled aggregate. Constr. Build. Mater. 2020, 259, 120657. [Google Scholar] [CrossRef]
- Shan, G.C.; Lu, C.; Chen, J.; Gao, N.X.; Qiao, M.; Ran, Q.P.; Liu, J.P. Effect of air entraining agent on properties of aqueous solutions and concretes. J. Chin. Chem. Soc. 2020, 48, 1256–1262. (In Chinese) [Google Scholar]
- Shan, G.C.; Wu, J.Z.; Qiao, M.; Gao, N.X.; Chen, J.; Ran, Q.P.; Hong, J.X.; Liu, J.P. Influence of defoamer on interface behavior and effect of air entraining agent. J. Chin. Chem. Soc. 2020, 48, 638–643. (In Chinese) [Google Scholar]
- Shao, J.J.; Lv, W.; Yang, Q.H. Self-assembly of graphene oxide at interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Shen, F.; Chen, J.; Qiao, M.; Shan, G.; Gao, N.; Wu, Q.; Ran, Q.; Liu, J.; Han, F.; Han, B.; et al. Effect of particle properties on the resulting bubble quality and stability: From solution foam to air-entrained cement mortars. Constr. Build. Mater. 2024, 432, 136596. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).