Abstract
The morphology and types of inclusion, as well as the microstructure, fundamentally affect the properties of high-strength peritectic steel. Rare earth elements not only modify inclusions but also act on the transformation of the microstructure. In this paper, the evolution mechanism of yttrium for the inclusions and microstructure in high-strength peritectic steel was investigated through experimental testing and thermodynamic analysis. The results show that yttrium treatment can modify the main large-sized irregular inclusions into spherical or near-spherical rare earth inclusions, accompanied by a reduction in the number density, area fraction, average diameter, and aspect ratio of inclusions. The evolution route for the inclusions follows Al2O3 + MnS + Al2O3-MnS→Y2O3 + Y-O-S + Y-S + Y-O-S-MnS with yttrium addition. The microstructural characteristics of yttrium-free steel show significant differences from those of yttrium-containing steel. Compared to yttrium-free steel, the yttrium-0.015 wt.% steel shows a refined austenite structure with more uniform size distribution and the absence of grain boundary ferrite films. The Y2O3 and Y2O2S inclusions mainly formed in liquid steel were found along the austenite grain boundary to prevent the grain growth and the formation of ferrite films. Additionally, after adding rare earth yttrium, the fraction of high-angle grain boundaries (HAGBs) increases, together with a decrease in the fraction of low-angle grain boundaries (LAGBs) in steel. The research results can provide a theoretical basis for the application of adding rare earth yttrium to high-strength peritectic steel.
1. Introduction
High-strength steels are widely used in various engineering structures such as ships, automobiles, bridges, buildings, and oil platforms due to their various advantages. Most of these series steels are produced by continuous casting, while the high incidence of crack defects on continuous casting slabs often acts as the key factor affecting efficient continuous casting production []. Especially for peritectic steel, which has a carbon content range from 0.08 wt.% to 0.17 wt.%, the liquid steel and δ phase almost simultaneously disappear and transform into austenite after peritectic reaction, causing significant volume shrinkage, increasing the gap between the casting slab and mold, and greatly increasing crack sensitivity []. The high-frequency surface cracks on continuous casting slabs of high-strength peritectic steel are mainly caused by the weakening of austenite grain boundaries due to element segregation, inclusions, grain boundary ferrite films, etc. [,,]. The inclusions in high-strength steel are predominantly irregular Al2O3 and elongated MnS [,], which tend to initiate microcracks in high-stress parts [,,].
Inclusion modification has been proven to be an effective method for eliminating harmful large-sized and irregular inclusions [,]. Rare earth elements, with an extremely high capability to combine with sulfur and oxygen in steel, are widely used in inclusion modification. The formation and modification of inclusions after the addition of Ce, La, or their mixtures have been extensively studied. Tie et al. [] demonstrated that Ce2O3, CeAlO3, and CeAl11O18 were identified after adding Ce to high-strength steel with different Al contents. Liang et al. [] investigated the effect of Ce on inclusions in high-strength oil casing steel and reported that the evolution route of inclusions in the Ce-containing steel was Al2O3 + xCaO·yAl2O3→Ca-Ce-Al-O→CeAlO3 + CeAl11O18→CeAlO3→CeAlO3 + Ce2O3. Gong et al. [] concluded that primarily La–S, La–O–S, and other complex rare earth inclusions were formed in the ultrahigh-strength low-alloy steel with the addition of La. Hao et al. [] suggested that with a 0.014 wt.% compound of La and Ce in spring steel, the main MnS and Al2O3 inclusions disappeared, replaced by rare earth inclusions.
Moreover, rare earth elements can also significantly act on the transformation of the microstructure in steel. Gao et al. [] experimentally revealed that the mixture of La and Ce in H13 steel could enhance its impact toughness by grain refining. The research results of Cheng et al. [] similarly showed that the presence of rare earth La and Ce significantly refined and optimized the microstructure of Nb-containing micro-alloyed steel. Zhao et al. [] found that 0.06 wt.% Ce played a crucial role in reducing grain size and inhibiting needle-shaped Widmanstätten structures in low-alloy cast steel. Su et al. [] suggested that the addition of rare earth Ce inhibited the formation of proeutectoid ferrite of overcooled austenite and strengthened the transformation of bainite ferrite in wear-resistant steel. Zhao et al. [] reported that rare earth (RE) addition promoted a more homogeneous microstructure and a larger fraction of HAGBs in a Twinning-Induced Plasticity (TWIP) steel.
As mentioned above, many studies have been completed on the influence of Ce and La on the inclusion and microstructure of different steels. However, the application of more rare earth elements such as yttrium still needs in-depth exploration. Zhang et al. [] observed Y2O3-TiN, Y2O2S, Y2O3, and Y2O2S-TiN inclusions after adding Y to a stainless steel, and concluded that Y2O2S could reduce the corrosion resistance of the stainless steel, while Y2O3 could improve the corrosion resistance of the steel. Wang et al. [] experimentally studied the effect of yttrium treatment on Al2O3 inclusions, proposing that the evolution route of inclusions in Y-containing high carbon steel was Al2O3→Y2S3 + YAlO3 + Al2O3→Y2S3 + YAlO3 + Y2O2S + YAlO3 + Al2O3→Y2S3 + Y2O2S. Guo et al. [] revealed that the main kinds of rare earth inclusions were Y2O2S, YS, and Y2S3 in Y-containing grain-oriented silicon steel. Liu et al. [] focused on the effect of Ce-Y composite addition on inclusion evolution, concluding that Mg-Al-O oxides and composite inclusions were modified into rare earth oxides, sulfides, and oxysulfides in T91 heat-resistant steel. In terms of the influence of rare earth yttrium on the microstructure of steel, Chen et al. [] suggested that the area fraction of δ-ferrite increased with the content of Y in ferritic/martensitic steels. Zhong et al. [] investigated the effects of yttrium on the microstructure of 20MnSi steel, demonstrating that yttrium reduced martensitic transformation temperature and obtained the refined pearlitic lamellae. Hu et al. [] clarified that the addition of rare earth yttrium effectively inhibited the coarsening of austenite grains in an austenitic stainless steel.
Apparently, rare earth elements can fundamentally affect the inclusions and microstructure in different steels. However, there are significant differences in the behavior of inclusions and evolution of the microstructure in different types of steel. For high-strength peritectic steel, the presence of large-sized irregular inclusions, coarsened austenite structure, and ferrite films distribute along grain boundaries can damage the mechanical properties of steel. Currently, there is limited research focusing on the effects of adding rare earth elements on inclusion, austenite, and ferrite evolution in high-strength peritectic steel, as well as their interrelationships. Rare earth yttrium has a smaller atomic radius than Ce and La, and the diffusion coefficients of Y are one order of magnitude larger than that of La in bcc Fe. Furthermore, the densities of the formed Y-containing inclusions are relatively smaller than those of Ce- or La-containing inclusions. It can be inferred that the effects of rare earth Y on the inclusions and microstructure of steel will be significantly different from those of La or Ce. In this paper, the influence of rare earth yttrium on the characteristics of the inclusions and microstructure in high-strength peritectic steel was systematically investigated. Firstly, the variations in types, number densities, area fractions, average diameters, aspect ratio, and size distributions of inclusions in the studied steel with and without rare earth yttrium were quantitatively measured. Secondly, the transformation mechanism of inclusions in liquid steel and the subsequent cooling process were clarified by thermodynamic calculations. Finally, an optical microscope (OM, Oberkochen, Germany) and scanning electron microscope (SEM, Oberkochen, Germany) with energy dispersive spectrometer (EDS, Karlsruhe, Germany) and electron backscatter diffraction (EBSD, Karlsruhe, Germany) were utilized to characterize the microstructure in the Y-free steel and Y-containing steel. Additionally, the effect of rare earth inclusions on the microstructure evolution was revealed. This work strengthens the theoretical foundation for reducing quality defects on continuous casting slabs and enhancing the mechanical properties of high-strength peritectic steel for rare earth yttrium addition.
2. Materials and Methods
The experimental steels with an ingot size of 150 × 150 × 300 mm3 were prepared by vacuum induction melting; more details of feeding, heating, sampling, alloying, and casting were described by Xing et al. []. The specific compositions are listed in Table 1. Clearly, the main variation between the two heats of steel is not added or added with 0.015 wt.% rare earth yttrium. The content of yttrium, silicon, manganese, phosphorus, aluminum, niobium, and vanadium were measured by an inductively coupled plasma optical emission spectrometer. Inert gas fusion pulse/infrared absorption spectroscopy was used to analyze the total oxygen content. The sulfur and carbon contents were determined by a sulfur/carbon analyzer.

Table 1.
Chemical composition of tested steels (wt.%).
Each tested specimen with a size of 15 mm × 15 mm × 15 mm was obtained from the steel ingot by wire cutting at 1/4 distance from the top in the height direction. The samples for inclusion and microstructure analysis were obtained by roughly grinding, polishing finely, and etching accurately with 4% nitrate alcohol solution at room temperature. Analysis of morphology, elemental composition, and type of inclusions was carried out by SEM-EDS. To further reveal the quantity characteristics of the inclusions in the tested samples, the automatic non-metallic inclusion particle detection system Phenom Particle X Steel (Eindhoven, The Netherlands) was adopted and operated with a scanning area of each sample larger than 25 mm2. Similarly, OM was used to characterize the microstructures. The microstructural characteristics of the steels were also explored by using EBSD. The specimens for EBSD were prepared by grinding through a series of sandpapers and finish polishing by electro polishing in 10% perchloric acid in absolute alcohol for 150 s at 5 V.
3. Results and Discussion
3.1. SEM-EDS Analysis of Chemical Compositions and Morphologies of Inclusions
The types of inclusions in steel I and II were revealed by analyzing the composition of typical inclusions via elemental mapping in conjunction with point scanning of energy dispersive spectroscopy. The elemental compositions and morphologies of the inclusions in the samples of RE-free steel I are shown in Figure 1. It is clear that the typical inclusions in steel I are mainly Al2O3, MnS, and Al2O3 + MnS. Figure 1a indicates that Al2O3 inclusion is blocky in shape and with a size larger than 4.3 μm. MnS inclusion is displayed in Figure 1b with a rod-like shape and a length of 2.9 μm. Figure 1c shows that the irregular Al2O3 + MnS complex inclusion consists of two parts with a size of 3.9 μm. The inner layer is Al2O3, while the outer layer is MnS.
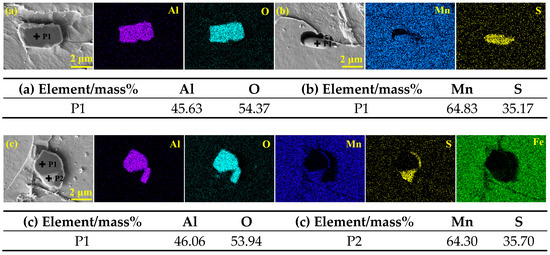
Figure 1.
SEM-EDS results of the typical inclusions observed in Y-free steel I: (a) Al2O3; (b) MnS; (c) Al2O3 + MnS.
The elemental compositions and morphologies of the inclusions in the samples of RE-containing steel II are shown in Figure 2. Generally, due to the presence of 0.015 wt.% rare earth yttrium in steel II, the typical inclusions are predominantly Y2O3, Y-O-S, Y-O-S + MnS, and Y-S. The spherical Y2O3 inclusion with a diameter of 1.6 μm is shown in Figure 2a. Figure 2b shows that Y-O-S inclusion is circular in shape and less than 1.3 μm in size. Similar to the complex inclusion in steel I, Figure 2c indicates that the Y-O-S + MnS complex inclusion also consists of two parts with a size of 4.6 μm. However, the Y-O-S + MnS complex inclusion forms a nearly circular structure, with the core of Y-O-S inclusion, which is surrounded by MnS. As presented in Figure 2d, the Y-S inclusion is circular with a diameter of 1.7 μm.
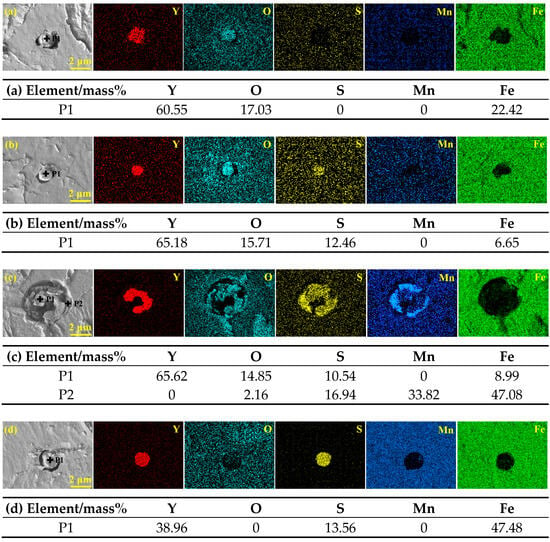
Figure 2.
SEM-EDS results of the typical inclusions detected in RE-containing steel II: (a) Y2O3; (b) Y-O-S; (c) Y-O-S + MnS; (d) Y-S.
3.2. Statistical Analysis of Quantities and Sizes of Inclusions
The type proportion, number density, area fraction, diameter, and aspect ratio of the inclusions in steel I and II were statistically analyzed through an automatic particle analysis system, and more than six hundred inclusions in each steel were analyzed three times. The types and total amount of inclusions in the steel with and without rare earth yttrium are shown in Figure 3. Consistent with the results observed through manual SEM-EDS analysis, the automatic particle analysis system also indicates that the primary inclusion types in steel I are Al2O3, MnS, and Al2O3 + MnS. The quantity proportions of each type of inclusion are 11.4%, 48.1%, and 40.5%, respectively, as shown in Figure 3a. Similarly, aside from minute amounts of Al2O3 and MnS, the major inclusions detected in steel II are Y2O3, Y-O-S, Y-O-S + MnS, and YS + Y2S3, with corresponding quantity proportions of 9.4%, 24.3%, 16.8%, and 40.5%. The inclusion number density decreases from 27.5 per mm2 in steel I to 25.2 per mm2 in steel II, with a reduction of 8.4%, as shown in Figure 3b. It is also clarified that the total area fraction of inclusions for steel I and II are 0.0109% and 0.0067%, a decrease of 38.5%. Obviously, the addition of rare earth yttrium fundamentally changed the types of inclusions in the steel, while the total amount of inclusions generated is also reduced greatly. The lower content of O and S in steel II is one of the main factors causing the decrease in the total amount of inclusions, as shown in Table 1.
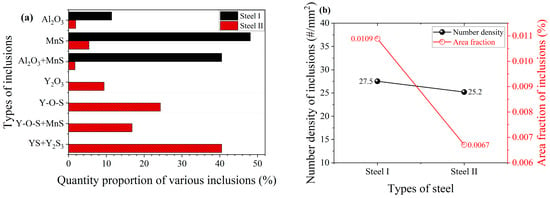
Figure 3.
Types and total amount of inclusions in the steels with and without rare earth yttrium. (a) Types of inclusions. (b) Total amount of inclusions.
Figure 4 shows the size variation of inclusions in the steel with and without rare earth yttrium. The average diameter of inclusions for steel I and II is 3.5 μm and 2.8 μm, while the aspect ratio of inclusions is 2.2 and 1.7, respectively, as shown in Figure 4a. This indicates that the size of inclusions becomes smaller and their shape tends to be more spherical. According to the size distribution of inclusions shown in Figure 4b, it is suggested that the decrease in the average diameter of inclusions in steel II is caused by an increase in the proportion of small-sized inclusions together with a decrease in the quantity of large-sized inclusions.
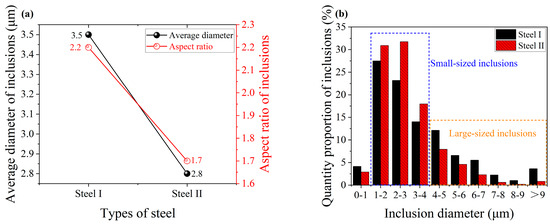
Figure 4.
Size variation of inclusions in the steels with and without rare earth yttrium. (a) Diameter and aspect ratio of inclusions. (b) Size distribution of inclusions.
Overall, the addition of rare earth yttrium reduced the total quantity of inclusions generated in the steel, and the types of inclusions changed from large-sized block-shaped Al2O3, elongated MnS, etc. to small spherical rare earth oxides, oxysulfides, and sulfides.
3.3. Thermodynamic Calculation for the Evolution Mechanism of Inclusions
Although the preliminary effects of rare earth yttrium on the modification of inclusions have been revealed, the detailed transformation processes of various inclusions need further analysis. Many researchers [,] have studied the modification mechanism of rare earths for different inclusions by thermodynamic calculations. The method that focuses on traditional Gibbs free energy calculation is widely used []. The reaction equation for the inclusion formation in steel together with the ΔG calculation can be represented by Equations (1)–(5).
x[A] + y[B] = AxBy (s)

Table 2.
Formation reactions of non-RE/RE inclusions and ΔGθ.
Figure 5 presents the calculated Gibbs free energies of various inclusions in the steels with different rare earth yttrium contents at 1600 °C. Zones I–VI represent changes in the types of inclusions with a Gibbs free energy of less than zero as the rare earth yttrium content increases. For instance, zone I indicates that the ΔG values of Al2O3, Y2O3, and Y2O2S are less than zero within the range of Y addition, while in zone VI, it turns to the inclusion YS and Y2S3, whose ΔG values are less than zero. As shown in Figure 5, the ΔG values of Y2O3 and Y2O2S are almost at the minimum values for the steel with 0.015% rare earth yttrium content. And the ΔG values of the two inclusions are significantly smaller than zero and lower than that of other inclusions, that is, Y2O3 and Y2O2S are most likely to be formed in steel II. The ΔG of Al2O3 significantly increases with the increase in Y addition; therefore, it is hard to generate Al2O3 inclusions in the steel with yttrium content exceeding 0.015 wt.% due to the ΔG value being larger than zero.
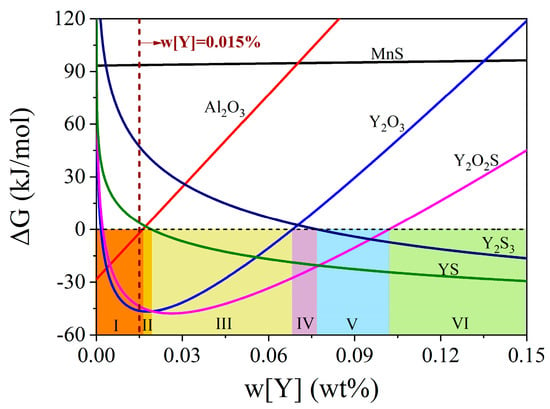
Figure 5.
Gibbs free energies of various inclusions in the steels with different rare earth yttrium contents at 1600 °C.
The evolution of inclusion formation in the steels with different rare earth yttrium contents at 1600 °C is presented in Figure 6. It clearly shows that there are four types of inclusions, namely, Al2O3, Y2O3, Y2O2S, and YS, within the yttrium content of less than 0.15%. Among them, the mass fraction of Al2O3 decreases with the increase in yttrium content. However, with the increase in yttrium content, the mass fraction of Y2O3 first increases and then decreases, as does the Y2O2S. For YS, its mass fraction increases with the increase in yttrium content. Y2O3 and Y2O2S inclusions are formed without Al2O3 in the steel with 0.015 wt.% yttrium content. It is suggested that the SEM/EDS results of inclusions in steel II presented in Figure 2 should contain Y2O3 and Y2O2S inclusions. However, the evolution of inclusions generated at lower temperatures, such as MnS, could not be explored at this fixed temperature. Therefore, as the yttrium content increases, the transformation route of inclusions is Al2O3→Al2O3 + Y2O3→Al2O3 + Y2O3 + Y2O2S→Y2O3 + Y2O2S→Y2O3 + Y2O2S + YS→Y2O2S + YS→YS, within the range of yttrium content less than 0.15 wt.% at 1600 °C.
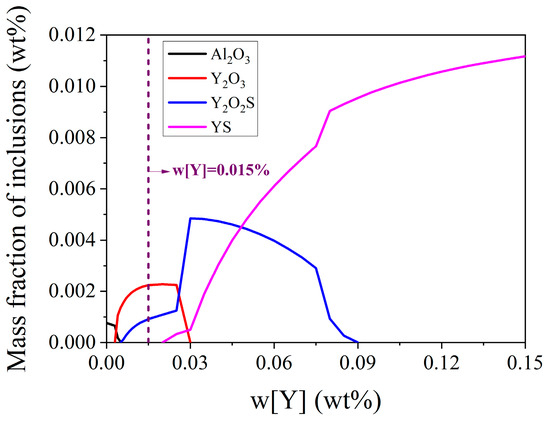
Figure 6.
Mass fraction of various inclusions in the steels with different rare earth yttrium contents at 1600 °C.
Figure 7 presents a stability diagram of rare earth inclusions at 1600 °C, and the content of each element used in the calculation is determined by the elemental composition of steel II.
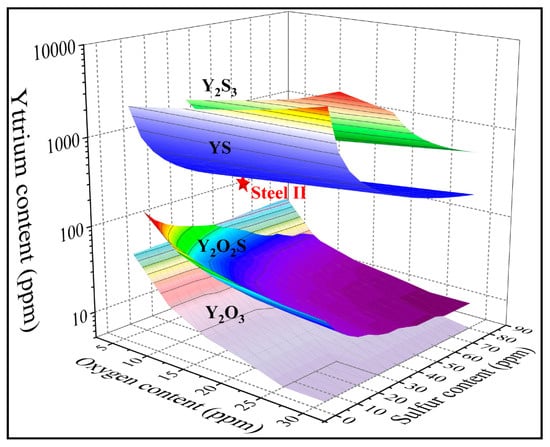
Figure 7.
Stability diagram of rare earth oxide, oxysulfide, and sulfide inclusions in the high-strength peritectic steel at 1600 °C.
As shown in Figure 7, the influence of yttrium, oxygen, and sulfur on inclusions can be studied simultaneously. Each point in the figure represents the inclusion of a certain composition; therefore, various information can be obtained. Firstly, it is easy to determine the generation sequence of various types of inclusions. For example, with a fixed O and S content, as the rare earth yttrium content increases, inclusions are generated in the following order: Y2O3, Y2O2S, YS, and then Y2S3. Secondly, the combination formula of Y, O, and S contents required for the initial generation of varying rare earth inclusions can be obtained. Thirdly, the stable regions of various inclusions are also clearly displayed. Finally, the types of rare earth inclusions for each point in the figure can also be clarified. For instance, at the point where steel II is located in the figure, it is suggested that the main inclusions generated in the steel are Y2O3 and Y2O2S, sequentially. For the line drawn parallel to the vertical axis through the red star point in the figure, the information presented along the line from high to low is the same as that shown in Figure 6.
The calculated equilibrium state and variation of inclusion composition in the studied steel I and II at a temperature from 600 °C to 1600 °C are shown in Figure 8. Therefore, the composition and generation temperature of inclusions, together with the transformation relationship among varying inclusions, can be clarified. As shown in Figure 8a, Al2O3 inclusions are first generated in the liquid Y-free steel I. As the temperature decreases to 1311 °C, Al2O3 reaches the equilibrium of precipitation with a mass fraction of 0.0032 wt.%. Subsequently, MnS precipitation commences at 913 °C and ends with a total amount of 0.0152 wt.%. Therefore, it is suggested that the types of inclusions in steel I obtained by calculation are consistent with the observed results, mainly Al2O3, MnS, and their composite inclusion. Figure 8b indicates that Y2O3 and Y2O2S inclusions already exist in the molten steel of Y-containing steel II at 1600 °C. As the temperature decreases, the precipitation amount of these two types of inclusions gradually increases and rarely precipitates after the steel solidifies. YS begins to precipitate in liquid steel at 1552 °C, and the precipitation amount rapidly increases with decreasing temperature until it reaches 1416 °C, at which point some of the YS will gradually transform into Y2S3. Y2S3 precipitation starts in the mushy zone and finishes with the largest amount of 0.01 wt.% at about 1200 °C. As the temperature further decreases, a small amount of MnS will also precipitate in steel II. Due to the presence of rare earth yttrium, its initial precipitation temperature decreases to 746 °C, and the total precipitation amount also significantly decreases to an extremely low value of 0.0011 wt.%. Moreover, there are no Al2O3 inclusions generated in steel II. Therefore, the main inclusions in steel II are Y2O3, Y2O2S, YS, Y2S3, and MnS. Among them, Y2O3 and Y2O2S are first generated, followed by YS, Y2S3, and then MnS in sequence.
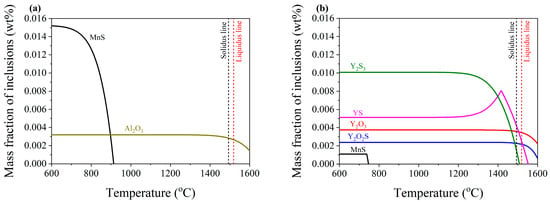
Figure 8.
Evolution of inclusions at different temperatures in the steels with and without rare earth yttrium. (a) Steel I. (b) Steel II.
Overall, the experimental results and thermodynamic calculations jointly demonstrate that the addition of rare earth yttrium not only effectively modified Al2O3 but also had a significant modification effect on MnS.
3.4. Microstructure Analysis
The microstructure of steel is directly related to its mechanical properties [,,]. The optical micrographs of the Y-free steel compared with those of Y-containing steel are presented in Figure 9.
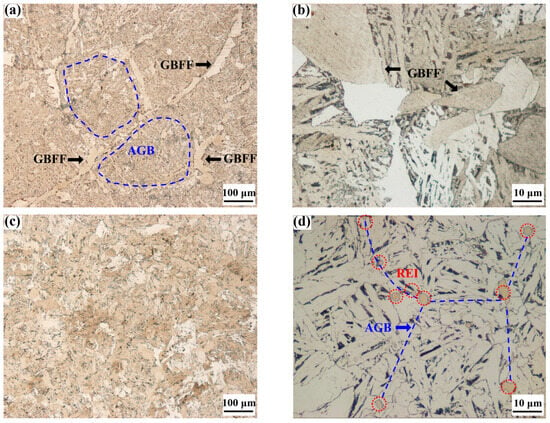
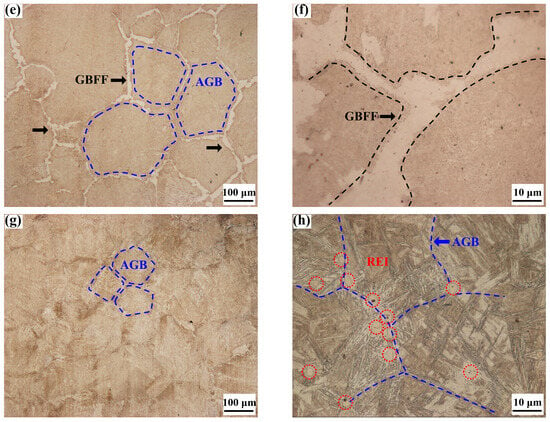
Figure 9.
Optical microstructures of the steels with and without rare earth yttrium: (a–d) ingot samples; (e–h) specimens quenched at 800 °C; RE-free steel I (a,b,e,f), RE-containing steel II (c,d,g,h); AGB: austenite grain boundary, GBFF: grain boundary ferrite film, REI: rare earth inclusion.
Ferrites and pearlites are the main microstructures for both the two experimental steels. It is shown in Figure 9a that there are large amounts of film-like ferrites distributed along the austenite grain boundaries of Y-free steel I. The thickness of the GBFF ranges from 6.7 μm to 35.8 μm with an average value of 18.5 μm. Figure 9b indicates that the ferrite films are composed of many large blocky ferrites. However, Figure 9c,d show that although many ferrites can also be observed in Y-containing steel II, the ferrites are uniformly distributed throughout the steel matrix, without massive ferrites being constructed into long strips. It can be inferred that the absence of grain boundary ferrite films in steel II is helpful for strengthening the mechanical properties of the steel. As thin ferrite films with a larger than 5 μm thick layer along the austenite grain boundaries could cause strain concentration on the ferrite film parts, which would cause poor hot ductility of the steel [,,]. In order to further study the formation process of ferrite films in the tested steels, specimens of steel I and II were heated at 1350 °C for 180 s, and then cooled to 800 °C at a 3 °C/s cooling rate, finally followed by water quenching after holding there for 10 min. The microstructure behavior for the specimens quenched at 800 °C of steel I and II varies significantly, as shown in Figure 9e–h. Figure 9e,f indicate that there is a proeutectoid ferrite layer formed along the austenite grain boundaries in the Y-free steel I. However, intergranular ferrite is rarely observed in the Y-containing steel II, as presented in Figure 9g,h. The average thickness of the grain boundary ferrite films in steel I is about 16.1 μm, with the thickest part reaching up to 33.7 μm. These results further indicate that the added rare earth yttrium in the experimental steel inhibits the formation of grain boundary ferrite films. Guo et al. [] suggested RE elements could play a double role of pinning carbon diffusion and changing grain boundary energy during the phase transformation of austenite to ferrite, making it difficult to form film-like ferrites. Li et al. [] similarly reported that RE element hindered the diffusion of carbon, significantly affecting the formation process of ferrite phase. Figure 9e,g also clearly show that the microstructure of austenite performs with significant differences in the steels with and without rare earth yttrium. The size distribution of austenite grain in steel I and II measured by the linear intercept method is shown in Figure 10. More than fifty photos were obtained by OM for the measurement of austenite grain size, and the statistics were repeated three times.
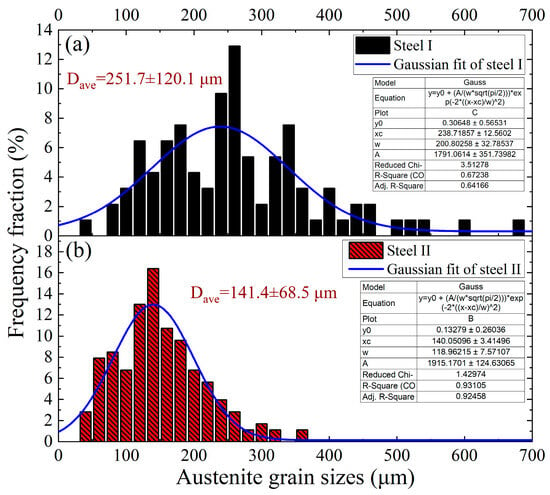
Figure 10.
Distribution of austenite grain sizes in the steels with and without rare earth yttrium. (a) steel with rare earth yttrium; (b) steel without rare earth yttrium.
The average austenite grain size apparently decreased from 251.7 μm of the Y-free steel I to 141.4 μm of the Y-containing steel II. Figure 10 reveals that the grain size distribution of steel I varies from about 30 μm to 700 μm, while it is about 25 μm to 350 μm for steel II. It implies that the distribution of austenite grain sizes in steel II is more dispersed. According to the literature, the uniformly distributed austenite structure with refined size is well known to enhance the strength of steel [,,]. Figure 9d,h also plainly show that there are certain amounts of rare earth inclusions distributed along the austenite grain boundaries in the Y-containing steel II. The SEM-EDS analysis results of the typical grain boundary inclusions are displayed in Figure 11.
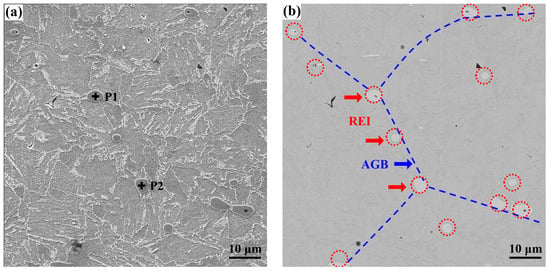
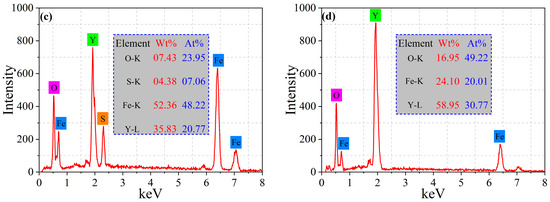
Figure 11.
SEM-EDS analysis of inclusions distributed along grain boundaries in RE-containing steel II: (a) secondary electron image; (b) backscattered electron image; (c,d) EDS results.
It is suggested that the inclusions distributed along the grain boundaries are mainly spherical or nearly spherical Y2O3 and Y2O2S, which were formed in the liquid steel. Therefore, during the formation process of austenite in the high-temperature zone, these rare earth inclusions make a critical impact on pinning of austenite grain boundaries and preventing their grain growth. As a result, a fine-grained austenite structure is obtained. Figure 12 depicts the EBSD maps of the microstructure in steel I and II, including the inverse pole figure (IPF) maps and the distribution of grain boundary misorientation. As can be seen in Figure 12a, there are oriented big blocks present in the Y-free steel I. While the small equiaxed blocks are presented in the Y-containing steel II, as shown in Figure 12b. The statistical result of the grain boundary misorientation distribution indicates that the relative frequency of grain boundaries with a misorientation angle of 2° < θ < 5° for steel I is larger than that of steel II, as presented in Figure 12c. However, for the grain boundary with a misorientation angle of θ > 5°, the relative frequency values for steel I are smaller than those of steel II. The total fraction of grain boundaries with a misorientation angle of θ > 5° increases from 17.2% in steel I to 31.3% in steel II. Usually, grain boundaries with a misorientation angle of 2° < θ < 15° are defined as LAGBs, while grain boundaries with a misorientation angle of θ > 15° are defined as HAGBs []. Apparently, the fraction of LAGBs decreases from 39.4% in the Y-free steel I to 34.5% in the Y-containing steel II; by contrast, the fraction of HAGBs increases from 6.8% in the Y-free steel I to 18.7% in the Y-containing steel II. It can be concluded that Y could reduce the fraction of LAGBs and increase the fraction of HAGBs in steel, which is consistent with the results of Huang et al. []. The high proportion of HAGBs in steel II is beneficial for strengthening the steel ductility, as the HAGBs could enhance energy absorption and prevent rapid crack propagation []. Wu et al. [] reported that the hardness of high-strength steel followed a near-linear positive relationship with the density of HAGBs.
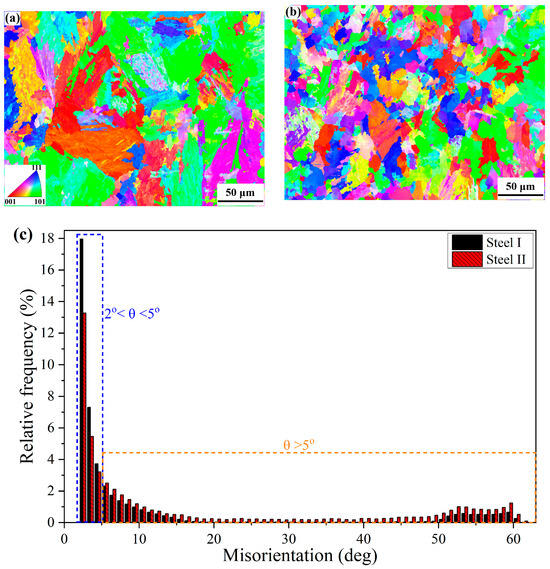
Figure 12.
EBSD analysis of microstructures in the experimental steels: (a) IPF map of steel I; (b) IPF map of steel II; (c) distribution of the grain boundary misorientation.
Generally, rare earth yttrium has three important effects on the microstructure of the tested steels. Firstly, the spherical Y2O3 and Y2O2S inclusions formed in liquid steel play a critical role in the pinning of austenite grain boundaries during the formation of austenite by peritectic reaction, resulting in refined austenite grains and a more uniform size distribution. Secondly, rare earth yttrium has a crucial impact on the formation of grain boundary ferrite, preventing large ferrite blocks from growing into strip-shaped films by hindering the diffusion of carbon distributed along austenite grain boundaries. Thirdly, it can reduce the fraction of LAGBs and increase the fraction of HAGBs in steel. All of these microstructural changes caused by rare earth yttrium can positively improve the mechanical properties of the steel.
3.5. Evolution of Inclusions and Microstructure After Yttrium Treatment
In summary, after adding rare earth yttrium, the differences in the evolution of the inclusions and microstructure during the cooling process are as described in Figure 13. In the initial stage of liquid steel, a larger number of small spherical Y2O3 and Y2O2S inclusions replaced the blocky Al2O3 inclusions. These rare earth inclusions play a crucial role in effectively pinning grain boundaries and refining austenite grain size during the peritectic reaction in molten steel. As the temperature further decreases, more rare earth sulfides take the place of the MnS inclusions. Finally, during the transformation of austenite to ferrite, rare earth yttrium hinders the diffusion of carbon along austenite grain boundaries, inhibiting the formation of long-strip ferrite films.
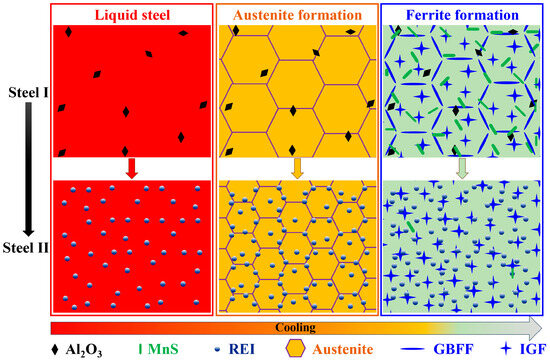
Figure 13.
Schematic illustration of evolution of inclusions and microstructure in the steels with and without rare earth yttrium during the cooling process. REI: rare earth inclusion, GBFF: grain boundary ferrite film, IGF: intragranular ferrite.
4. Conclusions
In this paper, metallographic observation, SEM-EDS and EBSD testing, an automatic inclusion particle analysis system, and thermodynamic calculation were jointly adopted to clarify the evolution of the inclusions and microstructure in steels with and without rare earth yttrium. The main conclusions are as follows:
- (1)
- The typical inclusions in Y-free steel are mainly block-shaped Al2O3, rod-like MnS, and their composites. However, in Y-containing steel, spherical or nearly spherical Y2O3, Y2O2S, Y2S3, YS, and Y-O-S + MnS dominate.
- (2)
- With the addition of rare earth yttrium, the average diameter of inclusions decreases from 3.5 μm to 2.8 μm, accompanied by a decrease in aspect ratio from 2.2 to 1.7; the inclusions are refined and spheroidized. In addition, the number density and area fraction of inclusions in the steels are reduced from 27.5 per mm2 and 0.0109% to 25.2 per mm2 and 0.0067%, respectively.
- (3)
- The thermodynamic theoretical calculation results are essentially in agreement with the experimental observations. After adding rare earth yttrium, the evolution route of inclusions in the steel at 1600 °C is Al2O3→Y2O3 + Y2O2S. As the temperature decreases, YS and Y2S3 gradually precipitate instead of MnS, with Y2S3 mainly precipitating after the steel solidifies.
- (4)
- Rare earth yttrium can significantly affect the microstructure evolution in the studied steel. The average austenite grain size is refined from 251.7 μm to 141.4 μm, with a more uniform size distribution. The rare earth inclusions distributed along grain boundaries have made critical contributions to the grain refinement.
- (5)
- Due to the presence of yttrium element in the steel, the grain boundary ferrite films with thickness ranging from 6.7 μm to 35.8 μm are suppressed. And the fraction of LAGBs decreases from 39.4% to 34.5%, together with the increasing of HAGB fraction from 6.8% to 18.7%.
Author Contributions
Conceptualization, X.Y., C.L. and W.L.; methodology, X.Y., C.L., K.L. and W.L.; formal analysis, M.L., K.L., Z.Z., Y.C. and X.Y.; investigation, M.L., K.L., Z.Z., Y.C. and X.Y.; writing—original draft preparation, M.L. and X.Y.; writing—review and editing, X.Y. and C.L.; funding acquisition, X.Y. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for support from the National Science Foundation China (Grant No. 52004110), Jiangxi Provincial Natural Science Foundation (Grant No. 20232BAB204033), and National Key R&D Program of China (Grant No. 2022YFC2905205).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
Author Min Liu was employed by the company Xinyu Iron and Steel Co., Ltd. Authors Xiaogang Yang and Weirong Li were employed by the company Dongguan Yian Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yang, X.; Zhang, L.; Lai, C.; Li, S.; Li, M.; Deng, Z. A Method to Control the Transverse Corner Cracks on a Continuous Casting Slab by Combining Microstructure Analysis with Numerical Simulation of the Slab Temperature Field. Steel Res. Int. 2018, 89, 1700480. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Yang, X.; Li, M. Control of Transverse Corner Cracks on Hypoperitectic Steel Slabs. Steelmak 2016, 32, 67–72. [Google Scholar]
- Miiko, R.; Fujda, M.; Fedakova, S.A.; Masek, V.; Duka, J.; Matvija, M. Under-solidus austenite grain growth and transverse cracking in hypoperitectic carbon steel. Metall. Res. Tech. 2017, 114, 118. [Google Scholar] [CrossRef]
- Banks, K.M.; Tuling, A.S.; Mintz, B. Influence of chemistry on transverse cracking during continuous casting of medium C high N steel billets. Mater. Sci. Tech. 2012, 28, 1254–1260. [Google Scholar] [CrossRef]
- Mintz, B.; Crowther, D. Hot ductility of steels and its relationship to the problem of transverse cracking in continuous casting. Int. Mater. Rev. 2010, 55, 168–196. [Google Scholar] [CrossRef]
- Wang, P.; Wang, B.; Liu, Y.; Zhang, P.; Luan, Y.; Li, D.; Zhang, Z. Effects of inclusion types on the high-cycle fatigue properties of high-strength steel. Scr. Mater. 2022, 206, 114232. [Google Scholar] [CrossRef]
- Zhao, L.; Zhai, G.; Wu, J.; Chen, X.; Zhai, Q. Evolution mechanism of inclusions and microstructure in low-alloy cast steel with cerium addition. J. Mater. Res. Technol. 2024, 33, 929–939. [Google Scholar] [CrossRef]
- Zarandi, F.; Yue, S. Mechanism for loss of hot ductility due to deformation during solidification in continuous casting of steel. ISIJ Int. 2004, 44, 1705–1713. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, Z.; Bao, Y. Hot ductility behavior of medium carbon sulfur-containing alloy steel. J. Mater. Res. Technol. 2022, 19, 1367–1378. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Li, B.; Chen, W.; Zhang, J.; Mao, J. Inclusions modification by rare earth in steel and the resulting properties: A review. J. Rare Earths 2024, 42, 431–445. [Google Scholar] [CrossRef]
- Miao, K.; Nabeel, M.; Dogan, N. Evaluation of calcium treatment on oxide and sulfide inclusions through modification indexes. Metall. Mater. Tran. B 2022, 53, 2897–2913. [Google Scholar] [CrossRef]
- Tie, Z.; Jiang, X.; Tang, H.; Li, G.; Wang, Y.; Zhang, J. Effect of Al content on modification behavior of non-metallic inclusions in high-strength steel treated with rare earth. J. Iron. Steel Res. Int. 2024, 31, 1886–1899. [Google Scholar] [CrossRef]
- Liang, Y.; Ni, P.; Liu, Q.; Li, Y. Effect of Rare Earth Metal Alloying on Inclusion Evolution in High-Strength Oil Casing Steel. Metall. Mater. Tran. B 2024, 55, 3158–3173. [Google Scholar] [CrossRef]
- Gong, W.; Wang, C.; Wang, P.; Jiang, Z.; Wang, R.; Li, H. Effect of La on inclusions and fracture toughness of low-alloy ultra-high-strength 40CrNi2Si2MoVA steel. J. Iron. Steel Res. Int. 2021, 28, 1408–1416. [Google Scholar] [CrossRef]
- Hao, C.; Yang, C.; Liu, P.; Luan, Y.; Sang, B. Effects of rare earth elements on inclusions, microstructure and impact toughness of spring steel. J. Iron. Steel Res. Int. 2024, 31, 933–944. [Google Scholar] [CrossRef]
- Gao, J.; Fu, P.; Liu, H.; Li, D. Effects of rare earth on the microstructure and impact toughness of H13 steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef]
- Cheng, S.; Hou, T.; Zheng, Y.; Yin, C.; Wu, K. Effect of Rare Earth Elements on Microstructure and Tensile Behavior of Nb-Containing Microalloyed Steels. Materials 2024, 17, 1701. [Google Scholar] [CrossRef]
- Su, C.; Feng, G.; Zhi, J.; Zhao, B.; Wu, W. The effect of rare earth cerium on microstructure and properties of low alloy wear-resistant steel. Metals 2022, 12, 1358. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Zhou, S.; Wang, X. Effects of rare earth addition on microstructure and mechanical properties of a Fe–15Mn–1.5 Al–0.6 C TWIP steel. Mater. Sci. Eng. A 2014, 608, 106–113. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhang, S.; Zhang, L. Effect of rare earth element yttrium modified inclusion on corrosion properties of stainless steel. Mater. Today Commun. 2024, 39, 109185. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, L.; Xiong, X.; Chen, L. Effect of yttrium treatment on alumina inclusions in high carbon steel. J. Iron. Steel Res. Int. 2022, 29, 655–664. [Google Scholar] [CrossRef]
- Guo, Z.; Li, X.; Liu, Y.; Zheng, Y.; Zhu, L.; Zhang, Y.; Sun, H.; Feng, J.; Cao, R. Effect of rare earth yttrium on inclusion characteristics of grain-oriented silicon steel. Crystals 2023, 13, 896. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Shi, C.; Tang, Z.; Jia, L.; Zhao, Y.; Wang, S.; He, X. Effect of Ce-Y Composite Addition on the Inclusion Evolution in T91 Heat-Resistant Steel. Materials 2025, 18, 1459. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Yan, Q.; Zhang, X.; Hong, Z. Microstructure characteristics of 12Cr ferritic/martensitic steels with various yttrium additions. J. Rare Earths 2019, 37, 547–554. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, Z.; Chen, R.; He, J. Effects of Yttrium on the Microstructure and Properties of 20MnSi Steel. Steel Res. Int. 2021, 92, 2100198. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, W.; Huang, C.; Qi, L.; Wang, Z.; Ge, Z. The beneficial effect of yttrium microalloying in the solid solution treatment of AISI 321 stainless steel: Grain refinement and inclusions modification. Mater. Today Commun. 2024, 38, 108077. [Google Scholar] [CrossRef]
- Liu, X.; Liang, J.L. Thermodynamic Analysis and Observation of Inclusions in H13 Die Steel with Rare Earth. Adv. Mater. Res. 2013, 690, 193–196. [Google Scholar] [CrossRef]
- Gan, W.; Huang, Z.; Liu, C.; Wang, Y.; Zhang, H.; Ni, H. Effect of Mg treatment on inclusion behaviors in 430 ferritic stainless steel during solidification. J. Mater. Res. Technol. 2024, 28, 3349–3364. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Duan, H.; Zhang, Y.; Luo, Y.; Conejo, A.N. Extraction, thermodynamic analysis, and precipitation mechanism of MnS-TiN complex inclusions in low-sulfur steels. Metall. Mater. Trans. A 2016, 47, 3015–3025. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, X.; Lai, C.; Tong, Z. Study on MnS inclusion aggregation along continuous casting slab thickness of medium carbon structural steel. Metals 2021, 12, 56. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Q.; Yan, Y. Processing, microstructure, mechanical properties, and hydrogen embrittlement of medium-Mn steels: A review. J. Mater. Res. Technol. 2024, 201, 44–57. [Google Scholar] [CrossRef]
- Seo, E.J.; Cho, L.; Estrin, Y.; De Cooman, B.C. Microstructure-mechanical properties relationships for quenching and partitioning (Q&P) processed steel. Acta Mater. 2016, 113, 124–139. [Google Scholar] [CrossRef]
- Zaefferer, S.; Ohlert, J.; Bleck, W. A study of microstructure, transformation mechanisms and correlation between microstructure and mechanical properties of a low alloyed TRIP steel. Acta Mater. 2004, 52, 2765–2778. [Google Scholar] [CrossRef]
- Yang, X.; Xie, L.; Lai, C.; Luo, D. Study on the mechanism of titanium improving the hot ductility of peritectic microalloyed steels in brittle zone III. Inter. J. Cast Metals Res. 2021, 34, 151–161. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Li, S.; Li, M. Influence of cooling conditions on the hot ductility of Nb-Ti-bearing steels. Metall. Res. Technol. 2015, 112, 604. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Li, M.; Ma, W. Control of Transverse Corner Cracks on Low-Carbon Steel Slabs. JOM 2014, 66, 1711–1720. [Google Scholar] [CrossRef]
- Fei, G.; Chengwu, Z.; Pei, W.; Dianzhong, L. Effect of Rare Earth Elements on Austenite-Ferrite Phase Transformation Kinetics of Low Carbon Steels. Chin. J. Mater. Res. 2023, 37, 495–501. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Chen, X.-Q.; Fu, P.; Luan, Y.; Hu, X.; Liu, H.; Sun, M.; Chen, Y.; Cao, Y. Low-oxygen rare earth steels. Nature Mater. 2022, 21, 1137–1143. [Google Scholar] [CrossRef]
- Sohn, S.S.; Song, H.; Suh, B.-C.; Kwak, J.-H.; Lee, B.-J.; Kim, N.J.; Lee, S. Novel ultra-high-strength (ferrite+ austenite) duplex lightweight steels achieved by fine dislocation substructures (Taylor lattices), grain refinement, and partial recrystallization. Acta Mater. 2015, 96, 301–310. [Google Scholar] [CrossRef]
- Kaijalainen, A.J.; Suikkanen, P.P.; Limnell, T.J.; Karjalainen, L.P.; Kömi, J.I.; Porter, D.A. Effect of austenite grain structure on the strength and toughness of direct-quenched martensite. J. Alloy. Compd. 2013, 577, S642–S648. [Google Scholar] [CrossRef]
- Ueji, R.; Tsuchida, N.; Terada, D.; Tsuji, N.; Tanaka, Y.; Takemura, A.; Kunishige, K. Tensile properties and twinning behavior of high manganese austenitic steel with fine-grained structure. Scr. Mater. 2008, 59, 963–966. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.; Wang, J.; Gao, C. Effect of welding heat input on microstructures and toughness in simulated CGHAZ of V–N high strength steel. Mater. Sci. Eng. A 2013, 577, 161–168. [Google Scholar] [CrossRef]
- Huang, F.; Li, J.; Zang, R. Effect of Ce on inclusion, microstructure and mechanical properties of Al-killed high-strength steel. Ironmak. Steelmak 2023, 50, 744–756. [Google Scholar] [CrossRef]
- Wang, L.; Qin, J.; Zhu, Y.; Chen, K.; Wang, Y.; Wang, Z. Microstructural optimization by two-step tempering treatment and quantitative analysis of microstructure effect on the toughness at− 40° C in CrNiMoV steel. J. Mater. Res. Technol. 2025, 35, 2781–2790. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Wang, X.; Zhao, J.; Shang, C.; Misra, R. Relationship between high angle grain boundaries and hardness after γ→ α transformation. Mater. Sci. Technol. 2019, 35, 1803–1814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).