Abstract
Zinc-substituted cobalt ferrites were obtained by a green method using a black grape extract as a reductant and fuel. XRD analysis confirmed the spinel structure of the synthesized ferrites. An increase in the lattice constant is explained by increased Zn content. SEM analysis confirmed changes in surface morphology, whereas FTIR spectra demonstrated the presence of organic species in the samples, which originated from grape extract. The content of Co(II) ions in octahedral sites as a function of the ratio between Fe(III) ions in A- and B-sites was calculated from Mössbauer data. pHPZC rose from 7.85 to 8.13 with an increase in zinc content, indicating a positive charge of the adsorbent surface at natural pH. The adsorption–catalytic properties of the spinel samples were investigated in terms of Congo Red (CR) dye removal. The mechanism of CR adsorption on the ferrite surface includes electrostatic and donor–acceptor interactions with the adsorbent surface. Furthermore, the sample with x(Zn) = 0.4 exhibited the highest degradation rate constant k = 0.102 min−1 in the peroxide oxidation of CR, whereas the sample with x(Zn) = 1.0 exhibited the highest adsorption capacity. The electron transfer between ferrite samples and hydrogen peroxide was evidenced using electrochemical tests. The green-synthesized Co-Zn ferrites demonstrate a big potential as adsorbents/catalysts for water treatment.
1. Introduction
Nowadays, numerous established physical and chemical methods exist for water purification [1,2]. However, a key challenge persists in identifying a portable adsorbent or catalyst to enhance the efficacy of existing purification techniques, followed by quick removal after purification. Magnetic spinel ferrite emerges as a highly promising material in this domain [3,4]. Research efforts are directed towards exploring novel synthesis methods with a predetermined cation composition [5]. The synthesis of spinel compounds involves diverse methods, including the sintering of oxides, a co-precipitation method [6,7], a biosynthetic method [8], hydrothermal synthesis [9,10,11], a sol–gel method [12,13], electrochemical synthesis, etc. Sol–gel synthesis is one of the most popular methods for the synthesis of nanoferrites. Usually, for the self-combustion sol–gel method, different fuel synthetic agents are used, such as acids, alcohols, carbohydrates [14], etc. In the case of the ‘green chemistry’ method, attention is paid to finding a green reducing agent and, at the same time, a fuel for the synthesis of cation-substituted cobalt ferrite. Plant-contained active compounds can reduce metal salts to NPs [15]. Thus, it is important to choose a plant that would contain sufficient substances to be used as a reductant. An example of such a plant is a grape. Grapes are commonly used in the food [16], cosmetic [17], and pharmaceutical [18] industries. The use of grapes as a green agent for nanoparticle synthesis is known. In particular, Krishnaswamy et al. [19] used grape waste to synthesize gold nanoparticles. Black grapes consist of carbohydrates, polyphenols, dextrose, and pectin. Tartaric, malic, and citric acids are also present. The peel and seeds of black grapes contain a large amount of resveratrol, which is known as a natural antioxidant. The ’green chemistry’ approach is also widely recognized for its efficacy in producing spinel ferrites. Among these ferrites, cobalt ferrite stands out for its effectiveness as a catalyst in environmental applications. For example, the study [20] demonstrated that introducing zinc ions into the structure of ferrites significantly enhanced their catalytic and adsorption properties.
This research aimed to synthesize Zn-substituted cobalt ferrites represented by the formula Co1−xZnxFe2O4, where 0.0 ≤ x ≤ 1.0 (with a step of 0.2), employing the ‘green chemistry’ approach utilizing an extract derived from a black grape. Grape extract contains compounds that are non-toxic compared to synthetic compounds, which, in turn, reduces potential hazards during the synthesis process. In addition, using grape extract could facilitate synthesis under milder conditions, e.g., lower temperature or water medium, which results in lower energy consumption. Moreover, the waste of grapes could be used as green fuel, making the principles of ‘green’ synthesis closer to the principles of green chemistry. Our earlier research demonstrated that CoFe2O4, synthesized using grape peel extract, is a more active catalyst for the decomposition of hydrogen peroxide compared to CoFe2O4, synthesized using grape pulp extract [21]. However, in contrast to [21], in this study, Co–Zn ferrites will be synthesized using cobalt(II) acetate as a source of Co(II) ions, which prevents the formation of nitrogen oxides during synthesis and also makes the process ‘greener’. Furthermore, the structure, morphology, and surface properties of Co1−xZnxFe2O4 samples will be comprehensively examined. In addition, the adsorption and catalytic properties of Zn-substituted cobalt ferrites will be assessed using Congo Red dye as a model pollutant.
2. Materials and Methods
2.1. Green Synthesis
2.1.1. Preparation of Black Grape Extract
Black grape peel extract was used as a ‘green’ agent. The grape peel was boiled with distilled water (grape-peel-to-water-mass ratio of 0.5) for 10 min and stored for 24 h.
2.1.2. Synthesis of Co1−xZnxFe2O4 Nanoparticles
Cobalt–zinc ferrites with the general formula Co1−xZnxFe2O4 (where x = 0; 0.2; 0.4; 0.6; 0.8; 1.0) were prepared by the self-combustion sol–gel method. The salts Co(CH3COO)2·4H2O, Zn(NO3)2·6H2O, and Fe(NO3)3·9H2O were used as sources of Co(II), Zn(II), and Fe(III) ions, respectively. The necessary amount of metal salts was taken according to stoichiometry (molar ratio of [Co(II) + Zn(II)]:Fe(III) = 1:2). The extract of the black grape peel was utilized as an eco-friendly fuel agent with reducing properties. Initially, the mixture of metal salts and 50 mL of distilled water was stirred for 30 min at a temperature of 45 °C for complete dissolution. Later, 50 mL of the grape extract was added to the solution. The resulting mixture was stirred at a constant temperature of 45 °C for 30 min. The solution underwent continuous evaporation, resulting in the formation of a viscous solution (sol). This sol progressively transitioned into a gel, after which the self-combustion of the gel was observed. The obtained ferrite powders were ground in a mortar to obtain powders for further characterization.
2.2. Characterization Techniques
X-ray diffraction patterns were obtained using a Shimadzu XRD–7000 X-ray diffractometer (Shimadzu Corporation, Kyoto, Japan) with a Cu-Kα monochromatic radiation source (λ = 1.5418 Å) under the operating conditions of 40 kV and 30 mA at room temperature. The reference sample LaB6 660 c was used for microstructural analysis. Crystal phases were identified using Match! 3.0/FullProf software. The Scherrer equation was used to determine the average sizes of the crystallites: , where FWHM is the full width at half maximum of the (311) peak. FTIR spectra were recorded using a Nicolet Nexus 470 spectrometer (Thermo, Waltham, MA, USA) in the range of 4000–400 cm−1, with the number of scans at 128 and at a resolution of 4 cm−1. The background was recorded relative to the optical element without the sample. The surface morphology of the samples was studied with a REMMA-102-02 scanning electron microscope (JCS SELMI, Sumy, Ukraine) with an attachment for energy-dispersive analysis (EDS). The accelerating voltage was approximately 20.00 kV. The texture characteristics of ferrites were studied using N2 adsorption–desorption porosimetry at 77 K. Before each measurement, the samples were degassed overnight at 180 °C. The measurements were performed on an automatic Quantachrome sorption analyzer (Quantachrome Instruments, Florida, FL, USA). Surface area, pore volume, pore size distribution, and pore diameter were calculated using Quantachrome NovaWin software (v.11.04). ⁵⁷Fe Mössbauer spectra were recorded using an MS1104EM spectrometer operating with a constant acceleration mode and a ⁵⁷Co source in a Cr matrix with an activity of approximately 10 mCi. Isomer shifts were calibrated relative to α-Fe at room temperature. Hyperfine interaction parameters were calculated using UNIVEM-MS software (v.701).
2.3. Congo Red Dye Elimination from Water
2.3.1. Adsorption Experiments
Adsorption studies were performed in batch mode. Congo Red (CR) dye was chosen as the model pollutant (Figure 1) to study the adsorption properties of ferrites. A total of 20 mg of the adsorbent was added to 20 mL of a dye solution with a certain concentration (10; 20; 50; 75; 100 mg/L). The mixture was shaken at 25 °C and left for 24 h to establish adsorption equilibrium. The adsorbent was removed using a magnet. The optical density of the solutions was measured by a spectrophotometric method using a ULAB-102UV spectrophotometer (Biobase Biolin Co., Ltd. Shandong, China) at a wavelength of λ = 500 nm. The equilibrium concentrations (Ceq) of the dye in the solutions were calculated using a calibration equation. The adsorption capacity (mg/g) was calculated using the following equation:
where C0 is the initial concentration of CR dye in the solution, mg/L; Ceq is the equilibrium concentration of CR dye in the solution, mg/L; m is the mass of the adsorbent, mg; V is the solution volume, L. The dye removal (in %) was calculated by the following formula [22]:
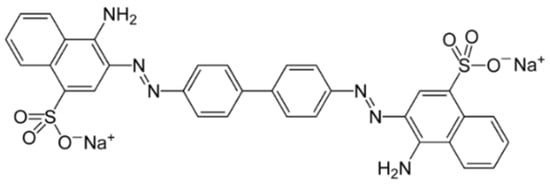
Figure 1.
Chemical structure of azo dye Congo Red (sodium salt of 3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid) (IUPAC name: disodium 4-amino-3-[4-[4-(1-amino-4-sulfonato-naphthalen-2-yl)diazenylphenyl]phenyl]diazenyl-naphthalene-1-sulfonate; chemical formula is C32H22N6Na2O6S2; molar mass is 696.7 g/mole).
2.3.2. Catalytic Wet Peroxide Oxidation Experiments
Kinetic experiments on the degradation of Congo Red in the presence of hydrogen peroxide were conducted utilizing 120 mg of the catalyst and 40 mL of CR dye solution with a concentration of 10 mg/L. The catalyst was initially stirred with the dye solution, and the first aliquot was withdrawn after 30 min to evaluate the adsorption of CR onto the catalyst surface. Subsequently, hydrogen peroxide at a concentration of 20 mM was introduced to initiate the process of the catalytic wet oxidation of CR. Thereafter, aliquots of the dye solution were collected at predetermined time intervals, and the optical density was measured using a ULAB-102UV spectrophotometer at a wavelength of λ = 500 nm to estimate the residual CR concentration. The residual concentration of hydrogen peroxide was also determined by UV-vis spectrophotometry at a wavelength of λ = 470 nm using the metavanadate method.
2.3.3. Electrochemical Tests
Amperometric I–τ curves were measured using an Autolab PGSTAT12 Eco Chemie galvanostat–potentiostat (Eco Chemie B.V., Utrecht, Netherlands). The electrode cell consisted of three electrodes: a saturated Ag/AgCl electrode as the reference electrode, Pt foil as the counter-electrode, and a ferrite-coated FTO-coated glass slide (fluorine-doped tin oxide-coated glass slide) as a working electrode. The working electrode was prepared using an aqueous suspension of ferrite, which was coated on the FTO glass (1.4 cm × 3 cm). After each coating, the electrode was sintered at 90 °C for 15 min. The solution of Congo Red dye ([CR] = 10 mg/L) was used as an electrolyte. Hydrogen peroxide solution ([H2O2] = 20 mM) was used as an activator of current transmission. H2O2 solution was added dropwise with continuous stirring, and I–τ curves were recorded.
3. Results
3.1. XRD Analysis
The structure of synthesized Co1−xZnxFe2O4 was validated through X-ray diffraction (XRD) analysis, as shown in Figure 2a. All Co–Zn ferrite samples were confirmed to be single-phase spinel materials classified under the space group Fd3m. The crystallite sizes, calculated using the Scherrer formula, demonstrate an increase from 10 nm (for x(Zn) = 0.0) to 18 nm (for x(Zn) = 1.0), indicating insignificant crystallinity and the influence of zinc on the microstructure (Figure 2b). With an increase in zinc doping, there is a significant expansion in the lattice parameter, escalating from 8.357 Å at x(Zn) = 0.0 to 8.418 Å at x(Zn) = 1.0 (Figure 2c). The notable shift in the linear dependence observed at x(Zn) = 0.4, illustrated in Figure 2c, is plausibly attributed to the transitions of Co(II), Zn(II), and Fe(III) ions between the tetra- and octahedral positions [23].
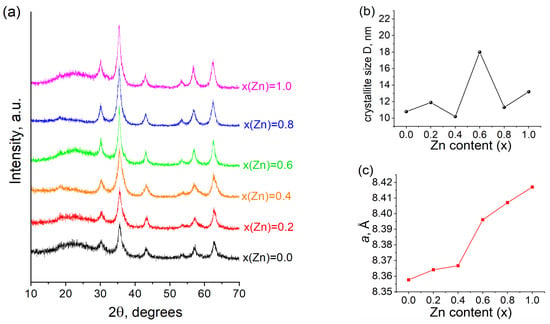
Figure 2.
(a) XRD patterns of ZnxCo1−xFe2O4 (0 ≤ x ≤ 1) ferrites; (b) average crystallite size vs. Zn content; (c) lattice constant as function of Zn content.
3.2. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy
The SEM images in Figure 3 depict the surface morphology of the Co1−xZnxFe2O4 samples, revealing a propensity for particle agglomeration. The x(Zn) = 0 sample exhibits substantial agglomeration and low porosity. Samples with x(Zn) = 0.2 and x(Zn) = 0.4 display higher porosity with agglomerated structures. Conversely, samples with x(Zn) = 0.6 and x(Zn) = 0.8 exhibit porous, lamellar structures. The sample with x(Zn) = 1 demonstrates numerous small pores and a more spherical, low-agglomeration structure, with agglomerate sizes averaging 2 µm.
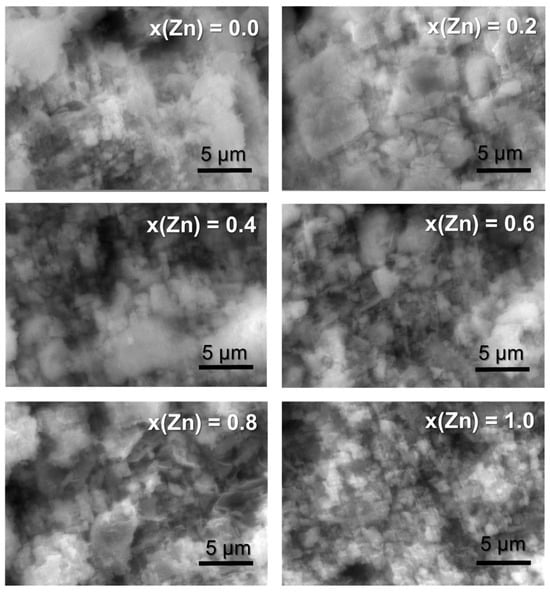
Figure 3.
The SEM images of Co1−xZnxFe2O4 ferrites, obtained using grape extract.
The presence of chemical elements O, Fe, Co, and Zn was confirmed by energy-dispersive spectroscopy (Figure 4, Table 1). It can be seen that the elemental composition of samples expressed in atomic and weight percent is close to theoretical calculations.
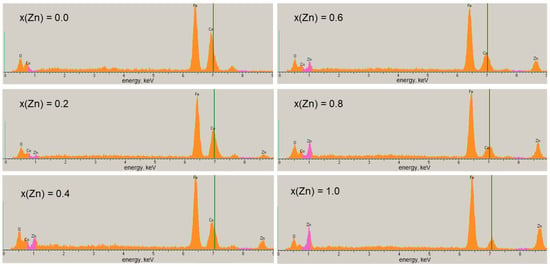
Figure 4.
The EDS spectra of Co-Zn ferrites.

Table 1.
The element composition of Co1−xZnxFe2O4 (calculated from EDS).
3.3. Mössbauer Spectroscopy
Mössbauer spectra obtained at room temperature (T = 293 ± 1 K) demonstrate the paramagnetic properties of ZnxCo1−xFe2O4 ferrites (Figure 5a). The relaxation character is evident in the Zn0.2Co0.8Fe2O4 sample, as indicated by the widening linewidth of the doublet component and a shift in the background. This trend persists in the CoFe2O4 sample, whose spectra feature a central doublet component along with broadened sextets that correspond to the magnetically ordered state of a portion of the material.
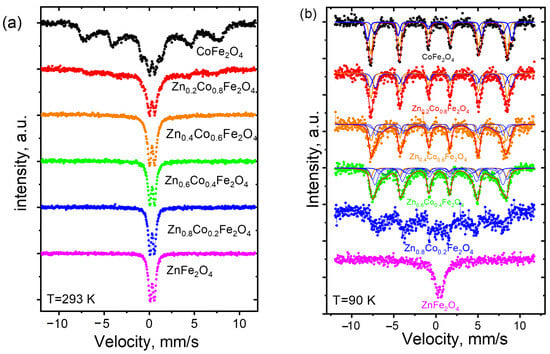
Figure 5.
Mössbauer spectra of ZnxCo1−xFe2O4 (0 ≤ x ≤ 1) ferrites at (a) 293 K and (b) 90 K.
The obtained results are explained by superparamagnetic phenomena. For ferromagnetic nanoparticles with an average size of less than 10 nm, a single-domain state is preferable. Thermal excitation leads to a magnetic moment flip between the directions of easy magnetization. When the average time between two flips (Néel relaxation time) becomes less than the time of data measurement (τm = 141.8 ns in our case as the lifetime of the 57Fe nucleus excited state), paramagnetic state particles will be observed.
The Néel time is calculated as , where τ0 is a characteristic time of the material (for spinel materials, it usually falls into the interval from 10−9 to 10−11 s), K is a magnetocrystalline anisotropy constant, V is the volume of a nanoparticle, and T is temperature (in K). An exponential decrease in characteristic time τ0 from 16.7∙10−10 s to 3.8∙10−10 s for CoFe2O4 nanoparticles was observed in [24]. For cobalt ferrite nanoparticles, the magnetocrystalline anisotropy values are about 7.5·105 J/m3 for particles 5 nm in size [25] and 3.1·105 J/m3 for particles 10–15 nm in size [26]. The dependence of temperature on magnetocrystalline anisotropy for cobalt ferrite can be expressed using the empirical Brukhatov–Kirensky expression: K(T) = K(0)exp(–BT2) for the temperature range of 20 K < T < 350 K, where K(0) = 1.96·106 J/m3, and B = 1.9·10−5 K−2 [27]. According to this calculation, under these conditions at room temperature (293 K), cobalt ferrite nanoparticles with an average size of less than about 7 nm will be observed as paramagnetic. The decreasing measurement temperature caused a shift in the threshold of the superparamagnetic/ferromagnetic transition to smaller particle sizes. The Mössbauer spectra for all samples were obtained at T = 90 K (Figure 5b). The disappearance of superparamagnetic relaxation was observed in all cases with an average particle size larger than approximately 4.0–4.5 nm, as estimated from the calculation of the Néel relaxation time. The spectra obtained at 90 K were optimally fitted by a superposition of five or four six-line magnetic subspectra. The association of iron ions to tetrahedrally and octahedrally coordinated positions was determined according to the isomeric shift values in Table 2. Typically, the isomeric shift for iron ions located in A-sites is smaller due to the stronger covalent FeA–O bonds.

Table 2.
The Mössbauer spectrum parameters (measured at 90 K) of ZnxCo1−xFe2O4 (0 ≤ x ≤ 0.6) ferrites (IS is an isomeric shift; QS is a quadrupole splitting; H is a hyperfine field; S is a relative integral intensity; G is a linewidth).
Zn(II) ions have a preference for tetrahedral sites due to their tendency to covalent bond with sp3-orbital formation [28]. Co(II) ions can be located in both A- and B-sites with a preference for octahedral surroundings [29]. Mixed cobalt–zinc ferrites can be described as (ZnxCo2+yFe3+1−x−y)A[Co2+zFe3+2−z]BO4, where z is an inversion degree. The distribution of Fe(III) ions between tetra- (A) and octahedrally (B) coordinated sites was calculated using Mössbauer spectrum analysis combined with EDS data (Table 3, Figure 6). The experimental values of Co/Fe (denoted as K1) and Zn/Fe (denoted as K2) molar ratios were used as well as the ratio between Fe(III) ions in the tetrahedral and octahedral positions (denoted as K3) in the spinel crystal lattice (FeA/FeB) obtained from Mössbauer data analysis. The systems of equations {(y + z)/(3–x–y–z) = K1; x/(3–x–y–z) = K2; (1–x–y)/(2–z) = K3} were solved for all samples. It was determined that an increase in Zn(II) ion contents leads to a decrease in ferrite’s average particle size. The Mössbauer spectrum obtained at 90 K for the sample with a predicted Zn content of 0.8 mol per formula unit exhibits a relaxation character. This is characterized by the superposition of broadened sextets and a doublet component. The observed continuous distribution of quadrupole splitting and hyperfine fields can be attributed to the lattice deformation of ultrafine particles, influenced by the Laplace pressure. The development of this process is observed for the sample without Co(II) ions (Figure 5b). This spectrum consists of only a broad doublet component due to imbalance between magnetically ordered and superparamagnetic states [30]. It can be argued that for this sample, the oscillation of magnetic moments at 90 K happens for the absolute majority of particles, and the upper threshold of the average size of these particles is about 4.0–4.5 nm. The changes in Co(II) ion content in the octahedral position as a function of the ratio between Fe(III) ions in the A- and B-positions calculated from Mössbauer data for different samples are presented in Figure 6. The calculated cation distributions are summarized in Table 4.

Table 3.
The theoretically predicted and experimentally obtained content of chemical elements for ZnxCo1−xFe2O4 (0 ≤ x ≤ 1) ferrites (based on EDS and Mössbauer data).
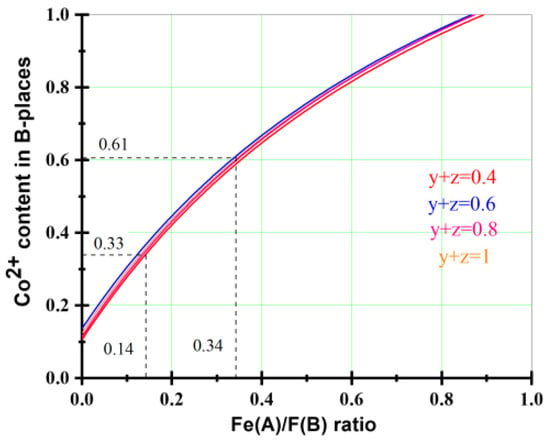
Figure 6.
The Co(II) ion contents in B-positions vs. the ratio between Fe(III) ions in A- and B-sites (denoted as K3) calculated from Mössbauer data.

Table 4.
The calculated cation distributions for ZnxCo1−xFe2O4 (0 ≤ x ≤ 1) ferrites.
3.4. FTIR Spectroscopy
Figure 7 shows the FTIR spectra of the Co-Zn ferrites. The band beginning at 408 cm−1 is characteristic of the vibration of the octahedral M–O bond. The strong bands at 526–538 cm−1 are characteristic of the vibration of the tetrahedral M–O bond. The shift in the tetrahedral peak to the left from 524 to 538 cm−1 indicates the substitution of Co by Zn ions with a larger radius. The broad band in the range of 3600–3300 cm−1 indicates the presence of water molecules. The band in the range 2923–2849 cm−1 belongs to aromatic C–H bonds. The absorption band at 2358–2333 cm−1 indicates the presence of adsorbed CO2. The region 1980–1350 cm−1 indicates the vibrational stretching of double bonds, which are characteristic of organic residues in the samples (originated from the grape extract). The stretching at 1650–1450 cm−1 indicates the presence of O–H bonds. The umbrella mode at 1340–1070 cm−1 indicates the presence of –CH3 groups. Thus, the FT-IR spectra demonstrate the formation of M–O bonds within the Co–Zn ferrites, as well as the presence of organic species, including aromatic C–H bonds, C=C bonds, and –CH3 groups, on the surface of the ferrites, which can be attributed to the incorporation of grape extract during the synthesis process [31].
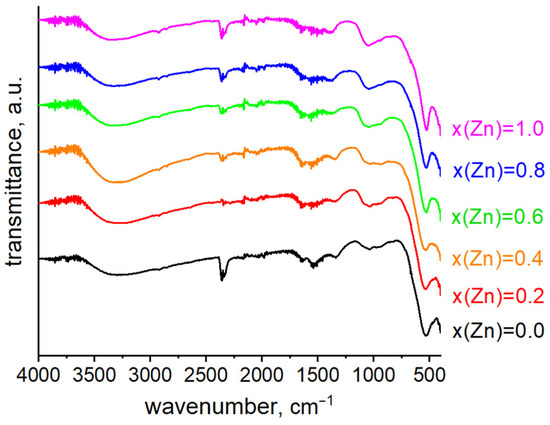
Figure 7.
FTIR spectra of Zn-substituted cobalt ferrites.
The force constant for the tetrahedral site was calculated using the following formula: , where c ≈ 2.99 × 1010 cm/s (the light speed), νT is the vibration frequency of the tetrahedral site, and µ ≈ 2.601 × 10−23 g (the reduced mass of the Fe3+ and O2− ions). Table 5 shows the variation in KT with x(Zn) content. It can be seen that the sample with x(Zn) = 0.4 exhibits the highest value of KT, indicating that it has the strongest bonds between A-cations and oxygen ions. It is expected that this sample will have the weakest bonds between B-cations and oxygen ions, which, in turn, should affect its catalytic activity. Thus, the introduction of Zn(II) ions into the spinel lattice affects the bonding strength between the tetrahedral cations and oxygen ions, resulting in changes in physicochemical properties.

Table 5.
IR absorption bands, which correspond to the tetrahedral site (νT), and the force constants (KT) for Co1−xZnxFe2O4 samples.
3.5. Surface Area and Pore Volume
Figure 8a presents the nitrogen adsorption–desorption isotherm and the pore size distribution of synthesized cobalt ferrite. According to the IUPAC classification [32], this isotherm is categorized as type IV, exhibiting a hysteresis loop that is indicative of mesoporous materials. Type IV isotherms are characterized by a hysteresis loop linked to capillary condensation in mesopores and show limiting uptake at high p/p°. The initial segment represents monolayer–multilayer adsorption and is commonly found in mesoporous adsorbents [32]. The BET surface area was determined to be 60 m2/g, which encompasses a micropore surface area of 9 m2/g and a mesopore surface area of 51 m2/g (Table 6). The calculated total pore volume is 0.12 cm3/g, comprising a micropore volume of 0.03 cm3/g and a mesopore volume of 0.09 cm3/g (Table 6). The BET method was utilized to assess the pore size distribution for the cobalt ferrite sample (Figure 8b), revealing a pore radius of 4.4 nm, thus confirming the mesoporous characteristics of the synthesized material.
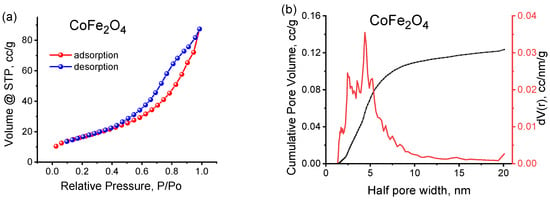
Figure 8.
(a) N2 adsorption–desorption isotherm for CoFe2O4 sample; (b) pore size distribution for CoFe2O4 sample.

Table 6.
The calculated textural parameters for the CoFe2O4 sample.
3.6. Adsorption Properties
To evaluate the capacity of the surface to electrostatically attract or repel ions and molecules, the surface charge of ferrites, identified as the point of zero charge (pHPZC), was measured in an aqueous solution. The results for the most representative samples are presented in Figure 9. It is evident that pHPZC increases with higher zinc content. Specifically, pHPZC for cobalt ferrite equals 7.85, whereas for zinc ferrite, it equals 8.13. This trend suggests that the substitution of cobalt with zinc in the spinel ferrite structure enhances the positive surface charge, resulting in an increased capacity for the adsorption of anionic pollutants.
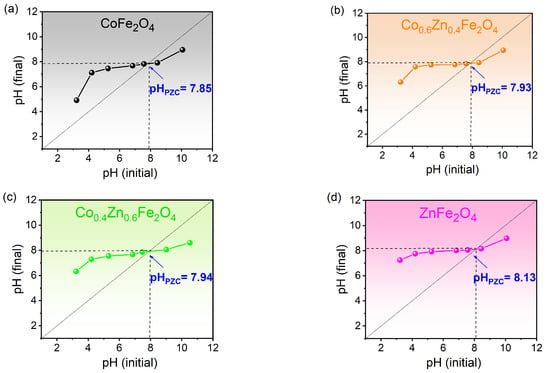
Figure 9.
pHPZC for Zn-substituted cobalt ferrites.
Figure 10a,b illustrate the adsorption isotherms and the adsorption capacity values of Co-Zn ferrites concerning zinc content when utilizing Congo Red dye as a model pollutant. The observed trend indicates that an increase in zinc content generally results in an enhancement in the adsorption capacity, with values ranging from 25.77 mg/g for CoFe2O4 to 50.88 mg/g for ZnFe2O4. It is important to note that the samples with x(Zn) = 0.2 and x(Zn) = 0.4 exhibit deviations from this established trend, which may be attributed to variations in the crystallite size of these particular samples.
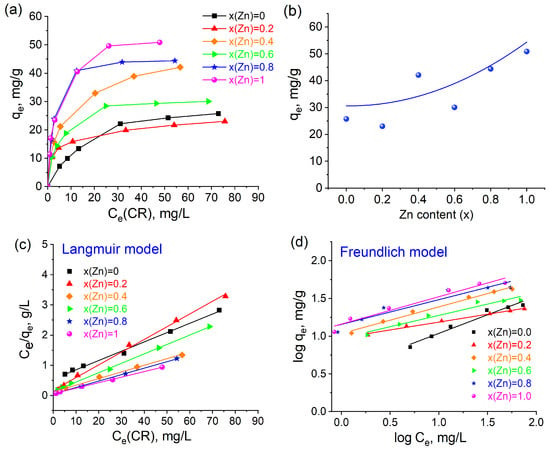
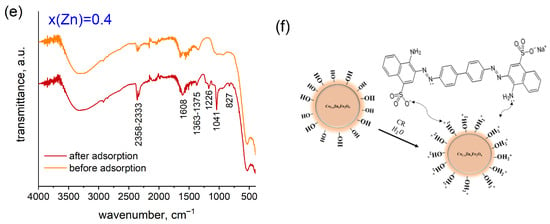
Figure 10.
CR adsorption on the Co1−xZnxFe2O4 NPs: (a) adsorption isotherms (conditions: m (adsorbent) = 20 mg; V (solution) = 20 mL); T = 298 K); (b) adsorption capacity vs. zinc content; (c) Langmuir model; (d) Freundlich model. (e) The FTIR spectra of the Co0.6Zn0.4Fe2O4 sample before and after CR adsorption; (f) the mechanism of Congo Red adsorption.
To elucidate the mechanism underlying the adsorption of Congo Red onto the ferrite surface, both the Langmuir and Freundlich models were used to fit the experimental data. The parameters associated with these models are presented in Table 7. The Langmuir constant (KL) quantifies the energy associated with the interaction between the adsorbate and the adsorbent [31]. A stronger interaction corresponds to an elevated value of the adsorption constant. The applicability of the Freundlich model in describing the adsorption of Congo Red dye suggests that the surface of the adsorbent may exhibit heterogeneity. Among the analyzed samples, the Langmuir constant KL is found to be the highest for Co0.2Zn0.8Fe2O4 and ZnFe2O4, with values of 0.40 and 0.29, respectively. In contrast, CoFe2O4 displays the lowest value of KL (0.06), indicating that the incorporation of zinc into cobalt ferrite enhances the interaction between Congo Red dye molecules and the adsorbent surface. Furthermore, Table 7 illustrates that the Freundlich constant KF is the largest for the ZnFe2O4 sample (KF = 14.49) and the smallest for the CoFe2O4 sample (KF = 3.51). In general, an increase in KF aligns with the increase in zinc content, except in the case where x(Zn) = 0.6. Therefore, the augmentation of zinc content leads to a strengthened interaction of Congo Red dye molecules with the adsorbent surface. The R2 value for the Langmuir model ranges from 0.98 to 0.99, while that for the Freundlich model is between 0.90 and 0.99, indicating the predominance of monomolecular interactions as characterized by the Langmuir model.

Table 7.
The parameters of the Langmuir and Freundlich adsorption models.
Figure 10e showcases a comparison of the FTIR spectra for the most representative Co0.6Zn0.4Fe2O4 sample, recorded before and after the adsorption of Congo Red. The lack of significant changes in the spectrum within the 600–400 cm−1 range indicates that the spinel structure was preserved after the adsorption process. Furthermore, the absorption bands observed between 1226 and 827 cm−1 correspond to the vibrational modes of the Congo Red molecule’s bonds. A pronounced band at 1041 cm−1 signifies S=O stretching vibration [33]. These results together provide strong evidence that the Congo Red molecules were effectively adsorbed onto the surface of the ferrite nanoparticles.
Figure 10f delineates the plausible mechanism underlying the adsorption of CR dye onto the ferrite surface. Several fundamental factors contribute to this adsorption process, with particular emphasis on surface area and electrostatic attraction [34]. The surface charge, specifically the point of zero charge of the adsorbents, is critical, as it contrasts with the charge of the ionized dye, thereby enhancing electrostatic attraction. In this context, the substitution of cobalt with zinc in the ferrites results in an increased positive surface charge, as corroborated by pHPZC measurements (Figure 9). It can thus be inferred that the mesoporosity and surface charge of Co-Zn ferrite nanoparticles are pivotal parameters for the adsorption of Congo Red. Furthermore, hydrogen bonding interactions between the functional groups of the dye and those present in the adsorbents play a significant role in this adsorption mechanism. Coordination between the acceptor species, namely the metal cations of the ferrites, and donor atoms, such as nitrogen in the –NH2 groups of the CR molecules, constitutes another vital factor in this process. The establishment of a mesoporous structure facilitates the increased availability of active sites and surface area essential for the effective adsorption of CR molecules through hydrogen bonding. Additionally, the surface –OH groups contribute to the hydrogen bonding interactions between the –NH2 groups of CR and the surface –OH groups, thereby further enhancing adsorption efficiency.
3.7. Catalytic Wet Peroxide Oxidation of Congo Red Dye
The catalytic wet peroxide oxidation of Congo Red dye was studied through two distinct ways: (I) hydrogen peroxide was introduced after a 30 min adsorption stage, during which the catalyst was initially mixed with the Congo Red dye solution to evaluate its capacity to absorb the dye molecules; (II) hydrogen peroxide was added directly to the dye solution without allowing the system to reach adsorption equilibrium. In the first case, the changes in the UV-Vis spectra of the Congo Red solutions during the catalytic wet peroxide oxidation process were monitored and are presented in Figure 11a–f.
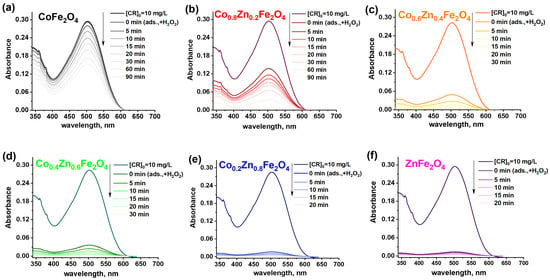
Figure 11.
(a–f) UV–Vis spectra of CR solutions obtained in presence of cobalt–zinc ferrite catalysts (conditions: m (catalyst) = 120 mg; V (solution) = 40 mL; C0(CR) = 10 mg/L; T = 298 K; C0(H2O2) = 20 mM).
Kinetic curves for the decomposition of Congo Red are presented in Figure 12a. The first 30 min is indicative of CR adsorption on the catalyst surface, while the subsequent 90 min pertains to the degradation of CR following the introduction of hydrogen peroxide (20 mM) into the solution via catalytic wet peroxide oxidation (CWPO). It is observed that the decolorization of CR is completed within 30 min after the addition of H2O2 for samples with compositions of x(Zn) = 0.6, x(Zn) = 0.8, and x(Zn) = 1.0. In contrast, the sample with x(Zn) = 0.2 necessitates 90 min for completion. Additionally, it is noted that CoFe2O4 achieves only a 40% decolorization of CR in 90 min. The data were analyzed using a first-order kinetic model (Figure 12b), and the corresponding rate constants for each catalyst were determined (Figure 12c). The sample with x(Zn) = 0.4 exhibits the highest rate constant of 0.102 min−1. The subsequent samples with x(Zn) = 0.6, x(Zn) = 0.8, and x(Zn) = 1.0 demonstrate rate constants of 0.084 min−1, 0.077 min−1, and 0.084 min−1, respectively. In comparison, the non-substituted cobalt ferrite displays the lowest rate constant of 0.008 min−1.
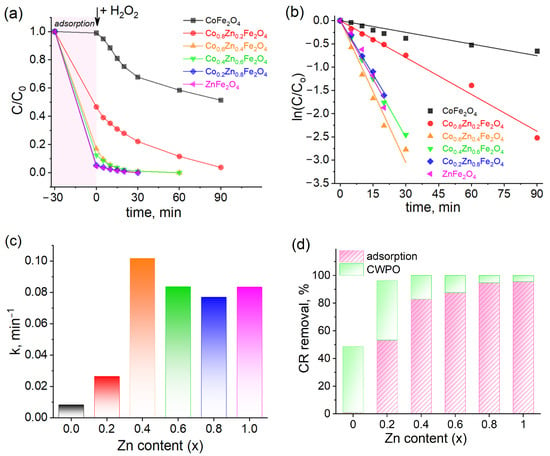
Figure 12.
The catalytic wet peroxide oxidation of Congo Red by Co-Zn ferrites. (a) The kinetics of Congo Red decolorization (conditions: m (catalyst) = 120 mg; V (solution) = 40 mL; C0(CR) = 10 mg/L; T = 298 K; C0(H2O2) = 20 mM). (b) First-order kinetic model. (c) The values of the rate constants. (d) The efficiency of CR removal by CWPO catalyzed by the Co1−xZnxFe2O4 samples. (e) The degree of H2O2 decomposition. (f) The efficiency of CR removal by direct CWPO (conditions: m (catalyst) = 3 mg; V = 10 mL; C0(CR) = 10 mg/L; T = 298 K; C0(H2O2) = 20 mM).
As illustrated in Figure 12d, the highest degree of CR adsorption was observed in samples with x(Zn) ranging from 0.4 to 1.0, achieving an adsorption rate exceeding 80%. The sample with x(Zn) = 0.2 showed a CR adsorption of 50%, while the sample with x(Zn) = 0.0 could absorb only a minimal fraction of CR within 30 min. These findings indicate that introducing zinc ions into the cobalt ferrite structure significantly enhances its catalytic and adsorption properties. The decomposition of hydrogen peroxide was monitored following the completion of the CWPO process, as illustrated in Figure 12e. It was observed that samples with zinc ion concentrations of x(Zn) = 0.2, x(Zn) = 0.4, and x(Zn) = 0.6 achieved the complete decomposition of hydrogen peroxide, exceeding 90% efficiency. Conversely, samples with x(Zn) = 0.8 and x(Zn) = 1.0 demonstrated the capability to decompose hydrogen peroxide by approximately 50%. These findings indicate that the presence of cobalt ions is crucial; in conjunction with iron ions, cobalt ions can generate redox pairs that engage in Fenton-like processes, facilitating the depletion of hydrogen peroxide via radical formation. The increase in zinc ion concentration possibly appears to inhibit this process.
Additionally, experiments were conducted without an adsorption stage, and hydrogen peroxide was introduced immediately into the solution containing CR dye and the catalyst. The concentration of hydrogen peroxide was subsequently monitored after one hour, as depicted in Figure 12f. The results indicate that the CWPO of CR dye increases with higher zinc content, nearing completion when utilizing catalysts with compositions of x(Zn) = 0.4, x(Zn) = 0.6, x(Zn) = 0.8, and x(Zn) = 1.0. By contrast, the CoFe2O4 catalyst achieves a 16% decolorization of CR. Consequently, it can be concluded that the CWPO of CR dye can effectively occur without the necessity for a preliminary adsorption stage, as the samples exhibited significant catalytic properties, enabling the direct degradation of CR azo dye simultaneously with its adsorption.
The degradation mechanism of CR dye by Co-Zn ferrite catalysts proceeds in two stages. The initial stage involves the adsorption of CR molecules onto the ferrite catalyst. This adsorption occurs through electrostatic interactions between the negatively charged anionic molecules of CR dye and the positively charged ferrite surface, as confirmed by the measurements of the point of zero charge (Figure 9). Additionally, hydrogen bonds may form between the hydrogen atoms of the CR molecules and the oxygen atoms present on the ferrite surface. Furthermore, the metal cations located on the ferrite surface can interact with the amine (–NH2) groups of CR dye through coordination bonding, thereby enhancing the adsorption process. The second stage involves the Fenton-like degradation of Congo Red by H2O2, activated by ferrite catalysts. Co(II) ions could act as donors of electrons and H2O2 as acceptors of electrons, resulting in the formation of •OH radicals: Co2+ + H2O2 → Co3+ + •OH + OH−. These radicals attack the CR molecules, resulting in their degradation: CR dye + •OH → intermediates.
The generation of radicals in the catalytic wet peroxide oxidation process is predominantly attributable to electron transfer mechanisms. To substantiate this hypothesis, electrochemical tests may be conducted [35]. In the current investigation, the I–τ response curve was employed to analyze the electron transfer interactions between ferrite catalysts and hydrogen peroxide (Figure 13a). Amperometric I–τ curves were measured using a galvanostat–potentiostat with platinum foil as the counter-electrode and a saturated Ag/AgCl electrode working as the reference electrode, where the working electrode was ferrite catalyst-coated FTO glass. Hydrogen peroxide was injected into the solution with continuous stirring at the hundredth second when the system was already in equilibrium. The catalytic decomposition of H2O2 led to the appearance of free species (radicals) in the solution, which caused a jump in current on the I–τ curve (Figure 13a). The current value was measured at the 200th second as the value of the system response, as shown in Figure 13b. The most intense response was observed for the sample with x(Zn) = 0.4, which corresponds to the highest rate constant observed for this sample (Figure 12c). The following order of current responses was recorded: x(Zn) = 0.4 > x(Zn) = 0.8 > x(Zn) = 0.6 > x(Zn) = 0.2 > x(Zn) = 1.0 > x(Zn) = 0.0. These results suggest that the x(Zn) = 0.4 sample demonstrates the enhanced decomposition of hydrogen peroxide into radicals more efficiently than the other samples.
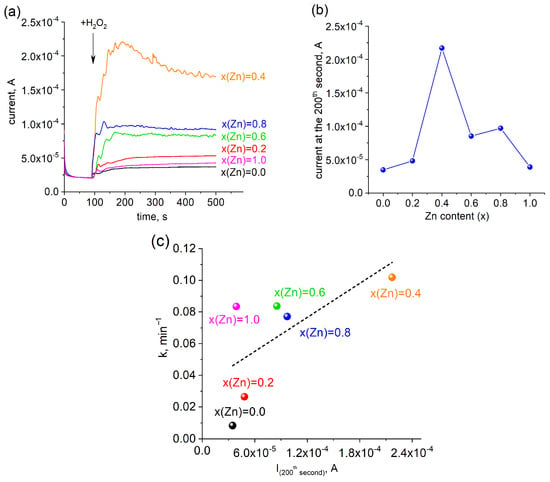
Figure 13.
(a) I–τ curve response for Zn-substituted cobalt ferrites ([CR]0 = 10 mg/L; T = 298 K; [H2O2]0 = 20 mM). (b) Current transmission vs. zinc content. (c) The relationship between the current and rate constant for the Co1−xZnxFe2O4 samples.
The data agreed well with the trend in CR degradation and H2O2 decomposition. There is a positive correlation between the measured values of the reaction rate constant and the magnitude of the current surge on the I–τ curves (Figure 13c). Furthermore, the I–τ response is affected by the size of the interface area, the specific surface area, and the microstructural characteristics of the catalysts.
4. Conclusions
Cobalt–zinc ferrites were synthesized through a ‘green’ synthesis method utilizing black grape extract. The characterization of the spinel structure was performed using X-ray diffraction and FTIR spectroscopy. The FTIR spectra revealed the presence of organic functional groups associated with the extract residues on the surface of the samples (aromatic C–H bonds, C=C bonds, and –CH3 groups). The cation distribution between the A- and B-positions was assessed via Mössbauer spectroscopy. Co(II) and Fe(III) ions were distributed simultaneously in the A- and B-positions, while the Zn(II) ions exclusively occupied the A-position. It was observed that the surface charge of the synthesized ferrites varied with increasing zinc content, indicating a rise in pHPZC from 7.85 for CoFe2O4 to 8.13 for ZnFe2O4. The increase in zinc concentration corresponded with an enhancement in adsorption capacity. The catalytic activity was primarily influenced by the material’s ability to form radicals. Electrochemical testing demonstrated a direct proportionality between the current response and catalytic activity. Upon the addition of hydrogen peroxide to the CR solution at the 100 s interval, a significant increase in current response was observed, indicative of the interaction between the ferrite catalyst and H2O2. The observed improvements in adsorption capacity and catalytic activity for the decomposition of hydrogen peroxide present a promising avenue for utilizing Co–Zn ferrites in water purification applications.
Author Contributions
Conceptualization, T.T.; methodology, T.T., V.K. and M.L.; formal analysis, T.T., V.K. and M.L.; investigation, M.L.; writing—original draft preparation, T.T. and M.L.; writing—review and editing, T.T. and M.L.; visualization, T.T. and M.L.; funding, T.T.; supervision, T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science of Ukraine, grant number 0121U109476.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reddy, D.H.K.; Yun, Y.-S. Spinel Ferrite Magnetic Adsorbents: Alternative Future Materials for Water Purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Qiu, B.; Deng, Y.; Du, M.; Xing, M.; Zhang, J. Ultradispersed Cobalt Ferrite Nanoparticles Assembled in Graphene Aerogel for Continuous Photo-Fenton Reaction and Enhanced Lithium Storage Performance. Sci. Rep. 2016, 6, 29099. [Google Scholar] [CrossRef] [PubMed]
- Hema, E.; Manikandan, A.; Karthika, P.; Antony, S.A.; Venkatraman, B.R. A Novel Synthesis of Zn2+-Doped CoFe2O4 Spinel Nanoparticles: Structural, Morphological, Opto-Magnetic and Catalytic Properties. J. Supercond. Nov. Magn. 2015, 28, 2539–2552. [Google Scholar] [CrossRef]
- Tatarchuk, T. Studying the Defects in Spinel Compounds: Discovery, Formation Mechanisms, Classification, and Influence on Catalytic Properties. Nanomaterials 2024, 14, 1640. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Y.; Zhao, L.; Jiang, Q. Adsorption Capability for Congo Red on Nanocrystalline MFe2O4 (M=Mn, Fe, Co, Ni) Spinel Ferrites. Chem. Eng. J. 2012, 181–182, 72–79. [Google Scholar] [CrossRef]
- Ibiyemi, A.A.; Akinrinola, O.; Yusuf, G.T. Photoelectric and Optoelectronic Effects of Hard Ferromagnetic Manganese Cobalt (Mn–Co) Ferrite Nanoparticles for High-Frequency Device Application. Appl. Phys. A Mater. Sci. Process. 2022, 128, 792. [Google Scholar] [CrossRef]
- Liu, H.; Li, A.; Ding, X.; Yang, F.; Sun, K. Magnetic Induction Heating Properties of Mg1-XZnxFe2O4 Ferrites Synthesized by Co-Precipitation Method. Solid State Sci. 2019, 93, 101–108. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Biological Synthesis of Cobalt Ferrite Nanoparticles. Nanotechnol. Dev. 2012, 2, 9. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.S.; Debnath, A.K.; Muthe, K.P.; Das, N.; Parhi, P. Cobalt Ferrite Nanoparticles Prepared by Microwave Hydrothermal Synthesis and Adsorption Efficiency for Organic Dyes: Isotherms, Thermodynamics and Kinetic Studies. Adv. Powder Technol. 2020, 31, 4552–4562. [Google Scholar] [CrossRef]
- Naidu, K.C.B.; Madhuri, W. Hydrothermal Synthesis of NiFe2O4 Nano-Particles: Structural, Morphological, Optical, Electrical and Magnetic Properties. Bull. Mater. Sci. 2017, 40, 417–425. [Google Scholar] [CrossRef]
- Sun, W.; Pan, W.; Wang, F.; Xu, N. Removal of Se(IV) and Se(VI) by MFe2O4 Nanoparticles from Aqueous Solution. Chem. Eng. J. 2015, 273, 353–362. [Google Scholar] [CrossRef]
- Jasso-Terán, R.; Cortés-Hernández, D.; Sánchez-Fuentes, H.; Reyes-Rodríguez, P.; de-León-Prado, L.; Escobedo-Bocardo, J.; Almanza-Robles, J. Synthesis, Characterization and Hemolysis Studies of Zn(1−x)CaxFe2O4 Ferrites Synthesized by Sol-Gel for Hyperthermia Treatment Applications. J. Magn. Magn. Mater. 2017, 427, 241–244. [Google Scholar] [CrossRef]
- Jadhav, S. Sol-Gel Auto Combustion Synthesis and Structural Analysis of Cobalt Ferrite Nanoparticles. Int. Res. J. Sci. Eng. 2018, A5, 116–118. [Google Scholar]
- Nesheva, D.; Dzhurkov, V.; Stambolova, I.; Blaskov, V.; Bineva, I.; Calderon Moreno, J.M.; Preda, S.; Gartner, M.; Hristova-Vasileva, T.; Shipochka, M. Surface Modification and Chemical Sensitivity of Sol Gel Deposited Nanocrystalline ZnO Films. Mater. Chem. Phys. 2018, 209, 165–171. [Google Scholar] [CrossRef]
- Shreyash, N.; Bajpai, S.; Khan, M.A.; Vijay, Y.; Tiwary, S.K.; Sonker, M. Green Synthesis of Nanoparticles and Their Biomedical Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 11428–11457. [Google Scholar] [CrossRef]
- Zhou, D.D.; Li, J.; Xiong, R.G.; Saimaiti, A.; Huang, S.Y.; Wu, S.X.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef]
- Serra, M.; Casas, A.; Teixeira, J.A.; Barros, A.N. Revealing the Beauty Potential of Grape Stems: Harnessing Phenolic Compounds for Cosmetics. Int. J. Mol. Sci. 2023, 24, 11751. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape Bioactive Molecules, and the Potential Health Benefits in Reducing the Risk of Heart Diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-Adding to Grape Waste: Green Synthesis of Gold Nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Farokhi, G.; Saidi, M. Catalytic Activity of Bimetallic Spinel Magnetic Catalysts (NiZnFe2O4, CoZnFe2O4 and CuZnFe2O4) in Biodiesel Production Process from Neem Oil: Process Evaluation and Optimization. Chem. Eng. Process.—Process Intensif. 2022, 181, 109170. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Danyliuk, N.; Shyichuk, A.; Kotsyubynsky, V.; Lapchuk, I.; Mandzyuk, V. Green Synthesis of Cobalt Ferrite Using Grape Extract: The Impact of Cation Distribution and Inversion Degree on the Catalytic Activity in the Decomposition of Hydrogen Peroxide. Emergent Mater. 2022, 5, 89–103. [Google Scholar] [CrossRef]
- Altowayti, W.A.; Salem, A.A.; Al-Fakih, A.M.; Bafaqeer, A.; Shahir, S.; Tajarudin, H.A. Optimization of As(V) Removal by Dried Bacterial Biomass: Nonlinear and Linear Regression Analysis for Isotherm and Kinetic Modelling. Metals 2022, 12, 1664. [Google Scholar] [CrossRef]
- Milutinović, A.; Lazarević, Z.Ž.; Šuljagić, M.; Andjelković, L. Synthesis-Dependent Structural and Magnetic Properties of Monodomain Cobalt Ferrite Nanoparticles. Metals 2024, 14, 833. [Google Scholar] [CrossRef]
- Torres, T.E.; Lima Jr, E.; Mayoral, A.; Ibarra, A.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Validity of the Néel-Arrhenius Model for Highly Anisotropic CoxFe3−xO4 Nanoparticles. J. Appl. Phys. 2015, 118, 183902. [Google Scholar] [CrossRef]
- Muscas, G.; Cobianchi, M.; Lascialfari, A.; Cannas, C.; Musinu, A.; Omelyanchik, A.; Peddis, D. Magnetic Interactions vs. Magnetic Anisotropy in Spinel Ferrite Nanoparticles. IEEE Magn. Lett. 2019, 10, 6110305. [Google Scholar] [CrossRef]
- Liu, B.H.; Ding, J.; Dong, Z.L.; Boothroyd, C.B.; Yin, J.H.; Yi, J.B. Microstructural Evolution and Its Influence on the Magnetic Properties of CoFe2O4 Powders during Mechanical Milling. Phys. Rev. B 2006, 74, 184427. [Google Scholar] [CrossRef]
- Hasz, K.; Ijiri, Y.; Krycka, K.L.; Borchers, J.A.; Booth, R.A.; Oberdick, S.; Majetich, S.A. Particle Moment Canting in CoFe2O4 Nanoparticles. Phys. Rev. B 2014, 90, 180405. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple Spinels; Crystallographic Parameters, Cation Radii, Lattice Energies, and Cation Distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Rao, K.; Choudary, G.; Rao, K.; Sujatha, C. Structural and Magnetic Properties of Ultrafine CoFe2O4 Nanoparticles. Procedia Mater. Sci 2015, 10, 19–27. [Google Scholar] [CrossRef]
- Maiti, D.; Mukhopadhyay, S.; Devi, P.S. Evaluation of Mechanism on Selective, Rapid, and Superior Adsorption of Congo Red by Reusable Mesoporous α-Fe2O3 Nanorods. ACS Sustain. Chem. Eng. 2017, 5, 11255–11267. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Daza, J.; Escoda, L.; Fiol, N.; Pineda, E.; Khitouni, M.; Suñol, J.-J. Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes. Metals 2023, 13, 474. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Aoopngan, C.; Nonkumwong, J.; Phumying, S.; Promjantuek, W.; Maensiri, S.; Noisa, P.; Pinitsoontorn, S.; Ananta, S.; Srisombat, L. Amine-Functionalized and Hydroxyl-Functionalized Magnesium Ferrite Nanoparticles for Congo Red Adsorption. ACS Appl. Nano Mater. 2019, 2, 5329–5341. [Google Scholar] [CrossRef]
- Castro, L.; Ayala, L.A.; Vardanyan, A.; Zhang, R.; Muñoz, J.Á. Arsenate and Arsenite Sorption Using Biogenic Iron Compounds: Treatment of Real Polluted Waters in Batch and Continuous Systems. Metals 2021, 11, 1608. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, X.; Wang, G.; Qian, B.; Liu, Y.; Hu, X.; He, B. Modulating Mesoporous Co3O4 Hollow Nanospheres with Oxygen Vacancies for Highly e Ffi Cient Peroxymonosulfate Activation. Chem. Eng. J. 2020, 400, 125869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).