Abstract
The use of recombinant human erythropoietin (rHuEPO) has been found to improve different cardiopulmonary-related variables that ultimately enhance endurance performance. The main goal of this systematic review was to analyze the hematological, physiological, and performance effects (both maximal and submaximal) of rHuEPO in well-trained endurance athletes. A literature search was conducted in three different databases (PubMed, Web of Science, and Scopus) on 20 January 2025; including studies published from 1 January 2010 to the search date. After analyzing 985 resultant articles and 5 records identified outside of the databases through citation tracking, 10 studies that met the inclusion criteria were included in the systematic review. We found that, regardless of the total dose of rHuEPO used, this substance improves the main hematological (total hemoglobin mass, hemoglobin concentration, and hematocrit) and physiological (maximal oxygen uptake and peak oxygen uptake) parameters, while the maximal performance-related parameters (mainly, maximal power output, and peak power output) also tend to increase. However, further research is needed to determine if rHuEPO can also improve submaximal parameters, which are also major determinants of performance in endurance sports.
1. Introduction
Erythropoietin (EPO) is the hematopoietic growth factor responsible for stimulating erythropoiesis in response to cellular hypoxia [1]. EPO is a glycoprotein hormone characterized by a protein skeleton of 165 amino acids with four carbohydrate chains, each containing two to four sialic acid residues [2]. Most EPO is produced in the peritubular interstitial cells of the renal cortex and medulla, with approximately 15% synthesized in the liver [3]. EPO plays a central role in regulating erythrocyte production by recruiting and differentiating erythroid progenitor cells in the bone marrow, supporting their maintenance and survival, and stimulating hemoglobin (Hb) synthesis [4]. In the 1980s, recombinant human erythropoietin (rHuEPO) was developed to address clinical conditions requiring this vital hormone, particularly anemia associated with chronic renal dysfunction [5].
Beyond its clinical applications, rHuEPO gained notoriety as a doping agent in endurance sports such as road cycling, swimming, middle- and long-distance running, and triathlon. These sports rely on complex physiological determinants of performance, including maximal oxygen uptake (VO2max), effort economy, blood lactate concentration at various intensities ([La−]), and time to exhaustion (TTE) [6,7]. VO2max, the primary indicator of aerobic capacity, reflects the maximum oxygen volume a person can absorb, transport, and utilize during maximal exertion [8]. However, other parameters, such as effort economy, that is, the energy consumed per unit of velocity, emerged as critical predictors of endurance performance [9]. Additionally, power output in cycling and running times in middle- and long-distance events are key performance markers [10,11,12,13].
The primary goal of rHuEPO use in sports is to increase erythrocyte count, elevate Hb levels, and enhance oxygen delivery to tissues, thereby improving VO2max and performance in aerobic exercise [14]. Despite its efficacy, rHuEPO belongs to the class of erythropoiesis-stimulating agents (ESAs) banned by the World Anti-Doping Agency (WADA) in 1990 [15,16].
Multiple reviews examined the impact of rHuEPO on endurance athletes. High-quality systematic reviews presented conflicting evidence: while some authors found no definitive performance-enhancing effects in elite cyclists despite increases in Hb, hematocrit (Hct), and VO2max [17], others concluded that rHuEPO could enhance hematological and pulmonary parameters, maximal power output (Pmax), and TTE [18]. These improvements were most evident during maximal intensities, which could be less relevant for cyclic endurance sports that require sustained submaximal efforts.
Conversely, narrative reviews with less rigorous methodologies highlighted significant enhancements in hematological, pulmonary, and performance parameters [19,20]. However, the lack of systematic approaches in these studies raises concerns about selection bias.
Nonetheless, rHuEPO remains a controversial substance in sports, both for its ethical implications and potential health risks. Considering the scientific evidence published to date, this systematic review aims to investigate the hematological, physiological, and performance effects (both maximal and submaximal) of rHuEPO use in well-trained endurance athletes. We hypothesize that rHuEPO will yield clear improvements in these parameters in this population.
2. Materials and Methods
2.1. Search Strategy
A comprehensive literature search was conducted on 20 January 2025, across three major scientific databases (PubMed, Web of Science, and Scopus) to identify relevant studies. The review included studies published between 1 January 2010 and 20 January 2025. The procedure adhered strictly to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and reproducibility. Further details, including the PRISMA 2020 checklists for the abstract (Supplementary Material File S1) and the main text (Supplementary Material File S2), can be found in the Supplementary Materials. The search in each database was performed in the areas of title, abstract, and keywords. For this purpose, combined with Boolean operators, different lines composed of keywords referring to different areas of the subject were used. Keywords in the same line were combined with the “OR” operator, while those in different lines were combined with the “AND” operator (Table 1).

Table 1.
Keywords and operators used in the search strategy.
Both authors (A.A.-G. and J.S.-C.) independently conducted a blinded search of study titles, abstracts, and keywords to ensure the accuracy and reproducibility of the results. After completing the initial selection, both researchers met to finalize the list of included studies. Subsequently, they independently conducted a full-text review of the eligible studies and reconvened to confirm the final selection. No discrepancies or ambiguities arose during the selection process, as both researchers reached a high level of agreement, unanimously confirming that all selected studies met the inclusion criteria.
Thus, the following combination of keywords and Boolean operators was used: (Erythropoietin OR EPO) AND (performance OR endurance) AND (athletes). Apart from the established date limits (1 January 2010–25 January 2025), no other limits or filters were used in the database searches.
2.2. Data Collection and Analysis
The results from the literature search were uploaded to Rayyan software (Rayyan Systems Inc., Doha, Qatar, web version), a powerful online tool designed to facilitate the methodology process in systematic reviews and meta-analyses [21]. The application allows us to analyze the abstract (introduction, objectives, methodology, results, and conclusions) of the general characteristics (type of publication, language, authors, etc.) of the studies found in the literature search. In addition, it offers the possibility of analyzing and classifying the studies one by one, being a valuable tool for carrying out systematic reviews [22].
2.3. Inclusion and Exclusion Criteria
Studies included in this systematic review met the following inclusion criteria: (a) studies with an experimental design (meta-analyses, systematic reviews, and other types of reviews are excluded); (b) studies conducted with human subjects; (c) studies in which participants were healthy, without any type of disease; (d) studies in which participants were endurance trained (VO2max = ≥45 mL·kg−1·min−1 or ≥3.5 L·min−1, a threshold established based on the limits defined in previous study [23]); (e) studies in which at least one performance-related parameter was reported; and (f) studies in which at least one of the performance-related parameters can be objectively measured. After applying the inclusion and exclusion criteria, the following data were extracted from each study: name of the first author, year of publication, intervention and placebo group characteristics, rHuEPO dose, duration of intervention, measured key variables, and rHuEPO administration effects on measured key variables.
2.4. Assessment of Methodological Quality
To assess the methodological quality of the studies included in this systematic review, the Oxford Level of Evidence scale [24] and the Physiotherapy Evidence Database (PEDro) scale were used [25]. The Oxford Level of Evidence scale measures the level of evidence of a study on a scale ranging from level 1a to level 5, with level 1a being systematic reviews of high-quality randomized controlled trials and level 5 being expert opinion. On the other hand, the PEDro scale is made up of 11 different items related to scientific precision [26]. Both authors (A.A.-G. and J.S.-C.) independently conducted the quality assessments of the selected studies, and consensus on the scores was achieved through meetings.
3. Results
3.1. Study Selection
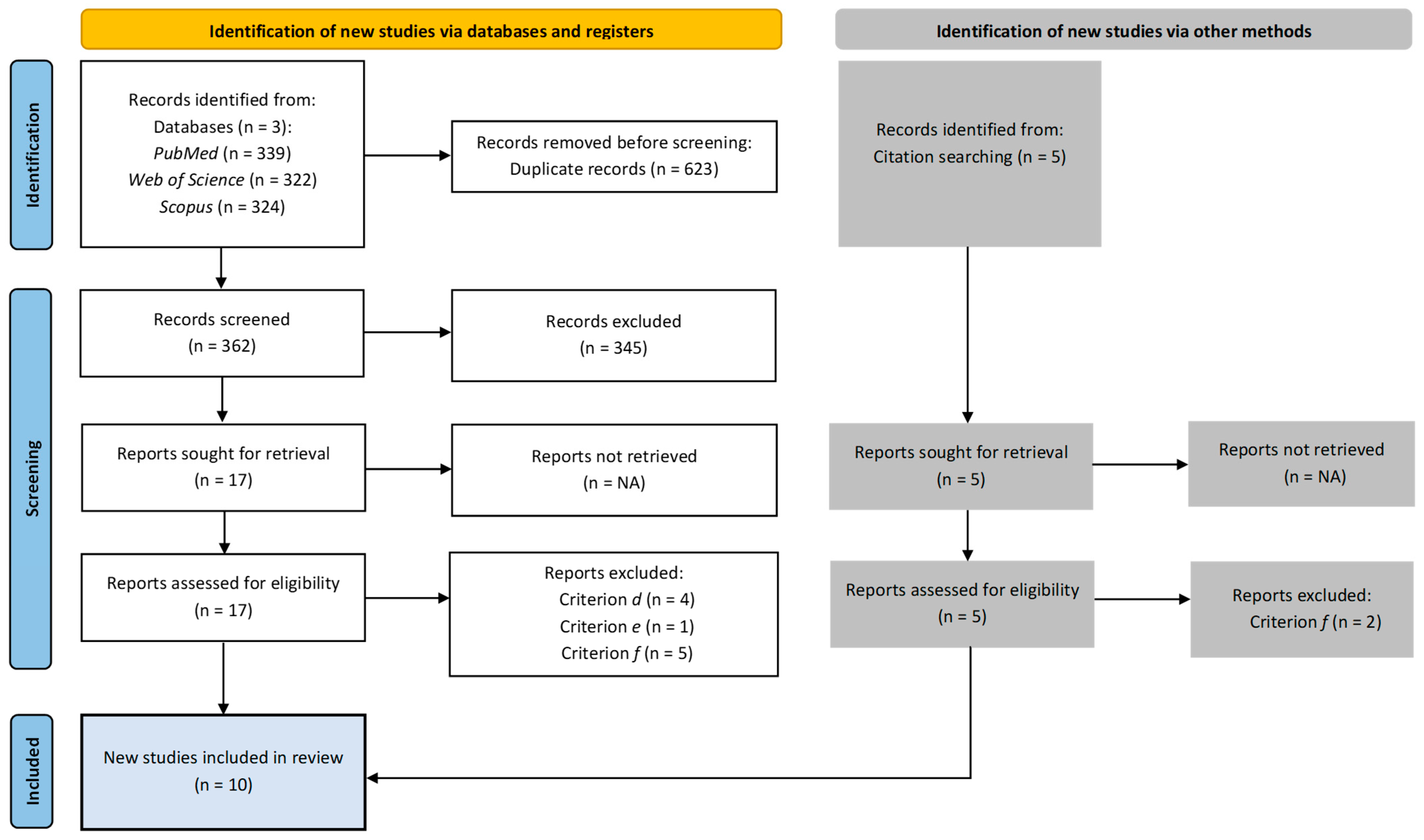
A total of ten papers were identified for inclusion in the review. Figure 1 presents detailed information on the study selection process.
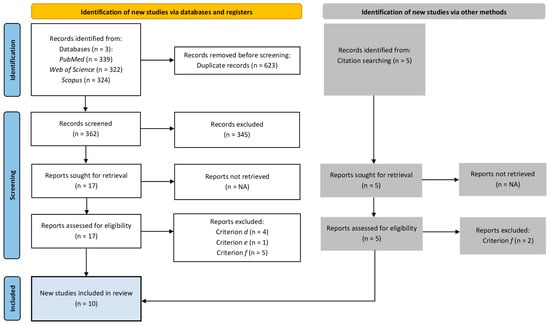
Figure 1.
Study selection process.
The search of the electronic databases provided a total of 985 citations. After duplication removal, the final number of citations was 362. Of these, 345 were eliminated after screening the titles, abstracts, and keywords. Seventeen full-text papers were examined for final confirmation of eligibility criteria. Additionally, five records were identified outside of the databases through citation tracking. In total, twelve studies did not meet the inclusion criteria.
3.2. Methodological Quality and Level of Evidence
Table 2 presents the evaluation of the methodological quality of the studies using the PEDro scale (also used to evaluate the risk of bias for each study) and the Oxford Level of Evidence framework. The average PEDro score was 7.2, reflecting high methodological standards overall. Six studies were categorized as level 1b, indicating high-quality randomized controlled trials, while four were classified as level 2b, representing cohort studies or lower-quality trials. Key strengths included robust randomization, allocation concealment, and blinding of outcome assessors. However, limitations such as incomplete blinding of therapists and variability in reporting key outcomes were noted in some studies. Despite these issues, the strong design and high evidence level of most studies ensure robustness in outcome measurement. Nonetheless, caution is warranted due to heterogeneity in study designs and intervention protocols, which may influence the generalizability of the findings.

Table 2.
Physiotherapy evidence database (PEDro) ratings and Oxford levels of the included studies *.
3.3. Tools and Protocols
To collect and analyze hematological variables, all studies included in this systematic review [10,11,12,13,27,28,29,30,31,32], employed blood gas analyzers as the primary measurement tool. These devices ensured accurate assessment of hematological parameters critical to evaluating the effects of rHuEPO administration.
In all the aforementioned studies, physiological variables were measured using a combination of tools and protocols. Specifically, a cycloergometer paired with a gas analyzer was used in conjunction with an incremental exercise protocol performed until exhaustion. This setup provided precise data on parameters such as oxygen consumption and ventilatory thresholds.
For studies focusing on maximal performance-related variables [11,12,13,28,30,31,32], a cycloergometer equipped with a power meter was employed. These measurements also relied on an incremental exercise protocol to exhaustion, ensuring reliable quantification of power output and related variables.
Finally, submaximal performance-related variables were assessed in several studies [10,11,12,13,27,30,31]. In this context, a cycloergometer or a bicycle equipped with a power meter was used, but the protocol differed. Instead of an incremental approach, a time trial protocol at submaximal intensity was implemented, allowing for the evaluation of sustained performance and efficiency under controlled conditions.
3.4. Study Characteristics
Table 3 indicates the characteristics of the included studies.

Table 3.
Characteristics of the included studies *.
3.5. Key Findings
The tools and protocols employed to obtain results have been consistent across the studies included in this systematic review. The primary differences between studies lie in the dosage of rHuEPO administered and the duration of treatment. This consistency in methodology allows for meaningful comparisons between studies and facilitates the identification of causal relationships in the observed outcomes.
Significant increases in hematological variables were reported in 9 out of the 10 studies analyzed [10,11,12,13,27,28,29,31,32]. Total hemoglobin mass (tHb) increased by 6.7% to 19.7%, Hb by 8.3% to 16.2%, and Hct by 2.6% to 18.8% relative to baseline values.
Similarly, physiological variables demonstrated significant increases in 9 out of 10 studies [10,11,12,13,27,28,29,31,32]. The reported improvements included increases in VO2max ranging from 2.6% to 10%, and peak oxygen uptake (VO2peak) ranging from 4.2% to 6.2% relative to baseline values.
Of the seven studies that analyzed maximal performance-related variables, four reported significant improvements [11,12,13,31]. Specifically, Pmax and peak power output (Ppeak) increased by 2.7% to 5.8% relative to baseline values. On the other hand, submaximal performance-related variables showed significant improvements in 3 out of 7 studies [10,11,13]. Notable increases were observed in the constant load test limit time (tlim) during time trials, ranging from 4.3% to 69.7%, and in the 3000 m race time, which improved by 4.6% to 5.7% relative to baseline values.
The systematic review reveals that the analyzed studies do not demonstrate a dose-dependent effect of rHuEPO on the investigated variables. Specifically, no greater effect, reflected as a higher percentage increase relative to baseline values, has been observed in studies administering higher doses of rHuEPO, nor has a diminished effect been evident with lower doses.
4. Discussion
4.1. Interpretation of Findings
This systematic review suggests that the administration of rHuEPO leads to significant increases in key hematological parameters, including tHb, Hb, and Hct. These effects are physiologically attributable to the synthetic stimulation of erythrocyte production induced by rHuEPO, a member of the ESAs group [33,34]. Similar increases in hematological parameters have been consistently reported in other reviews investigating the effects of rHuEPO on endurance-trained athletes [17,18]. These hematological enhancements facilitate greater oxygen delivery to tissues [35], which, as observed in this review, translates into improvements in key physiological variables, particularly VO2max and VO2peak. This aligns with findings from previous studies analyzing the same physiological outcomes in comparable populations and settings [17,20].
The importance of tHb as a key determinant of endurance performance has been further supported by recent findings [36], which establish reference values for tHb in well-trained endurance athletes. This study reports an average tHb of approximately 960 g in male athletes and 625 g in female athletes, aligning with the notion that endurance-trained individuals exhibit significantly higher values compared to non-athletes. These values are consistent with the increases observed in this systematic review, where rHuEPO administration led to significant enhancements in tHb across multiple studies. Given that tHb is more strongly correlated with VO2max than Hb alone [37], these reference values provide a useful framework to contextualize the hematological effects of rHuEPO. Notably, the increases in tHb following rHuEPO administration in the studies analyzed in this review approach or even exceed the upper range of natural variations reported in endurance athletes [36]. Consequently, these findings highlight the necessity of advanced monitoring strategies that integrate individual baseline tHb values to improve the detection of artificial hematological manipulations.
In addition to hematological and physiological parameters, maximal performance-related variables, such as Pmax and Ppeak, showed significant improvements in four out of the seven studies included in this review [11,12,13,31]. This indicates that increases in hematological and physiological variables can contribute to enhanced performance in over half of the studies, a finding corroborated by other reviews [20]. Conversely, the evidence for the effects of rHuEPO on submaximal performance variables, such as tlim and running times over specific distances, remains inconsistent. While significant improvements were observed in only three out of seven studies in this review [10,11,13], previous reviews reported both positive changes [20] and negligible changes [17,18].
The findings of this systematic review suggest that rHuEPO administration, regardless of the total dose, consistently enhances key hematological and physiological parameters and tends to improve maximal performance-related outcomes. However, further research is required to elucidate its effects on submaximal parameters, which are critical determinants of performance in cyclic endurance sports.
The ergogenic effects of rHuEPO, particularly its capacity to enhance hematological and physiological parameters, pose a significant challenge for anti-doping detection methods [38,39]. This difficulty arises because, while rHuEPO administration leads to measurable increases in tHb, Hb, Hct, VO2max, and VO2peak, its pharmacokinetics, including a variable elimination half-life influenced by renal clearance and dosing strategies, can complicate detection [40]. Additionally, the use of microdoses, as suggested by recent research [41], may further obscure detection by sustaining performance-enhancing effects while keeping hematological fluctuations within the individual reference ranges of the athlete biological passport [42]. Furthermore, the structural similarity between exogenous rHuEPO and endogenous EPO necessitates highly sensitive analytical techniques, such as isoelectric focusing and mass spectrometry, to differentiate their isoforms [43]. Consequently, the interplay between the physiological enhancements of rHuEPO and current anti-doping limitations underscores the need for continued advancements in detection methodologies.
Considering all this, some competitive athletes continue to exploit the ergogenic effects of rHuEPO, disregarding its ban by WADA since the 1990s [15], and exposing themselves to severe health risks, including thrombotic complications [17].
4.2. Limitations
The present review has certain limitations that must be acknowledged. Firstly, the treatment durations and total doses of rHuEPO administered varied across most studies, with only two investigations utilizing the same protocol [10,12]. This heterogeneity complicates direct comparisons of the analyzed variables under identical dosing conditions. However, the diversity in dosing protocols allows for broader conclusions on the effects of varying rHuEPO doses. Notably, as observed in this review, the ergogenic effects of rHuEPO appear to be independent of the total dose, at least within the range examined in the included studies.
Secondly, the sample sizes in 6 of the 10 studies included in this review were relatively small (ranging from 6 to 29 participants) [10,27,28,29,30,32]. In contrast, the remaining four studies had larger sample sizes (ranging from 39 to 48 participants) [11,12,13,31]. This discrepancy in sample sizes introduces variability that poses challenges when making meaningful comparisons across studies. Additionally, only 3 of 10 studies included female participants [13,28,30], limiting the ability to draw sex-specific conclusions and underscoring the need for more inclusive research designs in this area.
4.3. Future Directions
Future research should focus on investigating the effects of rHuEPO on submaximal performance variables in cyclic endurance sports and well-trained athletes. These variables are crucial determinants of performance in this type of sport, yet the current evidence is insufficient to draw clear conclusions regarding their responsiveness to rHuEPO administration.
Furthermore, studies dedicated exclusively to analyzing the effects of microdoses of rHuEPO in endurance athletes are warranted. Microdoses may provide an ergogenic advantage [41], while evading detection by standard anti-doping protocols, such as the biological passport [42]. Developing robust methods to reliably detect microdose usage is essential to prevent athletes from gaining illicit advantages and to ensure fair competition.
5. Conclusions
This systematic review highlights the varying effects of rHuEPO on hematological, physiological, and performance parameters in well-trained athletes involved in endurance sports. Irrespective of the total dose administered, rHuEPO consistently enhances key hematological markers such as tHb, Hb, and Hct, alongside physiological parameters including VO2max and VO2peak.
Regarding performance outcomes, rHuEPO administration tends to improve maximal performance variables, particularly Pmax and Ppeak. However, evidence for its impact on submaximal performance parameters remains inconclusive, warranting further research to clarify its role in metrics that are critical determinants of endurance performance.
The findings underscore rHuEPO’s capacity to artificially augment oxygen transport and delivery by increasing erythrocyte mass, conferring a physiological advantage to athletes who utilize it illicitly. This reinforces the ethical and health concerns surrounding its use, particularly in competitions where such practices are prohibited, as it provides an unfair advantage and poses significant health risks.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sports13030078/s1, File S1: PRISMA 2020 for Abstracts Checklist; File S2: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, A.A.-G. and J.S.-C.; methodology, A.A.-G. and J.S.-C.; formal analysis, A.A.-G.; investigation, A.A.-G.; data curation, A.A.-G.; writing—original draft preparation, A.A.-G.; writing—review and editing, A.A.-G. and J.S.-C.; visualization, A.A.-G. and J.S.-C.; supervision, J.S.-C.; project administration, J.S.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basque Government, grant number IT1726-22.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created or analyzed in this study. Data sharing is not applicable to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin, T.Y.; Lai, Y.F.; Chen, Y.H.; Lu, D.W. The latest evidence of erythropoietin in the treatment of glaucoma. Int. J. Mol. Sci. 2022, 23, 16038. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Recombinant human erythropoietin and its analogues. In Introduction to Biological and Small Molecule Drug Research and Development, 1st ed.; Ganellin, R., Roberts, S., Jefferis, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 307–326. [Google Scholar]
- Yasuoka, Y.; Izumi, Y.; Fukuyama, T.; Inoue, H.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; Shimada, Y.; Nagaba, Y.; et al. Effects of angiotensin II on erythropoietin production in the kidney and liver. Molecules 2021, 26, 5399. [Google Scholar] [CrossRef]
- Jelkmann, W. Regulation of erythropoietin production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, F.H.; Fliser, D. Erythropoietin and renoprotection. Curr. Opin. Nephrol. Hypertens. 2009, 18, 15–20. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Physiological and performance characteristics of male professional road cyclists. Sports Med. 2001, 31, 479–487. [Google Scholar] [CrossRef]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol 2008, 586, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.N.; Raven, P.B.; Snell, P.G.; Stray-Gundersen, J.; Levine, B.D. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med. Sci. Sports Exerc. 2007, 39, 103–107. [Google Scholar]
- Peyré-Tartaruga, L.A.; Machado, E.; Guimarães, P.; Borba, E.; Tartaruga, M.P.; Buzzachera, C.F.; Correale, L.; Lanferdini, F.J.; da Silva, E.S. Biomechanical, physiological and anthropometrical predictors of performance in recreational runners. PeerJ 2024, 12, e16940. [Google Scholar] [CrossRef]
- Durussel, J.; Daskalaki, E.; Anderson, M.; Chatterji, T.; Wondimu, D.H.; Padmanabhan, N.; Patel, R.K.; McClure, J.D.; Pitsiladis, Y.P. Haemoglobin mass and running time trial performance after recombinant human erythropoietin administration in trained men. PLoS ONE 2013, 8, e56151. [Google Scholar] [CrossRef]
- Annaheim, S.; Jacob, M.; Krafft, A.; Breymann, C.; Rehm, M.; Boutellier, U. RhEPO improves time to exhaustion by non-hematopoietic factors in humans. Eur. J. Appl. Physiol. 2016, 116, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Haile, D.W.; Durussel, J.; Mekonen, W.; Ongaro, N.; Anjila, E.; Mooses, M.; Daskalaki, E.; Mooses, K.; Mcclure, J.D.; Sutehall, S.; et al. Effects of EPO on blood parameters and running performance in Kenyan athletes. Med. Sci. Sports Exerc. 2019, 51, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.B.; Graae, J.; Bejder, J.; Bonne, T.C.; Seier, S.; Debertin, M.; Eibye, K.; Hostrup, M.; Nordsborg, N.B. Micro-doses of recombinant human erythropoietin enhance time trial performance in trained males and females. Med. Sci. Sports Exerc. 2023, 55, 311–321. [Google Scholar] [CrossRef]
- Perrey, S. Do we perform better when we increase red blood cells? Lancet Haematol. 2017, 4, e344–e345. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Konstantinopoulos, P.A.; Papailiou, J.; Kandarakis, S.A.; Andreopoulos, A.; Sykiotis, G.P. Erythropoietin abuse and erythropoietin gene doping: Detection strategies in the genomic era. Sports Med. 2005, 35, 831–840. [Google Scholar] [CrossRef]
- Robinson, N.; Giraud, S.; Saudan, C.; Baume, N.; Avois, L.; Mangin, P.; Saugy, M. Erythropoietin and blood doping. Br. J. Sports Med. 2006, 40, i30–i34. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.A.; Cohen Tervaert, J.M.; Schepers, F.M.; Vliegenthart, A.D.; Rotmans, J.I.; Daniels, J.M.; Burggraaf, J.; Cohen, A.F. Erythropoietin doping in cycling: Lack of evidence for efficacy and a negative risk-benefit. Br. J. Clin. Pharmacol. 2013, 75, 1406–1421. [Google Scholar] [CrossRef]
- Trinh, K.V.; Diep, D.; Chen, K.J.Q.; Huang, L.; Gulenko, O. Effect of erythropoietin on athletic performance: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2020, 6, e000716. [Google Scholar] [CrossRef]
- Bird, S.R.; Goebel, C.; Burke, L.M.; Greaves, R.F. Doping in sport and exercise: Anabolic, ergogenic, health and clinical issues. Ann. Clin. Biochem. 2016, 53, 196–221. [Google Scholar] [CrossRef]
- Sgrò, P.; Sansone, M.; Sansone, A.; Romanelli, F.; Di Luigi, L. Effects of erythropoietin abuse on exercise performance. Phys. Sportsmed. 2018, 46, 105–115. [Google Scholar] [CrossRef]
- Johnson, N.; Phillips, M. Rayyan for systematic reviews. J. Electron. Resour. Libr. 2018, 30, 46–48. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Edvardsen, E.; Hansen, B.H.; Holme, I.M.; Dyrstad, S.M.; Anderssen, S.A. Reference values for cardiorespiratory response and fitness on the treadmill in a 20-to 85-year-old population. Chest 2013, 144, 241–248. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The 2011 Oxford CEBM Level of Evidence. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 16 January 2025).
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Baz-Valle, E.; Fontes-Villalba, M.; Santos-Concejero, J. Total number of sets as a training volume quantification method for muscle hypertrophy: A systematic review. J. Strength Cond. Res. 2021, 35, 870–878. [Google Scholar] [CrossRef]
- Caillaud, C.; Connes, P.; Ben Saad, H.; Mercier, J. Erythropoietin enhances whole body lipid oxidation during prolonged exercise in humans. J. Physiol. Biochem. 2015, 71, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Woolford, S.M.; Eastwood, A.; Sharpe, K.; Barnes, P.G.; Gore, C.J. Temporal changes in physiology and haematology in response to high-and micro-doses of recombinant human erythropoietin. Drug Test. Anal. 2017, 9, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe-Grau, A.; Plenge, U.; Helbo, S.; Kristensen, M.; Andersen, P.R.; Fago, A.; Belhage, B.; Dela, F.; Helge, J.W. Effects of an 8-weeks erythropoietin treatment on mitochondrial and whole body fat oxidation capacity during exercise in healthy males. J. Sports Sci. 2015, 33, 570–578. [Google Scholar] [CrossRef]
- Haider, T.; Diaz, V.; Albert, J.; Alvarez-Sanchez, M.; Thiersch, M.; Maggiorini, M.; Hilty, M.P.; Spengler, C.M.; Gassmann, M. A single 60.000 IU dose of erythropoietin does not improve short-term aerobic exercise performance in healthy subjects: A randomized, double-blind, placebo-controlled crossover trial. Front. Physiol. 2020, 11, 537389. [Google Scholar] [CrossRef]
- Heuberger, J.A.; Rotmans, J.I.; Gal, P.; Stuurman, F.E.; van’t Westende, J.; Post, T.E.; Daniels, J.M.; Moerland, M.; van Veldhoven, P.L.; de Kam, M.L.; et al. Effects of erythropoietin on cycling performance of well trained cyclists: A double-blind, randomised, placebo-controlled trial. Lancet Haematol. 2017, 4, e374–e386. [Google Scholar] [CrossRef]
- Sutehall, S.; Martin-Rincon, M.; Wang, G.; Shurlock, J.; Durussel, J.; Mooses, M.; Wang, J.; Pitsiladis, Y.P. The performance effects of microdose recombinant human erythropoietin administration and carbon monoxide rebreathing. Curr. Sports Med. Rep. 2018, 17, 457–466. [Google Scholar] [CrossRef]
- Larsen, M.S.; Vissing, K.; Thams, L.; Sieljacks, P.; Dalgas, U.; Nellemann, B.; Christensen, B. Erythropoietin administration alone or in combination with endurance training affects neither skeletal muscle morphology nor angiogenesis in healthy young men. Exp. Physiol. 2014, 99, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.S.; Kahn, M.J. Blood doping: Then and now. A narrative review of the history, science and efficacy of blood doping in elite sport. Blood Rev. 2020, 39, 100632. [Google Scholar] [CrossRef]
- Totonchi, Z.; Noohi, F.; Futuhi, F.; Azarfarin, R.; Radbin, P. Effects of recombinant erythropoietin on hemoglobin levels and blood transfusion needs in patients with preoperative anemia undergoing cardiac surgery. Ann. Card. Anaesth. 2022, 25, 466–471. [Google Scholar] [CrossRef]
- Kasiak, P.; Kowalski, T.; Faiss, R.; Malczewska-Lenczowska, J. Hemoglobin mass is accurately predicted in endurance athletes. J. Sports Sci. 2025. ahead-of-print. [Google Scholar]
- Schmidt, W.; Prommer, N. Impact of alterations in total hemoglobin mass on VO2max. Exerc. Sport Sci. Rev. 2010, 38, 68–75. [Google Scholar] [CrossRef]
- Lundby, C.; Achman-Andersen, N.J.; Thomsen, J.J.; Norgaard, A.M.; Robach, P. Testing for recombinant human erythropoietin in urine: Problems associated with current anti-doping testing. J. Appl. Physiol. 2008, 105, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Durussel, J.; Haile, D.W.; Mooses, K.; Daskalaki, E.; Beattie, W.; Mooses, M.; Mekonen, W.; Ongaro, N.; Anjila, E.; Patel, R.K.; et al. Blood transcriptional signature of recombinant human erythropoietin administration and implications for antidoping strategies. Physiol. Genom. 2016, 48, 202–209. [Google Scholar] [CrossRef]
- Athanasiadou, I.; Dokoumetzidis, A.; Voss, S.C.; El Saftawy, W.; Al-Maadheed, M.; Valsami, G.; Georgakopoulos, C. Hyperhydration effect on pharmacokinetic parameters and detection sensitivity of recombinant human erythropoietin in urine and serum doping control analysis of males. J. Pharm. Sci. 2019, 108, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Ashenden, M.; Varlet-Marie, E.; Lasne, F.; Audran, M. The effects of microdose recombinant human erythropoietin regimens in athletes. Haematologica 2006, 91, 1143–1144. [Google Scholar]
- Ashenden, M.; Gough, C.E.; Garnham, A.; Gore, C.J.; Sharpe, K. Current markers of the Athlete Blood Passport do not flag microdose EPO doping. Eur. J. Appl. Physiol. 2011, 111, 2307–2314. [Google Scholar] [CrossRef]
- Agoston, R.; Izake, E.L.; Sivanesan, A.; Lott, W.B.; Sillence, M.; Steel, R. Rapid isolation and detection of erythropoietin in blood plasma by magnetic core gold nanoparticles and portable Raman spectroscopy. Nanomedicine 2016, 12, 633–641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).