Demographic and Functional Consequences of Secondary Host Selection in a Facultative Autoparasitoid, Encarsia sophia (Hymenoptera: Aphelinidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plants
2.2. Insects
2.3. Primary and Secondary Hosts
2.4. Life Table Study: Developmental Duration of Females and Males
2.5. Reproduction and Pest Control Capacity Assays
2.6. Data Analysis
3. Results
3.1. Life Table of E. sophia Reproduced by Different Secondary Hosts
3.2. Host-Feeding Rate and Pest-Killing Rate
3.3. Population Projection
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Baaren, J.; Wist, T.; Soroka, J.; Tougeron, K. Host-parasitoid network in extreme conditions: The case of cereal aphids in wheat crops in Saskatchewan, Canada. Entomologia 2020, 40, 63–77. [Google Scholar] [CrossRef]
- Schooler, S.S.; De Barro, P.; Ives, A.R. The potential for hyperparasitism to compromise biological control: Why don’t hyperparasitoids drive their primary parasitoid hosts extinct? Biol. Control 2011, 58, 167–173. [Google Scholar] [CrossRef]
- Segoli, M.; Kishinevsky, M.; Harvey, J.A. Climate change, temperature extremes, and impacts on hyperparasitoids. Curr. Opin. Insect Sci. 2024, 64, 101229. [Google Scholar] [CrossRef]
- Poelman, E.H.; Cusumano, A.; De Boer, J.G. The ecology of hyperparasitoids. Annu. Rev. Entomol. 2022, 67, 143–161. [Google Scholar] [CrossRef]
- Sullivan, D.J. Insect hyperparasitism. Annu. Rev. Entomol. 1987, 32, 49–70. [Google Scholar] [CrossRef]
- Sullivan, D.J. Hyperparasitism. In Encyclopedia of Insects, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 486–488. [Google Scholar] [CrossRef]
- Bourne, M.E.; Bottacini, D.; Cuny, M.A.; van Zadelhoff, K.; Cusumano, A.; Poelman, E.H. Hyperparasitoids exploit plant volatiles to locate their parasitoid host despite nonhost herbivore interference. Animal Behav. 2024, 209, 29–42. [Google Scholar] [CrossRef]
- Harvey, J.A.; Gols, R.; Vet, L.E.; Kruidhof, H.M. Development of a hyperparasitoid wasp in different stages of its primary parasitoid and secondary herbivore hosts. J. Insect Physiol. 2012, 58, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.X.; Stansly, P.A.; Gerling, D. Whitefly parasitoids: Distribution, life history, bionomics, and utilization. Annu. Rev. Entomol. 2015, 60, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.S.; Woolley, J.B. Evolution and behavioral ecology of heteronomous aphelinid parasitoids. Annu. Rev. Entomol. 2001, 46, 251–290. [Google Scholar] [CrossRef]
- Hunter, M.S.; Godfray, H. Ecological determinants of sex allocation in an autoparasitoid wasp. J. Animal Ecol. 1995, 64, 95–106. [Google Scholar] [CrossRef]
- Nguyen, R.; Sailer, R. Facultative hyperparasitism and sex determination of Encarsia smithi (Silvestri) (Hymenoptera: Aphelinidae). Annals Entomol. Soc. Am. 1987, 80, 713–719. [Google Scholar] [CrossRef]
- Harvey, J.A.; De Haan, L.; Verdeny-Vilalta, O.; Visser, B.; Gols, R. Reproduction and offspring sex ratios differ markedly among closely related hyperparasitoids living in the same microhabitats. J. Insect Behav. 2019, 32, 243–251. [Google Scholar] [CrossRef]
- Xu, H.Y.; Yang, N.W.; Chi, H.; Ren, G.D.; Wan, F.H. Comparison of demographic fitness and biocontrol effectiveness of two parasitoids, Encarsia sophia and Eretmocerus hayati (Hymenoptera: Aphelinidae), against Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2018, 74, 2116–2124. [Google Scholar] [CrossRef]
- Zang, L.S.; Liu, T.X.; Wan, F.H. Reevaluation of the value of autoparasitoids in biological control. PLoS ONE 2011, 6, e20324. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, L.; Ramirez-Romero, R.; Dai, P.; Yang, X.; Ruan, C.C.; Desneux, N.; Zang, L.S. Mating status of an autoparasitoid and sex of the secondary host impact the outcome of heteronomous hyperparasitism. Entomol. Gen. 2022, 42, 87–99. [Google Scholar] [CrossRef]
- Frago, E. Interactions between parasitoids and higher order natural enemies: Intraguild predation and hyperparasitoids. Curr. Opin. Insect Sci. 2016, 14, 81–86. [Google Scholar] [CrossRef]
- Heinz, K.M.; Nelson, J.M. Interspecific Interactions among Natural Enemies of Bemisiain an Inundative Biological Control Program. Biol. Control 1996, 6, 384–393. [Google Scholar] [CrossRef]
- Hunter, M.S.; Collier, T.R.; Kelly, S.E. Does an autoparasitoid disrupt host suppression provided by a primary parasitoid? Ecology 2002, 83, 1459–1469. [Google Scholar] [CrossRef]
- Giorgini, M.; Baldanza, F. Species status of two populations of Encarsia sophia (Girault & Dodd) (Hymenoptera: Aphelinidae) native to different geographic areas. Biol. Control 2004, 30, 25–35. [Google Scholar] [CrossRef]
- Kidane, D.; Ferrante, M.; Man, X.M.; Liu, W.X.; Wan, F.H.; Yang, N.W. Cold storage effects on fitness of the whitefly parasitoids Encarsia sophia and Eretmocerus hayati. Insects 2020, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Song, Y. Fitness evaluation of Encarsia sophia parasitizing Aleurocybotus indicus on two rice cultivars. Chil. J. Agric. Res 2020, 80, 209–218. [Google Scholar] [CrossRef]
- Pang, S.T.; Wang, L.; Hou, Y.H.; Shi, Z.H. Interspecific interference competition between Encarsia formosa and Encarsia sophia (Hymenoptera: Aphelinidae) in parasitizing Bemisia tabaci (Hemiptera: Aleyrodidae) on five tomato varieties. Insect Sci. 2011, 18, 92–100. [Google Scholar] [CrossRef]
- Zang, L.S.; Liu, T.X. Host-feeding of three parasitoid species on Bemisia tabaci biotype B and implications for whitefly biological control. Entomol. Exp. Appl. 2008, 127, 55–63. [Google Scholar] [CrossRef]
- Man, X.; Sun, L.Y.; Francis, F.; Yang, N.W.; Liu, W.X. Can heteronomous hyperparasitoids recognize host abundance and adjust offspring ratio? Entomol. Gen. 2024, 44, 1017–1025. [Google Scholar] [CrossRef]
- Dai, P.; Liu, L.; Ruan, C.; Zang, L.; Wan, F. Effect of the primary host for production of both sexes on the mating interaction in an autoparasitoid species. BioControl 2013, 58, 331–339. [Google Scholar] [CrossRef]
- Luo, C.; Liu, T.X. Fitness of Encarsia sophia (Hymenoptera: Aphelinidae) parasitizing Trialeurodes vaporariorum and Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Sci. 2011, 18, 84–91. [Google Scholar] [CrossRef]
- Avilla, J.; Copland, M. Effects of host stage on the development of the facultative autoparasitoid Encarsia tricolor (Hymenoptera: Aphelinidae). Ann. Appl. Biol. 1987, 110, 381–389. [Google Scholar] [CrossRef]
- Avilla, J.; Anadón, J.; Sarasúa, M.; Albajes, R. Egg allocation of the autoparasitoid Encarsia tricolor at different relative densities of the primary host (Trialeurodes vaporariorum) and two secondary hosts (Encarsia formosa and E. tricolor). Entomol. Exp. Appl. 1991, 59, 219–227. [Google Scholar] [CrossRef]
- Williams, T. Host selection and sex ratio in a heteronomous hyperparasitoid. Ecol. Entomol. 1991, 16, 377–386. [Google Scholar] [CrossRef]
- Bográn, C.E.; Heinz, K.M. Host selection by the heteronomous hyperparasitoid Encarsia pergandiella: Multiple-choice tests using Bemisia argentifolii as primary host. Entomol. Exp. Appl. 2002, 103, 11–21. [Google Scholar] [CrossRef]
- Huang, Y.; Loomans, A.J.; van Lenteren, J.C.; RuMei, X. Hyperparasitism behaviour of the autoparasitoid Encarsia tricolor on two secondary host species. BioControl 2009, 54, 411–424. [Google Scholar] [CrossRef]
- Chen, Q.; Li, N.; Wang, X.; Ma, L.; Huang, J.B.; Huang, G.H. Age-stage, two-sex life table of Parapoynx crisonalis (Lepidoptera: Pyralidae) at different temperatures. PLoS ONE 2017, 12, e0173380. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–735. [Google Scholar] [CrossRef]
- Sun, W.; Cui, M.; Xia, L.; Yu, Q.; Cao, Y.; Wu, Y. Age-stage, two-sex life tables of the predatory mite Cheyletus Malaccensis Oudemans at different temperatures. Insects 2020, 11, 181. [Google Scholar] [CrossRef]
- Chi, H.; Kara, H.; Özgökçe, M.S.; Atlihan, R.; Güncan, A.; Rişvanlı, M.R. Innovative application of set theory, Cartesian product, and multinomial theorem in demographic research. Entomol. Gen. 2022, 42, 863–874. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. Fujian Academy of Agricultural Sciences: Fuzhou, China. 2025. Available online: https://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000 (accessed on 14 March 2025).
- Chi, H. CONSUME-MSChart: A Computer Program for Age-Stage, Two-Sex Consumption Rate Analysis. Fujian Academy of Agricultural Sciences: Fuzhou, China. 2025. Available online: https://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000 (accessed on 14 March 2025).
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. Fujian Academy of Agricultural Sciences: Fuzhou, China. 2025. Available online: https://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000 (accessed on 14 March 2025).
- Jin, Y.; Wang, J.; Huang, D.L.; Shi, M.Z.; Chi, H.; Fu, J.W. Comparative demography of group-and individually-reared life tables of papaya mealybug with an innovative life table analysis for species in which females and males have a different number of stages. Entomol. Gen. 2024, 44, 727–735. [Google Scholar] [CrossRef]
- Lin, L.H.; Shi, M.Z.; Chi, H.; Li, J.Y.; Zheng, L.Z.; Fu, J.W. Demographic characteristics of Paracoccus marginatus on papaya fruit and potato tubers with an innovative method for efficient application of the multinomial theorem in demographic research. Entomol. Gen. 2024, 44, 949–959. [Google Scholar] [CrossRef]
- Rehman, S.U.; Zhou, X.; Ali, S.; Rasheed, M.A.; Islam, Y.; Hafeez, M.; Sohail, M.A.; Khurram, H. Predatory functional response and fitness parameters of Orius strigicollis Poppius when fed Bemisia tabaci and Trialeurodes vaporariorum as determined by age-stage, two-sex life table. PeerJ 2020, 8, e9540. [Google Scholar] [CrossRef]
- Zang, Z.Y.; Chen, Y.M.; Xu, W.; Zang, L.S. Evidence of two-sex life table analysis supporting Anastatus japonicus, a more effective biological control agent of Caligula japonica compared with other two Anastatus species. Biol. Control 2023, 180, 105188. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Wang, L.; Yang, X.; Wu, S.; Wu, Q.; Xu, H. Comparison of demography and biocontrol efficacy of Encarsia sophia reared on Hamiltonella-infected and Hamiltonella-free whitefly strains. Entomol. Gen. 2025, 45, 505–515. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.L.; Zhou, Y.; He, S.Y.; Lin, C.J.; Jiang, C.N.; Zhou, Z.Q.; Zhou, Y.C.; Chi, H.; Chen, L.L. Integrating temperature-dependent demography, predation rate, and computer simulation for using Arma custos as a biological control agent against Ectropis grisescens. Entomol. Gen. 2025, 45, 431–439. [Google Scholar] [CrossRef]
- Chi, H.; Getz, W.M. Mass rearing and harvesting based on an age-stage, two-sex life table: A potato tuberworm (Lepidoptera: Gelechiidae) case study. Environ. Entomol. 1988, 17, 18–25. [Google Scholar] [CrossRef]
- Smucker, M.D.; Allan, J.; Carterette, B. A comparison of statistical significance tests for information retrieval evaluation. In Proceedings of the Sixteenth ACM Conference on Conference on Information and Knowledge Management, Lisbon, Portugal, 6–10 November 2007. [Google Scholar] [CrossRef]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Xu, J.; De Barro, P.; Liu, S. Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull. Entomol. Res. 2010, 100, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, C.L.; Yang, X.; Chi, H.; Dai, P.; Desneux, N.; Benelli, G.; Zang, L.S. Yacon as an alternative host plant for Encarsia formosa mass-rearing: Validating a multinomial theorem for bootstrap technique in life table research. Pest Manag. Sci. 2021, 77, 2324–2336. [Google Scholar] [CrossRef]
- Mou, D.F.; Lee, C.C.; Smith, C.; Chi, H. Using viable eggs to accurately determine the demographic and predation potential of Harmonia dimidiata (C oleoptera: C occinellidae). J. Appl. Entomol. 2015, 139, 579–591. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Z.; Chi, H.; Chen, B.H. Comparison of the life tables and predation rates of Harmonia dimidiata (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Moore, D.S.; McCabe, G.P.; Duckworth, W.; Sclove, S. Bootstrap methods and permutation tests. In The Practice of Business Statistics: Using Data for Decisions; WH Freeman: New York, NY, USA, 2003. [Google Scholar]
- Naseri, B.; Golparvar, Z.; Razmjou, J.; Golizadeh, A. Age-stage, two-sex life table of Helicoverpa armigera (Lepidoptera: Noctuidae) on different bean cultivars. J. Agr. Sci. Technol. 2014, 16, 19–32. [Google Scholar]
- Chen, Q.; Zhang, J.; Tian, Y.; Li, J.; Ning, W.; Chen, G.; Zhang, X. Evaluating the effects of short-term low temperature on the growth and development of Trichopria drosophilae based on the age–stage two-sex life table. Parasites Vectors 2024, 17, 418. [Google Scholar] [CrossRef]
- Abbas, K.; Zaib, M.S.; Zakria, M.; Hani, U.-e.; Zaka, S.M.; Ane, M.N.-u. Cheilomenes sexmaculata (Coccinellidae: Coleoptera) as a potential biocontrol agent for aphids based on age-stage, two-sex life table. PLoS ONE 2020, 15, e0228367. [Google Scholar] [CrossRef]
- Pan, K.; Xin, T.; Chen, Y.; Wang, H.; Wen, K.; Liu, Y.; Li, Z.; Zou, Z.; Xia, B. Age-stage, two-sex life table and functional response of Amblyseius orientalis (Acari: Phytoseiidae) feeding on different nutrient sources. Insects 2022, 13, 983. [Google Scholar] [CrossRef]
- Iqbal, A.; Chen, Y.-M.; Hou, Y.Y.; Ruan, C.C.; Desneux, N.; Khan, M.Q.; Zang, L.S. Rearing Trichogramma ostriniae on the factitious host Antheraea pernyi via multiparasitism with Trichogramma chilonis facilitates enhanced biocontrol potential against Ostrinia furnacalis. Biol. Control 2021, 156, 104567. [Google Scholar] [CrossRef]
- Pan, M.Z.; Liu, T.X. Suitability of three aphid species for Aphidius gifuensis (Hymenoptera: Braconidae): Parasitoid performance varies with hosts of origin. Biol. Control 2014, 69, 90–96. [Google Scholar] [CrossRef]
- Xu, H.Y.; Yang, N.W.; Duan, M.; Wan, F.H. Functional response, host stage preference and interference of two whitefly parasitoids. Insect Sci. 2016, 23, 134–144. [Google Scholar] [CrossRef]
- Cao, K.; Lan, R.; Yang, X.; Gong, B.; Zhang, J.; Zhou, X.; Jin, L. Two-sex life table analysis of the predator Arma chinensis (Hemiptera: Pentatomidae) and the prediction of its ability to suppress populations of Scopula subpunctaria (Lepidoptera: Geometridae). Agriculture 2023, 13, 1254. [Google Scholar] [CrossRef]
- Park, J.; Mostafiz, M.M.; Hwang, H.S.; Jung, D.O.; Lee, K.Y. Comparing the life table and population projection of Gaeolaelaps aculeifer and Stratiolaelaps scimitus (Acari: Laelapidae) based on the age-stage, two-sex life table theory. Agronomy 2021, 11, 1062. [Google Scholar] [CrossRef]
- Araj, S.-E.; Wratten, S.; Lister, A.; Buckley, H. Floral diversity, parasitoids and hyperparasitoids—A laboratory approach. Basic Appl. Ecol. 2008, 9, 588–597. [Google Scholar] [CrossRef]
- Li, C.; Li, C.Q.; Chen, Z.B.; Liu, B.Q.; Sun, X.; Wei, K.H.; Li, C.Y.; Luan, J.B. Wolbachia symbionts control sex in a parasitoid wasp using a horizontally acquired gene. Curr. Biol. 2024, 34, 2359–2372.e2359. [Google Scholar] [CrossRef]
- Arikan, B.; Karaca, M.M.; Karut, K. Host-feeding capacity and parasitism performance of heteronomous hyperparasitoid Encarsia lutea Masi (Hym.: Aphelinidae) on different host plant species. Biocontrol Sci. Technol. 2024, 34, 257–268. [Google Scholar] [CrossRef]
- Katono, K.; Macfadyen, S.; Omongo, C.A.; Colvin, J.; Karungi, J.; Otim, M.H. Effect of Bemisia tabaci SSA1 host density and cassava genotype on host feeding capacity and parasitism by two Hymenoptera parasitoid species. Biocontrol Sci. Technol. 2023, 33, 19–34. [Google Scholar] [CrossRef]
- Ali, S.; Li, S.; Jaleel, W.; Musa Khan, M.; Wang, J.; Zhou, X. Using a two-sex life table tool to calculate the fitness of Orius strigicollis as a predator of Pectinophora gossypiella. Insects 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.S.; Liu, T.X.; Zhang, F.; Shi, S.S.; Wan, F.H. Mating and host density affect host feeding and parasitism in two species of whitefly parasitoids. Insect Sci. 2011, 18, 78–83. [Google Scholar] [CrossRef]
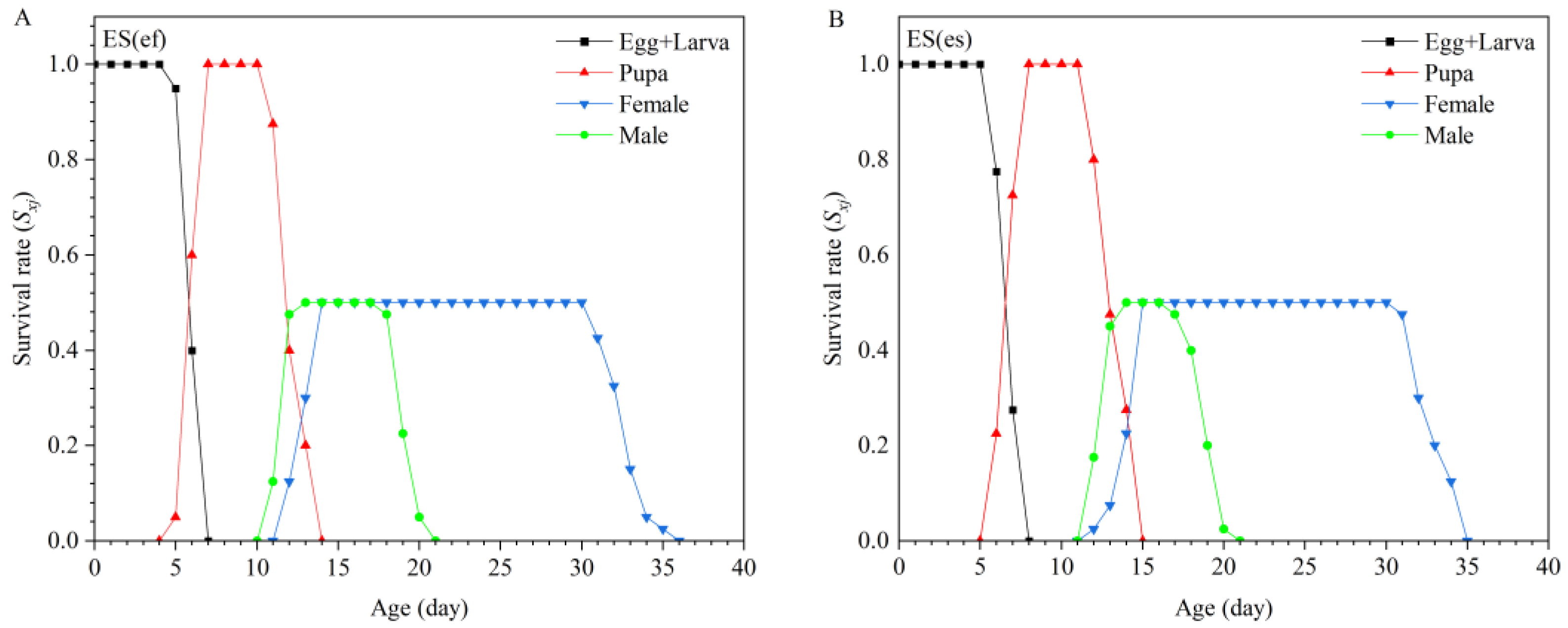
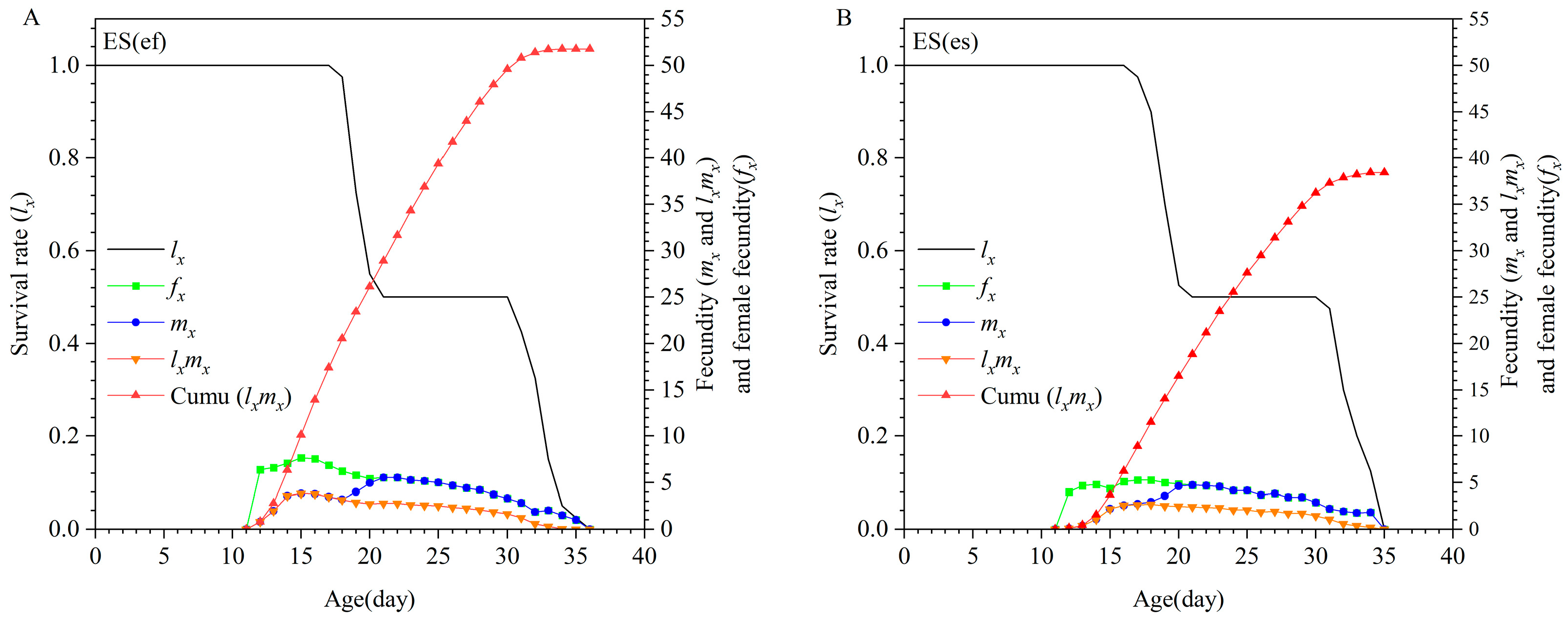
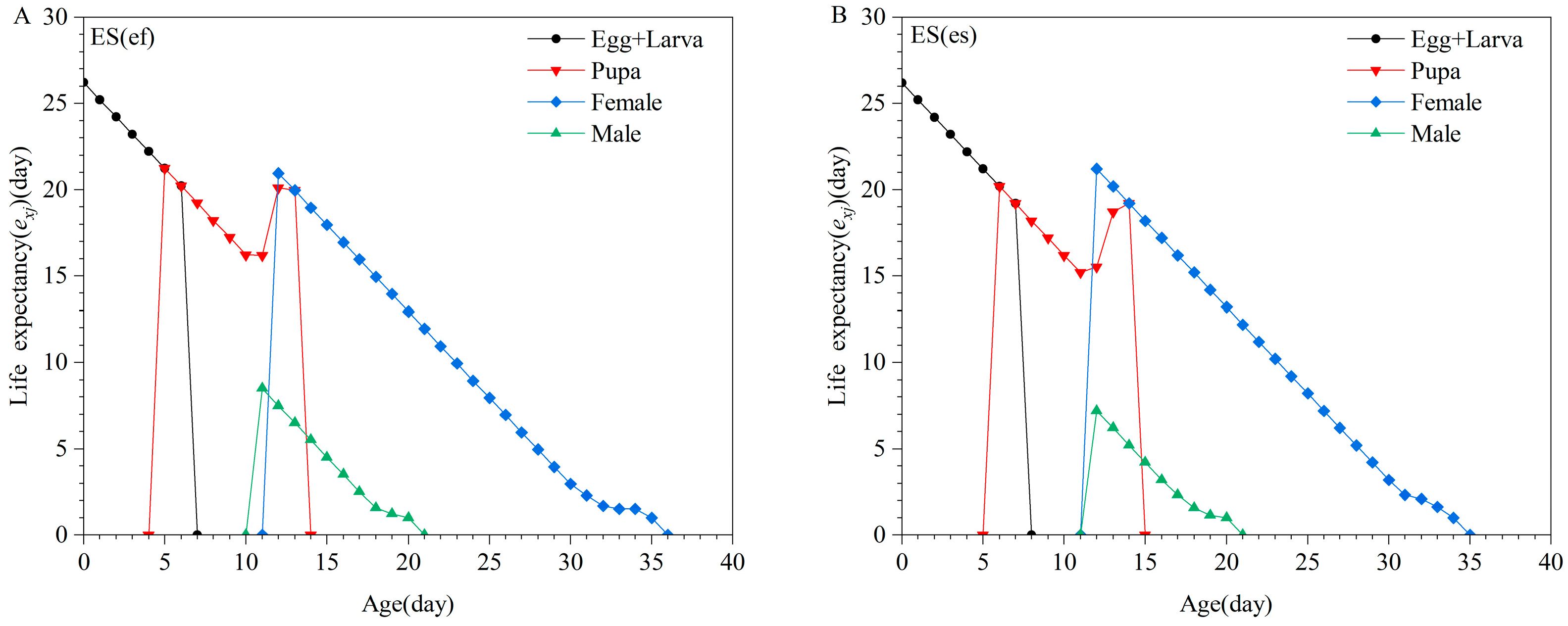
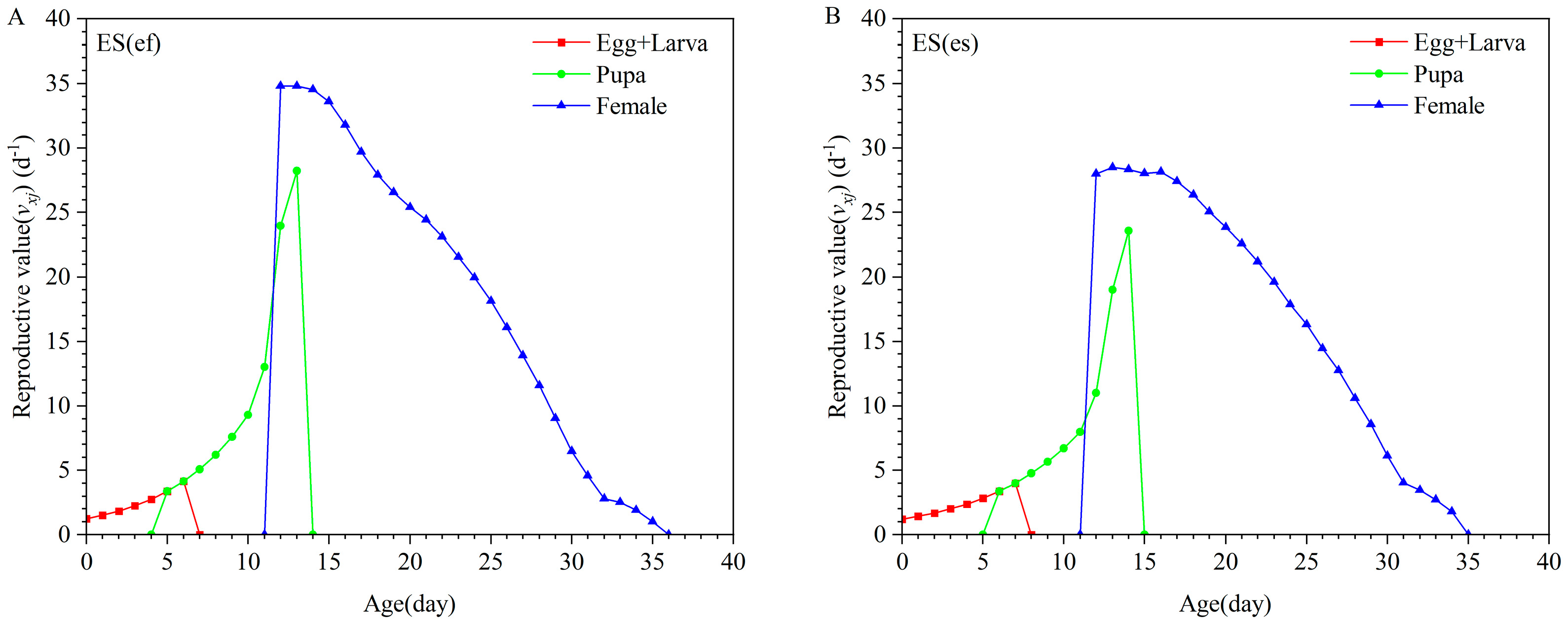
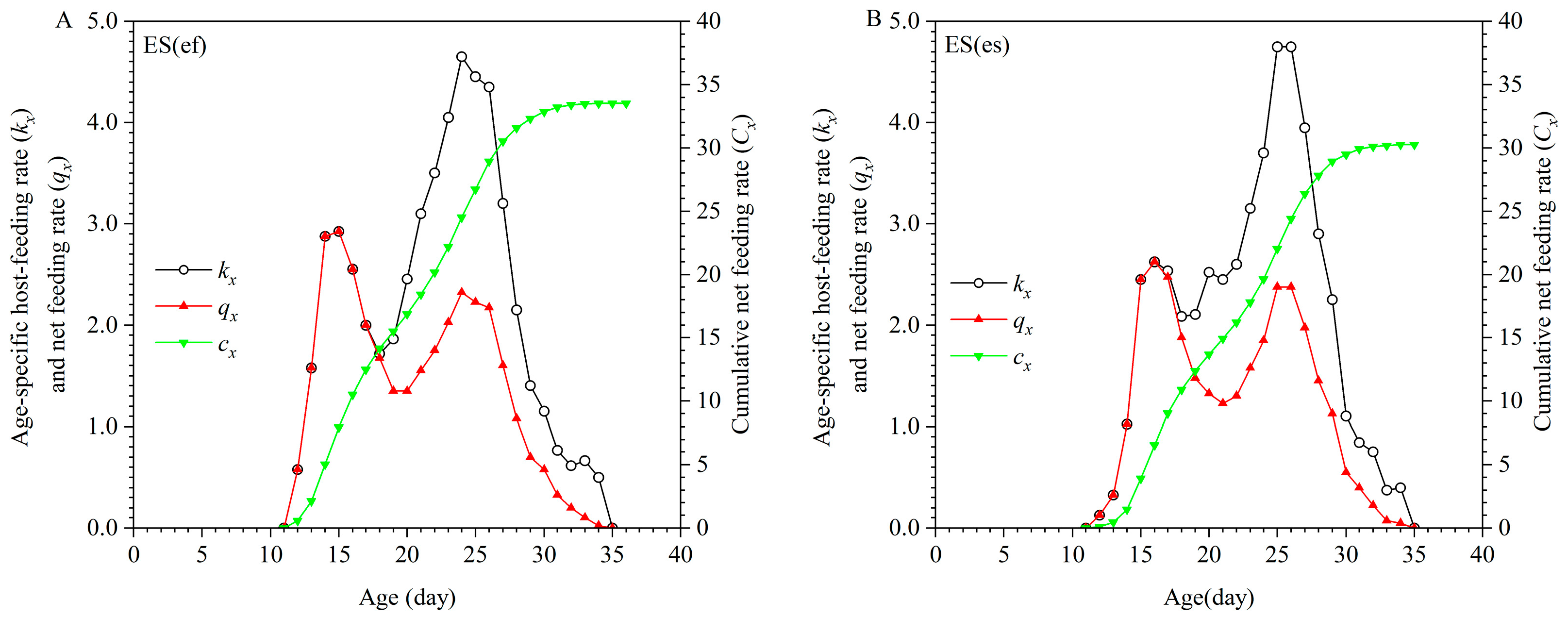

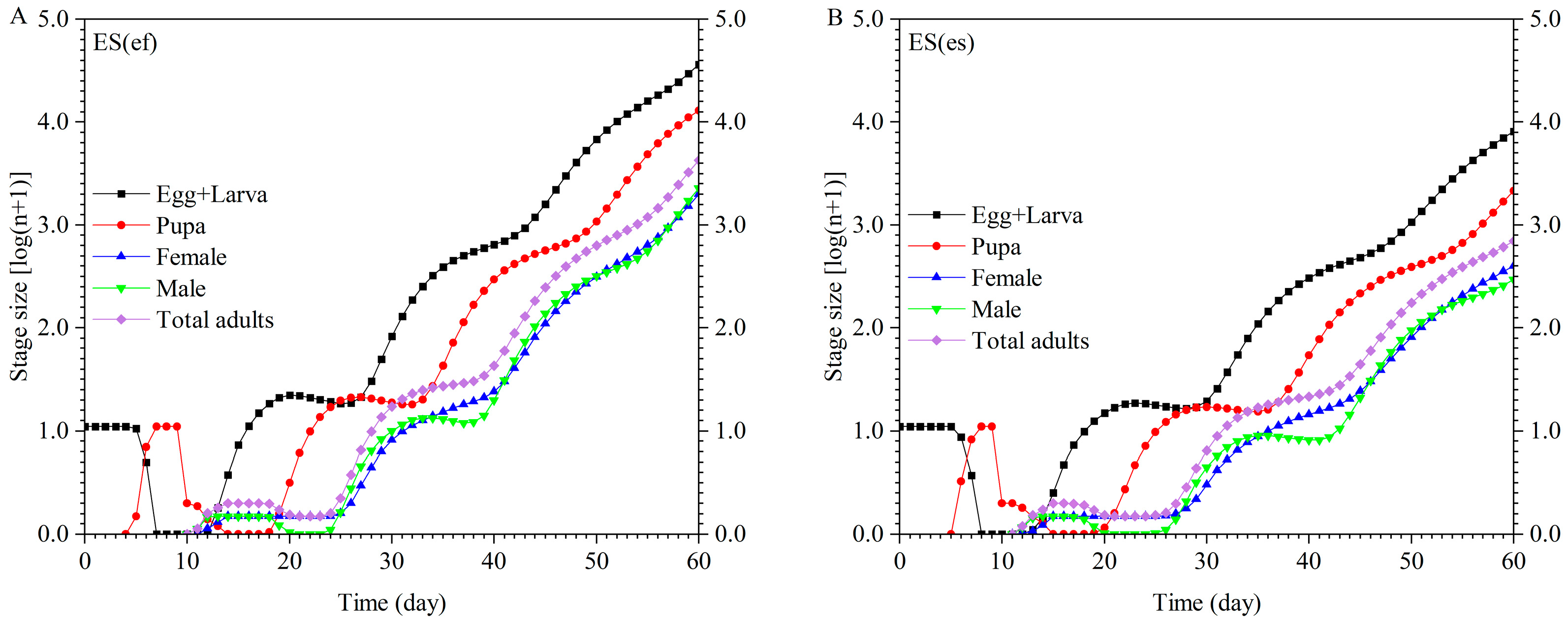
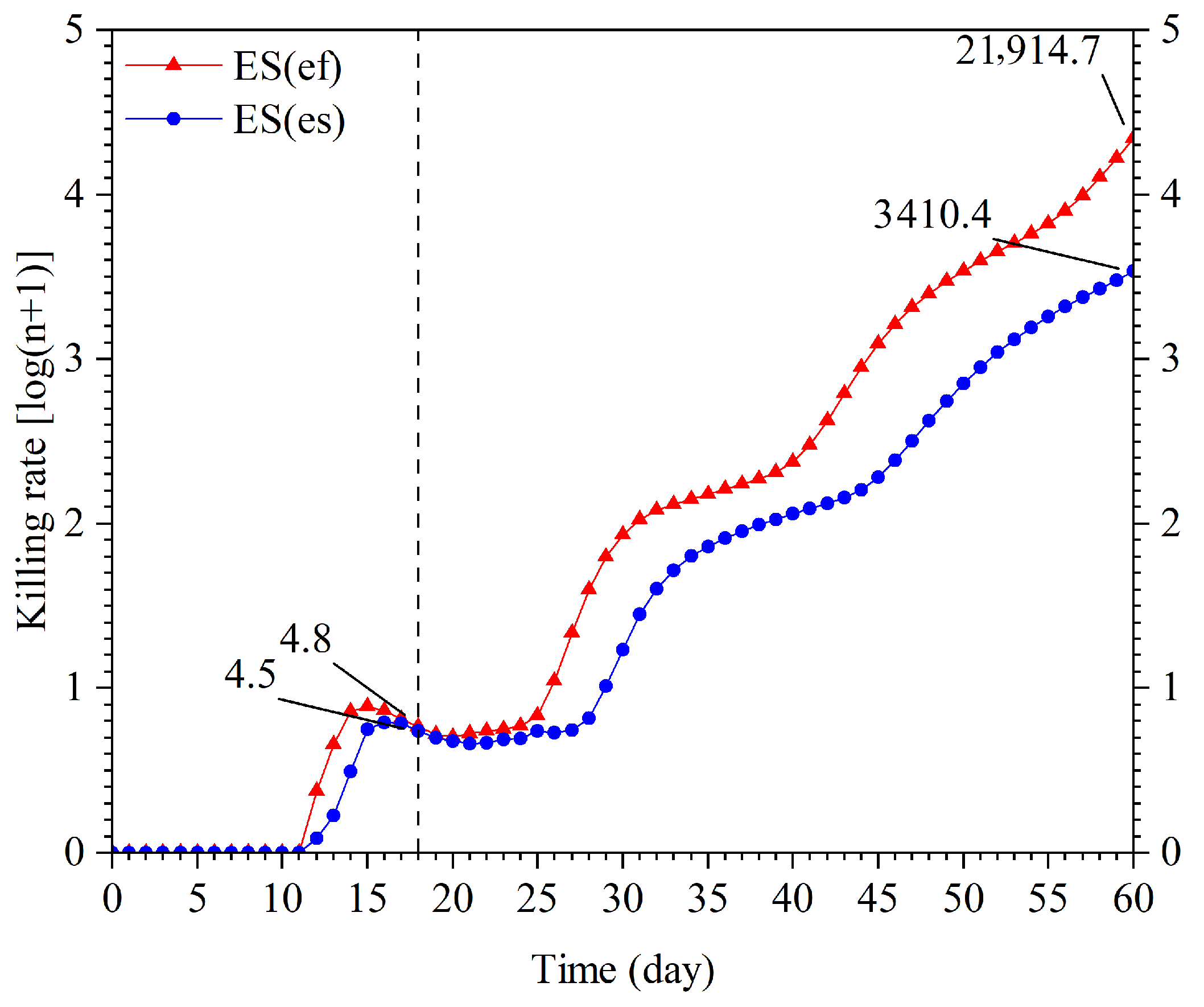
| Parameter | Population | p-Value | |||
|---|---|---|---|---|---|
| n | ES(ef) | n | ES(es) | ||
| Female egg–larva duration (days) | 20 | 6.70 ± 0.10 b | 20 | 7.45 ± 0.15a | <0.0001 |
| Male egg–larva duration (days) | 20 | 6.00 ± 0.10 b | 20 | 6.65 ± 0.11a | <0.0001 |
| Female pupa duration (days) | 20 | 6.45 ± 0.11 b | 20 | 6.90 ± 0.07a | 0.0008 |
| Male pupa duration (days) | 20 | 5.80 ± 0.09 b | 20 | 6.10 ± 0.07a | 0.0101 |
| Female total pre-adult duration (days) | 20 | 13.15 ± 0.18 b | 20 | 14.35 ± 0.19a | <0.0001 |
| Male total pre-adult duration (days) | 20 | 11.80 ± 0.12 b | 20 | 12.75 ± 0.14a | <0.0001 |
| Female adult longevity (days) | 20 | 19.80 ± 0.22 a | 20 | 18.85 ± 0.31b | 0.0136 |
| Male adult longevity (days) | 20 | 7.70 ± 0.13 a | 20 | 6.45 ± 0.17b | <0.0001 |
| Female total longevity (days) | 20 | 32.95 ± 0.29 a | 20 | 33.20 ± 0.29a | 0.5450 |
| Male total longevity (days) | 20 | 19.50 ± 0.18 a | 20 | 19.20 ± 0.21a | 0.2671 |
| APOP (days) 1 | 20 | 0.00 | 20 | 0.00 | 1.0000 |
| TPOP (days) 2 | 20 | 13.15 ± 0.19 a | 20 | 14.35 ± 0.19a | <0.0001 |
| Oviposition days (Od) | 20 | 19.60 ± 0.24 a | 20 | 18.50 ± 0.35b | 0.0092 |
| Fecundity (F) (eggs/female) | 20 | 103.55 ± 1.94 a | 20 | 76.90 ± 1.95b | <0.0001 |
| Parameter | Population | p-Value | |
|---|---|---|---|
| ES(ef) | ES(es) | ||
| Net reproduction rate (R0) (offspring) | 51.78 ± 8.29 a | 38.45 ± 6.18 a | 0.2004 |
| Intrinsic rate of increase (r) (day−1) | 0.20 ± 0.01 a | 0.17 ± 0.01 b | 0.0261 |
| Finite rate of increase (λ) (day−1) | 1.22 ± 0.01 a | 1.19 ± 0.01 b | 0.0258 |
| Mean generation time (T) (days) | 19.48 ± 0.22 b | 21.08 ± 0.24 a | <0.0001 |
| Parameter | Population | p-Value | |
|---|---|---|---|
| ES(ef) | ES(ef) | ||
| Net host-feeding rate (C0) | 33.53 ± 5.33 a | 30.25 ± 4.83 a | 0.6537 |
| Female host-feeding rate (whiteflies/parasitoid) | 67.05 ± 1.07 a | 60.50 ± 1.17 b | 0.0002 |
| Stable host-feeding rate (ψ) | 0.16 ± 0.02 a | 0.17 ± 0.02 a | 0.8183 |
| Finite host-feeding rate (ω) | 0.19 ± 0.03 a | 0.20 ± 0.03 a | 0.9384 |
| Net killing rate (Z0) (whiteflies/parasitoid) | 85.05 ± 13.56 a | 68.70 ± 10.96 a | 0.3519 |
| Female-killing rate (whiteflies/parasitoid) | 170.60 ± 2.79 a | 137.45 ± 2.55 b | 0.0001 |
| Stable killing rate (θ) | 0.38 ± 0.05 a | 0.36 ± 0.05 a | 0.7448 |
| Finite killing rate (υ) | 0.47 ± 0.07 a | 0.43 ± 0.06 a | 0.6547 |
| Transformation rate (Qp) | 1.64 ± 0.01 b | 1.79 ± 0.02 a | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, X.; Wang, J.; Gao, S.; Zhang, Z.; Li, Y.; Desneux, N.; Zhang, J.; Zhao, Y.; Ruan, C. Demographic and Functional Consequences of Secondary Host Selection in a Facultative Autoparasitoid, Encarsia sophia (Hymenoptera: Aphelinidae). Insects 2025, 16, 1165. https://doi.org/10.3390/insects16111165
Zhang S, Wang X, Wang J, Gao S, Zhang Z, Li Y, Desneux N, Zhang J, Zhao Y, Ruan C. Demographic and Functional Consequences of Secondary Host Selection in a Facultative Autoparasitoid, Encarsia sophia (Hymenoptera: Aphelinidae). Insects. 2025; 16(11):1165. https://doi.org/10.3390/insects16111165
Chicago/Turabian StyleZhang, Siteng, Xiaocong Wang, Jing Wang, Shuli Gao, Zhiqi Zhang, Yuning Li, Nicolas Desneux, Junjie Zhang, Yue Zhao, and Changchun Ruan. 2025. "Demographic and Functional Consequences of Secondary Host Selection in a Facultative Autoparasitoid, Encarsia sophia (Hymenoptera: Aphelinidae)" Insects 16, no. 11: 1165. https://doi.org/10.3390/insects16111165
APA StyleZhang, S., Wang, X., Wang, J., Gao, S., Zhang, Z., Li, Y., Desneux, N., Zhang, J., Zhao, Y., & Ruan, C. (2025). Demographic and Functional Consequences of Secondary Host Selection in a Facultative Autoparasitoid, Encarsia sophia (Hymenoptera: Aphelinidae). Insects, 16(11), 1165. https://doi.org/10.3390/insects16111165





