Simple Summary
This study tested the Farming with Alternative Pollinators (FAP) approach in small and large fields in Morocco, which combines a main crop with marketable habitat enhancement plants to support biodiversity and farmer income. Results showed that both small (300 m2) and large (1 ha) FAP fields compared to monoculture fields had more abundance and diversity in terms of pollinators, and natural pest enemies, and higher incomes, though benefits were stronger in smaller fields. Net income increased by 108% in small fields and 36% in large fields. Farmers also recognized the value of marketable habitat enhancement plants as a cost-effective, multi-benefit solution.
Abstract
Novel agro-ecosystem management practices are necessary to sustain biodiversity. In low- and middle-income countries, profitable marketable habitat enhancement plants (MHEPs) associated with a single main crop may be more efficient at supporting insect diversity and farmer income compared to monoculture fields. An approach known as “Farming with Alternative Pollinators” (FAP), which uses marketable habitat enhancement plants, was tested in Morocco. To date, the FAP approach has achieved success in fields measuring approximately 300 square meters, supporting the diversity and abundance of pollinators, natural enemies of pests, and farmer net income. However, the question remains: how efficient would this approach be in large fields of one hectare or more? We present a case study conducted using faba bean as the main crop and compared the development of the FAP approach in 300 square meter and one-hectare fields in Morocco. At the field level, compared to the control fields (i.e., monoculture), the diversity and abundance of pollinators and natural enemies were higher in FAP fields of both field sizes, but the difference was less in large fields. The difference in net income (108% vs. 36% in small vs. large fields) was significant, indicating a potential incentive for the farmers of small and large fields. A questionnaire conducted with farmers confirmed their recognition of the value of marketable habitat enhancement plants as a cost-efficient solution that can offer multiple benefits.
1. Introduction
The global decline in biodiversity has been documented in various groups of organisms, with insect pollinators being one of the most important groups [1,2]. The decline of insect populations poses a significant threat to ecosystem functioning and, ultimately, human well-being [3,4]. Pollination is a crucial ecosystem service provided mostly by insects, as it ensures plant sexual reproduction and hence the many ecosystem services associated with plants: clean air, clean water, shelter, fuel, food, and medicine [3,5,6]. Insect pollinators are primarily threatened by the reduction in floral resources and nesting sites [7,8,9]. Additionally, habitat fragmentation resulting from urban development and the excessive use of fertilizers and pesticides for crop production has exacerbated the threat [10]. Notably, pesticides have well-known adverse effects on pollinators [11]. Mitigation strategies aiming to halt the decline in pollinator populations have been tested [10]. In Europe, a shift from conventional to organic agricultural practices has been implemented without experiencing yield losses [12]. In low- and middle-income countries, farmers have little awareness of the threats to wild pollinators [13,14]. In Morocco, for example, one reason for this may be the dominance of cereal crops (i.e., wind-pollinated crops) that cover more than 50% of the arable land [15]. Despite this, pollinator-dependent agricultural production has been increasing for decades [10] and holds significant economic value. In Morocco, the estimated value of insect pollination to agricultural production was 1.85 × 109 € in 2019, approximately 1.7% of the Moroccan PIB in 2019 [16].
While shifting from conventional to organic practices could be seen as a challenging transition by farmers, biodiversity-friendly practices can be proposed as a stepping stone [17]. In the European Union and the USA, agri-environmental schemes offer incentives to promote ecological restoration and reward farmers for enhancing plant diversity by seeding wildflower strips [18,19]. However, in low- and middle-income countries like Morocco, planting wildflower strips is often met with resistance from farmers due to concerns about the spread of weeds in their fields [13,14]. An alternative approach known as “Farming with Alternative Pollinators” (FAP) aims to enhance crop diversity and support populations of pollinators and natural enemies of pests by using marketable habitat enhancement plants (MHEPs) [7,13,14,17,20]. The FAP approach considers local MHEPs such as oil seeds, spices, vegetables, berries, medicinal and aromatic plants, and forage plants. FAP can enhance farmers’ yields [13,14,20,21,22] without relying on pesticides and does not depend on governmental payments, in contrast to agri-environmental schemes [23]. The impacts on beneficial insects and income have been measured to provide evidence to farmers. This approach has been tested in Uzbekistan, Zimbabwe, and several countries in North Africa and the Near East [13,14,20,21,22,24,25,26]. The costs for FAP and control fields are similar on average [13,14,20,22]. FAP demonstrated to be beneficial for farmers in those countries. A recent study with smallholders in Morocco showed that and which MHEP in particular could substantially reduce the antagonism between scientists concerned with pollinator protection and farmers focusing on production; MHEP had a high adoption rate (68%) after the trials ended [21]. The research showed that farmers decide from economic and agronomic points of view, the authors recommended taking this into account when researchers suggest pollinator-protection approaches [22]. However, questions remain regarding the effects of field size (i.e., the FAP approach has mainly been tested on relatively small fields). Clough et al. [27] highlighted field size as a major driver of farm land biodiversity; smaller fields support more pollinators and natural enemies of pest species [28]. Furthermore, the effectiveness of ecological intensification was demonstrated to be higher in small fields [29].
In the present study, we used faba beans as the main crop to compare the efficiency of the FAP approach between small (0.03 ha) and large (1 ha) fields. There had already been trials with faba beans in small fields in Morocco, it is an important crop in the agro-ecosystems of this country [20,21,26,30]. The average annual production from 2013 to 2023 was 92,473 tons, as reported by the Food and Agriculture Organization (FAO).
This study has four primary objectives:
- Measure the impacts of the FAP approach on pollinator abundance and diversity by comparing of “FAP fields” vs. “control fields” at two spatial scales (i.e., small and large fields).
- Evaluate the diversity and abundance of pests and their natural enemies in FAP and control fields using the same approach.
- Compare net income between FAP and control fields in small and large fields.
- Assess farmer perceptions of FAP through a questionnaire survey.
Our central hypothesis was that implementing the FAP approach in large fields (i.e., one ha surface) would result in increases in pollinator diversity and abundance, natural enemy diversity and abundance, and farm net income. These impacts are expected to be lower in large fields compared to small fields because beneficial interactions (e.g., pollination, pest control) are more concentrated and efficient over a limited area [29]. We also hypothesized that this approach would have a positive impact on the farmers’ perception of the system, benefiting both small- and large-scale farmers, because it can enhance biodiversity, increase income, and reduce the dependency on chemical inputs, making the farming system more resilient and sustainable.
2. Materials and Methods
2.1. Study Sites
The study was carried out in two distinct agro-climatic zones of Morocco: the semi-arid Settat region and the sub-humid Kenitra region. The semi-arid Settat region, located in the Settat-Casablanca area (33°00′ N–7°36′ W, elevation 600 m), has temperate, dry summer, hot summer, and limited rainfall (approximately 300–400 mm per year). Large and small fields were established in the same locality. In the sub-humid Kenitra region (the Rabat-Sale-Kenitra region; 34°02′ N 6°50′ W, elevation 50 m, with annual rainfall of 400–450 mm per year), we selected fields in two different areas: one near Maamora forest (25 km from Kenitra, Morocco) characterized by small farms producing vegetables, fig trees, and avocados, and one farm in Sidi Slimane, where the fields are larger and known for fruit and legume production. The semi-arid region is characterized by flower scarcity resulting from a hot and dry climate and the prevalence of 90% cereal monocultures. In contrast, the sub-humid region is known for fruit production, particularly oranges, and legume crops, especially in Sidi Slimane, where our large field experiments were conducted. In both areas, we selected a total of 32 small (300 m2) fields in 2018–2019, and we selected 14 large (one ha) fields in 2021.
2.2. Experimental Design
A two-year study spanning from 2018 to 2019 was conducted in small experimental fields (i.e., 10 × 30 m) in both semi-arid and sub-humid zones. In the FAP fields, 75% of the total area was planted with faba beans (the main crop), and the outer one-meter perimeter (25%) was planted with MHEP encompassing oilseed rape, spices, vegetables, medicinal plants, and forage plants (Supplementary Figure S1 in Supplementary Materials S1). The control fields were planted with 100% faba beans. A randomized block design was employed, consisting of five FAP and three control fields within each zone per year. Each field was drip-irrigated, and all fields were located at least one km apart to sample different pollinator communities. The location within the farm was changed each year based on availability and irrigation facilities, but maintaining the same soil type. Both FAP and control fields were weeded periodically. The MHEPs in sub-humid Kenitra region comprised wild lupine (Fabaceae: Lupinus luteus), arugula (Brassicaceae: Eruca vesicaria), canola (Brassicaceae: Brassica napus), alfalfa (Fabaceae: Medicago sativa), chia (Lamiaceae: Salvia hispanica), cultivated lupine (Fabaceae: Lupinus albus), coriander (Apiaceae: Coriandrum sativum), celery (Apiaceae: Apium graveolens), and grass pea (Fabaceae: Lathyrus sativus). Wild lupine, grass pea, alfalfa, canola, coriander, arugula, chia, and flax (Linaceae: Linum usitatissimum) were planted in semi-arid Settat region.
In the small fields at both locations (i.e., Settat and Kenitra), the selection of MHEPs was based on landscape characteristics and the farmers’ recommendations [20,21]. However, we made changes to the selection of MHEPs in the second year to address various factors, including seed availability, flowering time, and farmer preferences. In the first year, we used a total of ten MHEPs in the small fields. However, this number was reduced to seven in the second year. This reduction was due to several species of plants not exhibiting the required features for attracting pollinators or natural enemies, such as high floral coverage and/or good plant height [21]. Additionally, some plants had short blooming periods. In the semi-arid region, lupine, grass pea, and alfalfa were replaced with other MHEPs such as coriander, chia, flax, and arugula [26]. In the sub-humid region, we removed alfalfa based on farmer feedback and because it was unsuitable for the region.
All the large FAP faba bean fields (1 ha) were planted in both regions in 2021 using just two MHEPs, canola and coriander. In Settat, eight fields (five FAP and three controls) were planted, and six fields (three FAP and three controls) were planted in Sidi Slimane (Supplementary Figure S2 in Supplementary Materials S1). MHEPs in large FAP fields were planted in seven parallel 1 m × 100 m strips, 15.5 m apart, covering a total area of 0.07 ha (7% of the field). The control fields were planted with 100% faba beans. In large fields, the selection of MHEPs was based on the benefits they provided to crops from FAP trials with small fields conducted in 2018–2019 [20,21].
2.3. Pollinator Sampling
Pollinators were sampled three times during the blooming season of both MHEP and the main crop in both small and large fields using sweep nets and pan traps. Each sampling event was carried out between 10 am and 4 pm under suitable weather conditions for foraging visitors (temperatures above 16 °C, clear skies, and calm winds). For each sampling round, data collection occurred over two consecutive days, with each field sampled once, contingent on weather conditions.
In the small fields, sweep netting was processed as follows: for faba beans (75% of the area of the field), two transects 28 m long × 2 m wide were surveyed for five minutes each, resulting in a total sampling time of ten minutes per field. In the other 25% area of each field (i.e., MHEP in FAP or main crop in control fields), pollinators were collected along an 80 m long, one-meter-wide transect for ten minutes.
We conducted sweep netting sampling in the large fields. Two transects were established within each field separately, measuring 100 m long × 15.5 m wide, one in the center and one along the edge. Main crop transects were sampled for ten minutes in both FAP and control fields separately. We sampled pollinators along a 100 × 1 m transect for ten minutes in 7% of the area of the control fields and 7% of the area of the FAP fields planted with MHEPs (five minutes in coriander and five minutes in canola). Sampling time was equal in both field scales (see Supplementary Figure S2 in Supplementary Materials S1).
Sampling primarily focused on three groups of pollinators: bees, hoverflies, and wasps. All insects were collected, except for honeybees (A. mellifera), Bombus terrestris, and Xylocopa pubescens, which were counted as they are identifiable in the field. Collected bees were anesthetized and killed using jars containing ethyl acetate.
Additionally, we captured entomofauna from all fields using three colored pan traps (500 mL, with a diameter of 145 mm and depth of 45 mm), which were painted white or with UV-reflecting yellow or blue (Rocol Top, Mons, Belgium). Each pan was filled halfway with odorless soapy water to capture entomofauna that may not have been caught by the sweep nets. Two sets were placed in the small fields (two sets were placed within the faba bean fields), and four sets were placed in the large fields (two in the center and two at the edges). The pan traps were set in each field at 10 am and collected after 30 h at the end of each sampling session, i.e., on the following day at 4 pm.
After all specimens were pinned and labeled, we identified them at the family level. For bees, these were Apidae (Ammobates, Anthophora, Apis, Bombus, Ceratina, Eucera, Melecta, Nomada, Thyreus, and Xylocopa), Megachilidae (Anthidium, Chelostoma, Hoplitis, Megachile, and Osmia), Andrenidae (Andrena, and Panurgus), Colletidae (Colletes, and Hylaeus), Halictidae (Ceylatulitus, Dufourea, Halictus, Lasioglossum, Seladonia, Sphecodes, and Systropha), and Melittidae (Melitta, and Dasypoda) [31]. For hoverflies, the families were Syrphidae (Ceriana, Episyrphus, Eristalis, Eristalinus, Eupeodes, Myolepta, Melanostoma, Meliscaeva, Platynochaetus, Scaeva, Sphaerophoria, and Syritta), and for wasps, the families were Chrysididae (Chrysura, and Pseudochrysis), Crabronidae (Cerceris, Diodontus, Ectemnius, Lindenius, Pseudochrysis, Lindenius, Liris, Oxybelus, Philanthus, and Tachysphex), Scoliidae (Campsomeriella, Dasyscolia, and Megascolia), Tiphiidae (Tiphia), Vespidae (Euodynerus, Polistes, Vespa, Vespula, Vespida, and Eumenes), Tenthredinidae (Tenthredo and Tabidus). All insect specimens were then sent to specialists for identification to species level (see the Acknowledgements Section).
2.4. Pests and Natural Enemies
Pest and natural enemy sampling were conducted in both the main crop and MHEP areas of “FAP fields” vs. “control fields”. The sampling were performed on three occasions for each field in conjunction with the pollinator sampling. We employed the plant beating method to collect pests and natural enemies. In the FAP fields, ten plants were randomly selected from the main crop across the entire field, as well as ten plants from each MHEP. In the control fields, sampling was limited to the main crop, with thirty plants selected at random from the entire field. To ensure consistency in the sampling, each plant was struck ten times with a stick fitted with a rubber cover at the end. The insects that fell into the trap were collected in a plastic bag. After sorting the samples using binocular dissecting scopes [32], the collected samples were pooled into 2 mL Eppendorf tubes containing 70% ethanol. Afterwards, all specimens were sent to specialists for identification (see the Acknowledgements Section).
2.5. Assessing Average Net Income from FAP and Control Fields
The calculation of net income for large fields followed the methods developed for small fields [13,19] see for details. Also for large fields, the net income of the main crop was determined by considering the number of faba bean pods and their weight. The number of pods and the weight of faba beans were measured within ten randomly chosen 1 m2 quadrats. Then, we extrapolated the count and weight of faba bean pods for the entire field (0.93 ha). The income generated from the 93% zone of the main crop was computed by multiplying the total weight by the local market price per kilogram. Farmers recorded the total yield weight of MHEP (7% zones) within FAP fields, as well as the corresponding control area (7% zones) (faba beans). The total income for the 7% zone of both treatments was calculated by multiplying the total weight by the prevailing per-kilogram market price. To arrive at the final income, we accounted for the respective investment expenses in seeds and the additional labor required for cultivating the MHEP within the 7% zones of FAP fields. This labor cost was estimated at 200 MAD, equivalent to two person-days per field, specifically for MHEP harvesting. The labor expenses for harvesting the 7% zones in control fields were omitted, considering that they could be efficiently harvested with the main crop and would not need extra labor (see Supplementary Tables S3 and S4 in Supplementary Materials S1).
2.6. Farmers’ Perceptions of the FAP Approach
Between 12 February and 17 March 2023, a comprehensive set of standardized face-to-face interviews was conducted, encompassing a diverse group of farmers from Morocco (see questionnaire in Supplementary Table S2), all of whom had participated in FAP research by cultivating a FAP field at least once, and in some cases, multiple times, between 2018 and 2021. The survey involved 18 small-scale farmers and 28 larger-scale farmers, comprising 45 male farmers and one female farmer from two distinct agro-ecosystems. The interviews were conducted in Arabic. In terms of educational background, the participants exhibited diverse levels of education: 28% were illiterate, 26% had completed primary education, 30% had finished high school, and 15% received a university education. On average, the farmers owned 6.07 hectares of land, with individual holdings ranging from 0.3 to 20 hectares.
2.7. Statistical Analysis
The data analysis was performed using R version 4.4.0 [33]. To assess the attractiveness of different field layouts (FAP and control) to various pollinator groups, we pooled the data of all FAP fields and of all control fields for each scale and employed a Generalized Linear Model (GLM) to compare the abundance of the assessed functional groups of pollinators (short-tongued bees, long-tongued bees, hoverflies, and wasps). A negative binomial was used for the data distribution. The model incorporated the treatments “FAP fields” vs. “control fields” as the primary explanatory variables. After analyzing the abundance of functional pollinator groups in MHEP, we performed a pairwise post hoc comparison using Type-3 ANOVA. The packages used for the analysis were lme4 [34] for fitting the GLM, Emmeans [35] for calculating estimated marginal means, and AER [36] for additional support.
For the following analyses, we used a one-way ANOVA to evaluate whether there was a significant interaction between the independent variables in combination. We tested “Treatment” for its effect on the mean abundance and diversity of natural enemies and pests. QQ plots and the Shapiro–Wilk test were used to check for normality, and homogeneity of variance was assessed by Levene’s test from the car package (version 3.1-2) [37]. For the comparison of abundance and diversity between treatment types, we used the Emmeans test function from the rstatix package (version 0.7.2) [38]. Tukey’s test (PAST 4.13) was used to analyze the collected data of the survey.
3. Results
3.1. Pollinator Abundance and Diversity in Small and Large Fields
3.1.1. Field Level
In the small fields planting incorporated a mosaic design comprising four to six MHEPs in the 25% zone of FAP fields, with two MHEPs in the 7% area of FAP fields in large fields. The FAP fields provided nectar and pollen for a longer period on average, 75 days in FAP fields and 47.5 days in control fields in small fields. In large fields, this period lasted from 64 days in FAP fields to 58 days in control fields. Beneficial insects, including pollinators, were only attracted to the control fields during the flowering period of the main crop. However, FAP fields were attractive forage sites both before and after the main crop flowering period due to the flowering of the MHEPs before, during, and after the main crop, as indicated by the insect sampling (Table 1). During the blooming periods of the main crop and MHEPs, sweep netting and pan traps recorded a diverse array of flower-visiting insects, resulting in a total of 6502 specimens collected and observed across small and large fields (Table 2 and Table 3).

Table 1.
Marketable habitat enhancement plants used in sub-humid and semi-arid regions at different field scales, including their blooming duration.

Table 2.
The flower visitors of faba bean (Vicia faba) and marketable habitat enhancement plants (MEEP) from different semi-arid and sub-humid regions in small fields.

Table 3.
The flower visitors of faba bean (Vicia faba) and marketable habitat enhancement plants (MHEP) from different semi-arid and sub-humid regions in large fields.
In the small fields, we collected and observed a total of 3925 specimens from both of “FAP fields” and “control fields”. In the semi-arid region [26], short-tongued bees (52% Andrenidae, 44% Halictidae, 3% Colletidae, and 1% Melittidae), the long-tongued bee group (91% Apidae and 9% Megachilidae); wasps (53% Tiphiidae, 42% Scoliidae, 3% Crabronidae, 1% Vespidae, and 1% Chrysididae), and diptera (100% were in the family Syrphidae). In the sub-humid region, of 2280 specimens collected, long-tongued bees (97% Apidae, 3% Megachilidae); short-tongued bees (51% Andrenidae, 38% Halictidae, 10% Colletidae, and 1% Melittidae); wasps (59% Vespidae, 27% Crabronidae, 12% Scoliidae, 1% Chrysididae, and 1% Tiphiidae), and diptera (100% were in the family Syrphidae). Overall, we found a significant difference between FAP and control fields in pollinator richness (p < 0.05). (see Supplementary Table S1 in Supplementary Materials S1).
In the large fields, a total of 2577 specimens were captured from of “FAP fields” and “control fields” in both regions. In the semi-arid region, long-tongued bees (99% Apidae and 1% Megachilidae); short-tongued bees (91% Andrenidae and 9% Halictidae), diptera (100% in the family Syrphidae), and wasps (37% Tiphiidae, 28% Tenthredinidae, 24% Scoliidae, 6% Vespidae, and 5% Crabronidae). In the sub-humid region, long-tongued bees (99% Apidae and 1% Megachilidae); short-tongued bees (80% Andrenidae, 14% Halictidae, 6% Colletidae); diptera (100% were Syrphidae), and wasps (82% Tenthredinidae, 9% Scoliidae, 6% Vespidae, and 3% Crabronidae). Overall, we found a significant difference between FAP and control fields in pollinator richness (p < 0.05) (see Supplementary Table S2 in Supplementary Materials S1).
3.1.2. MHEP Level
In small fields, 53% of the hoverfly specimens were attracted to coriander, 25% to canola, and 22% to arugula. The long-tongued bees demonstrated a clear preference for canola, with 78% of the specimens recorded, followed by arugula with 13% and coriander with 9%. Short-tongued bees were attracted to canola (49%), followed by coriander (30%) and arugula (21%). Similarly, wasps were attracted to coriander (59%), canola (31%), and flax (10%). The statistical analysis revealed a highly significant difference in the species richness of pollinators among the MHEPs (p < 0.001) (Figure 1). In large fields, the pollinators captured on canola comprised 50% of the specimens, while coriander also attracted 50% of the specimens. There was no significant difference between MHEPs in terms of species diversity (p > 0.05) (Figure 2).
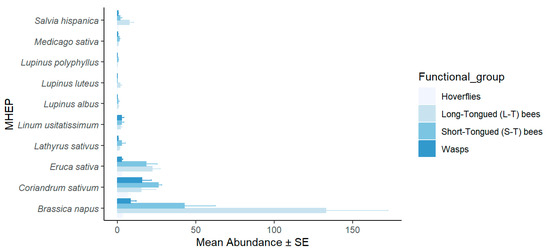
Figure 1.
Mean abundance (±SE) of pollinator functional groups visiting each marketable habitat enhancement plant used in small fields in 2018–2019.
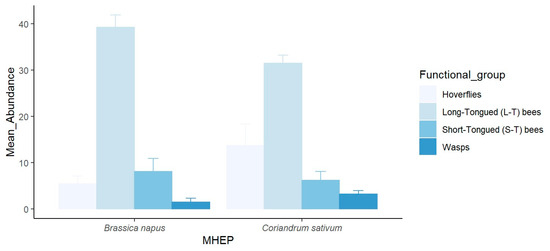
Figure 2.
Mean abundance (±SE) of pollinator functional groups visiting each marketable habitat enhancement plant in large fields in 2021.
3.1.3. FAP Versus Control
The number of pollinators in small fields was significantly higher in “FAP fields” vs. “control fields” (p < 0.05). Moreover, the FAP fields exhibited considerably higher species richness, in comparison to the species observed in the control fields (p < 0.01) (Figure 3). In large fields, the abundance of pollinators was significantly higher in “control fields” vs. “FAP fields” (p < 0.001). Regarding species richness, there was a significant difference in “FAP fields” vs. “control fields” (p < 0.05) (Figure 4).
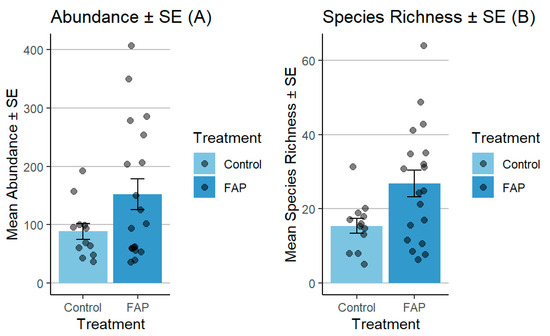
Figure 3.
Comparison of pollinator abundance (A) and species richness (B) between FAP and control fields in small fields during 2018–2019. There were significant differences between FAP and control fields in terms of pollinator abundance (p < 0.05) and species richness (p < 0.01).
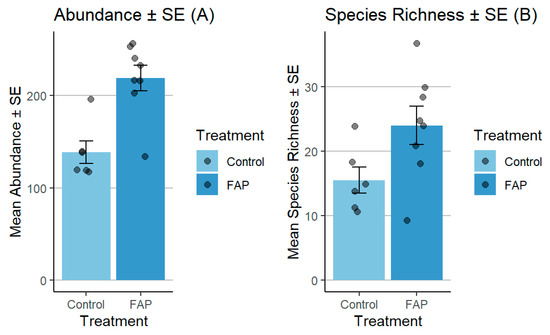
Figure 4.
Comparison of pollinator abundance (A) and species richness (B) between FAP and control fields in large fields in 2021. There were significant differences between FAP and control fields in terms of flower visitor abundance (p < 0.001), and species richness (p < 0.05).
3.2. Pests and Natural Enemy Abundance and Diversity in Small and Large Fields
In small fields, earlier research demonstrated a highly significant difference in the pest abundance and diversity of “FAP fields” vs. “control fields” [13,14,17,20,21,25,39]. However, in large fields, a total of 272 natural enemies and 1202 pest specimens were collected in FAP fields, whereas in control fields, 16 natural enemies and 1520 pest specimens were collected. This finding showed a significantly higher abundance of harmful insect populations in the control fields compared to FAP fields (p < 0.05). However, in contrast to the results from small fields [14,17,20], there was no significant difference in beneficial insects of “FAP fields” vs. “control fields”. (p > 0.05). Additionally, there were no significant differences in the diversity of pests (p > 0.05) or natural enemies (p > 0.05) of “FAP fields” vs. “control fields” (Figure 5).
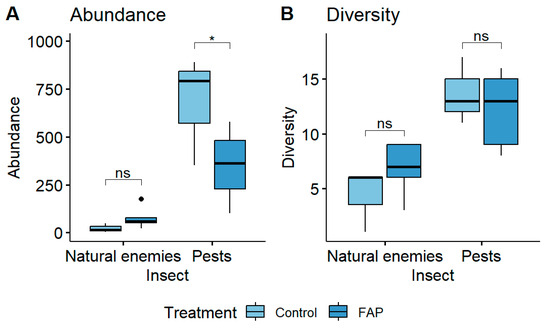
Figure 5.
The abundance (A) and diversity (B) of natural enemies and pests of eight FAP (100% FAP field areas) and six control (100% control field areas) large fields. No significant differences in natural enemy abundance and diversity between FAP and control fields, but a significant difference in pest abundance in control fields (Emmeans). ns = not significant. Statistical analysis was performed by Emmeans tests, with asterisk indicating level of significance as follow: * p < 0.05.
3.3. Net Incomes from FAP and Control Fields in Small and Large Fields
In large fields, the net income was significantly higher in FAP compared to control fields in both regions. The average net income was calculated based on the weight of faba bean pods and in FAP fields, the yield of MHEP. We counted the weight of faba bean pods in ten randomly selected 1 × 1 m2 quadrants. Based on these results, we calculated the weight of the faba bean pods across each field. The income from the 93% zone of the main crop was calculated by multiplying total weight by the market price per kg in 2021. For MHEP (7% zones) in FAP fields and the equivalent area (7% zones) in control fields, FAP farmers weighed and recorded the yield (seeds) of each MHEP, and control farmers weighed and recorded the total weight of faba bean. Income was calculated by multiplying total weight by the market price per kg in 2021. We deducted respective investment costs for seeds and extra-work required for MHEP cultivation in the 7% zones of FAP fields, estimated to be 200 MAD (two persons per day per field) as labor costs for MHEP harvesting. We did not take into consideration labor costs for harvesting the 7% zones in control fields as they can easily be harvested together with the main crop. We calculated the MHEP income of farmers per hectare for FAP fields in comparison to monoculture fields. Based on the economic assessment calculations, the income from FAP fields in both regions is higher by 36% compared to control fields (see Supplementary Figure S3 in Supplementary Materials S1). There was a significant difference between FAP and control fields (t = 2.24, one-tailed p = 0.02 and two-tailed p = 0.04) (see Supplementary Table S5 in Supplementary Materials S1).
Similar analyses for small fields in earlier research showed income was 108% higher in FAP versus control fields in both regions due to the increase in primary crop production [20] (Table 4).

Table 4.
Economic assessment calculation of faba bean small fields in semi-arid Settat and sub-humid Kenitra regions in 2018 and 2019.
3.4. Farmers’ Perceptions of FAP Practice
We interviewed both small and large-scale farmers who had established the FAP approach and had experience with different crops (Supplementary Table S2). Of 28 large-scale farmers, the majority cultivated melons, faba beans, and wheat. The 18 small-scale farmers grew faba beans, zucchini, eggplant, melons, and tomatoes. After trials had ended, among the 46 participating farmers, only five small and three large-scale farmers could not establish FAP without assistance from the scientific team. Farmers attributed different benefits to MHEPs. Among these attributes, “induces higher productivity” emerged as the top priority for farmers. When they were asked to rank five different attributes and label them as high, medium, or low importance, the farmers regarded MHEPs as financially efficient instruments that brought advantages to both the field and the entire farm. Most of the farmers ranked “increase quality of the main crop” as “high”, while some farmers ranked “increase in productivity of the main crop” as “high”. In terms of investment costs in MHEPs, the farmers ranked this as “low”. Additionally, farmers ranked “generating income from MHEP” as “medium”. Furthermore, all farmers ranked “lower pest abundance in MHEP” as “medium” attributes.
In response to the question “Would you be ready to seed weeds instead of MHEP to conserve insects?”, all farmers responded with “no”. In contrast, they agreed to use MHEP corridors in pollinator-independent crops to contribute to pollinator protection and benefit from pest control.
When asked about the lessons learned from FAP-trials (an open-ended question with multiple answers possible; see Figure 6), most of the farmers reported that they had discovered how to increase their income through FAP planting designs. Additionally, some learned about the role of protecting pollinators in their fields; 22% gained insights about crop diversification; some became more aware of pollinators and their importance, and some acquired knowledge about safeguarding plants.
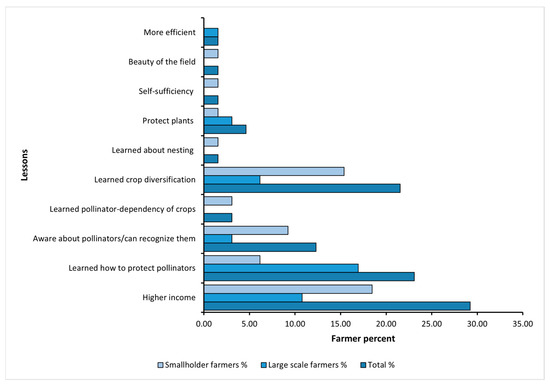
Figure 6.
The lessons learned by the farmers from the trials implemented in small fields (2018–2019) and large fields (2021).
All the farmers interviewed acknowledged that Morocco is prone to drought and faces water scarcity due to climate change. A total of 83% of small-scale and 93% of large-scale farmers expressed their readiness to dedicate parts of their fields every 2 km to cultivate perennial crops such as cacti, fruit trees, olives, and medicinal plants. This would ensure a conducive environment for nesting and the offspring of pollinators [7]. However, 17% of small-scale and 7% of large-scale farmers opposed this idea. Overall, most farmers seemed aware of or were interested in the FAP approach. Their main objectives were to increase income, protect pollinators, and diversify crops, rather than relying on pesticides.
4. Discussion
4.1. FAP Enhances the Abundance and Diversity of Pollinators in Faba Bean Fields
FAP in small and large fields was much more attractive to pollinators compared to control fields, consistent with the findings of previous studies [13,14,20,25,26,30,39,40]. This positive impact can be explained, at least in part, by the prolonged flowering duration in FAP fields achieved through diverse MHEPs and their staggered phenology [13,14,20,26,30,39,40]. Moreover, the diversity of floral traits, such as nectar accessibility, apparently matched the requirements of different functional groups [41]. Our results are in accord with literature on other FAP trials [7,13,14,17,20,26], also reported that FAP in small fields hosted more diverse and abundant pollinators. Overall, this demonstrates that the FAP approach has a positive contribution to species abundance and richness, in both larger fields as well as in the smaller fields. These findings suggest that the FAP approach may be effective and adaptable in different agricultural landscapes, corroborating the results of FAP-cucumber trials in Uzbekistan [13] and Morocco [20] and trials from earlier work in four highly differing agroecosystems (semi-arid, sub-humid, mountainous, and oasis) in Morocco [20,24]. There were also trials in Zimbabwe [24].
4.2. Habitat Enhancement Plants and Different Functional Groups
In both small and large fields, we considered farmers’ recommendations for MHEP selection. Coriander and canola were identified as the most promising options due to their ability to attract pollinators and natural enemies of pests, while also providing a reasonable income, although not the highest. Previous studies [20,21] demonstrated that coriander had the highest beneficial potential and was recommended by pollinator specialists, experts on plant protection, and farmers as well; therefore, in Morocco it is considered the MHEP with highest potential to bridge the gap between biologists concerned with pollinator protection and farmers focusing on income [21]. Coriander is highly effective in attracting pollinating insects [42], while canola is noted for its attractiveness to Apis mellifera [43] and solitary bees [44]. MHEPs such as coriander, canola, and arugula are highly attractive to pollinators in both regions in small fields, with the sub-humid region showing a higher level of attraction [21,22,39,40,45,46]. This combination extends the bloom period, as the plants offer various types of nectar and pollen. Their tall growth habit (ranging from 0.5 to 1 m) makes them suitable for attracting a greater abundance and diversity of pollinators to the main crop, faba beans. Faba bean plants are primarily visited by long-tongued bees, particularly Anthophora fulvitarsis and Eucera nigrilabris [26].
4.3. Pest and Natural Enemy Abundance and Diversity
The FAP approach, whether in small and large fields, has gained attraction in recent years as a biological control strategy [13,14,17,20,21,39]. The utilization of natural enemies for pest management is of paramount importance for the agricultural sector as a means of increased agricultural production [47]. Controlling pests through natural enemies is not only ecologically significant but also economically practical [48]. The present study demonstrated that pest control was more effective in small and large FAP fields compared to control fields due to the higher pest population in the former. This positive effect of FAP at both spatial scales is likely attributable to the variety of flowers provided by MHEP and/or the diversity of floral nectar. This finding aligns with previous studies [49,50,51] that also examined diversity in the field. Some studies, such as [52], reported that a diverse array of non-crop habitats can improve ecological control of pests. This is because herbaceous and wooded habitats can increase the populations of natural enemies by up to 71% [52]. However, farmers employing alternative farming methods such as FAP do so because this aligns with their land as their most valuable asset [13,20,21]. Our research indicates that planting diverse MHEPs could be an effective way to enhance natural enemies of pests, thus warranting consideration within the framework of integrated pest management for faba bean crops. MHEPs could also be used as push-pull crops [17,39] to reduce pest damage. To ensure the effectiveness of this approach, it is essential to conduct thorough testing over several years in diverse farming systems. Such studies will provide the necessary data to verify the approach’s efficacy and encourage its adoption. It is important to note that it takes time for natural enemies to become established and to start controlling pests [53]. Furthermore, the effect of biodiversity on agroecosystem functioning may not be immediate and can often be observed in the responses of species to predation, competition, or parasitism [54].
4.4. Income Benefits from FAP Fields
In this study, faba bean field income increased by 36% in one-hectare FAP fields compared to monoculture fields within just one year. This is a substantial increase, but much lower than the increase described for small fields in earlier research (112% increases in four highly different agro-ecosystems in Morocco) [14,20,21]. Our findings demonstrate the success of FAP regarding farmers’ incomes across diverse agro-ecosystems in Morocco [20,30], despite variation in bee fauna [55]. Moreover, the greater visitation diversity of wild pollinators resulted in higher yields [13,14,20,21,56]. Previous research has also shown the positive impact of FAP on income from fields with various main crops [20,21,22,25,30,39,40]. For example, after cucumber and sour cherry FAP trials in Uzbekistan and Morocco, other farmers adopted FAP methods [13,14,20,21]. Compared to wildflower strips, MHEPs generated income that provided insurance in case the main crop failed due to flooding or other unexpected events [3,13,14,20,21]. Given that small-scale farmers in low- and middle-income countries typically lack access to subsidies that encourage biodiversity, the FAP approach’s emphasis on generating income from marketable crops is a promising strategy for realizing the dual goals of maintaining biodiversity and economic stability [3,13,14,20,21,22].
4.5. Farmers’ Point of View Concerning the FAP Approach
This study provides valuable insights into farmer perceptions of the FAP approach in Morocco and confirms earlier results of studies concerned with smallholder farmers [13,14,20,21]. The findings reveal that Moroccan farmers primarily value the agronomic and economic benefits of FAP rather than its ecological or ethical dimensions. This is consistent with other studies in Uzbekistan [13] and Morocco that have shown that improvements in immediate livelihood often take precedence over environmental concerns [57]. The high satisfaction rates reported by both small-scale (89%) and large-scale (57%) farmers suggest that FAP is generally perceived as beneficial. However, satisfaction does not necessarily guarantee long-term adoption. The primary challenges to farming, especially drought and water scarcity, remain pivotal factors concerning how infrastructure and climatic conditions impact the scalability of agroecological innovations such as FAP. All farmers are aware of the challenges posed by climate and water scarcity. Interestingly, while most of the farmers rejected the idea of sowing wildflowers due to concerns over weed proliferation, they expressed strong support for MHEPs, as they avoid opportunity costs; this result is in line with earlier research [13,14,20,24]. This suggests a preference of Moroccan farmers for interventions perceived as controllable and beneficial to production rather than those primarily serving ecological functions and confirms results with Uzbekistan farmers [13]. The result also emphasizes the importance of carefully framing conservation-related components in a way that resonates with farmers’ priorities [21]. Furthermore, the dual use of MHEPs, both for household consumption and market sale, demonstrates the potential of FAP to contribute to both food security and income generation [14,20,21]. This multifunctionality could be an additional incentive for broader adoption, especially among smallholders. Nevertheless, farmers acknowledged the critical issue of pollinator extinction and recognized the economic value of pollination ecosystem services, as pointed out by [16,58], as well as the losses in yield associated with pollinator decline [59]. According to Anougmar [16], the awareness among Moroccan farmers and consumers concerning the value of pollinators increases the more arid the region is. However, a lack of knowledge remains a constraint in Morocco and other low- and middle-income countries [14]. Increasing the accessibility of information to stakeholders is crucial in facilitating the transition to more sustainable practices. It is noteworthy that FAP farmers have voluntarily banned the use of insecticides [20,21]. These results underscore farmers’ perception of MHEPs as a valuable and cost-efficient tool, providing multiple benefits to their fields. Moreover, the questionnaire results indicated that over 72% of small-scale and 64% of large-scale farmers have reduced their pesticide usage due to the positive impact of FAP on the diversity and abundance of pollinators, as well as pest control [14]. The lower pest abundance in the main crop of FAP small fields (64% reduction) demonstrates that FAP can contribute to reducing pesticide dependency among farmers [17,20,21]. A similar trend was observed in large fields, where FAP fields exhibited a reduction in pest abundance compared to control fields. This may encourage farmers to conduct more FAP trials in large fields. However, it is important to note that natural enemies require time to establish and effectively suppress pest populations [53]. Therefore, minimizing pesticide usage is crucial for long-term success. However, a lack of knowledge about alternative methods may still pose a challenge. FAP emerges as a valuable alternative, since it helps protect pollinators and positively impacts pest control, even for pollinator-independent crops [39]. In the farmers’ responses to the questionnaire, faba bean was the most recommended main crop (31%). This preference is likely influenced by the higher income generated by FAP fields. FAP fields yielded 108% and 36% higher income in small and large fields, respectively, compared to control fields [20] (Table 4 and Table 5).

Table 5.
Economic assessment calculation of faba bean large fields in semi-arid Settat and sub-humid Sidi Slimane regions in 2021.
5. Conclusions
This comparative analysis should encourage more large-scale FAP on-farm trials investigating other main crops and in more countries, notably in low- and middle-income countries. FAP appears to be economically self-sustaining also in large fields of pollinator-dependent crops, as demonstrated by the examples of melons [40], faba beans (the present study), and sorry cherry [13]. Farmers’ responses have been predominantly favorable. This is important, as in the end, researchers can only make suggestions, while farmers decide whether pollinator protection is worthwhile [21]. A survey on the capacity of agricultural advisors could also offer valuable insights for future pollinator-protection programs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects16111164/s1, Figure S1: (a) Experimental design of faba bean in semi-arid region (Settat) 2018. (b) Experimental design of faba bean in sub-humid region (Kenitra) 2018. (c) Experimental design of faba bean in semi-arid region (Settat) 2019. Experimental design of faba bean in sub-humid region (Kenitra) 2019; Figure S2: (a) Experimental design of faba bean in large scale fields in semi-arid region (Settat) 2021. (b) Experimental design of faba bean in large scale fields in sub-humid region (Sidi Slimane) 2021. (c) Sampling method in faba bean in large scale fields in semi-arid and sub-humid region 2021: Blue line = 5 min of transect in field edge, orange line = 5 min of transect in field center in both FAP and control fields. Black line 5 min of transect in canola, and 5 min of transect in coriander in FAP field. black lines in 7% of main crop of control field; Figure S3: Average net income from entire fields (1 ha) with faba bean as main crop in semi-arid and sub-humid regions; Table S1: The flower visitors of faba bean (Vicia faba) and marketable habitat enhancement plants (MHEP) from different semi-arid and sub-humid regions in small fields; Table S2: The flower visitors of faba bean (Vicia faba) and marketable habitat enhancement plants (MHEP) from different semi-arid and sub-humid regions in large fields; Table S3: (a): Economic assessment calculation of faba bean large fields in semi-arid Settat region in 2021. (b) Economic assessment calculation of faba bean large fields in sub-humid Sidi Slimane region in 2021; Table S4: (a) Economic assessment calculation of faba bean small fields in semi-arid Settat region in 2018. (b) Economic assessment calculation of faba bean small fields in semi-arid Settat region in 2019. (c) Economic assessment calculation of faba bean small fields in sub-humid Kenitra region in 2018. (d) Economic assessment calculation of faba bean small fields in sub-humid Kenitra region in 2019; Table S5: T-Test: Two-Sample Assuming Unequal Variances (FAP vs. control) in the term of the income; Table S6: Abundance and species richness of functional group visited marketable habitat enhancement plants used in small fields 2018–2019, ns = not significant (p > 0.05); *** = very highly significant; Table S7: Abundance and species richness of functional group visited each habitat enhancement plants in large fields, 2021, ns = not significant (p > 0.05); Table S8: Comparison of pollinators abundance and species richness between FAP and control fields in small fields 2018–2019, * = significant (p ≤ 0.05); ** = highly significant (p ≤ 0.01); Table S9: Comparison of pollinators abundance and species richness between FAP and control fields in large fields, 2021, * = significant (p ≤ 0.05); *** = very highly significant.
Author Contributions
Y.B.: writing—original draft, writing—review and editing, conceptualization, data curation, formal analysis. D.M.: writing—review and editing, conceptualization, supervision; resources. P.L.: writing—review and editing, data curation; methodology. S.R.S.: writing—review and editing; formal analysis. O.I.: writing—review and editing, investigation; data curation. A.S.: writing—review and editing, investigation; data curation. I.E.A.: writing—review and editing, investigation; data curation. L.H.: writing—review and editing, investigation; data curation. M.C.S.: writing—review and editing; investigation. A.A.-H.: writing—review and editing; investigation. P.R.: writing—review and editing, data curation; resources. S.C.: writing—review and editing, methodology, conceptualization, supervision, project administration, funding acquisition, and resources. All authors have read and agreed to the published version of the manuscript.
Funding
The work is part of an ICARDA project funded by the German Federal Ministry for the Environment, Nature Protection and Nuclear Safety (BMU) within the International Climate Initiative (IKI, 17_IV_065).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank all funders who supported this research through their contributions to the CGIAR Trust Fund: https://www.cgiar.org/funders/, accessed on 9 June 2025. The authors wish to thank the following for their support: Jakub Straka (University of Prague, Czechia) (Sphecodes and Nomada), Andreas Müller (freelancer, Zürich, Switzerland) (Osmiini), Achik Dorchin (University of Mons, Belgium) (Eucera), Holger Dathe (Humboldt Universität, Berlin, Germany) and Romain Le Divelec (University of Mons, Belgium) (Hylaeus), Thomas Wood (University of Mons, Belgium) (Andrena), Pierre Rasmont (University of Mons, Belgium) (Anthophora), Simone Flaminio (University of Mons, Belgium) (Lasioglossum, Halictus, Seladonia and Nomioides). Pests and natural enemies were identified by a co-author (Moulay Chrif Smaili). Hoverflies were identified by Axel Ssymank (Federal Agency for Nature Conservation, Germany). Wasps were identified by Christian Schmid-Egger (Ökoteam Institute for Animal Ecology and Landscape Planning, Berlin, Germany). We would like to thank Mohamed Kabli for his help with field sampling and data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Christmann, S. Do we realize the full impact of pollinator loss on other ecosystem services and the challenges for any restoration in terrestrial areas? Restor. Ecol. 2019, 27, 720–725. [Google Scholar] [CrossRef]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Casas, J. Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst. Serv. 2019, 35, 109–115. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; The Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Christmann, S. Regard and protect ground-nesting pollinators as part of soil biodiversity. Ecol. Appl. 2022, 32, e2564. [Google Scholar] [CrossRef]
- Deguines, N.; Jono, C.; Baude, M.; Henry, M.; Julliard, R.; Fontaine, C. Large-scale trade-off between agricultural intensification and crop pollination services. Front. Ecol. Environ. 2014, 12, 212–217. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Entling, M.H.; Jacot, K. High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151369. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Singh, A.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Keulemans, W.; Bylemans, D.; De Coninck, B. Farming Without Plant Protection Products: Can We Grow Without Using Herbicides, Fungicides and Insecticides? European Parliament: Panel for the Future of Science and Technology: Brussels, Belgium, 2019; p. 44. [Google Scholar] [CrossRef]
- Christmann, S.; Aw-Hassan, A.; Rajabov, T.; Khamraev, A.S.; Tsivelikas, A. Farming with alternative pollinators increases yields and incomes of cucumber and sour cherry. Agron. Sustain. Dev. 2017, 37, 24. [Google Scholar] [CrossRef]
- Christmann, S.; Aw-Hassan, A.; Güler, Y.; Sarisu, H.C.; Bernard, M.; Smaili, M.C.; Tsivelikas, A. Two enabling factors for farmer-driven pollinator protection in low- and middle-income countries. Int. J. Agric. Sustain. 2021, 20, 54–67. [Google Scholar] [CrossRef]
- Schouten, C.; Calon, M.; Tweel, V.D.T. NL Pollinator Strategy “Bed & Breakfast for Bees”; Ministry of Agriculture, Nature and Food Quality Bezuidenhoutseweg: The Hague, The Netherlands, 2018. [Google Scholar]
- Anougmar, S. Economics of Pollination in Drylands: Farmers’ and Consumers’ Perspectives in a Middle-Income Country. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2021. [Google Scholar]
- Christmann, S.; Smaili, M.C.; Bencharki, Y. Synergies of Farming with Alternative Pollinators (FAP) and Certified Organic Farming for Transformative Change of Agriculture; CABI: Wallingford, UK, 2025; pp. 138–145. [Google Scholar] [CrossRef]
- Kleijn, D.; Bommarco, R.; Fijen, T.P.M.; Garibaldi, L.A.; Potts, S.G.; van der Putten, W.H. Ecological Intensification: Bridging the Gap between Science and Practice. Trends Ecol. Evol. 2019, 34, 154–166. [Google Scholar] [CrossRef]
- Kremen, C. Ecological intensification and diversification approaches to maintain biodiversity, ecosystem services and food production in a changing world. Emerg. Top Life Sci. 2020, 4, 229–240. [Google Scholar] [CrossRef]
- Christmann, S.; Bencharki, Y.; Anougmar, S.; Rasmont, P.; Smaili, M.C.; Tsivelikas, A.; Aw-Hassan, A. Farming with Alternative Pollinators benefits pollinators, natural enemies, and yields, and offers transformative change to agriculture. Sci. Rep. 2021, 11, 18206. [Google Scholar] [CrossRef]
- Christmann, S.; Bencharki, Y.; Sentil, A.; Smaili, M.C.; Ssymank, A.; Tsivelikas, A.; Aw-Hassan, A. Advantages of marketable habitat enhancement plants for pollinator protection notably in low-and middle-income countries. J. Environ. Manag. 2025, 389, 126088. [Google Scholar] [CrossRef]
- Christmann, S.; Aw-Hassan, A. Ecosystems and Environment Farming with alternative pollinators (FAP)—An overlooked win-win-strategy for climate change adaptation. Agric. Ecosyst. Environ. 2012, 161, 161–164. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef]
- Allebone-webb, A.S.; Gossrau, F.; Orland, C.; Bara, G.; Fioekou, C. Farming with Alternative Pollinators for Increased Biodiversity and Smallholder Incomes in Zimbabwe. Front. Agron. 2025, 7, 1646610. [Google Scholar] [CrossRef]
- Bencharki, Y.; Christmann, S.; Lhomme, P.; Ihsane, O.; Sentil, A.; El-Abdouni, I.; Hamroud, L.; Rasmont, P.; Michez, D. ‘Farming with alternative pollinators’ approach supports diverse and abundant pollinator community in melon fields in a semi-arid landscape. Renew. Agric. Food Syst. 2022, 38, e6. [Google Scholar] [CrossRef]
- Sentil, A.; Lhomme, P.; Michez, D.; Reverté, S.; Rasmont, P.; Christmann, S. “Farming with Alternative Pollinators” approach increases pollinator abundance and diversity in faba bean fields. J. Insect Conserv. 2021, 26, 401–414. [Google Scholar] [CrossRef]
- Clough, Y.; Kirchweger, S.; Kantelhardt, J. Field sizes and the future of farmland biodiversity in European landscapes. Conserv. Lett. 2020, 13, e12752. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Loreau, M.; Benzaquen, M. Farm Size Matters: A Spatially Explicit Ecological-Economic Framework for Biodiversity and Pest Management. arXiv 2025, arXiv:2505.17687. Available online: http://arxiv.org/abs/2505.17687 (accessed on 9 June 2025). [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmill-herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Åström, J.; et al. Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef]
- El-Abdouni, I.; Lhomme, P.; Christmann, S.; Dorchin, A.; Sentil, A.; Pauly., A.; Hamroud, L.; Ihsane, O.; Reverté, S.; Patiny, S.; et al. Diversity and Relative Abundance of Insect Pollinators in Moroccan Agroecosystems. Front. Ecol. Evol. 2022, 10, 866581. [Google Scholar] [CrossRef]
- Michez, D.; Rasmont, P.; Terzo, M.; Vereecken, N.J. Bees of Europe; NAP Editions: Paris, France, 2019; Volume 1, p. 548. ISBN 978-2-913688-34-6. [Google Scholar]
- Bonsignore, C.P.; Vacante, V. Natural enemies. Integr. Control Citrus Pests Mediterr. Reg. 2012, 2016, 66–87. [Google Scholar] [CrossRef]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Stat Computing: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; Riebl, H.; et al. Package ‘emmeans’: Estimated Marginal Means, aka Least-Squares Means. Am. Stat. 2023, 34, 216–221. [Google Scholar] [CrossRef]
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–9. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- Kassambara, A. Practical Statistics in R II-Comparing Groups: Numerical Variables; Datanovia: Montpellier, France, 2019; p. 129. Available online: https://www.datanovia.com/en (accessed on 9 June 2025).
- Bencharki, Y.; Michez, D.; Smaili, M.C.; Ihsane, O.; Aw-hassan, A.; Ssymank, A. Beyond biodiversity: Does “Farming with Alternative Pollinators” also boost farmers’ income in wheat (Triticum aestivum L.) fields? A case study in Morocco. Front. Ecol. Evol. 2025, 13, 1551190. [Google Scholar] [CrossRef]
- Bencharki, Y.; Michez, D.; Ihsane, O.; Sara, R.; Aw-Hassan, A.; Smaili, M.C.; Ssymank, A.; Rasmont, P.; Christmann, S. “Farming with alternative pollinators” provides benefits also in large-scale fields. Acta Oecologica 2024, 122, 103978. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Bartomeus, I.; Bommarco, R.; Klein, A.M.; Cunningham, S.A.; Aizen, M.A.; Boreux, V.; Garratt, M.P.D.; Carvalheiro, L.G.; Kremen, C.; et al. Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J. Appl. Ecol. 2015, 52, 1436–1444. [Google Scholar] [CrossRef]
- Ranjitha, M.; Koteswara, S.R.S.; Rajesh, A.; Reddi Shekhar, M.; Revanasidda. Insect pollinator fauna of coriander (Coriandrum sativum L.) ecosystem. J. Entomol. Zool. Stud. 2019, 7, 1609–1616. [Google Scholar]
- Kral-O’Brien, K.C.; Hovick, T.J.; Harmon, J.P. Quid Pro Quo? A Review on Bee Utilization of Pollinator-Independent Crops. Ann. Entomol. Soc. Am. 2022, 115, 1–9. [Google Scholar] [CrossRef]
- Beyer, N.; Kirsch, F.; Gabriel, D.; Westphal, C. Identity of mass-flowering crops moderates functional trait composition of pollinator communities. Landsc. Ecol. 2021, 36, 2657–2671. [Google Scholar] [CrossRef]
- Masierowska, M.L. Floral nectaries and nectar production in brown mustard (Brassica juncea) and white mustard (Sinapis alba) (Brassicaceae). Plant Syst. Evol. 2003, 238, 97–107. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Martin, E.A.; Reineking, B.; Seo, B.; Steffan-Dewenter, I. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc. Natl. Acad. Sci. USA 2013, 110, 5534–5539. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Albrecht, M.; Tschumi, M.; Blaauw, B.R. Global synthesis of the effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield. Environ. Sci. Agric. Food Sci. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Herrera, A.R.; Cotes, B.; Agustí, N.; Tasin, M.; Porcel, M. Using flower strips to promote green lacewings to control cabbage insect pests. J. Pest Sci. 2022, 95, 669–683. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Dubsky, V.; Herzog, F.; Jacot, K. Les bandes fleuries pour auxiliaires limitent les ravageurs dans les grandes cultures. Rech. Agron. Suisse 2016, 7, 260–267. [Google Scholar]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Jonsson, M.; Kaartinen, R.; Straub, C.S. Relationships between natural enemy diversity and biological control. Curr. Opin. Insect Sci. 2017, 20, 1–6. [Google Scholar] [CrossRef]
- Gaba, S.; Bretagnolle, F.; Rigaud, T.; Philippot, L. Managing biotic interactions for ecological intensification of agroecosystems. Front. Ecol. Evol. 2014, 2, 29. [Google Scholar] [CrossRef]
- Lhomme, P.; Michez, D.; Christmann, S.; Scheuchl, E.; El-Abdouni, I.; Hamroud, L.; Ihsane, O.; Sentil, A.; Smaili, M.C.; Schwarz, M.; et al. The wild bees (Hymenoptera: Apoidea) of Morocco. Zootaxa 2020, 4892, 1–159. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Scheper, J.A.; Boom, T.M.; Janssen, N.; Raemakers, I.; Kleijn, D. Insect pollination is at least as important for marketable crop yield as plant quality in a seed crop. Ecol. Lett. 2018, 21, 1704–1713. [Google Scholar] [CrossRef]
- Sabbahi, R.; El Abdouni, I.; Lhomme, P.; Boubker, O.; Azzaoui, K.; Hammouti, B.; Neffa, M.; Hock, V. Public Attitudes towards Insect Pollinators in Morocco: Insights from a Pilot Study with Broader Applications. Diversity 2024, 16, 383. [Google Scholar] [CrossRef]
- Porto, R.G.; de Almeida, R.F.; Cruz-Neto, O.; Tabarelli, M.; Viana, B.F.; Peres, C.A.; Lopes, A.V. Pollination ecosystem services: A comprehensive review of economic values, research funding and policy actions. Food Secur. 2020, 12, 1425–1442. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Scheper, J.A.; Vogel, C.; van Ruijven, J.; Kleijn, D. Insect pollination is the weakest link in the production of a hybrid seed crop. Agric. Ecosyst. Environ. 2020, 290, 106743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).