Simple Summary
This study examined how gamma radiation at a substerilizing dose of 300 Gy affects the invasive tomato pest Tuta absoluta, focusing on male moths and their F1 offspring. An age-stage, two-sex life table approach was used to assess the F1 generation’s development, survival, and reproduction. Compared to non-irradiated groups, the irradiated F1 moths experienced longer development times, lower survival rates, shorter lifespans, and fewer offspring. Key population metrics also declined, with some even turning negative, indicating population decline may occur. Additionally, transcriptomic analysis identified 232 differentially expressed genes between irradiated and non-irradiated groups. These genes are involved in crucial processes such as metabolism, hormone production, and immune response. The expression levels of 13 key genes related to male fertility were reduced in irradiated males. Overall, this study shows that irradiated male T. absoluta can pass harmful effects to their offspring, impairing growth and reproduction. This provides a theoretical basis for controlling T. absoluta through the sterile insect technique and highlights potential genetic targets for a genetic sterile insect technique strategy.
Abstract
The tomato leaf miner, Tuta absoluta, is a major pest affecting economically important crops like tomatoes, causing significant global economic losses and exhibiting increasing resistance to pesticides. The sterile insect technique (SIT) is an environmentally friendly control method that is sustainable for both ecosystems and human health. This study used age-stage, two-sex life tables, transcriptomics, and bioinformatics to analyze how irradiation affects the reproductive capacity of male T. absoluta. Compared to the control group, the irradiated offspring showed reduced total lifespan, pre-adult survival rate, net reproductive rate, and intrinsic growth rate. Transcriptomic analysis identified 232 differentially expressed genes (DEGs). GO and KEGG enrichment analyses revealed that irradiation impacted biological processes in male adults related to key biomolecules, hormone metabolism and synthesis, and immune responses. Of the 14 selected genes validated through RT-qPCR, 13 were identified as potential regulators of male reproductive capacity, offering possible targets for controlling T. absoluta using inherited sterility-based SIT strategies. Overall, this study provides a theoretical basis for applying SIT in field control and identifies potential genetic targets for managing T. absoluta populations through a genetic sterile insect technique.
1. Introduction
The tomato leaf miner, Tuta absoluta, also known as the South American tomato moth (Lepidoptera: Gelechiidae), was first identified in 1917 in Huancayo, Peru. By the 1950s, it had become a significant pest impacting the tomato industry in South America [1,2]. From the 1990s onwards, the insect spread and established itself in nearly all South American countries [1]. Currently, T. absoluta has successfully invaded over 110 countries and regions worldwide [3,4]. In China, this pest was first detected damaging open-field fresh tomatoes in Xinjiang in August 2017. To date, it has been reported in multiple provinces and municipalities, including Xinjiang, Yunnan, Shaanxi, Ningxia, and Beijing, causing varying degrees of damage to local tomato production and posing a serious threat to the healthy development of China’s tomato industry [5].
Currently, chemical control remains the primary method for managing T. absoluta [6], despite its several unintended effects on beneficial insects [7]. However, these chemical insecticides break down to sublethal concentrations through biotic and abiotic factors, which can cause both sublethal/hormetic effects and resistance development [8,9]. The widespread use of insecticides may lead to insecticide resistance in the pest population. Moreover, T. absoluta larvae exhibit strong concealment behaviors by mining into host plant tissues such as leaves, axillary buds, tender stems, and immature fruits. This feeding habit creates a natural protective barrier that greatly reduces the effectiveness of insecticides. Therefore, there is an urgent need to develop effective, eco-friendly green management technologies specifically targeting invasive insect pests [10]. The Sterile Insect Technique (SIT) employs ionizing radiation to induce male sterility, followed by sustained mass releases in infested areas. By competing with wild males for mating opportunities with fertile females, this method can significantly reduce or even eradicate target pest populations. As an environmentally safe method, SIT is a vital component of Integrated Pest Management (IPM) strategies. Currently, SIT has been successfully used in controlling and eradicating several major invasive pests, including Ceratitis capitata, Zeugodacus cucurbitae, and Cydia pomonella [11,12]. Notably, this technique substantially provides significant environmental benefits as an eco-friendly and harmless strategy for humans and non-target organisms. These qualities make SIT a promising green solution for managing T. absoluta [13].
Previous studies on radiation-induced sterility in lepidopteran pests have shown that females are more sensitive to radiation than males. When treated with substerilizing doses, male moths display increased mating competitiveness while passing sterility to their offspring. Inherited Sterility (IS) is a key strategy for managing lepidopteran pests, with substerilizing doses causing partial sterility in males and full sterility in females. Importantly, the genetic damage caused by radiation is inherited by the F1 generation through chromosomes, resulting in offspring sterility. Recent progress has been made in developing IS techniques for T. absoluta. The use of X-ray irradiation at 200 Gy produced a dose-dependent sterilizing effect, causing complete sterility in females and partial sterility in males [14]. Field cage experiments with a 15:1 release ratio (irradiated to non-irradiated moths at a balanced sex ratio) showed that this treatment significantly lowered the number of offspring compared to control groups [15]. Zhou et al. found that gamma-irradiation at 300 Gy causes full female sterility and partial male sterility in T. absoluta. This dose maintains normal eclosion rates and mating competitiveness in the irradiated male group while effectively inducing F1 sterility, leading to a notable reduction in offspring numbers [16].
The two-sex life table considers how male individuals influence population dynamics [17]. Using life table analysis is important for further exploring its effectiveness in pest control. Since most pest species show sexual dimorphism and age-stage developmental traits, limited knowledge of their developmental stages can delay pest management [18]. Both male and female adults, as well as larvae of T. absoluta, can damage host plants. Using a two-sex life table analysis provides a more complete assessment of this damage, which is crucial for understanding the growth of irradiated offspring and developing more effective control strategies. This leads to the question: which genes are linked to the inherited sterility effects caused by substerilizing doses? To explore this, our study used transcriptome sequencing to compare gene expression differences between F1 generation male adults irradiated at 300 Gy and normal male adults. This approach aims to uncover the molecular mechanisms behind sterility, identify male-specific genes associated with inherited sterility in T. absoluta, and suggest potential molecular targets for designing inherited sterility-based SIT strategies against this damaging pest.
2. Materials and Methods
2.1. Insect Rearing
The T. absoluta population used in this study was collected from tomato fields in Yuxi, Yunnan Province, in June 2019. The collected population was maintained in the laboratory on tomato host plant under controlled environmental conditions (25 ± 1 °C, 60–70% RH, 8:16 h L:D) to establish an experimental colony. Throughout the rearing process, the T. absoluta population remained unexposed to any environmental stressors.
2.2. Irradiation
Pupae of T. absoluta were collected 2–3 days before emergence and sexed based on genital positioning (with female and male genital pores located on the 8th and 9th abdominal segments, respectively) [19]. Male pupae were selected as the F0 generation and irradiated with a 137Cs-γ radiation source (model xN658, activity 7.77 × 1014 Bq, Radiochemistry Center, Buckinghamshire, UK) at a dose of 300 Gy, with a dose rate of 1 Gy/min. Non-irradiated male pupae served as controls. For each irradiation replicate, 5 male pupae were placed in glass tubes (1.5 cm diameter × 8.0 cm height), with ten replicates processed per irradiation session. The irradiated pupae were housed individually housed in 10 mL centrifuge tubes with ventilation holes. Virgin male adults (2 days old) from the F1 generation were collected from irradiated males × normal females (IR group) and non-irradiated males × normal females (CK group). All emerged adults were maintained on 10% honey–water-soaked cotton as a nutritional source. For transcriptome analysis, 30 male adults were collected from each group (CK and IR), with three biological replicates per group.
2.3. Life Table Study
Virgin F0 male adults that successfully emerged from irradiated pupae were individually paired with untreated virgin females in glass tubes (3.0 cm diameter × 10.0 cm height) containing fresh tomato leaves. In total, ten irradiated male adults and ten untreated female adults were copulated, and 10% honey–water was supplied to provide supplementary nutrition. Tomato leaves were replaced daily until oviposition ceased, at which point the total egg production per female was recorded. Eggs on each leaf were transferred daily onto fresh tomato leaves in Petri dishes (9.0 cm diameter × 1.5 cm height). From the oviposition clusters, 820 eggs from the treatment group and 320 eggs from the control group were randomly selected for subsequent analysis. The following parameters were documented for the F1 generation: egg hatch rate, larval-to-adult survival rate (from egg to adult emergence), adult sex ratio, and developmental duration at each stage. These metrics were analysed to assess the effect of 300 Gy gamma irradiation on F1 progeny development. Daily records were kept for both male and female adult survival rates and fecundity until mortality. In cases where individual moths died prior to mating, replacements were chosen from the same population cohorts to maintain the experiment. Data from deceased individuals were excluded from life table analysis. For both control (CK) and treatment (IR) groups, F1 female adults were mated with non-irradiated male adults, with oviposition records maintained for each pairing.
2.4. Life Table Data Analysis
Raw data of T. absoluta were analysed using the TWOSEX-MSChart program (https://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000) “URL (accessed on 25 July 2025)” [20], incorporating both sexual dimorphism and individual developmental rate variations. The following demographic parameters for T. absoluta were calculated: developmental duration, fecundity, mortality rate, net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T).
The lx and mx were determined using Equations (1) and (2):
where sxj represents the possibility that a newly born nymph will survive to age x and stage j. β shows number of stages, while fxj represents age-stage specific fecundity of the individual at age x and stage j.
The standard errors for developmental duration, female adult pre-oviposition period (APOP), total pre-oviposition period (TPOP), oviposition, fecundity, and life table parameters were estimated using bootstrap resampling [21]. All standard errors were calculated through 100,000 bootstrap iterations using the bootstrap technique implemented in the TWOSEX-MSChart software [22].
2.5. RNA Extraction and Transcriptome Sequencing
Total RNA was extracted from 2-day-old F1 male adults (both irradiated and non-irradiated groups) using the Trizol method. RNA purity and concentration were measured with a NanoDrop 2000 spectrophotometer (Thermo, Wilmington, DE, USA), while RNA integrity was assessed using an Agilent 2100 Bioanalyzer/LabChip GX system (PerkinElmer, Waltham, MA, USA). The eukaryotic mRNA was enriched using oligo(dT)-coupled magnetic beads, followed by fragmentation in Fragmentation Buffer. Using the fragmented mRNA as template, first-strand cDNA was synthesized and subsequently converted to double-stranded cDNA. After purification, the double-stranded cDNA underwent end repair, A-tailing, and sequencing adapter ligation. Fragment size selection was performed using AMPure XP beads (Yeasen, Shanghai, China), followed by PCR amplification to construct the final cDNA library. Following library construction, the cDNA library was first quantified using a Qubit 3.0 Fluorometer (Invitrogen, Carlsbad, CA, USA), with concentration requirements exceeding 1 ng/μL. Subsequently, the insert size distribution was verified using the Qsep400 High-throughput Analysis System (Bioptic, Changzhou, China). Upon confirmation of expected insert sizes, the library’s effective concentration (>2 nM) was precisely quantified by RT-qPCR to ensure quality. Finally, qualified libraries were sequenced on a high-throughput sequencing platform in PE150 mode. The clean data were mapped to the reference Tuta_absoluta genome version IBG_00819 (http://v2.insect-genome.com/Tabs) “URL (accessed on 20 March 2023)”. The selected gene fragments were subjected to functional enrichment analysis using Gene Ontology (GO) and pathway enrichment analysis through the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
2.6. Quantitative Real-Time PCR
RT-qPCR analysis was performed for the 14 candidate genes using the PrimeScript™ RT Master Mix (Perfect Real Time) cDNA synthesis kit (Takara, Dalian, China) and TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, Dalian, China), with TaEF1-α serving as the reference gene. Three biological replicates and three technical replicates were included for each treatment, with primers listed in Table 1.

Table 1.
Primer sequences for RT-qPCR of the 14 target genes.
2.7. Statistical Analysis
The experiment included three biological replicates for each treatment group, with each sample analyzed in three technical replicates. Gene relative expression levels were calculated using the 2−ΔΔCT method based on RT-qPCR results. Statistical analysis was performed using SPSS software (version 21.0; IBM, Armonk, NY, USA). The normality of the data was verified using Shapiro–Wilk tests. After confirming the normal distribution, gene expression differences between irradiated and non-irradiated groups were compared using independent samples t-tests. Data visualization and curve fitting were conducted using GraphPad Prism statistical software (version 8, GraphPad Software, San Diego, CA, USA, www.graphpad.com).
3. Results
3.1. Development Time, Survival, and Oviposition Period
Significant differences in egg-to-pupation developmental duration, egg hatch rate, adult pre-oviposition period (APOP) and total pre-oviposition period (TPOP) were analyzed between the control and treatment groups (Table 2). Compared to the control group, the egg hatch rate of offspring produced by irradiated males showed an extremely significant reduction (p < 0.001). The egg hatching duration was slightly shortened, showing no statistically significant difference between treatments (p = 0.21129). Compared to the F1 generation of the control group, the irradiated F1 larvae showed significantly prolonged developmental durations across all stages (the 1st instar larvae, 2nd instar larvae, 3rd instar larvae, 4th instar larvae, and pupal stage showed p < 0.001, p < 0.001, p = 0.0155, p < 0.001, and p < 0.001, respectively). The APOP of the F1 generation in the irradiated treatment group was 1.24 days longer than that in the non-irradiated group, with a significant difference (p = 0.0219). Moreover, the TPOP of the non-irradiated offspring was significantly lower than that of the irradiated offspring (p < 0.001).

Table 2.
Effects of 300 Gy irradiation doses administered to male parent on their offspring fitness parameters in Tuta absoluta.
The differences in pre-adult duration, pre-emergence survival rate, adult longevity, and total longevity between the offspring without irradiation and those irradiated with 300 Gy are shown in Table 3. Compared to the non-irradiated offspring with a pre-adult developmental duration, the irradiated offspring exhibited a significantly prolonged pre-adult developmental period (p < 0.001). The pre-emergence survival rate of the F1 generation showed a dramatic reduction following irradiation treatment (p < 0.001). The longevity of both female and male adults in the F1 generation of the irradiation treatment group was significantly shorter than the control group (p < 0.001).

Table 3.
Effects of 300 Gy of gamma radiation administered to male parents on the offspring longevity and survival in Tuta absoluta.
The survival rates (sxj) of the F1 generation of T. absoluta in both control and 300 Gy irradiated groups are shown in Figure 1. The daily larval survival rate was significantly lower in the irradiated treatment group compared to the control. A shorter x-axis span of the sxj curve indicates a shorter instar duration and faster developmental rate for individual insects. The overlapping sxj curves at different ages of T. absoluta suggest generational overlap, where various developmental stages (instars) occur simultaneously due to individual differences in growth rates. Both control and irradiated groups displayed gradually declining survival rates, but the irradiated treatment group showed significantly lower survival rates during the 2nd, 3rd, and 4th instar larval stages, as well as reduced pupal and adult survival. These findings demonstrate that 300 Gy of gamma radiation markedly inhibits the growth and development of the F1 generation T. absoluta.
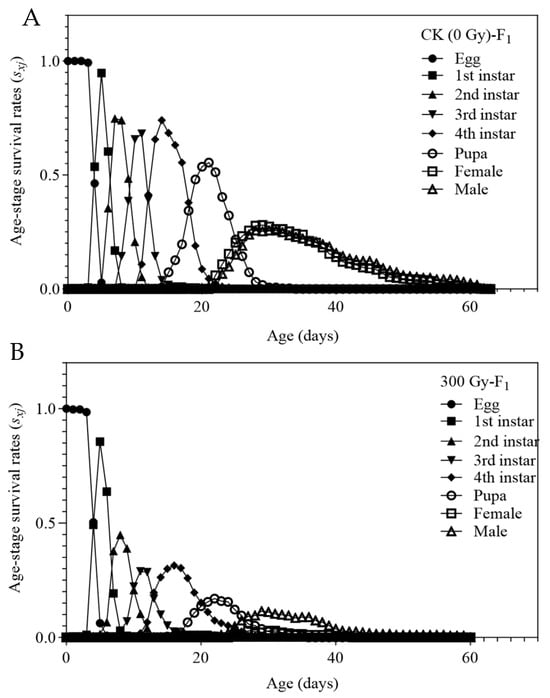
Figure 1.
Age-stage -specific survival rate (sxj) of Tuta absoluta F1 generation. (A) Survival rate of offspring of male parent treated with no irradiation. (B) Survival rate of offspring of male parent treated with 300 Gy gamma irradiation doses.
3.2. Life Table Parameters
The population parameters and fecundity of T. absoluta calculated using the age-stage, two-sex life table program under different treatments are presented in Table 4. The number of eggs laid per female in the F1 generation after irradiation was only 36.8% of that in the control group, with significant reductions in fecundity (p < 0.001). Compared with the control group, both the intrinsic rate of increase (r) (p < 0.001) and the finite rate of increase (λ) (p < 0.001) in the 300 Gy of gamma radiation group were extremely significantly lower than those in the control group. The mean generation time (T) of the irradiation treatment group was 2.8 days longer than that of the control group, also showing an extremely significant difference (p < 0.001).

Table 4.
Life table parameters showing the effects of 300 Gy of gamma ray-irradiated male parent on their offspring in Tuta absoluta.
After 300 Gy of gamma radiation, the ratio of female adults to male adults in the F1 generation (0.25) was significantly lower than that in the control group (1.127). This indicates that 300 Gy of gamma radiation has a greater effect on female individuals in the offspring than on males, which is unfavourable for population growth. R0 can be calculated as follows: R0 = Nf × N × F, where F is the average fecundity [23]. Therefore, the trend in R0 in the control group and the treatment groups (p < 0.001) is similar to that of the average fecundity (p < 0.001).
The age-specific survival rate (lx) and the age-stage-specific fecundity curve (fx,7, where 7 indicates the adult stage in T. absoluta’s developmental staging system) of the F1 generation are shown in Figure 2. The survival curve (lx) of the F1 generation in the control group initially fluctuated, then stabilised during mid-development, and gradually declined towards the end. Conversely, the irradiated group displayed an initially stable lx curve that rapidly declined before slowing down. Combining the developmental duration parameters and the (sxj) curve suggests that the larval stage and pupal stages of the F1 generation of T. absoluta in the 300 Gy of gamma radiation group face a higher risk of mortality. The (fx,7) curve in the control group shows a rising slope in the early stage and a declining slope falling in the later stage, with minimal fluctuation. In contrast, the (fx,7) curve of the gamma radiation group fluctuates more irregularly, indicating that the female adults in the F1 generation of the control group have relatively consistent daily fecundity, while those in the gamma radiation group differ in egg production and the egg-laying period.
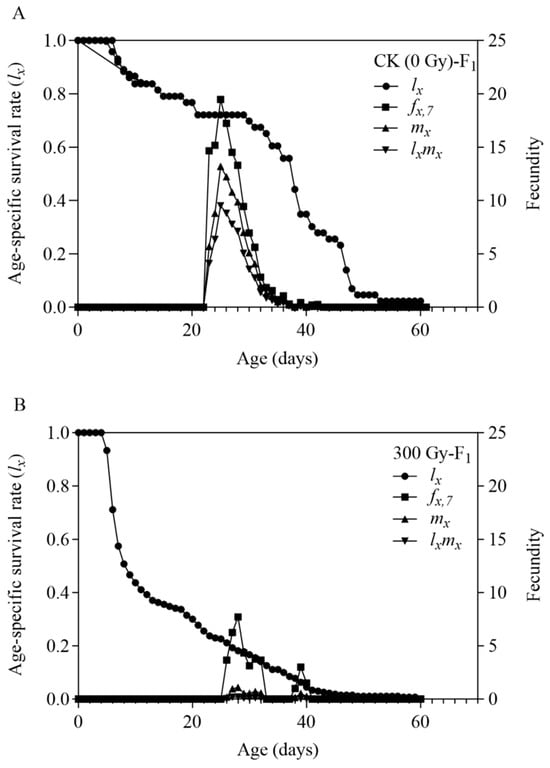
Figure 2.
Age-specific survival rate (lx), age-specific fecundity (fx,7), age-specific fecundity (mx) and age-specific net fecundity (lxmx) of the F1 population of Tuta absoluta. (A) Offspring of male parent treated with no irradiation. (B) Offspring of male parent treated with 300 Gy gamma irradiation doses.
3.3. RNA Quality Control Data Statistics
To further explore the molecular mechanism of inherited sterility in T. absoluta and identify its target genes, the transcriptome sequencing was used. Sequencing data are shown in Table 5. After filtering and quality control of the raw data, the CK group produced 19,546,077–26,314,076 clean reads, while the IR group generated 22,573,216–25,163,786 clean reads. Eukaryotic reference-based transcriptome (RNA-seq) analysis of the six samples yielded a total of 41.19 Gb of clean data, with each sample contributing at least 5.82 Gb. The percentage of Q30 bases was 97.70% or higher, and the GC content of the sample sequences ranged from 42.95% to 43.89%. The efficiency of aligning the reads from each sample to the reference genome was between 76.94% and 81.71%. These results demonstrate the high-quality metrics of the transcriptome sequencing data, confirming their suitability for subsequent bioinformatics analyses.

Table 5.
Summary of Illumina RNA-sequencing data.
3.4. Transcriptome Sequencing Analysis
The FPKM distribution of genes under different irradiation doses was assessed on a global level. All biological replicates exhibited highly consistent gene expression patterns (Figure 3A), indicating that irradiation treatment did not significantly alter transcriptional profiles. The expression trends demonstrated remarkable reproducibility across all samples. Principal component analysis (PCA) among samples from various treatment groups is shown in Figure 3B. There were notable differences between the two treatment samples. A total of 232 DEGs were detected, with 107 being up-regulated and 125 down-regulated (Figure 3C).
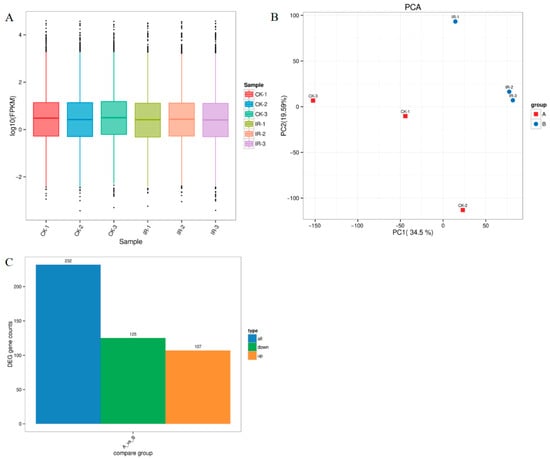
Figure 3.
Transcriptome analysis after 300 Gy of gamma radiation. (A) Violin plot of gene expression in different treatment groups. (B) PCA analysis of the number of expressed samples among different irradiation treatment groups. (C) Number of DEGs in different irradiation groups.
3.5. GO Enrichment Analysis
In the GO enrichment analysis of the IR vs. CK group, the significantly enriched GO terms with many DEGs are as follows: Cellular Component: integral component of membrane; Molecular Function: nucleic acid binding; Biological Process: biological process; Molecular Function: molecular function; Cellular Component: cellular component; Molecular Function: hydrolase activity; Cellular Component: cytoplasm; Cellular Component: obsolete cell; Molecular Function: metal ion binding; Molecular Function: serine-type endopeptidase activity; Cellular Component: obsolete cell part; and Biological Process: cellular process (Figure 4). These results show the widespread involvement of various biological processes, cellular components, and molecular functions in the development of F1 male offspring from crosses between irradiated T. absoluta males and non-irradiated females.
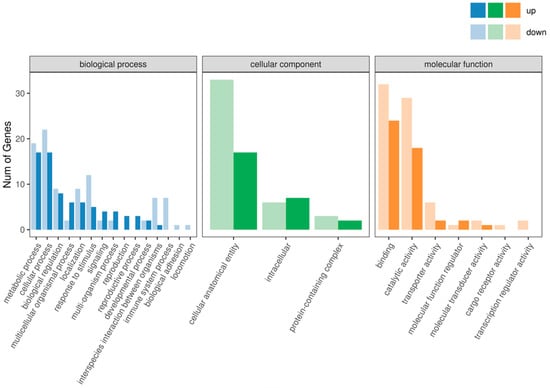
Figure 4.
GO function annotation analysis.
3.6. KEGG Pathway Analysis
KEGG analysis was conducted on the DEGs obtained from the IR/CK group, identifying a total of 40 signaling pathways. The top 20 pathways are shown in Figure 5. These DEGs are associated with core metabolic pathways such as glucose metabolism, lipid metabolism, and amino acid metabolism. These findings indicate that, compared to non-irradiated male adults, the anabolic metabolism of essential biological substances in the irradiated F1 generation male adults of T. absoluta was impacted.
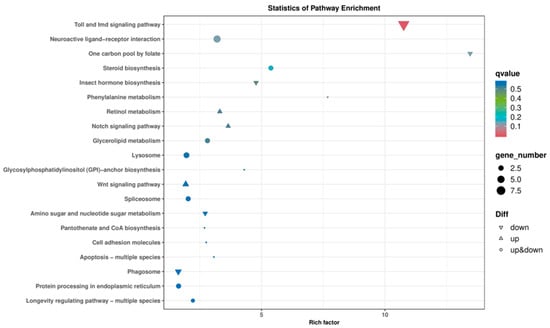
Figure 5.
KEGG enrichment analysis.
3.7. Identification and Screening of Differentially Expressed Genes (DEGs) Associated with Sterility
Based on the results above, 14 target genes potentially related to male fertility were identified. TaTREX1 is involved in replication, recombination, and repair processes. TaCarE1 functions in carbohydrate transport and metabolism. TaCYP405D1 and TaMdr49 are connected to the biosynthesis, transport, and breakdown of secondary metabolites, as well as lipid transport and metabolism. TaNrf-6 belongs to the acyltransferase family and participates in lipid metabolism. Trypsins TaCLSP16 and TaCLSP2, along with TaAtt1, TaAtt2, and TaLys2, are linked to post-translational modification, protein turnover, and chaperoning. Taα-SNAP is involved in intracellular transport, secretion, and vesicle trafficking. Transferrin TaTsf relates to energy production and conversion. TaDef plays a role in signal transduction. TaLeb-4 is an antimicrobial peptide key to host defence. RT-qPCR validated the expression of these genes. The findings (Figure 6) indicated that 13 genes were significantly downregulated in the irradiated progeny. These genes will be further studied to explore their association with decreased male fertility in T. absoluta.
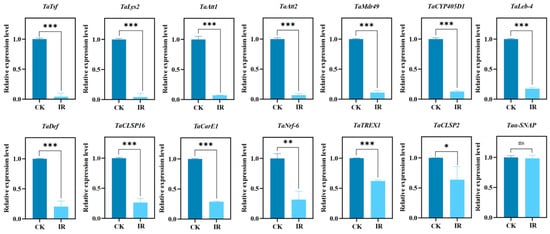
Figure 6.
Quantitative verification of key genes of Tuta absoluta related to male fertility by RT-qPCR. Data are shown as mean ± SE. Statistical significance was analyzed by independent-samples t-test. ns: no significance, (p > 0.05), * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
In this study, by comparing the differences in development, survival, reproduction, and gene expression between the F1 generation of T. absoluta irradiated with a sub-sterile dose (300 Gy) of gamma rays and the non-irradiated F1 generation, we systematically evaluated the biological and transcriptomic effects of this irradiation dose on the F1 generation of T. absoluta. The results showed that the detrimental effects caused by irradiation can be inherited by the F1 generation, affecting their growth, development, reproduction, and the expression of related genes.
The F1 generation after irradiation treatment showed significant delays in egg hatching and developmental stages of each larval instar, pupal stage, and pre-adult period, along with a substantial decrease in pre-emergence survival rate. This effect has also been seen in other lepidopteran pests such as Spodoptera frugiperda and Ephestia elutella [24,25]. It may be related to irradiation-induced DNA damage and the suppression of cell division [26,27]. Previous studies suggest that ionizing radiation can cause apoptosis either by directly damaging DNA or by generating reactive oxygen species (ROS) [28,29,30,31], with increased sensitivity during the rapidly developing larval stage. Additionally, the decrease in pupal survival might result from irradiation disrupting hormone synthesis pathways involved in metamorphosis, like juvenile hormone and ecdysone [32,33], which is supported by the finding that the “Insect hormone biosynthesis” pathway was significantly enriched in KEGG analysis.
The number of eggs laid per female in the F1 generation after irradiation was only 36.8% of that in the control group, with extremely significant reductions in both the intrinsic rate of increase and the finite rate of increase. A combination of factors may cause this reproductive inhibition. The female-to-male ratio in the irradiated group was significantly lower than that in the control group, which aligns with the results of Zhou [16], indicating that irradiation exerts stronger selection pressure on the development or survival of female individuals. A similar phenomenon has also been observed in other lepidopteran pests such as S. frugiperda [25]. Lepidopteran insects have a WZ/ZZ (female/male) sex determination system [34], and irradiation-induced lethal mutations occur on the Z sex chromosome in the current generation, thereby causing the death of female offspring [35]. In the transcriptome analysis, it was found that 125 genes were down-regulated in the irradiated group. Among them, genes involved in the “Steroid biosynthesis” and “Retinol metabolism” pathways may affect the synthesis of sex hormones [33,36]. Additionally, the enrichment of the Toll and Imd signalling pathway suggests that immune deficiency may further reduce the lifespan and fecundity of adults [37,38]. Another possible reason for the developmental delay and decreased survival rate of the F1 generation after irradiation is that energy is prioritized for maintaining survival rather than reproduction [39,40], which manifests as a significant reduction in the lifespan and fecundity of female adults.
GO analysis showed that the differentially expressed genes were mainly involved in metabolic processes and binding functions, with notable enrichment of “One carbon pool by folate” and “Pantothenate and CoA biosynthesis” in KEGG pathways. Folate metabolism is a key pathway for DNA synthesis and methylation, and its disruption can directly lead to abnormal embryonic development, such as a lower egg hatching rate. Moreover, folate is crucial for germ cell proliferation [41]. Coenzyme A participates in energy metabolism and lipid synthesis [42], which may affect energy storage during the pupal stage and influence adult fecundity.
Furthermore, among the 13 down-regulated genes verified by RT-qPCR, several may be linked to insect sperm function [43]. Some genes are related to sperm production, transfer and storage. Chymotrypsin-like serine proteases (CLSPs) have been reported to play a crucial role in sperm migration and sperm–egg interaction to regulate male fertility [44]. Transferrin (Tsf), with abundant expressed in the testis at sexual maturity stages, may be crucial for spermatogenesis and sperm formation in Bactrocera dorsalis [45]. Knocking down the transferrin gene (Tsf) in Nilaparvata lugens (brown planthopper) significantly affects its fecundity, hatching rate, and survival rate, and this gene may also be involved in molting [46]. SeTsf1 in Spodoptera exigua (beet armyworm) plays a dual role in regulating antimicrobial immunity and reproductive processes through tissue-specific iron redistribution [47]. Both NSF and αSNAP play essential roles in cellular transport and fusion; the absence of these proteins can lead to apoptosis [48]. α-SNAP/NSF serves a key function in the acrosome reaction, a process essential for fertilization [49]. The carboxylesterase EST 6 modulated male reproductive functions, which may be consequences of its action on sperm transfer, storage and utilization [50]. TaCYP405D1 is significantly upregulated in tetraniliprole-resistant populations, which is associated with resistance-related fitness costs [51]. Cytochrome P450 (CYP) was found to be associated with sperm morphology and sperm function [52]. The knockout of MRP9 and MRP5 resulted in abonormal sperm mitochondria and decreased fertilization rates [53]. Mdr49 also regulates reproductive functions in Aedes aegypti [54]. We speculate that these genes may serve as core factors that ensure male fertility by directly regulating spermatogenesis.
Nevertheless, for successful sperm delivery and fertilization to occur, it is imperative for sperm to overcome challenges present in the external environment, most notably the host immune system [55]. Several genes have been reported to be involved in the immune system. The queen of Apis mellifera ligustica (Western honeybee) can store sperm in her specialized sperm storage organ for many years. Lysozyme, a conserved immune effector, has been found to be associated with lower fertility rates, which correlates with higher levels of the immune effector lysozyme, consistent with a trade-off between immunity and fertility [56]. Wolbachia, a symbiotic bacterium widely present in insects, can manipulate host reproduction and alter their immune systems [54]. After infecting Bombyx mori (silkworm) with two Wolbachia strains, wKue and wCauB, and treating them with PGN, analysis of four antimicrobial peptide (AMP) genes—cecropin B, defensin B, attacin, and lebocin 3—showed that Wolbachia infection enhances the host’s ability to produce AMPs in response to PGN immune stimulation [57]. The main function of TREX1 is to degrade cytoplasmic DNA and prevent the activation of innate immune responses, playing an important immune role in many mammals [58]. The rupture of the micronuclear membrane, which exposes DNA contents to nucleases, is a possible mechanism for DNA degradation [59]. We speculate that a similar function may exist in insects, which requires further exploration. The resource competition between immunity and reproduction highlights a fundamental trade-off within organisms [60]. Therefore, these immune-related genes may impact fertility through resource allocation.
Every physiological process, including spermatogenesis and immune responses, rests ultimately on a sustained energy supply [61]. According to life history theory, a trade-off happens when two or more traits compete for the same resources, requiring organisms to balance energy-intensive traits such as reproduction, growth, and longevity [62]. The decrease in longevity and fecundity of the irradiated progeny of T. absoluta may result from an energy trade-off between adaptability and life history traits under stress from treatment [63]. In humans and Drosophila melanogaster, upregulation of TORC1 can enhance the oxidative stress response under stress conditions, which is controlled by cnc, a transcription factor homologous to the human Nrf gene [64]. Ubiquitinated proteins and damaged mitochondria activate cnc/nrf-2 via p62, leading to the activation of oxidative response genes with the support of TORC1 upregulation [65,66]. The downregulation of these genes may further explain the decrease in fertility of the irradiated progeny. Thus, we speculate that these genes may modulate the development and reproduction by energy metabolism.
However, all experiments were carried out under laboratory conditions focusing on a single population and radiation dose, which is a limitation of our study. Different populations may vary in radiosensitivity, and radiation dose is closely related to sterility level and competitiveness [67]. Therefore, to apply the sterile insect technique under real field conditions, further research involving the effect of different populations and dose ranges, the field performance of sterile males, and the effectiveness under a semi-field setting is essential. In addition, very small sample sizes were used for the APOP and TPOP parameters in the irradiated group due to the extremely high mortality of its F1 generation. More biological replications need to be performed to confirm this finding.
For the direct application of the sterile insect technique (SIT), although laboratory experiments are essential for isolating the effects of radiation, the behavior and interactions of irradiated progeny under semi-field or field conditions, with factors like predator presence, temperature fluctuations, and host plant quality variation, can lead to divergent outcomes. At the molecular level, transcriptomic studies have identified a strong association between the dysregulation of specific genes and reduced fertility. However, confirming a direct causal link requires future research using gene-editing tools such as CRISPR/Cas9 or RNAi to functionally clarify the roles of these candidate genes. In addition, the current study lacks tissue-level examination, particularly regarding reproductive tissues such as the testis. The effects of irradiation on the morphology and gene expression of reproductive organs will be further explored.
5. Conclusions
In summary, exposing male T. absoluta to a substerilizing dose (300 Gy) can pass on adverse effects to their offspring. It may impede the offspring’s growth, development, and reproduction by altering the expression of key genes and associated signaling pathways, thereby effectively reducing offspring population growth. This study provides a theoretical basis for applying the sterile insect technique (SIT) in field control and identifies potential targets for preventing and managing T. absoluta through the genetic sterile insect technique strategy.
Author Contributions
Investigation, experimental design, methodology, software, data curation, and writing—original draft preparation, Y.P. (Yuhan Pan), H.Z., F.U. and S.Z.; investigation, experimental operation and revising article, Y.P. (Yuhan Pan), Q.Z., Y.P. (Yiming Pan) and Y.W.; experimental design, writing and editing, and supervision, F.U., Y.W., S.Z., X.L., L.C. and J.Z.; experimental design, writing and editing, funding acquisition, project administration, and supervision, Y.H., Y.L., X.L. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ22C140004; Major Science and Technoogy Projects in Xinjiang (2023A02006); Zhejiang High-level Talents Special Support Program (2023R5249); and the Joint Funds of the National Natural Science Foundation of China (No. U22A20489, 32361143791).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Han, P.; Desneux, N.; Becker, C.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Zhang, J.; Lavoir, A.-V. Bottom-up effects of irrigation, fertilization and plant resistance on Tuta absoluta: Implications for integrated pest management. J. Pest Sci. 2019, 92, 1359–1370. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; He, T.; Li, P.; Liu, Y.; Zhou, S.; Wu, Q.; Chen, T.; Lu, Y.; Hou, Y. Comparative biochemical and transcriptome analyses in tomato and eggplant reveal their differential responses to Tuta absoluta infestation. Genomics 2021, 113, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Güncan, A.; Gul, H.; Hafeez, M.; Zhou, S.; Wang, Y.; Zhang, Z.; Huang, J.; Ghramh, H.A.; Guo, W.; et al. Spinosad-induced intergenerational sublethal effects on Tuta absoluta: Biological traits and related gene expressions. Entomol. Gen. 2024, 44, 395–404. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Ullah, F.; Güncan, A.; Abbas, A.; Gul, H.; Guedes, R.N.C.; Zhang, Z.; Huang, J.; Khan, K.A.; Ghramh, H.A.; Chavarín-Gómez, L.E.; et al. Sublethal effects of neonicotinoids on insect pests. Entomol. Gen. 2024, 44, 1145–1160. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Chen, L.; Jinchao, W.; Zhang, Z.; Huang, J.; Li, X.; Lu, Y. Cyantraniliprole resistance in Tuta absoluta: Selection, comparative transcriptomes, and nanocarrier-mediated RNAi studies. Entomol. Gen. 2025, 45, 565–575. [Google Scholar] [CrossRef]
- Ullah, F.; Guru-Pirasanna-Pandi, G.; Murtaza, G.; Sarangi, S.; Gul, H.; Li, X.; Chavarín-Gómez, L.E.; Ramírez-Romero, R.; Guedes, R.N.C.; Desneux, N.; et al. Evolving strategies in agroecosystem pest control: Transitioning from chemical to green management. J. Pest Sci. 2025. [Google Scholar] [CrossRef]
- Marec, F.; Vreysen, M.J.B. Advances and challenges of using the sterile insect technique for the management of pest Lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-insect methods for control of mosquito-borne diseases: An analysis. Vector Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K.; et al. Tuta absoluta continues to disperse in Asia: Damage, ongoing management and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Cagnotti, C.L.; Viscarret, M.M.; Riquelme, M.B.; Botto, E.N.; Carabajal, L.Z.; Segura, D.F.; López, S.N. Effects of X-rays on Tuta absoluta for use in inherited sterility programmes. J. Pest Sci. 2012, 85, 413–421. [Google Scholar] [CrossRef]
- Candás, L.; Cagnotti, C.L.; López, S.N. Integration of inherited sterility and inoculative releases of a miridae predator for the control of the tomato leaf miner, Tuta absoluta. BioControl 2024, 69, 29–37. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhang, J.; Liu, C.; Huang, J.; Zhang, Z.; Ren, X.; Chen, L.; Hafeez, M.; Han, P.; et al. Screening the optimal dose of gamma radiation for Tuta absoluta sterility: Paving the way for sterile insect technique programs. Entomol. Gen. 2024, 44, 415–422. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Huang, H.-W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Genç, H. The tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): Pupal key characters for sexing individuals. Turk. J. Zool. 2016, 40, 801–805. [Google Scholar] [CrossRef]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Salih Özgökçe, M.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Roya, T. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Akkopru, E.P.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Bradley, E.; Tibshirani, R.J. Introduction to the Bootstrap, 1st ed.; CRC Press: New York, NY, USA, 1994; p. 456. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Xu, L.; Li, C.; Li, Q.; Dewer, Y.; Wu, K. Effects of X-ray irradiation on biological parameters and induced sterility of Ephestia elutella: Establishing the optimum irradiation dose and stage. Front. Physiol. 2022, 13, 895882. [Google Scholar] [CrossRef]
- Hao, Z.; Jin, T.; Yang, S.Y.; Lin, Y.Y.; Zhong, H.; Peng, Z.Q.; Ma, G.C. Exploring the hormetic effects of radiation on the life table parameters of Spodoptera frugiperda. Pest Manag. Sci. 2024, 80, 1533–1546. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Wen, J.; Zeng, Y.; Deng, Y.; Hu, Q.; Weng, Q. Molecular mechanism of male sterility induced by 60Co γ-rays on Plutella xylostella (Linnaeus). Molecules 2023, 28, 5727. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Hossain, M.A.; Athanassiou, C.G. Improved quality management of the Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) for enhanced efficacy of the sterile insect technique. Insects 2023, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, G.; Hahn, D.A. Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. J. Exp. Biol. 2012, 215, 2150–2161. [Google Scholar] [CrossRef]
- Ayvaz, A.; Albayrak, S.; Karaborklu, S. Gamma radiation sensitivity of the eggs, larvae and pupae of Indian meal moth Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Pest Manag. Sci. 2008, 64, 505–512. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Deng, Y.; He, R.; Zhang, X.; Zhong, G.; Hu, Q.; Weng, Q. Effects of 60Co-γ radiation on testis physiological aspects of Plutella xylostella (Linnaeus). Ecotoxicol. Environ. Saf. 2019, 169, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction–oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, J.; Zhang, G.; Singh, D.; Zhang, X.; Liang, Z. Mamestra brassicae multiple nucleopolyhedroviruses prevents pupation of Helicoverpa armigera by regulating juvenile hormone titer. Insects 2024, 15, 202. [Google Scholar] [CrossRef]
- Vengatharajuloo, V.; Goh, H.-H.; Hassan, M.; Govender, N.; Sulaiman, S.; Afiqah-Aleng, N.; Harun, S.; Mohamed-Hussein, Z.-A. Gene co-expression network analysis reveals key regulatory genes in Metisa plana hormone pathways. Insects 2023, 14, 503. [Google Scholar] [CrossRef]
- Traut, W.; Sahara, K.; Marec, F. Sex chromosomes and sex determination in Lepidoptera. Sex. Dev. 2007, 1, 332–346. [Google Scholar] [CrossRef]
- Marec, F.; Kollárová, I.; Pavelka, J. Radiation-induced inherited sterility combined with a genetic sexing system in Ephestia kuehniella (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 1999, 92, 250–259. [Google Scholar] [CrossRef]
- Toyofuku, M.; Fujinaga, D.; Inaba, K.; Funahashi, T.; Fujikawa, Y.; Inoue, H.; Kataoka, H.; Niwa, R.; Ono, H. The plant-derived triterpenoid, cucurbitacin B, but not cucurbitacin E, inhibits the developmental transition associated with ecdysone biosynthesis in Drosophila melanogaster. J. Insect Physiol. 2021, 134, 104294. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Killiny, N. In silico characterization and gene expression analysis of toll signaling pathway-related genes in Diaphorina citri. Insects 2022, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Rice, A.; Li, T.; Wang, J.; Yang, X.; Yuan, H.; Graham, R.I.; Wilson, K. Partiti-like viruses from African armyworm increase larval and pupal mortality of a novel host: The Egyptian cotton leafworm. Pest Manag. Sci. 2022, 78, 1529–1537. [Google Scholar] [CrossRef]
- Leyria, J.; Fruttero, L.L.; Paglione, P.A.; Canavoso, L.E. How insects balance reproductive output and immune investment. Insects 2025, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Niu, R.; Gao, X.; Luo, J.; Cui, J.; Wang, L.; Zhu, X. Pseudomonas infection affects the growth and development of Aphis gossypii by disrupting energy metabolism and reproductive processes. Insects 2025, 16, 238. [Google Scholar] [CrossRef]
- Walker, A.K. Germ cells need folate to proliferate. Dev. Cell 2016, 38, 8–9. [Google Scholar] [CrossRef]
- Zapata, R.; Piulachs, M.D.; Bellés, X. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase lower fecundity in the German cockroach: Correlation between the effects on fecundity in vivo with the inhibition of enzymatic activity in embryo cells. Pest Manag. Sci. 2003, 59, 1111–1117. [Google Scholar] [CrossRef]
- Park, H.-G.; Kim, B.-Y.; Kim, J.-M.; Choi, Y.-S.; Yoon, H.-J.; Lee, K.-S.; Jin, B.-R. Upregulation of transferrin and major royal jelly proteins in the spermathecal fluid of mated honeybee (Apis mellifera) queens. Insects 2021, 12, 690. [Google Scholar] [CrossRef]
- Shang, X.; Shen, C.; Liu, J.; Tang, L.; Zhang, H.; Wang, Y.; Wu, W.; Chi, J.; Zhuang, H.; Fei, J.; et al. Serine protease PRSS55 is crucial for male mouse fertility via affecting sperm migration and sperm–egg binding. Cell. Mol. Life Sci. 2018, 75, 4371–4384. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, M.-Y.; Gu, P.-M.; Smagghe, G.; Wang, J.-J. Label-free based quantitative proteomic analysis identifies proteins involved in the testis maturation of Bactrocera dorsalis (Hendel). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Xu, C.; Jiao, Q.; Ye, C.; Chen, T.; Yu, X.; Pang, K.; Hao, P. Rice defense against brown planthopper partially by suppressing the expression of transferrin family genes of brown planthopper. J. Agric. Food Chem. 2022, 70, 2839–2850. [Google Scholar] [CrossRef]
- Lin, K.; Zhou, Y.; Tian, H.; Du, X.; Yue, L. Iron-binding transferrins regulate immunity and reproduction via tissue-specific iron redistribution in Spodoptera exigua. Int. J. Biol. Macromol. 2025, 310, 143310. [Google Scholar] [CrossRef]
- Chae, T.H.; Kim, S.; Marz, K.E.; Hanson, P.I.; Walsh, C.A. The hyh mutation uncovers roles for αSnap in apical protein localization and control of neural cell fate. Nat. Genet. 2004, 36, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tomes, C.N.; De Blas, G.A.; Michaut, M.A.; Farré, E.V.; Cherhitin, O.; Visconti, P.E.; Mayorga, L.S. α-SNAP and NSF are required in a priming step during the human sperm acrosome reaction. Mol. Hum. Reprod. 2005, 11, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.G.; Richmond, R.C. Esterase 6 in Drosophila melanogaster. Reproductive function ofactive and null males at low temperature. Proc. Natl. Acad. Sci. USA 1982, 79, 2962–2966. [Google Scholar] [CrossRef]
- Ullah, F.; Ullah, Z.; Gul, H.; Li, X.; Pan, Y.; Zhang, H.; Zhang, Z.; Huang, J.; Emmanouil, R.; Guedes, R.N.C.; et al. Proactive resistance management studies highlight the role of cytochrome P450 genes in the resistance of Tuta absoluta against tetraniliprole. Int. J. Mol. Sci. 2025, 26, 5180. [Google Scholar] [CrossRef]
- Liu, Y.; Dettin, L.E.; Folmer, J.; Zirkin, B.R.; Papadopoulos, V. Abnormal morphology of spermatozoa in cytochrome P450 17α-hydroxylase/17, 20-lyase (CYP17) deficient mice. J. Androl. 2013, 28, 453–460. [Google Scholar] [CrossRef]
- Chambers, I.G.; Kumar, P.; Lichtenberg, J.; Wang, P.; Yu, J.; Phillips, J.D.; Kane, M.A.; Bodine, D.; Hamza, I. MRP5 and MRP9 play a concerted role in male reproduction and mitochondrial function. Proc. Natl. Acad. Sci. USA 2022, 119, e2111617119. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Lan, J.; Zhang, L.; Chen, H.; Jiang, L.; Yu, H.; Liu, N.; Liao, C.; Han, Q. MDR49 coding for both P-glycoprotein and TMOF transporter functions in ivermectin resistance, trypsin activity inhibition, and fertility in the yellow fever mosquito, Aedes aegypti. Pestic. Biochem. Physiol. 2024, 201, 105899. [Google Scholar] [CrossRef]
- McGraw, K.; Dowling, D.K.; Simmons, L.W. Ejaculate economics: Testing the effects of male sexual history on the trade-off between sperm and immune function in Australian crickets. PLoS ONE 2012, 7, e30172. [Google Scholar] [CrossRef]
- McAfee, A.; Chapman, A.; Pettis, J.S.; Foster, L.J.; Tarpy, D.R. Trade-offs between sperm viability and immune protein expression in honey bee queens (Apis mellifera). Commun. Biol. 2021, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yoshimura, S.; Sasaki, T.; Furukawa, S. Influence of Wolbachia infection on antimicrobial peptide gene expressions in a cell line of the silkworm, Bombyx mori (Lepidoptera: Bombycidae). J. Asia-Pac. Entomol. 2021, 24, 1164–1169. [Google Scholar] [CrossRef]
- Lehtinen, D.A.; Harvey, S.; Mulcahy, M.J.; Hollis, T.; Perrino, F.W. The TREX1 double-stranded DNA degradation activity is defective in dominant mutations associated with autoimmune disease. J. Biol. Chem. 2008, 283, 31649–31656. [Google Scholar] [CrossRef]
- Mohr, L.; Toufektchan, E.; von Morgen, P.; Chu, K.; Kapoor, A.; Maciejowski, J. ER-directed TREX1 limits cGAS activation at micronuclei. Mol. Cell 2021, 81, 724–738.e9. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction–immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Y.; Gao, D.; Lin, D.; Ma, W.; Chang, D. Metabolic pathways and male fertility: Exploring the role of Sertoli cells in energy homeostasis and spermatogenesis. Am. J. Physiol.-Endocrinol. Metab. 2025, 329, E160–E178. [Google Scholar] [CrossRef] [PubMed]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Monroy Kuhn, J.M.; Meusemann, K.; Korb, J. Disentangling the aging gene expression network of termite queens. BMC Genomics 2021, 22, 339. [Google Scholar] [CrossRef]
- Ichimura, Y.; Waguri, S.; Sou, Y.-s.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef]
- Aramburu, J.; Carmen Ortells, M.; Tejedor, S.; Buxadé, M.; López-Rodríguez, C. Transcriptional regulation of the stress response by mTOR. Sci. Signal. 2014, 7, re2. [Google Scholar] [CrossRef] [PubMed]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 355–398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).