Polyandry in Noctuid Moths: Taxonomic, Bionomic, and Evolutionary Implications
Simple Summary
Abstract
1. Introduction: Facts and Hypotheses Regarding Polyandry
2. Materials and Methods
3. Hypotheses and Results
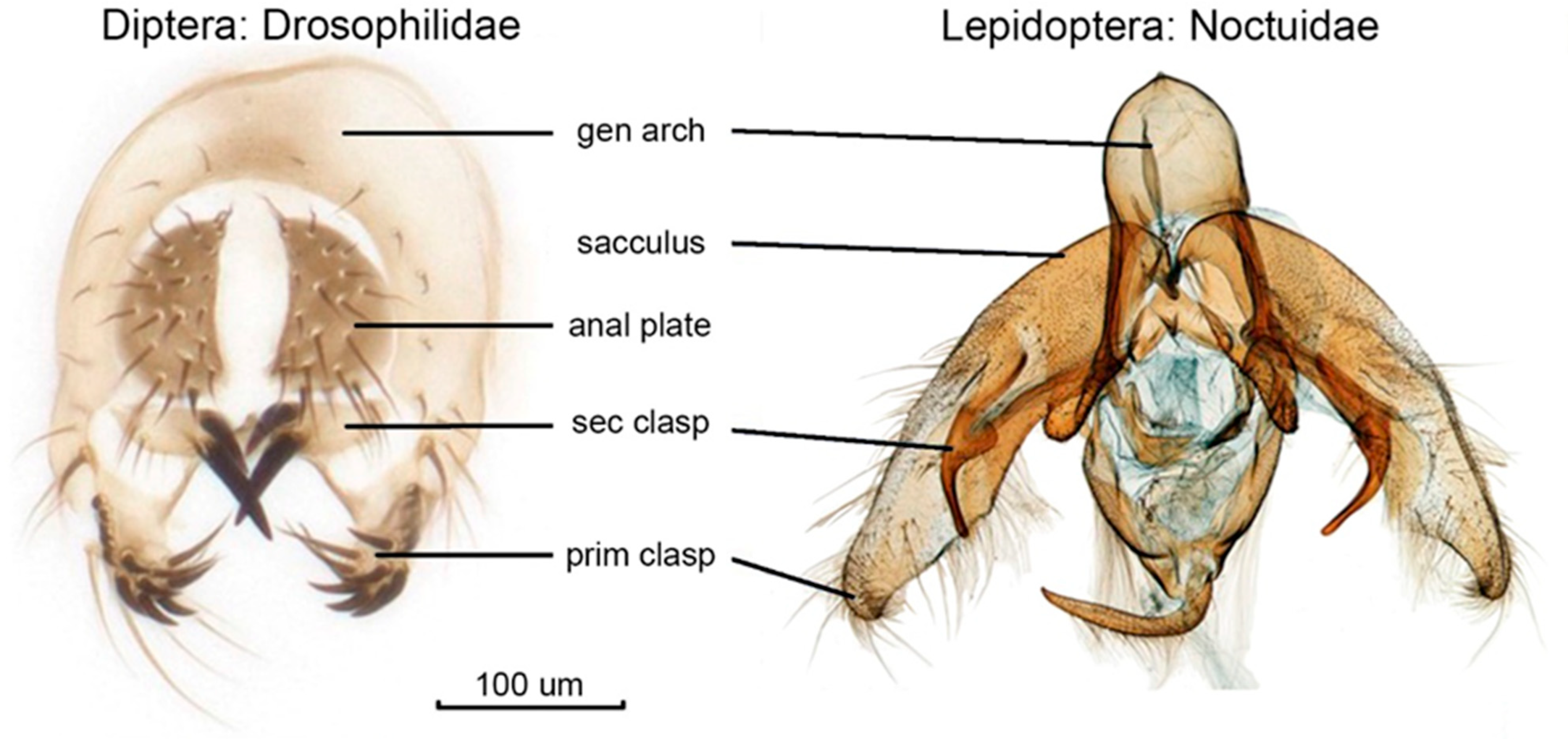
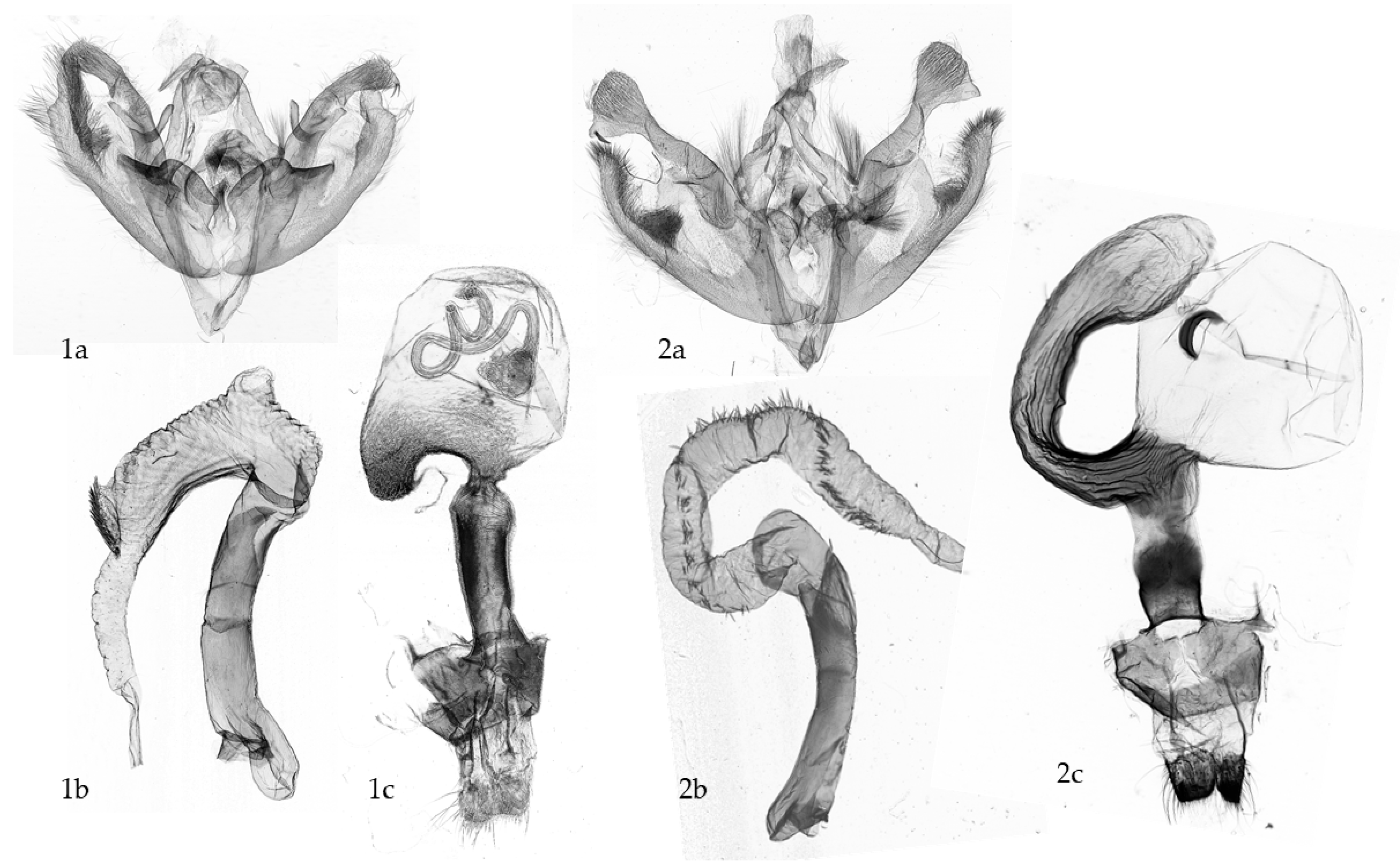
3.1. Trade-Offs in Copulation: Structures, Functions, and New Working Hypotheses

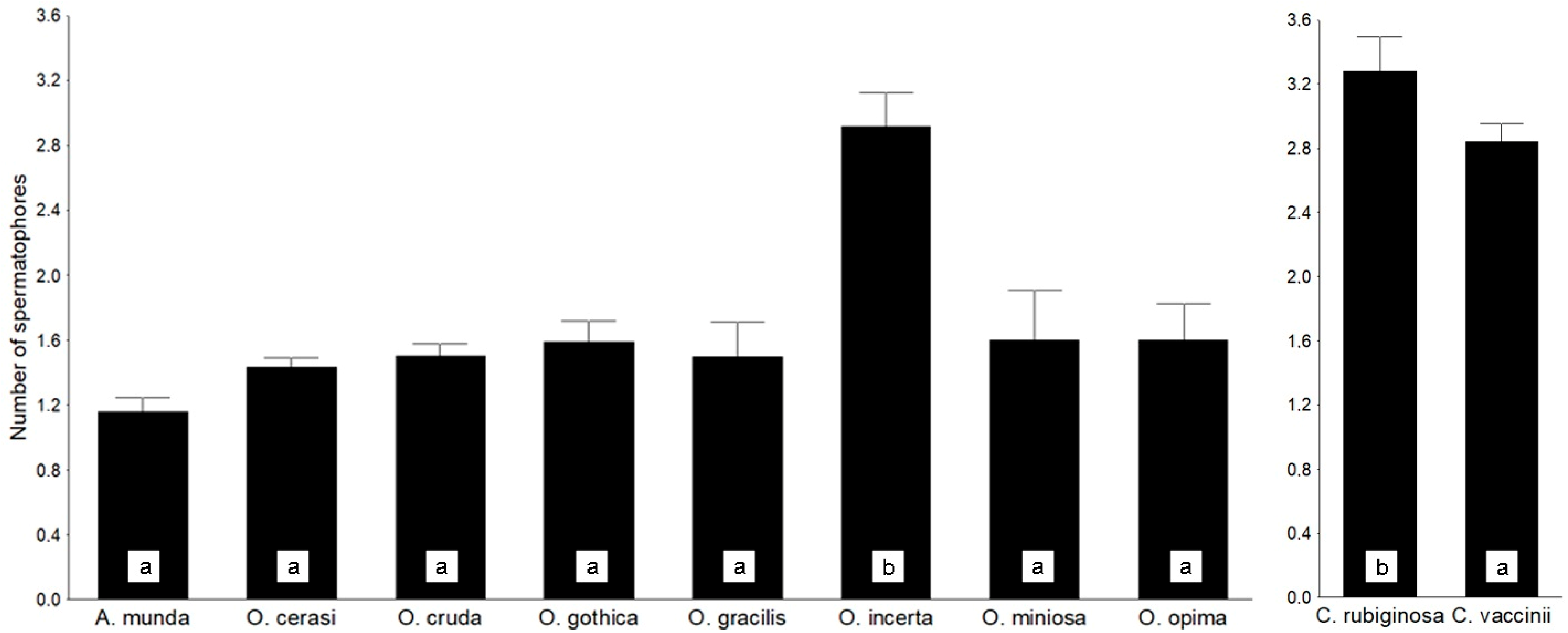
3.2. Preliminary Results: Correlates and Consequences of Polyandry
4. Discussion and Remaining Questions
4.1. Connection of Polyandry to Life Cycle: Hypotheses and Preliminary Results
4.2. Questions and Suggestions for Further Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, M.L.; Price, T.A.R.; Wedell, N. Polyandry in Nature: A Global Analysis. Trends Ecol. Evol. 2014, 29, 376–383. [Google Scholar] [CrossRef]
- Torres-Vila, L.M. Polyandry-fecundity relationship in insects: Methodological and conceptual problems. J. Evol. Biol. 2012, 26, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, G.; Nilsson, T. The Evolution of Polyandry: Multiple Mating and Female Fitness in Insects. Anim. Behav. 2000, 60, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W. The evolution of polyandry: Sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 125–146. [Google Scholar] [CrossRef]
- Kvarnemo, C.; Simmons, L.W. Polyandry as a mediator of sexual selection before and after mating. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120042. [Google Scholar] [CrossRef]
- Fromonteil, S.; Marie-Orleach, L.; Winkler, L.; Janicke, T. Sexual selection in females and the evolution of polyandry. PLoS Biol. 2023, 21, e3001916. [Google Scholar] [CrossRef]
- Collet, J.; Richardson, D.S.; Worley, K.; Pizzaria, T. Sexual selection and the differential effect of polyandry. Proc. Natl. Acad. Sci. USA 2012, 109, 8641–8645. [Google Scholar] [CrossRef]
- Arnqvist, G.; Rowe, L. Sexual Conflict; Princeton University Press: Princeton, NJ, USA, 2005; xii+330p. [Google Scholar]
- Drummond, B.A. Multiple mating and sperm competition in the Lepidoptera. In Sperm Competition and the Evolution of Animal Mating Systems; Smith, R.L., Ed.; Academic Press: New York, NY, USA, 1984; pp. 291–370. [Google Scholar]
- Simmons, L.W.; Siva-Jothy, M.T. Sperm competition in insects: Mechanisms and the potential for selection. In Sperm Competition and Sexual Selection; Birkhead, T.R., Møller, A.P., Eds.; Academic Press: Cambridge, MA, USA, 1998; pp. 783–826. [Google Scholar]
- Underwood, D.L.A.; Shapiro, A.M. A male-biased primary sex ratio and larval mortality in Eucheira socialis (Lepidoptera: Pieridae). Evol. Ecol. Res. 1999, 1, 703–717. [Google Scholar]
- Simmons, L.W. Sperm Competition and Its Evolutionary Consequences in the Insects; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Cordero, C.; Baixeras, J. Sexual selection within the female genitalia in Lepidoptera. In Cryptic Female Choice in Arthropods: Patterns, Mechanisms and Prospects; Peretti, V.A., Aisenberg, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 325–350. [Google Scholar]
- Boggs, C.L.; Gilbert, L.E. Male Male contribution to egg production in butterflies: Evidence for transfer nutrients at mating. Science 1979, 206, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Boggs, C.L. Male nuptial gifts: Phenotypic consequences and evolutionary implications. In Insect Reproduction; Leather, S.R., Hardie, J., Eds.; CRC Press: New York, NJ, USA, 1995; pp. 215–242. [Google Scholar]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Jennions, M.D. Polyandry and fecundity in the Lepidoptera: Can methodological and conceptual approaches bias outcomes? Behav. Ecol. Sociobiol. 2004, 55, 315–324. [Google Scholar] [CrossRef]
- Callahan, P.S.; Chapin, J.B. Morphology of the reproductive systems and mating in two representative members of the family Noctuidae, Pseudaletia unipuncta and Peridroma margaritosa, with comparison to Heliothis zea (Boddie). Ann. Entomol. Soc. Am. 1960, 53, 763–782. [Google Scholar] [CrossRef]
- Seth, R.K.; Kaur, J.J.; Rao, D.K.; Reynolds, S.E. Sperm transfer during mating, movement of sperm in the female reproductive tract, and sperm precedence in the common cutworm Spodoptera litura. Physiol. Entomol. 2002, 27, 1–14. [Google Scholar] [CrossRef]
- Wanke, D. Interaction of the extenal and internal genitalia during copulation in Eupithecia abbreviata Stephens, 1831 (Lepidoptera: Geometridae: Larentiinae). Integr. Syst. Stuttg. Contrib. Nat. Hist. 2022, 5, 137–143. [Google Scholar] [CrossRef]
- Arnquist, G. Comparative evidence for the evolution of genitalia by sexual selection. Nature 1998, 393, 784–786. [Google Scholar] [CrossRef]
- Eberhard, W.G. Sexual Selection and Animal Genitalia; Harvard University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Eberhard, W.G. Females Control: Sexual Selection by Cryptic Female Choice; Princeton University Press: Princeton, NJ, USA, 1996. [Google Scholar]
- Ward, K.E.; Landolt, P.J. Influence of multiple matings on fecundity and longevity of female cabbage looper moths (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1995, 88, 768–772. [Google Scholar] [CrossRef]
- Unnithan, G.C.; Paye, S.O. Factors involved in mating, longevity, fecundity and egg fertility in the maize stem-borer, Busseola fusca (Fuller) (Lep., Noctuidae). J. Appl. Entomol. 1990, 109, 295–301. [Google Scholar] [CrossRef]
- Svärd, L.; McNeil, J.N. Female benefit, male risk: Polyandry in the true armyworm Pseudaletia unipuncta. Behavoural Ecol. Sociobiol. 1994, 35, 319–326. [Google Scholar] [CrossRef]
- Hou, M.; Sheng, C. Fecundity and Longevity of Helicoverpa armigera (Lepidoptera: Noctuidae): Effects of Multiple Mating. J. Econ. Entomol. 1999, 92, 569–573. [Google Scholar] [CrossRef]
- LaMunyon, C.W. Sperm storage by females of the polyandrous noctuid moth Heliothis virescens. Anim. Behav. 2000, 59, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Gragera, J.; Bielza-Lino, P. Polyandry in Lepidoptera: A heritable trait in Spodoptera exigua Hübner. Heredity 2001, 86, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Nakagawa, S.; Coltman, D.W.; Slate, J.; Sheldon, B.C. A quantitative review of heterozygosity–fitness correlations in animal populations. Mol. Ecol. 2009, 18, 2746–2765. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Jennions, M.D.; Backwell, P.R.Y. Polyandry Occurs Because Females Initially Trade Sex for Protection. Anim. Behav. 2012, 83, 1203–1206. [Google Scholar] [CrossRef]
- Santostefano, F.; Galarza, J.A.; Mappes, J. Testing the direct and genetic benefit hypotheses of polyandry in the wood tiger moth. Behav. Ecol. Sociobiol. 2018, 72, 1–10. [Google Scholar] [CrossRef]
- LaMunyon, C.W.; Huffman, T.S. Determinants of sperm transfer by males of the noctuid moth Heliothis virescens. J. Insect Behav. 2001, 14, 187–199. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. A polyandrous female moth discriminates against previous mates to gain genetic diversity. Anim. Behav. 2009, 78, 1309–1315. [Google Scholar] [CrossRef]
- Montezano, D.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, W.F.; Bortolin, T.M.; Fronza, E.; Pezzi, P.; Luz, P.C.; Barros, N.M. Biotic potential, fertility and life-table of Spodoptera albula (Walker) (Lepidoptera, Noctuidae) under controlled conditions. Ann. Bras. Acad. Sci. 2014, 86, 723–732. [Google Scholar]
- Taylor, M.L.; Price, T.A.R.; Skeats, A.; Wedell, N. Temperature can shape a cline in polyandry, but only genetic variation can sustain it over time. Behav. Ecol. 2016, 27, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.S. The preparation of slides of Lepidoptera genitalia with special reference to the microlepidoptera. Entomol. Gaz. 1976, 27, 127–132. [Google Scholar]
- Lafontaine, J.D. Noctuoidea: Noctuidae (part): Noctuinae (Part Euxoa). In The Moths of North America, Fascicle 27.2; Dominick, R.B., Ferguson, D.C., Franclemont, J.G., Hodges, R.W., Munroe, E.G., Eds.; The Wedge Entomological Research Foundation: Washington, DC, USA, 1978; 237p. [Google Scholar]
- Xu, J.; Zhan, S.; Chen, S.; Zeng, B.; Li, Z.; James, A.A.; Tan, A.; Huang, Y. Sexually Dimorphic Traits in the Silkworm, Bombyx mori, Are Regulated by Doublesex. Insect Biochem. Mol. Biol. 2017, 80, 42–51. [Google Scholar] [CrossRef]
- Callahan, P.S. Serial morphology as a technique for determination of reproductive patterns in the corn earworm, Heliothis zea (Boddie). Ann. Entomol. Soc. Am. 1958, 51, 413–428. [Google Scholar] [CrossRef]
- Mikkola, K. The lock-and-key mechanisms of the internal genitalia of the Noctuidae (Lepidoptera): How are they selected for? Eur. J. Entomol. 2008, 105, 13–25. [Google Scholar] [CrossRef]
- Dufour, L. Anatomie générale des diptères. Ann. Des Sci. Nat. 1844, 1, 244–264. [Google Scholar]
- Dougherty, L.R.; Shuker, D.M. Variation in pre- and post-copulatory sexual selection on male genital size in two species of lygaeid bug. Behav. Ecol. Sociobiol. 2016, 70, 625–637. [Google Scholar] [CrossRef]
- De Wilde, J. Reproduction. In Physiology of Insecta; Rockstein, M., Ed.; Academic Press: New York, NY, USA, 1964; pp. 9–58, 404. [Google Scholar]
- Eberhard, W.G. Species isolation, genital mechanics, and the evolution of species-specific genitalia in three species of Macrodactylus beetles (Coleoptera, Scarabeidae, Melolonthinae). Evolution 1992, 46, 1774–1783. [Google Scholar] [CrossRef]
- Hosken, D.J.; Stockley, P. Sexual selection and genital evolution. Trends Ecol. Evol. 2004, 19, 87–93. [Google Scholar] [CrossRef]
- Masly, J.P. 170 Years of “Lock-and-Key”: Genital Morphology and Reproductive Isolation. Int. J. Evol. Biol. 2012, 1–10. [Google Scholar] [CrossRef]
- Varga, Z.; Ronkay, G.; Ronkay, L. Revised taxonomic check list of the Eurasiatic species of the subtribe Poliina (Noctuidae, Noctuinae, Hadenini). Dtsch. Entomol. Z. 2017, 64, 133–160. [Google Scholar] [CrossRef]
- Varga, Z.; Gyulai, P.; Ronkay, G.; Ronkay, L. Review of the species groups of the genus Ctenoceratoda Varga, 1992 with description of four new species and a new subspecies (Lepidoptera, Noctuidae). Acta Zool. Acad. Sci. Hung. 2018, 64, 51–74. [Google Scholar] [CrossRef]
- Varga, Z.; Ronkay, G.; Ronkay, L. Phylogenetic Trends in the Dissymmetrisation of Genitalia in Hadenini (Lepidoptera, Noctuidae). Diversity 2024, 16, 248. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Yu, J.-F.; Lu, Q.; Xu, J.; Ye, H. Female and Male Moths Display Different Reproductive Behavior When Facing New versus Previous Mates. PLoS ONE 2014, 9, e109564. [Google Scholar] [CrossRef]
- Lafontaine, J.D.; Mikkola, K. Lock-and-key systems in the inner genitalia of Noctuidae (Lepidoptera) as a taxonomic character. Entomol. Meddelelser 1987, 55, 161–167, [In Swedish, with English abstr.]. [Google Scholar]
- Lees, D.C. The genome sequence of the Chestnut, Conistra vaccinii (Linnaeus, 1761). Wellcome Open Res. 2023, 8, 532. [Google Scholar] [CrossRef]
- Spitzer, K.; Rejmánek, M.; Soldán, T. The Fecundity and Long-Term Variability in Abundance of Noctuid Moths (Lepidoptera, Noctuidae). Oecologia 1984, 62, 91–93. [Google Scholar] [CrossRef]
- Varga, Z.; Ronkay, L. New and revised taxa of the genera Chersotis Boisduval, 1840 and Dichagyris Lederer, 1857 from Central Asia (Lep.: Noctuidae). Esperiana 1996, 5, 175–214. [Google Scholar]
- Sten, T.H.; Li, R.; Hollunder, F.; Eleazer, S.; Ruta, V. Male-male interactions shape mate selection in Drosophila. bioRxiv 2023. [Google Scholar] [CrossRef]
- Oku, T.; Chiba, T.; Saito, O.; Kobayashi, T. Preliminary notes on the aestivation of a cutworm moth, Euxoa sibirica Boisduval, at high altitude in Tohoku District (Lepidoptera: Noctuidae). Kontyû 1972, 40, 265–269. [Google Scholar]
- Larsdotter Mellström, H.; Wiklund, C. What affects mating rate? Polyandry is higher in the directly developing 436 generation of the butterfly Pieris napi. Anim. Behav. 2010, 80, 413–418. [Google Scholar] [CrossRef]
- Szalárdi, T.; Szanyi, Sz.; Szarukán, I.; Tóth, M.; Nagy, A. Semiochemical baited traps of lepidopteran pests of economic importance can deliver reliable data also on wide range of non-target species: Case study in the Hajdúság Region of East Pannonian Lowland (East Hungary). Biodivers. Data J. 2021, 9, e72305. [Google Scholar] [CrossRef] [PubMed]
- Batalias, R.E.; Evenden, M.L. Fermented or Floral? Developing a Generalized Food Bait Lure to Monitor Cutworm and Armyworm Moths (Lepidoptera: Noctuidae) in Field Crops. Insects 2023, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.L., Jr. Trapping noctuid moths with synthetic floral volatile lures. Entomol. Exp. Appl. 2002, 103, 219–226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, Z.; Nagy, A.; Kovács, C.L.; Szanyi, S. Polyandry in Noctuid Moths: Taxonomic, Bionomic, and Evolutionary Implications. Insects 2025, 16, 1063. https://doi.org/10.3390/insects16101063
Varga Z, Nagy A, Kovács CL, Szanyi S. Polyandry in Noctuid Moths: Taxonomic, Bionomic, and Evolutionary Implications. Insects. 2025; 16(10):1063. https://doi.org/10.3390/insects16101063
Chicago/Turabian StyleVarga, Zoltán, Antal Nagy, Csenge Lelle Kovács, and Szabolcs Szanyi. 2025. "Polyandry in Noctuid Moths: Taxonomic, Bionomic, and Evolutionary Implications" Insects 16, no. 10: 1063. https://doi.org/10.3390/insects16101063
APA StyleVarga, Z., Nagy, A., Kovács, C. L., & Szanyi, S. (2025). Polyandry in Noctuid Moths: Taxonomic, Bionomic, and Evolutionary Implications. Insects, 16(10), 1063. https://doi.org/10.3390/insects16101063







