Current Status and Clinical Characteristics of Familial Hypercholesterolemia Patients in Korea: A Multicenter, Real-World Experience
Abstract
1. Introduction
2. Methods
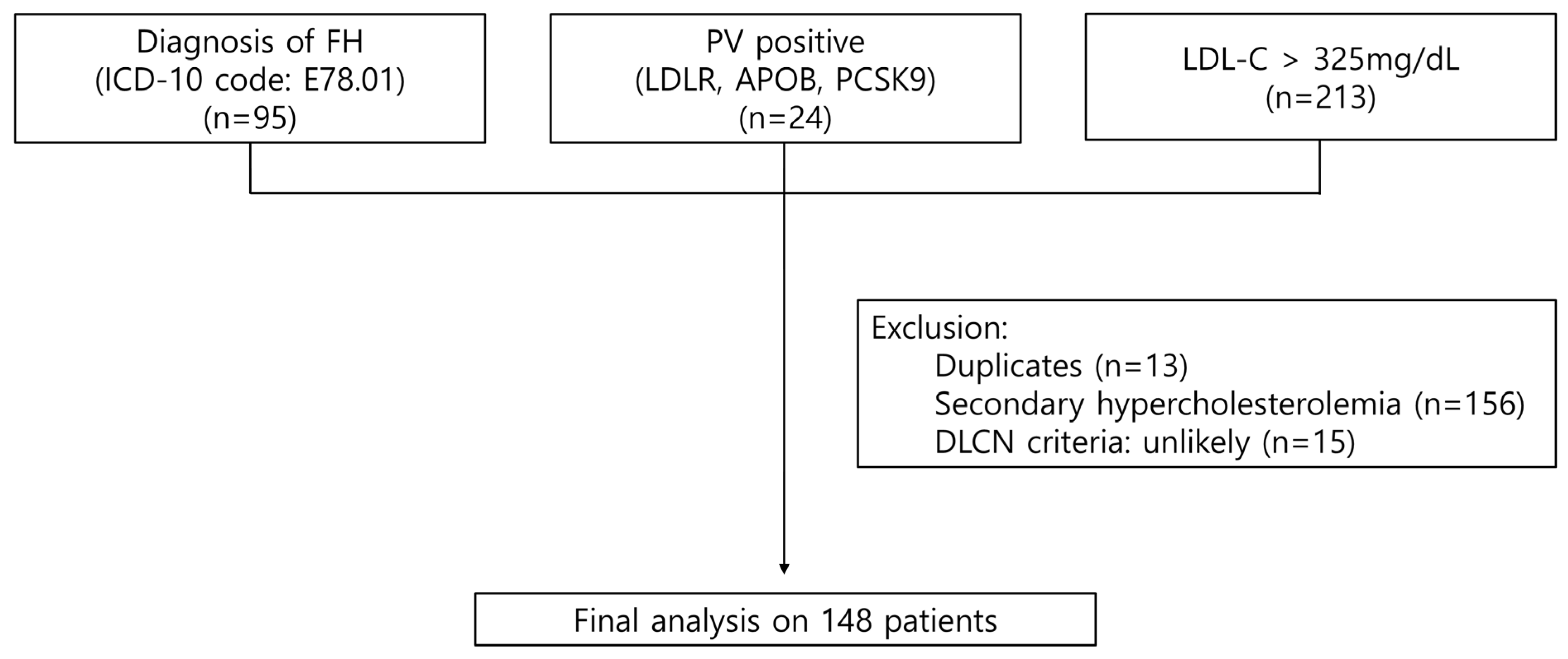
2.1. Study Design and Study Participants
2.2. Definitions
2.3. Genetic Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Presentation and Reasons for Referral
3.3. Pathologic Variant Testing
3.4. Lipid Profiles and Lipid Lowering Treatments
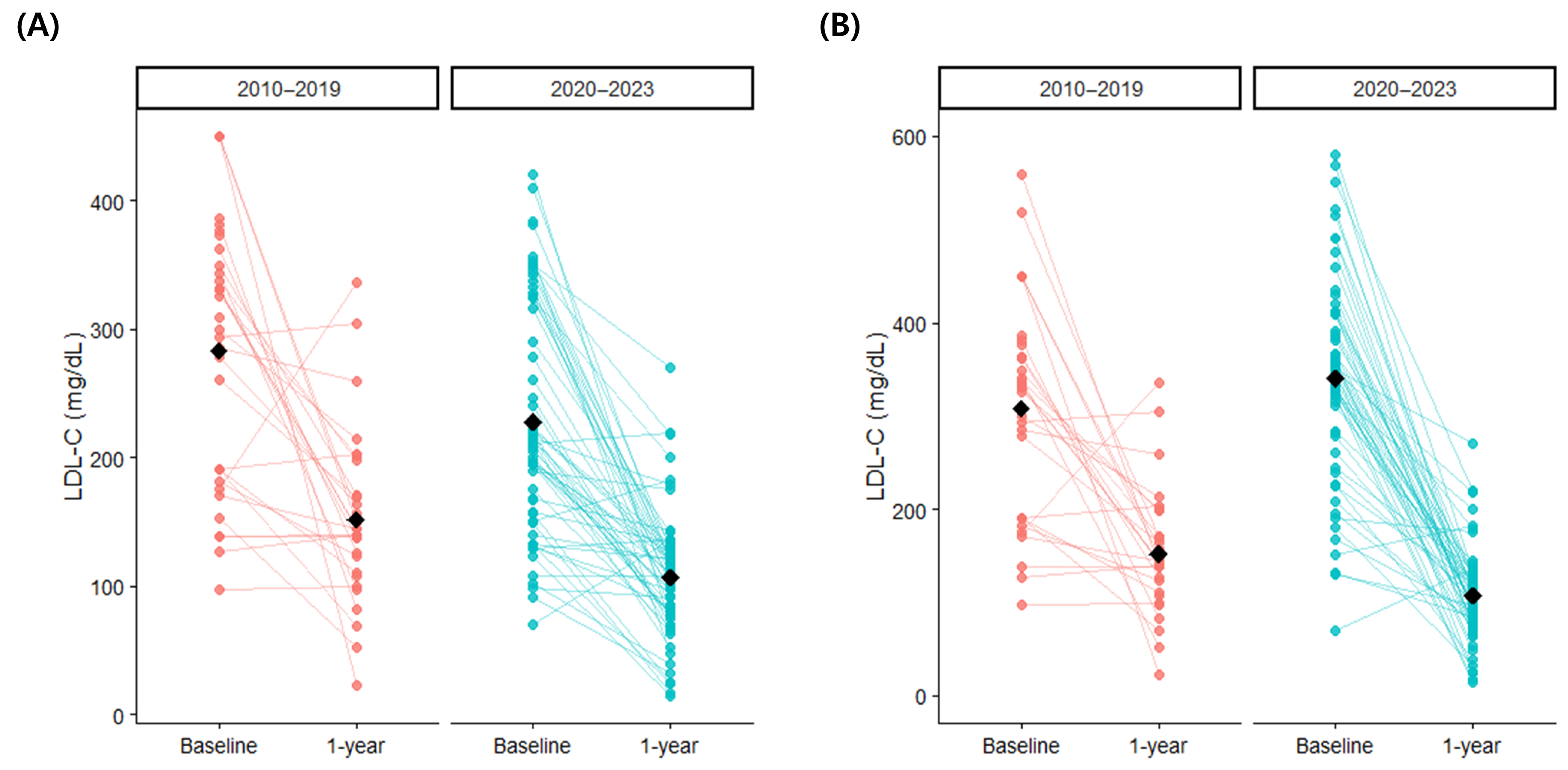
3.5. Clinical Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 2017, 3, 17093. [Google Scholar] [CrossRef]
- Lee, C.J.; Yoon, M.; Kang, H.J.; Kim, B.J.; Choi, S.H.; Jeong, I.K.; Lee, S.H.; Task Force Team for Familial Hypercholesterolemia; Korean Society of Lipid and Atherosclerosis. 2022 Consensus Statement on the Management of Familial Hypercholesterolemia in Korea. J. Lipid Atheroscler. 2022, 11, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; ESC Comm Practice Guidelines CPG; ESC Natl Cardiac Societies; Mach, F.; Baigent, C.; Taskinen, M.-R. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Benn, M.; Watts, G.F.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Mutations causative of familial hypercholesterolaemia: Screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur. Heart J. 2016, 37, 1384–1394. [Google Scholar] [CrossRef]
- Wald, D.S.; Bestwick, J.P.; Morris, J.K.; Whyte, K.; Jenkins, L.; Wald, N.J. Child-Parent Familial Hypercholesterolemia Screening in Primary Care. N. Engl. J. Med. 2016, 375, 1628–1637. [Google Scholar] [CrossRef]
- Kalra, S.; Chen, Z.; Deerochanawong, C.; Shyu, K.G.; Tan, R.S.; Tomlinson, B.; Yeh, H.I. Familial Hypercholesterolemia in Asia Pacific: A Review of Epidemiology, Diagnosis, and Management in the Region. J. Atheroscler. Thromb. 2021, 28, 417–434. [Google Scholar] [CrossRef]
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A HuGE prevalence review. Am. J. Epidemiol. 2004, 160, 407–420. [Google Scholar] [CrossRef]
- Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ 1991, 303, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, C.J.; Kim, S.H.; Kim, J.Y.; Choi, S.H.; Kang, H.J.; Park, K.S.; Cho, B.R.; Kim, B.J.; Sung, K.C.; et al. Phenotypic and Genetic Analyses of Korean Patients with Familial Hypercholesterolemia: Results from the KFH Registry 2020. J. Atheroscler. Thromb. 2022, 29, 1176–1187. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Liu, Z.; Cui, K.; Zhang, Y.; Zhang, Y.; Zhao, K.; Yin, K.; Li, W.; Zhou, Z. Targeted Genetic Analysis in a Chinese Cohort of 208 Patients Related to Familial Hypercholesterolemia. J. Atheroscler. Thromb. 2020, 27, 1288–1298. [Google Scholar] [CrossRef]
- Futema, M.; Whittall, R.A.; Kiley, A.; Steel, L.K.; Cooper, J.A.; Badmus, E.; Leigh, S.E.; Karpe, F.; Neil, H.A.; Simon Broome Register, G.; et al. Analysis of the frequency and spectrum of mutations recognised to cause familial hypercholesterolaemia in routine clinical practice in a UK specialist hospital lipid clinic. Atherosclerosis 2013, 229, 161–168. [Google Scholar] [CrossRef]
- Matsunaga, K.; Mizobuchi, A.; Ying Fu, H.; Ishikawa, S.; Tada, H.; Kawashiri, M.A.; Yokota, I.; Sasaki, T.; Ito, S.; Kunikata, J.; et al. Universal Screening for Familial Hypercholesterolemia in Children in Kagawa, Japan. J. Atheroscler. Thromb. 2022, 29, 839–849. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.A.; Nohara, A.; Inazu, A.; Mabuchi, H.; Yamagishi, M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur. Heart J. 2017, 38, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, C.J.; Pak, H.; Kim, D.I.; Rhee, M.Y.; Lee, B.K.; Ahn, Y.; Cho, B.R.; Woo, J.T.; Hur, S.H.; et al. GENetic characteristics and REsponse to lipid-lowering therapy in familial hypercholesterolemia: GENRE-FH study. Sci. Rep. 2020, 10, 19336. [Google Scholar] [CrossRef]
- Li, B.V.; Laurie, A.D.; Reid, N.J.; Leath, M.A.; King, R.I.; Chan, H.K.; Florkowski, C.M. Association of Clinical Characteristics With Familial Hypercholesterolaemia Variants in a Lipid Clinic Setting: A Case-Control Study. J. Lipid Atheroscler. 2024, 13, 29–40. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490a. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Vaz, A.J.; Stevens, C.A.; Lyons, A.R.; Dharmayat, K.I.; Freiberger, T.; Hovingh, G.K.; Mata, P.; Raal, F.J.; Santos, R.D.; Soran, H.; et al. Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021, 398, 1713–1725. [Google Scholar] [CrossRef]
- Kayikcioglu, M.; Basaran, O.; Dogan, V.; Mert, K.U.; Mert, G.O.; Ozdemir, I.H.; Rencuzogullari, I.; Karadeniz, F.O.; Tekinalp, M.; Askin, L.; et al. Misperceptions and management of LDL-cholesterol in secondary prevention of patients with familial hypercholesterolemia in cardiology practice: Real-life evidence from the EPHESUS registry. J. Clin. Lipidol. 2023, 17, 732–742. [Google Scholar] [CrossRef]
- Yang, J.H.; Cho, K.H.; Hong, Y.J.; Kim, J.H.; Kim, H.Y.; Shin, M.H. Enhancing Familial Hypercholesterolemia Detection in South Korea: A Targeted Screening Approach Integrating National Program and Genetic Cascade Screening. Korean Circ. J. 2024, 54, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H. Advancing Familial Hypercholesterolemia Detection and Management in South Korea. Korean Circ. J. 2024, 54, 739–742. [Google Scholar] [CrossRef]
- Ruel, I.; Aljenedil, S.; Sadri, I.; de Varennes, E.; Hegele, R.A.; Couture, P.; Bergeron, J.; Wanneh, E.; Baass, A.; Dufour, R.; et al. Imputation of Baseline LDL Cholesterol Concentration in Patients with Familial Hypercholesterolemia on Statins or Ezetimibe. Clin. Chem. 2018, 64, 355–362. [Google Scholar] [CrossRef]
- Roberts, W.C. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am. J. Cardiol. 1997, 80, 106–107. [Google Scholar] [CrossRef]
- Ose, L.; Budinski, D.; Hounslow, N.; Arneson, V. Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Curr. Med. Res. Opin. 2009, 25, 2755–2764. [Google Scholar] [CrossRef]
- Morrone, D.; Weintraub, W.S.; Toth, P.P.; Hanson, M.E.; Lowe, R.S.; Lin, J.; Shah, A.K.; Tershakovec, A.M. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: A pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012, 223, 251–261. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Koenig, W. Familial Hypercholesterolemia: Pitfalls and Challenges in Diagnosis and Treatment. Rev. Cardiovasc. Med. 2023, 24, 236. [Google Scholar] [CrossRef]
- Groselj, U.; Wiegman, A.; Gidding, S.S. Screening in children for familial hypercholesterolaemia: Start now. Eur. Heart J. 2022, 43, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Won, H.H.; Peloso, G.M.; Lawson, K.S.; Bartz, T.M.; Deng, X.; van Leeuwen, E.M.; Natarajan, P.; Emdin, C.A.; Bick, A.G.; et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients with Severe Hypercholesterolemia. J. Am. Coll. Cardiol. 2016, 67, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, K.; Nishibukuro, T.; Ogiwara, Y.; Ikegawa, K.; Tada, H.; Yamagishi, M.; Kawashiri, M.A.; Ochi, A.; Toyoda, J.; Nakano, Y.; et al. Genetic Analysis of Japanese Children Clinically Diagnosed with Familial Hypercholesterolemia. J. Atheroscler. Thromb. 2022, 29, 667–677. [Google Scholar] [CrossRef]
- Taylor, A.; Wang, D.; Patel, K.; Whittall, R.; Wood, G.; Farrer, M.; Neely, R.D.; Fairgrieve, S.; Nair, D.; Barbir, M.; et al. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin. Genet. 2010, 77, 572–580. [Google Scholar] [CrossRef]
- Umans-Eckenhausen, M.A.; Defesche, J.C.; Sijbrands, E.J.; Scheerder, R.L.; Kastelein, J.J. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001, 357, 165–168. [Google Scholar] [CrossRef]
- Mahdieh, N.; Heshmatzad, K.; Rabbani, B. A systematic review of LDLR, PCSK9, and APOB variants in Asia. Atherosclerosis 2020, 305, 50–57. [Google Scholar] [CrossRef]
- Henderson, R.; O’Kane, M.; McGilligan, V.; Watterson, S. The genetics and screening of familial hypercholesterolaemia. J. Biomed. Sci. 2016, 23, 39. [Google Scholar] [CrossRef]
- Plon, S.; Jarvik, G. Ten Years of Incidental, Secondary, and Actionable Findings. N. Engl. J. Med. 2023, 389, 1813–1814. [Google Scholar] [CrossRef]
- Harada-Shiba, M.; Arai, H.; Ohmura, H.; Okazaki, H.; Sugiyama, D.; Tada, H.; Dobashi, K.; Matsuki, K.; Minamino, T.; Yamashita, S.; et al. Guidelines for the Diagnosis and Treatment of Adult Familial Hypercholesterolemia 2022. J. Atheroscler. Thromb. 2023, 30, 558–586. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, C.J.; Kim, D.I.; Rhee, M.Y.; Lee, B.K.; Ahn, Y.; Cho, B.R.; Woo, J.T.; Hur, S.H.; Jeong, J.O.; et al. Target achievement with maximal statin-based lipid-lowering therapy in Korean patients with familial hypercholesterolemia: A study supported by the Korean Society of Lipid and Atherosclerosis. Clin. Cardiol. 2017, 40, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Masuda, D.; Harada-Shiba, M.; Arai, H.; Bujo, H.; Ishibashi, S.; Daida, H.; Koga, N.; Oikawa, S. Effectiveness and Safety of Lipid-Lowering Drug Treatments in Japanese Patients with Familial Hypercholesterolemia: Familial Hypercholesterolemia Expert Forum (FAME) Study. J. Atheroscler. Thromb. 2022, 29, 608–638. [Google Scholar] [CrossRef]
- Perez de Isla, L.; Alonso, R.; Watts, G.F.; Mata, N.; Saltijeral Cerezo, A.; Muniz, O.; Fuentes, F.; Diaz-Diaz, J.L.; de Andres, R.; Zambon, D.; et al. Attainment of LDL-Cholesterol Treatment Goals in Patients With Familial Hypercholesterolemia: 5-Year SAFEHEART Registry Follow-Up. J. Am. Coll. Cardiol. 2016, 67, 1278–1285. [Google Scholar] [CrossRef]
- Kim, K.A.; Park, H.J. New Therapeutic Approaches to the Treatment of Dyslipidemia 2: LDL-C and Lp(a). J. Lipid Atheroscler. 2023, 12, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Duell, P.B.; Banach, M.; Catapano, A.L.; Laufs, U.; Mancini, G.B.J.; Ray, K.K.; Broestl, C.; Zhang, Y.; Lei, L.; Goldberg, A.C. Efficacy and safety of bempedoic acid in patients with heterozygous familial hypercholesterolemia: Analysis of pooled patient-level data from phase 3 clinical trials. J. Clin. Lipidol. 2024, 18, e153–e165. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Arroyo-Olivares, R.; Muniz-Grijalvo, O.; Diaz-Diaz, J.L.; Munoz-Torrero, J.S.; Romero, M.J.; de Andres, R.; Zambon, D.; Manas, M.D.; Fuentes-Jimenez, F.; et al. Persistence with long-term PCSK9 inhibitor treatment and its effectiveness in familial hypercholesterolaemia: Data from the SAFEHEART study. Eur. J. Prev. Cardiol. 2023, 30, 320–328. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Entire Population (n = 148) |
|---|---|
| Demographics and medical history | |
| Age (years) | 49.3 ± 17.2 |
| Sex | |
| Male (%) | 58 (39.2) |
| Female (%) | 90 (60.8) |
| BMI (kg/m2) | 23.7 ± 3.9 |
| History of smoking | |
| Non-smoker | 105 (70.9) |
| Ex-smoker | 31 (20.9) |
| Current smoker | 12 (8.1) |
| Diabetes (%) | 20 (13.5) |
| Hypertension (%) | 34 (23.0) |
| Previous MI (%) | 9 (6.1) |
| Previous PCI (%) | 16 (10.8) |
| Previous Stroke (%) | 5 (3.4) |
| Family History | |
| MI in 1st degree relative (%) | 16 (10.8) |
| MI in 2nd degree relative (%) | 11 (7.4) |
| Xanthoma in 1st degree relative (%) | 2 (1.4) |
| Xanthoma in 2nd degree relative (%) | 2 (1.4) |
| Dyslipidemia in 1st degree relative (%) | 37 (25.0) |
| Dyslipidemia in 2nd degree relative (%) | 13 (8.8) |
| Clinical and Laboratory Characteristics | |
| Systolic blood pressure (mmHg) | 126.0 ± 18.5 |
| Diastolic blood pressure (mmHg) | 78.2 ± 13.7 |
| Total cholesterol (mg/dL) | 337.6 ± 130.3 |
| HDL-cholesterol (mg/dL) | 54.6 ± 17.7 |
| LDL-cholesterol (mg/dL) | 242.0 ± 97.9 |
| Conversion to treatment-naïve (mg/dL) | 343.3 ± 140.9 |
| Triglycerides (mg/dL) | 192.3 ± 161.5 |
| Hemoglobin (mg/dL) | 13.9 ± 1.9 |
| WBC count (109/L) | 6.7 ± 2.3 |
| Platelet count (109/L) | 248.6 ± 80.1 |
| hs-CRP (mg/dL) | 0.4 ± 1.0 |
| Glucose (mg/dL) | 108.3 ± 42.8 |
| HbA1c (%) | 6.1 ± 1.4 |
| eGFR (mL/min/1.73m2) | 94.7 ± 24.9 |
| Uric acid (mg/dL) | 5.5 ± 4.8 |
| Medical treatment | |
| Glucose-lowering medication (%) | 16 (10.8) |
| Antihypertensive medication (%) | 39 (26.3) |
| Lipid-lowering medication (%) | 71 (48.0) |
| Antiplatelet (%) | 18 (12.2) |
| Diagnosis | |
| PV tested | 47 (31.8) |
| PV-positive | 24 (16.2) |
| DLCN criteria | |
| Definite (%) | 64 (43.2) |
| Probable (%) | 57 (38.5) |
| Possible (%) | 27 (18.2) |
| ICD diagnosis of FH (%) | 61 (41.2) |
| n (%) | |
|---|---|
| 1. CAD | 26 (17.6) |
| (1) Asymptomatic CAD | 5 (3.4) |
| (2) Angina or equivalent | 17 (11.5) |
| (3) Myocardial infarction | 4 (2.7) |
| 2. Embolic event | 7 (4.7) |
| (1) Stroke | 5 (3.4) |
| (2) Retinal artery occlusion | 2 (1.4) |
| 3. Dyslipidemia | 55 (37.2) |
| (1) Without previous lipid-lowering treatment | 30 (20.3) |
| (2) With previous lipid-lowering treatment | 25 (16.9) |
| 4. Xanthoma | 2 (1.1) |
| 5. Family History | 3 (2.0) |
| 6. During in-hospital evaluation for other medical conditions | 48 (32.4) |
| (1) Arrhythmia | 6 (4.1) |
| (2) Hypertension | 1 (0.7) |
| (3) Diabetes | 3 (2.0) |
| (4) Malignancy | 7 (4.7) |
| (5) Health examination | 6 (4.1) |
| (6) Genetic testing | 2 (1.4) |
| (7) Pre-operative evaluation | 4 (2.7) |
| (8) Other | 19 (12.8) |
| 7. Undiagnosed | 7 (4.7) |
| c.DNA Change | Protein Change | Type | |
|---|---|---|---|
| 1 | LDLR c.2054C>T | p.Pro685Leu | Pathogenic |
| 2 | LDLR c.361T>G | p.Cys121Gly | Pathogenic |
| 3 | LDLR c.361T>G | p.Cys121Gly | Likely pathogenic |
| 4 | LDLR c.1448G>A | p.Trp483* | Pathogenic |
| 5 | LDLR c.682G>T | p.Glu228* | Pathogenic |
| 6 | LDLR c.313 + G>A | splicing error | Pathogenic |
| 7 | LDLR c.361T>G | p.Cys121Gly | Likely pathogenic |
| 8 | LDLR c.361T>G | p.Cys121Gly | Likely pathogenic |
| 9 | LDLR c.301G>A | p.Glu101Lys | Pathogenic |
| 10 | LDLR c.395G>A | p.Arg132Gln | VUS |
| 11 | LDLR c.1195G>A | p.Ala339Thr | Likely pathogenic |
| 12 | LDLR c.661G>A APOB c.1599_1611del | p.Asp221Asn p.Met534ThrfsTer28 | Pathogenic Likely pathogenic |
| 13 | LDLR c.280G>A | p.Asp94Asn | Likely pathogenic |
| 14 | LDLR c.661G>A | p.Asp221Asn | Pathogenic |
| 15 | LDLR c.241C>A | p.Arg81Ser | Likely pathogenic |
| 16 | LDLR c.337G>T | p.Glu113Ter | Pathogenic |
| 17 | LDLR c.280G>A LDLR c.352G>A | p.Asp94Asn p.Asp118Asn | Likely pathogenic VUS |
| 18 | LDLR c.418G>A LDLR c.2333G>T | p.Glu140Lys p.Arg778Ile | Pathogenic VUS |
| 19 | LDLR c.2416dup | p.Val806Glyfs*11 | Pathogenic |
| 20 | LDLR c.621C>T | p.Gly207* | Likely pathogenic |
| 21 | LDLR c.361T>G | p.Cys121Gly | Pathogenic |
| 22 | LDLR c.2054C>T | p.Pro685Leu | Pathogenic |
| 23 | LDLR c.-84G>A | ? | VUS |
| 24 | LDLR c.682G>T | p.Glu228* | Pathogenic |
| Characteristics | Entire Population (n = 96) | According to Period of Diagnosis | ||
|---|---|---|---|---|
| Diagnosed 2010-2019 (n = 33) | Diagnosed 2020-2023 (n = 63) | p-Value | ||
| Initial visit | ||||
| Total cholesterol (mg/dL) | 349.1 ± 139.7 | 408.7 ± 190.7 | 318.8 ± 92.9 | 0.028 |
| HDL-cholesterol (mg/dL) | 53.9 ± 16.6 | 55.0 ± 17.7 | 53.4 ± 16.1 | 0.379 |
| LDL-cholesterol (mg/dL) | 246.6 ± 96.4 | 283.2 ± 102.4 | 228.0 ± 88.4 | 0.008 |
| Conversion to treatment-naïve (mg/dL) | 347.1 ± 133.9 | 328.9 ± 139.4 | 356.3 ± 131.2 | 0.348 |
| Triglycerides (mg/dL) | 210.9 ± 136.7 | 336.1 ± 224.5 | 147.3 ± 120.4 | 0.065 |
| Statin (%) | 43 (44.8) | 5 (18.2) | 38 (61.3) | <0.001 |
| Dose (mg) a | 20 [0, 40] | 10 [0, 20] | 20 [5, 40] | 0.002 |
| High-intensity (%) b | 35 (58.3) | 4 (36.4) | 31 (63.3) | 0.195 |
| Ezetimibe (%) | 29 (30.2) | 4 (12.1) | 25 (39.7) | 0.010 |
| Fibrate (%) | 3 (3.2) | 2 (6.1) | 1 (1.6) | 0.271 |
| PCSK9 inhibitor (%) | 3 (3.2) | 0 (0.0) | 3 (4.8) | 0.549 |
| Followed up at 1 year | ||||
| Total cholesterol (mg/dL) | 208.4 ± 65.4 | 246.8 ± 69.6 | 188.6 ± 53.8 | <0.001 |
| HDL-cholesterol (mg/dL) | 56.4 ± 12.9 | 57.4 ± 14.8 | 56.0 ± 12.1 | 0.662 |
| LDL-cholesterol (mg/dL) | 121.6 ± 60.0 | 152.1 ± 68.3 | 106.8 ± 49.8 | 0.003 |
| Change from initial visit (mg/dL) | −119.4 ± 101.1 | −118.4 ± 127.0 | −119.9 ± 87.7 | 0.955 |
| Change from treatment-naïve level (mg/dL) | −222.3 ± 153.7 | −161.4 ± 163.3 | −250.7 ± 141.7 | 0.010 |
| Target level achievement (%) c | 11 (11.5) | 2 (6.9) | 9 (15.0) | 0.321 |
| Triglycerides (mg/dL) | 129.6 ± 94.2 | 170.5 ± 132.8 | 109.2 ± 58.6 | 0.021 |
| Statin (%) | 77 (80.2) | 26 (77.4) | 51 (81.6) | 0.800 |
| Dose (mg) a | 40 [10, 80] | 20 [5, 40] | 40 [10, 80] | 0.143 |
| High-intensity (%) | 50 (54.3) | 14 (45.2) | 36 (59.0) | 0.298 |
| Ezetimibe (%) | 61 (64.9) | 14 (43.8) | 47 (75.8) | 0.004 |
| Fibrate (%) | 7 (7.4) | 6 (18.8) | 1 (1.6) | 0.010 |
| PCSK9 inhibitor (%) | 11 (11.7) | 0 (0.0) | 11 (17.7) | 0.028 |
| Covariates | Uncorrected Baseline LDL-C | Treatment-Naïve Baseline LDL-C | ||
|---|---|---|---|---|
| β Coefficient (95% CI) | p-Value | β Coefficient (95% CI) | p-Value | |
| Overall LDL-C reduction after 1 year | −130.6 (−165.5, −94.6) | <0.001 | −339.7 (−408.3, −279.2) | <0.001 |
| Overall LDL-C levels according to periods | 0.002 | 0.401 | ||
| 2010–2019 | reference | reference | ||
| 2020–2023 | −53.7 (−90.6, −23.4) | 19.8 (−29.9, 65.1) | ||
| Difference in 1-year LDL-C reduction between periods | 0.688 | 0.026 | ||
| 2010–2019 | reference | reference | ||
| 2020–2023 | 9.5 (−35.0, 55.9) | −73.2 (−134.8, −12.2) | ||
| Age (per 1 year) | −0.73 (−1.60, 0.03) | 0.075 | −0.33 (−1.50, −0.62) | 0.532 |
| Male sex | 7.0 (−16.3, 28.1) | 0.562 | 21.6 (−11.4, 58.3) | 0.173 |
| Prior ASCVD | −35.1 (−73.6, 5.5) | 0.103 | 20.6 (−32.1, 78.0) | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.A.; Jung, M.-k.; Kim, E.-S.; Kim, D.; Kim, J.; Kim, H.S.; Youn, J.-C. Current Status and Clinical Characteristics of Familial Hypercholesterolemia Patients in Korea: A Multicenter, Real-World Experience. Diagnostics 2025, 15, 3062. https://doi.org/10.3390/diagnostics15233062
Kim KA, Jung M-k, Kim E-S, Kim D, Kim J, Kim HS, Youn J-C. Current Status and Clinical Characteristics of Familial Hypercholesterolemia Patients in Korea: A Multicenter, Real-World Experience. Diagnostics. 2025; 15(23):3062. https://doi.org/10.3390/diagnostics15233062
Chicago/Turabian StyleKim, Kyung An, Moon-kyung Jung, Eui-Soon Kim, Dongwoo Kim, Joonseok Kim, Hoon Seok Kim, and Jong-Chan Youn. 2025. "Current Status and Clinical Characteristics of Familial Hypercholesterolemia Patients in Korea: A Multicenter, Real-World Experience" Diagnostics 15, no. 23: 3062. https://doi.org/10.3390/diagnostics15233062
APA StyleKim, K. A., Jung, M.-k., Kim, E.-S., Kim, D., Kim, J., Kim, H. S., & Youn, J.-C. (2025). Current Status and Clinical Characteristics of Familial Hypercholesterolemia Patients in Korea: A Multicenter, Real-World Experience. Diagnostics, 15(23), 3062. https://doi.org/10.3390/diagnostics15233062






