Obstructive Sleep Apnea and Recovery in Athletes: BMI and Neck Circumference and Their Impact on Recovery Capacity and Injury Risk
Abstract
1. Introduction
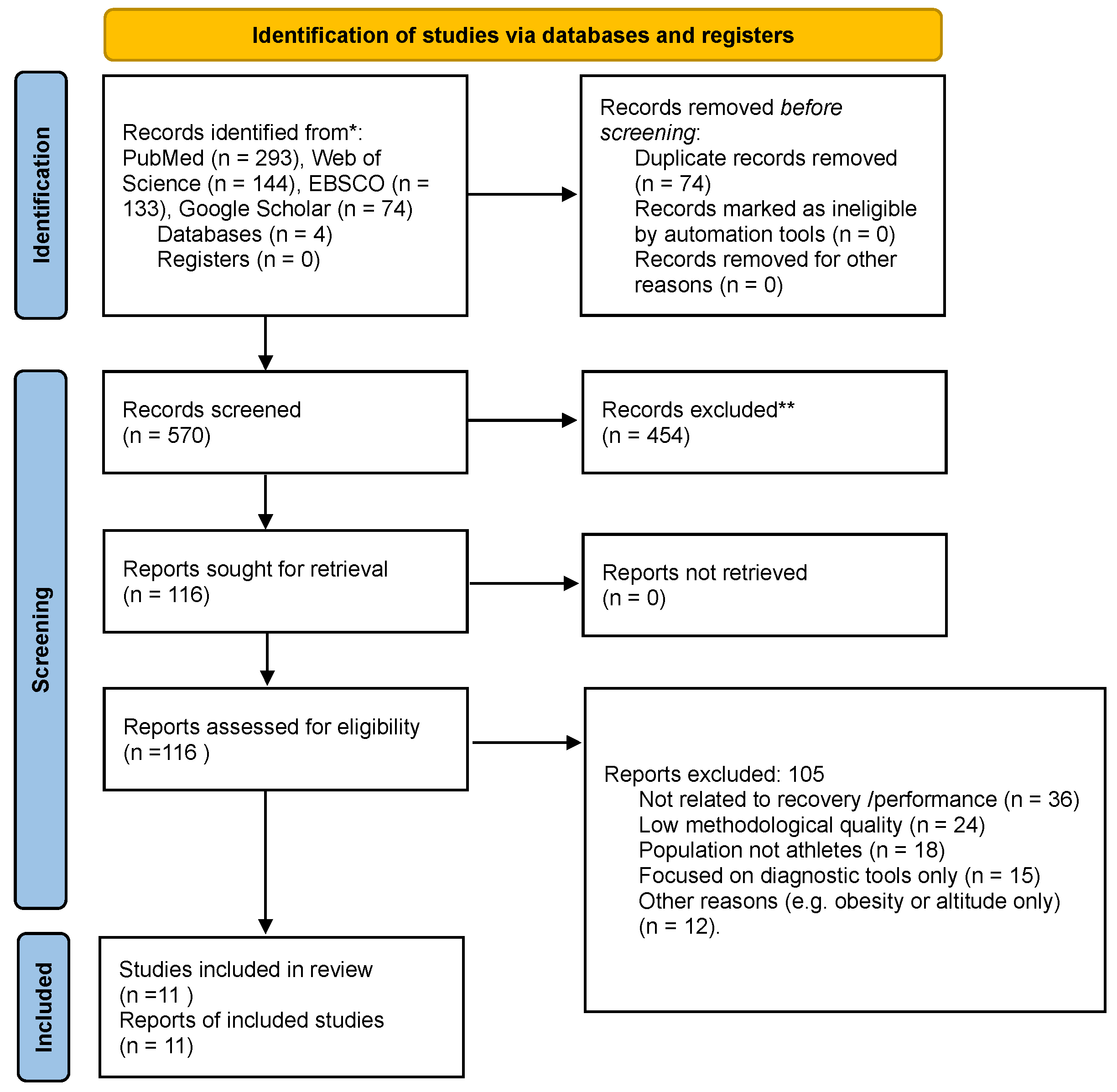
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Time range: Articles published between 2015 and 2025.
- Language of publication: English language publications only, to avoid errors in interpretation.
- Study population: Studies on amateur and professional athletes.
- Type of research: Systematic reviews, meta-analyses, clinical and observational studies included.
- OSA diagnostic methods: Polysomnography, polygraphy, STOP-BANG questionnaire (Charité–Universitätsmedizin Berlin, Berlin, Germany), Berlin Questionnaire (Charité–Universitätsmedizin Berlin, Berlin, Germany). The Apnea–Hypopnea Index (AHI), which quantifies the number of apneas and hypopneas per hour of sleep, is commonly used to assess the severity of OSA. An AHI of ≥5 is generally considered diagnostic for mild OSA.
- Accessibility: Studies available in full text or obtainable through institutional databases were preferred.
- Anthropometric data: Studies reporting anthropometric parameters such as body mass index (BMI), neck circumference or body composition indicators were prioritized where available.
- Population: Studies involving the general population and non-sports-related OSA patients were excluded.
- Scope: Papers focusing exclusively on psychological aspects of OSA, non-recovery-related pharmacological interventions, or validation of diagnostic tools were not included.
- Case studies: Case studies of individual athletes with limited statistical value were excluded.
- Methodological evaluation: Articles rated negatively in CASP in terms of methodological soundness were excluded.
2.3. Evaluation of the Quality of the Reviewed Articles
2.4. Data Extraction
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charest, J.; Grandner, M.A. Sleep and Athletic Performance: Impacts on Physical Performance, Mental Performance, Injury Risk and Recovery, and Mental Health. Sleep Med. Clin. 2020, 15, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Morgan, K.; Gilchrist, S. Does Elite Sport Degrade Sleep Quality? A Systematic Review. Sports Med. 2017, 47, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, D.; Ehrlenspiel, F.; Oa, A.; El-Din, H.G. Sleep Habits in German Athletes before Important Competitions or Games. J. Sports Sci. 2011, 29, 859–866. [Google Scholar] [CrossRef]
- Fullagar, H.H.K.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and Athletic Performance: The Effects of Sleep Loss on Exercise Performance, and Physiological and Cognitive Responses to Exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between Food Intake and Sleep Pattern in Healthy Individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Nutrients 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef]
- Chang, A.-M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening Use of Light-Emitting eReaders Negatively Affects Sleep, Circadian Timing, and Next-Morning Alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- George, C. Sleep and Breathing in Professional Football Players. Sleep Med. 2003, 4, 317–325. [Google Scholar] [CrossRef]
- Albuquerque, F.N.; Sert, K.F.H.; Calvin, A.D.; Sierra-Justo, R.; Romero-Corral, A.; Lopez-Jimenez, F.; George, C.F.; Rapoport, D.M.; Vogel, R.A.; Khandheria, B.; et al. Sleep-Disordered Breathing, Hypertension, and Obesity in Retired National Football League Players. J. Am. Coll. Cardiol. 2010, 56, 1432–1433. [Google Scholar] [CrossRef]
- Ryan, S. Mechanisms of Cardiovascular Disease in Obstructive Sleep Apnoea. J. Thorac. Dis. 2018, 10, S4201–S4211. [Google Scholar] [CrossRef]
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J.; et al. Recovery and Performance in Sport: Consensus Statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakayama, T.; Sawa, A.; Yagi, T.; Iwata, Y.; Takeuchi, H.; Motoyoshi, M.; Chow, C.-M.; Komiyama, O. Mandibular Advancement Device Therapy in Japanese Rugby Athletes with Poor Sleep Quality and Obstructive Sleep Apnea. Life 2022, 12, 1299. [Google Scholar] [CrossRef]
- Mendelson, M.; Bailly, S.; Marillier, M.; Flore, P.; Borel, J.; Vivodtzev, I.; Doutreleau, S.; Verges, S.; Tamisier, R.; Pépin, J. Obstructive Sleep Apnea Syndrome, Objectively Measured Physical Activity and Exercise Training Interventions: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2018, 42, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.; Renzi, T.; Peach, H.; Gaultney, J.; Marino, J. Examination of Risk for Sleep-Disordered Breathing Among College Football Players. J. Clin. Sleep Med. 2019, 15, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hollowed, C.; Irwin-Weyant, M.; Patel, K.; Hosny, K.; Aida, H.; Gowani, Z.; Sher, S.; Gleason, P.; Shoop, J.L.; et al. Sleep-Disordered Breathing and Cardiovascular Correlates in College Football Players. Am. J. Respir. Crit. Care Med. 2019, 199, A2963. [Google Scholar] [CrossRef]
- Morssinkhof, M.W.L.; van Wylick, D.W.; Priester-Vink, S.; van der Werf, Y.D.; den Heijer, M.; van den Heuvel, O.A.; Broekman, B.F.P. Associations between Sex Hormones, Sleep Problems and Depression: A Systematic Review. Neurosci. Biobehav. Rev. 2020, 118, 669–680. [Google Scholar] [CrossRef]
- Caia, J.; Halson, S.L.; Scott, A.; Kelly, V.G. Obstructive Sleep Apnea in Professional Rugby League Athletes: An Exploratory Study. J. Sci. Med. Sport 2020, 23, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Dobrosielski, D.A.; Nichols, D.; Ford, J.; Watts, A.; Wilder, J.N.; Douglass-Burton, T. Estimating the Prevalence of Sleep-Disordered Breathing Among Collegiate Football Players. Respir. Care 2016, 61, 1144–1150. [Google Scholar] [CrossRef]
- Morris, T.P.; McCracken, C.; Baggish, A.; Weisskopf, M.; Zafonte, R.; Taylor, H.A.; Nadler, L.M.; Speizer, F.E.; Pascual-Leone, A. Multisystem Afflictions in Former National Football League Players. Am. J. Ind. Med. 2019, 62, 655–662. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme (CASP) Checklists. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 6 March 2025).
- Kölling, S.; Duffield, R.; Erlacher, D.; Venter, R.; Halson, S.L. Sleep-Related Issues for Recovery and Performance in Athletes. Int. J. Sports Physiol. Perform. 2019, 14, 144–148. [Google Scholar] [CrossRef]
- Kawada, T. Validation Study of STOP-Bang Score for Screening Sleep-Disordered Breathing. Sleep Breath. 2016, 20, 1093. [Google Scholar] [CrossRef]
- Framnes, S.N.; Arble, D.M. The Bidirectional Relationship Between Obstructive Sleep Apnea and Metabolic Disease. Front. Endocrinol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Kinsman, T.; Gore, C.; Hahn, A.; Hopkins, W.; Hawley, J.; McKenna, M.; Clark, S.; Aughey, R.; Townsend, N.; Chow, C.-M. Sleep in Athletes Undertaking Protocols of Exposure to Nocturnal Simulated Altitude at 2650 m. J. Sci. Med. Sport 2005, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Dunican, I.C.; Walsh, J.; Higgins, C.C.; Jones, M.J.; Maddison, K.; Caldwell, J.A.; David, H.; Eastwood, P.R. Prevalence of Sleep Disorders and Sleep Problems in an Elite Super Rugby Union Team. J. Sports Sci. 2019, 37, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Surda, P.; Putala, M.; Siarnik, P.; Walker, A.; De Rome, K.; Amin, N.; Sangha, M.S.; Fokkens, W. Sleep in Elite Swimmers: Prevalence of Sleepiness, Obstructive Sleep Apnoea and Poor Sleep Quality. BMJ Open Sport Exerc. Med. 2019, 5, e000673. [Google Scholar] [CrossRef]
- Ortiz-Naretto, A.E.; Pereiro, M.P.; Ernst, G.; Aramburo, J.M.; Tovo, A.M.; Vázquez-Fernández, A.; Borsini, E. Effect of Mild Obstructive Sleep Apnea in Mountaineers during the Climb to Mount Aconcagua. Sleep Sci. 2020, 13, 138–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suppiah, H.T.; Swinbourne, R.; Wee, J.; Tay, V.; Gastin, P. Sleep Characteristics of Elite Youth Athletes: A Clustering Approach to Optimize Sleep Support Strategies. Int. J. Sports Physiol. Perform. 2021, 16, 1225–1233. [Google Scholar] [CrossRef]
- Swinbourne, R.; Gill, N.; Vaile, J.; Smart, D. Prevalence of Poor Sleep Quality, Sleepiness and Obstructive Sleep Apnoea Risk Factors in Athletes. Eur. J. Sport Sci. 2016, 16, 850–858. [Google Scholar] [CrossRef]
- Nabhan, D.; Lewis, M.; Taylor, D.; Bahr, R. Expanding the Screening Toolbox to Promote Athlete Health: How the US Olympic & Paralympic Committee Screened for Health Problems in 940 Elite Athletes. Br. J. Sports Med. 2021, 55, 226–230. [Google Scholar] [CrossRef]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the Athlete: Narrative Review and 2021 Expert Consensus Recommendations. Br. J. Sports Med. 2021, 55, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.J.; Xia, K.; Soe, K.; Sexias, A.; Sogade, F.; Hutchinson, B.; Vieira, D.; McFarlane, S.I.; Jean-Louis, G. Obstructive Sleep Apnea among Players in the National Football League: A Scoping Review. J. Sleep Disord. Ther. 2017, 6, 278. [Google Scholar] [CrossRef]
- Sforza, E.; Roche, F. Sleep Apnea Syndrome and Cognition. Front. Neurol. 2012, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; McSharry, D.; Malhotra, A. Adult Obstructive Sleep Apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Venkataraman, S.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep Apnea, Hypertension and the Sympathetic Nervous System in the Adult Population. J. Clin. Med. 2020, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Pack, A.I. Obstructive Sleep Apnea and Cognitive Impairment: Addressing the Blood–Brain Barrier. Sleep Med. Rev. 2014, 18, 35–48. [Google Scholar] [CrossRef]
- Beebe, D.W.; Gozal, D. Obstructive Sleep Apnea and the Prefrontal Cortex: Towards a Comprehensive Model Linking Nocturnal Upper Airway Obstruction to Daytime Cognitive and Behavioral Deficits. J. Sleep Res. 2002, 11, 1–16. [Google Scholar] [CrossRef]
- Huang, K.; Ihm, J. Sleep and Injury Risk. Sports Med. 2021, 51, 1581–1593. [Google Scholar] [CrossRef]
- Milewski, M.D.; Skaggs, D.L.; Bishop, G.A.; Pace, J.L.; Ibrahim, D.A.; Wren, T.A.L.; Barzdukas, A. Chronic Lack of Sleep Is Associated with Increased Sports Injuries in Adolescent Athletes. J. Pediatr. Orthop. 2014, 34, 129–133. [Google Scholar] [CrossRef]
- Epstein, L.; Kristo, D.; Strollo, P.; Friedman, N.; Malhotra, A.; Patil, S.; Ramar, K.; Rogers, R.; Schwab, R.; Weaver, E.; et al. Clinical Guideline for the Evaluation, Management and Long-Term Care of Obstructive Sleep Apnea in Adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [CrossRef] [PubMed]
- McDaid, C.; Griffin, S.; Weatherly, H.; Durée, K.; van der Burgt, M.; van Hout, S.; Akers, J.; Davies, R.J.O.; Sculpher, M.; Westwood, M. Continuous Positive Airway Pressure Devices for the Treatment of Obstructive Sleep Apnoea-Hypopnoea Syndrome: A Systematic Review and Economic Analysis. Health Technol. Assess. 2009, 13, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Samuels, C. Sleep, Recovery, and Performance: The New Frontier in High-Performance Athletics. Sports Med. Open 2012, 48, 41–53. [Google Scholar]
| Study (First Author, Year) | Tool Used | Study Design | Risk of Bias (Overall) | Key Comments |
|---|---|---|---|---|
| Suzuki et al., 2022 | CASP | Intervention (MAD) | Low | Well-designed intervention, small sample |
| Peck et al., 2019 | RoB 2.0 | Observational (comparative) | Moderate | No randomization, different comparison groups |
| Dunican et al., 2019 | CASP | Observational (PSG) | Low | Lab PSG, solid control and diagnostics |
| Surda et al., 2019 | CASP | Observational | Low | Objective measures, good design |
| Ortiz-Naretto et al., 2020 | Newcastle–Ottawa | Observational (altitude) | Moderate | Extreme setting, low sample size |
| Caia et al., 2020 | Newcastle–Ottawa | Observational | Moderate | Home PSG, positional variability |
| Swinbourne et al., 2016 | CASP | Cross-sectional | Moderate | Subjective data, adequate sample |
| Nabhan et al., 2020 | CASP | Retrospective (survey) | Low | Large sample, standardized tool |
| Kölling et al., 2019 | N/A | Narrative review | N/A | Narrative review—no bias rating |
| Dobrosielski et al., 2016 | RoB 2.0 | Observational | Moderate | Subjective risk stratification, limited method |
| Suppiah et al., 2021 | CASP | Observational (survey) | Moderate | Self-report PSQI, no objective data |
| L.p. | Authors, (Year) | Tittle | DOI | Link to Article |
|---|---|---|---|---|
| 1 | Dunican et al. (2019) | Prevalence of sleep disorders and sleep problems in an elite super rugby union team. | 10.1080/02640414.2018.1537092 | https://doi.org/10.1080/02640414.2018.1537092 |
| 2 | Swinbourne et al. (2016) | Prevalence of poor sleep quality, sleepiness and obstructive sleep apnoea risk factors in athletes | 10.1080/17461391.2015.1120781 | https://doi.org/10.1080/17461391.2015.1120781 |
| 3 | Peck et al. (2019) | Examination of Risk for Sleep-Disordered Breathing Among College Football Players | 10.1123/jsr.2017-0127 | https://doi.org/10.1123/jsr.2017-0127 |
| 4 | Dobrosielski et al. (2016) | Estimating the Prevalence of Sleep-Disordered Breathing Among Collegiate Football Players | 10.4187/respcare.04520 | https://doi.org/10.4187/respcare.04520 |
| 5 | Caia et al. (2020) | Obstructive sleep apnea in professional rugby league athletes: An exploratory study | 10.1016/j.jsams.2020.04.014 | https://doi.org/10.1016/j.jsams.2020.04.014 |
| 6 | Suzuki et al. (2022) | Mandibular Advancement Device Therapy in Japanese Rugby Athletes with Poor Sleep Quality and Obstructive Sleep Apnea | 10.3390/life12010032 | https://doi.org/10.3390/life12010032 |
| 7 | Suppiah et al. (2021) | Sleep Characteristics of Elite Youth Athletes: A Clustering Approach to Optimize Sleep Support Strategies | 10.1123/ijspp.2020-0675 | https://doi.org/10.1123/ijspp.2020-0675 |
| 8 | Surda et al. (2019) | Sleep in elite swimmers: prevalence of sleepiness, obstructive sleep apnoea and poor sleep quality | 10.1136/bmjsem-2019-000566 | https://doi.org/10.1136/bmjsem-2019-000566 |
| 9 | Ortiz-Naretto et al. (2020) | Effect of mild obstructive sleep apnea in mountaineers during the climb to Mount Aconcagua | 10.5935/1984-0063.20200019 | https://doi.org/10.5935/1984-0063.20200019 |
| 10 | Kölling et al. (2019) | Sleep-Related Issues for Recovery and Performance in Athletes | 10.1123/ijspp.2017-0746 | https://doi.org/10.1123/ijspp.2017-0746 |
| 11 | Nabhan et al. (2020) | Expanding the screening toolbox to promote athlete health: how the US Olympic & Paralympic Committee screened for health problems in 940 elite athletes | 10.1136/bjsports-2020-102756 |
| Author (Year) | Study Characteristics | Key Findings | Key Observations |
|---|---|---|---|
| Caia et al. (2020) | 22 male professional rugby league athletes; age 23.8 ± 3.6 years; BMI: 30.0 ± 2.2 kg/m2; neck circumference: 41.1 ± 4.0 cm; sum of 8 skinfolds: 74.3 ± 17.0 mm; positional groups: forwards (n = 13) vs. backs (n = 9); home-based polysomnography used to diagnose OSA. | OSA was diagnosed in 45% of subjects. Rugby players with OSA were more likely to report poorer recovery-related outcomes between matches. | OSA is common in professional rugby players, with a prevalence comparable to other contact sports. Ethnic differences in OSA prevalence were observed, with players of Polynesian descent more likely to have OSA. Higher BMI and neck circumference were associated with increased AHI. No significant correlation was found between neck circumference and AHI. |
| Dobrosielski et al. (2016) | 56 male Division I collegiate American football players; age 19–23 years; BMI: 33.0 ± 5.4 vs. 27.6 ± 3.6 kg/m2 (high- vs. low-risk); neck circumference: 44.6 ± 2.2 vs. 41.4 ± 2.8 cm; body composition assessed using DXA (total fat mass, trunk fat mass, abdominal visceral fat). | Sleep-disordered breathing (SDB) was identified in approximately 8% of collegiate American football players. Athletes classified as high-risk for SDB reported poorer sleep quality and higher levels of daytime sleepiness. Reduced aerobic capacity was reported in football players classified as high-risk for SDB. | Higher BMI and greater neck circumference were more frequently observed among athletes classified as high-risk for SDB. Athletes with SDB exhibited higher total and central fat mass, although differences were not statistically significant. Mean total sleep duration was 4.2 ± 1.1 h, and 35% of athletes reported clinically significant daytime sleepiness (ESS ≥ 10). |
| Dunican et al. (2019) | 25 male elite rugby union players; age 25 ± 4 years; BMI: 30 ± 3 kg/m2 (forwards: 31 ± 3; backs: 29 ± 2); neck circumference: 43 ± 4 cm (above OSA risk threshold); full-night in-laboratory polysomnography; OSA prevalence: 24%. | OSA was identified in 24% of elite rugby players. Players with OSA reported higher levels of daytime sleepiness and fatigue, as well as poorer sleep-related recovery outcomes. Short sleep duration was observed across the cohort, with all players reporting excessive daytime sleepiness (ESS ≥ 10). | No significant associations were observed between BMI, neck circumference, or playing position and apnea–hypopnea index (AHI), despite higher BMI values among forwards. Questionnaire-based screening tools were reported to be ineffective in identifying OSA, whereas polysomnography remained the reference diagnostic method. Reduced oxygen-related exercise tolerance was reported in players with OSA. |
| Kölling et al. (2019) | Narrative review of sleep in elite athletes synthesizing evidence from team and individual sports; focus on sleep quantity, sleep quality, recovery, and performance; discusses prevalence of poor sleep and self-reported symptoms suggestive of sleep-disordered breathing; no original anthropometric or body composition data reported. | The reviewed literature indicated that insufficient sleep was associated with impaired recovery-related processes, altered immune function, and changes in cognitive performance. Sleep deprivation was reported to affect coordination, reaction time, and decision-making, although strength and endurance were not consistently reduced. | Poor sleep quality was reported in approximately 50% of elite athletes, characterized by low sleep efficiency and frequent awakenings. Daytime sleepiness was frequently reported and associated with impaired performance-related outcomes. Self-reported snoring (38%) and apnea episodes (8%) were noted as potential indicators of undiagnosed OSA. External factors such as training schedules, competition timing, travel across time zones, pre-competition stress, and evening exposure to electronic devices were identified as common contributors to sleep disruption. |
| Nabhan et al. (2020) | 940 elite athletes (683 Olympians, 257 Paralympians); 462 males and 478 females; representing 36 Olympic and Paralympic sports; OSA risk and sleep quality assessed using the Berlin Questionnaire and the Pittsburgh Sleep Quality Index (PSQI); no objective anthropometric measures (BMI, neck circumference, or body composition) reported. | Poor sleep quality was identified in more than 20% of the athletes. A higher prevalence of OSA risk was reported among Paralympic athletes compared with Olympians (8.6% vs. 3.5%). Paralympic athletes classified as high-risk for OSA reported higher levels of fatigue and poorer recovery-related outcomes. | Females more frequently reported poor sleep quality than males (28.3% vs. 22.2%), while no significant sex differences were observed for OSA risk, anxiety, or depression. Daytime sleepiness and poor sleep quality were associated with higher prevalence of psychological symptoms, including anxiety and depression. Screening questionnaires identified OSA risk in a limited proportion of athletes, highlighting potential underdetection in elite sport populations. |
| Ortiz-Naretto et al. (2020) | 8 amateur mountaineers (4 females; mean age 36 years) assessed during a high-altitude expedition; respiratory polygraphy used to identify mild asymptomatic OSA; baseline BMI was higher in the OSA group (27.5 vs. 22.3 kg/m2); no data on neck circumference or body composition reported. | Participants with OSA exhibited lower nocturnal oxygen saturation and greater difficulty adapting to hypoxic conditions during ascent. A greater decline in oxygen saturation was observed with increasing altitude in the OSA group compared with controls (SpO2: 80% at 746 m vs. 52.5% at 4900 m). | Altitude exposure was associated with an increased number of central apneas and hypopneas in all participants, while obstructive events did not increase. All participants developed high-altitude periodic breathing, with a higher frequency of central apneas observed in individuals with OSA. Participants with OSA more frequently exhibited elevated systolic blood pressure at higher altitudes and showed less favorable physiological adaptation, including higher post-expedition BMI and metabolic markers. None of the participants with OSA reached the summit, and cases of severe altitude sickness required medical treatment. |
| Peck et al. (2019) | 21 male Division I American football linemen and 19 track athletes (age 18–22 years); sleep-disordered breathing (SDB) risk assessed using the MAP Index and Epworth Sleepiness Scale (ESS); anthropometry and body composition assessed using DXA; football linemen exhibited higher BMI (35.87 ± 4.77 kg/m2), neck circumference (44.36 ± 2.89 cm), and body fat percentage (29.2%) compared with track athletes. | American football players demonstrated a higher SDB risk index (MAP) and a higher prevalence of OSA risk compared with non-football athletes. Football players classified as high-risk for SDB reported greater post-training fatigue and earlier onset of fatigue during match play. | Anthropometric and adiposity-related variables differed substantially between football players and controls (BMI: 35.87 vs. 23.07 kg/m2; neck circumference: 44.36 vs. 36.92 cm; body fat: 29.2% vs. 13.1%). Neck circumference, BMI, and body fat percentage were correlated with SDB risk indices. Football players exhibited higher Mallampati scores, longer sleep latency, and greater visceral fat accumulation. Despite elevated SDB risk, ESS scores did not differ between groups, suggesting potential under-recognition of sleep-related symptoms in this population. |
| Suppiah et al. (2021) | 135 elite national youth athletes (male and female); mean age 15.5 ± 2.0 years; participants from individual and team sports; mean BMI: 20.9 ± 2.7 kg/m2 (range approximately 19.7–25.1 depending on sport); OSA-related symptoms and sleep quality assessed using questionnaires; neck circumference and body composition not reported. | Poor sleep quality was reported in 45.2% of participants (PSQI > 5). Athletes reporting sleep deficiency or OSA-related symptoms more frequently reported fatigue and reduced recovery-related outcomes. | Team-sport athletes exhibited poorer sleep quality than individual-sport athletes, characterized by later bedtimes, lower sleep efficiency, and a higher prevalence of snoring (38.7% vs. 28.8%). No significant associations were observed between BMI and snoring or between snoring and overall sleep quality. Daytime naps were commonly used to compensate for sleep deficits. Caffeine use was identified as a potential contributor to impaired sleep quality, particularly in youth athletes. |
| Surda et al. (2019) | 101 elite swimmers (with additional comparison groups including non-elite swimmers, non-swimming athletes, and controls); mean age approximately 18–21 years; mean BMI in elite swimmers: 22.8 ± 3.1 kg/m2; OSA assessed using overnight pulse oximetry (oxygen desaturation index, ODI ≥ 5 events/h); actigraphy and pulse oximetry conducted in a subgroup (n = 20); neck circumference and body composition not reported. | OSA was identified in approximately 30% of elite swimmers (ODI ≥ 5), representing a higher prevalence compared with young adult control populations. Elite swimmers reported shorter sleep duration on training days, and shorter sleep duration was associated with reduced aerobic exercise tolerance. | OSA prevalence in swimmers was independent of BMI, with no significant BMI differences between swimmers with and without OSA. Daytime sleepiness was more frequently reported among swimmers and other athletes than in controls. Screening with the Epworth Sleepiness Scale demonstrated limited sensitivity, whereas objective assessment using overnight pulse oximetry identified a greater number of OSA cases. Sleep duration was consistently shorter on training days compared with rest days, particularly before early-morning training sessions. Non-allergic rhinitis was identified as a potential contributor to sleep-related disturbances. |
| Suzuki et al. (2022) | 42 male professional Japanese rugby union athletes; mean age 26.3 ± 3.7 years; BMI: 26.7 ± 3.2 kg/m2; neck circumference: 41.5 ± 2.1 cm; OSA assessed using a level III portable sleep test (respiratory event index, REI); subgroup of six athletes underwent mandibular advancement device (MAD) therapy. | OSA was identified in 64.3% of professional rugby players. Poor sleep quality (PSQI > 5.5) was reported in 69% of athletes, excessive daytime sleepiness (ESS > 10) in 35.7%, and mean sleep duration was 6.5 h. Following MAD therapy, improvements in REI, ESS scores, sleep quality, and selected cognitive performance measures were reported in the treated subgroup. | Athletes with moderate to severe OSA exhibited larger neck circumference and higher BMI compared with those without OSA. Neck circumference ≥ 42 cm, higher BMI, and increasing age were more frequently observed among athletes classified as high-risk for OSA. Improvements observed following MAD therapy were based on a small intervention subgroup and short-term follow-up. |
| Swinbourne et al. (2016) | 175 highly trained male and female team-sport athletes (rugby union, rugby sevens, cricket); age ≥ 18 years; sleep quality, daytime sleepiness, and OSA risk factors assessed using the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and OSA risk questionnaires; no objective anthropometric measures (BMI, neck circumference, or body composition) reported. | Poor sleep quality (PSQI > 5) was reported in approximately 50% of athletes, with mean sleep duration of 7.9 ± 1.3 h. Daytime sleepiness was common (mean ESS 8.5 ± 4.4), and 28% of athletes reported clinically significant sleepiness (ESS ≥ 10). Self-reported snoring (38%) and witnessed apnea episodes (8%) indicated the presence of OSA-related symptoms in this population. | Sleep quality varied across the competitive season, with poorer sleep reported during the pre-season compared with the post-season. Athletes training before 8:00 a.m. exhibited shorter sleep duration and higher levels of daytime sleepiness than those training later in the day. Younger athletes (<20 years) reported higher ESS scores. Rugby union players more frequently reported OSA-related symptoms compared with athletes from other team sports. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sikora, M.; Panek, M.; Łakomy, O.; Siatkowski, S.; Głowacka, E.; Żebrowska, A. Obstructive Sleep Apnea and Recovery in Athletes: BMI and Neck Circumference and Their Impact on Recovery Capacity and Injury Risk. Life 2026, 16, 76. https://doi.org/10.3390/life16010076
Sikora M, Panek M, Łakomy O, Siatkowski S, Głowacka E, Żebrowska A. Obstructive Sleep Apnea and Recovery in Athletes: BMI and Neck Circumference and Their Impact on Recovery Capacity and Injury Risk. Life. 2026; 16(1):76. https://doi.org/10.3390/life16010076
Chicago/Turabian StyleSikora, Marcin, Mariusz Panek, Olga Łakomy, Szymon Siatkowski, Emilia Głowacka, and Aleksandra Żebrowska. 2026. "Obstructive Sleep Apnea and Recovery in Athletes: BMI and Neck Circumference and Their Impact on Recovery Capacity and Injury Risk" Life 16, no. 1: 76. https://doi.org/10.3390/life16010076
APA StyleSikora, M., Panek, M., Łakomy, O., Siatkowski, S., Głowacka, E., & Żebrowska, A. (2026). Obstructive Sleep Apnea and Recovery in Athletes: BMI and Neck Circumference and Their Impact on Recovery Capacity and Injury Risk. Life, 16(1), 76. https://doi.org/10.3390/life16010076








