Abstract
Mood disorders, including major depressive disorder (MDD) and bipolar disorder (BD), are among the leading causes of disability worldwide and are frequently associated with treatment resistance, functional impairment, and high comorbidity with metabolic dysfunction. Increasing evidence implicates insulin resistance (IR) as a key pathophysiological factor linking metabolic and psychiatric illness. IR is associated with chronic low-grade inflammation, hypothalamic–pituitary–adrenal (HPA) axis dysregulation, impaired neuroplasticity, mitochondrial dysfunction, and altered reward processing mechanisms that may contribute to core depressive features such as anhedonia, cognitive slowing, and emotional dysregulation. These processes are further exacerbated by the metabolic side effects of many psychotropic medications, creating a self-perpetuating cycle that worsens both psychiatric and physical health outcomes. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), initially developed for type 2 diabetes and obesity, have emerged as promising candidates to address this metabolic–psychiatric interface. Beyond improving glycemic control and promoting weight loss, GLP-1 RAs exert central actions relevant to mood disorders, including modulation of dopaminergic reward pathways, enhancement of hippocampal neurogenesis, attenuation of neuroinflammation, and regulation of appetite and energy balance. Preclinical studies demonstrate that GLP-1 RAs reduce microglial activation, promote hippocampal neurogenesis, and normalize stress-induced behavioral changes. Early clinical trials in patients with metabolic disorders suggest improvements in depressive symptoms, quality of life, and cognitive function, with some effects independent of weight loss or glycemic outcomes. Observational evidence also indicates reduced antidepressant use and psychological distress in diabetic and obese populations receiving GLP-1 RAs. While these findings are promising, large randomized controlled trials in primary psychiatric populations are lacking. Key challenges include clarifying dose–response relationships, disentangling central from peripheral effects, and addressing safety and adherence concerns in individuals with comorbid psychiatric conditions. Future research should focus on biomarker-informed stratification, comparative trials with standard treatments, and integration of GLP-1 RAs into multimodal care frameworks. Overall, GLP-1 RAs represent a biologically plausible and clinically relevant approach to bridging metabolic and psychiatric care, with the potential to improve outcomes in patients with mood disorders who carry a high metabolic burden.
1. Introduction
Mood disorders, including major depressive disorder (MDD) and bipolar disorder (BD), remain a major cause of disability worldwide, with lifetime prevalence exceeding 15% for depression and up to 3% for bipolar disorder, with more than 400 million people affected by depression [1]. These conditions are often persistent, associated with reduced functional capacity, and respond only partially to available treatments. Even with recent advances in pharmacotherapy, many individuals continue to experience symptoms, particularly anhedonia, cognitive slowing or fatigue, and emotional instability [2], and up to one-third of patients develop treatment resistance [3]. These limitations highlight the urgent need for innovative approaches that integrate metabolic and psychiatric dimensions to improve outcomes and quality of life.
Increasing evidence implicates insulin resistance (IR) as an important factor in the pathophysiology of mood disorders [2,4,5]. IR is frequently accompanied by chronic, low-grade systemic inflammation, driven by elevated concentrations of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, produced by adipose tissue and immune cells in the context of metabolic dysfunction [6,7]. This inflammatory state not only disrupts metabolic regulation but also impacts brain function. Circulating cytokines can cross the blood–brain barrier and activate microglia, which subsequently release additional inflammatory mediators, including IL-1, IL-6, and TNF-α. Neuroinflammation in turn, interferes with neuroplasticity by reducing neurogenesis, altering synaptic architecture, and dysregulating neurotransmitter systems [7,8], changes that are closely linked to mood disturbance, cognitive impairment, and affective lability in individuals with IR [4,6,9].
Alterations of the hypothalamic–pituitary–adrenal (HPA) axis, a hallmark of stress-related psychiatric disorders, are also more pronounced in patients with insulin resistance [2,9]. Hyperglycemia and pro-inflammatory cytokines can exacerbate this dysregulation, contributing to heightened stress reactivity and mood instability [10]. Several studies describe a bidirectional association between IR, HPA axis dysfunction, and depression, particularly among patients with type 2 diabetes [5,9]. Hyperinsulinemia, the defining metabolic feature of IR, may further drive both metabolic and affective disturbances. Notably, comorbid mood disorders appear more prevalent and severe in people with IR, with a particularly strong link observed in obese adolescents with neuroendocrine abnormalities [11,12].
In addition to inflammatory and neuroendocrine mechanisms, IR has been associated with mitochondrial dysfunction and impaired brain energy metabolism, particularly in regions central to mood regulation, such as the prefrontal cortex and limbic system [2,4,6]. Adding to these vulnerabilities; many psychotropic drugs have adverse effects on metabolic health; increasing the risk of obesity; type 2 diabetes; and non-alcoholic fatty liver disease [13,14,15]. This underscores the complex, bidirectional relationship between psychiatric and metabolic disorders.
It is important to acknowledge, however, that the relationship between insulin resistance and mood disorders is likely bidirectional. Depression itself can contribute to metabolic dysregulation through mechanisms such as physical inactivity, altered dietary patterns, sleep disruption, and chronic stress physiology, all of which can promote insulin resistance. Lifestyle factors, therefore, act both as confounders and as mediators, complicating the directionality of causality. This emphasizes the need for longitudinal and mechanistic studies that resolve the cause and consequence of the relationship between IR and depression.
In this context, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have emerged as potential therapeutic agents. Initially developed for type 2 diabetes and obesity, these drugs also exert central effects relevant to mood regulation, including modulation of reward circuits, appetite control, and attenuation of neuroinflammation [8,16,17]. Early studies suggest that GLP-1 RAs may reduce depressive symptoms, either via direct neurobiological effects or indirectly through improvements in metabolic health and body weight [18].
This narrative review examines the potential application of GLP-1 RAs in mood disorders, with a particular focus on their dual effects on insulin resistance and mood-related symptoms. We outline the biological mechanisms linking IR and mood dysregulation, discuss the metabolic consequences of psychotropic medications, and summarize the current evidence for GLP-1 RAs in psychiatric populations. Finally, we consider the clinical implications of integrating metabolic-targeted strategies into psychiatric care, advocating for a more individualized, metabolically informed approach.
2. Materials and Methods
The purpose of this narrative review is to synthesize the current theoretical and empirical evidence on the role of glucagon-like peptide-1 receptor agonists in mood disorders, with a particular focus on their impact on insulin resistance and affective symptoms. We aimed to integrate findings from multiple domains, including psychiatry, endocrinology, and neurobiology, to provide a comprehensive overview of potential translational applications.
A literature search was conducted in the electronic databases PubMed and Scopus up to July 2025. The search strategy included combinations of MeSH terms and free-text terms such as “GLP-1 receptor agonists,” “insulin resistance,” “major depressive disorder,” “bipolar disorder,” “mood disorders,” “neuroinflammation,” and “reward system.” Articles published in English were considered, and we included clinical trials, observational studies, systematic and narrative reviews, meta-analyses, preclinical animal studies, and cellular models relevant to GLP-1 RAs in the context of psychiatric and metabolic disorders. Case reports, editorials, and non-peer-reviewed material were excluded.
Reference lists of eligible publications were also screened to identify additional studies. Given the narrative design, no formal systematic assessment of study quality was undertaken; instead, we prioritized peer-reviewed research that directly addressed psychiatric or mechanistic outcomes of GLP-1 receptor agonists.
3. Psychiatric Relevance of GLP-1 Signaling
Glucagon-like peptide-1 receptor agonists have shown effects that extend beyond glucose regulation, impacting several neurobiological systems involved in mood disorders [15,19]. At the peripheral level, they support glycemic control and insulin sensitivity by promoting glucose-dependent insulin release from pancreatic β-cells, inhibiting excess glucagon secretion, and slowing gastric emptying [20,21]. These combined actions lead to improved postprandial glucose control, reduce appetite, and lead to weight loss, outcomes that directly oppose insulin resistance, a condition frequently seen in individuals with mood disorders and known to worsen psychiatric prognosis [2,22].
In the brain, GLP-1 receptors are present in regions critical for mood and motivation, including the hypothalamus, prefrontal cortex, ventral tegmental area (VTA), and nucleus accumbens [23]. Among these, the arcuate and paraventricular nuclei of the hypothalamus, as well as the VTA and nucleus accumbens, exhibit particularly high receptor density [24]. Activation of hypothalamic GLP-1 receptors enhances satiety signaling by influencing neurons that express pro-opiomelanocortin (POMC) and neuropeptide Y (NPY), mechanisms that reduce excessive food intake and the metabolic load tied to obesity [17,24]. In addition, GLP-1 RAs appear to modulate dopamine pathways within mesolimbic circuit networks that play a key role in processing reward and motivation. This effect may be particularly relevant in individuals with depression marked by anhedonia or poor treatment response [25].
Importantly, GLP-1 RAs also possess anti-inflammatory and neuroprotective properties, which are increasingly seen as relevant in the context of mood disorders [15,26]. Several preclinical studies have shown that these compounds reduce central neuroinflammation by lowering levels of pro-inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α) and by limiting oxidative stress [27,28]. Since these processes are linked to impaired neurogenesis and synaptic plasticity, their modulation may support neuronal health and resilience, particularly in stress-related mood symptoms. While anti-inflammatory and neuroprotective effects of GLP-1 receptor agonists have been described, it remains unclear to what extent these benefits are attributable to peripheral metabolic improvements (e.g., weight loss, improved insulin sensitivity) versus direct central actions on brain circuits. The relative contribution of these mechanisms likely varies across patients, depending on their metabolic status. The ability of GLP-1 RAs to penetrate the blood–brain barrier and mediate central effects (e.g., appetite suppression) can differ based on the molecular structure of the agonist and individual patient characteristics such as blood–brain barrier permeability, obesity, and metabolic health. Additionally, the magnitude of peripheral metabolic effects, like insulin secretion, glucagon suppression, and modulation of gut hormones, can also be influenced by a patient’s existing metabolic status and degree of insulin resistance [29,30].
Overall, the combined metabolic, neurochemical, and immunomodulatory effects of GLP-1 RAs suggest they could offer therapeutic benefits in mood disorders, especially for patients with coexisting metabolic dysfunction or resistance to standard treatments [15,19,20]. Their ability to address both physiological and psychiatric dimensions supports a more integrated view of mood disorders as systemic conditions, rather than disorders confined to brain circuits alone [31,32]
4. Mood Disorders and Metabolic Comorbidity: The Bidirectional Interface
Insulin resistance, long considered a defining feature of metabolic syndrome and a risk factor for cardiovascular disease, is increasingly recognized as a key contributor to the pathophysiology of mood disorders [21,33,34]. Far from being a consequence of poor lifestyle or medication side effects alone, IR has been detected in young, drug-naïve, and non-obese psychiatric patients, suggesting it may represent an intrinsic biological vulnerability in affective illness [12,35].
Numerous studies have documented a high prevalence of IR in individuals with major depressive disorder and bipolar disorder, even in the absence of obesity or overt metabolic disease [36,37]. A large meta-analysis of over 240,000 participants found elevated insulin levels and HOMA-IR indices in those with acute depression, independent of antidepressant use and body weight [22]. Similarly, cross-sectional studies in drug-naïve BD patients have shown a significantly higher risk of IR compared to healthy controls, with no correlation to BMI or comorbidities [12,38]. These findings challenge the view of IR as merely a metabolic byproduct and instead position it as a potential core component of mood pathology [34].
This has led to the identification of a “metabolic subtype” of depression, marked by IR and clinical features such as anhedonia, low energy, cognitive slowing, and poor response to first-line antidepressant treatments [25,33]. These symptoms persist even after controlling for physical activity and BMI, pointing to an independent role for metabolic dysfunction in shaping affective symptomatology. IR has also been identified as a predictor of non-response to selective serotonin and noradrenaline reuptake inhibitors (SNRIs), further reinforcing its association with treatment-resistant depression [35].
Neurobiological studies support this link, showing that IR is associated with structural and functional brain changes, including reduced volumes in regions involved in mood regulation and cognition. These alterations may underlie the clinical features of cognitive slowing and diminished reward sensitivity observed in patients with IR [39,40]. Additionally, higher levels of IR predict slower improvement in symptoms such as anhedonia during antidepressant treatment and are associated with worse longitudinal outcomes, including increased relapse risk and hospitalization [35,41,42]. For example, Watson et al. found that even mild increases in IR over a nine-year period were associated with an 89% greater risk of developing MDD [5].
Prospective epidemiological studies reinforce the relationship between metabolic dysfunction and mood disorders [21,43]. A large Dutch cohort study showed that moderate elevations in IR markers, such as increased waist circumference, fasting glucose, and elevated triglyceride-to-HDL ratios, were linked to a significantly higher risk of developing MDD over time [3]. Every 5 cm increase in waist circumference, for instance, was associated with an 11% greater risk of incident depression, and higher fasting glucose conferred a 37% increased risk. In that study, 14% of participants without prior depression developed MDD over nine years, with those exhibiting higher baseline IR measures at significantly higher risk [5].
IR appears to be particularly relevant in bipolar disorder, where its prevalence exceeds 50% in some samples [37,44,45]. Patients with BD and comorbid IR or type 2 diabetes are more likely to experience rapid cycling, a chronic course of illness, and poor response to lithium or other mood stabilizers [34,46]. IR in BD has been associated with neuroprogression, cognitive decline, and treatment resistance, independent of age, BMI, or exposure to antipsychotics [42,44].
Mechanistically, IR represents a critical biological interface between peripheral metabolic dysfunction and central nervous system changes. It contributes to low-grade inflammation, mitochondrial dysfunction, altered energy metabolism, impaired neuroplasticity, and dysregulation of the HPA axis [47,48]. Peripheral IR may also exacerbate central insulin resistance, disrupting dopaminergic signaling and further impairing brain function [49].
Compounding the issue, many commonly prescribed psychotropic medications, including second-generation antipsychotics, mood stabilizers, and certain antidepressants, are known to worsen metabolic parameters [50,51,52,53]. This creates a vicious cycle in which psychiatric symptoms and metabolic dysfunction reinforce one another, further complicating treatment outcomes [42].
Taken together, the evidence positions insulin resistance not only as a metabolic concern but also as a central player in the onset, severity, and treatment resistance of mood disorders. Understanding IR as a shared pathophysiological mechanism opens the door to more targeted, integrated approaches to treatment, particularly in patients with atypical features, poor therapeutic response, or evidence of metabolic dysregulation [54].
5. Psychotropic Medications and Metabolic Dysregulation
Psychotropic medications, including antipsychotics, mood stabilizers, and certain classes of antidepressants, are major contributors to metabolic dysfunction in individuals with mood disorders [55,56]. Among these, second-generation antipsychotics (SGAs) such as olanzapine, clozapine, and risperidone exhibit the highest metabolic liability [57,58,59]. These agents are known to induce weight gain, dyslipidemia, and IR through mechanisms that involve the antagonism of histaminergic (H1), serotonergic (5-HT2C), and muscarinic (M3) receptors [52]. This receptor profile interferes with hypothalamic appetite regulation, reduces energy expenditure, and impairs peripheral insulin signaling. (Table 1).

Table 1.
Main classes of psychotropic medications, metabolic impact, and effects on mood.
Mood stabilizers like lithium and valproate, although less potent in this regard, are also associated with increased weight and impaired glucose metabolism, especially with long-term use [50,53]. Certain antidepressants, particularly tricyclic antidepressants and some SSRIs (e.g., paroxetine), have been linked to modest increases in adiposity and reduced insulin sensitivity [51,60]. Crucially, these pharmacologically induced changes compound an underlying vulnerability: individuals with psychiatric disorders show a higher baseline prevalence of insulin resistance, metabolic syndrome, and type 2 diabetes, even in young, non-obese populations [45,57]. This predisposition is likely multifactorial, involving shared genetic, inflammatory, and neuroendocrine mechanisms [61].
Beyond physical health, IR appears to exert a detrimental effect on psychiatric outcomes [62]. Several studies have linked insulin resistance to residual depressive symptoms, particularly anhedonia, fatigue, and cognitive slowing, which are often resistant to standard antidepressant treatments [19,63,64]. Functional neuroimaging suggests that IR affects reward-related brain regions such as the ventral striatum, potentially mediating these symptoms [40,65,66]. This bidirectional relationship between psychotropic medications and metabolic dysfunction creates a self-reinforcing pathogenic cycle: psychotropic medications worsen insulin resistance, which contributes to reduced treatment efficacy, often leading to dose escalation or polypharmacy. This, in turn, intensifies the metabolic burden, further undermining both psychiatric and somatic health [55,67,68].
Disrupting this cycle is a clinical priority. Current guidelines recommend regular metabolic monitoring in patients on psychotropics, along with early implementation of lifestyle interventions [51,57]. Moreover, pharmacologic strategies such as metformin or GLP-1 receptor agonists are gaining interest not only for their metabolic benefits but also for their potential to improve mood and cognitive outcomes in patients with insulin resistance and treatment-resistant depression [62,69].
6. Clinical Studies in Psychiatric Populations
A growing body of clinical research suggests that GLP-1 receptor agonists may offer antidepressant benefits, particularly in individuals with comorbid metabolic disturbances [13,58]. Initial evidence has come from studies in patients with type 2 diabetes, where agents like liraglutide and semaglutide have not only improved glycemic control but also been associated with reductions in depressive symptoms [63,64,69]. In some trials, improvements in mood appeared independently of changes in HbA1c, pointing to potential neuropsychiatric effects that go beyond glycemic normalization [8,65,66].
This therapeutic effect has also been explored in patients with obesity and major depressive disorder, a population often affected by overlapping pathophysiological processes such as insulin resistance, inflammation, and altered reward system function [33,42,70]. Preliminary findings from both randomized controlled trials and observational studies suggest that treatment with GLP-1 RAs leads to significant weight loss and metabolic improvements, alongside decreases in depressive symptoms and enhancements in health-related quality of life [19,62]. Although sample sizes remain small, the consistency of results across studies underscores the potential of integrated metabolic-psychiatric approaches [41].
Weight reduction and improved insulin sensitivity may indirectly alleviate depressive symptoms by reducing systemic inflammation and restoring metabolic balance [21,43]. Modulation of leptin and adiponectin signaling, as well as a reduction in HPA axis overactivation, may further contribute to mood stabilization [47,48].
In addition, early preclinical and clinical studies have demonstrated direct central nervous system effects of GLP-1 Ras [71,72]. These actions have been associated with improvements in mood and cognition [39]. When used alongside standard antidepressant or antipsychotic treatments, GLP-1 RAs may offer additional benefit, particularly for patients with mood disorders and coexisting metabolic issues [34,42]. Psychotropic medications, especially second-generation antipsychotics and some mood stabilizers, are well known for their metabolic side effects, including weight gain, insulin resistance, and dyslipidemia. In this setting, GLP-1 RAs present a unique therapeutic option by targeting both residual mood symptoms and medication-induced metabolic burden [51,62,69].
By improving insulin sensitivity, promoting weight loss, and restoring glucose metabolism, GLP-1 RAs engage biological pathways relevant to both mood dysregulation and metabolic syndrome [8,63,72]. Their inclusion in psychiatric treatment plans could address an often-overlooked clinical need: managing both psychiatric symptoms and their metabolic comorbidities, which significantly affect long-term prognosis and quality of life. Table 2 summarizes characteristics and main findings of studies of GLP-1 Ras in the psychiatric population.
Although data in psychiatric populations are still limited, safety and tolerability findings are encouraging [15,36,54]. Reported side effects are largely gastrointestinal, such as nausea, vomiting, and appetite suppression, and tend to be dose-dependent and transient. However, long-term adherence remains a significant challenge. High dropout rates in obesity and diabetes trials highlight tolerability issues, which may be further compounded in psychiatric populations where comorbid anxiety, cognitive impairment, or poor illness insight can interfere with consistent medication use. Practical barriers such as injectable administration, cost, and access may also limit feasibility. These factors underline the need for careful patient selection, structured behavioral support, and real-world studies to assess persistence and tolerability in psychiatric contexts. Importantly, there is no evidence of increased risk for suicidality, psychosis, or affective destabilization, key concerns in mood disorders [73]. Cognitive impairment and sedation have also not been reported, supporting their suitability as adjunctive agents [39]. That said, careful monitoring remains essential, especially in patients with complex medical conditions or polypharmacy [37,44].
By complementing monoaminergic mechanisms with modulation of reward-related pathways and inflammatory tone, GLP-1 RAs may work synergistically with conventional antidepressants, particularly in subtypes characterized by anhedonia or treatment resistance [63,66]. Overall, integrating GLP-1 RAs into the management of mood disorders represents a biologically sound and clinically promising strategy. These agents have the potential to improve treatment outcomes, lower cardiometabolic risk, and enhance functional recovery by addressing both the metabolic and psychiatric components of illness [21,42,62]. Larger, long-term randomized controlled trials are still needed to define optimal patient profiles, dosing strategies, and long-term safety in psychiatric care.

Table 2.
Characteristics and main findings of studies of GLP-1 RAs in the psychiatric population.
Table 2.
Characteristics and main findings of studies of GLP-1 RAs in the psychiatric population.
| Study (Author/Year and Ref) | Study Design and Population | Intervention | Key Outcomes on Mood and Cognition | Notes |
|---|---|---|---|---|
| Mansur et al., 2017 [74] | A 4-week, open-label pilot; n = 19 adults with MDD or BD and executive dysfunction | Liraglutide 1.8 mg/day adjunctive | Improved executive function (Trail Making Test-B; composite cognition z-score). No significant change in mood scales | First proof-of-concept in a mood-disorder cohort; cognitive benefits independent of glycemic changes; small sample |
| Bezin et al., 2025 [73] | Nationwide case-time-control (France); n = 1102 suicide/attempt cases, with psychiatric subgroups | GLP-1 RAs (various) | No increased risk of suicide/attempt (OR 0.62); consistent across psychiatric-history subgroups | Strong real-world evidence of psychiatric safety, including high-risk populations |
| Ueda et al., 2024 [75] | Active-comparator cohort (Sweden and Denmark); n = 298,553 T2D patients | GLP-1 RAs vs. SGLT2i | No excess risk of suicide, self-harm, incident depression or anxiety | Large-scale pharmacoepidemiology confirming reassuring safety |
| Farr et al., 2016 [76] | Randomized, placebo-controlled crossover; n = 18 T2D | Liraglutide up to 1.8 mg/day | Reduced brain activation to palatable food cues (parietal cortex, insula, putamen) | Demonstrates central GLP-1R activity; relevant to reward and anhedonia pathways |
| van Bloemendaal et al., 2014 [77] | Randomized crossover with GLP-1R blockade; n = 48 obese ± T2D | Exenatide ± GLP-1R antagonist | Decreased food-cue responses (insula, amygdala, OFC); reduced caloric intake | Confirms central GLP-1R mediation of reward-circuit responses |
| Coveleskie et al., 2017 [78] | Double-blind crossover fMRI; n = 19 women (obese vs. lean) | Exenatide 5 μg SC | In obese women: increased connectivity (NTS–thalamus/hypothalamus); reduced hunger ratings | Suggests obesity moderates central GLP-1 signaling; implications for satiety/reward |
| Fanelli et al., 2025 [9] | UK Biobank; n = 30,919 with MDD | Insulin resistance (HOMA-IR, proxies) | IR+ patients had greater antidepressant resistance, longer treatment duration, and more severe profiles | Supports targeting metabolic dysfunction in depression |
| Li et al., 2024 [12] | Cross-sectional; n = 125 drug-naïve BD vs. 85 controls | Assessment of insulin resistance | BD patients had 2–3× higher prevalence of IR; IR linked to hypersomnia | Confirms IR as a core metabolic abnormality in BD |
| Watson et al., 2021 [5] | NESDA longitudinal cohort; n = 601, 9-year follow-up | Baseline IR markers (TG/HDL, glucose, waist) | IR predicted incident MDD (HR 1.3–1.9); prediabetes doubled risk | Strong prospective evidence linking IR and depression onset |
| Shomaker et al., 2010 [11] | Cross-sectional; n = 136 adolescents | Psychological symptoms + insulin sensitivity | Depressive symptoms were inversely correlated with insulin sensitivity | Provides early-life evidence of mood–metabolic coupling |
| Weiss et al., 2024 [38] | Retrospective chart review; elderly MDD/BD (n≈100) | Naturalistic follow-up with/without T2D | T2D associated with greater manic morbidity in BD and cognitive decline | Highlights adverse psychiatric impact of metabolic comorbidity |
7. Mechanistic Hypotheses for Psychotropic Action
GLP-1 receptor agonists have demonstrated a range of central nervous system (CNS) effects that align with key neurobiological pathways implicated in mood regulation, motivation, stress responsiveness, and cognitive function [74,76,79]. Several interrelated mechanisms have been proposed to explain their potential psychotropic effects [80,81].
A leading hypothesis centers on the modulation of neuroinflammation. Chronic low-grade inflammation, characterized by elevated levels of cytokines such as TNF-α and IL-6, is frequently observed in patients with mood disorders and metabolic dysregulation [26,82,83]. Both preclinical and clinical studies suggest that GLP-1 RAs reduce systemic and central inflammation, potentially restoring immune homeostasis in brain regions critical for emotional regulation and reward processing, such as the prefrontal cortex and limbic structures [26,82,84]. Beyond a general reduction in inflammatory cytokines, GLP-1 RAs appear to exert targeted effects on key inflammatory pathways. Experimental models show that GLP-1 receptor activation inhibits NF-κB signaling, thereby reducing transcription of pro-inflammatory mediators including IL-1β, IL-6, and TNF-α. GLP-1 RAs also attenuate activation of the NLRP3 inflammasome, a central driver of neuroinflammation and microglial reactivity in depression [85]. In parallel, modulation of the JAK/STAT pathway has been observed, further dampening cytokine signaling and promoting an anti-inflammatory ambient [86].
Another key pathway involves neurotrophic signaling. GLP-1 RAs have been shown to promote the expression of brain-derived neurotrophic factor (BDNF), enhancing synaptic plasticity and neurogenesis, particularly in the hippocampus and prefrontal cortex [84]. These regions are consistently implicated in the pathophysiology of depression, and such neurotrophic effects may underlie symptom improvements in domains like anhedonia, fatigue, and cognitive slowing [74,84]. Furthermore, GLP-1 RAs exhibit neuroprotective properties observed in models of neurodegenerative diseases, including Alzheimer’s and Parkinson’s, which may extend to the treatment of psychiatric conditions [26,79].
Preclinical studies in rodent models further support these mechanisms. Chronic administration of exendin-4 or liraglutide has been shown to enhance hippocampal neurogenesis, reduce anxiety and depression-like behaviors, and attenuate neuroinflammatory cascades through downregulation of IL-6 and TNF-α [63]. At the cellular level, GLP-1 receptor activation promotes neuroprotective signaling via cAMP-CREB-BDNF pathways, reduces oxidative stress, and prevents apoptosis in neuronal cultures exposed to hyperglycemic or inflammatory conditions [84,87]. Additional rodent studies have confirmed that GLP-1 RAs reduce depressive- and anxiety-like behaviors via modulation of hippocampal BDNF and suppression of neuroinflammation [88,89,90]. Systematic reviews and meta-analyses further support improvements in mood outcomes, underscoring the translational potential of these agents even if the heterogeneity of the studies included was high [15,91].
Importantly, GLP-1 RAs influence central reward circuitry. They enhance dopaminergic tone in the mesolimbic pathway, particularly the ventral tegmental area and nucleus accumbens, thereby improving motivational salience and hedonic responsiveness [80,81,92]. This may help alleviate anhedonia, a core symptom of depression [83]. While GLP-1 RAs reduce food intake by dampening hyperactive reward responses to palatable cues, they appear to preserve general reward sensitivity, differentiating them from medications that blunt dopaminergic tone nonspecifically [81,92]. Enhancing central insulin signaling, especially in individuals with insulin resistance, may further normalize dopaminergic function and contribute to the reversal of anhedonic phenotypes [40,41].
GLP-1 RAs have also been shown to alter brain functional connectivity [78]. Neuroimaging studies report modulation of networks such as the dorsal default mode network (DMN), salience network, and visuospatial attention circuits [76,78]. Specific compounds (e.g., exenatide, liraglutide) differentially affect connectivity in regions like the hypothalamus, thalamus, nucleus tractus solitarius, and hippocampus [78,93]. These effects may contribute to observed improvements in mood and cognitive domains [74].
A final and increasingly relevant mechanism involves the gut–brain axis [94,95]. GLP-1 RAs modulate gut microbiota composition, promote vagal afferent signaling, and influence gut-derived neuropeptides such as ghrelin and peptide YY (PYY), all of which can impact CNS function [96,97,98]. For example, liraglutide has been shown to activate vagal pathways and reduce postprandial triglyceride levels via a gut–brain–liver reflex [96]. Additionally, chronic treatment with GLP-1 RAs increases the abundance of anti-inflammatory microbial taxa (e.g., Bacteroidetes), which has been associated with improvements in both mood and glycemic control [94,95,99]. Preclinical studies confirm that long-term GLP-1 RA administration enhances hippocampal neurogenesis and reduces anxiety- and depression-like behaviors in rodent models [84].
In addition, GLP-1 RAs play a central role in the regulation of appetite and feeding behavior, which may indirectly influence mood [76,77]. Central GLP-1R activation in the amygdala also suppresses food intake, indicating a direct CNS mechanism linked to mood regulation [100]. Moreover, GLP-1 receptors are expressed in the brainstem and hypothalamus, key regions involved in energy homeostasis [76,77]. Activation of these receptors, either endogenously via oral or intraduodenal nutrient stimuli or through pharmacological administration, activates vagal afferents, a neural relay known to suppress appetite and improve glucose metabolism. Experimental knockdown of GLP-1Rs in the vagal pathway increases food intake and reduces insulin secretion in animal models, emphasizing the functional importance of this circuit [97]. Additionally, GLP-1 RAs modulate gut peptides such as ghrelin and can enhance PYY secretion, reinforcing satiety signaling [98]. These effects on appetite regulation may also contribute to improvements in self-perception, energy levels, and overall psychological well-being, especially in individuals with comorbid obesity or binge-related behaviors [74,77].
Taken together, these pleiotropic effects, ranging from anti-inflammatory, neurotrophic actions to dopaminergic and gut-mediated modulation and appetite-regulating effects, support a multimodal model of GLP-1 RA activity relevant to psychiatric disorders [26,83]. Their pleiotropic actions may be particularly beneficial in mood disorder patients with metabolic dysregulation, treatment resistance, or reward-processing deficits [74,83].
Figure 1 summarizes the main hypotheses for the psychotropic action of GLP-1 RAs.
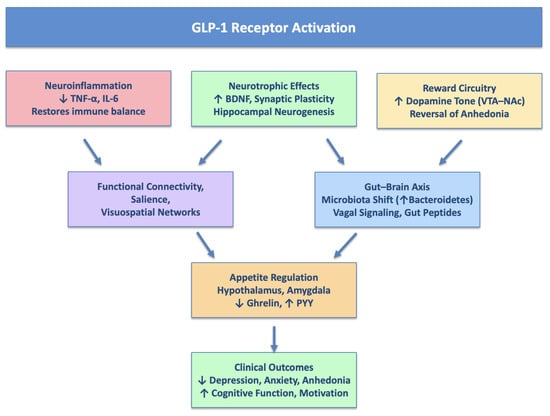
Figure 1.
Main hypotheses for psychotropic action of GLP-1 Ras.
8. Clinical Challenges and Research Priorities
Despite encouraging mechanistic and preliminary clinical findings, the integration of GLP-1 RAs into psychiatric treatment remains in an early and exploratory stage [18]. To date, most studies investigating their psychotropic potential have been conducted in populations with type 2 diabetes or obesity, where mood improvements may partially reflect metabolic normalization [101]. Consequently, the generalizability of these results to individuals with primary mood disorders, particularly those without overt metabolic disease, remains uncertain [18]. Large-scale, placebo-controlled randomized clinical trials specifically designed for psychiatric populations are critically needed to determine the efficacy, optimal dosing, duration, and long-term safety of GLP-1 RAs in treating mood symptoms [101]. Future trials should stratify patients by metabolic phenotype, incorporate mechanistic biomarkers, and include long-term follow-up to evaluate both psychiatric and metabolic endpoints. Comparative effectiveness studies with established antidepressants and mood stabilizers will also be essential. The integration of GLP-1 RAs into multimodal frameworks, including psychotherapy and lifestyle interventions, may represent a more effective precision psychiatry approach.
An additional challenge lies in the considerable metabolic heterogeneity observed among patients with mood disorders [102]. Individuals with MDD or bipolar disorder (BD) may vary widely in terms of insulin sensitivity, inflammatory status, and body composition, even in the absence of clinical metabolic syndrome [102,103]. This heterogeneity highlights the need for metabolic phenotyping in psychiatric research. Identifying biologically distinct subgroups, such as patients with central adiposity, insulin resistance, or elevated inflammatory markers, may enable the development of stratified treatment approaches, improving the likelihood of clinical benefit from GLP-1 RAs and similar agents [102].
Emerging data also point to a broader neuropsychiatric profile of GLP-1 RAs, with preliminary evidence suggesting benefits across multiple domains, including cognitive function, suicidality, anxiety, substance use, and binge-related eating behaviors. Some GLP-1 analogues may influence glutamatergic and GABAergic pathways, potentially contributing to therapeutic effects in conditions such as epilepsy, autism spectrum disorders, and addiction, although further validation is required. These findings underscore the potential for GLP-1 RAs to act on shared neurobiological substrates across psychiatric and neurological disorders [104,105].
Nevertheless, important safety considerations remain. Post-marketing surveillance and pharmacovigilance data have reported psychiatric adverse effects, including anxiety, mood lability, and suicidal ideation, in some cases, although these findings are agent-specific and not consistently observed in controlled studies [75,106,107]. Individualized risk–benefit assessment and clinical monitoring are warranted, particularly in vulnerable populations or those with complex psychiatric comorbidities. Although GLP-1 RAs are considered safe as monotherapy, their glucose-dependent mechanism of insulin secretion means that hypoglycemia is rare unless combined with insulin or sulfonylureas. Psychiatric patients with comorbid diabetes on such therapies require close monitoring. Currently, no specific clinical guidelines exist regarding the psychiatric use of GLP-1 RAs, highlighting the need for expert consensus and real-world implementation frameworks [18,103]. Current clinical studies have used standard antidiabetic or anti-obesity doses (e.g., liraglutide 1.8 mg/day; semaglutide 1.0–2.4 mg/week). Determining optimal doses for psychiatric indications, balancing efficacy against tolerability and metabolic safety, remains a key priority for future trials [15].
A promising avenue for improving precision in treatment selection involves the use of biomarker-informed strategies. Markers such as fasting insulin, HOMA-IR, leptin/adiponectin ratio, and high-sensitivity C-reactive protein (hs-CRP) may help identify patients with metabolic-inflammation-driven subtypes of depression or bipolar disorder [102,105]. Incorporating such markers into trial design and clinical workflows could enhance predictive accuracy and treatment responsiveness, though standardization and validation in prospective cohorts are needed.
Finally, translational studies aimed at disentangling the relative contributions of peripheral metabolic improvements versus central neuromodulatory effects (e.g., anti-inflammatory action, synaptic plasticity, dopamine regulation) are essential. These investigations will help clarify the mechanistic basis of GLP-1 RA efficacy in psychiatry and guide the development of next-generation incretin-based compounds with enhanced CNS specificity [102,103,105]. In parallel, future research should also address the integration of GLP-1 RAs into multimodal treatment frameworks, combining pharmacological, psychotherapeutic, and lifestyle-based interventions. The combination of pharmacological innovations with psychotherapeutic approaches leads to the best possible results in the treatment of mood disorders. This principle also applies to treatment with GLP-1 receptor agonists (GLP-1 RA). Essential support for patients starting GLP-1 RA treatment comes from behavioral therapy providers such as psychologists, therapists and specialist nurses who help patients with lifestyle changes, emotional adjustments and adherence challenges. Research studies show that behavioral interventions along with GLP-1 RA therapy help patients maintain lifestyle changes while treating eating disorders and improving their ability to control mood and metabolism [108]. Structured psychotherapeutic support for patients with mood disorders reinforces the mood and quality of life benefits of GLP-1 RAs by helping patients adhere to their treatment plan and promoting behavioral activation and reduction in weight- or metabolism-related avoidance or demoralization. Future clinical care models need to develop integrated approaches that combine GLP-1 RAs with personalized behavioral interventions. The implementation of such multidisciplinary approaches would optimize both metabolic and psychiatric outcomes while creating a more comprehensive, patient-centered model of care. This approach may maximize the impact of GLP-1 RAs by not only targeting metabolic and neurobiological dysfunctions but also reinforcing adherence, behavioral activation, and long-term recovery trajectories. Further exploration of dose–response relationships, long-term maintenance strategies, and the potential preventive role of GLP-1 RAs in high-risk populations (e.g., insulin-resistant youth with depressive symptoms) will be key to translating preliminary findings into clinical practice.
In summary, while GLP-1 RAs hold substantial promise as dual-action agents addressing both metabolic dysfunction and neuropsychiatric symptoms, their integration into psychiatric care demands rigorous clinical trials, biomarker-guided stratification, and mechanistic validation. As research progresses, these agents may help close the gap between psychiatry and metabolic medicine, offering a novel therapeutic avenue for complex mood disorders with a systemic basis.
9. Conclusions
The growing recognition of the bidirectional interplay between metabolic dysfunction and mood disorders underscores the need for integrated therapeutic strategies capable of addressing both domains concurrently. GLP-1 RAs, originally developed for glycemic control in type 2 diabetes, have demonstrated mechanistic plausibility and emerging clinical efficacy in modulating key pathophysiological processes implicated in depression, including insulin resistance, neuroinflammation, and reward system dysregulation.
Their pleiotropic effects, targeting both metabolic dysfunction and neuropsychiatric symptoms, position GLP-1 RAs as promising candidates for future therapeutic integration in mood disorders. However, the current evidence base is limited by small sample sizes, heterogeneous study populations, and a paucity of randomized controlled trials in non-diabetic psychiatric cohorts.
Future research should prioritize biomarker-informed, stratified clinical trials that integrate metabolic phenotyping and neuropsychiatric outcome measures. This precision medicine approach may facilitate the identification of subgroups most likely to benefit from GLP-1 RAs and contribute to the development of targeted interventions at the metabolic-psychiatric interface. In conclusion, while GLP-1 RAs are not yet established as standard components of psychopharmacological treatment algorithms, these multimodal properties make GLP-1 RAs a biologically coherent addition to the evolving landscape of psychiatric therapeutics. As our understanding of the metabolic–psychiatric interface deepens, GLP-1 RAs may come to represent a paradigm shift in the management of mood disorders, one that moves beyond symptomatic control to address the systemic basis of treatment resistance.
Author Contributions
P.C.: conceptualization, methodology, writing—original draft, project administration. Supervision. A.C.: writing—review and editing. M.B.R.: writing—original draft and editing. A.F.: conceptualization, validation, writing—review, and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The Lancet Psychiatry. Global Burden of Disease 2021: Mental health messages. Lancet Psychiatry 2024, 11, 573. [Google Scholar] [CrossRef]
- Krupa, A.J.; Dudek, D.; Siwek, M. Consolidating evidence on the role of insulin resistance in major depressive disorder. Curr. Opin. Psychiatry 2024, 37, 23. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry Off. J. World Psychiatr. Assoc. WPA 2023, 22, 394–412. [Google Scholar] [CrossRef]
- Calkin, C.; McClelland, C.; Cairns, K.; Kamintsky, L.; Friedman, A. Insulin Resistance and Blood-Brain Barrier Dysfunction Underlie Neuroprogression in Bipolar Disorder. Front. Psychiatry 2021, 12, 636174. [Google Scholar] [CrossRef]
- Watson, K.T.; Simard, J.F.; Henderson, V.W.; Nutkiewicz, L.; Lamers, F.; Nasca, C.; Rasgon, N.; Penninx, B.W.J.H. Incident Major Depressive Disorder Predicted by Three Measures of Insulin Resistance: A Dutch Cohort Study. Am. J. Psychiatry 2021, 178, 914–920. [Google Scholar] [CrossRef]
- Erichsen, J.M.; Fadel, J.R.; Reagan, L.P. Peripheral versus central insulin and leptin resistance: Role in metabolic disorders, cognition, and neuropsychiatric diseases. Neuropharmacology 2022, 203, 108877. [Google Scholar] [CrossRef]
- Sălcudean, A.; Bodo, C.-R.; Popovici, R.-A.; Cozma, M.-M.; Păcurar, M.; Crăciun, R.-E.; Crisan, A.-I.; Enatescu, V.-R.; Marinescu, I.; Cimpian, D.-M.; et al. Neuroinflammation—A Crucial Factor in the Pathophysiology of Depression—A Comprehensive Review. Biomolecules 2025, 15, 502. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Fanelli, G.; Bralten, J.; Franke, B.; Roth Mota, N.; Atti, A.R.; De Ronchi, D.; Monteleone, A.M.; Grassi, L.; Serretti, A.; Fabbri, C.; et al. Insulin resistance and poorer treatment outcomes in depression: Evidence from UK Biobank primary care data. Br. J. Psychiatry J. Ment. Sci. 2025, 1–10. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Shomaker, L.B.; Tanofsky-Kraff, M.; Young-Hyman, D.; Han, J.C.; Yanoff, L.B.; Brady, S.M.; Yanovski, S.Z.; Yanovski, J.A. Psychological symptoms and insulin sensitivity in adolescents. Pediatr. Diabetes 2010, 11, 417–423. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Yang, T.; Lin, Y.; Liao, Y.; Gan, Z. Prevalence of insulin resistance and its associated factors in drug-naïve patients with bipolar disorder among Han Chinese population. BMC Psychiatry 2024, 24, 388. [Google Scholar] [CrossRef]
- Sepúlveda-Lizcano, L.; Arenas-Villamizar, V.V.; Jaimes-Duarte, E.B.; García-Pacheco, H.; Paredes, C.S.; Bermúdez, V.; Rivera-Porras, D. Metabolic Adverse Effects of Psychotropic Drug Therapy: A Systematic Review. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 1505–1520. [Google Scholar] [CrossRef]
- Khan, M.M.; Khan, Z.A.; Khan, M.A. Metabolic complications of psychotropic medications in psychiatric disorders: Emerging role of de novo lipogenesis and therapeutic consideration. World J. Psychiatry 2024, 14, 767. [Google Scholar] [CrossRef]
- Meshkat, S.; Luciano, C.; Swiderski, A.; Li, G.; Aguilar, R.; Dunkley, B.; Reichelt, A.; Zhang, Y.; Greenshaw, A.; Vermetten, E.; et al. Efficacy and Safety of Glucagon-Like Peptide-1 Agonists for Psychiatric Symptoms: A Systematic Review. Brain Behav. 2025, 15, e70661. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Hubl, D. The effect of GLP-1RAs on mental health and psychotropics-induced metabolic disorders: A systematic review. Psychoneuroendocrinology 2025, 176, 107415. [Google Scholar] [CrossRef] [PubMed]
- Tempia Valenta, S.; Nicastri, A.; Perazza, F.; Marcolini, F.; Beghelli, V.; Atti, A.; Petroni, M. The Impact of GLP-1 Receptor Agonists (GLP-1 RAs) on Mental Health: A Systematic Review. Curr. Treat. Options Psychiatry 2024, 11, 310–357. [Google Scholar] [CrossRef]
- Gammoh, O.; Qnais, E.; Aljabali, A.A.A.; Hataet, T.; Alqudah, A. Metabolic resilience: Liraglutide’s potential in alleviating depressive symptoms. Mol. Biol. Rep. 2025, 52, 550. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Salagre, E.; Enduru, N.; Grande, I.; Vieta, E.; Zhao, Z. Insulin resistance in depression: A large meta-analysis of metabolic parameters and variation. Neurosci. Biobehav. Rev. 2022, 139, 104758. [Google Scholar] [CrossRef]
- Trammell, T.S.; Henderson, N.L.; Madkour, H.S.; Stanwood, G.D.; Graham, D.L. GLP-1R activation alters performance in cognitive tasks in a sex-dependent manner. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 2911–2919. [Google Scholar] [CrossRef]
- Anderberg, R.; Richard, J.; Eerola, K.; Ferreras, L.; Nordbeck, E.; Hansson, C.; Nissbrandt, H.; Bergquist, F.; Gribble, F.; Reimann, F.; et al. Glucagon-Like Peptide 1 and Its Analogs Act in the Dorsal Raphe and Modulate Central Serotonin to Reduce Appetite and Body Weight. Diabetes 2017, 66, 1062–1073. [Google Scholar] [CrossRef]
- Hamer, J.A.; Testani, D.; Mansur, R.B.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Brain insulin resistance: A treatment target for cognitive impairment and anhedonia in depression. Exp. Neurol. 2019, 315, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Du, P.; Bai, B.; Feng, P.; Lian, X.; Hölscher, C.; Wang, Y.; Xue, G. The GLP-1 receptor agonist liraglutide inhibits necroptosis and neuroinflammation in a mouse model of Parkinson’s disease with diabetes co-morbidity. Front. Neurosci. 2025, 19, 1596506. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef] [PubMed]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef]
- Mosili, P.; Mkhize, B.C.; Sibiya, N.H.; Ngubane, P.S.; Khathi, A. Review of the direct and indirect effects of hyperglycemia on the HPA axis in T2DM and the co-occurrence of depression. BMJ Open Diabetes Res. Care 2024, 12, e003218. [Google Scholar] [CrossRef]
- Sarwar, H.; Rafiqi, S.I.; Ahmad, S.; Jinna, S.; Khan, S.A.; Karim, T.; Qureshi, O.; Zahid, Z.A.; Elhai, J.D.; Levine, J.C.; et al. Hyperinsulinemia Associated Depression. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 11795514221090244. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- SayuriYamagata, A.; Brietzke, E.; Rosenblat, J.D.; Kakar, R.; McIntyre, R.S. Medical comorbidity in bipolar disorder: The link with metabolic-inflammatory systems. J. Affect. Disord. 2017, 211, 99–106. [Google Scholar] [CrossRef]
- Salviati, M.; Valeriani, G.; Terlizzi, S.; Maurizi, A.; Melcore, C.; Romano, G.F.; Panico, R.; Biondi, M. The bidirectional relationship between insulin resistance and psychiatric disorders: Considerations for using HOMA-IR index. Clin. Ter. 2013, 164, e549–e561. [Google Scholar] [CrossRef] [PubMed]
- Starostina, E. Comorbidity Between Severe Mental Disorders and Metabolic Disease. In Comorbidity Between Mental and Physical Disorders; Springer: Cham, Switzerland, 2025; pp. 181–202. ISBN 978-3-031-81801-1. [Google Scholar]
- Regan, A.S.; Valcourt, S.C. Metabolic Syndrome in Bipolar Disorder: Review and Management. Psychiatr. Ann. 2020, 50, 334–339. [Google Scholar] [CrossRef]
- Weiss, F.; Brancati, G.E.; Elefante, C.; Petrucci, A.; Gemmellaro, T.; Lattanzi, L.; Perugi, G. Type 2 diabetes mellitus is associated with manic morbidity in elderly patients with mood disorders. Int. Clin. Psychopharmacol. 2024, 39, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Maksyutynska, K.; Stogios, N.; Prasad, F.; Gill, J.; Hamza, Z.; De, R.; Smith, E.; Horta, A.; Goldstein, B.I.; Korczak, D.; et al. Neurocognitive correlates of metabolic dysregulation in individuals with mood disorders: A systematic review and meta-analysis. Psychol. Med. 2024, 54, 1245–1271. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Veit, R.; Ketterer, C.; Schick, F.; Häring, H.-U.; Fritsche, A.; Preissl, H. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 2011, 33, 1052. [Google Scholar] [CrossRef]
- Colomer, L.; Anmella, G.; Vieta, E.; Grande, I. Physical health in affective disorders: A narrative review of the literature. Rev. Bras. Psiquiatr. 2021, 43, 621–630. [Google Scholar] [CrossRef]
- Jones, B.D.M.; Farooqui, S.; Kloiber, S.; Husain, M.O.; Mulsant, B.H.; Husain, M.I. Targeting Metabolic Dysfunction for the Treatment of Mood Disorders: Review of the Evidence. Life 2021, 11, 819. [Google Scholar] [CrossRef]
- Pan, A.; Hu, F.B. Response to Comment on: Pan et al. Bidirectional Association Between Depression and Metabolic Syndrome: A Systematic Review and Meta-analysis of Epidemiological Studies. Diabetes Care 2012;35:1171–1180. Diabetes Care 2013, 36, e28. [Google Scholar] [CrossRef]
- McElroy, S.L.; Keck, P.E. Metabolic syndrome in bipolar disorder: A review with a focus on bipolar depression. J. Clin. Psychiatry 2014, 75, 46–61. [Google Scholar] [CrossRef]
- Vancampfort, D.; Correll, C.U.; Galling, B.; Probst, M.; De Hert, M.; Ward, P.B.; Rosenbaum, S.; Gaughran, F.; Lally, J.; Stubbs, B. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: A systematic review and large scale meta-analysis. World Psychiatry Off. J. World Psychiatr. Assoc. WPA 2016, 15, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.; MacQueen, G. Associations between bipolar disorder and metabolic syndrome: A review. J. Clin. Psychiatry 2006, 67, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Martins, L.B.; Berk, M.; Bauer, M.E. Severe psychiatric disorders and general medical comorbidities: Inflammation-related mechanisms and therapeutic opportunities. Clin. Sci. 2022, 136, 1257–1280. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Cai, X.; Zheng, C.; Qiu, S.; Ke, H.; Huang, Y. Update on the Relationship Between Depression and Neuroendocrine Metabolism. Front. Neurosci. 2021, 15, 728810. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Chan, L.C.; Borreginne, K.; Kale, R.P.; Hu, C.; Tye, S.J. A review of brain insulin signaling in mood disorders: From biomarker to clinical target. Neurosci. Biobehav. Rev. 2018, 92, 7–15. [Google Scholar] [CrossRef]
- Correll, C.U.; Lencz, T.; Malhotra, A.K. Antipsychotic drugs and obesity. Trends Mol. Med. 2011, 17, 97–107. [Google Scholar] [CrossRef]
- Scheen, A.J. Metabolic disorders induced by psychotropic drugs. Ann. Endocrinol. 2023, 84, 357–363. [Google Scholar] [CrossRef]
- Gp, R.; Sl, K. Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacol. Ther. 2010, 125, 169–179. [Google Scholar] [CrossRef]
- Kong, L.; Wang, H.; Yan, N.; Xu, C.; Chen, Y.; Zeng, Y.; Guo, X.; Lu, J.; Hu, S. Effect of antipsychotics and mood stabilisers on metabolism in bipolar disorder: A network meta-analysis of randomised-controlled trials. eClinicalMedicine 2024, 71, 102581. [Google Scholar] [CrossRef] [PubMed]
- Cermolacce, M.; Belzeaux, R.; Adida, M.; Azorin, J.-M. Affective disorders: Endocrine and metabolic comorbidities. L’Encephale 2014, 40 (Suppl. 3), S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Mazereel, V.; Detraux, J.; Vancampfort, D.; Van Winkel, R.; De Hert, M. Impact of Psychotropic Medication Effects on Obesity and the Metabolic Syndrome in People With Serious Mental Illness. Front. Endocrinol. 2020, 11, 573479. [Google Scholar] [CrossRef]
- Filaković, P.; Erić, A.P.; Radanović-Grgurić, L. Metabolic syndrome and psychotropic medications. Med. Glas. Off. Publ. Med. Assoc. Zenica-Doboj Cant. Bosnia Herzeg. 2012, 9, 180–188. Available online: https://pubmed.ncbi.nlm.nih.gov/22926348/ (accessed on 1 August 2025).
- De Hert, M.; Detraux, J.; van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2011, 8, 114–126. [Google Scholar] [CrossRef]
- Yuen, J.W.Y.; Kim, D.D.; Procyshyn, R.M.; Panenka, W.J.; Honer, W.G.; Barr, A.M. A Focused Review of the Metabolic Side-Effects of Clozapine. Front. Endocrinol. 2021, 12, 609240. [Google Scholar] [CrossRef]
- Gahr, M. Metabolic adverse drug reactions related to psychotropic drugs. Fortschr. Neurol. Psychiatr. 2025, 93, 95–103. [Google Scholar] [CrossRef]
- Serretti, A.; Mandelli, L. Antidepressants and body weight: A comprehensive review and meta-analysis. J. Clin. Psychiatry 2010, 71, 979. [Google Scholar] [CrossRef]
- Mukherjee, S.; Im, S.-S. Decoding Health: Exploring Essential Biomarkers Linked to Metabolic Dysfunction-Associated Steatohepatitis and Type 2 Diabetes Mellitus. Biomedicines 2025, 13, 359. [Google Scholar] [CrossRef]
- Qassab, M.A.; Mneimneh, M.; Jradi, A.; Derbas, B.; Dabboussi, D.; Baini, J.K.; Katrib, N.; Chaarani, N.; Attieh, P.; Kanaan, A.; et al. The Expanding Role of GLP-1 Receptor Agonists: Advancing Clinical Outcomes in Metabolic and Mental Health. Curr. Issues Mol. Biol. 2025, 47, 285. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fu, X.; Liu, Y.; Wang, T.; Zhao, X.; Cui, R.; Yang, W. Exploring the Effect and Mechanism of Liraglutide in Treating Depression Based on Network Pharmacology and Experimental Analysis. J. Cell Mol. Med. 2025, 29, e70630. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, P.; Wang, W.; Guo, L.; Pan, Q. The Antidepressant Effects of GLP-1 Receptor Agonists: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2024, 32, 117–127. [Google Scholar] [CrossRef]
- Monney, M.; Jornayvaz, F.; Gariani, K. GLP-1 receptor agonists effect on cognitive function in patients with and without type 2 diabetes. Diabetes Metab. 2023, 49, 101470. [Google Scholar] [CrossRef] [PubMed]
- Kabahizi, A.; Wallace, B.; Lieu, L.; Chau, D.; Dong, Y.; Hwang, E.; Williams, K.W. Glucagon-like peptide-1 (GLP-1) signalling in the brain: From neural circuits and metabolism to therapeutics. Br. J. Pharmacol. 2022, 179, 600–624. [Google Scholar] [CrossRef]
- Ress, C.; Tschoner, A.; Kaser, S.; Ebenbichler, C.F. Psychotropic drugs and diabetes. Wien. Med. Wochenschr. 2011, 161, 531–542. [Google Scholar] [CrossRef]
- Bolger, A.; Verdolini, N.; Agius, M. Can metabolic side effects of antipsychotics be reversed by lifestyle changes? Psychiatr. Danub. 2014, 26 (Suppl. 1), 330–335. [Google Scholar]
- Kopp, K.O.; Glotfelty, E.J.; Li, Y.; Greig, N.H. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol. Res. 2022, 186, 106550. [Google Scholar] [CrossRef]
- Nousen, E.K.; Franco, J.G.; Sullivan, E.L. Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology 2013, 98, 254–266. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Bezin, J.; Bénard-Laribière, A.; Hucteau, E.; Tournier, M.; Montastruc, F.; Pariente, A.; Faillie, J.-L. Suicide and suicide attempt in users of GLP-1 receptor agonists: A nationwide case-time-control study. eClinicalMedicine 2025, 80, 103029. [Google Scholar] [CrossRef]
- Mansur, R.B.; Ahmed, J.; Cha, D.S.; Woldeyohannes, H.O.; Subramaniapillai, M.; Lovshin, J.; Lee, J.G.; Lee, J.-H.; Brietzke, E.; Reininghaus, E.Z.; et al. Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: A pilot, open-label study. J. Affect. Disord. 2017, 207, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ueda, P.; Söderling, J.; Wintzell, V.; Svanström, H.; Pazzagli, L.; Eliasson, B.; Melbye, M.; Hviid, A.; Pasternak, B. GLP-1 Receptor Agonist Use and Risk of Suicide Death. JAMA Intern. Med. 2024, 184, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar] [CrossRef] [PubMed]
- van Bloemendaal, L.; IJzerman, R.G.; ten Kulve, J.S.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 Receptor Activation Modulates Appetite- and Reward-Related Brain Areas in Humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef]
- Coveleskie, K.; Kilpatrick, L.A.; Gupta, A.; Stains, J.; Connolly, L.; Labus, J.S.; Sanmiguel, C.; Mayer, E.A. The effect of the GLP-1 analogue Exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes. Sci. Pract. 2017, 3, 434–445. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides 2018, 71, 70–80. [Google Scholar] [CrossRef]
- Anderberg, R.H.; Richard, J.E.; Hansson, C.; Nissbrandt, H.; Bergquist, F.; Skibicka, K.P. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 2016, 65, 54–66. [Google Scholar] [CrossRef]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The Glucagon-Like Peptide 1 (GLP-1) Analogue, Exendin-4, Decreases the Rewarding Value of Food: A New Role for Mesolimbic GLP-1 Receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef]
- Wang, R.-F.; Xue, G.-F.; Hölscher, C.; Tian, M.-J.; Feng, P.; Zheng, J.-Y.; Li, D.-F. Post-treatment with the GLP-1 analogue liraglutide alleviate chronic inflammation and mitochondrial stress induced by Status epilepticus. Epilepsy Res. 2018, 142, 45–52. [Google Scholar] [CrossRef]
- Gruber, J.; Hanssen, R.; Qubad, M.; Bouzouina, A.; Schack, V.; Sochor, H.; Schiweck, C.; Aichholzer, M.; Matura, S.; Slattery, D.A.; et al. Impact of insulin and insulin resistance on brain dopamine signalling and reward processing—An underexplored mechanism in the pathophysiology of depression? Neurosci. Biobehav. Rev. 2023, 149, 105179. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Liu, X.-X.; An, Z.-G.; Luo, X.; Zhang, L.-L.; Yan, P.; Jin, L.; Cai, R.; Yi, Q.-Z. GLP-1 plays a protective role in hippocampal neuronal cells by activating cAMP-CREB-BDNF signaling pathway against CORT+HG-induced toxicity. Heliyon 2023, 9, e18491. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Q.; Feng, J.; Xiao, Z.; Zhang, X.; Zhao, L. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway. J. Int. Med. Res. 2021, 49, 0300060521992981. [Google Scholar] [CrossRef]
- Zitman-Gal, T.; Einbinder, Y.; Ohana, M.; Katzav, A.; Kartawy, A.; Benchetrit, S. Effect of liraglutide on the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway in diabetic kidney disease in db/db mice and in cultured endothelial cells. J. Diabetes 2019, 11, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, Y.; Wang, S.; Zhang, L.; Guo, L. GLP-1 Inhibits High-Glucose-Induced Oxidative Injury of Vascular Endothelial Cells. Sci. Rep. 2017, 7, 8008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Cao, X.; Ma, H.; White, P.F.; Xu, X.; Jiang, Y.; Sun, X.; Cui, Y. Exendin-4 improves behaviorial deficits via GLP-1/GLP-1R signaling following partial hepatectomy. Brain Res. 2019, 1706, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, C.; Turan, İ.; Özaçmak, H.S. The effect of glucagon like peptide-1 receptor agonist on behavioral despair and anxiety-like behavior in ovariectomized rats: Modulation of BDNF/CREB, Nrf2 and lipocalin 2. Behav. Brain Res. 2022, 435, 114053. [Google Scholar] [CrossRef]
- de Paiva, I.H.R.; da Silva, R.S.; Mendonça, I.P.; de Souza, J.R.B.; Peixoto, C.A. Semaglutide Attenuates Anxious and Depressive-Like Behaviors and Reverses the Cognitive Impairment in a Type 2 Diabetes Mellitus Mouse Model Via the Microbiota-Gut-Brain Axis. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2024, 19, 36. [Google Scholar] [CrossRef]
- Pozzi, M.; Mazhar, F.; Peeters, G.G.A.M.; Vantaggiato, C.; Nobile, M.; Clementi, E.; Radice, S.; Carnovale, C. A systematic review of the antidepressant effects of glucagon-like peptide 1 (GLP-1) functional agonists: Further link between metabolism and psychopathology: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J. Affect. Disord. 2019, 257, 774–778. [Google Scholar] [CrossRef]
- Wakabayashi, K.T.; Baindur, A.N.; Feja, M.; Suarez, M.; Chen, K.; Bernosky-Smith, K.; Bass, C.E. Synthetic exendin-4 disrupts responding to reward predictive incentive cues in male rats. Front. Behav. Neurosci. 2024, 18, 1363497. [Google Scholar] [CrossRef] [PubMed]
- Deden, L.N.; Booij, J.; Grandjean, J.; Homberg, J.R.; Hazebroek, E.J.; Gotthardt, M.; Boss, M. Brain Imaging of the GLP-1 Receptor in Obesity Using 68Ga-NODAGA-Exendin-4 PET. Brain Sci. 2021, 11, 1647. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Lu, H.-C.; Chou, Y.-H.; Liu, P.-Y.; Chen, H.-Y.; Huang, M.-C.; Lin, C.-H.; Tsai, C.-N. Gut Microbial Signatures for Glycemic Responses of GLP-1 Receptor Agonists in Type 2 Diabetic Patients: A Pilot Study. Front. Endocrinol. 2022, 12, 814770. [Google Scholar] [CrossRef]
- Feng, J.; Teng, Z.; Yang, Y.; Liu, J.; Chen, S. Effects of semaglutide on gut microbiota, cognitive function and inflammation in obese mice. PeerJ 2024, 12, e17891. [Google Scholar] [CrossRef]
- Hoffman, S.; Alvares, D.; Adeli, K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol. Metab. 2022, 65, 101590. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef]
- Lindqvist, A.; Shcherbina, L.; Fischer, A.-H.T.; Wierup, N. Ghrelin Is a Regulator of Glucagon-Like Peptide 1 Secretion and Transcription in Mice. Front. Endocrinol. 2017, 8, 135. [Google Scholar] [CrossRef]
- Shang, J.; Liu, F.; Zhang, B.; Dong, K.; Lu, M.; Jiang, R.; Xu, Y.; Diao, L.; Zhao, J.; Tang, H. Liraglutide-induced structural modulation of the gut microbiota in patients with type 2 diabetes mellitus. PeerJ 2021, 9, e11128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Cutts, E.J.; Lopez, C.B.; Kaur, S.; Duran, M.; Virkus, S.A.; Hardaway, J.A. Anatomical and Functional Characterization of Central Amygdala Glucagon-Like Peptide 1 Receptor Expressing Neurons. Front. Behav. Neurosci. 2021, 15, 724030. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P. Risk of depression and dementia among individuals treated with sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists. Curr. Opin. Psychiatry 2025, 38, 368–375. [Google Scholar] [CrossRef]
- Gill, H.; Lieberman, J.; DiVincenzo, J.; Rodrigues, N.; Mansur, R.; McKenzie, A.; Phan, L.; Rosenblat, J.; McIntyre, R. Evaluating the Use of Glucagon-Like Peptide 1 for the Treatment of Cognitive Dysfunction in Individuals with Mood Disorders. Curr. Treat. Options Psychiatry 2022, 9, 331–345. [Google Scholar] [CrossRef]
- Ali, M.; Ahmed, A.; Khan, B.A.; Shah, S.T.; Naveed, S.; Ashraf, N. Efficacy of GLP-1 Agonists in Psychiatric Illnesses: A Scoping Review. Prim. Care Companion CNS Disord. 2025, 27, 24nr03828. [Google Scholar] [CrossRef] [PubMed]
- VI, N.B.; Tressler, E.H.; Vendruscolo, L.F.; Leggio, L.; Farokhnia, M. IUPHAR review—Glucagon-like peptide-1 (GLP-1) and substance use disorders: An emerging pharmacotherapeutic target. Pharmacol. Res. 2024, 207, 107312. [Google Scholar] [CrossRef]
- Xiang, L.; Peng, Y. Impact of Glucagon-like Peptide-1 Receptor Agonists on Mental Illness: Evidence from a Mendelian Randomization Study. Int. J. Mol. Sci. 2025, 26, 2741. [Google Scholar] [CrossRef]
- Tobaiqy, M.; Elkout, H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: A pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int. J. Clin. Pharm. 2024, 46, 488–495. [Google Scholar] [CrossRef]
- Kim, J.A.; Yoo, H.J. Exploring the Side Effects of GLP-1 Receptor Agonist: To Ensure Its Optimal Positioning. Diabetes Metab. J. 2025, 49, 525–541. [Google Scholar] [CrossRef]
- Reilly-Harrington, N.A.; Himes, S.M.; Lauretti-Robbins, J.; Sogg, S. One Size Does Not Fit All: Special Considerations for Behavioral Health Providers Treating Patients Using GLP-1RA Medications for Obesity. Cogn. Behav. Pract. 2025, in press. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).