Evaluation of Dry Eye Disease Signs, Symptoms, and Vision-Related Quality of Life in Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Approval
2.3. Ophthalmological Examination Procedures
- Distant and near visual acuity test on Snellen charts with the best spectacle correction (BCDVA and BCNVA) in the same light conditions
- Evaluation of the anterior and posterior segment of the eyeball in a slit biomicroscope (Haag-Streit, Switzerland) with a 90D lens ( Volk Optical Inc., Mentor, OH, USA)
- Examination of the retina using Optical Coherence Tomography (Zeiss, Germany)
- Evaluation of the eyeball using an ultrasonographic device (ultrasound of the eye with a 10 MHz probe).
- Evaluation of the refractive error with an RM-8100 autorefractometer (Topcon, Japan)
- Intraocular pressure (IOP) measurement with a Goldmann applanation tonometer (Haag-Streit, Switzerland)
- Evaluation of tear film stability using the Tear film Break-Up Time (T-BUT) test.
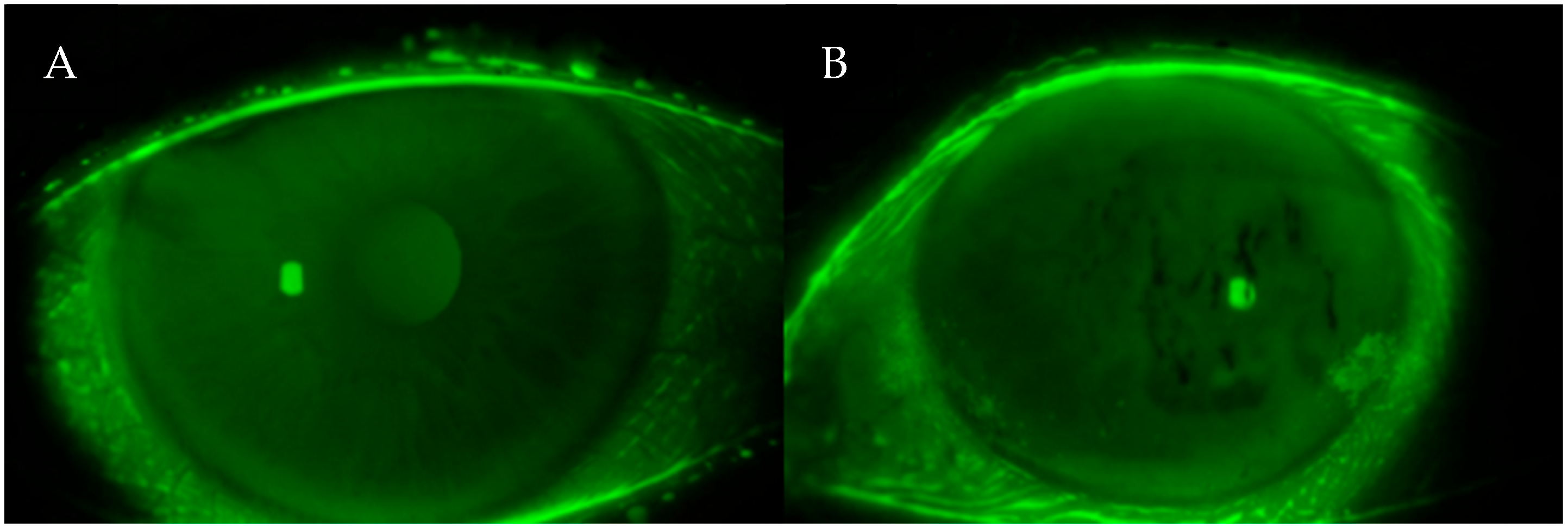
- Fluorescein staining test and anterior segment assessment using both the Oxford and Bijsterveld scale
- Evaluation of tear secretion using the Schirmer test I without anesthesia
- Evaluation of ocular surface dysfunction parameters using the Ocular Surface Disease Index (OSDI) questionnaire
2.4. Ocular Surface Disease Index (OSDI) Questionnaire
- 0–12: Normal
- 13–22: Mild DED
- 23–32: Moderate DED
- 33–100: Severe DED
2.5. Tear Film Break-Up Time (T-BUT) Test
- T-BUT of >10 s was considered normal tear film stability;
- T-BUT of <10 s indicated decreased tear film stability, often associated with lipid layer dysfunction;
- T-BUT of <5 s was interpreted as indicative of significant ocular surface lubrication deficiency and marked tear film instability, typically resulting from meibomian gland dysfunction or lipid layer insufficiency.
2.6. Schirmer I Test
- Wetting of >15 mm: Normal aqueous tear secretion
- Wetting of 10–15 mm: Early or borderline aqueous deficiency
- Wetting of 5–10 mm: Moderate aqueous tear deficiency, suggestive of evolving dry eye
- Wetting of <5 mm: Severe aqueous-deficient dry eye, indicating advanced lacrimal hyposecretion and significant tear film insufficiency
2.7. Ocular Surface Staining Assessment
2.8. Diagnostic Criteria for Dry Eye Disease
- Schirmer I test: ≤5 mm of wetting after 5 min
- Tear Break-Up Time (T-BUT): <10 s
- van Bijsterveld score: >3
2.9. Statistical Analysis
3. Results
3.1. Characteristics and Clinical Findings
- Lens opacification in 8 patients (22.9%);
- Hyaloid degeneration in 12 patients (34.3%);
- Retinal degenerations, including dry (atrophic) changes in 4 patients (11.4%) and wet (exudative) changes in 1 patient (2.9%);
- Hypertensive or microangiopathic retinal angiopathy in 8 patients (22.9%);
- Episcleritis in 1 patient (2.9%).
3.2. Tear Film Parameters and Quality of Life
- Patients with DED exhibited significantly lower values in the Schirmer I test (p = 0.0003) and
- Significantly reduced tear break-up time (T-BUT) (p = 0.0002) compared with non-DED counterparts.
- van Bijsterveld scores were significantly higher among DED patients (p = 0.0000);
- The Oxford grading scale demonstrated a trend toward statistical significance (p = 0.080), indicating a possible association.
- Schirmer I Test: 10.5 mm (SD = 4.17)
- Tear Break-Up Time (T-BUT): 8.5 s (range: 6.5–11.5 s)
- van Bijsterveld score: Median of 3 (range: 1–4)
4. Discussion
“A disorder of the ocular surface characterized by tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities, leading to symptoms of discomfort, visual disturbance, and reduced quality of life.” [27]
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| ANOVA | Analysis of Variance |
| BCDVA | Best-Corrected Distant Visual Acuity |
| BCNVA | Best-Corrected Near Visual Acuity |
| DED | Dry Eye Disease |
| HCQ | Hydroxychloroquine |
| IOP | Intraocular Pressure |
| KCS | Keratoconjunctivitis Sicca |
| OSDI | Ocular Surface Disease Index |
| QoL | Quality of Life |
| SLE | Systemic Lupus Erythematosus |
| T-BUT | Tear Break-up Time |
| TFOS DEWS III | Tear Film and Ocular Surface Society Dry Eye Workshop III |
References
- Shaikh, M.F.; Jordan, N.; D’Cruz, D.P. Systemic lupus erythematosus. Clin. Med. 2017, 17, 78–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narváez, J. Systemic lupus erythematosus 2020. Med. Clin. 2020, 155, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Dörner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Olesińska, M.; Saletra, A. Quality of life in systemic lupus erythematosus and its measurement. Reumatologia 2018, 56, 45–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aringer, M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 2020, 110, 102374. [Google Scholar] [CrossRef] [PubMed]
- Gergianaki, I.; Bortoluzzi, A.; Bertsias, G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2018, 32, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Woo, J.M.P.; Parks, C.G.; Costenbader, K.H.; Jacobsen, S.; Bernatsky, S. Systemic Lupus Erythematosus Risk: The Role of Environmental Factors. Rheum. Dis. Clin. N. Am. 2022, 48, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532, Erratum in Nat. Rev. Rheumatol., 2021, 17, 642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stojan, G.; Petri, M. Epidemiology of systemic lupus erythematosus: An update. Curr. Opin. Rheumatol. 2018, 30, 144–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ocampo-Piraquive, V.; Nieto-Aristizábal, I.; Cañas, C.A.; Tobón, G.J. Mortality in systemic lupus erythematosus: Causes, predictors and interventions. Expert Rev. Clin. Immunol. 2018, 14, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.M.P.; Parks, C.G.; Jacobsen, S.; Costenbader, K.H.; Bernatsky, S. The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J. Intern. Med. 2022, 291, 755–778. [Google Scholar] [CrossRef] [PubMed]
- Karvonen-Gutierrez, C.A.; Leis, A. Impact of menopause on women with systemic lupus erythematosus. Maturitas 2021, 154, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Guéry, J.C. Why Is Systemic Lupus Erythematosus More Common in Women? Jt. Bone Spine 2019, 86, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Luboń, W.; Luboń, M.; Kotyla, P.; Mrukwa-Kominek, E. Understanding Ocular Findings and Manifestations of Systemic Lupus Erythematosus: Update Review of the Literature. Int. J. Mol. Sci. 2022, 23, 12264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arevalo, J.F.; Lowder, C.Y.; Muci-Mendoza, R. Ocular manifestations of systemic lupus erythematosus. Curr. Opin. Ophthalmol. 2002, 13, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Stone, D.U.; Kaufman, C.E.; Hefner, K.S.; Fram, N.R.; Siatkowski, R.L.; Huang, A.J.W.; Chodosh, J.; Rasmussen, P.T.; Fife, D.A.; et al. Reproducibility of Ocular Surface Staining in the Assessment of Sjögren Syndrome-Related Keratoconjunctivitis Sicca: Implications on Disease Classification. ACR Open Rheumatol. 2019, 1, 292–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitcher, J.P.; Shiboski, C.H.; Shiboski, S.C.; Heidenreich, A.M.; Kitagawa, K.; Zhang, S.; Hamann, S.; Larkin, G.; McNamara, N.A.; Greenspan, J.S.; et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am. J. Ophthalmol. 2010, 149, 405–415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, K.; Bunya, V.; Maguire, M.; Asbell, P.; Ying, G.S.; Dry Eye Assessment and Management Study Research Group. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology 2021, 128, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Inomata, T.; Iwata, N.; Sung, J.; Fujimoto, K.; Fujio, K.; Midorikawa-Inomata, A.; Miura, M.; Akasaki, Y.; Murakami, A. A Review of Dry Eye Questionnaires: Measuring Patient-Reported Outcomes and Health-Related Quality of Life. Diagnostics 2020, 10, 559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozulken, K.; Aksoy Aydemir, G.; Tekin, K.; Mumcuoğlu, T. Correlation of Non-invasive Tear Break-Up Time with Tear Osmolarity and Other Invasive Tear Function Tests. Semin. Ophthalmol. 2020, 35, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Brott, N.R.; Ronquillo, Y. Schirmer Test. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- de A F Gomes, B.; Santhiago, M.R.; de Azevedo, M.N.; Moraes, H.V., Jr. Evaluation of dry eye signs and symptoms in patients with systemic sclerosis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, O.; Norheim, K.B.; Rødahl, E.; Jonsson, R.; Omdal, R. Primary Sjögren’s syndrome and the eye. Surv. Ophthalmol. 2020, 65, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Dutta, D.; Jones, L.; Craig, J.P. TFOS DEWS III: Definition and Classification of Dry Eye Disease. Ocul. Surf. 2023, 27, 45–56. [Google Scholar]
- Manoussakis, M.N.; Georgopoulou, C.; Zintzaras, E.; Spyropoulou, M.; Stavropoulou, A.; Skopouli, F.N.; Moutsopoulos, H.M. Sjögren’s syndrome associated with systemic lupus erythe-matosus: Clinical and laboratory profiles and comparison with primary sjögren’s syndrome. Arthritis Rheum. 2004, 50, 882–891. [Google Scholar] [CrossRef]
- Hsu, C.S.; Hsu, C.W.; Lu, M.C.; Koo, M. Risks of ophthalmic disorders in patients with systemic lupus erythematosus—A secondary cohort analysis of population-based claims data. BMC Ophthalmol. 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Dias-Santos, A.; Tavares Ferreira, J.; Pinheiro, S.; Cunha, J.P.; Alves, M.; Papoila, A.L.; Moraes-Fontes, M.F.; Proença, R. Ocular involvement in systemic lupus erythematosus patients: A paradigm shift based on the experience of a tertiary referral center. Lupus 2020, 29, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, H.T.; Hwang, Y.H.; Chen, Y.T.; Hsiao, C.H.; Chen, H.C. Severity of dry eye syndrome is related to anti-dsDNA autoantibody in systemic lupus erythematosus patients without secondary Sjogren syndrome: A cross-sectional analysis. Medicine 2016, 95, e4218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, A.; Gu, Z.; Liao, R.; Shuai, Z. Dry Eye Indexes Estimated by Keratograph 5M of Systemic Lupus Erythematosus Patients without Secondary Sjögren’s Syndrome Correlate with Lupus Activity. J. Ophthalmol. 2019, 2019, 8509089. [Google Scholar] [CrossRef]
- Bartlett, J.; Keith, M.; Sudharshan, L.; Snedecor, S. Associations between signs and symptoms of dry eye disease: A systematic review. Clin. Ophthalmol. 2015, 9, 1719–1730. [Google Scholar] [CrossRef]
- Jensen, J.L.; Bergem, H.O.; Gilboe, I.M.; Husby, G.; Axéll, T. Oral and ocular sicca symptoms and findings are prevalent in systemic lupus erythematosus. J. Oral Pathol. Med. 1999, 28, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Dammacco, R.; Procaccio, P.; Racanelli, V.; Vacca, A.; Dammacco, F. Ocular Involvement in Systemic Lupus Erythematosus: The Experience of Two Tertiary Referral Centers. Ocul. Immunol. Inflamm. 2018, 26, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Lu, Q.; Zhang, A.; Shuai, Z.W.; Liao, R. Analysis of Ocular Surface Characteristics and Incidence of Dry Eye Disease in Systemic Lupus Erythematosus Patients Without Secondary Sjögren’s Syndrome. Front. Med. (Lausanne) 2022, 9, 833995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tankunakorn, J.; Sawatwarakul, S.; Vachiramon, V.; Chanprapaph, K. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis-Like Lupus Erythematosus. J. Clin. Rheumatol. 2019, 25, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Cresce, N.D.; Ameri, M.; Martin, A.A.; Patterson, J.W.; Kimpel, D.L. Systemic lupus erythematosus presenting as Stevens–Johnson syndrome/toxic epidermal necrolysis. J. Clin. Rheumatol. 2014, 20, 167–171. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Dana, R.; Sullivan, R.M.; Krenzer, K.L.; Sahin, A.; Arica, B.; Liu, Y.; Kam, W.R.; Papas, A.S.; Cermak, J.M. Meibomian Gland Dysfunction in Primary and Secondary Sjögren Syndrome. Ophthalmic. Res. 2018, 59, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xiao, Y.; Zhang, S.; Liu, L.; Chen, K. Elevated Rheumatoid Factor Associates with Dry Eye in Patients with Common Autoimmune Diseases. J. Inflamm. Res. 2022, 15, 2789–2794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Xie, Y.; Deng, Y. Prevalence of dry eye in patients with systemic lupus erythematosus: A meta-analysis. BMJ Open 2021, 11, e047081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sayegh, R.R.; Yu, Y.; Farrar, J.T.; Kuklinski, E.J.; Shtein, R.M.; Asbell, P.A.; Maguire, M.G.; Dry Eye Assessment and Management (DREAM) Study Research Group. Ocular Discomfort and Quality of Life Among Patients in the Dry Eye Assessment and Management Study. Cornea 2021, 40, 869–876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Liu, X.; Han, J.; Shao, T.; Wang, Y. Association Between Sleep Quality, Mood Status, and Ocular Surface Characteristics in Patients With Dry Eye Disease. Cornea 2019, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Guo, O.D.L.W.; Akpek, E. The negative effects of dry eye disease on quality of life and visual function. Turk. J. Med. Sci. 2020, 50, 1611–1615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomes, J.A.P.; Santo, R.M. The impact of dry eye disease treatment on patient satisfaction and quality of life: A review. Ocul. Surf. 2019, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Asfuroğlu, E.; Bicakcigil, M.; Toker, E. Hydroxychloroquine improves dry eye symptoms of patients with primary Sjogren’s syndrome. Rheumatol. Int. 2011, 31, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Utheim, T.P.; Bootsma, H.; Hammond, C.J. Advances, limitations and future perspectives in the diagnosis and management of dry eye in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 301–309. [Google Scholar] [PubMed]
- Jacobi, C.; Jacobi, A.; Kruse, F.E.; Cursiefen, C. Tear film osmolarity measurements in dry eye disease using electricalimpe-dance technology. Cornea 2011, 30, 1289–1292. [Google Scholar] [CrossRef]
- Kozikowska, M.; Luboń, W.; Kucharz, E.J.; Mrukwa-Kominek, E. Ocular manifestations in patients with systemic sclerosis. Reumatologia 2020, 58, 401–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Ophthalmic Abnormalities | Number of Patients (%) |
|---|---|
| Lens opacification | 8 (22.9%) |
| Cataract | 1 (2.9%) |
| Degenerative changes in the vitreous body | 12 (34.3%) |
| Retinoschisis/retinal detachment | 0 |
| Degenerative “dry” changes in the retina | 4 (11.4%) |
| Exudative or hemorrhagic changes in the retina | 1 (2.9%) |
| Abnormalities in the course and size of blood vessels (narrowing or widening of veins and arteries) | 8 (22.9%) |
| Visual field defects | 5 (14.3%) |
| Episcleritis | 1 (2.9%) |
| Refractive error: astigmatism (>0.50 D cyl) | 21 (60.0%) |
| DED (n = 13) | Non-DED (n = 22) | p | Total (n = 35) | |
|---|---|---|---|---|
| Schirmer Test [mm/5 min] | 7.4 (3.11) | 12.3 (3.64) | 0.0003 a,* | 10.5 (4.17) |
| Oxford scale | 2.4 (0.79) | 1.7 (1.27) | 0.0818 a | 1.9 (1.16) |
| T-BUT [s] | 6 (5.5–7.5) | 11.3 (7–12) | 0.0002 b,* | 8.5 (6.5–11.5) |
| Bijsterveld staining | 4 (4–4) | 2 (1.0–2) | 0.0000 b,* | 3 (1–4) |
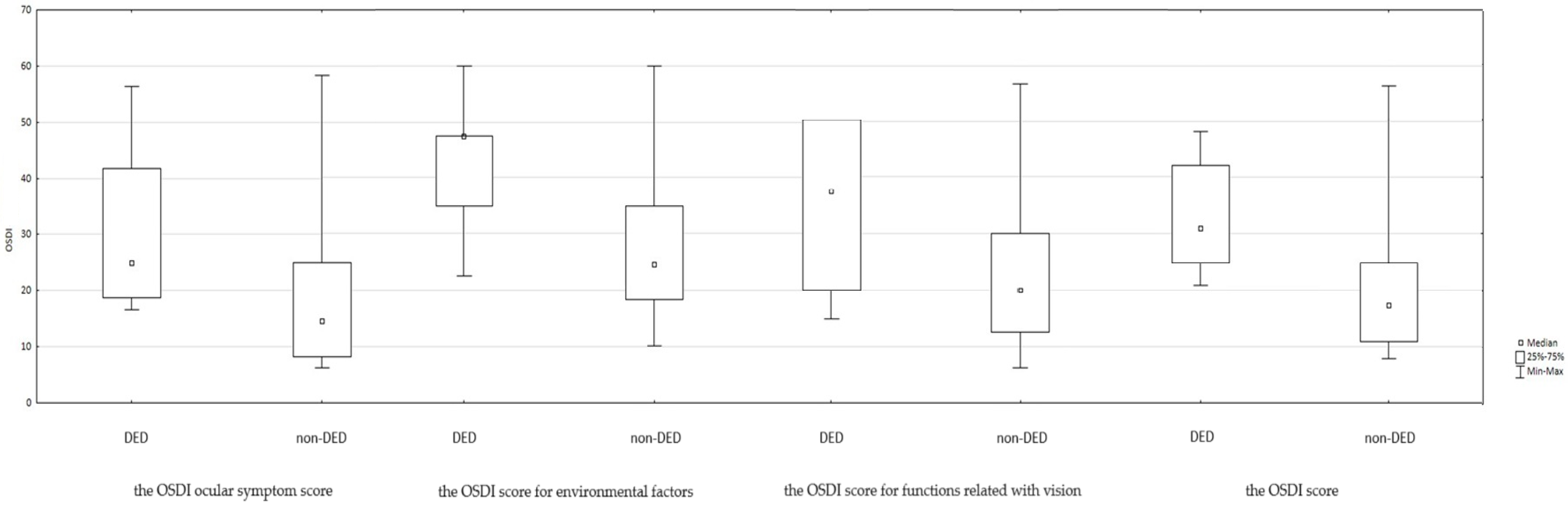
| OSDI | 31 (25–42) | 17.5 (11–25) | 0.0007 b,* | 25 (14–31) |
| OSDI vision-related function | 37 (20–50) | 20 (12.5–30) | 0.0123 b,* | 25 (15–40) |
| OSDI ocular symptoms | 25 (18.8–41.7) | 14.6 (8.3–25) | 0.0036 b,* | 18.5 (12.5–25) |
| OSDI environmental triggers | 37.5 (25–37.5) | 14.6 (8.3–25) | 0.0049 b,* | 25 (12.5–37.5) |
| OSDI | Non-DED | DED | Sum—Rows | Fisher’s Exact Test |
|---|---|---|---|---|
| Normal | 6 27% | 0 0% | 6 17% | p = 0.0645 |
| Mild | 9 41% | 2 15% | 11 31% | p = 0.1501 |
| Moderate | 5 23% | 5 38% | 10 29% | p = 0.4437 |
| Severe | 2 9% | 6 46% | 8 23% | p = 0.3209 |
| Sum—Columns | 22 | 13 | 35 |
| Schirmer Test | T-BUT | Bijsterveld | Oxford Scale | |||||
|---|---|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | ρ | p | |
| OSDI | −0.4286 | 0.0102 * | −0.7203 | 0.0000 * | 0.6072 | 0.0001 * | 0.7844 | 0.0000 * |
| Vision-related function | −0.2925 | 0.0882 | −0.5939 | 0.0002 * | 0.4069 | 0.0153 | 0.6958 | 0.0000 * |
| Ocular symptoms | −0.4596 | 0.0055 * | −0.6365 | 0.0000 * | 0.5207 | 0.0013 * | 0.6068 | 0.0001 * |
| Environmental triggers | −0.4021 | 0.0167 | −0.5798 | 0.0003 * | 0.5998 | 0.0001 * | 0.7262 | 0.0000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luboń, W.; Agaś-Lange, A.; Mrukwa-Kominek, E.; Smędowski, A.; Wyględowska-Promieńska, D. Evaluation of Dry Eye Disease Signs, Symptoms, and Vision-Related Quality of Life in Patients with Systemic Lupus Erythematosus. Life 2025, 15, 1423. https://doi.org/10.3390/life15091423
Luboń W, Agaś-Lange A, Mrukwa-Kominek E, Smędowski A, Wyględowska-Promieńska D. Evaluation of Dry Eye Disease Signs, Symptoms, and Vision-Related Quality of Life in Patients with Systemic Lupus Erythematosus. Life. 2025; 15(9):1423. https://doi.org/10.3390/life15091423
Chicago/Turabian StyleLuboń, Wojciech, Anna Agaś-Lange, Ewa Mrukwa-Kominek, Adrian Smędowski, and Dorota Wyględowska-Promieńska. 2025. "Evaluation of Dry Eye Disease Signs, Symptoms, and Vision-Related Quality of Life in Patients with Systemic Lupus Erythematosus" Life 15, no. 9: 1423. https://doi.org/10.3390/life15091423
APA StyleLuboń, W., Agaś-Lange, A., Mrukwa-Kominek, E., Smędowski, A., & Wyględowska-Promieńska, D. (2025). Evaluation of Dry Eye Disease Signs, Symptoms, and Vision-Related Quality of Life in Patients with Systemic Lupus Erythematosus. Life, 15(9), 1423. https://doi.org/10.3390/life15091423






