Abstract
Background: Melioidosis is a severe infectious disease caused by Burkholderia pseudomallei, with high mortality rates, particularly in severe cases complicated by acute kidney injury (AKI). Objective: The objective of this study was to systematically review and quantitatively synthesize the impact of AKI on mortality and other clinical outcomes—including ICU admission and the need for renal replacement therapy (RRT)—in patients with melioidosis. Methods: A systematic search was conducted in PubMed, Scopus, and Embase up to 16 May 2025. Studies reporting mortality, ICU admission, or RRT use in patients with AKI were included. A random-effects meta-analysis was performed to estimate the odds ratio (OR) for mortality associated with AKI. Results: Twenty-nine studies (380 patients) were included. AKI occurred in 123 patients (32.4%). The pooled analysis revealed that AKI patients had a significantly higher mortality risk than non-AKI patients (OR = 23.37; 95% CI: 13.97–39.10; p = 0.0082), with no significant heterogeneity (I2 = 0%). Sensitivity analysis confirmed the robustness of this association. ICU admission and RRT data were frequently reported but were not suitable for meta-analysis due to insufficient data. Conclusions: AKI is a serious complication in melioidosis, significantly increasing the risk of mortality. Early recognition and aggressive management of AKI in melioidosis may be critical to improving clinical outcomes.
1. Introduction
Burkholderia pseudomallei is a motile, Gram-negative, facultative intracellular bacillus and the causative agent of melioidosis, a disease of increasing global importance. It is an environmental saprophyte that thrives in soil and stagnant water, particularly in tropical and subtropical regions. Human infection occurs primarily via percutaneous inoculation, inhalation, or ingestion, especially during the rainy season or extreme weather events such as typhoons or floods [1]. Melioidosis is endemic in Southeast Asia and Northern Australia but has also been reported in South Asia, the Middle East, Africa, and more recently in the Americas [2]. Clinical manifestations are highly variable, ranging from asymptomatic infection and localized skin ulcers to fulminant septicemia with multi-organ failure. Diabetes mellitus is the most common risk factor, followed by chronic kidney disease, hazardous alcohol use, and immunosuppression [3].
One of the severe and underrecognized complications of melioidosis is acute kidney injury (AKI). AKI can result from direct renal invasion by B. pseudomallei, systemic inflammation, septic shock, or nephrotoxic treatments (such as aminoglycosides) used during management [4]. Clinical manifestations of AKI in melioidosis range from transient elevations in serum creatinine to oliguric or anuric renal failure, and in some cases, the need for renal replacement therapy (RRT). Patients with AKI often have concurrent complications, such as pneumonia, hepatic dysfunction, or hematologic abnormalities, which exacerbate disease severity. The requirement for renal replacement therapy (RRT) has been reported in several cases, highlighting the burden of critical illness in this patient population [5]. Despite its clinical importance, the true incidence and impact of AKI in melioidosis remain unclear. Studies have used a range of diagnostic criteria, including the Acute Kidney Injury Network (AKIN) and the Kidney Disease: Improving Global Outcomes (KDIGO) definitions, leading to considerable heterogeneity in reported outcomes [6]. In the broader context of sepsis, AKI is a well-established predictor of poor prognosis. Patients with sepsis-associated AKI experience higher mortality, longer ICU and hospital stays, and a greater likelihood of long-term renal dysfunction [7]. Similarly, limited data from melioidosis studies suggest a strong association between AKI and increased mortality. For example, Chou et al. (2007) reported that 47.4% of melioidosis patients with AKI died, while none without AKI experienced mortality [4]. Prabhu et al. (2021) also found that AKI was independently associated with increased risk of death and ICU admission [5]. However, to date, no systematic review or meta-analysis has quantified the association between AKI and clinical outcomes in melioidosis.
We hypothesized that AKI is associated with increased risk of mortality and adverse clinical outcomes in patients with melioidosis. Therefore, we conducted a systematic review and meta-analysis to assess whether the presence of acute kidney injury (AKI) in patients with melioidosis is associated with increased mortality. Understanding the prognostic implications of AKI in melioidosis may support early identification, risk stratification, and targeted interventions to reduce morbidity and mortality in this high-risk group.
2. Materials and Methods
2.1. Protocol Registration
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8]. The review protocol was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD420251052619).
2.2. Search Strategy
We systematically searched three major electronic databases—PubMed, Scopus, and Embase—from their inception to 16 May 2025. The search terms included combinations of Medical Subject Headings (MeSH) and keywords related to Burkholderia pseudomallei, melioidosis, acute kidney injury, and clinical outcomes. The complete search strategies for each database are provided in Supplementary Table S1.
To ensure comprehensive coverage, we also examined the reference lists of all included studies and relevant reviews. Additionally, we searched gray literature sources, such as Google Scholar and reference lists from papers, to identify any potentially eligible reports not indexed in the traditional databases. Only studies published in English were included in this review.
2.3. Inclusion and Exclusion Criteria
We included studies that met the following criteria. Population: Human subjects diagnosed with culture-confirmed or clinically diagnosed melioidosis. Exposure: Presence of acute kidney injury, as defined by the authors of the included studies. Comparator: Patients with melioidosis without AKI. Outcomes: At least one of the following reported outcomes—mortality (in-hospital or 30-day), ICU admission, or requirement for renal replacement therapy (RRT). Study design: Case reports, case series, retrospective cohort studies, or prospective studies that provided extractable individual or grouped outcome data.
We excluded review articles, animal studies, editorials, conference abstracts without sufficient data, and non-English publications. Case reports were excluded only if they lacked outcome data relevant to AKI.
2.4. Study Selection
All identified records were imported into a reference management software program (EndNote), and duplicates were removed. Two independent reviewers (W.K.K. and A.P.) screened titles and abstracts against the eligibility criteria. Full texts of potentially relevant studies were then retrieved and assessed for inclusion. Discrepancies were resolved by discussion or by consultation with a third reviewer.
2.5. Data Extraction
Data were extracted independently by two reviewers, using a standardized data extraction form. Extracted variables included first author, year of publication, country, sample size, number of AKI cases, definition of AKI, mortality in AKI and non-AKI groups, ICU admission rates, RRT use, and relevant clinical characteristics (e.g., comorbidities and organ dysfunction). Definitions of AKI varied across studies, with some using standardized criteria (e.g., AKIN or KDIGO) and others relying on clinical judgment or unspecified parameters. Where data were unclear or missing, authors were contacted when possible. Any disagreements were resolved by consensus.
2.6. Quality Assessment
We assessed study quality using the Joanna Briggs Institute (JBI) critical appraisal tools, selecting checklists based on study design. The appropriate JBI checklists were applied to case reports (n = 19; 8 items), case series (n = 8; 10 items), and cohort studies (n = 2; 11 items), evaluating factors such as patient selection, diagnostic clarity, outcome reporting, and risk of bias.
Two reviewers (W.K.K. and A.P.) independently rated each item as “Yes,” “No,” “Unclear,” or “Not applicable,” with disagreements resolved by discussion. Studies were not excluded based on quality, but ratings were used to support the interpretation of results. Based on the number of criteria met, studies were classified as having low, moderate, or high risk of bias.
2.7. Statistical Analysis
Pooled odds ratios (ORs) were calculated using a random-effects model. Log-transformed ORs and their standard errors were used to compute 95% confidence intervals (CIs). The between-study variance (Tau2) was estimated to account for variability across studies. The I2 statistic was used to assess the degree of heterogeneity between studies. Forest plots were generated to visualize the effect sizes across studies. All analyses were performed using R software with the “meta” package (R Foundation for Statistical Computing, Vienna, Austria).
For outcomes with insufficient data (e.g., ICU admission and RRT use), a narrative synthesis was conducted. A sensitivity analysis was performed by excluding individual studies to assess the robustness of the primary outcome. The results of these sensitivity tests, which confirmed the consistency of the primary findings, are provided in Supplementary Figures S1 and S2.
2.8. Bayesian Re-Analysis of Risk Difference (RD)
To address concerns regarding studies with zero events in both treatment and control arms, we re-analyzed the data using a Bayesian Generalized Linear Model (GLM) with the stan_glm function from the rstanarm package in R. A Gaussian family with an identity link was used to model the Risk Difference (RD) across studies, specifying the formula rd~1 and generating 4000 posterior samples via Markov Chain Monte Carlo (MCMC) sampling. This approach effectively handled zero-event studies, offering more reliable RD estimates [9,10].
3. Results
3.1. Included Studies
A total of 86 records were identified through database searches: 10 from PubMed, 17 from Scopus, and 44 from Embase. An additional 15 records were identified through Google Scholar and references. After removing 23 duplicate records, 63 records remained for screening. Of these, 12 were excluded based on title and abstract review. Full-text reports were sought for 51 records, but 4 reports could not be retrieved. After assessing the eligibility of 47 reports, 8 were excluded due to lack of outcome data, 4 were review articles, 4 were conference abstracts, 1 had a duplicate population, and 1 was a systematic review. Ultimately, 29 studies were included in the systematic review and meta-analysis. The PRISMA flow diagram is presented in Figure 1. Additionally, as shown in Supplementary Table S2, 18 studies were excluded from the final analysis for reasons such as duplicate populations, lack of outcome data, review articles, and conference abstracts.
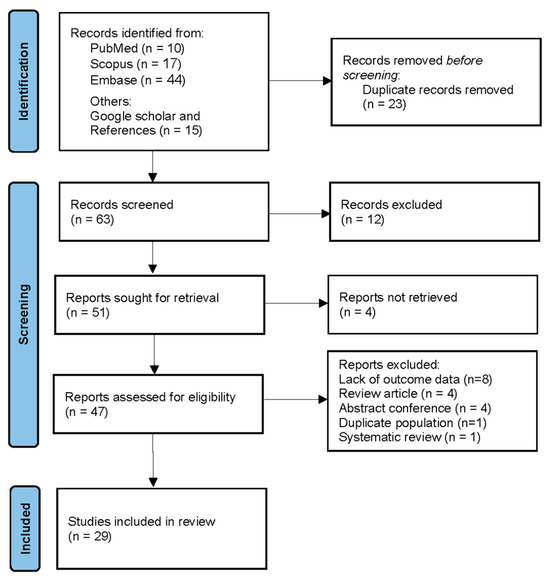
Figure 1.
PRISMA flow diagram.
3.2. Characteristics of Included Studies
A total of 29 studies were included in this systematic review and meta-analysis, comprising 380 patients with melioidosis, of whom 123 (32.4%) developed AKI. The studies were conducted across a wide geographical distribution, including Southeast Asia (e.g., Thailand, Malaysia, Singapore, and Vietnam), South Asia (India and Sri Lanka), the Middle East (Saudi Arabia and Oman), and Western countries (United States, Australia, and the Netherlands).
Study designs included case reports (n = 19), case series (n = 8), and retrospective cohort studies (n = 2). The sample sizes varied from single-patient case reports to a large retrospective study with 164 patients. The reported incidence of AKI ranged widely from 3.7% to 100%, with AKI being more common in studies involving critically ill patients.
Mortality among patients with AKI varied by study and ranged from 0% to 100%, with several reports noting markedly higher mortality in the AKI group compared to those without AKI. The need for RRT was reported in several studies, reflecting the severity of renal dysfunction. Organ dysfunction beyond the kidneys was common, including pulmonary, hepatic, and neurological involvement.

Table 1.
Characteristics of included studies.
Table 1.
Characteristics of included studies.
| Author (Year) [Ref.] | Country | Sample Size (n = 380) | AKI Cases (n = 123) | AKI % | Mortality (AKI) | Mortality (no AKI) | ICU Admission | RRT Required | Organ Dysfunction | Risk Factor | AKI Definition | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alhatmi (2020) [11] | Saudi Arabia | 2 | 1 | 50 | 100% (Case 1) | 0% (Case 2) | 1 | 1 | Multi-organ failure | Travel to Thailand (Case 1), India (Case 2) | Not explicitly defined, clinical evidence | Case 1: Fulminant sepsis, ECMO support; Case 2: Treated successfully with antibiotics. |
| Amali (2024) [12] | Singapore | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes (ECMO support) | Yes | Multi-organ dysfunction, including lungs, spleen, liver (hepatosplenic abscesses) | CASP4 mutation (R344W), no diabetes or other comorbidities | Acute presentation with renal dysfunction requiring ECMO support | Persistent B. pseudomallei infection, treated with recombinant IFN-γ leading to successful outcome and discharge. |
| Arya (2021) [13] | United States | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Multiple organ involvement, including lungs, spleen, kidneys (severe sepsis) | Type-2 diabetes, hyperlipidemia, non-alcoholic fatty liver disease, prior SARS-CoV-2 infection, recent travel to Bangladesh | Clinical evidence of acute renal dysfunction | Complex case with delayed diagnosis, multiple organ abscesses, treated with meropenem and TMP-SMX, eventual recovery. |
| Boyle (2024) [14] | Australia | 8 | 2 | 25 | 0/2 (0%) | 0/5 (0%) | 6/8 (75%) | Not specified | Multiple organ systems, including aortic, renal, pulmonary, splenic involvement | Vascular disease, diabetes, chronic kidney disease, chronic lung disease, alcohol use | Not explicitly defined, clinical diagnosis of acute renal dysfunction | Complex cases of mycotic aneurysm due to Burkholderia pseudomallei, delayed diagnosis common, management includes surgical intervention and long-term antibiotics, high morbidity and mortality noted. |
| Chang (2020) [15] | Malaysia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Not specified | No | Splenic abscess, renal dysfunction, sepsis | Pregnancy, no other comorbidities | Mild renal impairment with raised serum creatinine at 129 µmol/L | Case of a young pregnant woman with bacteraemic melioidosis and splenic abscesses, eventual spontaneous abortion. |
| Chanvitan (2019) [16] | Thailand | 27 | 1 | 3.7 | 100% (1/1) | 9/26 (34.6%) | Not specified | Not specified | Sepsis, pneumonia, soft tissue infection, splenic and hepatic abscesses | Diabetes, thalassemia, renal disease in a minority of cases | Increase in serum creatinine ≥ 0.3 mg/dL or 1.5-fold from baseline | Pediatric cohort, significant hepatic and splenic abscesses as diagnostic clues, AKI rare but fatal in one case. |
| Che Rahim (2019) [17] | Malaysia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (dialysis) | Septic shock, respiratory failure, hepatic dysfunction, renal dysfunction | Systemic lupus erythematosus, immunosuppression | Acute renal failure requiring dialysis | Young female patient with SLE, developed severe sepsis and multi-organ failure, successfully recovered with intensive treatment including immunosuppression and antibiotics. |
| Chou (2007) [4] | Taiwan | 30 | 19 | 63.3 | 9/19 (47.4%) | 0% (0/11) | 14/30 (46.7%) | Not specified | Sepsis, pneumonia, respiratory failure, renal failure | Diabetes mellitus, chronic renal disease, excessive alcohol consumption, malignancy, cardiovascular disease | Reduction in estimated creatinine clearance of 50% or need for RRT | Study of bacteremic melioidosis in Taiwan post-typhoon outbreak; high mortality in patients with AKI, often associated with pneumonia and septic shock. |
| Cossaboom (2020) [18] | United States | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (CRRT) | Respiratory failure, sepsis | Type 2 diabetes, unilateral renal agenesis, rural water exposure | Clinical evidence with renal failure | Melioidosis acquired from environmental exposure in Texas; patient recovered with treatment. |
| Fairhead (2020) [19] | Australia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Pneumonia, discitis, osteomyelitis | Rheumatoid arthritis, chronic lung disease, immunosuppression with etanercept | Elevated creatinine on admission | Polymicrobial bacteremia (melioidosis and Acinetobacter), improved with antibiotics. |
| Ganesan (2019) [20] | India | 7 | 6 | 85.7 | 50% (3/6) | 0% (0/1) | Not specified | Not specified | Renal dysfunction, hepatic dysfunction, sepsis, metabolic derangements | Diabetes, alcoholism | Elevated renal parameters and clinical evidence | Case series of melioidosis with high mortality among AKI cases; slow microbiological clearance and persisting radiological abnormalities were noted. |
| Gouse (2017) [21] | India | 24 | 2 | 8.3 | 50% (1/2) | 0% (0/22) | Not specified | Not specified | Musculoskeletal involvement, septic arthritis, osteomyelitis, intramuscular abscesses | Diabetes, thalassemia, sickle cell anemia, chronic renal disease | Clinical evidence with acute renal failure | Largest musculoskeletal melioidosis series from India, surgical management resulted in good outcomes. |
| Gulati (2022) [22] | United States (Vietnam origin) | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | No (died before) | Sepsis, multi-organ dysfunction, ARDS | Diabetes, HIV infection, history of travel to Vietnam | Acute renal failure with sepsis | First reported case of latent melioidosis activation by COVID-19; rapid deterioration despite treatment. |
| Gunasena (2023) [23] | Sri Lanka | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (intermittent hemodialysis) | Pulmonary hemorrhage, sepsis, jaundice | Farmer, environmental exposure | Acute renal dysfunction, oliguric AKI | Co-infection with leptospirosis and melioidosis, recovered with antibiotics, dialysis, and plasma exchange. |
| Gupta (2021) [24] | India | 11 | 3 | 27.3 | 33.3% (1/3) | 0% (0/8) | Not specified | Not specified | Osteoarticular melioidosis with systemic involvement | Diabetes, trauma, immunosuppression | Clinical evidence of acute renal dysfunction | Combination of osteomyelitis and arthritis, some patients had pulmonary involvement, treated with meropenem/ceftazidime and cotrimoxazole. |
| Hin (2012) [25] | Malaysia | 4 | 1 | 25 | 100% (1/1) | 66.7% (2/3) | Yes | Yes (hemodialysis) | Sepsis, multi-organ failure, pneumonia, hepatic dysfunction | Diabetes, leptospirosis co-infection, rescue operation exposure | Acute renal failure with sepsis | Cluster of cases among rescuers exposed to contaminated water, co-infection with leptospirosis confirmed by PCR. |
| Jagtap (2017) [26] | India | 9 | 1 | 11.1 | 100% (1/1) | 14.3% (1/7) | Not specified | Not specified | Liver abscess, splenic abscess, pancreatic abscess, empyema, SBP | Diabetes, alcoholism | Acute-on-chronic liver failure with renal dysfunction | GI manifestations of melioidosis, unusual presentation including pancreatitis and SBP, high mortality with severe liver disease. |
| Jang (2015) [27] | Korea | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (due to worsening renal function) | Mycotic aneurysm with multi-organ embolism | Travel to Thailand, IgA nephropathy, gout, hypertension | Acute renal dysfunction during hospital course | Identified B. pseudomallei by 16S rRNA sequencing, surgical intervention with aortic repair, good recovery. |
| Lim (2022) [28] | Malaysia | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | Yes (hemodialysis) | Multi-organ failure, sepsis, ARDS, hepatic dysfunction | Uncontrolled diabetes, co-infection with leptospirosis, panhypopituitarism | Acute renal failure with sepsis and shock | Late diagnosis due to non-specific presentation, death from multi-organ failure despite aggressive treatment. |
| Liu (2014) [29] | Singapore | 74 | 9 | 12.2 | 15.9% (Approx.) | 3.3% (Approx.) | Not specified | Not specified | Sepsis, hypotension, respiratory distress, renal impairment | Type II diabetes, sulfonylurea treatment | Renal impairment with need for RRT | Sulfonylurea usage linked to severe septic complications and immune suppression. |
| Loh (2017) [30] | Australia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (dialysis) | Sepsis, respiratory failure, cerebral abscess, temporal lobe involvement | Pig hunting, exposure to soil and environmental pathogens | Acute renal failure with raised creatinine (241 µmol/L) | Complex case with delayed diagnosis, eventually recovered with prolonged antibiotics. |
| Meraj (2019) [31] | United States (Filipino origin) | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (meropenem failure) | Persistent bacteremia sepsis | Travel to endemic areas, ceftazidime-resistant strain | Persistent bacteremia with renal involvement | Prolonged meropenem treatment required, eventual recovery. |
| Morelli (2015) [32] | Netherlands (Gambia travel) | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (eventual hemodialysis) | Sepsis, prostatic abscess, ESRD | Travel to Gambia, environmental exposure | Acute renal failure progressing to ESRD | Prostatic abscess, recovery with ceftazidime, but renal failure persisted. |
| Prabhu (2021) [5] | India | 164 | 59 | 35.98 | 32.2% | 5.7% | 37.3% (AKI), 13.3% (non-AKI) | 8/59 (13.6%) | Sepsis, bacteremia, shock, multi-organ dysfunction | CKD, bacteremia, shock | AKIN criteria | AKI associated with higher mortality and ICU care; survivors showed kidney recovery. |
| Stewart (2021) [33] | United States (Arizona) | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Sepsis, pneumonia, multiple abscesses | Unknown environmental exposure | Not directly defined, clinical evidence | First autochthonous case in the U.S., delayed diagnosis, recovery with antibiotics. |
| Tamtami (2017) [34] | Oman | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | Yes | Severe sepsis, ARDS, multi-organ failure | Occupational exposure in Laos/Cambodia, diabetes mellitus | Acute renal failure with multi-organ dysfunction | Imported case of melioidosis with B. pseudomallei isolated from blood; rapid deterioration despite intensive therapy. |
| Tran (2022) [35] | Vietnam | 3 | 2 | 66.7 | 100% (2/2) | 100% (1/1) | Yes (for all) | Not specified | Liver dysfunction, renal dysfunction, sepsis | Contaminated borehole water (B. pseudomallei ST541) | Elevated creatinine, clinical evidence | Cluster of 3 children from 1 family; B. pseudomallei traced to household borehole water; severe outcomes with liver and kidney involvement. |
| Wadwekar (2018) [36] | India | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Sepsis, multiple abscesses, splenic rupture | Diabetes mellitus, soil exposure | Acute renal dysfunction with sepsis | Case report of melioidosis presenting with sepsis and splenic rupture, survived after aggressive treatment. |
| Warapitiya (2021) [37] | Sri Lanka | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | Yes (dialysis) | Severe sepsis; multi-organ failure, including renal and hepatic failure | Cut injury in paddy field, environmental exposure | Acute renal failure requiring dialysis | Severe sepsis with melioidosis, leading to multi-organ failure and death despite ICU support and broad-spectrum antibiotics. |
AKI, acute kidney injury; RRT, renal replacement therapy; CKD, chronic kidney disease; ST, sequence type; ICU, intensive care unit.
Risk factors associated with AKI in melioidosis included diabetes mellitus, chronic kidney disease, sepsis, and environmental exposures such as travel to endemic areas or contaminated water sources. AKI definitions varied, with some studies applying standard clinical criteria, such as serum creatinine elevation [15,16] or AKIN/KDIGO guidelines [23,25], while others relied on clinical judgment or did not provide explicit definitions.
Overall, the included studies demonstrate the heterogeneity of AKI presentation in melioidosis and its significant association with increased morbidity and mortality, particularly in cases requiring ICU care or RRT (see Table 1 for detailed study characteristics).
3.3. Pooled Odd and Heterogeneity
Data extraction was completed for mortality outcomes, but ICU admission and RRT data were insufficient for pooled analysis. The pooled odds of death in melioidosis patients with AKI are 23 times higher than in patients without AKI (OR = 23.37, 95% CI: 13.97–39.10, p = 0.0082), as shown in Figure 2 with the forest plot. This result is statistically significant, and the confidence interval does not cross 1, confirming the strength of the association. I2 for mortality was 0%, indicating low heterogeneity across included studies. In addition, Tau2 = 0 also indicates low between-study variance. However, only two studies contributed usable data: Prabhu (2021) [5] dominated 89% of the weight, while Chou (2007) [4] contributed ~11%, with a very wide CI.
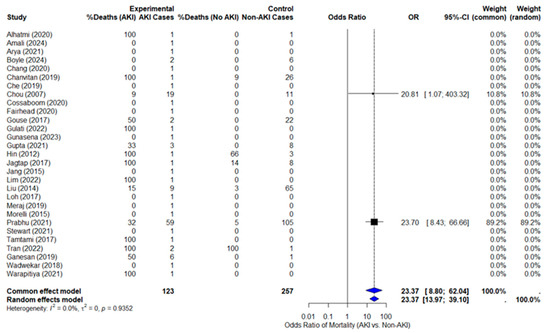
Figure 2.
The forest plot.
To further address concerns regarding studies with zero events in both treatment and control arms, a Bayesian re-analysis was conducted. The results are presented in Supplementary Figure S3. The estimates indicate that the posterior mean for the intercept is 0.5, with no variability (SD = 0.0), suggesting that the RD is consistent across the two studies. The sigma value, representing the variability in RD, is 0.0, further supporting the absence of variability between the studies. The mean posterior predictive distribution (mean_PPD) is also 0.5, reflecting the predicted RD from the posterior distribution. The MCMC diagnostics show convergence with Rhat values of 1.0, and the effective sample sizes for the parameters are satisfactory, ranging from 677 for the intercept to 1173 for the mean_PPD. These findings suggest that the Risk Difference (RD) is consistent across the studies and can be used for decision-making in research. However, the absence of variability (sigma = 0.0) indicates that the data from the two studies by Chou (2007) [4] and Prabhu (2021) [5] are highly consistent.
3.4. Sensitivity Analysis
To assess the influence of individual studies on the overall estimate, a sensitivity analysis was performed by sequentially excluding individual studies to test the robustness of the primary outcome. Initially, we excluded the study by Prabhu et al. (2021) [5], which contributed 89.2% of the weight in the primary meta-analysis. When only the study by Chou et al. (2007) [4] was included, the odds ratio remained elevated (OR = 20.81; 95% CI: 1.07–403.32). The forest plot for this analysis is shown in Supplementary Figure S1. Further sensitivity analyses were conducted by excluding the study by Chou et al. (2007) [4]. The odds ratio (OR = 23.0; 95% CI: 8.43–66.66) remained similar to the pooled OR, as shown in Supplementary Figure S2. While the estimate remained consistent, the wide confidence interval and reliance on a single study limit the interpretability of this result. Together, these analyses suggest that the primary outcome remains relatively robust, but the uncertainty in the estimates, particularly when based on a small number of studies, warrants caution in interpreting the findings.
3.5. Qualities of Included Studies
The methodological quality of all 29 included studies was evaluated using JBI critical appraisal tools appropriate to each study design: case reports (n = 19), case series (n = 8), and cohort studies (n = 2). Most studies were rated as having a low-to-moderate risk of bias. Among case reports, 68% (n = 13) scored ≥ 7/8, with clear reporting of clinical presentation, diagnosis, and outcomes. Some lacked detail on adverse events or follow-up. Of the case series, 63% (n = 5) met at least 80% of quality criteria, though several had unclear inclusion methods or incomplete reporting of baseline data. Both cohort studies were of relatively high quality. Prabhu (2021) [5] scored 10/11, with well-defined AKI criteria and adjustment for confounders, while Chou (2007) [4] scored 9/11 but lacked detailed control of confounding. Full quality ratings and comments are presented in Supplementary Table S3.
4. Discussion
This systematic review and meta-analysis provide a comprehensive synthesis of current evidence regarding the impact of AKI on outcomes in patients with melioidosis. Across 29 studies involving a total of 380 patients, AKI was identified in approximately one-third of cases (32.4%). Importantly, the presence of AKI was associated with a significantly increased risk of mortality, with a pooled odds ratio of 23.37 (95% CI: 13.97–39.10). This finding emphasizes the severity of AKI as a complication of melioidosis and highlights its prognostic importance.
Our findings align with the broader literature on sepsis-associated AKI, which consistently demonstrates that AKI independently predicts worse outcomes, including higher mortality, prolonged ICU stays, and greater dependence on organ support [7]. In melioidosis, AKI may result from several pathophysiological mechanisms, such as direct bacterial invasion, septic shock, systemic inflammation, nephrotoxic medications, and pre-existing renal conditions. These mechanisms resemble those observed in other tropical infectious diseases, like leptospirosis and dengue, where AKI is caused by a combination of hemodynamic instability, tubular toxicity, and immune-mediated injury [5,38,39,40,41]. Some studies in our analysis reported cases requiring renal replacement therapy, particularly in patients with multi-organ failure, underscoring the critical nature of AKI in this population.
Despite consistent evidence of increased mortality, there was substantial heterogeneity in study design, AKI definitions, and outcome reporting. Definitions of AKI varied considerably—only a minority of studies applied standardized criteria like AKIN [5], while others relied on clinical judgment or did not specify criteria at all. This variability likely introduced misclassification bias and limited the comparability of findings across studies. Additionally, data on ICU admission and RRT use were inconsistently reported, precluding pooled analysis of these secondary outcomes. Nonetheless, the low statistical heterogeneity observed in our mortality analysis (I2 = 0%) suggests a robust association across diverse study settings. Subgroup sensitivity analyses by region, AKI severity, or comorbidities were not possible due to insufficient or inconsistent data reporting. Future studies should focus on standardizing reporting practices to enable more detailed and accurate analyses of AKI in melioidosis.
This study has several limitations. Firstly, the majority of included studies were case reports or small observational studies, which are prone to bias and lack generalizability. Secondly, AKI definitions varied significantly across studies, with few adhering to standardized criteria such as AKIN or KDIGO, potentially leading to misclassification. Thirdly, most studies lacked data on long-term renal outcomes, recovery from AKI, and the timing of renal replacement therapy initiation. Additionally, only two studies provided suitable data for meta-analysis, with one (Prabhu et al., 2021) contributing approximately 89% of the statistical weight [5]. This heavy reliance on a single study limits generalizability and may introduce bias, suggesting that the findings should be interpreted with caution. Fourth, the exclusion of non-English articles may have led to language bias, omitting potentially relevant regional data. We acknowledge this limitation and suggest that future reviews consider multilingual inclusion or translation support to minimize bias. Finally, long-term mortality data (e.g., 60-day or 90-day mortality) were not available, so our analysis focused on in-hospital and 30-day mortality, potentially underestimating the true mortality burden associated with AKI.
While the overall quality of the included studies was assessed as low to moderate using JBI criteria, many studies lacked sufficient detail on adverse events or follow-up, limiting the ability to draw strong conclusions. Inconsistent reporting of clinical risk factors, such as diabetes, chronic kidney disease, and sepsis, further complicates the interpretation of results. The absence of multivariable analysis prevented the adjustment for potential confounders, suggesting that the observed association between AKI and mortality should be interpreted with caution due to unmeasured or uncontrolled biases.
Our findings align with established research on sepsis-associated AKI, which identifies renal impairment as a key predictor of mortality. The current KDIGO guidelines emphasize early identification, fluid resuscitation, and renal function monitoring in critically ill patients [6,42], which may be particularly relevant in melioidosis-endemic regions. For clinicians, early detection of AKI in melioidosis patients, especially those with sepsis or multi-organ dysfunction, is crucial. Routine renal function monitoring; cautious use of nephrotoxic agents; and timely initiation of supportive therapies, including renal replacement therapy, when necessary, should be prioritized. Given the high mortality risk linked to AKI, patients should be considered for early ICU referral and multidisciplinary care.
This review also highlights critical gaps in the current literature. Prospective studies using standardized definitions and outcome measures for AKI in melioidosis are urgently needed. There is also a limited understanding of long-term renal outcomes and recovery in survivors of melioidosis-associated AKI. Additionally, variations in healthcare infrastructure between endemic and non-endemic regions may significantly influence management, and outcomes were not adequately addressed in the studies reviewed.
5. Conclusions
Acute kidney injury is a prevalent and severe complication in melioidosis, significantly increasing mortality risk. Our findings highlight the critical need for early detection, prompt intervention, and vigilant monitoring of renal function in melioidosis patients, especially those with sepsis or pre-existing risk factors for renal impairment. Future research should focus on standardizing AKI definitions in melioidosis, enhancing the quality of prospective data, and investigating interventions to reduce kidney injury and improve survival in this high-risk group.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/life15071108/s1, Figure S1: The forest plot that excludes Prabhu (2021) [5] study; Figure S2: The forest plot that excludes Chou (2007) [4] study; Figure S3: A Bayesian model analysis of Risk Difference (RD) calculated using data from two studies (Chou 2007 [4] and Prabhu 2021 [5]); Table S1: Search strategies; Table S2: Eighteen studies were excluded from the final analysis; Supplementary Table S3: Quality assessment of included studies, using JBI checklists. References [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] are cited in the supplementary materials.
Author Contributions
Conceptualization, W.K.K. and A.P.; methodology, W.K.K. and A.P.; software, W.K.K.; validation, W.K.K. and A.P.; formal analysis, W.K.K., M.C., S.-n.L., P.W., P.P., J.T. (Jitabanjong Tangpong), J.T. (Jongkonnee Thanasai), S.K., C.C. and A.P.; investigation, W.K.K., M.C., S.-n.L., P.W., P.P., J.T. (Jitabanjong Tangpong), J.T. (Jongkonnee Thanasai), S.K., C.C. and A.P.; resources, W.K.K. and A.P.; data curation, W.K.K., M.C. and A.P.; writing—original draft preparation, W.K.K. and A.P.; writing—review and editing, W.K.K. and A.P.; visualization, W.K.K. and A.P.; supervision, W.K.K. and A.P.; project administration, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is a systematic review and meta-analysis based solely on previously published data. As it did not involve the enrollment of human participants or the use of animal subjects, ethical approval was not required. The Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University, waived the requirement for ethical review, as this study met the criteria for exemption (Approval No. REC. 68-232-14-1; approval date: 6 June 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AKI | acute kidney injury |
| AKIN | Acute Kidney Injury Network |
| ARDS | Acute Respiratory Distress Syndrome |
| CKD | chronic kidney disease |
| ECMO | Extracorporeal Membrane Oxygenation |
| ICU | intensive care unit |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| MeSH | Medical Subject Heading |
| OR | odds ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SLE | Systemic Lupus Erythematosus |
References
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2018, 378, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Ward, L.; Cheng, A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl. Trop. Dis. 2010, 4, e900. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.W.; Chung, K.M.; Chen, C.H.; Cheung, B.M.H. Bacteremic melioidosis in southern Taiwan: Clinical characteristics and outcome. J. Formos. Med. Assoc. 2007, 106, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.A.; Shaw, T.; Rao, I.R.; Kalwaje Eshwara, V.; Nagaraju, S.P.; Shenoy, S.V.; Mukhopadhyay, C. Acute kidney injury and its outcomes in melioidosis. J. Nephrol. 2021, 34, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105 (Suppl. S4), S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Priyanka, P.; Wang, S.; Smith, A.; Singbartl, K.; Palevsky, P.M.; Chawla, L.S.; Yealy, D.M.; Angus, D.C.; Kellum, J.A. ProCESS and ProGReSS-AKI investigators. Sepsis-associated acute kidney disease. Kidney Int. Rep. 2020, 5, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An up-dated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Heath, A.; Harhay, M.O. Bayesian statistics for clinical research. Lancet 2024, 404, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Casey, J.D.; Shankar-Hari, M.; Harrell, F.E., Jr.; Harhay, M.O. Using bayesian methods to augment the interpretation of critical care trials. An overview of theory and example reanalysis of the alveolar recruitment for acute respiratory distress syndrome trial. Am. J. Respir. Crit. Care Med. 2021, 203, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Alhatmi, H.; Alharbi, A.; Bosaeed, M.; Aldosary, O.; Aljohani, S.; Alalwan, B.; Alsaeedi, A.; Almahmoud, S.; Alothman, A. Melioidosis: Case reports of confirmed Burkholderia pseudomallei in Saudi Arabia. J. Infect. Public Health 2020, 13, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Amali, A.A.; Ravikumar, S.; Chew, W.L.; Tan, Z.; Sam, Q.H.; Chen, K.W.; Boucher, D.; MacLaren, G.; Chai, L.Y.A. Extracorporeal membrane oxygenation-dependent fulminant melioidosis from caspase 4 mutation reversed by interferon gamma therapy. Clin. Infect. Dis. 2024, 78, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Shaikh, H.; Weber, D.; Pettengill, M.; Moss, S. Fever in a returning traveler: A case and literature review of melioidosis. IDCases 2021, 26, e01340. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.; Withey, G.; Smith, S.; Hanson, J. Mycotic aneurysms due to Burkholderia pseudomallei in Far North Queensland, tropical Australia: A case series and review of the literature. Acta Trop. 2024, 260, 107480. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Lau, N.L.J.; Currie, B.J.; Podin, Y. Disseminated melioidosis in early pregnancy—An unproven cause of foetal loss. BMC Infect. Dis. 2020, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Chanvitan, S.; Geater, A.; Laoprasopwattana, K. Hepatic/splenic abscess and/or skin and soft tissue infection as predictors of melioidosis in children. J. Infect. Dev. Ctries. 2019, 13, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Che Rahim, M.J.; Mohammad, N.; Kamaruddin, M.I.; Wan Ghazali, W.S. Systemic lupus erythematosus and melioidosis. BMJ Case Rep. 2019, 12, e229974. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Marinova-Petkova, A.; Strysko, J.; Rodriguez, G.; Maness, T.; Ocampo, J.; Gee, J.E.; Elrod, M.G.; Gulvik, C.A.; Liu, L.; et al. Melioidosis in a resident of texas with no recent travel history, united states. Emerg. Infect. Dis. 2020, 26, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Fairhead, L.; Vardanega, J.; Pandey, R.; Smith, S. Polymicrobial community-acquired Acinetobacter baumannii and Burkholderia pseudomallei bacteremia: Opportunistic infections with similar risk factors in northern Australia. IDCases 2020, 21, e00833. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Sundaramoorthy, R.; Subramanian, S. Melioidosis-Series of seven cases from Madurai, Tamil Nadu, India. Indian J. Crit. Care Med. 2019, 23, 149–151. [Google Scholar] [PubMed]
- Gouse, M.; Jayasankar, V.; Patole, S.; Veeraraghavan, B.; Nithyananth, M. Clinical outcomes in musculoskeletal involvement of Burkholderia pseudomallei infection. Clin. Orthop. Surg. 2017, 9, 386–391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gulati, U.; Nanduri, A.C.; Juneja, P.; Kaufman, D.; Elrod, M.G.; Kolton, C.B.; Gee, J.E.; Garafalo, K.; Blaney, D.D. Case report: A fatal case of latent melioidosis activated by COVID-19. Am. J. Trop. Med. Hyg. 2022, 106, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Gunasena, J.B.; De Silva, S.T. Double-trouble: A rare case of co-infection with melioidosis and leptospirosis from Sri Lanka. Trop. Doct. 2023, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Bhat, S.N.; Reddysetti, S.; Kadavigere, R.; Godkhindi, V.M.; Mukhopadhyay, C.; Saravu, K. Osteoarticular melioidosis: A retrospective cohort study of a neglected disease. Infez. Med. 2021, 29, 574–582. [Google Scholar] [PubMed]
- Hin, H.S.; Ramalingam, R.; Chunn, K.Y.; Ahmad, N.; Ab Rahman, J.; Mohamed, M.S. Fatal co-infection--melioidosis and leptospirosis. Am. J. Trop. Med. Hyg. 2012, 87, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, N.; Shah, H.; Kancharla, A.; Tandan, M.; Pal, P.; Lakhtakia, S.; Ramchandani, M.; Reddy, D.N. Gastrointestinal manifestations of melioidosis: A single center experience. Indian J. Gastroenterol. 2017, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Lee, C.W.; Ok, S.J.; Kim, M.J.; Bae, M.J.; Song, S.; Yi, J.; Kim, K.H. Melioidosis presenting as a mycotic aneurysm in a Korean patient, diagnosed by 16S rRNA sequencing and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Int. J. Infect. Dis. 2015, 38, 62–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, K.; Shukeri, W.; Mazlan, M.; Rafiqi, M.; Abidin, H. Fulminant septic shock from melioidosis and leptospirosis co-infections. Anaesth. Pain Intensive Care 2022, 26, 257–259. [Google Scholar] [CrossRef]
- Liu, X.; Foo, G.; Lim, W.P.; Ravikumar, S.; Sim, S.H.; Win, M.S.; Goh, J.G.; Lim, J.H.; Ng, Y.H.; Fisher, D.; et al. Sulphonylurea usage in melioidosis is associated with severe disease and suppressed immune response. PLoS Negl. Trop. Dis. 2014, 8, e2795. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.L.; Latis, S.; Crossland, G.; Patel, H. Disseminated melioidosis in the head and neck. BMJ Case Rep. 2017, 2017, bcr2016218606. [Google Scholar] [CrossRef] [PubMed]
- Meraj, S.; Rodenberg, B.; Thannum, S.; Sheley, J.; Foreman, J. Persistent Burkholderia pseudomallei Bacteremia in A Filipino Immigrant to the United States: A Case Report. Trop. Med. Infect. Dis. 2019, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Smeets, L.; Hobijn, M.; Boom, H. Melioidosis and renal failure in a Dutch man after a trip to Gambia. Neth. J. Med. 2015, 73, 296–298. [Google Scholar] [PubMed]
- Stewart, T.; Engelthaler, D.M.; Blaney, D.D.; Tuanyok, A.; Wangsness, E.; Smith, T.L.; Pearson, T.; Komatsu, K.K.; Keim, P.; Currie, B.J.; et al. Epidemiology and investigation of melioidosis, Southern Arizona. Emerg. Infect. Dis. 2011, 17, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Tamtami, N.A.; Khamis, F.; Al-Jardani, A. Imported Case of Melioidosis in Oman: Case Report. Oman. Med. J. 2017, 32, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.L.; Phan, P.H.; Bui, L.N.H.; Bui, H.T.V.; Hoang, N.T.B.; Tran, D.M.; Trinh, T.T. Child melioidosis deaths caused by Burkholderia pseudomallei-Contaminated borehole water, Vietnam, 2019. Emerg. Infect. Dis. 2022, 28, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Wadwekar, B.; Suresh Ninan, R.; Bhat, S.; Sheela, D.; Ramaya, S.; Kanungo, R. Lid abscess: An unusual presentation of melioidosis. Australas. Med. J. 2018, 11, 322–325. [Google Scholar] [CrossRef]
- Warapitiya, D.S.; Subasinghe, S.; de Silva, R.F.; Piyarisi, D.L.; Jayatilleke, K. Severe sepsis with multiorgan failure due to melioidosis: A lesson to learn. Case Rep. Med. 2021, 2021, 5563214. [Google Scholar] [CrossRef] [PubMed]
- Daher, E.D.F.; de Abreu, K.L.; da Silva Junior, G.B. Leptospirosis-associated acute kidney injury. J. Bras. Nefro. 2010, 32, 400–407. [Google Scholar]
- Osorio-Rodríguez, E.; Rodelo-Barrios, D.; Rebolledo-Maldonado, C.; Polo-Barranco, A.; Patiño-Patiño, J.; Aldana-Roa, M.; Sánchez-Daza, V.; Sierra-Ordoñez, E.; Bettin-Martínez, A. Acute kidney injury associated with severe leptospirosis: Fatal re-emerging disease in Latin America. Kidney Dial. 2024, 4, 78–92. [Google Scholar] [CrossRef]
- Bignardi, P.R.; Pinto, G.R.; Boscarioli, M.L.N.; Lima, R.A.A.; Delfino, V.D.A. Acute kidney injury associated with dengue virus infection: A review. J. Bras. Nefro. 2022, 44, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Burdmann, E.A. Dengue-associated acute kidney injury. Clin. Kidney J. 2015, 8, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Levi, T.M.; de Souza, S.P.; de Magalhães, J.G.; de Carvalho, M.S.; Cunha, A.L.; Dantas, J.G.; Cruz, M.G.; Guimarães, Y.L.; Cruz, C.M. Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev. Bras. Ter. Intensiv. 2013, 25, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.R.; Shaw, T.; Prabhu, R.A.; Eshwara, V.K.; Nagaraju, S.P.; Rangaswamy, D.; Shenoy, S.V.; Bhojaraja, M.V.; Mukhopadhyay, C. Hyponatremia in Melioidosis: Analysis of 10-year Data from a Hospital-Based Registry. J. Glob. Infect. Dis. 2022, 14, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.W.J. The Great Mimicker or the Great Masquerader? Am. J. Med. 2022, 135, e27–e30. [Google Scholar] [CrossRef] [PubMed]
- Luvira, U.; Sukahatya, M.; Alano, F.A.; Danguilan, R.A.; Thang, N.T.; Lin, C.H.; Lee, G.; Morad, Z.; Thirakhupt, P. Clinical features of renal diseases in South-East Asia. Nephrology 1998, 4, S9–S11. [Google Scholar] [CrossRef]
- Mackintosh, D.; Mantha, M.; Oliver, K. Goodpasture disease as a consequence of melioidosis. Intern. Med. J. 2016, 46, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.E.; Bramwell, J.; Gadil, E.; Woerle, C.; Ewin, T.; Davies, J.; Janson, S.; Currie, B.J. Adverse reactions to trimethoprim/sulfamethoxazole for melioidosis eradication therapy: An evaluation of frequency and risk factors. Int. J. Infect. Dis. 2025, 150, 107283. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Lee, K.H.; Tambyah, P.A. Bacteraemic melioidosis pneumonia: Impact on outcome, clinical and radiological features. J. Infect. 2004, 48, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Nandurkar, D.; Lau, K. Melioidosis as a cause of multifocal osteomyelitis. Clin. Nucl. Med. 2006, 31, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Laklaeng, S.N.; Songsri, J.; Wisessombat, S.; Mala, W.; Phothaworn, P.; Senghoi, W.; Nuinoon, M.; Tangphatsornruang, S.; Wongtawan, T.; Hayakijkosol, O.; et al. Multi-locus sequence typing and genetic diversity of antibiotic-resistant genes and virulence-associated genes in Burkholderia pseudomallei: Insights from whole genome sequencing of animal and environmental isolates in Thailand. Vet. Microbiol. 2024, 298, 110236. [Google Scholar] [CrossRef] [PubMed]
- Patamatamkul, S.; Klungboonkrong, V.; Praisarnti, P.; Jirakiat, K. A case-control study of community-acquired Acinetobacter baumannii pneumonia and melioidosis pneumonia in northeast Thailand: An emerging fatal disease with unique clinical features. Diagn. Microbiol. Infect. Dis. 2017, 87, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.; Currie, B.J. Melioidosis and the kidney. Nephrology 2013, 18, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Currie, B.J. Approach to melioidosis. CMI Commun. 2024, 1, 100008. [Google Scholar] [CrossRef]
- Norman, F.F.; Blair, B.M.; Chamorro-Tojeiro, S.; González-Sanz, M.; Chen, L.H. The Evolving Global Epidemiology of Human Melioidosis: A Narrative Review. Pathogens 2024, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S. Melioidotic septic arthritis: A case report and literature review. J. Microbiol. Immunol. Infect. 2007, 40, 178–182. [Google Scholar] [PubMed]
- Keragala, K.A.R.K.; Gunathilaka, M.G.R.S.S.; Senevirathna, R.M.I.S.K.; Jayaweera, J.A.A.S. Efficacy and safety of co-trimoxazole in eradication phase of melioidosis; systematic review. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Aravan, L.; Mohamad, N.I. Pos-165 Acute kidney injury and its outcome among melioidosis patients in a tertiary hospital of a north-eastern state of Malaysia. Kidney Int. Rep. 2022, 7, S71–S72. [Google Scholar] [CrossRef]
- Grace, S.; Currie, B.; Kumar, S. Parathyroid Hormone Independent Hypercalcaemia Secondary to Granulomatous Inflammation: Could This Be Melioidosis? J. Endocr. Soc. 2022, 6, A172. [Google Scholar] [CrossRef]
- Janelle, P.; Maeve, O.; Robert, H.; Anoushka, K.; Hemant, K.; Rajalingam, S.; Sze, A.W. Atypical anti-gbm disease in association with systemic melioidosis. In Proceedings of the Australian and New Zealand Society of Nephrology (ANZSN) 2023, Christchurch, New Zealand, 2–6 September 2023. [Google Scholar]
- Tan, Y.L.; Ming, L.J.; Wei, K.W. Burkholderia pseudomallei (melioidosis) peritoneal dialysis peritonitis. In Proceedings of the World Congress of Nephrology WCN′22. WCN′22, Kuala Lumpur, Malaysia, 24–27 February 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).