Oculoplastic Interventions in the Management of Ocular Surface Diseases: A Comprehensive Review
Abstract
1. Introduction
2. Methods
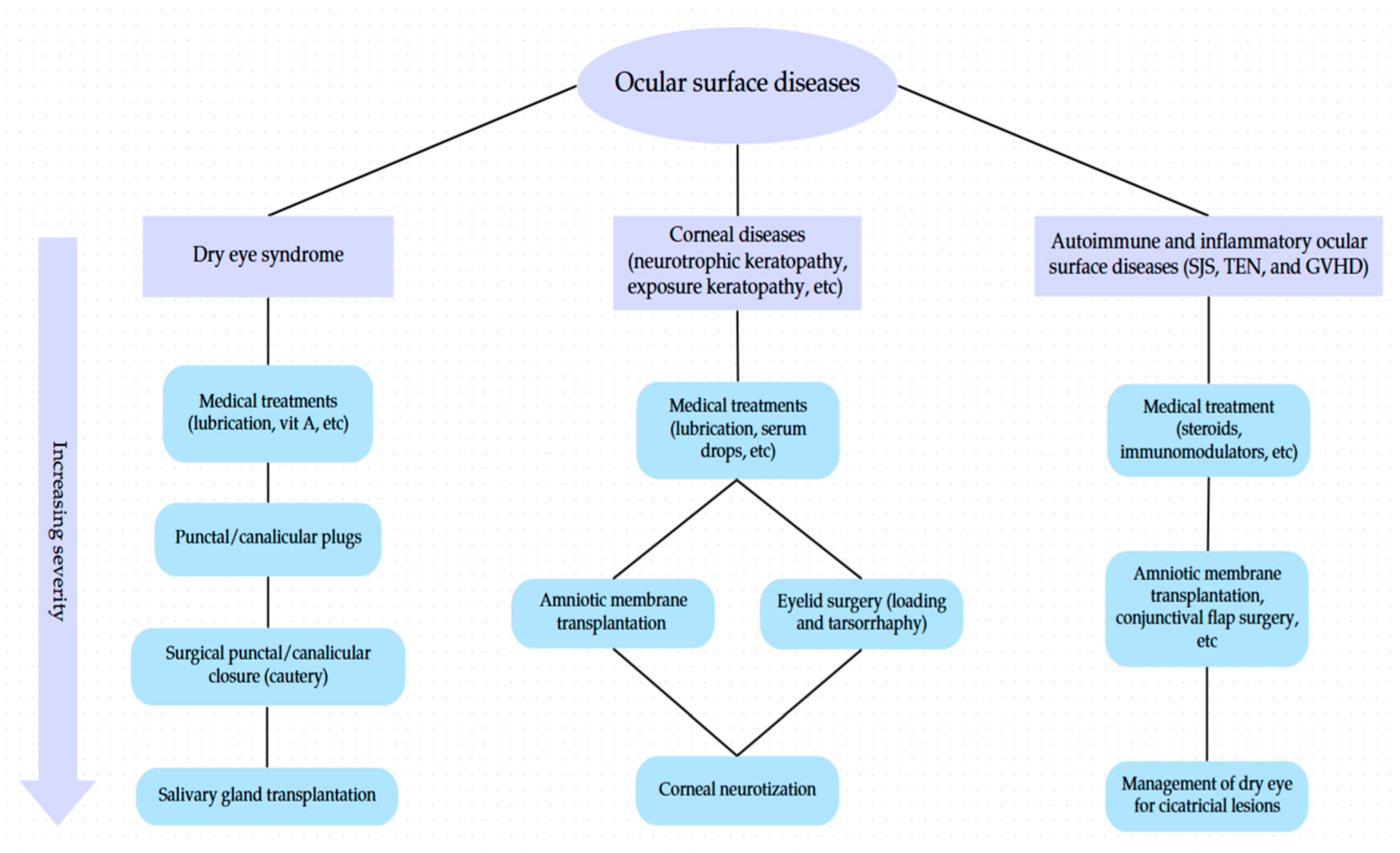
3. Oculoplastic Interventions
3.1. Punctal Occlusion
3.2. Eyelid Fissure Narrowing Techniques
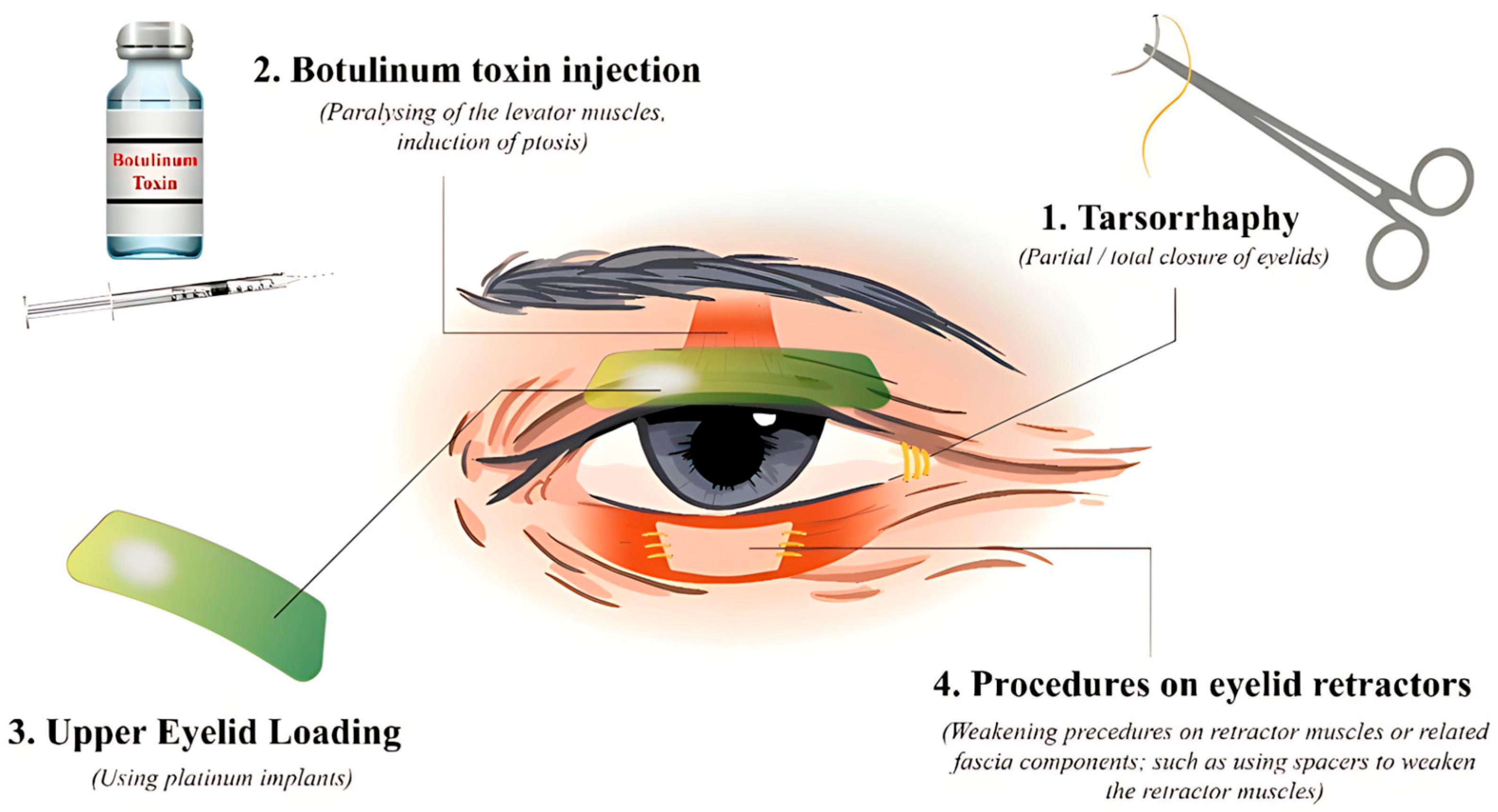
3.2.1. Tarsorrhaphy
3.2.2. Botulinum Toxin Injection
3.2.3. Upper Eyelid Loading
3.2.4. Upper Eyelid Retractor Weakening
3.2.5. Lower Eyelid Retractor Weakening
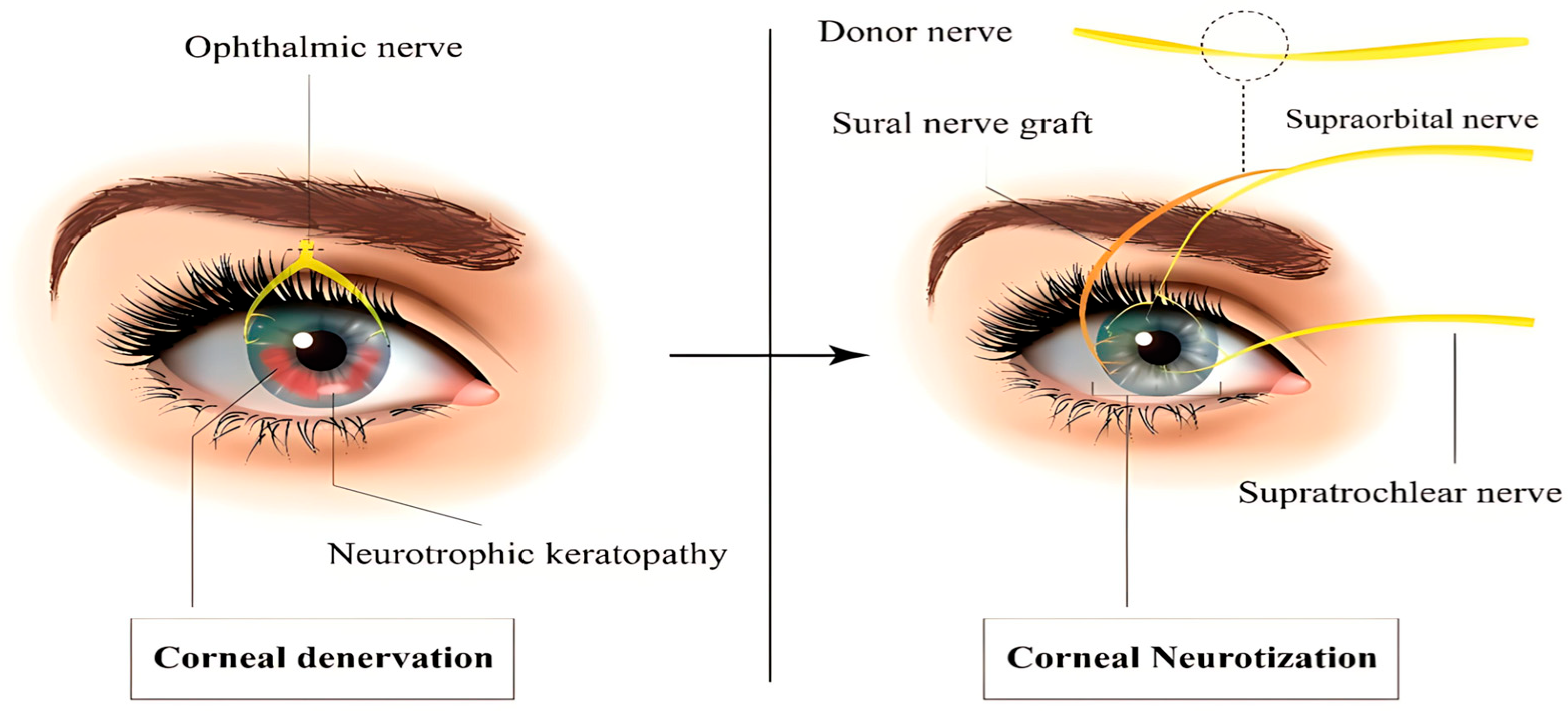
3.3. Corneal Neurotization
3.4. Amniotic Membrane Transplantation
3.5. Conjunctival Flap Surgery
3.6. Salivary Gland Transplantation
3.7. Future Direction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mathenge, W. Emergency management: Exposure keratopathy. Community Eye Health 2018, 31, 69. [Google Scholar] [PubMed]
- Kalogeropoulos, C.D.; Bassukas, I.D.; Moschos, M.M.; Tabbara, K.F. Eye and Periocular Skin Involvement in Herpes Zoster Infection. Med. Hypothesis Discov. Innov. Ophthalmol. 2015, 4, 142–156. [Google Scholar] [PubMed]
- Lee, S.; Lew, H. Ophthalmologic Clinical Features of Facial Nerve Palsy Patients. Korean J. Ophthalmol. 2019, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Tomić, M.; Salopek-Rabatić, J.; Novak, B. Diagnostic procedures and management of dry eye. Biomed. Res. Int. 2013, 2013, 309723. [Google Scholar] [CrossRef] [PubMed]
- Wolkow, N.; Chodosh, J.; Freitag, S.K. Innovations in Treatment of Lagophthalmos and Exposure Keratopathy. Int. Ophthalmol. Clin. 2017, 57, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Scanzera, A.C.; Ahmad, A.; Shorter, E.; Mittal, R.; Patel, S.; Galor, A. Adjunct Use of Therapeutic Scleral Lens for Exposure Keratopathy after Severe Chemical BurnAlternative therapies for dry eye disease. Case Rep. Ophthalmol. 2021, 12, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yoonesi, S.; Abedi Azar, R.; Arab Bafrani, M.; Yaghmayee, S.; Shahavand, H.; Mirmazloumi, M.; Limoudehi, M.M.; Rahmani, M.; Hasany, S.; Idjadi, F.Z.; et al. Facial expression deep learning algorithms in the detection of neurological disorders: A systematic review and meta-analysis. Biomed. Eng. Online 2025, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Momenaei, B.; Baradaran-Rafii, A.; Cheraqpour, K.; An, S.; Ashraf, M.J.; Abedi, F.M.D.; Javadi, M.A.M.D.; Djalilian, A.R.M.D. Mustard gas–induced ocular surface disorders: An update on the pathogenesis, clinical manifestations, and management. Cornea 2023, 42, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Tabatabaei, S.A.; Mahmoudzadeh, R. Use of autologous serum tears for the treatment of ocular surface disease from patients with systemic autoimmune diseases. Am. J. Ophthalmol. 2019, 199, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Raghu Raj Singh, T. Punctal plug: A medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov. Today 2015, 20, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Best, A.L.; Labetoulle, M.; Legrand, M.; M’Garrech, M.; Barreau, E.; Rousseau, A. Punctal and canalicular plugs: Indications, efficacy and safety. J. Fr. Ophtalmol. 2019, 42, e95–e104. [Google Scholar] [CrossRef] [PubMed]
- Jehangir, N.; Bever, G.; Mahmood, S.M.; Moshirfar, M. Comprehensive Review of the Literature on Existing Punctal Plugs for the Management of Dry Eye Disease. J. Ophthalmol. 2016, 2016, 9312340. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Behjati Najafabadi, O.; Atighehchian, M.; Razavi, A.; Abedinifar, Z.; Tabatabaei, S.A.; Asadigandomani, H. Microbial contamination of therapeutic contact lenses after photorefractive keratectomy: A prospective analysis. J. Ophthalmic Inflamm. Infect. 2025, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.E.; Ahn, H.; Jun, I.; Kim T-i Seo, K.Y. Causes of Punctal plug loss in Sjögren’s syndrome. Yonsei Med. J. 2023, 64, 505. [Google Scholar] [CrossRef] [PubMed]
- Ohba, E.; Dogru, M.; Hosaka, E.; Yamazaki, A.; Asaga, R.; Tatematsu, Y.; Ogawa, Y.; Tsubota, K.; Goto, E. Surgical punctal occlusion with a high heat-energy releasing cautery device for severe dry eye with recurrent punctal plug extrusion. Am. J. Ophthalmol. 2011, 151, 483–487.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Carreno-Galeano, J.T.; Singh, R.B.; Dana, R.; Yin, J. Long-term Outcomes of Punctal Cauterization in the Management of Ocular Surface Diseases. Cornea 2021, 40, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kruoch, Z.; Ting, D.S.J.; McCann, P.; Kemp, A.; Gonzales, M.; Kuo, I.C. Punctal occlusion for the dry eye. Three-year revision. Am. Acad. Ophthalmol. Surg. Interv. Neurotrophic keratopathy. Ophthalmol. 1997, 104, 1521–1524. [Google Scholar]
- Geerling, G.; Tost, F.H.W. Surgical occlusion of the lacrimal drainage system. Dev Ophthalmol. 2008, 41, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, S.; Ogawa, Y.; Kamoi, M.; Uchino, M.; Tatematsu, Y.; Ban, Y.; Ohba, E.; Okamoto, S.; Goto, E.; Tsubotal, K. Surgical management of lacrimal punctal cauterization in chronic GVHD-related dry eye with recurrent punctal plug extrusion. Bone Marrow Transplant. 2012, 47, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Rajak, S.; Rajak, J.; Selva, D. Performing a tarsorrhaphy. Community Eye Health 2015, 28, 10–11. [Google Scholar] [PubMed]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-Del-Castillo, J.M.; Hossain, P.N.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C.; et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018, 66, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Cosar, C.B.; Cohen, E.J.; Rapuano, C.J.; Maus, M.; Penne, R.P.; Flanagan, J.C.; Laibson, P.R. Tarsorrhaphy: Clinical experience from a cornea practice. Cornea 2001, 20, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.H.; Riaz, K.M.; Karamichos, D. Treatment of Non-Infectious Corneal Injury: Review of Diagnostic Agents, Therapeutic Medications, and Future Targets. Drugs 2022, 82, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.H.; Bartlett, J.D. Ophthalmic Procedures for Treatment of Advanced Ocular Surface Diseases. Optom. Vis. Sci. 2015, 92, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Thaller, V.T.; Vahdani, K. Tarsal suture tarsorrhaphy: Quick, safe and effective corneal protection. Orbit 2016, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Valim, V.; Trevisani, V.F.; de Sousa, J.M.; Vilela, V.S.; Belfort, R., Jr. Current Approach to Dry Eye Disease. Clin. Rev. Allergy Immunol. 2015, 49, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Arıcı, C.; Tabatabaei, S.A.; Soleimani, M.; Behrouz, M.J.; Torkashvand, A.; Anvari, P.; Yaseri, M. Use of Botulinum Neurotoxin in OphthalmologyA randomized clinical trial to evaluate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Turk. J. Ophthalmol. 2016, 46, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.W.; Fang, P.C.; Chang, C.H.; Liu, Y.P.; Kuo, M.T. A Review of Periocular Botulinum Neurotoxin on the Tear Film Homeostasis and the Ocular Surface Change. Toxins 2019, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, S.; Chen, E.; Kaugesaar, T.; Almqvist, H.; Kjellberg, K.; Lennerstrand, G. Effect of eyelid botulinum toxin injection on lacrimal drainage. Am. J. Ophthalmol. 2000, 129, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, S.; Linderoth, R. Eyelid botulinum toxin injections for the dry eye. Dev Ophthalmol. 2008, 41, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A.H.; Eid, S.A. Botulinum toxin injection and tear production. Curr. Opin. Ophthalmol. 2018, 29, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.G.S.; Pérez, L.A.; Picazo, C.C.; Sanz, L.S.; Ontiveros, A.H.; Gregori, E.E. Acute calcareous corneal degeneration in a patient with chronic graft-versus-host disease. Rom. J. Ophthalmol. 2024, 68, 53. [Google Scholar] [PubMed]
- Portelinha, J.; Passarinho, M.P.; Costa, J.M. Neuro-ophthalmological approach to facial nerve palsy. Saudi J. Ophthalmol. 2015, 29, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Harrisberg, B.P.; Singh, R.P.; Croxson, G.R.; Taylor, R.F.; McCluskey, P.J. Long-term outcome of gold eyelid weights in patients with facial nerve palsy. Otol. Neurotol. 2001, 22, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Amer, T.A.; El-Minawi, H.M.; El-Shazly, M.I. Low-level versus high-level placement of gold plates in the upper eyelid in patients with facial palsy. Clin Ophthalmol. 2011, 5, 891–895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soleimani, M.; Baharnoori, S.M.; Massoumi, H.; Cheraqpour, K.; Asadigandomani, H.; Mirzaei, A.; Ashraf, M.J.; Koganti, R.; Chaudhuri, M.; Ghassemi, M.; et al. A deep dive into radiation keratopathy; going beyond the current frontierss. Exp. Eye Res. 2025, 251, 110234. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C. Controversies in periocular reconstruction for facial nerve palsy. Curr. Opin. Ophthalmol. 2018, 29, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Gospodarowicz, I.; Gospodarowicz, M.; Rękas, M.; Chalfin, J.; Putterman, A.M. Factors influencing medical expenditures in patients with unresolved facial palsy and pharmacoeconomic analysis of upper eyelid lid loading with gold and platinum weights compared to tarsorrhaphyMüller’s muscle excision and levator recession in retracted upper lid. Treatment of thyroid-related retraction. Health Econ. Rev. 2024, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, J.; Chen, L.; Liu, L. Lid loading for treatment of paralytic lagophthalmos. Aesthetic Plast. Surg. 2011, 35, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Kilduff, C.L.S.; Casswell, E.J.; Imonikhe, R.; Marjanovic, B. Type IV Hypersensitivity to Gold Weight Upper-Eyelid Implant: Case Report and Review of the Literature. Ocul. Immunol. Inflamm. 2018, 26, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; James, C.; Sutton-Smith, P.; Dodd, T.; Selva, D. Histological evidence of tissue reaction to platinum eyelid chain. Arch. Ophthalmol. 2011, 129, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Ghodke, A.; Miller, K.M.; Miller, M.Q. Symptomatic and Histological Tissue Reaction to Upper Eyelid Platinum Weight. Ann. Otol. Rhinol. Laryngol. 2023, 132, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.J. Management of the Eye in Facial Paralysis. Facial Plast. Surg. Clin. N. Am. 2016, 24, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Asadigandomani, H.; Rajabi, M.T.; Aghajani, A.; Mousavi, S.A.; Rahmanikhah, E.; Abedinifar, Z.; Afshar, P.; Rafizadeth, S.M. The continuous rise in orbital subperiosteal abscess incidence in the Iranian pediatric population. Sci. Rep. 2024, 14, 23205. [Google Scholar] [CrossRef] [PubMed]
- Irawati, Y.; Natalia, M.E.R.; Gondhowiardjo, T.D.; Dachlan, I.; Soebono, H. Modified tarsorrhaphy versus gold weight implant technique for paralytic lagophthalmos treatment in patients with leprosy: One-year observation of a randomized controlled trial study. Front. Med. 2022, 9, 941082. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Kwong, T.Q.; Malhotra, R. Non-aesthetic indications for periocular hyaluronic acid filler treatment: A review. Br. J. Ophthalmol. 2018, 102, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Grisolia, A.B.D.; Couso, R.C.; Matayoshi, S.; Douglas, R.S.; Briceño, C.A.; Wolkow, N.; Chodosh, J.; Freitag, S.K. Non-surgical treatment for eyelid retraction in thyroid eye disease (TED) Innovations in Treatment of Lagophthalmos and Exposure Keratopathy. Br. J. Ophthalmol. 2017, 57, 85–103. [Google Scholar] [CrossRef]
- Martín-Oviedo, C.; García, I.; Lowy, A.; Scola, E.; Aristegui, M.; Scola, B. Hyaluronic acid gel weight: A nonsurgical option for the management of paralytic lagophthalmos. Laryngoscope 2013, 123, E91–E96. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.; Taban, M.; Lowinger, A.; Nakra, T.; Tsirbas, A.; Douglas, R.S.; Shorr, N.; Goldbetrg, R.A. Use of hyaluronic Acid gel in the management of paralytic lagophthalmos: The hyaluronic Acid gel “gold weight”. Ophthalmic Plast. Reconstr. Surg. 2009, 25, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F.; Asadigandomani, H.; Bafrani, M.A.; Nozarian, Z.; Abedinifar, Z.; Pirmarzdashti, N.; Jafari, B.; Siami, Z. Late infection after peri-orbital autologous micro-fat graft: A case presentation and literature review. Int. J. Ophthalmol. 2024, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Cochran, M.L.; Lopez, M.J.; Czyz, C.N. Anatomy, Head and Neck: Eyelid. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Putterman, A.M. Surgical treatment of thyroid-related upper eyelid retraction. Graded Müller’s muscle excision and levator recession. Ophthalmology 1981, 88, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Asadigandomani, H.; Rajabi, M.T.; Mohsenzadeh Kermani, N.; Nozarian, Z.; Ghaedamini, M.; Rafizadeh, S.M. Muller’s muscle fibrosis is a possible predictive factor in the outcome of Muller’s muscle-conjunctival resection. BMC Ophthalmol. 2025, 25, 284. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Frueh, B.R.; Elner, V.M. Müllerectomy for upper eyelid retraction and lagophthalmos due to facial nerve palsy. Arch. Ophthalmol. 2005, 123, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Ueland, H.O.; Uchermann, A.; Rødahl, E. Levator recession with adjustable sutures for correction of upper eyelid retraction in thyroid eye disease. Acta Ophthalmol. 2014, 92, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Guastella, C.; di Furia, D.; Torretta, S.; Ibba, T.M.; Pignataro, L.; Accorona, R. Upper Eyelid Retraction in Graves’ Ophthalmopathy: Our Surgical Experience on 153 Cases of Full-Thickness Anterior Blepharotomy with Mullerectomy. Aesthetic Plast. Surg. 2022, 46, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, G.J.; Mansury, A.M.; Schwarcz, R.M.; Modjtahedi, S.; McCann, J.D.; Goldberg, R.A. Transconjunctival Müller muscle recession with levator disinsertion for correction of eyelid retraction associated with thyroid-related orbitopathy. Am. J. Ophthalmol. 2005, 140, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I. Recession of the levator muscle for lagophthalmos in exophthalmic goiter. Arch. Ophthalmol. 1934, 11, 389–393. [Google Scholar] [CrossRef]
- Henderson, J.W. A surgical procedure for retraction of eyelids in endocrine exophthalmos (a moving picture). Trans. Am. Ophthalmol. Soc. 1965, 63, 70–74. [Google Scholar]
- Kazim, M.; Gold, K.G. A review of surgical techniques to correct upper eyelid retraction associated with thyroid eye disease. Curr. Opin. Ophthalmol. 2011, 22, 391–393. [Google Scholar] [CrossRef]
- Putterman, A.M.; Urist, M. Surgical treatment of upper eyelid retraction. Arch. Ophthalmol. 1972, 87, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Chalfin, J.; Putterman, A.M. Müller’s muscle excision and levator recession in retracted upper lid. Treatment of thyroid-related retraction. Arch. Ophthalmol. 1979, 97, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Elner, V.M.; Hassan, A.S.; Frueh, B.R. Graded full-thickness anterior blepharotomy for upper eyelid retraction. Trans. Am. Ophthalmol. Soc. 2003, 101, 67–73; discussion 73–75. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.H.; Monteiro, L.G.; Osaki, M.H.; Swanson, M.A.; Swanson, R.D.; Kotha, V.S.; Cai, Y.; Clark, R.; Jin, A.; Kumar, A.R.; et al. Management of eyelid retraction related to thyroid eye diseaseCorneal Neurotization: A Meta-analysis of Outcomes and Patient Selection Factors. Taiwan J. Ophthalmol. 2022, 12, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hintschich, C.; Haritoglou, C. Full thickness eyelid transsection (blepharotomy) for upper eyelid lengthening in lid retraction associated with Graves’ disease. Br. J. Ophthalmol. 2005, 89, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Shams, P.N.; Katori, N.; Kinoshita, S.; Selva, D. Turn-over orbital septal flap and levator recession for upper-eyelid retraction secondary to thyroid eye disease. Eye 2013, 27, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, B.; Ghadimi, H.; Nikdel, M. Levator recession and minimal lateral tarsorrhaphy for the management of lagophthalmos and corneal exposure in facial palsy. Eur. J. Ophthalmol. 2021, 31, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.F.; Garcia, D.M.; Leal, V.; Faria-Correia, F.; Rocha-Sousa, A.; Falcão-Reis, F.; Velasco e Cruz, A.A. Graded müllerectomy for correction of graves upper eyelid retraction: Effect on eyelid movements. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.N.; Walvekar, P.; Vasanthapuram, V.H.; Shankar, L. Eyelid Surgery in Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2023, 39, S92–S104. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lewis, K.; Alghoul, M.S. Comparison of Efficacy and Complications Among Various Spacer Grafts in the Treatment of Lower Eyelid Retraction: A Systematic Review. Aesthetic Surg. J. 2017, 37, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Rafizadeh, S.M.; Mirghorbani, M.; Tavakoli, M.; Haydar, A.A. Surgical Correction of Cicatricial Lower Eyelid Retraction: A Systematic Review. Semin. Ophthalmol. 2024, 39, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Dryer, M.M.; Bodner, B.I. Corneal neurotization: A novel solution to neurotrophic keratopathy. Plast. Reconstr. Surg. 2009, 123, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, K.; Tajdaran, K.; Mirmoeini, K.; Daeschler, S.C.; Henriquez, M.A.; Stevens, K.E.; Mulenga, C.M.; Hussain, A.; Hamrah, P.; Ali, A.; et al. The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal. Int. J. Mol. Sci. 2023, 24, 12615. [Google Scholar] [CrossRef] [PubMed]
- Roumeau, S.; Dutheil, F.; Sapin, V.; Baker, J.S.; Watson, S.L.; Pereira, B.; Chiambaretta, F.; Navel, V. Efficacy of treatments for neurotrophic keratopathy: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Kruoch, Z.; Ting, D.S.J.; McCann, P.; Kemp, A.; Gonzales, M.; Kuo, I.C. Medical and surgical interventions for neurotrophic keratopathy. Cochrane Database Syst Rev. 2023, 2023, CD015723. [Google Scholar]
- Turkoglu, E.; Celik, E.; Alagoz, G. A comparison of the efficacy of autologous serum eye drops with amniotic membrane transplantation in neurotrophic keratitis. Semin. Ophthalmol. 2014, 29, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Najafabadi, S.J.; Razavi, A.; Tabatabaei, S.A.; Mirmoosavi, S.; Asadigandomani, H. Clinical characteristics, predisposing factors, and management of moraxella keratitis in a tertiary eye hospital. J. Ophthalmic Inflamm. Infect. 2024, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Tabatabaei, S.A.; Bahadorifar, S.; Mohammadi, A.; Asadigandomani, H. Unveiling the landscape of post-keratoplasty keratitis: A comprehensive epidemiological analysis in a tertiary center. Int. Ophthalmol. 2024, 44, 230. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Rezaeimanesh, N.; Owji, M.; Arab Bafrani, M.; Mohammadi Lapevandani, M.; Naser Moghadasi, A. Current treatment and management of neuromyelitis optica spectrum disorder: Areas for improvement. Expert. Rev. Ophthalmol. 2025, 1–11. [Google Scholar] [CrossRef]
- Kasbi, N.A.; Jahani, S.; Ezabadi, S.G.; Kohandel, K.; Khodaie, F.; Sahraian, A.H.; Bafrani, M.A.; Almasi-Hashiani, A.; Eskandarieh, S.; Sahraian, M.A. Environmental risk factors of late-onset multiple sclerosis: A population-based case-control study. J. Clin. Neurosci. 2025, 135, 111146. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Khoshdooz, S.; Bafrani, M.A.; Bakhshimoghaddam, F.; Abbasi, H.; Doaei, S. Uncovering the Causal Link Between Obesity-Associated Genes and Multiple Sclerosis: A Systematic Literature Review. Brain Behav. 2025, 15, e70439. [Google Scholar] [CrossRef] [PubMed]
- Bafrani, M.A.; Rios, V.; Kim, M.J.; Balan, A.; Bove, R. Gynecological health: A missing link in comprehensive treatment monitoring for multiple sclerosis. Mult. Scler. 2025, 13524585251346371. [Google Scholar] [CrossRef] [PubMed]
- Dragnea, D.C.; Krolo, I.; Koppen, C.; Faris, C.; Van den Bogerd, B.; Ní Dhubhghaill, S.; Bulut, O.; Palamar, M.; Yaman, B.; Egrilmez, S.; et al. Corneal Neurotization-Indications, Surgical Techniques and OutcomesAmniotic Membrane Transplantation for Reconstruction of Ocular Surface Lesion Excisions in Pediatric Population. J. Clin. Med. 2023, 12, 2214. [Google Scholar] [CrossRef]
- NaPier, E.; Camacho, M.; McDevitt, T.F.; Sweeney, A.R. Neurotrophic keratopathy: Current challenges and future prospects. Ann. Med. 2022, 54, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, J.; Wu, Y.; Wang, W.; Shao, C.; Li, J. Evaluation of Corneal Nerve Regeneration After Minimally Invasive Corneal Neurotization. Asia Pac. J. Ophthalmol. 2023, 12, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, U.; Bains, R.; Zuker, R.M.; Borschel, G.H.; Ali, A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: A new approach to a difficult problem. JAMA Ophthalmol. 2014, 132, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Daeschler, S.C.; Woo, J.H.; Hussein, I.; Ali, A.; Borschel, G.H.; Rafizadeh, S.M.; Mirghorbani, M.; Tavakoli, M.; Haydar, A.A. Corneal Neurotization: Preoperative Patient Workup and Surgical Decision-makingSurgical Correction of Cicatricial Lower Eyelid Retraction: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5334. [Google Scholar] [CrossRef] [PubMed]
- Leyngold, I.; Weller, C.; Leyngold, M.; Espana, E.; Black, K.D.; Hall, K.L.; Tabor, M. Endoscopic Corneal Neurotization: Cadaver Feasibility Study. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.A.; Swanson, R.D.; Kotha, V.S.; Cai, Y.; Clark, R.; Jin, A.; Kumar, A.R.; Davidson, E.H. Corneal Neurotization: A Meta-analysis of Outcomes and Patient Selection Factors. Ann. Plast. Surg. 2022, 88, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Kalia, A.; Jain, A.K.; Gaba, S.; Malhotra, C.; Gupta, A.; Soni, T.; Saini, K.; Gupta, P.C.; Singh, M.; et al. Clinical outcomes of corneal neurotization using sural nerve graft in neurotrophic keratopathy. PLoS ONE 2023, 18, e0294756. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, A.R.; Wang, M.; Weller, C.L.; Burkat, C.; Kossler, A.L.; Lee, B.W.; Yen, M.T. Outcomes of corneal neurotisation using processed nerve allografts: A multicentre case series. Br. J. Ophthalmol. 2022, 106, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Leyngold, I.M.; Yen, M.T.; Tian, J.; Leyngold, M.M.; Vora, G.K.; Weller, C. Minimally invasive corneal neurotization with acellular nerve allograft: Surgical technique and clinical outcomes. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Catapano, J.; Fung, S.S.; Halliday, W.; Jobst, C.; Cheyne, D.; Ho, E.S.; Zuker, R.M.; Borschel, G.H.; Ali, A. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: Long-term clinical outcomes and evidence of corneal reinnervation. Br. J. Ophthalmol. 2019, 103, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Daeschler, S.C.; Mireskandari, K.; Borschel, G.H.; Ali, A. Minimally invasive corneal neurotization provides sensory function, protects against recurrent ulceration, and improves visual acuity. Am. J. Ophthalmol. 2022, 241, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Elalfy, M.; Maqsood, S.; Hau, S.; Kannan, R.Y.; Nduka, C.; Hamada, S.; Malhotra, R. Functional and structural changes following corneal neurotisation in the management of neurotrophic keratopathy: UK single centre series. Clin. Ophthalmol. 2021, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Meller, D.; Pauklin, M.; Thomasen, H.; Westekemper, H.; Steuhl, K.P. Amniotic membrane transplantation in the human eye. Dtsch. Arztebl. Int. 2011, 108, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ganger, A.; Vanathi, M.; Mohanty, S.; Tandon, R. Long-Term Outcomes of Cultivated Limbal Epithelial Transplantation: Evaluation and Comparison of Results in Children and Adults. Biomed. Res. Int. 2015, 2015, 480983. [Google Scholar] [CrossRef] [PubMed]
- Muraine, M.; Gueudry, J.; Toubeau, D.; Gardea, E.; Verspyck, E.; Menguy, E.; Brasseur, G. Advantages of amniotic membrane transplantation in eye surface diseases. J. Francais D Ophtalmol. 2006, 29, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Burman, S.; Tejwani, S.; Mahesh, S.P.; Murthy, R. Amniotic membrane transplantation: A review of current indications in the management of ophthalmic disorders. Indian. J. Ophthalmol. 2007, 55, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Musayeva, G.; Selver, O.B. Impact of adjuvant amniotic membrane transplantation in infectious ulcerative keratitis. Int. Ophthalmol. 2023, 43, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Thatte, S. Amniotic membrane transplantation: An option for ocular surface disorders. Oman J. Ophthalmol. 2011, 4, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, A.; Pop, M.; Burcea, M.; Serban, M. New clinical application of amniotic membrane transplant for ocular surface disease. J. Med. Life 2016, 9, 177–179. [Google Scholar] [PubMed]
- Muraine, M.; Descargues, G.; Franck, O.; Villeroy, F.; Toubeau, D.; Menguy, E.; Martin, J.; Brasseur, G. Amniotic membrane graft in ocular surface disease. Prospective study with 31 cases. J. Fr. Ophtalmol. 2001, 24, 798–812. [Google Scholar] [PubMed]
- Tabatabaei, S.A.; Soleimani, M.; Behrouz, M.J.; Torkashvand, A.; Anvari, P.; Yaseri, M. A randomized clinical trial to evaluate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Ocul. Surf. 2017, 15, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Prabhasawat, P.; Tesavibul, N.; Komolsuradej, W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br. J. Ophthalmol. 2001, 85, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.G.; Di Pascuale, M.A.; Liu, D.T.-S.; Gao, Y.Y.; Baradaran-Rafii, A. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology 2005, 112, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, H.; Aketa, N.; Shimizu, E.; Takano, Y.; Kawakita, T.; Uchino, Y.; Matsumoto, Y.; Ogawa, J.; Tsubota, K.; Ogawa, Y. Two case reports of continued progression of chronic ocular graft-versus-host disease without concurrent systemic comorbidities treated by amniotic membrane transplantation. BMC Ophthalmol. 2021, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Lavaris, A.; Elanwar, M.F.M.; Al-Zyiadi, M.; Xanthopoulou, P.T.; Kopsachilis, N.; Al-Zyadi, M.; Xanthopoulou, P. Glueless and sutureless multi-layer amniotic membrane transplantation in a patient with pending corneal perforation. Cureus 2021, 13, e16678. [Google Scholar] [CrossRef] [PubMed]
- Zemba, M.; Stamate, A.C.; Tataru, C.P.; Branisteanu, D.C.; Balta, F. Conjunctival flap surgery in the management of ocular surface disease (Review). Exp. Ther. Med. 2020, 20, 3412–3416. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; How, A.C.; Ang, L.P.; Tan, D.T. Gundersen flaps in the management of ocular surface disease in an Asian population. Cornea 2009, 28, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Tuli, S.S.; Schultz, G.S.; Downer, D.M. Science and strategy for preventing and managing corneal ulceration. Ocul. Surf. 2007, 5, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Sandinha, T.; Zaher, S.; Roberts, F.; Devlin, H.; Dhillon, B.; Ramaesh, K. Superior forniceal conjunctival advancement pedicles (SFCAP) in the management of acute and impending corneal perforations. Eye 2006, 20, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Khodadoust, A.; Quinter, A.P. Microsurgical approach to the conjunctival flap. Arch. Ophthalmol. 2003, 121, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Bernabei, F.; Barbato, F.; Arpinati, M.; Giannaccare, G.; Versura, P.; Bonifazi, F. Incidence, risk factors and complications of ocular graft-versus-host disease following hematopoietic stem cell transplantation. Am. J. Ophthalmol. 2021, 227, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.-M.; Sun, Z.-T.; Yang, X.-L.; Zhuang, X.-Y.; Ren, Y.-R.; Chen, Y.-J.; Chen, F.; Ma, X.; Tang, X.-W.; et al. Corneal perforation associated with ocular graft-versus-host disease. Front. Oncol. 2022, 12, 962250. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Z.; Zheng, B.; Wang, Z.; Liu, X.J.; Cai, Z.G.; Zhang, L.; Peng, X.; Wu, J.; Liu, X.-H.; Lv, L.; et al. Submandibular Gland Transplantation vs Minor Salivary Glands Transplantation for Treatment of Dry Eye: A Retrospective Cohort Study. Am. J. Ophthalmol. 2022, 241, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Raus, P.; Murube, J. Minor salivary gland transplantation. Dev. Ophthalmol. 2008, 41, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Z.; Zheng, B.; Liu, X.J.; Xie, Z.; Sun, D.; Cai, Z.G.; Lv, L.; Yu, G.-Y. Quality of life and patient satisfaction after submandibular gland transplantation in patients with severe dry eye disease. Ocul. Surf. 2019, 17, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, T.; Su, J.; Cong, X.; Lv, L.; Tong, L.; Yu, H.; Feng, Y.; Yu, G. Salivary Gland Transplantation as a Promising Approach for Tear Film Restoration in Severe Dry Eye Disease. J. Clin. Med. 2024, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Bhalekar, S.; Amescua, G.; Singh, S.; Basu, S. Minor salivary gland transplantation for severe dry eye disease due to cicatrising conjunctivitis: Multicentre long-term outcomes of a modified technique. Br. J. Ophthalmol. 2021, 105, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, J.Z.; Cai, Z.G.; Lv, L.; Zou, L.H.; Liu, X.J.; Wu, J.; Zhu, Z.-H.; Mao, C.; Wang, Y.; et al. Factors influencing the long-term results of autologous microvascular submandibular gland transplantation for severe dry eye disease. Int. J. Oral. Maxillofac. Surg. 2019, 48, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.H.; SantʼAnna, A.; Cristovam, P.C.; Alves, V.A.F.; Wakamatsu, A.; Gomes, J.A.P. Minor Salivary Gland Transplantation for Severe Dry Eyes. Cornea 2017, 36 (Suppl. S1), S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Fuest, M.; Yam, G.H.-F.; Peh, G.S.-L.; Mehta, J.S. Advances in corneal cell therapy. Regen Med. 2016, 11, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Thomasen, H.; Hestermann, K.; Dekowski, D.; Steuhl, K.-P.; Meller, D. Long-term results of autologous transplantation of limbal epithelium cultivated ex vivo for limbal stem cell deficiency. Der. Ophthalmol. 2016, 113, 321–329. [Google Scholar]
- Borderie, V.M.; Ghoubay, D.; Georgeon, C.; Borderie, M.; Sousa, C.; Legendre, A.; Rouard, H. Long-term results of cultured limbal stem cell versus limbal tissue transplantation in stage III limbal deficiency. Stem Cells Transl. Med. 2019, 8, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mohanty, S.; Jhanji, V.; Vajpayee, R.B. Amniotic membrane transplantation with or without autologous cultivated limbal stem cell transplantation for the management of partial limbal stem cell deficiency. Clin. Ophthalmol. 2018, 12, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Bobba, S.; Chow, S.; Watson, S.; Di Girolamo, N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res. Ther. 2015, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- González-Andrades, M.; Mata, R.; del Carmen González-Gallardo, M.; Medialdea, S.; Arias-Santiago, S.; Martínez-Atienza, J.; Ruiz-Garcia, A.; Perez-Fajardo, L.; Lizana-Moreno, A.; Garzon, I.; et al. A study protocol for a multicentre randomised clinical trial evaluating the safety and feasibility of a bioengineered human allogeneic nanostructured anterior cornea in patients with advanced corneal trophic ulcers refractory to conventional treatment. BMJ Open 2017, 7, e016487. [Google Scholar] [CrossRef] [PubMed]
- Prabhasawat, P.; Ekpo, P.; Uiprasertkul, M.; Chotikavanich, S.; Tesavibul, N.; Pornpanich, K.; Luemsamran, P. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank 2016, 17, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Kushnerev, E.; Shawcross, S.G.; Sothirachagan, S.; Carley, F.; Brahma, A.; Yates, J.M.; Hillarby, M.C. Regeneration of corneal epithelium with dental pulp stem cells using a contact lens delivery system. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5192–5199. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, A.; Ilmarinen, T.; Uusitalo, H.; Skottman, H. Small-molecule induction promotes corneal epithelial cell differentiation from human induced pluripotent stem cells. Stem Cell Rep. 2014, 2, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.C.; Abedi, F.; Chang, A.Y.; Shen, X.; Soleimani, M.; Araujo, I.; Jung, R.; Kwon, J.; Anwar, K.N.; Arabpour, Z.; et al. Safety of Subconjunctival Injection of Mesenchymal Stromal Cells in Persistent Corneal Epithelial Disease–A Phase 1b Clinical Trial. Ocul. Surf. 2025, 38, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Møller-Hansen, M.; Larsen, A.-C.; Toft, P.B.; Lynggaard, C.D.; Schwartz, C.; Bruunsgaard, H.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J.; Heegaard, S. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul. Surf. 2021, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Du, X.; Geng, S.; Peng, Y.; Wang, Z.; Lu, Z.; Wu, S.J.; Luo, C.W.; Guo, R.; Ling, W.; et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transpl. 2010, 45, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; He, C.; Lai, P.; Luo, C.; Guo, R.; Wu, S.; Geng, S.; Xiangpeng, A.; Liu, X.; Du, X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol. Ther. 2012, 20, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Mirshahi, R.; Cheraqpour, K.; Baharnoori, S.M.; Massoumi, H.; Chow, C.; Shahjahan, S.; Momenaei, B.; Ashraf, M.J.; Koganti, R.; et al. Intrastromal versus subconjunctival injection of mesenchymal stem/stromal cells for promoting corneal repair. Ocul. Surf. 2023, 30, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Masoumi, A.; Momenaei, B.; Cheraqpour, K.; Koganti, R.; Chang, A.Y.; Ghassemi, M.; Djalilian, A.R. Applications of mesenchymal stem cells in ocular surface diseases: Sources and routes of delivery. Expert Opin. Biol. Ther. 2023, 23, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W.; et al. miR-204–containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef] [PubMed]

| Intervention | Main Indications | Advantages | Limitations/Complications | Comparison Notes |
|---|---|---|---|---|
| Punctal Occlusion (Punctal plug, Canalicular plug, and Surgical occlusion) | DES, Sjögren’s, SJS, NK, SLK, contact lens-related dryness | Non-invasive, improves tear retention, can serve as drug delivery system | Plug loss/extrusion, irritation, canaliculitis, biofilm, epiphora | Compared to surgery, plugs are easier to apply but have higher extrusion rates. |
| Tarsorrhaphy | Persistent CED, exposure keratopathy, facial nerve palsy | Simple, protective, effective in both short- and long-term settings | Cosmetic dissatisfaction, prevents eye drop use in complete closure | More invasive than BoNT or eyelid loading, but more durable in severe exposure. |
| Botulinum Toxin Injection | DES, epiphora due to NLDO, hemifacial spasm, eyelid retraction | Minimally invasive, dual role in tear modulation | Short duration (6–12 weeks), potential for undesired ptosis or undercorrection | Less invasive than tarsorrhaphy; suitable for temporary disease. |
| Upper Eyelid Loading | Paralytic lagophthalmos, facial nerve palsy, eyelid retraction | Cosmetically favorable, reversible, avoids visual field restriction | Implant migration, extrusion, astigmatism, allergic reaction (esp. gold) | Better cosmesis and vision preservation than tarsorrhaphy; more durable than BoNT. |
| Upper Eyelid Retractor Weakening | Upper eyelid retraction (TED, facial palsy) | Preserves cosmesis and visual field, avoids foreign implants | Contour defects, asymmetry, ptosis/undercorrection, requires surgical expertise | More cosmetic and cost-effective than tarsorrhaphy or implants |
| Lower Eyelid Retractor Weakening | LER > 3 mm, severe lagophthalmos | Effective for severe cases with use of spacers, transconjunctival route preferred | Spacer-dependent results, graft-related issues (contracture, extrusion) | Better than tarsorrhaphy for functional/aesthetic outcomes in lower lid |
| Corneal Neurotization | NK unresponsive to conservative therapy | Addresses root cause by restoring corneal sensation, improves long-term epithelial healing | Technically demanding, longer healing time, risk of donor nerve disturbance | Superior in etiology-targeted treatment compared to AMT/tarsorrhaphy; MICN and ICN minimize invasiveness |
| Amniotic Membrane Transplantation | Persistent epithelial defects, chemical burns, LSCD, corneal ulcers | Anti-inflammatory, promotes epithelialization, useful in multilayer for stromal thinning | Risk of neovascularization, detachment, less effective in older burns or tumors | Preferred over conjunctival flap for healing but may require adjunctive stem cell transplant in LSCD |
| Conjunctival Flap Surgery | Deep ulcers, neurotrophic/infectious keratitis, non-healing corneal perforations | Effective tectonic support in absence of grafts, avoids evisceration, accessible technique | Reduced visual potential, flap retraction, cysts, not suitable in Mooren’s or autoimmune ulcers | Useful when AMT unavailable; inferior in optical outcomes but superior in structural preservation |
| Salivary Gland Transplantation | Severe refractory DES (non-Sjögren), especially with cicatricial disease (e.g., SJS, MMP) | Long-term lubrication, cost-effective, high symptom relief rates (~80%) | Contraindicated in xerostomia/Sjögren; excess secretion, duct issues may occur | Provides continuous lubrication unlike drops/plugs; best for end-stage DES where other methods fail |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafizadeh, S.M.; Asadigandomani, H.; Khannejad, S.; Hasanzade, A.; Rezaei, K.; Zhou, A.W.; Soleimani, M. Oculoplastic Interventions in the Management of Ocular Surface Diseases: A Comprehensive Review. Life 2025, 15, 1110. https://doi.org/10.3390/life15071110
Rafizadeh SM, Asadigandomani H, Khannejad S, Hasanzade A, Rezaei K, Zhou AW, Soleimani M. Oculoplastic Interventions in the Management of Ocular Surface Diseases: A Comprehensive Review. Life. 2025; 15(7):1110. https://doi.org/10.3390/life15071110
Chicago/Turabian StyleRafizadeh, Seyed Mohsen, Hassan Asadigandomani, Samin Khannejad, Arman Hasanzade, Kamran Rezaei, Avery Wei Zhou, and Mohammad Soleimani. 2025. "Oculoplastic Interventions in the Management of Ocular Surface Diseases: A Comprehensive Review" Life 15, no. 7: 1110. https://doi.org/10.3390/life15071110
APA StyleRafizadeh, S. M., Asadigandomani, H., Khannejad, S., Hasanzade, A., Rezaei, K., Zhou, A. W., & Soleimani, M. (2025). Oculoplastic Interventions in the Management of Ocular Surface Diseases: A Comprehensive Review. Life, 15(7), 1110. https://doi.org/10.3390/life15071110






