Effect of Different Feeds on the Fungi Microbiome of Suffolk Crossed with Tibetan Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Suffolk Cross with Tibetan Sheep Microbiome Analysis
2.3. Statistical Analysis
3. Results
3.1. Generated Sequencing Data in the ST Sheep in Different Feeding Groups
3.2. Comparing Analyses of the Fungal Microbiota in ST Sheep in Different Feeding Groups
3.3. Revealing Marker Fungi Species in ST Sheep in Different Feeding Groups
3.4. Functional Analysis of Fungal Microbiota in the ST Sheep in Different Feeding Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, R.; Cui, X.; Zhang, S.; Xu, C. Effects of Regular Water Replenishment on Enzyme Activities and Fungal Metabolic Function of Sheep Manure Composting on the Qinghai–Tibet Plateau. Int. J. Environ. Res. Public Health 2022, 19, 12143. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Guo, Y.; Zheng, W.; Cai, Y.; Qi, X.; Zhao, S. A comparative analysis of differentially expressed mRNAs, miRNAs and circRNAs provides insights into the key genes involved in the high-altitude adaptation of yaks. BMC Genom. 2021, 22, 744. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Z.; Yuan, C.; Wang, F.; Yang, B. Phylogeography and Phylogenetic Evolution in Tibetan Sheep Based on MT-CYB Sequences. Animals 2020, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, W.; Degen, A.; Guo, Y.; Kang, J.; Liu, P.; Ding, L.; Shang, Z.; Fievez, V.; Zhou, J.; et al. Tibetan sheep have a high capacity to absorb and to regulate metabolism of SCFA in the rumen epithelium to adapt to low energy intake. Brit. J. Nutr. 2020, 123, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, E.; Zhang, N.; Duan, C. Mitochondrial DNA diversity, origin, and phylogenic relationships of three Chinese large-fat-tailed sheep breeds. Trop. Anim. Health Prod. 2011, 43, 1405–1410. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2, 104. [Google Scholar] [CrossRef]
- Lapiere, A.; Richard, M.L. Bacterial-fungal metabolic interactions within the microbiota and their potential relevance in human health and disease: A short review. Gut Microbes 2022, 14, 2105610. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Das, A.; Herlihy, E.O.; Shanahan, F.; Toole, P.W.O.; Jeffery, I.B. The fecal mycobiome in patients with irritable bowel syndrome. Sci. Rep. 2021, 11, 124. [Google Scholar] [CrossRef]
- Li, X.V.; Leonardi, I.; Putzel, G.G.; Semon, A.; Fiers, W.D.; Kusakabe, T.; Lin, W.; Gao, I.H.; Doron, I.; Gutierrez-Guerrero, A.; et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 2022, 603, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Schnabl, B. Roles for the mycobiome in liver disease. Liver Int. 2022, 42, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. Biomed. Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef] [PubMed]
- Kiarie, E.; Romero, L.F.; Nyachoti, C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013, 26, 71–88. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Chen, N.; Xiang, Y.; Wang, Y.; Jin, M. Characteristics of gut microbiota in pigs with different breeds, growth periods and genders. Microb. Biotechnol. 2022, 15, 793–804. [Google Scholar] [CrossRef]
- Li, Z.; Zu, C.; Wang, C.; Yang, J.; Yu, H.; Wu, H. Different responses of rhizosphere and non-rhizosphere soil microbial communities to consecutive Piper nigrum L. monoculture. Sci. Rep. 2016, 6, 35825. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01 (accessed on 2 November 2023).
- Gao, B.; Chi, L.; Zhu, Y.; Shi, X.; Tu, P.; Li, B.; Yin, J.; Gao, N.; Shen, W.; Schnabl, B. An Introduction to Next Generation Sequencing Bioinformatic Analysis in Gut Microbiome Studies. Biomolecules 2021, 11, 530. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, X.; Li, X.; Guo, L.; Yang, F.; Ni, K. Exploring the rumen microbiota of Hu lambs in response to diet with paper mulberry. Appl. Microbiol. Biotechnol. 2023, 107, 4961–4971. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e201279. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Z.; Yan, T.; Chang, S.; Wang, H.; Hou, F. Rumen bacterial diversity of Tibetan sheep (Ovis aries) associated with different forage types on the Qinghai-Tibetan Plateau. Can. J. Microbiol. 2019, 65, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Zhang, J.B.; Liang, Z.; Yang, C.; Kalwar, Q.; Shah, T.; Du, M.; Muhammad, I.; Zheng, J.; Yan, P.; et al. Dynamics of rumen bacterial composition of yak (Bos grunniens) in response to dietary supplements during the cold season. PeerJ 2021, 9, e11520. [Google Scholar] [CrossRef] [PubMed]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2016, 8, 352–358. [Google Scholar] [CrossRef]
- Szóstak, N.; Handschuh, L.; Samelak-Czajka, A.; Tomela, K.; Schmidt, M.; Pruss, Ł.; Milanowska-Zabel, K.; Kozlowski, P.; Philips, A.; Sangwan, N. Host Factors Associated with Gut Mycobiome Structure. mSystems 2023, 8, e98622. [Google Scholar] [CrossRef]
- Zhu, Y.; Cidan, Y.; Sun, G.; Li, X.; Shahid, M.A.; Luosang, Z.; Suolang, Z.; Suo, L.; Basang, W. Comparative analysis of gut fungal composition and structure of the yaks under different feeding models. Front. Vet. Sci. 2023, 10, 1193558. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Zhang, Z.; Zhou, Y.; Zhang, X.; Sun, W.; Yang, Z.; Zhao, J.; Jiang, H. Altered gut bacterial–fungal interkingdom networks in children and adolescents with depression. J. Affect. Disord. 2023, 332, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, J.; Hou, Q.; Xu, X.; Ren, J.; Ma, L.; Yan, X. Core-predominant gut fungus Kazachstania slooffiae promotes intestinal epithelial glycolysis via lysine desuccinylation in pigs. Microbiome 2023, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, X.; Zhang, X.; Chen, X.; Rui, X.; Zhang, Q.; Dong, M.; Li, W. Simulated digestion and fecal fermentation behaviors of exopolysaccharides from Paecilomyces cicadae TJJ1213 and its effects on human gut microbiota. Int. J. Biol. Macromol. 2021, 188, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, X.; Tang, C.; Wang, X.; Zhang, X.; Xiao, L.; Li, W. Protective effect of Paecilomyces cicadae TJJ11213 exopolysaccharide on intestinal mucosa and regulation of gut microbiota in immunosuppressed mice. Food Res. Int. 2023, 165, 112477. [Google Scholar] [CrossRef]
- Hof, H. Rhodotorula spp. in the gut—Foe or friend? GMS Infect. Dis. 2019, 7, 6734584. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Kong, Q.; Liu, S.; Li, A.; Wang, Y.; Zhang, L.; Iqbal, M.; Jamil, T.; Shang, Z.; Suo, L.; Li, J. Characterization of fungal microbial diversity in healthy and diarrheal Tibetan piglets. BMC Microbiol. 2021, 21, 204. [Google Scholar] [CrossRef]
- Lu, S.; Zou, W.; Chen, X.; Sun, G.; Cidan, Y.; Almutairi, M.H.; Dunzhu, L.; Nazar, M.; Mehmood, K.; Zhu, Y.; et al. Effects of Cryptosporidium parvum infection on intestinal fungal microbiota in yaks (Bos grunniens). Microb. Pathog. 2023, 183, 106322. [Google Scholar] [CrossRef]
- Cao, Q.; Li, R.; Fu, R.; Zhang, X.; Yue, B.; Wang, J.; Sun, Z.; Niu, R. Intestinal fungal dysbiosis in mice induced by fluoride. Chemosphere 2020, 245, 125617. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Yu, B.; He, J.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; Tian, G.; et al. The fungal community and its interaction with the concentration of short-chain fatty acids in the faeces of Chenghua, Yorkshire and Tibetan pigs. Microb. Biotechnol. 2019, 13, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.G.; Barka, T.A.; Tayeb, F.A.E.F. Effect of ginger powder (Zingiber officinale) on acid-base balance, rumen and blood constituents in healthy Egyptian sheep. Int. J. Vet. Sci. 2021, 10, 55–58. [Google Scholar] [CrossRef]
- Oraby, M.I.; Baraka, T.A.; Rakha, G.H. Impact of cadmium intoxication on health status, rumen and blood constituents in Egyptian Ossimi sheep. Int. J. Vet. Sci. 2021, 10, 102–106. [Google Scholar] [CrossRef]
- Naibaho, F.G.; Hartanto, A.; Bintang, M.; Jamilah, I.; Priyani, N.; Putra, E.D. GC-MS analysis antimicrobial activity of the aqueous extract from the bulbs of Allium chinense G. Don. cultivated in North Sumatra, Indonesia. Asian J. Agric. Biol. 2021, 2, 201912562. [Google Scholar] [CrossRef]
- Van Thiel, I.A.M.; Rahman, S.; Hakvoort, T.B.M.; Davids, M.; Verseijden, C.; van Hamersveld, P.H.P.; Bénard, M.V.; Lodders, M.H.; Boekhout, T.; van den Wijngaard, R.M.; et al. Fecal Filobasidium Is Associated with Clinical Remission and Endoscopic Response following Fecal Microbiota Transplantation in Mild-to-Moderate Ulcerative Colitis. Microorganisms 2022, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Novak Babič, M.; Gunde-Cimerman, N.; Breskvar, M.; Džeroski, S.; Brandão, J. Occurrence, Diversity and Anti-Fungal Resistance of Fungi in Sand of an Urban Beach in Slovenia—Environmental Monitoring with Possible Health Risk Implications. J. Fungi 2022, 8, 860. [Google Scholar] [CrossRef]
- Undugoda, L.; Kannangara, S. Nature and activities of microfungi associated with the decomposition of rice straw in Sri Lanka. Asian J. Agric. Biol. 2022, 2022, 202103115. [Google Scholar] [CrossRef]
- Chafai, W.; El Gabardi, S.; Douira, A.; Khalid, A. Diversity and mycorrhizal potential of arbuscular mycorrhizal fungi in two natural soils in the eastern region of Morocco. Asian J. Agric. Biol. 2022, 2022, 202102101. [Google Scholar] [CrossRef]
- He, M.; Sun, W.; Cui, S.; Mu, G.; Liu, L.; Guo, W. Analysis of Microbial Diversity and Community Structure of Peanut Pod and Its Surrounding Soil in Peanut Rot Epidemic Area. Curr. Microbiol. 2021, 78, 2173–2182. [Google Scholar] [CrossRef]





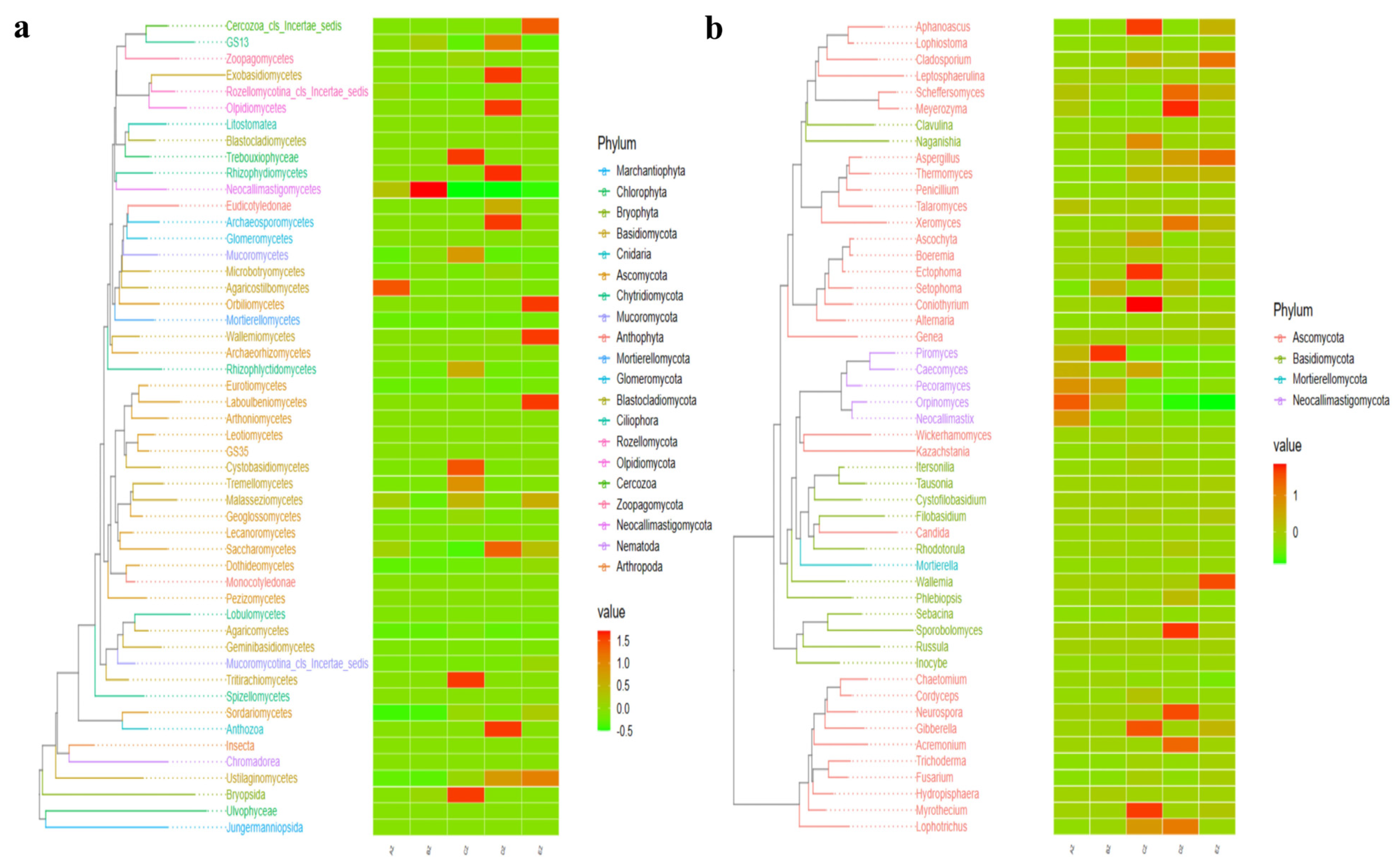
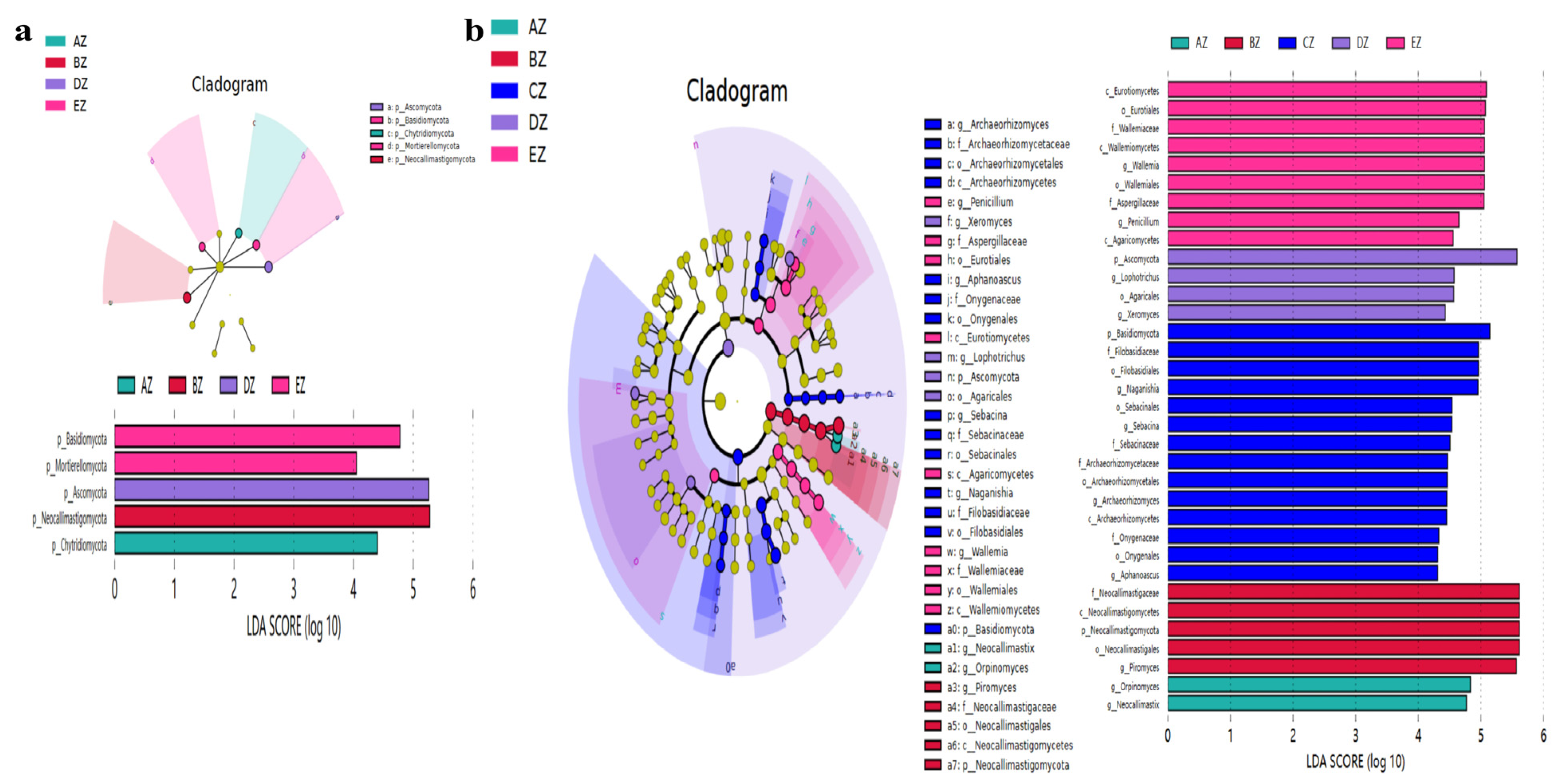

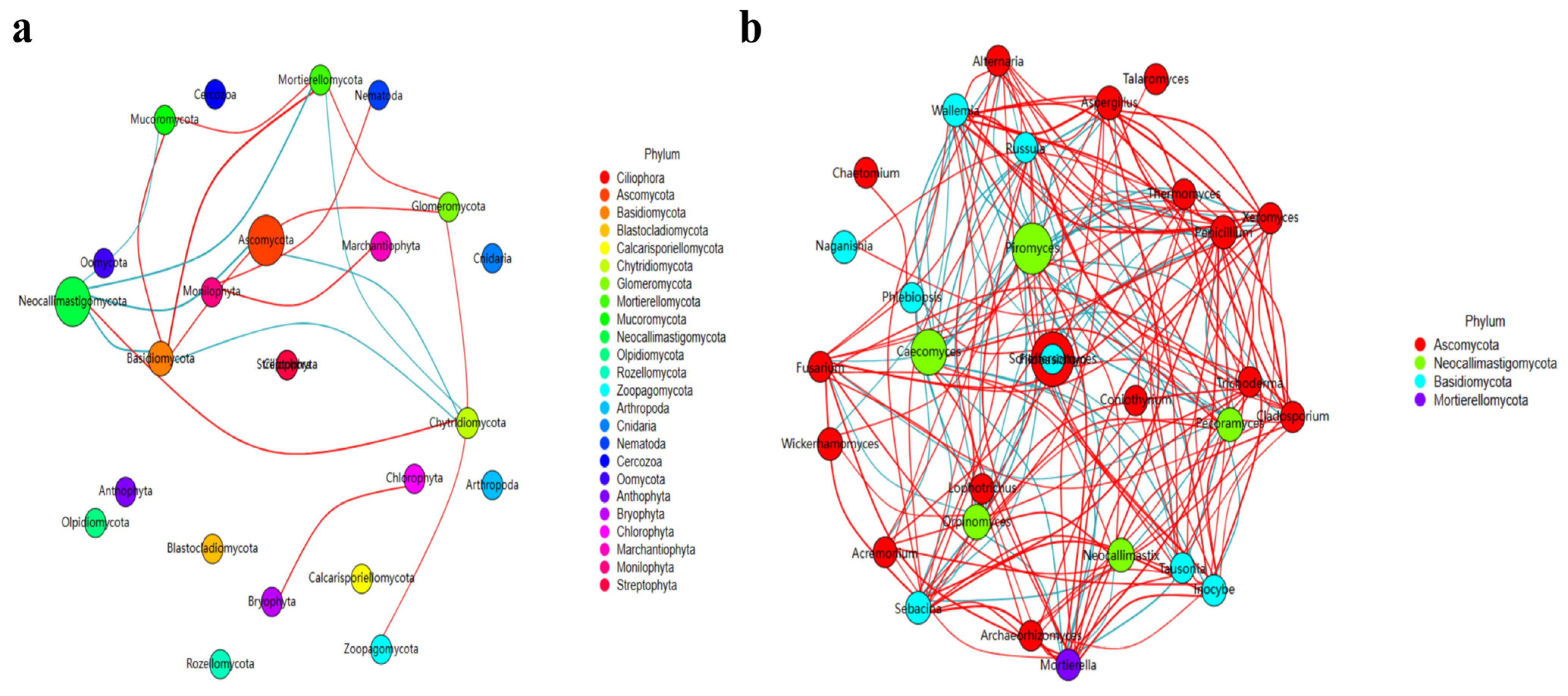
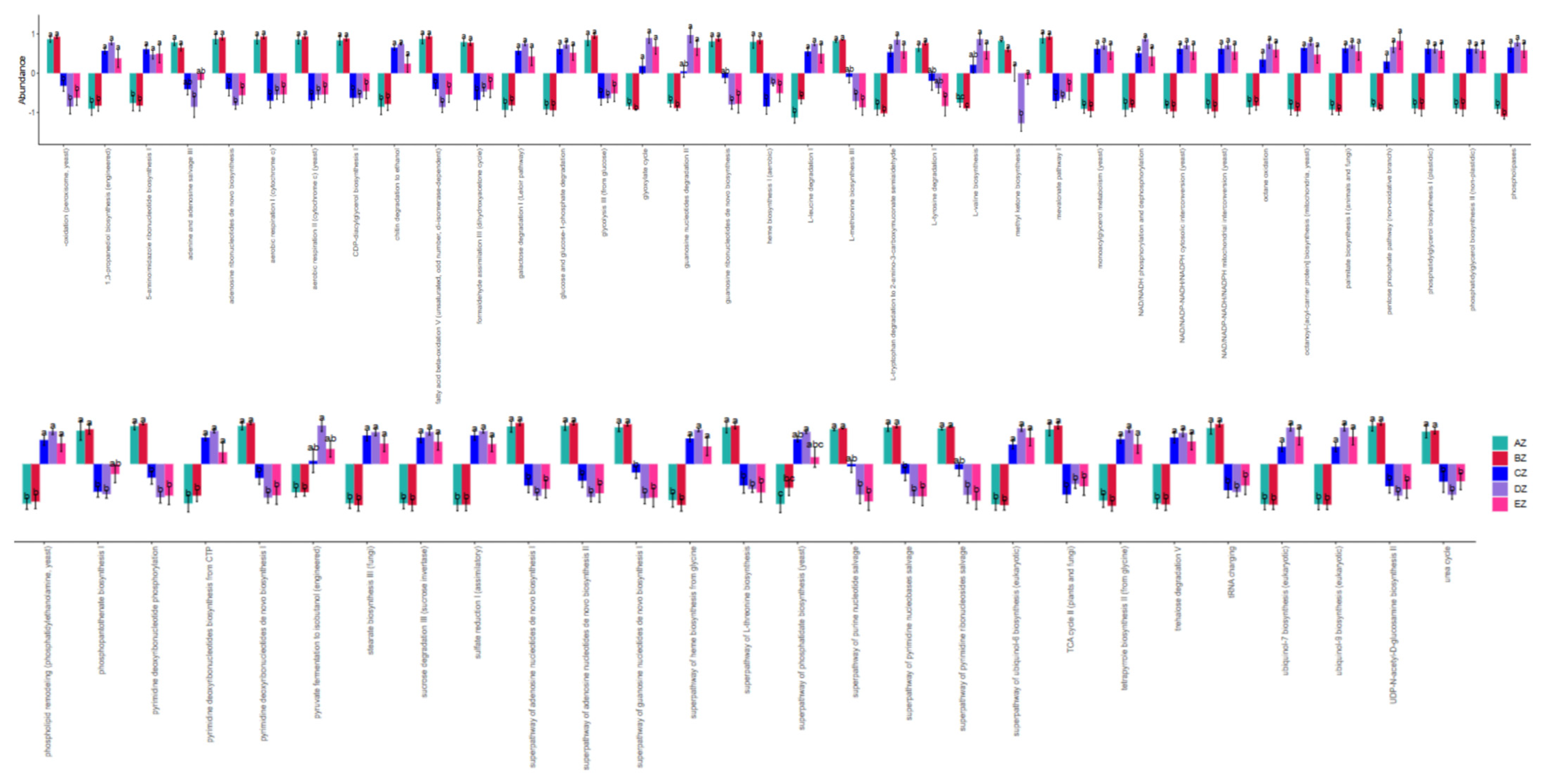
| Taxonomy | Group | Species | Percent (%) |
|---|---|---|---|
| Phylum | AZ | Ascomycota | 22.81% |
| AZ | Neocallimastigomycota | 48.51% | |
| AZ | Chytridiomycota | 7.10% | |
| BZ | Ascomycota | 14.09% | |
| BZ | Neocallimastigomycota | 57.76% | |
| BZ | Chytridiomycota | 4.55% | |
| CZ | Ascomycota | 33.32% | |
| CZ | Neocallimastigomycota | 22.24% | |
| CZ | Basidiomycota | 14.57% | |
| DZ | Ascomycota | 73.28% | |
| DZ | Neocallimastigomycota | 4.29% | |
| DZ | Basidiomycota | 5.90% | |
| EZ | Ascomycota | 51.40% | |
| EZ | Neocallimastigomycota | 4.29% | |
| EZ | Basidiomycota | 13.26% | |
| Class | AZ | Neocallimastigomycetes | 48.51% |
| AZ | Saccharomycetes | 16.30% | |
| AZ | Sordariomycetes | 1.36% | |
| BZ | Neocallimastigomycetes | 57.76% | |
| BZ | Saccharomycetes | 8.50% | |
| BZ | Sordariomycetes | 1.23% | |
| CZ | Neocallimastigomycetes | 22.24% | |
| CZ | Eurotiomycetes | 9.73% | |
| CZ | Sordariomycetes | 9.50% | |
| DZ | Saccharomycetes | 44.07% | |
| DZ | Eurotiomycetes | 9.43% | |
| DZ | Sordariomycetes | 7.43% | |
| EZ | Saccharomycetes | 22.15% | |
| EZ | Eurotiomycetes | 11.17% | |
| EZ | Sordariomycetes | 8.11% | |
| Order | AZ | Neocallimastigales | 48.51% |
| AZ | Saccharomycetales | 16.30% | |
| AZ | Eurotiales | 9.60% | |
| BZ | Neocallimastigales | 57.76% | |
| BZ | Saccharomycetales | 8.50% | |
| BZ | Pleosporales | 0.46% | |
| CZ | Neocallimastigales | 22.24% | |
| CZ | Filobasidiales | 9.15% | |
| CZ | Eurotiales | 8.79% | |
| DZ | Saccharomycetales | 44.07% | |
| DZ | Eurotiales | 9.08% | |
| DZ | Hypocreales | 3.40% | |
| EZ | Saccharomycetales | 22.15% | |
| EZ | Eurotiales | 10.84% | |
| EZ | Wallemiales | 8.59% | |
| Family | AZ | Neocallimastigaceae | 48.51% |
| AZ | Debaryomycetaceae | 16.28% | |
| AZ | Aspergillaceae | 0.59% | |
| BZ | Neocallimastigaceae | 57.76% | |
| BZ | Debaryomycetaceae | 8.02% | |
| BZ | Phaffomycetaceae | 0.37% | |
| CZ | Neocallimastigaceae | 22.24% | |
| CZ | Filobasidiaceae | 9.15% | |
| CZ | Aspergillaceae | 7.25% | |
| DZ | Debaryomycetaceae | 43.14% | |
| DZ | Aspergillaceae | 7.57% | |
| DZ | Wallemiaceae | 1.63% | |
| EZ | Debaryomycetaceae | 21.76% | |
| EZ | Aspergillaceae | 10.26% | |
| EZ | Wallemiaceae | 8.59% | |
| Genera | AZ | Piromyces | 18.77% |
| AZ | Scheffersomyces | 16.12% | |
| AZ | Caecomyces | 9.65% | |
| BZ | Scheffersomyces | 7.97% | |
| BZ | Orpinomyces | 4.14% | |
| BZ | Piromyces | 4.12% | |
| CZ | Caecomyces | 10.57% | |
| CZ | Naganishia | 9.04% | |
| CZ | Aspergillus | 5.29% | |
| DZ | Scheffersomyces | 42.80% | |
| DZ | Aspergillus | 3.26% | |
| DZ | Xeromyces | 2.85% | |
| EZ | Scheffersomyces | 21.55% | |
| EZ | Wallemia | 8.59% | |
| EZ | Aspergillus | 6.12% |
| Taxonomy | Group | Species | p-Value | Significance |
|---|---|---|---|---|
| Phylum | AZ | Chytridiomycota | <0.01 | ↑ |
| BZ | Neocallimastigomycota | <0.01 | ↑ | |
| DZ | Ascomycota | <0.01 | ↑ | |
| EZ | Basidiomycota | <0.05 | ↑ | |
| EZ | Mortierellomycota | <0.05 | ↑ | |
| Genera | AZ | Orpinomyces | <0.01 | ↑ |
| AZ | Neocallimastix | <0.01 | ↑ | |
| BZ | Neocallimastigaceae | <0.01 | ↑ | |
| BZ | Neocallimastigomycetes | <0.01 | ↑ | |
| BZ | Neocallimastigales | <0.01 | ↑ | |
| BZ | Neocallimastigomycota | <0.01 | ↑ | |
| BZ | Piromyces | <0.001 | ↑ | |
| CZ | Sebacina | <0.05 | ↑ | |
| CZ | Archaeorhizomycetales | <0.05 | ↑ | |
| CZ | Basidiomycota | <0.05 | ↑ | |
| CZ | Archaeorhizomyces | <0.05 | ↑ | |
| CZ | Archaeorhizomycetes | <0.05 | ↑ | |
| CZ | Sebacinales | <0.05 | ↑ | |
| CZ | Aphanoascus | <0.05 | ↑ | |
| CZ | Onygenaceae | <0.05 | ↑ | |
| CZ | Archaeorhizomycetaceae | <0.05 | ↑ | |
| CZ | Naganishia | <0.05 | ↑ | |
| CZ | Filobasidiales | <0.05 | ↑ | |
| CZ | Sebacinaceae | <0.05 | ↑ | |
| CZ | Filobasidiaceae | <0.05 | ↑ | |
| CZ | Onygenales | <0.05 | ↑ | |
| DZ | Ascomycota | <0.01 | ↑ | |
| DZ | Agaricales | <0.05 | ↑ | |
| DZ | Lophotrichus | <0.05 | ↑ | |
| DZ | Xeromyces | <0.05 | ↑ | |
| EZ | Eurotiales | <0.05 | ↑ | |
| EZ | Eurotiomycetes | <0.05 | ↑ | |
| EZ | Wallemiales | <0.05 | ↑ | |
| EZ | Wallemia | <0.05 | ↑ | |
| EZ | Penicillium | <0.05 | ↑ | |
| EZ | Aspergillaceae | <0.05 | ↑ | |
| EZ | Wallemiomycetes | <0.05 | ↑ | |
| EZ | Wallemiaceae | <0.05 | ↑ | |
| EZ | Agaricomycetes | <0.01 | ↑ |
| Taxonomy | Group | Species | p-Value | Significance |
|---|---|---|---|---|
| Phylum | CZ | Basidiomycota | <0.01 | ↑ |
| CZ | Mucoromycota | <0.01 | ↑ | |
| CZ | Chytridiomycota | <0.0001 | ↓ | |
| DZ | Mortierellomycota | <0.0001 | ↑ | |
| DZ | Mucoromycota | <0.05 | ↑ | |
| DZ | Ascomycota | <0.05 | ↑ | |
| DZ | Rozellomycota | <0.05 | ↑ | |
| DZ | Chytridiomycota | <0.0001 | ↓ | |
| DZ | Neocallimastigomycota | <0.0001 | ↓ | |
| DZ | Streptophyta | <0.05 | ↓ | |
| EZ | Basidiomycota | <0.001 | ↑ | |
| EZ | Mortierellomycota | <0.001 | ↑ | |
| EZ | Chytridiomycota | <0.001 | ↓ | |
| EZ | Chytridiomycota | <0.01 | ↓ | |
| Genera | CZ | Phaeosphaeria | <0.0001 | ↑ |
| CZ | Aphanoascus | <0.0001 | ↑ | |
| CZ | Gibberella | <0.0001 | ↑ | |
| CZ | Plenodomus | <0.0001 | ↑ | |
| CZ | Thelonectria | <0.0001 | ↑ | |
| CZ | Mucor | <0.001 | ↑ | |
| CZ | Sebacina | <0.001 | ↑ | |
| CZ | Xeromyces | <0.001 | ↑ | |
| CZ | Kazachstania | <0.001 | ↑ | |
| CZ | Cordyceps | <0.001 | ↑ | |
| CZ | Chrysosporium | <0.001 | ↑ | |
| CZ | Bjerkandera | <0.001 | ↑ | |
| CZ | Lophotrichus | <0.001 | ↑ | |
| CZ | Naganishia | <0.001 | ↑ | |
| CZ | Archaeorhizomyces | <0.001 | ↑ | |
| CZ | Cephaliophora | <0.01 | ↑ | |
| CZ | Scopulariopsis | <0.01 | ↑ | |
| CZ | Clavispora | <0.01 | ↑ | |
| CZ | Amphinema | <0.01 | ↑ | |
| CZ | Fusarium | <0.01 | ↑ | |
| CZ | Lophiostoma | <0.01 | ↑ | |
| CZ | Penicillium | <0.05 | ↑ | |
| CZ | Phoma | <0.05 | ↑ | |
| CZ | Aspergillus | <0.05 | ↑ | |
| CZ | Pecoramyces | <0.001 | ↓ | |
| CZ | Occultifur | <0.001 | ↓ | |
| CZ | Tuber | <0.01 | ↓ | |
| CZ | Papiliotrema | <0.05 | ↓ | |
| CZ | Saitozyma | <0.05 | ↓ | |
| DZ | Xeromyces | <0.0001 | ↑ | |
| DZ | Hyphopichia | <0.0001 | ↑ | |
| DZ | Lophotrichus | <0.0001 | ↑ | |
| DZ | Pichia | <0.0001 | ↑ | |
| DZ | Sebacina | <0.001 | ↑ | |
| DZ | Chrysosporium | <0.001 | ↑ | |
| DZ | Penicillium | <0.001 | ↑ | |
| DZ | Clonostachys | <0.01 | ↑ | |
| DZ | Rhodotorula | <0.01 | ↑ | |
| DZ | Cortinarius | <0.01 | ↑ | |
| DZ | Sporisorium | <0.01 | ↑ | |
| DZ | Fusarium | <0.01 | ↑ | |
| DZ | Amphinema | <0.01 | ↑ | |
| DZ | Clavispora | <0.01 | ↑ | |
| DZ | Verticillium | <0.01 | ↑ | |
| DZ | Clavulina | <0.05 | ↑ | |
| DZ | Ilyonectria | <0.05 | ↑ | |
| DZ | Cordyceps | <0.05 | ↑ | |
| DZ | Setophoma | <0.05 | ↑ | |
| DZ | Acremonium | <0.05 | ↑ | |
| DZ | Lecanicillium | <0.05 | ↑ | |
| DZ | Pecoramyces | <0.0001 | ↓ | |
| DZ | Caecomyces | <0.0001 | ↓ | |
| DZ | Neocallimastix | <0.0001 | ↓ | |
| DZ | Piromyces | <0.0001 | ↓ | |
| DZ | Occultifur | <0.0001 | ↓ | |
| DZ | Stephanonectria | <0.001 | ↓ | |
| DZ | Papiliotrema | <0.01 | ↓ | |
| DZ | Tuber | <0.05 | ↓ | |
| DZ | Colletotrichum | <0.05 | ↓ | |
| DZ | Saitozyma | <0.05 | ↓ | |
| EZ | Xeromyces | <0.0001 | ↑ | |
| EZ | Clavispora | <0.0001 | ↑ | |
| EZ | Penicillium | <0.0001 | ↑ | |
| EZ | Mortierella | <0.0001 | ↑ | |
| EZ | Plectosphaerella | <0.0001 | ↑ | |
| EZ | Gibberella | <0.0001 | ↑ | |
| EZ | Russula | <0.0001 | ↑ | |
| EZ | Wallemia | <0.0001 | ↑ | |
| EZ | Sebacina | <0.001 | ↑ | |
| EZ | Archaeorhizomyces | <0.001 | ↑ | |
| EZ | Hirsutella | <0.001 | ↑ | |
| EZ | Tomentella | <0.001 | ↑ | |
| EZ | Aphanoascus | <0.001 | ↑ | |
| EZ | Pseudallescheria | <0.001 | ↑ | |
| EZ | Rhodotorula | <0.01 | ↑ | |
| EZ | Verticillium | <0.01 | ↑ | |
| EZ | Lophiostoma | <0.01 | ↑ | |
| EZ | Filobasidium | <0.05 | ↑ | |
| EZ | Aspergillus | <0.05 | ↑ | |
| EZ | Thelonectria | <0.05 | ↑ | |
| EZ | Ilyonectria | <0.05 | ↑ | |
| EZ | Inocybe | <0.05 | ↑ | |
| EZ | Neocallimastix | <0.0001 | ↓ | |
| EZ | Occultifur | <0.001 | ↓ | |
| EZ | Stephanonectria | <0.001 | ↓ | |
| EZ | Phlebiopsis | <0.01 | ↓ | |
| EZ | Setophoma | <0.05 | ↓ | |
| EZ | Xenopolyscytalum | <0.05 | ↓ | |
| EZ | Tuber | <0.05 | ↓ | |
| EZ | Zopfiella | <0.05 | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Ciwang, R.; Wang, J.; Mehmood, K.; Ataya, F.S.; Li, K. Effect of Different Feeds on the Fungi Microbiome of Suffolk Crossed with Tibetan Sheep. Life 2023, 13, 2210. https://doi.org/10.3390/life13112210
Ren Y, Ciwang R, Wang J, Mehmood K, Ataya FS, Li K. Effect of Different Feeds on the Fungi Microbiome of Suffolk Crossed with Tibetan Sheep. Life. 2023; 13(11):2210. https://doi.org/10.3390/life13112210
Chicago/Turabian StyleRen, Yue, Renzeng Ciwang, Jia Wang, Khalid Mehmood, Farid Shokry Ataya, and Kun Li. 2023. "Effect of Different Feeds on the Fungi Microbiome of Suffolk Crossed with Tibetan Sheep" Life 13, no. 11: 2210. https://doi.org/10.3390/life13112210
APA StyleRen, Y., Ciwang, R., Wang, J., Mehmood, K., Ataya, F. S., & Li, K. (2023). Effect of Different Feeds on the Fungi Microbiome of Suffolk Crossed with Tibetan Sheep. Life, 13(11), 2210. https://doi.org/10.3390/life13112210








