Traditional Meat Products—A Mycotoxicological Review
Abstract
1. Introduction
2. Traditional Dry-Cured Products—Common Fungi
3. Mycotoxins in Dry Sausages and Dry-Cured Meat Products
4. Prevention Methods
- Physical methods:
- Washing grain with water or sodium carbonate;
- Manual sorting of contaminated grains based on the physical aspect of grains or using fluorescence to detect the presence of mycotoxins;
- Exposure to high temperatures, UV, X-rays or microwave irradiation;
- Solvent extraction of toxins;
- Dilution of contaminated feed with non-infected feed;
- Supplementation of binding agents which bind mycotoxins in order to decrease the bioavailability of these compounds in animals; hydrated sodium calcium aluminosilicates (HSCAS) and phyllosilicates derived from natural zeolites have a high affinity to AFB1; zeolites (hydrated aluminosilicates of alkaline cations), bind with AFB1 and ZEN [145,146]; bentonites bind with AFB1 and T-2 [147]. Kaolin, sepiolite, activated carbon and montmorillonite bind AFB1. Activated carbon is obtained by pyrolysis and the activation of organic compounds. It has a more heterogeneous porous structure. Activated carbon is also able to bind mycotoxins [148]. Resins such as cholestyramine and polyvinylpolypyrrolidoxynivalenol are also able to bind OTA and AFB1 [146]. However, all of them show an adverse effect to bioavailability of minerals and vitamins. Namely, they reduce the bioavailability of vitamins and minerals, and some of them are potential heavy-metals and dioxin carriers [149].
- Chemicals: acids, bases (ammonia, caustic soda), oxidants (hydrogen peroxide, ozone), reducing agents (bisulphites), chlorinated agents, and formaldehyde have been reportedly successful in degradation of mycotoxins [143].
- Microbiological methods: lactic acid bacteria, propionibacteria, and bifidobacteria cell wall structure are efficient in binding mycotoxins [150,151]. Mycotoxins are then eliminated in the feces without significant detrimental effects on the animals or any risk for toxic residues to be found in edible animal products. Glucomannans found in the cell wall of Saccharomyces cerevisiae bind to AFs’, FUM, ZEN, T-2, CIT, DAS, DON, OTA, NIV, and fusariotoxin. Corynebacterium rubrum can biotransform mycotoxins in contaminated feed [152].
5. Future Prospects and Legislation
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Langsrud, S.; Omer, M.K.; Nesbakken, T.; Skaar, I. A HACCP plan for mycotoxigenic hazards associated with dry-cured meat production processes. Food Control 2011, 22, 831–837. [Google Scholar] [CrossRef]
- Matrella, R.; Monaci, L.; Milillo, M.A.; Palmisano, F.; Tantillo, M.G. Ochratoxin A determination in paired kidneys and muscle samples from swines slaughtered in southern Italy. Food Control 2006, 17, 114–117. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Gualla, A.; Piva, G. Occurrence of ochratoxin A in raw ham muscles and in pork products from Northern Italy. Ital. J. Food Sci. 2006, 18, 99–106. [Google Scholar]
- Battilani, P.; Pietri, V.A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007, 70, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Omer, M.K.; Langsrud, S.; Nesbakken, T.; Skaar, I. Fungal growth pattern, sources and factors of mould contamination in a dry-cured meat production facility. Int. J. Food Microbiol. 2010, 140, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Chiesa, L.; Boscolo, D.; Manzano, M.; Cantoni, C.; Orli´c, S.; Comi, G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009, 26, 65–70. [Google Scholar] [CrossRef]
- Lusky, K.; Tesch, D.; Gobel, R. Influence of the mycotoxin ochratoxin A on animal health and formation of residues in pigs and different types of sausages derived from these animals. Arch. Lebensmittelhyg. 1993, 44, 131–134. [Google Scholar]
- Roncada, P.; Altafini, A.; Fedrizzi, G.; Guerrini, A.; Polonini, G.L.; Caprai, E. Ochratoxin A contamination of the casing and the edible portion of artisan salamis produced in two Italian regions. World Mycotoxin J. 2020, 13, 553–562. [Google Scholar] [CrossRef]
- Perrone, G.; Rodriguez, A.; Magistà, D.; Magan, N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr. Opin. Food Sci. 2019, 29, 20–27. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364/5, 5–24.
- Nunez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sanchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.; Gidlund, A.; Sulyok, M. Experimental mould growth and mycotoxin diffusion in different food items. World Mycotoxin J. 2017, 10, 153–161. [Google Scholar] [CrossRef]
- Singh, P.; Cotty, P.J. Aflatoxin contamination of dried red chilies: Contrasts between the United States and Nigeria, two markets differing in regulation enforcement. Food Control 2017, 80, 374–379. [Google Scholar] [CrossRef]
- Sunesen, L.O.; Stahnke, L.H. Mould starter cultures for dry sausages—Selection, application and effects. Meat Sci. 2003, 65, 935–948. [Google Scholar] [CrossRef]
- Mizáková, A.; Pipová, M.; Turek, P. The occurence of moulds in fermented raw meat products. Czech J. Food Sci. 2002, 20, 89–94. [Google Scholar] [CrossRef]
- Strzelecki, E.L.; Badura, L. Occurrence of Aflatoxinogenic Molds on Dry Cracower Sausage. Acta Microbiol. Pol. Ser. B-Microbiol. Appl. 1972, 4, 233–239. [Google Scholar]
- Andersen, S.J. Compositional Changes in Surface Mycoflora during Ripening of Naturally Fermented Sausages. J. Food Protec. 1995, 58, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, I.; Cantoni, C.; Papa, A. Microflora of Carnian dry sausages. Ind. Aliment. 1991, 30, 842–844. [Google Scholar]
- Feofilova, E.P.; Kuznetsova, L.S.; Sergeeva, Y.E.; Galanina, L.A. Species composition of food-spoiling mycelial fungi. Microbiology 2009, 78, 112–116. [Google Scholar] [CrossRef]
- Grazia, L.; Romano, P.; Bagni, A.; Roggiani, D.; Guglielmi, G. The role of moulds in the ripening process of salami. Food Microbiol. 1986, 3, 19–25. [Google Scholar] [CrossRef]
- Jircovsky, M.; Galgóczy, J. Investigations into the mould flora of Hungarian Winter salami. Die Fleischwirtsch. 1966, 46, 128. [Google Scholar]
- Leistner, L.; Ayres, J.C. Mold fungi and meat products. Die Fleischwirtsch. 1967, 47, 1320–1326. [Google Scholar]
- Leistner, L.; Eckardt, C. Occurence of toxinogenic Penicillia in meat products. Die Fleischwirtsch. 1979, 59, 1892–1896. [Google Scholar]
- Lopez-Diaz, T.M.; Santos, J.A.; Garcia-Lopez, M.L.; Otero, A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int. J. Food Microbiol. 2001, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.J.S.; Jensen, B.B.; Bernardo, F.M.A.; Barreto, A.H.S.; Hojberg, O. Mycoflora of two types of Portuguese dry-smoked sausages and inhibitory effect of sodium benzoate, potassium sorbate, and methyl p-hydroxybenzoate on mold growth rate. J. Food Prot. 2007, 70, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Mutti, P.; Previdi, M.P.; Quintavalla, S.; Spotti, E. Toxigenity of mould strains isolated from salami as a function of culture medium. Ind. Conserve 1988, 63, 142–145. [Google Scholar]
- Papagianni, M.; Ambrosiadis, I.; Filiousis, G. Mould growth on traditional Greek sausages and penicillin production by Penicillium isolates. Meat Sci. 2007, 76, 653–657. [Google Scholar] [CrossRef]
- Racovita, A.; Racovita, A.; Constantinescu, T. The importance of mould layers on salami. Die Fleischwirtsch. 1969, 49, 461–466. [Google Scholar]
- Skrinjar, M.; Horvar-Skenderovic, T. Contamination of dry sausage with moulds, aflatoxin, achratoxin and zearalenone. Tehnol. Mesa 1989, 30, 53–59. [Google Scholar]
- Tabuc, C.; Bailly, J.D.; Bailly, S.; Querin, A.; Guerre, P. Toxigenic potential of fungal mycoflora isolated from dry cured meat products: Preliminary study. Rev. Med. Vet. 2004, 155, 287–291. [Google Scholar]
- Asefa, D.T.; Gjerde, R.O.; Sidhu, M.S.; Langsrud, S.; Kure, C.F.; Nesbakken, T.; Skaar, I. Moulds contaminants on Norwegian dry-cured meat products. Int. J. Food Microbiol. 2009, 128, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Casado, M.K.; Borrás, M.D.; Aguilar, R.V. Fungal flora present on the surface of cured Spanish ham. Die Fleischwirtsch. 1991, 71, 1300–1302. [Google Scholar]
- Comi, G.; Orlic, S.; Redzepovic, S.; Urso, R.; Iacumin, L. Moulds isolated from Istrian dried ham at the pre-ripening and ripening level. Int. J. Food Microbiol. 2004, 96, 29–34. [Google Scholar] [CrossRef]
- Dragoni, I.; Marino, C.; Cantoni, C. “Bresaole” and raw hams surface moulds. Ind. Aliment. 1980, 19, 405–407. [Google Scholar]
- Monte, E.; Villanueva, J.R.; Domínquez, A. Fungal profiles of Spanish country-cured hams. Int. J. Food Microbiol. 1986, 3, 355–359. [Google Scholar] [CrossRef]
- Peintner, U.; Geiger, J.; Pöder, R. The Mycobiota of Speck, a Traditional Tyrolean Smoked and Cured Ham. J. Food Protec. 2000, 63, 1399–1403. [Google Scholar] [CrossRef]
- Spotti, E.; Mutti, P.; Campanini, M. Occurence of moulds on hams during preripening and ripening: Contamination of the environment and growth on the muscle portion of hams. Ind. Conserve 1989, 64, 110–113. [Google Scholar]
- Spotti, E.; Chiavaro, E.; Lepiani, A.; Colla, F. Mould and ochratoxin A contamination of pre-ripened and fully ripened hams. Ind. Conserve 2001, 76, 341–354. [Google Scholar]
- Sutic, M.; Ayres, J.C.; Koehler, P.E. Identification and Aflatoxin Production of Molds Isolated from Country Cured Hams. Appl. Microbiol. 1972, 23, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Rojas, F.J.; Jodral, M.; Gosalvez, F.; Pozo, R. Mycoflora and toxinogenic Aspergillus flavus in Spanish dry-cured ham. Int. J. Food Microbiol. 1991, 13, 249–256. [Google Scholar] [CrossRef]
- Huerta, T.; Sanchis, V.; Hernandez, J.; Hernandez, E. Mycoflora of dry-salted Spanish ham. Microbiol. Aliment. Nutr. 1987, 5, 247–252. [Google Scholar]
- Filtenborg, O.; Frisvad, J.C.; Samson, R.A. Specific association of fungi to foods and influence of physical environmental factors. In Introduction to Food- and Airborne Fungi, 6th ed.; Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O., Eds.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; pp. 306–320. [Google Scholar]
- Leistner, L.; Eckardt, C. Schimmelpilze und Mykotoxine in Fleisch und Fleischerzeugnissen. In Mykotoxine in Lebensmitteln; Reiss, J., Ed.; Gustav Fisher Verlag: Stuttgartt, Germany, 1981; pp. 297–341. [Google Scholar]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–173. [Google Scholar]
- Sørensen, L.M.; Frisvad, J.C.; Nielsen, P.V.; Lametsch, R.; Koch, A.G.; Jacobsen, T. Filamentous Fungi on Meat Products, Their Ability to Produce Mycotoxins and a Proteome Approach to Study Mycotoxin Production; Technical University of Denmark (DTU): Kongens Lyngby, Denmark, 2009. [Google Scholar]
- Pizzolato Montanha, F.; Anater, A.; Burchard, J.F.; Luciano, F.B.; Meca, G.; Manyes, L.; Pimpão, C.T. Mycotoxins in dry-cured meats: A review. Food Chem. Toxicol. 2018, 111, 494–502. [Google Scholar] [CrossRef]
- Rocha, M.E.B.; Freire, F.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Perši, N.; Pleadin, J.; Kovačević, D.; Scortichini, G.; Milone, S. Ochratoxin A in raw materials and cooked meat products made from OTA treated pigs. Meat Sci. 2014, 96, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Iacumin, L. Ecology of moulds during the pre-ripening and ripening of San Daniele dry cured ham. Food Res. Int. 2013, 54, 1113–1119. [Google Scholar] [CrossRef]
- Rodríguez, A.; Medina, A.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Gualla, A.; Morlacchini, M.; Pietri, A. Direct and indirect contamination with ochratoxin A of ripened pork products. Food Control 2013, 34, 79–83. [Google Scholar] [CrossRef]
- Lippolis, V.; Ferrara, M.; Cervellieri, S.; Damascelli, A.; Epifani, F.; Pascale, M.; Perrone, G. Rapid prediction of ochratoxin A-producing strains of Penicillium on dry-cured meat by MOS-based electronic nose. Int. J. Food Microbiol. 2016, 218, 71–77. [Google Scholar] [CrossRef]
- Lusky, K.; Tesch, R.; Gobel, R. The effect of natural and crystalline ochratoxin A in pigs after 28 day-feeding period and the residues of the mycotoxin in the body fluids organs and meat products. Arch. Lebensmittelhyg. 1995, 46, 45–48. [Google Scholar]
- Iacumin, L.; Milesi, S.; Pirani, S.; Comi, G.; Chiesa, L.M. Ochratoxigenic mold and ochratoxin a in fermented sausages from different areas in northern Italy: Occurrence, reduction or prevention with ozonated air. J. Food Saf. 2011, 31, 538–545. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Malir, J.; Toman, J.; Malir, F. Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.H. Factors Affecting the Growth of Microorganisms in Food. In Progress in Food Preservation; Bhat, R., Alias, A.K., Paliyath, G., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 405–427. [Google Scholar]
- Mandeel, Q.A. Fungal contamination of some imported spices. Mycopathologia 2005, 159, 291–298. [Google Scholar] [CrossRef]
- Farghaly, R.M. Occurrence and significance of moulds and their mycotoxins in spices as meat additives. Ben. Vet. Med. J. 2006, 17, 35–46. [Google Scholar]
- Bokhari, F.M. Spices mycobiota and mycotoxins available in Saudi Arabia and their abilities to inhibit growth of some toxigenic fungi. Myco J. 2007, 35, 47–53. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.D.; Dimić, G.R.; Karalic, D. Contamination of spices with moulds potential producers of sterigmatocystine. Acta Period. Technol. 2007, 38, 29–35. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin producing fungi. Saudi Biol. Sci. J. 2010, 17, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Martins, H.M.; Bernardo, F. Aflatoxins in spices marketed in Portugal. Food Addit. Contam. 2001, 18, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Jinap, S. Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control 2012, 24, 160–164. [Google Scholar] [CrossRef]
- Gambacorta, L.; Magistà, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotox J. 2018, 11, 159–174. [Google Scholar] [CrossRef]
- Zahra, N.; Khan, M.; Mehmood, Z.; Saeed, M.; Kalim, I.; Ahmad, I.; Malik, K. Determination of aflatoxins in spices and dried fruits. J. Sci. Res. 2018, 10, 315–321. [Google Scholar] [CrossRef]
- Jacxsens, L.; Yogendrarajaha, P.; Meulenaer, B. Risk assessment of mycotoxins and predictive mycology in Sri Lankan spices: Chilli and pepper. Procedia Food Sci. 2016, 6, 326–330. [Google Scholar] [CrossRef][Green Version]
- Pleadin, J.; Kovačević, D.; Perši, N. Ochratoxin A contamination of the autochthonous dry-cured meat product “Slavonski Kulen” during a six-month production process. Food Control 2015, 57, 377–384. [Google Scholar] [CrossRef]
- Karan, D.D.; Vukojević, J.B.; Ljaljević-Grbić, M.V.; Miličević, D.R.; Janković, V.V. Presence of moulds and mycotoxins in spices. Proc. Nat. Sci. Matica Srp. 2005, 108, 77–84. [Google Scholar] [CrossRef]
- Pleadin, J.; Malenica Staver, M.; Vahčić, N.; Kovačević, D.; Milone, S.; Saftić, L.; Scortichini, G. Survey of aflatoxin B1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Pleadin, J.; Kovačević, D.; Perković, I. Impact of casing damaging on aflatoxin B1 concentration during the ripening of dryfermented sausages. J. Immunoass. Immunochem. 2015, 36, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Olsen, M. (Eds.) Mycotoxins in Food: Detection and Control; Woodhead Publishing: Abington, MA, USA, 2004. [Google Scholar]
- Bui-Klimke, T.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2014, 55, 1860–1869. [Google Scholar] [CrossRef]
- Tolosa, J.; Ruiz, M.J.; Ferrer, E.; Vila-Donat, P. Ochratoxin A: Occurrence and carry-over in meat a nd meat by-products. A Review. Toxicology 2020, 37, 106–110. [Google Scholar]
- Bhat, R.; Rai, R.; Karim, A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe-Irimia, R.A.; Tăpăloagă, D.; Tăpăloagă, P.R.; Ilie, L.I.; Șonea, C.; Serban, A.I. Mycotoxins and Essential Oils—From a Meat Industry Hazard to a Possible Solution: A Brief Review. Foods 2022, 11, 3666. [Google Scholar] [CrossRef] [PubMed]
- Gai, F.; Pattono, D. Ochratoxin A (OTA) Occurence in Meat and Dairy Products: Prevention and Remediation Strategies; Nova Sience Publisher: New York, NY, USA, 2020; pp. 1–31. [Google Scholar]
- Koteswara, V.; Girisham, S.; Reddy, S. Inhibitory effect of essential oils on growth and ochratoxin a production by Penicillium species. Res. J. Microbiol. 2015, 10, 222–229. [Google Scholar] [CrossRef]
- Álvarez, M.; Delgado, J.; Núñez, F.; Roncero, E.; Andrade, M. Proteomic approach to unveil the ochratoxin A repression by Debaryomyces hansenii and rosemary on Penicillium nordicum during dry-cured fermented sausages ripening. Food Control 2022, 137, 108695. [Google Scholar] [CrossRef]
- Pleadin, J.; Lešić, T.; Milićević, D.; Markov, K.; Šarkanj, B.; Vahčić, N.; Kmetič, I.; Zadravec, M. Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes 2021, 9, 2122. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1987; Volume 1–42, pp. 83–87. [Google Scholar]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Chemical Agents and Related Occupations: A Review of Human Carcinogens; IARC Press: Lyon, France, 2012; Volume 100F, pp. 225–248. [Google Scholar]
- Elzupir, A.; Abdulkhair, B. Health risk from aflatoxins in processed meat products in Riyadh, KSA. Toxicon 2020, 181, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zohri, A.; Abdel-Gawad, K.; Saber, S. Antibacterial, antidermatophytic and antitoxigenic activities of onion (Allium cepa L.) oil. Microbiol. Res. 1995, 150, 167–172. [Google Scholar] [CrossRef]
- Tzanidi, C.; Proestos, C.; Markaki, P. Saffron (Crocus sativus L.) inhibits aflatoxin B1 production by Aspergillus parasiticus. J. Adv. Microbiol. 2012, 2, 310–316. [Google Scholar] [CrossRef]
- Fakour, M.; Alameh, A.; Rasouli, I.; Mazaheri, M. Antifungal effects of Zataria multiflora Boiss. and Thymus eriocalyx (ronniger) Jalas essential oils on aflatoxin producing Aspergillus parasiticus. Iran. J. Med. Aromat. Plants 2007, 23, 269–277. [Google Scholar]
- Alinezhad, S.; Kamalzadeh, A.; Rezaee, M.; Jaimand, K.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Inhibitory effects of some native medicinal plants on Aspergillus parasiticus growth and aflatoxin production. Acta Hortic. 2012, 963, 207–210. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Dubey, N. Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm.) Sweet essential oil and its major component 1,8-cineole against fungal isolates from chickpea seeds. Food Control 2012, 25, 27–33. [Google Scholar] [CrossRef]
- Atanda, O.; Akpan, I.; Oluwafemi, F. The potential of some spice essential oils in the control of A. parasiticus CFR 223 and aflatoxin production. Food Control 2007, 18, 601–607. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.; Aly, S. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J. Appl. Toxicol. 2005, 25, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Soud, N.; Deabes, M.; Abou El-Kassem, L.; Khalil, M. Antifungal activity of family Apiaceae essential oils. J. Appl. Sci. Res. 2012, 8, 4964–4973. [Google Scholar]
- Prakash, B.; Singh, P.; Kedia, A.; Dwivedy, A.; Singh, A.; Dubey, N. Mycoflora and aflatoxin analysis of Arachis hypogaeal and assessment of Anethum graveolensl seed and leaf essential oils against isolated fungi, aflatoxin production and their antioxidant activity. J. Food Saf. 2012, 32, 481–491. [Google Scholar] [CrossRef]
- El-Nagerabi, S.; Elshafie, A.; AlKhanjari, S.; Al-Bahry, S.; Elamin, M. Biological activities of Boswellia sacra extracts on the growth and aflatoxins secretion of two aflatoxigenic species of Aspergillus species. Food Control 2013, 34, 763–769. [Google Scholar] [CrossRef]
- Chang, H.; Kim, W.; Park, J.-H.; Kim, D.; Kim, C.-R.; Chung, S.; Lee, C. The Occurrence of Zearalenone in South Korean Feedstuffs between 2009 and 2016. Toxins 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- WHO/IARC: Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. P R E A M B L E. 2006. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/CurrentPreamble.pdf (accessed on 1 August 2023).
- Mirocha, C.; Robison, T.; Pawlosky, R.; Allen, N. Distribution and residue determination of [3H]zearalenone in broilers. Toxicol. Appl. Pharmacol. 1982, 66, 77–87. [Google Scholar] [CrossRef]
- Jonker, M.; van Egmond, H.; Stephany, R. Mycotoxins in Food of Animal Origin: A Review. Available online: https://www.rivm.nl/bibliotheek/digitaaldepot/389002_095.pdf (accessed on 13 July 2023).
- Velluti, A.; Sanchis, V.; Ramos, A.; Turon, C.; Marin, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004, 96, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalek, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.; Patiño, B. Mycotoxins|Toxicology, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 887–892. [Google Scholar]
- Pan, T.; Hsu, W. Monascus-Fermented products. In Encyclopedia of Food Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 815–825. [Google Scholar]
- Macholz, R. Some naturally occurring and synthetic food components, furocoumarins and ultraviolet radiation. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Int. Agency Res. Cancer 1986, 32, 150. [Google Scholar]
- Sarı, F.; Öztaş, E.; Özden, S.; Özhan, G. Liquid chromatographic determination of citrinin residues in various meat products: A pioneer survey in Turkey. J. Fac. Pharm. Istanb. Univ. 2020, 50, 195–202. [Google Scholar] [CrossRef]
- Silva, L.; Pereira, A.; Pena, A.; Lino, C. Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ayres, J.; Koehler, P. Production of citrinin by Penicillium viridicatum on country-cured ham. J. Appl. Microbiol. 1974, 27, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Meerpoel, C.; Vidal, A.; Tangni, E.; Huybrechts, B.; Couck, L.; De Rycke, R.; De Bels, L.; De Saeger, S.; Van den Broeck, W.; Devreese, M.; et al. A study of carry-over and histopathological effects after chronic dietary intake of citrinin in pigs, broiler chickens and laying hens. Toxins 2020, 12, 719. [Google Scholar] [CrossRef]
- Kharayat, B.; Singh, Y. Mycotoxins in foods: Mycotoxicoses, detection, and management. In Microbial Contamination Food Degradation; Academic Press: Cambridge, MA, USA, 2018; pp. 395–421. [Google Scholar]
- Bullerman, L. Mycotoxins|Classifications. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4080–4089. [Google Scholar]
- Bailly, J.; Tabuc, C.; Quérin, A.; Guerre, P. Production and stability of patulin, ochratoxin a, citrinin, and cyclopiazonic acid on dry cured ham. Rev. Anal. Chem. 2005, 68, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Nurme, J.; Piecková, E.; Viegas, S. Sterigmatocystin in foodstuffs and feed: Aspects to consider. Mycology 2018, 11, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, X.; Li, J.; Niu, Y.; Shi, L.; Fang, Z.; Zhang, T.; Ding, H. Quantitative determination of carcinogenic mycotoxins in human and animal biological matrices and animal-derived foods using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. J. Chromatogr. B 2018, 1073, 191–200. [Google Scholar] [CrossRef]
- Aupanun, S.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Poapolathep, A. An overview of the toxicology and toxicokinetics of fusarenon-X, a type B trichothecene mycotoxin. J. Vet. Med. Sci. 2017, 79, 6–13. [Google Scholar] [CrossRef]
- Saito, M.; Horiuchi, T.; Ohtsubo, K.; Hatakana, Y.; Ueno, Y. Low tumor-incidence in rats with long-term feeding of fusarenon X, a cytotoxic trichothecene produced by Fusarium nivale. Jpn. J. Exp. Med. 1980, 50, 293–302. [Google Scholar]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.; Kaushik, N.; Choi, E. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Q.; Kuča, K.; Dohnal, V.; Tian, Z. Deoxynivalenol: Signaling pathways and human exposure risk assessment—An update. Arch. Toxicol. 2014, 88, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Toman, J.; Grosse, Y.; Malir, F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018, 11, 135–148. [Google Scholar] [CrossRef]
- Frisvald, J.C.; Samson, A. Mycotoxins produced by species of Penicillium and Aspergillus occurring in cereals. In Cereal Grain; Chelkwski, J., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 441–476. [Google Scholar]
- Pitt, J.I.; Leistner, L. Toxigenic Penicillium species. In Mycotoxins and Animal Foods; Smith, J.E., Henderson, R.S., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1991; pp. 81–100. [Google Scholar]
- Sørensen, L.M.; Mogensen, J.; Nielsen, K.F. Simultaneous determination of ochratoxin A, mycophenolic acid and fumonisin B2 in meat products. Anal. Bioanal. Chem. 2010, 398, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Rodriguez, M.; Martin, A.; Delgado, J.; Cordoba, J.J. Presence of ochratoxin A on the surface of dry-cured Iberian ham after initial fungal growth in the drying stage. Meat Sci. 2012, 92, 728–734. [Google Scholar] [CrossRef]
- Zadravec, M.; Vahčić, N.; Brnić, D.; Markov, K.; Frece, J.; Beck, R.; Lešić, T.; Pleadin, J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int. J. Food Microbiol. 2020, 317, 108459. [Google Scholar] [CrossRef]
- Vulić, A.; Lešić, T.; Kudumija, N.; Zadravec, M.; Kiš, M.; Vahčić, N.; Pleadin, J. The development of LC-MS/MS method of determination of cyclopiazonic acid in dry-fermented meat products. Food Control 2021, 123, 107814. [Google Scholar] [CrossRef]
- Kudumija, N.; Vulić, A.; Lešić, T.; Vahčić, N.; Pleadin, J. Aflatoxins and ochratoxin A in dry-fermented sausages in Croatia, by LC-MS/MS. Food Addit. Contam. A 2020, 13, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.; Rodríguez, M.M.; Bermúdez, M.E.; Córdoba, J.J.; Asensio, M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996, 32, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.; Rodríguez-Martín, A.; Martín, A.; Núñez, F.; Asensio, M.A. Selection of antifungal protein-producing molds from dry-cured meat products. Int. J. Food Microbiol. 2009, 135, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Stoll, D.A.; Geisen, R. Fungicides effectively used for growth inhibition of several fungi could induce mycotoxin biosynthesis in toxigenic species. Int. J. Food Microbiol. 2013, 166, 407–412. [Google Scholar] [CrossRef]
- Bernáldez, V.; Rodríguez, A.; Martín, A.; Lozano, D.; Córdoba, J.J. Development of a multiplex qPCR method for simultaneous quantification in dry-cured ham of an antifungal-peptide Penicillium chrysogenum strain used as protective culture and aflatoxin-producing moulds. Food Control 2014, 36, 257–265. [Google Scholar] [CrossRef]
- Rodríguez, A.; Bernáldez, V.; Rodríguez, M.; Andrade, M.J.; Núñez, F.; Córdoba, J.J. Effect of selected protective cultures on ochratoxin A accumulation in dry-cured Iberian ham during its ripening process. Food Sci. Technol-LEB 2015, 60, 923–928. [Google Scholar] [CrossRef]
- Rodríguez, A.; Capela, D.; Medina, Á.; Córdoba, J.J.; Magan, N. Relationship between ecophysiological factors, growth and ochratoxin A contamination of dry-cured sausage based matrices. Int. J. Food Microbiol. 2015, 194, 71–77. [Google Scholar] [CrossRef]
- Domijan, A.M.; Pleadin, J.; Mihaljevié, B.; Vahčić, N.; Frece, J.; Markov, K. Reduction of ochratoxin A in dry-cured meat products using gammairradiation. Food Addit. Contam. Part. A 2015, 32, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of applications—A review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef]
- Virgili, R.; Simoncini, N.; Toscani, T.; Leggieri, M.C.; Formenti, S.; Battilani, P. Biocontrol of Penicillium nordicum growth and ochratoxin A production by native yeasts of dry cured ham. Toxins 2012, 4, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Bernáldez, V.; Córdoba, J.J.; Rodríguez, M.; Cordero, M.; Polo, L.; Rodríguez, A. Effect of Penicillium nalgiovense as protective culture in processing of dry-fermented sausage “salchichón”. Food Control 2013, 32, 69–76. [Google Scholar] [CrossRef]
- Andrade, M.J.; Thorsen, L.; Rodríguez, A.; Córdoba, J.J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2014, 170, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, N.; Virgili, R.; Spadola, G.; Battilani, P. Autochthonous yeasts as potential biocontrol agents in dry-cured meat products. Food Control 2014, 46, 160–167. [Google Scholar] [CrossRef]
- Delgado, J.; Acosta, R.; Rodríguez-Martín, A.; Bernúdez, E.; Núñez, F.; Asensio, M.A. Growth inhibition and stability of PgAFP from Penicillium chrysogenum against fungi common on dry-ripened meat products. Int. J. Food Microbiol. 2015, 205, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Jinap, S.; Noranizan, A. Aflatoxins and ochratoxin A reduction in black and white pepper by gamma radiation. Radiat. Phys. Chem. 2012, 81, 1786–1788. [Google Scholar] [CrossRef]
- Aquino, K.A.S. Sterilization by gamma irradiation. In Gamma Radiation; Adrovic, F., Ed.; InTech: Vienna, Austria, 2012; pp. 171–206. [Google Scholar]
- Kovačević, D. Tehnologija Kulena i Drugih Fermentiranih Kobasica; Prehrambeno—Tehnološki Fakultet Osijek: Osijek, Croatia, 2014. [Google Scholar]
- Gareis, M.; Scheuer, R. Prevention of mycotoxin contamination of meat and meat products. Int. Symp. Mycotoxicol. 1999, 1999, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pelhate, J. La microbiologie des foins. In Les Fourrages Secs: Récolte, Traitement, Utilisation; Demarquilly, C., Ed.; INRA Editions: Paris, France, 1987; pp. 63–81. [Google Scholar]
- Scott, P.M. Industrial and farm detoxification processes for mycotoxins. Rev. Méd. Vét. 1998, 149, 543–548. [Google Scholar]
- Yiannikouris, A.; Jouany, J.P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef]
- Piva, G.; Galvano, F.; Pietri, A.; Piva, A. Detoxification methods of aflatoxins. Ann. Rev. Nutr. Res. 1995, 15, 767–776. [Google Scholar]
- Piva, A.; Galvano, F. Managing mycotoxin impact: Nutritional approaches to reduce the impact of mycotoxins. In Alltech’s 15th Annual Symposium; Lyons, T.P., Jacques, K.A., Eds.; Nottingham University Press: Nottingham, UK, 1999; pp. 381–399. [Google Scholar]
- Ramos, A.J.; Fink-Gremmels, J.; Hernandez, E. Prevention of toxic effect of mycotoxins by means of non-nutritive adsorbent compounds. J. Food Prot. 1996, 59, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and stability of aflatoxin M1 in milk and milk products: A worldwide review. J. Food Prot. 1996, 59, 1079–1090. [Google Scholar] [CrossRef]
- Devegowda, G. Mettre les mycotoxines sur la touche: D’où viennent les glucomannanes esterifies. Feed. Times 2000, 4, 12–14. [Google Scholar]
- Yoon, Y.; Baeck, Y.J. Aflatoxin binding and antimutagenic activities of Bifidobacterium bifidum HY strains and their genotypes. Korean J. Dairy. Sci. 1999, 21, 291–298. [Google Scholar]
- El-Nemazi, H.; Kankaanpää, P.; Salinen, S.; Mykkänen, H.; Ahokas, J. Use of probiotic bacteria to reduce aflatoxin uptake. Rev. Méd. Vét. 1998, 149, 570. [Google Scholar]
- Nakazato, M.; Morozumi, S.; Saito, K.; Fujinuma, K.; Nishima, T.; Kasai, N. Interconversion of aflatoxin B1 and aflatoxicol by several fungi. Appl. Environ. Microbiol. 1990, 56, 1465–1470. [Google Scholar] [CrossRef]
- Eckhardt, J.C.; Santurio, J.M.; Zanette, R.A.; Rosa, A.P.; Scher, A.; Dal Pozzo, M.; Alves, S.H.; Ferreiro, L. Efficacy of a Brazilian calcium montmorillonite against toxic effects of dietary aflatoxins on broilers reared to market weight. Br. Poult. Sci. 2014, 55, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chu, F. Mycotoxins|Toxicology. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 4096–4108. [Google Scholar]
- Gupta, R.; Srivastava, A.; Lall, R. Ochratoxins and Citrinin. In Veterinary Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1019–1027. [Google Scholar]
- Tam, J.; Pantazopoulos, P.; Scott, P.; Moisey, J.; Dabeka, R.; Richard, I. Application of isotope dilution mass spectrometry: Determination of ochratoxin A in the Canadian Total Diet Study. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; GraslKraupp, B.; et al. Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 µg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA J. 2018, 16, e05175. [Google Scholar] [PubMed]
- Food and Agriculture Organization of the United Nations on Food Control (Maximum Levels of Aflatoxins in Food) Regulations. Available online: http://faolex.fao.org/docs/pdf/BOT196888.pdf (accessed on 11 August 2023).
- European Comission Scientific Comitee on Food—Opinion of the Scientific Committee for Food on Aflatoxins, Ochratoxin A and Patulin. 1996. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out14_en.pdf (accessed on 11 August 2023).
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113.
- Dhanasekaran, D.; Shanmugapriya, S.; Thajuddin, N.; Panneerselvam, A. Aflatoxins and Aflatoxicosis in Human and Animals. In Aflatoxins—Biochemistry and Molecular Biology; InTechOpen: London, UK, 2011. [Google Scholar]
- Benkerroum, N. Aflatoxins: Producing-molds, structure, health issues and incidence in southeast asian and sub-saharan african countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. [Google Scholar] [CrossRef]
- Aziz, N.; Youssef, Y. Occurrence of aflatoxins and aflatoxin-producing moulds in fresh and processed meat in Egypt. Food Addit. Contam. 1991, 8, 321–331. [Google Scholar] [CrossRef]
- Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA J. 2014, 12, 3699.
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research progress of safety of zearalenone: A review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- El-Hoshy, S. Occurrence of zearalenone in milk, meat and their products with emphasis on influence of heat treatments on its level. Arch. Lebensmittelhyg. 1999, 50, 140–143. [Google Scholar]
- Pleadin, J.; Mihaljević, Ž.; Barbir, T.; Vulić, A.; Kmetič, I.; Zadravec, M.; Brumen, V.; Mitak, M. Natural incidence of zearalenone in croatian pig feed, urine and meat in 2014. Food Addit. Contam. Part B Surveill. 2015, 8, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; World Health Organization: Geneva, Switzerland, 1976; Volume 10, p. 248.
- Zouagui, Z.; Asrar, M.; Lakhdissi, H.; Abdennebi, E. Prevention of mycotoxin effects in dairy cows by adding an anti-mycotoxin product in feed. J. Mater. Environ. Sci. 2017, 8, 3766–3770. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- European Comission Scientific Comitee on Food—Opinion of the Scientific Committee on Food on Fusarium toxins. Part 6: Group Evaluation of T-2 Toxin, HT-2 Toxin, Nivalenol and Deoxynivalenol. Available online: https://food.ec.europa.eu/system/files/2016-10/cs_contaminants_catalogue_fusarium_out123_en.pdf (accessed on 12 August 2023).
- Food and Agriculture Organization of the United Nations/WHO Joint FAO/WHO Expert Comitee on FOOD ADDITIVES—93 Meeting—Summary and Conclusions. 2022. Available online: https://www.fao.org/3/cb9478en/cb9478en.pdf (accessed on 13 August 2023).
- Zou, Z.; He, Z.; Li, H.; Han, P.; Tang, J.; Xi, C.; Li, Y.; Zhang, L.; Li, X. Development and application of a method for the analysis of two trichothecenes: Deoxynivalenol and T-2 toxin in meat in China by HPLC–MS/MS. Meat Sci. 2012, 90, 613–617. [Google Scholar] [CrossRef] [PubMed]
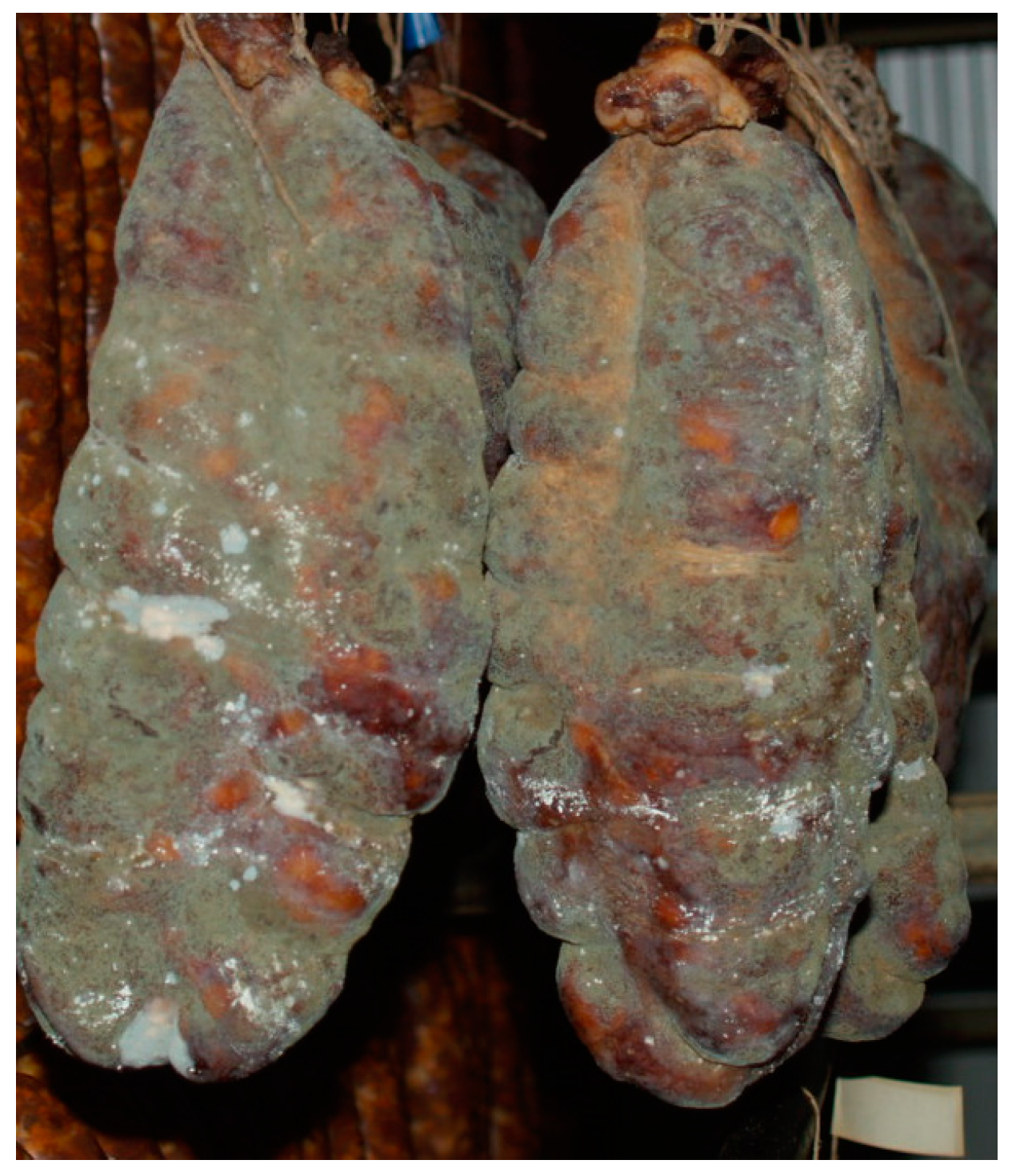
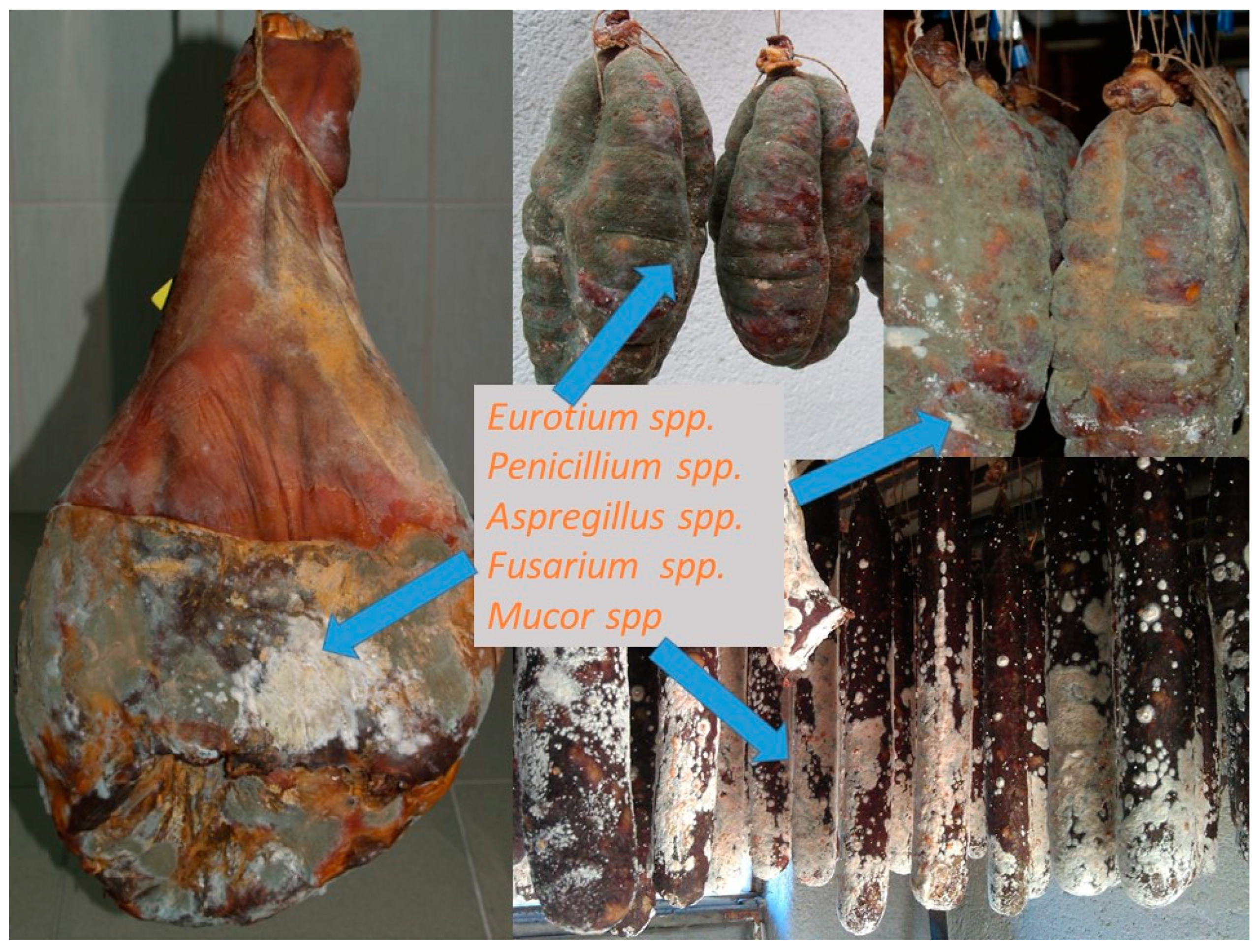

| Fungi | Mycotoxin |
|---|---|
| Aspergillus spp. | |
| A. flavus | Aflatoxin B1, cyclopiazonic acid, 3-nitropropionic acid |
| A. niger | Ochratoxin A, fumonisin B2 |
| A. ochraceus | Ochratoxin A, penicillic acid, xanthomegnin, viomellein, vioxanthin |
| A. versicolor | Sterigmatocystin |
| Penicillium spp. | |
| P. aurantiogriseum | Penicillic acid, verrucosidin, terrestric acid, nephrotoxic glycopeptides |
| P. brevicompactum | Botryodiploidin |
| P. chrysogenum | Secalonic acid, PR toxin, roquefortine C |
| P. citrinum | Citrinin |
| P. commune | Cyclopiazonic acid |
| P. crustosum | Terrestric acid, penitrems, roquefortine C |
| P. expansum | Patulin, citrinin, chaetoglobosins, communesins, roquefortine C |
| P. glabrum | Citromycetin |
| P. griseofulvum | Patulin, griseofulvins, roquefortine C, cyclopiazonic acid |
| P. nordicum | Ochratoxin A, viridic acid |
| P. oxalicum | Secalonic acids, roquefortine C |
| P. palitans | Cyclopiazonic acid |
| P. roquefortii | PR toxin, roquefortine C |
| P. rugulosum | Rugulosin |
| P. variabile | Rugulosin |
| P. verrucosum | Ochratoxin A, citrinin |
| P. viridicatum | Penicillic acid, xanthoemegnins, viridic acid |
| Product | Mycotoxin | Concentration μg/kg | Country | Source |
|---|---|---|---|---|
| Parma (retail product) | OTA | 56.0, 158.0, 113.0 | Denmark | [120] |
| Dry-cured Iberian ham | OTA | 2–160.9 | Spain | [121] |
| Fermented meat products | OTA, CIT, AFB1 | <0.05–7.83 <1.0–1.3 <1.0–3.0 | Croatia | [100] |
| Traditional meat products | AFB1, OTA | <1.0–1.2 2.03–2.31 | Croatia | [70,122] |
| Dry-fermented sausages | OTA, CPA | 0.48 2.55–59.80 | Croatia | [123,124] |
| Agent | |
|---|---|
| Protein | Fungi |
| AFP | Aspergillus giganteus |
| Anafp | Aspergillus niger |
| AcAFP | Aspergillus clavatus |
| NFAP | Neosartorya fischeri |
| PAF | Penicillium chrysogenum |
| PgAFP | Penicillium chrysogenum |
| Pc-Arctin | Penicillium chrysogenum |
| Mycotoxin | Regulatory Body | Total Daily Intake | Health Risk | Identified in | Concentration | Source |
|---|---|---|---|---|---|---|
| OTA | EFSA | 120 ng/kg bw/week | Nephrotoxicity hepatotoxicity, immunotoxicity, neurotoxicity, teratogenicity, and carcinogenicity | Sausage | 0.12 µg/kg | [3,100,154,155,156] |
| Joint FAO/WHO | 100 ng/kg bw */week | Dry-meat products | <LOQ- ≤ 7.83 µg/kg | |||
| Scientific Committee of Food (SCF) of the European Union | 5 ng/kg bw */day | Ham | ≤28.42 µg/kg | |||
| Salami | ≤0.08 µg/kg | |||||
| Pig muscle | ≤0.04–0.06 µg/kg ≤0.14 µg/kg | |||||
| Aflatoxin B1 | EFSA | 4 µg/kg to 10 µg/kg for total aflatoxin | Genotoxicity, hepatotoxicity, immunotoxicity, teratogenicity, carcinogenicity | Sausage | 1.5 µg/kg | [3,100,112,157,158,159,160,161] |
| Joint FAO/WHO | Not more than 10 µg/kg for total aflatoxin of which aflatoxin B1 shall not be more than 5 µg/kg | Dry-meat products | <LOQ-3.0 µg/kg | |||
| Scientific Committee of Food (SCF) of the European Union | 5–10 µg/kg for total aflatoxin | Ham | 0.95–1.06 µg/kg | |||
| Pig muscle | 0.46–0.74 µg/kg | |||||
| Aflatoxin B2 | EFSA | 4 µg/kg to 10 µg/kg for total aflatoxins | Hepatotoxicity, carcinogenicity, weak mutagenic effects | Sausage | 3 µg/kg | [157,158,159,162,163] |
| Joint FAO/WHO | Not more than 10 µg/kg for total aflatoxin of which aflatoxin B1 shall not be more than 5 µg/kg | |||||
| Scientific Committee of Food (SCF) of the European Union | 5–10 µg/kg for total aflatoxins | |||||
| Zearlenone | EFSA | 0.25 µg/kg body weight | Reproductive toxicity, hepatotoxicity, immunotoxicity, genotoxicity and carcinogenicity, intestinal toxicity, endocrine disruption | Sausage | 2.1–8.9 µg/kg | [101,164,165,166,167] |
| Joint FAO/WHO | 0.5 µg/kg bw * | Pig muscle | ≤4.31 µg/kg | |||
| Citrinin | EFSA | 0.2 µg/kg bw * per day | Necrotic changes of parenchyma organs ephrotoxicity, gastrointestinal ailments, fetal malformations, and lymphoid tissue damage (additively, synergistically, or antagonistically to OTA) | Sausage | 1.0 µg/kg | [100,102,105] |
| Dry-meat products | <LOQ-1.3 µg/kg | |||||
| Sterigmatocystin | EFSA | Not established | Possible carcinogen, immunotoxic and immunomodulatory activity, together with mutagenic effect | Pig muscle | 0.76–1.23 µg/kg | [111,112,168,169] |
| Joint FAO/WHO | ||||||
| Scientific Committee of Food (SCF) of the European Union | ||||||
| T-2 Toxin | EFSA | 100 ng/kg bw * for T-2 toxins and HT-2 toxins | Anorexia, emesis, carcinogenicity, haematotoxicity, neurotoxicity and immunotoxicity | Pig muscle | 0.0240–0.4515 µg/kg | [154,170,171,172,173,174] |
| Joint FAO/WHO | 25 ng/kg bw * for T-2, HT-2 and DAS, alone or in combination | |||||
| Scientific Committee of Food (SCF) of the European Union | 0.06 g/kg bw */day. for T-2 toxins and HT-2 toxins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastanjević, K.; Kovačević, D.; Nešić, K.; Krstanović, V.; Habschied, K. Traditional Meat Products—A Mycotoxicological Review. Life 2023, 13, 2211. https://doi.org/10.3390/life13112211
Mastanjević K, Kovačević D, Nešić K, Krstanović V, Habschied K. Traditional Meat Products—A Mycotoxicological Review. Life. 2023; 13(11):2211. https://doi.org/10.3390/life13112211
Chicago/Turabian StyleMastanjević, Krešimir, Dragan Kovačević, Ksenija Nešić, Vinko Krstanović, and Kristina Habschied. 2023. "Traditional Meat Products—A Mycotoxicological Review" Life 13, no. 11: 2211. https://doi.org/10.3390/life13112211
APA StyleMastanjević, K., Kovačević, D., Nešić, K., Krstanović, V., & Habschied, K. (2023). Traditional Meat Products—A Mycotoxicological Review. Life, 13(11), 2211. https://doi.org/10.3390/life13112211









