Abstract
The new mineral achyrophanite (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5 was found in high-temperature sublimates of the Arsenatnaya fumarole at the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka, Russia. It is associated with aphthitalite-group sulfates, hematite, alluaudite-group arsenates (badalovite, calciojohillerite, johillerite, nickenichite, hatertite, and khrenovite), ozerovaite, pansnerite, arsenatrotitanite, yurmarinite, svabite, tilasite, katiarsite, yurgensonite, As-bearing sanidine, anhydrite, rutile, cassiterite, and pseudobrookite. Achyrophanite occurs as long-prismatic to acicular or, rarer, tabular crystals up to 0.02 × 0.2 × 1.5 mm, which form parallel, radiating, bush-like, or chaotic aggregates up to 3 mm across. It is transparent, straw-yellow to golden yellow, with strong vitreous luster. The mineral is brittle, with (001) perfect cleavage. Dcalc is 3.814 g cm–3. Achyrophanite is optically biaxial (+), α = 1.823(7), β = 1.840(7), γ = 1.895(7) (589 nm), 2V (meas.) = 60(10)°. Chemical composition (wt.%, electron microprobe) is: Na2O 3.68, K2O 9.32, CaO 0.38, MgO 1.37, MnO 0.08, CuO 0.82, ZnO 0.48, Al2O3 2.09, Fe2O3 20.42, SiO2 0.12, TiO2 7.35, P2O5 0.14, V2O5 0.33, As2O5 51.88, SO3 1.04, and total 99.40. The empirical formula calculated based on 22 O apfu is Na1.29K2.15Ca0.07Mg0.34Mn0.01Cu0.11Zn0.06Al0.44Fe3+2.77Ti1.00Si0.02P0.02S0.14V0.04As4.90O22. Achyrophanite is orthorhombic, space group P2221, a = 6.5824(2), b = 13.2488(4), c = 10.7613(3) Å, V = 938.48(5) Å3 and Z = 2. The strongest reflections of the PXRD pattern [d,Å(I)(hkl)] are 5.615(59)(101), 4.174(42)(022), 3.669(31)(130), 3.148(33)(103), 2.852(43)(141), 2.814(100)(042, 202), 2.689(29)(004), and 2.237(28)(152). The crystal structure of achyrophanite (solved from single-crystal XRD data, R = 4.47%) is unique. It is based on the octahedral-tetrahedral M-T-O pseudo-framework (M = Fe3+ with admixed Ti, Al, Mg, Na; T = As5+). Large-cation A sites (A = K, Na) are located in the channels of the pseudo-framework. The achyrophanite structure can be described as stuffed, with the defect heteropolyhedral pseudo-framework derivative of the orthorhombic Fe3+AsO4 archetype. The mineral is named from the Greek άχυρον, straw, and φαίνομαι, to appear, in allusion to its typical straw-yellow color and long prismatic habit of crystals.
1. Introduction
Diverse and rich arsenate mineralization is one of the most interesting mineralogical and geochemical features of the high-temperature fumaroles of the oxidizing type born by the active Tolbachik volcano at Kamchatka (Russian Far East). Almost sixty H-free arsenates found here involve in their chemical composition many cations as species-defining constituents: Cu, Zn, Mg, Mn, Al, Fe3+, Ti, Zr, Sn, Ca, Na, and K; some fumarolic arsenates contain additional anions: (SO4), (VO4), (PO4), O, F, or Cl. The uniqueness of this mineralization is brightly highlighted by the great number of arsenate minerals first discovered in exhalations of Tolbachik fumaroles, namely forty new mineral species ([,] and references therein).
In this paper, we describe the new mineral achyrophanite (Cyrillic: ахирoфанит) which has a unique crystal structure. The mineral is named from the Greek (in both ancient and modern Greek languages) άχυρον, straw, and φαίνομαι, to appear, in allusion to its straw-yellow color and long prismatic habit of crystals: typical aggregates of the mineral visually resemble straw bunches.
Both the new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification, IMA2018–011. The type specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue number 96254.
2. Occurrence and Mineral Association
Achyrophanite was found in the Arsenatnaya fumarole situated at the second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976 (NB GTFE), Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N 160°14′E, 1200 m asl). This scoria cone is a monogenetic basalt volcano formed in 1975. Many fumaroles of the oxidizing type, including several richly mineralized ones, are still active here ([,,] and references therein). Arsenatnaya is the largest fumarole at the second scoria cone of the NB GTFE. It is an outstanding mineralogical occurrence due to the greatest mineral diversity and originality: more than 200 mineral species have been reliably identified here, including 72 new minerals. The detailed data on the Arsenatnaya fumarole have been reported in [,].
The type material of achyrophanite was collected by us in July 2016 from the medium (about 2 m under the day surface) zone of Arsenatnaya. The temperature in this area measured using a chromel-alumel thermocouple during collection was ca. 430 °C. In July 2018, additional specimens were collected.
Achyrophanite is associated with aphthitalite-group sulfates (metathénardite, belomarinaite and aphthitalite), hematite, alluaudite-group arsenates (badalovite, calciojohillerite, johillerite, nickenichite, hatertite, and khrenovite forming a continuous solid-solution system), ozerovaite, pansnerite, arsenatrotitanite, yurmarinite, svabite, tilasite, katiarsite, yurgensonite, As-bearing sanidine, anhydrite, rutile (Sn-, Fe3+-, and Sb5+-enriched variety), cassiterite, and pseudobrookite.
3. Methods
The Raman spectrum of achyrophanite was obtained using an EnSpectr R532 spectrophotometer (Dept. of Mineralogy, Lomonosov Moscow State University) with a green laser (532 nm) at room temperature. The radiation power at the output of the laser source was about 16 mW. The spectrum was processed in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 mm−1 and a resolution equal to 6 cm−1. The diameter of the laser spot on the sample was about 10 μm with a 40× objective. The spectrum was acquired on a randomly oriented crystal. The mineral is stable under a laser beam.
Scanning electron microscopic (SEM) studies in secondary electron (SE) mode were carried out, and chemical composition was determined for all studied samples using a JEOL JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Lomonosov Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA, and a 3 μm beam diameter. The standards used are as follows: Na, Al, Si (jadeite), K, P (KTiOPO4), Ca (CaSiO3), Mg (olivine), Mn, Ti (MnTiO3), Cu (Cu), Zn, S (ZnS), Fe (FeS2), V (V), and As (GaAs). Contents of other elements with atomic numbers > 6 are below detection limits.
Powder X-ray diffraction (XRD) data were collected using a Rigaku RAXIS Rapid II diffractometer (Rigaku Corporation, Tokyo, Japan) with a curved image plate detector and rotating anode with VariMAX microfocus optics, using CoKα radiation in Debye-Scherrer geometry at an accelerating voltage of 40 kV, current of 15 mA, and exposure time 15 min. The distance between the sample and the detector was 127.4 mm. The data were processed using osc2xrd software [].
Single-crystal XRD studies were carried out using an Xcalibur S diffractometer equipped with a CCD detector (Oxford Diffraction, Oxford, UK) (MoKα radiation).
4. Results
4.1. General Appearance and Physical Properties
In the type material, achyrophanite occurs as long-prismatic (lath-, sword-, or spear-like) to acicular crystals elongated along [100] and usually flattened on [001], up to 0.02 × 0.2 × 1.5 mm, which form parallel, radiating, bush-like, or chaotic aggregates up to 3 mm across (Figure 1a–d and Figure 2). Rarely, they are combined in interrupted, open-work encrustations on the surface of basalt scoria altered by fumarolic gas. In the specimens collected in 2018, achyrophanite crystals (up to 0.5 mm) demonstrate another morphology: they are flattened prismatic to tabular (Figure 1e,f). The pinacoid {001} is the major form of achyrophanite crystals; lateral faces are typically represented by the pinacoid {010}; on some crystals the pinacoidal face {100} and/or non-indexed faces of the prismatic zone {hk0} are observed.

Figure 1.
Morphology of achyrophanite: (a–d): clusters of acicular crystals from type specimen [parallel intergrowth of two crystals (a), radiating crystal cluster (b), and bush-like crystal groups], (e,f): flattened prismatic to tabular crystals. SEM (SE) images.

Figure 2.
Radiating (a) and chaotic (b) aggregates of straw-yellow achyrophanite crystals with iron-black hematite, whitish aphthitalite-group sulfates and minor fine-grained orange cassiterite on basalt scoria altered by fumarolic gas. FOV width: (a)—4.4 mm, (b)—3.6 mm. Photo: I.V. Pekov and A.V. Kasatkin.
Achyrophanite is transparent, with strong vitreous luster and a white streak. The mineral is straw-yellow to golden yellow; the thinnest crystals are almost colorless. Achyrophanite is brittle; however, parallel intergrowths of flattened acicular crystals are flexible and elastic. Perfect cleavage on (001) was observed under the microscope. The fracture is uneven across a crystal elongation (observed under the microscope). Density could not be measured because crystals are thin and their aggregates are open-work. The density value calculated using the empirical formula and unit-cell volume found from single-crystal XRD data is 3.814 g cm–3.
4.2. Optical Data
In plane-polarized transmitted light, achyrophanite is very weakly pleochroic, with the following absorption scheme: Z (very pale yellow) > Y = X (colorless). It is optically biaxial (+), α = 1.823(7), β = 1.840(7), γ = 1.895(7) (589 nm), 2V (meas.) = 60(10)°, and 2V (calc.) = 60°. Dispersion of optical axes is weak, r > v. Orientation is as follows: X = c, Y = a, Z = b. Extinction is straight, and elongation is negative.
4.3. Raman Spectroscopy
The Raman spectrum of achyrophanite (Figure 3) was interpreted according to []. The bands in the region between 1000 and 700 cm−1 correspond to As5+–O stretching vibrations of AsO43− anions. The presence of two strong bands with maxima at 865 and 777 cm−1 and a distinct band at 921 cm−1 is caused by significant distortion of AsO4 tetrahedra marked as T(1)O4, T(2)O4, T(4)O4, and, especially, T(5)O4 (see structure data below). Bands in the region between 600 and 350 cm−1 are assigned to bending vibrations of AsO4 tetrahedra, Fe3+–O, Ti–O, Al–O, and Mg–O stretching vibrations. Bands with frequencies lower than 300 cm−1 correspond to lattice modes. The absence of bands with frequencies higher than 1000 cm−1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O, and B–O bonds in achyrophanite.

Figure 3.
The Raman spectrum of the achyrophanite.
4.4. Chemical Data
Chemical composition of achyrophanite (type specimen) is given in Table 1.

Table 1.
Chemical composition (wt.%) of achyrophanite.
The empirical formula calculated on the basis of 22 oxygen atoms per formula unit (apfu) is Na1.29K2.15Ca0.07Mg0.34Mn0.01Cu0.11Zn0.06Al0.44Fe3+2.77Ti1.00Si0.02P0.02S0.14V0.04As4.90O22 or, after the grouping of constituents; or, after the formal grouping of cations between T, M and A sites: A(K2.15Na1.29Ca0.07)Σ3.51M(Fe3+2.57Ti1.00Al0.44Mg0.34Cu0.11Zn0.06Mn0.01)Σ4.53(As4.90S0.14V0.04Si0.02P0.02)Σ5.12O22 (Z = 2). Some surplus of T and (M + A) constituents over the values required by the idealized formula, 5 and 8 apfu, respectively, is caused by the existence of additional low-occupied positions T’ and M’ in the structure of achyrophanite. The surplus of (K + Na + Ca) over the idealized value for A cations = 3 apfu is caused by the location of Na not only at the A sites but also at the M(4) and M’ sites (see below).
The simplified formula of achyrophanite is (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5. The idealized formula with whole-number coefficients (K2Na)(Fe3+4Ti)O2(AsO4)5 requires Na2O 2.82, K2O 8.57, Fe2O3 29.06, TiO2 7.27, As2O5 52.28, total 100 wt%.
The Gladstone–Dale compatibility index [] for achyrophanite is −0.029 (excellent).
4.5. X-Ray Crystallography and Crystal Structure
Powder XRD data of achyrophanite are given in Table 2. The orthorhombic unit-cell parameters calculated from the powder data are as follows: a = 6.579(2), b = 13.255(3), c = 10.763(4) Å, and V = 938.6(8) Å3.

Table 2.
Powder X-ray diffraction data (d in Å) of achyrophanite.
Single-crystal XRD studies of achyrophanite, including the obtaining of the dataset used for the structure determination, were carried out at room temperature. A full sphere of three-dimensional data was collected. Data reduction was performed using CrysAlisPro Version 1.171.39.46 []. The data were corrected for Lorentz factor and polarization effects. The crystal structure was solved by direct methods and refined using the SHELX software package (version SHELXL-97) [] to R = 0.0447 for 2088 independent reflections with I > 2σ(I). The crystal data and the experimental details are presented in Table 3, atom coordinates and equivalent displacement parameters in Table 4, selected interatomic distances in Table 5, and bond valence calculations in Table 6.

Table 3.
Crystal data, data collection information, and structure refinement details for achyrophanite.

Table 4.
Coordinates and equivalent displacement parameters (Ueq, in Å2) of atoms, site occupancy factors (s.o.f.) and site multiplicities (Q) for achyrophanite.

Table 5.
Selected interatomic distances (Å) in the structure of achyrophanite.

Table 6.
Bond valence calculations for achyrophanite.
5. Discussion
5.1. Structure Description
The crystal structure of achyrophanite (Figure 4a–c) is unique. It is based on the heteropolyhedral (octahedral-tetrahedral) M-T-O pseudo-framework. Cations M centre five crystallographically non-equivalent octahedrally coordinated sites: four of them are fully occupied and Fe3+-dominant [M(1–4)] with admixed Ti, Al, and Mg [and Na in M(4)], whereas the fifth site (M’) is 25% occupied and assumed to contain Cu2+ with subordinate Na and Zn. The cation distribution (Table 4) is based on the refined numbers of electrons, interatomic distances (Table 5) and bond valence calculations (Table 6) and takes into account electron-microprobe data (Table 1). The M(1)O6 and M(4)O6 octahedra share edges to form dimers as well as M(3)O6 and M’O6 octahedra. The neighboring dimers are connected with each other via the M(2)-centered octahedra [each M(2)O6 octahedron shares four oxygen vertices with the octahedra of the dimers] to form an octahedral pseudo-framework (Figure 5a).
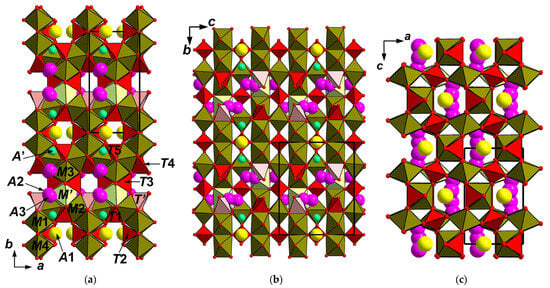

Figure 4.
The crystal structures of achyrophanite (a–c) and synthetic Fe3+AsO4 (d–f): drawn after [], both in three projections. The unit cells are outlined.

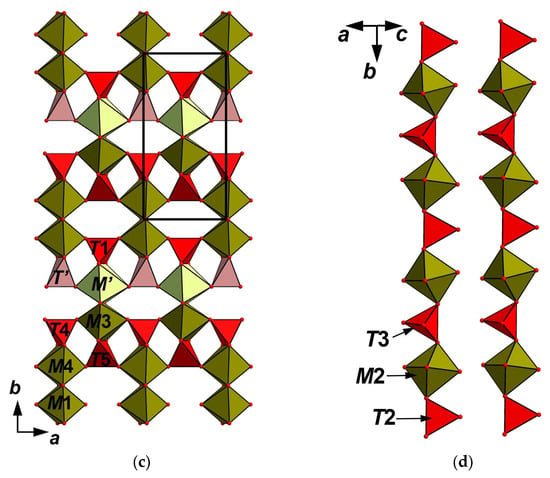
Figure 5.
The octahedral pseudo-framework (a) and heteropolyhedral pseudo-framework (b) built by the heteropolyhedral layers; (c) linked with each other via heteropolyhedral chains; (d) in the structure of achyrophanite. The unit cell is outlined.
Interatomic distances (Table 5) and bond valence calculations (Table 6) undoubtedly confirm the trivalent state of iron in achyrophanite.
The structure of achyrophanite contains six crystallographically non-equivalent tetrahedrally coordinated sites T. Four of them [T(1,2,3,5)] are fully occupied by As5+. The T(4) site is filled by As with admixed lighter components; according to electron-microprobe data, there are S (prevailing), V, P, and Si (Table 1); it is refined as As0.68S0.32, assuming that “S” means all these admixtures together. The T’ site is predominantly vacant: ☐0.75As0.25. TO4 tetrahedra share with MO6 octahedra all vertices [T(2,3)] or two vertices and one edge [T(1,4,5) and T’] and, thus, “reinforce” the linkages in the octahedral pseudo-framework forming the heteropolyhedral pseudo-framework (Figure 5b). This pseudo-framework could also be described as a building unit formed by the (001) layers of octahedral dimers linked by common edges to the T(1,4,5)- and T’-centered tetrahedra (Figure 5c). Adjacent layers are linked with each other via the chains of alternating M(2)O6 octahedra and T(2,3)O4 tetrahedra (Figure 5d) sharing vertices with each other and with polyhedra of the layers.
Large-cation sites are located in the channels of the pseudo-framework (Figure 4a–c). Almost fully occupied site A(1) [K0.47Na0.47☐0.06] and low-occupied site A’ [☐0.94Ca0.03Na0.03] are located at the distance of 2.47 Å from one another and, thus, cannot be filled simultaneously. We do not also exclude that a minor amount of Ca could also be located in the A(1) site. The A(2) [☐0.75K0.25] and A(3) [K0.50☐0.50] sites are located at a distance of 1.24 Å from one another and also cannot be filled simultaneously. These sites are also close to T’- and M’-centered polyhedra (both 25% filled), and, thus, the fragment with T’O4 tetrahedron and M’O6 octahedron and the fragment with A(2)/A(3) sites cannot be occupied jointly.
5.2. Comparative Crystal Chemistry
The above-described heteropolyhedral M-T-O pseudo-framework can be considered as a base of the crystal structure of achyrophanite. The simplified structural formula of this pseudo-framework can be written as M(1−5)M10M’(☐0.75M0.25O1.5)2{T(1−5)(AsO4)10T’[☐0.75(AsO4)0.25]2} (Z = 1). Topologically the same pseudo-framework forms the crystal structure of the synthetic orthorhombic (space group Imam) modification of Fe3+AsO4 (Figure 4d–f) with the following unit-cell dimensions: a = 13.468(2), b = 6.5255(11), c = 10.7678(18) Å, V = 946.3(3) Å3 and Z = 12 [,]. The unit-cell parameters of this compound and achyrophanite are very close. As it is shown in Figure 4, the structure of achyrophanite is in fact a stuffed derivative of the structure of this modification of Fe3+AsO4. The porous character of the latter was emphasized by Bazán et al. [], who reported it as “the new textural porous orthorhombic Fe(AsO4) phase”. Indeed, the pseudo-framework contains two systems of broad channels. In the synthetic Fe3+AsO4, these channels are empty (Figure 4d,f), whereas in achyrophanite, they are stuffed by A cations (Figure 4a–c). This is accompanied by the transition from a non-defect, full-cationic heteropolyhedral pseudo-framework MFe3+12(TAsO4)12 (Z = 1) of the orthorhombic Fe3+AsO4 to the pseudo-framework (M10.5☐1.5)Σ12O3[(AsO4)10.5☐1.5]Σ12 (Z = 1) with vacancy defects in both T and M positions in achyrophanite.
Besides the orthorhombic modification of Fe3+AsO4 [,], a similar octahedral-tetrahedral framework was found in α-Cr3+PO4 [,,], α-Cr3+AsO4 [], and Rh3+PO4 [].
Thus, achyrophanite demonstrates a novel structure type: stuffed, with the defect heteropolyhedral pseudo-framework derivative of the orthorhombic Fe3+AsO4 archetype.
The general topology of the heteropolyhedral M-T-O pseudo-framework in achyrophanite corresponds to the space group Imma (in standard setting) as well as one in the synthetic Fe3+AsO4—[,]. However, our attempt to refine the structure of achyrophanite in this space group was not successful. The symmetry lowering to space group P2221 found for achyrophanite is caused by significantly different occupancies of several cationic sites in the M-T-O pseudo-framework. Perhaps it is related to the presence of large-cation sites in the mineral in contrast with Fe3+AsO4.
Achyrophanite is microtwinned: twinning by merohedry Class I [] with the twin domains ratio 53/47 was found in the studied crystal. Probably the symmetric relationship between the structure of achyrophanite and the archetype structure of the orthorhombic Fe3+AsO4 is a cause of this merohedral twinning.
In unit-cell dimensions, achyrophanite and the orthorhombic Fe3+AsO4 are close to katiarsite, KTiO(AsO4), a mineral recently discovered in the same Arsenatnaya fumarole []. Katiarsite is an analogue of the well-studied synthetic compound named KTA, which belongs to the well-known KTP [KTiO(PO4)] structure type. The crystal structure of KTA is based on undulating chains of corner-linked TiO6 octahedra. These chains are cross-linked by isolated AsO4 tetrahedra to form a three-dimensional mixed framework containing K+ cations in channels [,,]. However, the structures of achyrophanite, Fe3+AsO4, and KTP-type compounds, including katiarsite, are quite different, although the undulating octahedral chains of the same configuration could be identified in the octahedral pseudo-framework of achyrophanite. Single octahedral chains in KTA lie in the bc plane and are parallel to [011] and [01-1] (Figure 6a); in achyrophanite such chains lying in the ac plane are interconnected (Figure 6b). These structural units are presumably responsible for the similarity of the a and c unit-cell parameters of achyrophanite with the b and c, respectively, of KTA. In achyrophanite there are two such layer fragments formed by octahedral chains and AsO4 tetrahedra per unit cell (Figure 6d). In KTA there are also two separate (100) “layers” containing the octahedral chains per unit cell (Figure 6c). These peculiarities determine the analogy of the b unit-cell parameter of achyrophanite (13.249 Å) with the a parameter of KTA (13.174 Å). The arrangement of AsO4 tetrahedra is also close in both achyrophanite and KTA (Figure 7). It is worthy to note that orthorhombic Fe3+AsO4 was obtained from the synthetic (NH4)[Fe3+(AsO4)F] as the result of its heating in air at 525 °C. (NH4)[Fe3+(AsO4)F] is related to KTA in stoichiometry, space group, unit-cell dimensions, and the structure of the heteropolyhedral Fe-As-O-F framework []. Their comparison highlights the relationship between all the above-discussed compounds. Table 7 presents unit-cell dimensions of achyrophanite and these structurally related compounds.
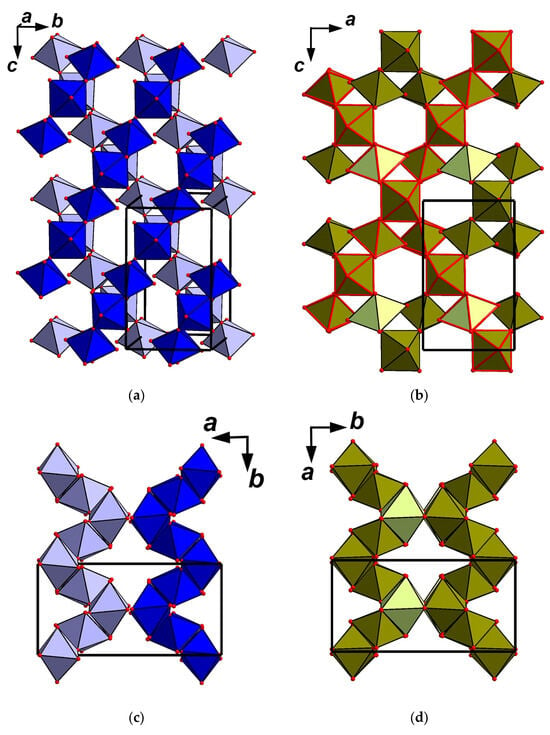
Figure 6.
Undulating octahedral chains in the structure of KTiO(AsO4) = KTA [the fragment is slightly tilted to show no polymerization of the chains; the octahedra of the lower level are light blue] (a): drawn after [] and intersecting octahedral chains of the same configuration (polyhedra of two chains are shown with red edges) in the structure of achyrophanite (b). The arrangement of the octahedral chains in the structure of KTA along the c axis (c) and the fragment of the octahedral pseudo-framework in the structure of achyrophanite along the c axis (d). The unit cells are outlined.
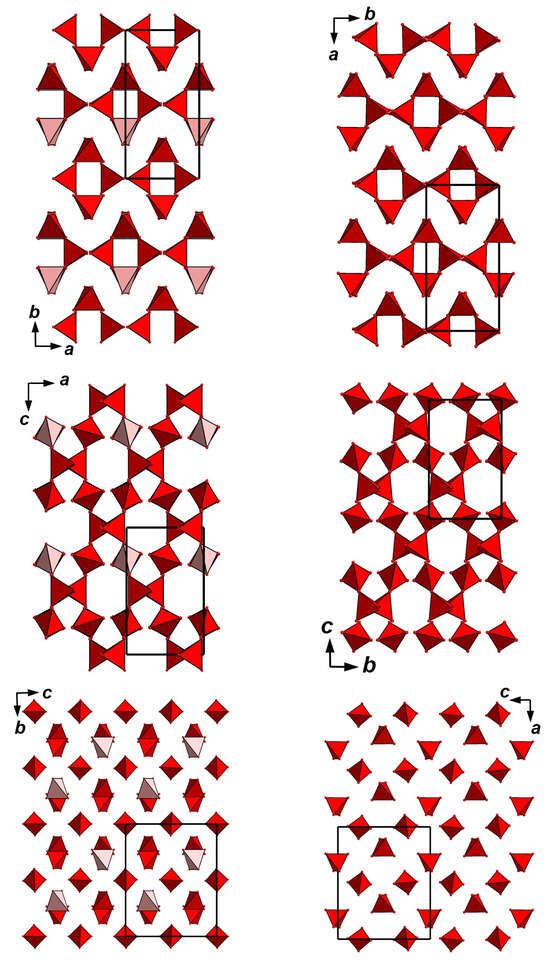
Figure 7.
The arrangement of AsO4 tetrahedra in the structures of achyrophanite (left column) and KTiO(AsO4) = KTA (right column: drawn after []). The unit cells are outlined. For legend see Figure 4a.

Table 7.
Unit-cell dimensions of achyrophanite and structurally related compounds.
5.3. Genetic Notes
Achyrophanite is a fumarolic mineral crystallized at temperatures not lower than 430–450 °C. It was deposited directly from the gas phase as a volcanic sublimate or, more probably, formed as a result of the interaction between fumarolic gas and basalt scoria. The latter could be a source of Mg, Al, and Ti, which have low volatilities in such post-volcanic systems at temperatures up to 400–500 °C [,].
Evolution of gas composition in the fumarole mineral-forming system results in alteration of achyrophanite. We observed partial or complete pseudomorphs of arsenatrotitanite, ideally NaTiO(AsO4), sometimes together with bradaczekite NaCu4(AsO4)3, after crystals of achyrophanite (Figure 8). They are associated with sylvite, halite, tenorite, hematite, lehmannite, badalovite, johillerite, rutile, and pseudobrookite. Gas evolution is probably accompanied by Na activity increase.

Figure 8.
Pseudomorph of arsenatrotitanite after achyrophanite crystals. SEM (SE) image.
The discovery of achyrophanite seems interesting firstly from the crystal chemical viewpoint: this mineral demonstrates an original structure type and unusual cation substitution schemes that are probably caused by its origin—in a volcanic fumarole system with non-stationary physical and chemical parameters.
Author Contributions
Conceptualization, I.V.P., N.V.Z. and D.Y.P.; methodology, I.V.P., N.V.Z., N.N.K. and S.N.B.; fieldworks: I.V.P., A.A.A., A.G.T. and E.G.S.; investigation, I.V.P., N.V.Z., D.I.B., M.F.V., A.A.A., A.G.T. and P.S.Z.; original manuscript draft preparation, I.V.P. and N.V.Z.; manuscript review and editing, I.V.P., N.V.Z., N.N.K., S.N.B. and D.Y.P.; figures, I.V.P., N.V.Z., N.N.K. and M.F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work in part of mineralogical studies, crystal chemical analysis, crystal structure solution and Raman spectroscopy was supported by the Russian Science Foundation, grant no. 25-17-00005 (I.V.P., D.Y.P., N.V.Z., M.F.V.). The powder XRD study was performed at the Center for X-Ray Diffraction Research of the Science Park of St. Petersburg State University within the framework of project 125021702335-5.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pekov, I.V.; Koshlyakova, N.N.; Zubkova, N.V.; Lykova, I.S.; Britvin, S.N.; Yapaskurt, V.O.; Agakhanov, A.A.; Shchipalkina, N.V.; Turchkova, A.G.; Sidorov, E.G. Fumarolic Arsenates—A Special Type of Arsenic Mineralization. Eur. J. Mineral. 2018, 30, 305–322. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Agakhanov, A.A.; Vigasina, M.F.; Yapaskurt, V.O.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; Zhitova, E.S.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. XX. Evseevite, Na2Mg(AsO4)F, the first natural arsenate with antiperovskite structure. Mineral. Mag. 2023, 87, 839–848. [Google Scholar] [CrossRef]
- The Great Tolbachik Fissure Eruption; Fedotov, S.A., Markhinin, Y.K., Eds.; Cambridge University Press: New York, NY, USA, 1983. [Google Scholar]
- Vergasova, L.P.; Filatov, S.K. A study of volcanogenic exhalation mineralization. J. Volcanol. Seismol. 2016, 10, 71–85. [Google Scholar] [CrossRef]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.V.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik Volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual Silicate Mineralization in Fumarolic Sublimates of the Tolbachik Volcano, Kamchatka, Russia—Part 1: Neso-, Cyclo-, Ino- And Phyllosilicates. Eur. J. Mineral. 2020, 32, 101–119. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zap. Ross. Mineral. Obs. 2017, 146, 104–107. (In Russian) [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Mandarino, J.A. The Gladstone-Dale relationship, Part IV. The compatibility concept and its application. Can. Mineral. 1981, 19, 441–450. [Google Scholar]
- Agilent Technologies. CrysAlisPro Software System; Version 1.171.37.34; Agilent Technologies UK Ltd.: Oxford, UK, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Bazán, B.; Mesa, J.L.; Pizarro, J.L.; Aguayo, A.T.; Arriortua, M.I.; Rojo, T. Fe(AsO4): A new iron(III) arsenate synthesized from thermal treatment of (NH4)[Fe(AsO4)F]. Chem. Commun. 2003, 5, 622–623. [Google Scholar] [CrossRef]
- Bazán, B.; Mesa, J.L.; Pizarro, J.L.; Rodríguez-Fernández, J.; Sánchez-Marcos, J.; Roig, A.; Molins, E.; Arriortua, M.I.; Rojo, T. Thermal transformation of (NH4)[Fe(AsO4)F] into the new textural porous orthorhombic Fe(AsO4) phase. Crystal structures, thermal behavior, spectroscopic and magnetic properties. Chem. Mater. 2004, 16, 5249–5259. [Google Scholar] [CrossRef]
- Glaum, R.; Gruehn, R.; Möller, M. Darstellung und Struktur von α-CrPO4. Beiträge zum thermischen Verhalten von wasserfreien Phosphaten. I. Z. Anorg. Allg. Chem. 1986, 543, 111–116. [Google Scholar] [CrossRef]
- Attfield, J.P.; Cheetham, A.K.; Cox, D.E.; Sleight, A.W. Synchrotron X-ray and neutron powder diffraction studies of the structure of α-CrPO4. J. Appl. Crystallogr. 1988, 21, 452–457. [Google Scholar] [CrossRef]
- Attfield, J.P.; Battle, P.D.; Cheetham, A.K.; Johnson, D.C. Magnetic structures and properties of α-CrPO4 and α-CrAsO4. Inorg. Chem. 1989, 28, 1207–1213. [Google Scholar] [CrossRef]
- Rittner, P.; Glaum, R. Kristallzüchtung und Einkristallstrukturverfeinerungen der Rhodium(III)-phosphate RhPO4 und RhP3O9. Z. Krist. 1994, 209, 162–169. [Google Scholar] [CrossRef]
- Nespolo, M.; Ferraris, G. Twinning by syngonic and metric merohedry. Analysis, classification and effects on the diffraction pattern. Z. Krist. 2000, 215, 77–81. [Google Scholar] [CrossRef]
- Pekov, I.V.; Yapaskurt, V.O.; Britvin, S.N.; Zubkova, N.V.; Vigasina, M.F.; Sidorov, E.G. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. V. Katiarsite, KTiO(AsO4). Mineral. Mag. 2016, 80, 639–646. [Google Scholar] [CrossRef]
- Mayo, S.C.; Thomas, P.A.; Teat, S.J.; Loiacono, G.M.; Loiacono, D.N. Structure and nonlinear optical properties of KTiOAsO4. Acta Crystallogr. 1994, 50, 655–662. [Google Scholar] [CrossRef]
- Northrup, P.A.; Parise, J.B.; Cheng, L.K.; Cheng, L.T.; McCarron, E.M. High-temperature single-crystal X-ray diffraction studies of potassium and (cesium, potassium) titanyl arsenates. Chem. Mater. 1994, 6, 434–440. [Google Scholar] [CrossRef]
- Novikova, N.E.; Verin, I.A.; Sorokina, N.I.; Alekseeva, O.A.; Tseitlin, M.; Roth, M. Structure of KTiOAsO4 single crystals at 293 and 30 K. Crystallogr. Rep. 2010, 55, 412–423. [Google Scholar] [CrossRef]
- Symonds, R.B.; Reed, M.H. Calculation of multicomponent chemical equilibria in gas-solid-liquid systems: Calculation methods, thermochemical data, and applications to studies of high-temperature volcanic gases with examples from Mount St. Helens. Am. J. Sci. 1993, 293, 758–864. [Google Scholar] [CrossRef]
- Churakov, S.V.; Tkachenko, S.I.; Korzhinskii, M.A.; Bocharnikov, R.E.; Shmulovich, K.I. Evolution of composition of high-temperature fumarolic gases from Kudryavy volcano, Iturup, Kuril Islands: The thermodynamic modeling. Geochem. Int. 2000, 38, 436–451. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).