Medieval Pb (Cu-Ag) Smelting in the Colline Metallifere District (Tuscany, Italy): Slag Heterogeneity as a Tracer of Ore Provenance and Technological Process
Abstract
1. Introduction
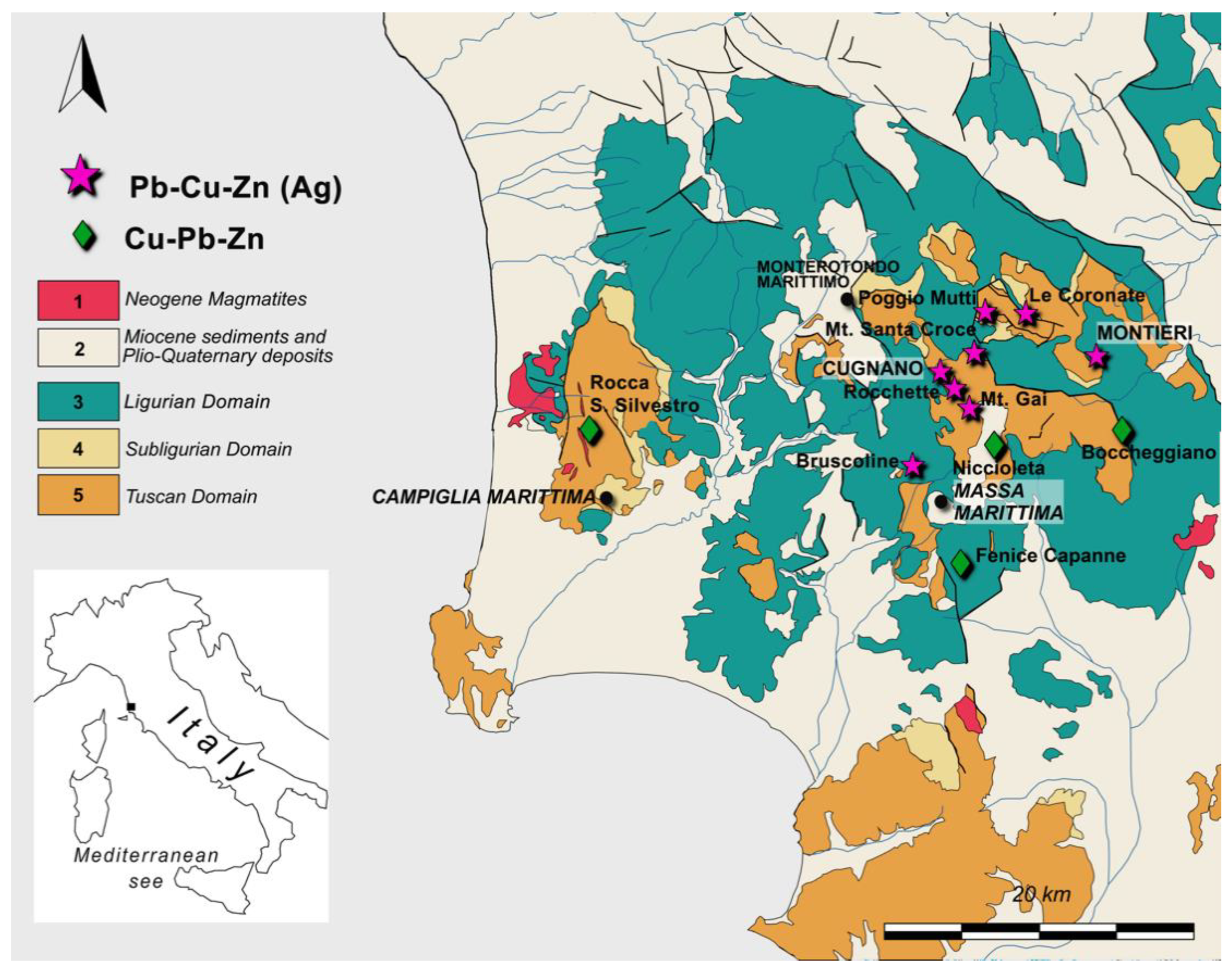
2. Geology and Ore Mineralogy
3. Materials and Methods
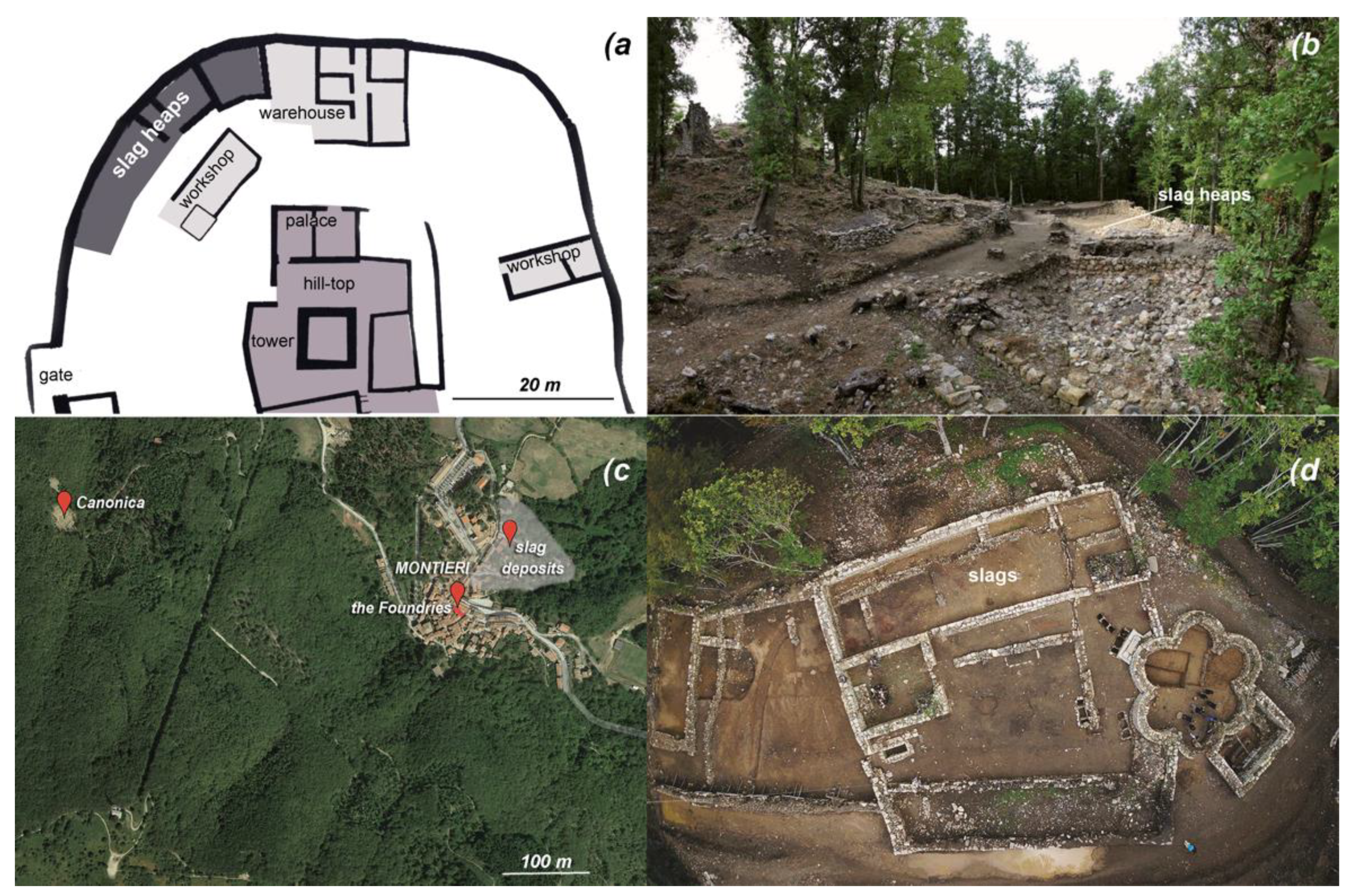
3.1. Slag-Heap Sampling: Cugnano Site
3.2. Slag-Heap Sampling: Montieri Site
3.3. Analytical Methods
4. Results
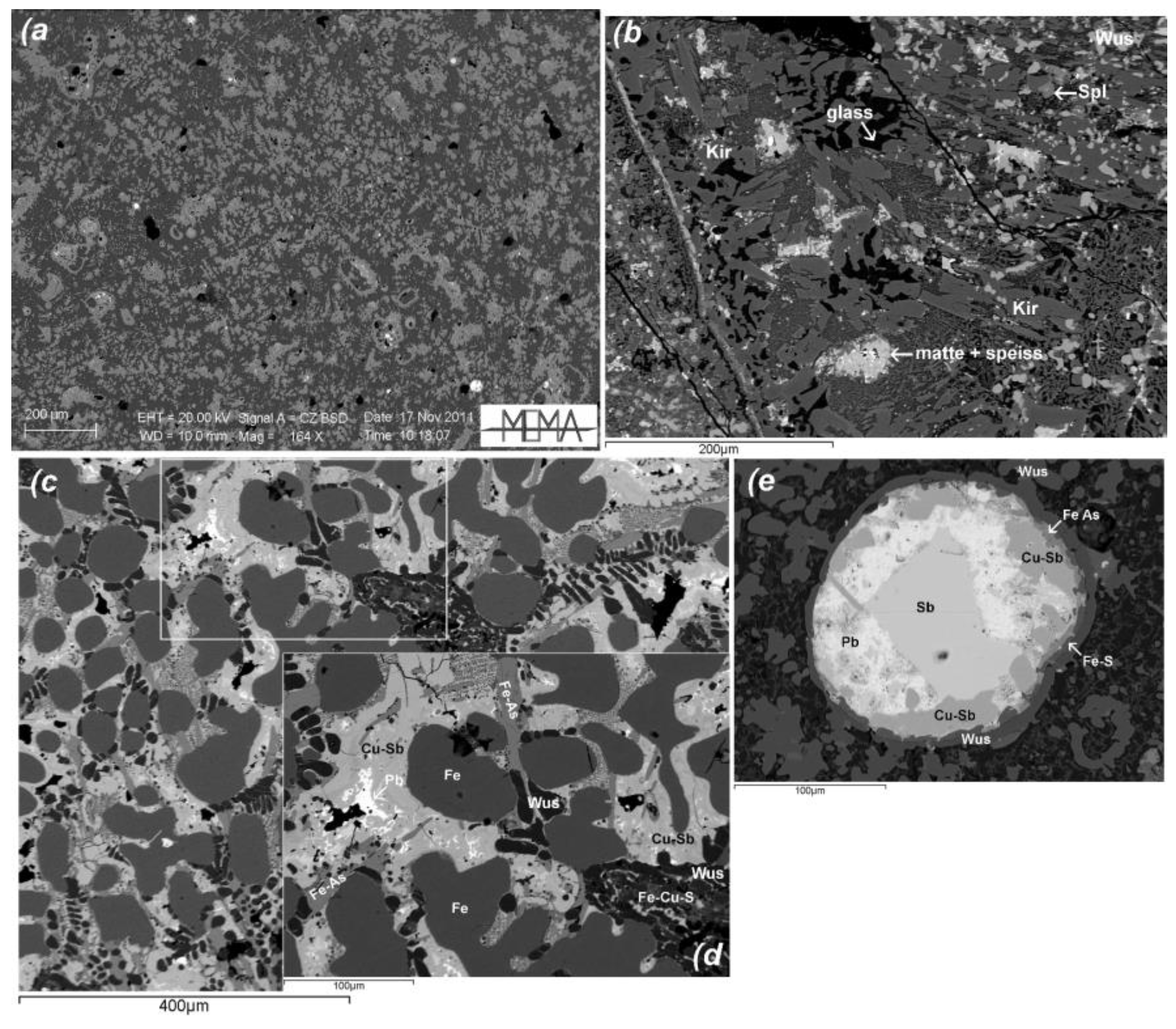
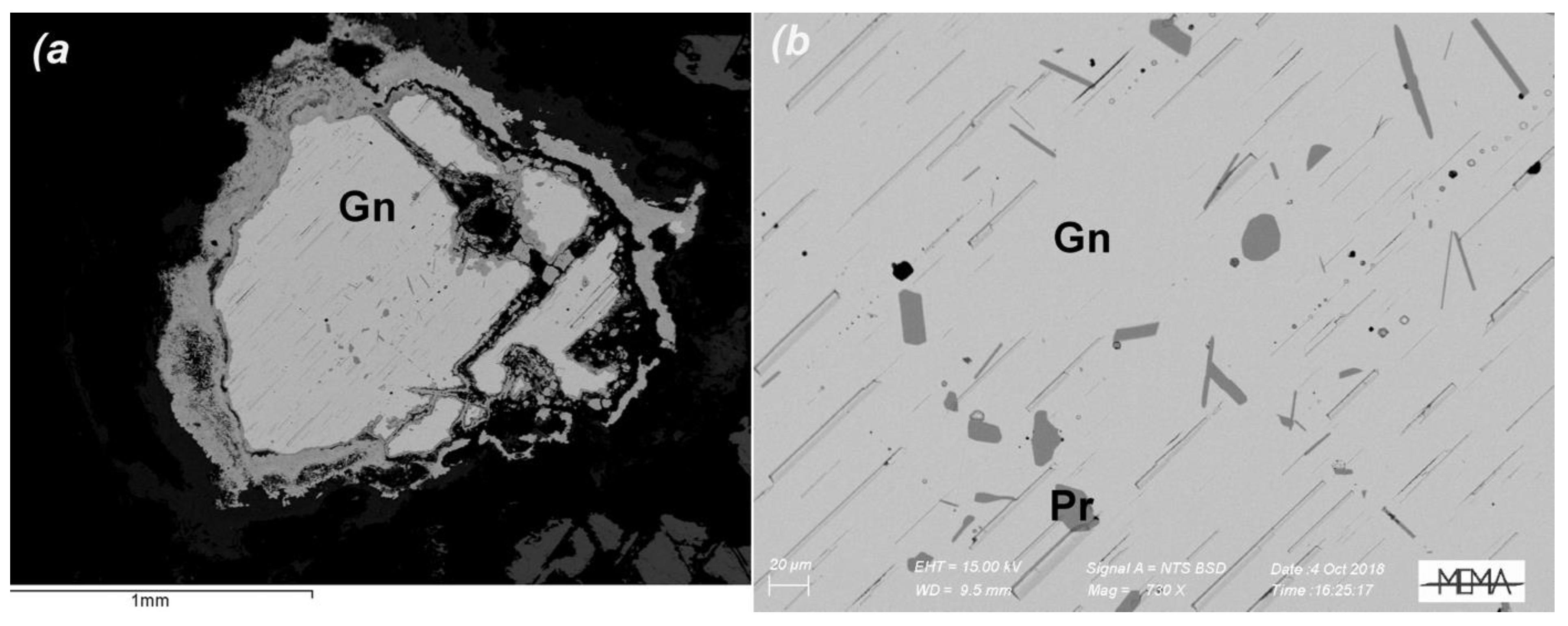
4.1. Slags from Cugnano: Chemical Composition and Mineralogy
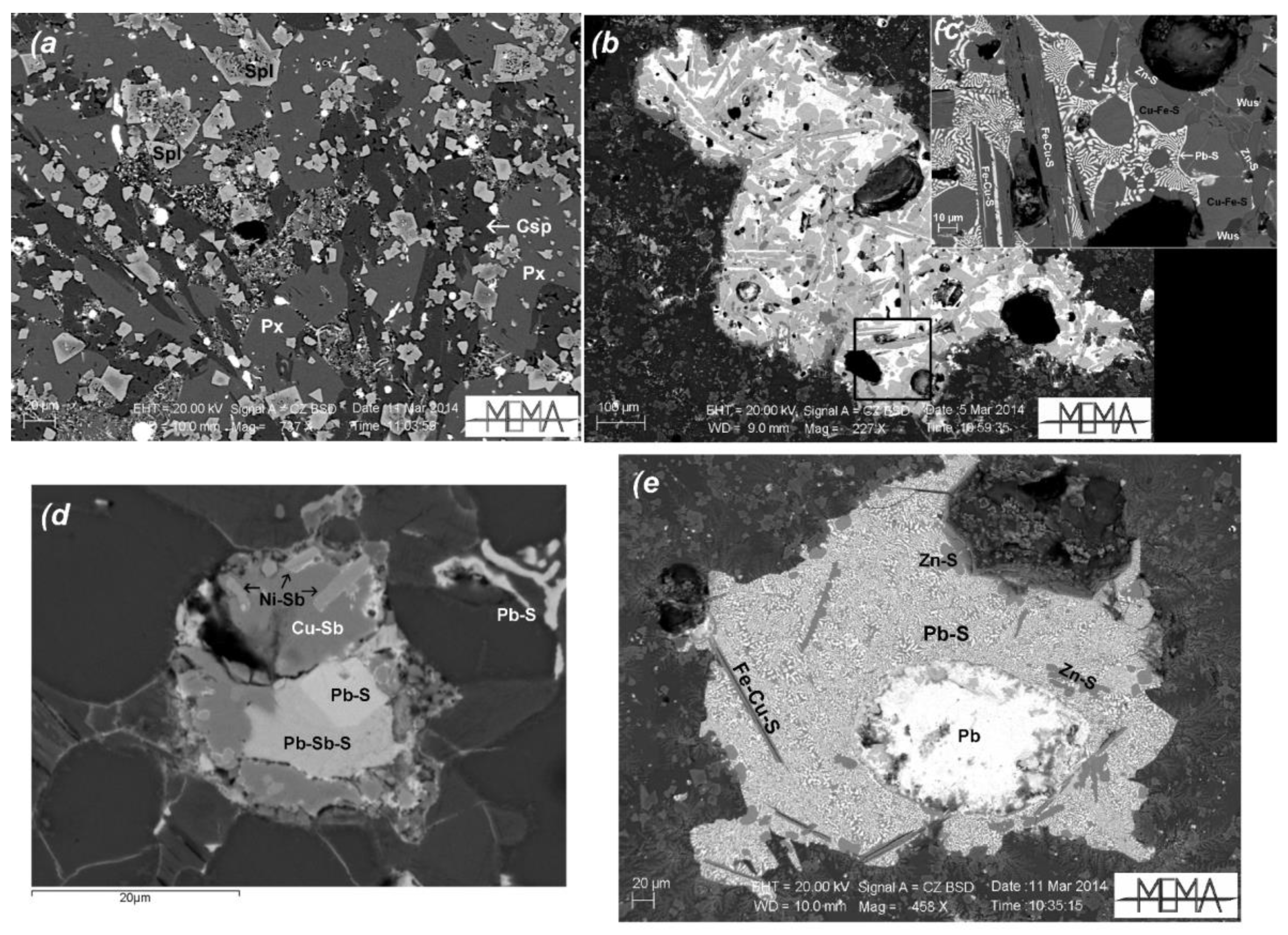
4.2. Slags from Montieri: Chemical Composition and Mineralogy
5. Discussion
5.1. Matte Slags
5.2. Matte-Poor Slags
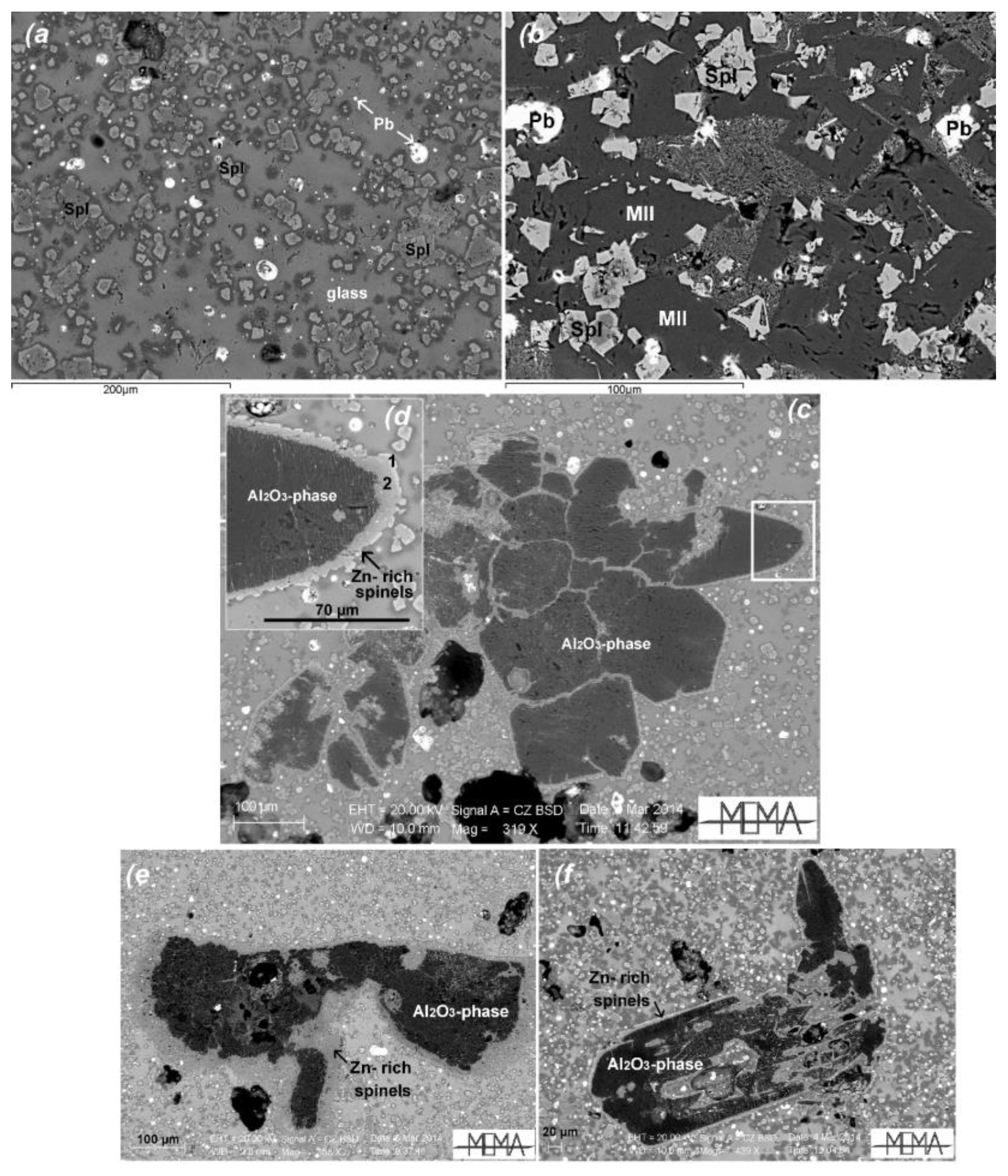
5.3. Al2O3 Aggregates as Tracers of Depuration Process
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francovich, R.; Parenti, R. Rocca San Silvestro e Campiglia. Prime Indagini Archeologiche; All’Insegna del Giglio: Florence, Italy, 1987; p. 208. ISBN 887-814-098-8. [Google Scholar]
- Mascaro, I.; Benvenuti, M.; Tanelli, G. Mineralogy Applied to Archaeometallurgy: An Investigation of Medieval Slags from Rocca San Silvestro (Campiglia M.Ma, Tuscany). Sci. Technol. Cult. Herit. 1995, 4, 87–98. [Google Scholar]
- Benvenuti, M.; Bianchi, G.; Bruttini, J.; Buonicontri, M.; Chiarantini, L.; Dallai, L.; Di Pasquale, G.; Donati, A.; Grassi, F.; Pescini, V. Studying the Colline Metallifere Mining Area in Tuscany: An Interdisciplinary Approach. In Proceedings of the Research and Preservation of Ancient Mining Areas, Yearbook of the Institute Europa Subterranea, 9th International Symposium on Archaeological Mining History, MuSe-Trento, Trento, Italy, 5–8 June 2014; pp. 5–8. [Google Scholar]
- Bianchi, G. Dominare e gestire un territorio. Ascesa e sviluppo delle ‘signorie forti’ nella Maremma toscana del Centro Nord tra X e metà XII secolo. Archeol. Mediev. 2010, XXXVII, 93–103. [Google Scholar]
- Bianchi, G.; Bruttini, J.; Grassi, F. Lo scavo della Canonica di san Niccolò a Montieri (Gr). Not. Soprintend. Beni Archeol. Toscana 2012, 8, 564–567. [Google Scholar]
- Bianchi, G.; Dallai, L.; Guideri, S. Indicatori di produzione per la ricostruzione dell’economia di un paesaggio minerario: Le Colline Metallifere nella Toscana Medievale. In Proceedings of the V Congresso nazionale di Archeologia Medievale, Foggia-Manfredonia, Italy, 30 September–3 October 2009; pp. 638–643. [Google Scholar]
- Dallai, L.; Francovich, R. Archaeologia di miniera ed insediamenti minerari delle Colline Metallifere grossetane nel Medioevo. In Il Calore Della Terra: Contributo Alla Storia Della Geotermia in Italia; Cataldi, R., Ciardi, M., Eds.; ETS: Pisa, Italy, 2005; pp. 126–142. ISBN 978-884-671-407-7. [Google Scholar]
- Bianchi, G.; Bruttini, J.; Dallai, L. Sfruttamento e ciclo produttivo dell’allume e dell’argento nel territorio delle Colline Metallifere grossetane. In Risorse Naturali e Attività Produttive: Ferento a Confronto con Altre Realtà, Proceedings of the II Convegno di Studi in Memoria di Gabriella Maetzke, Viterbo, Italy, 27–28 April 2010; De Minicis, E., Pavolini, C., Eds.; Tipografia Agnesotti: Viterbo, Italy, 2011; pp. 249–281. [Google Scholar]
- Dallai, L. L’allumiera Di Monteleo Nel Territorio Di Monterotondo Marittimo. Mélanges l’École Française Rome-Moyen Âge 2014, 126, 245–258. [Google Scholar] [CrossRef]
- Dallai, L. Lo scavo dell’Allumiera di Monteleo. Nuovi dati per la produzione dell’allume alunitico nel tardo Medioevo, In I Paesaggi Dell’allume Archeologia Della Produzione Ed Economia Di Rete (Alum Landscapes Archaeology of Production and Network Economy); Dallai, L., Bianchi, G., Romana Stasolla, F., Eds.; All’Insegna del Giglio: Sesto Fiorentino, Italy, 2020; pp. 115–129. ISBN 978-88-7814-989-2. [Google Scholar]
- Lattanzi, P.; Benvenuti, M.; Costagliola, P.; Tanelli, G. An overview on recent research on the metallogeny of Tuscany, with special reference to the Apuane Alps. Mem. Soc. Geol. It. 1994, 48, 613–625. [Google Scholar]
- Pratellesi, G. Studio Giacimentologico Delle Mineralizzazioni Argentifere Della Zona di Massa Marittima—Montieri (Grosseto). Unpublished Thesis, University of Florence, Florence, Italy, 1984. [Google Scholar]
- Benvenuti, M.; Chiarantini, L.; Cicali, C.; Villa, I.M.; Volpi, V. La produzione d’argento nel distretto minerario di Montieri-Massa Marittima (Colline Metallifere, Toscana meridionale) Alcune considerazioni su dati recenti. In Les Métaux Précieux en Méditerranée Médiévale: Exploitations, Transformations, Circulations, Proceedings of the Colloque International d’Aix-en-Provence, France, 6–8 October 2016; Minvielle Larousse, N., Bailly-Maître, M.C., Bianchi, C., Eds.; Presses Universitaires de Provence: Aix-Marseille, France, 2019; pp. 41–51. [Google Scholar]
- Haupt, T. Esperienze Dell’interno dei Monti e Della Industria Delle Miniere, Annali di Miniere, 1; Le Monnier: Florence, Italy, 1851; pp. 1–35. [Google Scholar]
- Lotti, B. Il Poggio di Montieri (provincia di Grosseto). Boll. Regio Com. Geol. 1976, 7, 111–122. [Google Scholar]
- Lotti, B. Descrizione Geologico-Mineraria dei Dintorni di Massa Marittima in Toscana, Memorie Descrittive Della Carta Geologica D’italia; Tipografia nazionale: Rome, Italy, 1983. [Google Scholar]
- Bruttini, J.; Fichera, G.; Grassi, F. Un insediamento a vocazione mineraria nella Toscana medievale: Il caso di Cugnano nelle Colline Metallifere. In Proceedings of the V Congresso nazionale di Archeologia Medievale, Foggia-Manfredonia, Italy, 30 September–3 October 2009; pp. 306–312. [Google Scholar]
- Bianchi, G.; Bruttini, J.; Quiros Castillo, J.A.; Ceres, F.; Lorenzini, S.M. La lavorazione del metallo monetabile nel castello di Cugnano (Monterotondo M.mo): Lo studio delle aree produttive dei secoli centrali (XI-XII secolo). In Proceedings of the VI Congresso Nazionale di Archaeologia Medievale, L’Aquila, Italy, 12–15 September 2012; pp. 644–649. [Google Scholar]
- Lisini, A. Le monete e le zecche di Volterra, Montieri, Berignone a Casole. Riv. Numis. Ital. 1909, 22, 253–302. [Google Scholar]
- Villoresi, R. Classificazione cronologica delle emissioni medievali dei vari tipo monetali della zecca di Volterra. Rass. Volterrana 1994, 70, 153–170. [Google Scholar]
- Volpe, G. Medio evo Italiano; Sansoni: Florence, Italy, 1961. [Google Scholar]
- Bruttini, J.; Grassi, F. Archeologia Urbana a Montieri: Lo Scavo Dell’edificio de “Le Fonderie” in via Delle Fonderie. FOLD&R FastiOnLine Doc. Res. 2010, 199, 1–25. [Google Scholar]
- Donaldson, C.H. An experimental investigation of olivine morphology. Contrib. Mineral. Petrol. 1976, 57, 187–213. [Google Scholar] [CrossRef]
- Ströbele, F.; Wenzel, T.; Kronz, A.; Hildebrandt, L.H.; Markl, G. Mineralogical and Geochemical Characterization of High-Medieval Lead–Silver Smelting Slags from Wiesloch near Heidelberg (Germany)—An Approach to Process Reconstruction. Archaeol. Anthropol. Sci. 2010, 2, 191–215. [Google Scholar] [CrossRef][Green Version]
- Manasse, A.; Mellini, M. Chemical and Textural Characterisation of Medieval Slags from the Massa Marittima Smelting Sites (Tuscany, Italy). J. Cult. Herit. 2002, 187–198. [Google Scholar] [CrossRef]
- Costagliola, P.; Benvenuti, M.; Chiarantini, L.; Bianchi, S.; Di Benedetto, F.; Paolieri, M.; Rossato, L. Impact of Ancient Metal Smelting on Arsenic Pollution in the Pecora River Valley, Southern Tuscany, Italy. Appl. Geochem. 2008, 23, 1241–1259. [Google Scholar] [CrossRef]
- Santarelli, S. Archeometallurgia delle scorie: Il sito medievale di Rocchette Pannocchieschi nel Territorio di Massa Marittima. Unpublished Thesis, University of Siena, Siena, Italy, 2000. [Google Scholar]
- Hauptmann, A.; Pernicka, E.; Wagner, G.A. Untersuchungen zur Prozesstechnik und zum Alter der frühen Blei-Silbergewinnung auf Thasos. Der. Anschnitt. 1988, 6, 88–112. [Google Scholar]
- Kresten, P. Melting points and viscosities of ancient slags: A discussion. Hist. Metall. 1986, 20, 43–45. [Google Scholar]
- Ettler, V.; Cervinka, R.; Johan, Z. Mineralogy of Medieval slags from lead and silver smelting (Bohutín, PrÍbram District, Czech Republic): Towards Estimation Of Historical Smelting Conditions. Archaeometry 2009, 51, 987–1007. [Google Scholar] [CrossRef]
- Hauptmann, A. The Archaeometallurgy of Copper; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; ISBN 978-3-540-72237-3. [Google Scholar]
- Osborn, E.F.; Muan, A. Phase Equilibrium Diagrams of Oxide Systems; Ceramic Foundation: Columbus, GA, USA, 1960. [Google Scholar]
- Bachmann, H.G. The Identification of Slags from Archaeological Sites; Routledge: London, UK, 1982; ISBN 978-090-585-310-9. [Google Scholar]
- Bachmann, H.G.; Lutz, C.; Thiemann, U. Schlackenviskositäten. Der. Anschnitt. 1989, 7, 137–140. [Google Scholar]
- Rehren, T.; Schneider, J.; Bartels, C. Medieval lead-silver smelting in the Siegerland, West Germany. Hist. Metall. 1999, 33, 73–84. [Google Scholar]
- Flament, J. Les Métallurgies Associées de la fin du XIIIe Siècle au XVe Siècle L’argent, les Cuivres et le Plomb à Castel-Minier (Ariège, France). Ph.D. Thesis, University of Orleans, Orléans, France, 2017. Available online: https://tel.archives-ouvertes.fr/tel-01956682/document (accessed on 1 December 2020).
- Ettler, V.; Johan, Z.; Zavřel, J.; Selmi Wallisová, M.; Mihaljevič, M.; Šebek, O. Slag Remains from the Na Slupi Site (Prague, Czech Republic): Evidence for Early Medieval Non-Ferrous Metal Smelting. J. Archaeol. Sci. 2015, 53, 72–83. [Google Scholar] [CrossRef]
- Martinón-Torres, M.; Freestone, I.C.; Hunt, A.; Rehren, T. Mass-Produced Mullite Crucibles in Medieval Europe: Manufacture and material properties. J. Am. Ceram. Soc. 2008, 91, 2071–2074. [Google Scholar] [CrossRef]
- Lee, W.E.; Zhang, S. Melt corrosion of oxide-carbon refractories. Int. Mater. Rev. 1999, 44, 77–104. [Google Scholar] [CrossRef]
- Zhao, B.; Hayes, P.C.; Jak, E. Phase Equilibria Studies in the System ZnO-“FeO”-Al2O3-CaO-SiO2 Relevant to Imperial Smelting Furnace Slags: Part I. Metall. Mater. Trans. B 2010, 41, 374–385. [Google Scholar] [CrossRef]
- Zhao, B.; Hayes, P.C.; Jak, E. Phase Equilibria Studies in the System ZnO-“FeO”-Al2O3-CaO-SiO2 Relevant to Imperial Smelting Furnace Slags: Part II. Metall. Mater. Trans. B 2010, 41, 386–395. [Google Scholar] [CrossRef]
- Craddock, P.T. Early Metal Mining and Production; Edinburgh University: Edinburgh, UK, 1995; ISBN 074-860-498-7/978-074-860-498-2. [Google Scholar]
- Bayley, J. Medieval precious metal refining: Archaeology and contemporary texts compared. In Archaeology, History and Science: Integrating Approaches to Ancient Materials; Martinón-Torres, M., Rehren, T., Eds.; Routledge: London, UK, 2008; pp. 131–150. ISBN 159-874-340-6/978-1598743401. [Google Scholar]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Percy, J. The Metallurgy of Lead: Including Desilverisartion and Cupellation; Jhon Murray: London, UK, 1870. [Google Scholar]
- Tylecote, R.F. A History of Metallurgy; CRC Press: Boca Raton, FL, USA, 1992; ISBN 978-0-901462-88-6. [Google Scholar]
- Cuteri, F.; Mascaro, I. Colline Metallifere, Inventario del Patrimonio Minerario e Mineralogico, Aspetti Naturalistici e Storico-Archeologici; Edizioni Regione Toscana: Florence, Italy, 1995. [Google Scholar]
- Dill, H.G. The geology of aluminium phosphates and sulphates of the alunite group minerals: A review. Earth Sci. Rev. 2001, 53, 35–93. [Google Scholar] [CrossRef]
- Agricola, G. De Re Metallica, Translated from the First Latin Edition of 1556 by Herbert Clark Hoover and Lou Henry Hoover; Dover Publications Inc.: New York, NY, USA, 1950. [Google Scholar]
- Özacar, M.; Ayhan Sengil, I. Adsorption of reactive dyes on calcined alunite from aqueous solutions. J. Hazard. Mater. 2003, 98, 211–224. [Google Scholar] [CrossRef]
- Fink, W.L.; Van Horn, K.R.; Pazour, H.A. Thermal decomposition of Alunite. Ind. Eng. Chem. 1931, 23, 1248–1249. [Google Scholar] [CrossRef]



| Site | Gn | Ttr | Py | Sp | Ccp | Cv | Fe-Oxhydroxides | Cu Carbonates | Gangue |
|---|---|---|---|---|---|---|---|---|---|
| Montieri | XX | XX | X | XX | tr | tr | X | X | Cal, Fl, Qz, Sm, Mn-ochre |
| Cugnano | XX | XX | XX | X | X | XX | X | Cal |
| Site | Cugnano | Montieri | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich | Matte Fe Rich |
| Sample | A1-13011 | A2-13019 | A3-13009 | A3-13028 | B2-13019 | B3-13016 | C1-13009 | C1-13011 | C1-13022 | C1-13028 | AG-13011 | E-13022 | S1-13022 | BO2 | DO1 ° | BO1 * | CO1 * |
| SiO2 | 22.75 | 15.96 | 15.98 | 18.51 | 18.75 | 11.11 | 19.02 | 20.28 | 15.46 | 16.20 | 21.41 | 22.66 | 13.91 | 23.68 | 18.36 | 28.0 | 23.5 |
| TiO2 | 0.44 | 0.27 | 0.35 | 0.35 | 0.33 | 0.24 | 0.40 | 0.38 | 0.28 | 0.24 | 0.36 | 0.49 | 0.39 | 0.43 | 0.40 | n.d. | n.d. |
| Al2O3 | 7.68 | 5.24 | 5.83 | 5.75 | 6.25 | 4.18 | 7.26 | 6.44 | 5.39 | 4.60 | 5.80 | 10.26 | 5.67 | 7.64 | 5.59 | 8.7 | 7.0 |
| Fe2O3 | 52.58 | 64.10 | 55.09 | 57.63 | 58.51 | 65.53 | 54.62 | 55.37 | 63.96 | 63.98 | 58.63 | 52.01 | 53.29 | 47.79 | 54.19 | n.d. | n.d. |
| as FeO | 47.31 | 57.68 | 49.57 | 51.86 | 52.65 | 58.96 | 49.15 | 49.82 | 57.55 | 57.57 | 52.76 | 46.80 | 47.95 | 43.00 | 50.56 | 42.2 | 37.1 |
| MnO | 0.39 | 0.32 | 0.35 | 0.36 | 0.24 | 0.20 | 0.46 | 0.32 | 0.33 | 0.24 | 0.23 | 0.47 | 0.19 | 0.67 | 0.44 | n.d. | 0.4 |
| MgO | 1.52 | 0.94 | 1.15 | 1.23 | 0.98 | 0.75 | 1.29 | 1.16 | 0.96 | 0.91 | 1.10 | 1.62 | 0.80 | 1.33 | 1.04 | 0.9 | 1.1 |
| CaO | 9.73 | 12.88 | 17.08 | 15.31 | 7.41 | 5.89 | 11.99 | 9.48 | 11.70 | 15.11 | 10.43 | 7.56 | 4.56 | 14.25 | 10.98 | 5.9 | 14.2 |
| K2O | 2.01 | 1.18 | 1.60 | 1.26 | 1.34 | 1.06 | 1.95 | 2.00 | 1.15 | 1.15 | 1.97 | 1.59 | 1.49 | 1.68 | 1.81 | 2.1 | 1.6 |
| Na2O | 0.26 | 0.22 | 0.21 | 0.24 | 0.19 | 0.16 | 0.30 | 0.34 | 0.17 | 0.20 | 0.31 | 0.22 | 0.15 | 0.48 | 0.69 | n.d. | n.d. |
| P2O5 | 0.25 | 0.22 | 0.26 | 0.22 | 0.23 | 0.22 | 0.30 | 0.18 | 0.23 | 0.19 | 0.29 | 0.26 | 0.21 | 0.57 | n.d. | n.d. | n.d. |
| TOT/S | 1.90 | 1.53 | 1.04 | 1.45 | 1.06 | 3.01 | 2.85 | 2.88 | 2.29 | 0.94 | 1.72 | 1.18 | 1.94 | 2.04 | 2.14 | 6.4 | 5.7 |
| F * | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.6 |
| L.O.I. | −6.29 | −6.54 | −4.05 | −6.23 | −3.18 | −10.74 | −6.33 | −6.27 | −7.88 | −6.30 | −6.05 | −4.80 | 6.30 | −4.90 | n.d. | n.d. | n.d. |
| As | 0.02 | 0.06 | 0.10 | 0.04 | 0.73 | 2.72 | 0.14 | 0.14 | 0.09 | 0.07 | 0.09 | 0.03 | 1.25 | 0.03 | 0.07 | 0.1 | 1.1 |
| Ba | 0.77 | 1.03 | 0.16 | 1.04 | 0.58 | 0.07 | 0.61 | 0.07 | 1.52 | 0.21 | 0.53 | 0.80 | 0.31 | 0.38 | <100 ppm | n.d. | n.d. |
| Cu | 0.33 | 0.55 | 0.46 | 0.40 | 1.30 | 4.16 | 0.86 | 1.33 | 0.59 | 0.40 | 0.62 | 0.35 | 1.47 | 0.59 | 0.43 | 0.1 | 0.6 |
| Pb | 1.18 | 1.72 | 1.47 | 1.18 | 1.98 | 2.19 | 1.83 | 2.30 | 1.05 | 1.39 | 2.22 | 2.47 | 6.21 | 0.46 | 1.77 | 3.8 | 0.9 |
| Sr | 0.02 | 0.04 | 0.02 | 0.04 | 0.02 | 0.01 | 0.03 | 0.02 | 0.04 | 0.02 | 0.03 | 0.02 | 0.01 | 0.37 | 0.60 | n.d. | n.d. |
| Sb | 0.13 | 0.56 | 0.37 | 0.43 | 2.33 | 5.45 | 0.65 | 0.49 | 0.42 | 0.46 | 0.61 | 0.24 | 1.95 | 0.14 | 0.58 | n.d. | n.d. |
| Zn | 2.01 | 0.41 | 0.45 | 0.19 | 0.16 | 0.81 | 1.30 | 0.69 | 0.33 | 0.26 | 0.35 | 1.94 | 0.65 | 2.17 | 1.26 | 1.8 | 3.3 |
| Ag ppm | 3 | 7 | 7 | 5 | 62 | 99 | 33 | 53 | 7 | 7 | 11 | 27 | 303 | 14 | <5 | n.d. | n.d. |
| Cr ppm | 80 | 70 | 50 | 70 | 80 | 60 | 80 | 80 | 80 | 70 | 70 | 100 | 50 | 90 | <10 | n.d. | n.d. |
| Ni ppm | 30 | 40 | 40 | 30 | 190 | 520 | 40 | 40 | 40 | 30 | 80 | 30 | 210 | 20 | 80 | n.d. | n.d. |
| TOT | 97.69 | 100.69 | 97.93 | 99.39 | 99.21 | 97.02 | 99.52 | 97.60 | 98.07 | 100.26 | 100.64 | 99.37 | 100.75 | 99.80 | 100.34 | 100 | 100 |
| V.I | 2.01 | 3.45 | 3.21 | 2.90 | 2.51 | 4.38 | 2.48 | 2.36 | 3.45 | 3.61 | 2.45 | 1.77 | 2.82 | 1.96 | 2.74 | 1.39 | 1.79 |
| Kz | 2.18 | 3.60 | 3.32 | 3.00 | 2.63 | 4.72 | 2.69 | 2.58 | 3.60 | 3.72 | 2.60 | 1.94 | 3.25 | 2.09 | 2.97 | 1.72 | 2.11 |
| ln (η) 1250 °C | 0.38 | 0.79 | 0.52 | 0.32 | 0.25 | 2.80 | 0.25 | 0.25 | 0.78 | 0.93 | 0.25 | 0.54 | 0.46 | 0.43 | 0.30 | 0.75 | 0.41 |
| Site | Montieri | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Matte Ca Rich | Matte Ca Rich | Matte Ca Rich | Matte Ca Rich | Matte Ca Rich | Matte Ca Rich | Matte Ca Rich | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor I | Matte Poor II |
| Sample | AM-01 ° | 7a ° | OG1 * | S1 * | OG2 * | 7b * | 7c * | CO2 ° | VO1 ° | F2 * | EO2 * | S2 * | AO2 * | AO-M2 * | 4 * | 5 * | GO2 |
| SiO2 | 21.82 | 19.30 | 28.7 | 28.4 | 29.3 | 31.2 | 26.0 | 21.05 | 28.67 | 36.3 | 37.5 | 30.3 | 34.1 | 34.4 | 32.9 | 34.0 | 16.33 |
| TiO2 | 0.42 | 0.47 | 0.2 | 0.5 | 0.2 | 0.2 | n.d. | 0.50 | 0.57 | 0.2 | 0.7 | n.d. | 0.3 | 0.3 | 0.2 | n.d. | 0.58 |
| Al2O3 | 8.98 | 8.28 | 6.3 | 9.8 | 7.8 | 9.3 | 6.6 | 9.67 | 12.91 | 12.6 | 14.5 | 9.4 | 14.9 | 11.9 | 8.6 | 8.4 | 11.45 |
| Fe2O3 | 26.88 | 26.17 | n.d. | n.d. | n.d. | n.d. | n.d. | 29.31 | 22.73 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 11.68 |
| as FeO | 24.19 | 23.54 | 13.9 | 19.6 | 16.6 | 15.5 | 27.7 | 26.37 | 20.46 | 21.6 | 18.4 | 23.5 | 21.8 | 23.1 | 22.7 | 21.8 | 10.51 |
| MnO | 1.72 | 1.51 | 1.2 | 1.6 | 0.8 | 0.8 | 1.3 | 1.60 | 0.87 | 0.4 | 1.0 | 1.1 | 1.0 | 1.0 | 1.0 | 2.2 | 12.01 |
| MgO | 1.72 | 1.51 | 1.7 | 1.8 | 1.4 | 1.8 | 2.1 | 1.19 | 1.36 | 1.8 | 1.9 | 2.3 | 1.3 | 1.5 | 1.8 | 2.3 | 1.73 |
| CaO | 22.96 | 22.68 | 29.8 | 29.7 | 32.2 | 27.1 | 22.1 | 21.98 | 14.98 | 16.5 | 15.7 | 20.8 | 16.4 | 15.7 | 24.8 | 17.4 | 13.58 |
| K2O | 1.21 | 1.08 | 1.9 | 1.0 | 1.2 | 2.5 | 0.1 | 0.72 | 2.05 | 2.4 | 2.6 | 1.8 | 2.5 | 2.4 | 1.7 | 3.7 | 0.58 |
| Na2O | 0.19 | 0.13 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.20 | 0.27 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.14 |
| P2O5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.3 | n.d. | n.d. | 0.4 | n.d. | n.d. | 0.77 |
| TOT/S | 0.73 | 0.25 | 2.6 | 1.4 | 2.1 | 2.0 | 0.4 | 0.57 | 0.11 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.7 | n.d. | 0.07 |
| F * | n.d. | 6.0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.2 |
| L.O.I. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 10.15 |
| As | 0.11 | 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.11 | 0.04 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.6 | 0.90 |
| Ba | <100 ppm | <100 ppm | n.d. | n.d. | n.d. | n.d. | n.d. | <100 ppm | <100 ppm | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.03 |
| Cu | 0.74 | 0.7 | 2.0 | 0.4 | 0.5 | n.d. | 1.1 | 0.88 | 0.47 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.9 | 0.90 |
| Pb | 3.09 | 5.2 | 4.8 | 1.0 | 8.0 | n.d. | 3.1 | 4.38 | 6.63 | 2.0 | 3.1 | 2.1 | 2.5 | 2.9 | 2.1 | 6.5 | 10.30 |
| Sr | <0.1 | <0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | < 0.1 | < 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.03 |
| Sb | 0.38 | 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.13 | 0.06 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.14 |
| Zn | 7.36 | 5.7 | 6.9 | 4.8 | n.d. | 9.6 | 9.6 | 6.89 | 6.43 | 6.2 | 4.3 | 8.7 | 5.2 | 6.3 | 3.6 | 2.3 | 5.55 |
| Ag ppm | <5 | <5 | n.d. | n.d. | n.d. | n.d. | n.d. | <5 | <5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 15 |
| Cr ppm | 30 | 80 | n.d. | n.d. | n.d. | n.d. | n.d. | 80 | 90 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 110 |
| Ni ppm | <50 | <50 | n.d. | n.d. | n.d. | n.d. | n.d. | <50 | <50 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 190 |
| TOT | 98.29 | 99.21 | 100 | 100 | 100 | 100 | 100 | 99.20 | 98.14 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100.10 |
| V.I | 1.69 | 1.83 | 1.39 | 1.41 | 1.41 | 1.18 | 1.63 | 1.69 | 0.96 | 0.87 | 0.76 | 1.25 | 0.88 | 0.94 | 1.26 | 1.12 | 1.39 |
| Kz | 2.06 | 2.25 | 1.80 | 1.61 | 1.68 | 1.47 | 2.04 | 2.10 | 1.29 | 1.04 | 0.91 | 1.52 | 1.04 | 1.14 | 1.41 | 1.33 | 1.93 |
| ln (η) 1250 °C | 0.40 | 0.34 | 0.67 | 0.88 | 0.79 | 1.05 | 0.47 | 0.40 | 0.30 | 1.76 | 2.02 | 1.00 | 1.77 | 1.59 | 1.14 | 1.27 | 0.60 |
| Cugnano | Main Phases | Minor Phases | Additional Phases | ||||
|---|---|---|---|---|---|---|---|
| Sample | Silicates | Oxides | Matte | Metals | Speiss | ||
| matte Fe rich | A1-13011 | Kir, Mtc | Wus | Fe-S; Pb-S; Zn-S | Pb | Sb; Cu-Sb | Pb oxides |
| matte Fe rich | A2-13019 | Kir, Mll | Wus, (Mag) | Fe-S | Pb | Cu-Sb | Pb oxides |
| matte Fe rich | A3-13009 | Kir, Mll | Wus, (Mag) | Fe-S | Pb | Cu-Sb | Pb oxides |
| matte Fe rich | A3-13028 | Kir, (Mll) | Wus, (Mag) | Pb | Sb; Cu-Sb | ||
| matte Fe rich | B2-13019 | Kir | Wus | Fe-S | Pb | Fe-As; Cu-Sb | Pb oxides |
| matte Fe rich | B3-13016 | Kir | Wus, (Hc) | Fe-S; Cu-Fe-S; Zn-S | Pb | Fe; Fe-As; Cu-Sb | Pb oxides, hematite |
| matte Fe rich | C1-13009 | Kir | Wus, Mag | Cu-Fe-S; Pb-Zn-S | Pb; Cu | Cu-Sb | |
| matte Fe rich | C1-13011 | Kir | Wus, (Mag) | Cu-Fe-S | Pb; Cu | Cu-Sb | Qz, |
| matte Fe rich | C1-13022 | Kir, Mll | Wus | Cu-Fe-S; Fe-S | Pb | Sb; Fe-As; Cu-Sb | Pb oxides, Fe-hydroxides, Qz |
| matte Fe rich | C1-13028 | Kir, Mll | Wus, (Mag) | Pb | Sb; Cu-Sb | ||
| matte Fe rich | AG-13011 | Kir | Wus | Fe-S | Pb | Sb; Cu-Sb | Pb oxides |
| matte Fe rich | E-13022 | Kir | Hc, (Wus) | Pb-S; Zn-S | Pb | Sb; Cu-Sb | |
| matte Fe rich | S1-13022 | Kir | Mag, (Wus) | Cu-S; Fe-S | Pb | Fe-As; Cu-Sb | Pb-Sb oxides, Fe-hydroxides, Cls |
| Montieri | |||||||
| matte Fe rich | BO2 | Kir, (Aug) | Wus, (Spl) | Cu-Fe-S; Fe-S; (Zn-S) | (Pb) | Fe; Sb; Fe-As; Cu-Sb | Pb oxides |
| matte Fe rich | DO1 | Kir, Mll | Wus | Cu-Fe-S; Fe-S; Pb-S | Pb | Fe; Sb; Fe-As; Cu-Sb | Pb oxides, Pb carbonates |
| matte Fe rich | BO1 | Kir | Wus, (Slp) | Cu-Fe-S; Fe-S | Pb | Fe-As | Pb oxides; Kfs |
| matte Fe rich | CO1 | Wus, (Spl) | Cu-Fe-S; Fe-S; (Zn-S) | (Pb) | Fe-As | Pb oxides | |
| matte Ca rich | AM-01 | Mll, Kir, (Aug) | Wus, (Spl) | Cu-Fe-S; Zn-S; Pb-S; Pb-Sb-S | (Pb) | Cu-Sb; Ni-Sb | Qz, Al2O3-phase |
| matte Ca rich | 7a | Mll | Spl, (Wus) | Cu-Fe-S | (Pb) | Qz, Al2O3-phase | |
| matte Ca rich | OG1 * | Px, Csp | Spl, (Wus) | Cu-Fe-S; Cu-S; Zn-S; Pb-S | Pb | Cu-Sb; Ni-Sb; Sb | Al2O3-phase |
| matte Ca rich | S1 | Px, (Csp) | Spl, (Wus) | Cu-Fe-S; Cu-S; Zn-S; Pb-S | (Pb) | Cu-Sb; Sb | Fe-hydroxides, calcite, Qz |
| matte Ca rich | OG2 | Csp, Mll | Spl, (Wus) | Cu-Fe-S; Zn-S; Pb-S | (Pb) | Cu-Sb; Ni-Sb | Al2O3-phase |
| matte Ca rich | 7b | Mll, (Csp) | Spl | Cu-Fe-S; Fe-S | (Pb) | Cu-Sb | Qz, Al2O3-phase; ZnO |
| matte Ca rich | 7c | Mll | Spl, (Wus) | Cu-Fe-S | (Pb) | Qz | |
| matte poor I | CO2 | (Mll) | Spl | (Cu-S) | Pb | Al2O3-phase | |
| matte poor I | VO1 | (Px) | Spl | (Cu-S, Cu-Fe-S) | Pb | Sb | Al2O3-phase |
| matte poor I | F2 | (Px) | Spl | (Cu-S, Cu-Fe-S) | Pb | Al2O3-phase | |
| matte poor I | EO2 | Spl | (Cu-Fe-S) | (Pb) | (Ni-Sb) | Al2O3-phase | |
| matte poor I | S2 | (Mll) | Spl | (Cu-Fe-S; Zn-S) | Pb | Sb; Ni-Sb | Al2O3-phase |
| matte poor I | AO2 | Spl | (Pb) | Al2O3-phase | |||
| matte poor I | AO-M2 | Spl | (Cu-S) | Pb | As-Sb | Al2O3-phase | |
| matte poor I | 4 | (Px) | Spl | (Cu-Fe-S; ZnS) | Pb | Al2O3-phase | |
| matte poor I | 5 | (Px) | Spl | (Cu-Fe-S; Cu-S) | Pb | Sb | Al2O3-phase |
| matte poor II | GO2 | Csp, Lrn, (Aug) | Spl, (Mn-oxide) | (Cu-S) | Pb (Cu) | Al2O3-phase | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarantini, L.; Benvenuti, M.; Bianchi, G.; Dallai, L.; Volpi, V.; Manca, R. Medieval Pb (Cu-Ag) Smelting in the Colline Metallifere District (Tuscany, Italy): Slag Heterogeneity as a Tracer of Ore Provenance and Technological Process. Minerals 2021, 11, 97. https://doi.org/10.3390/min11020097
Chiarantini L, Benvenuti M, Bianchi G, Dallai L, Volpi V, Manca R. Medieval Pb (Cu-Ag) Smelting in the Colline Metallifere District (Tuscany, Italy): Slag Heterogeneity as a Tracer of Ore Provenance and Technological Process. Minerals. 2021; 11(2):97. https://doi.org/10.3390/min11020097
Chicago/Turabian StyleChiarantini, Laura, Marco Benvenuti, Giovanna Bianchi, Luisa Dallai, Vanessa Volpi, and Rosarosa Manca. 2021. "Medieval Pb (Cu-Ag) Smelting in the Colline Metallifere District (Tuscany, Italy): Slag Heterogeneity as a Tracer of Ore Provenance and Technological Process" Minerals 11, no. 2: 97. https://doi.org/10.3390/min11020097
APA StyleChiarantini, L., Benvenuti, M., Bianchi, G., Dallai, L., Volpi, V., & Manca, R. (2021). Medieval Pb (Cu-Ag) Smelting in the Colline Metallifere District (Tuscany, Italy): Slag Heterogeneity as a Tracer of Ore Provenance and Technological Process. Minerals, 11(2), 97. https://doi.org/10.3390/min11020097








