Abstract
Oily wastewater treatment is crucial for protecting the environment and ensuring sustainable water use. The current study examines the effectiveness of electrocoagulation in treating oily wastewater by conducting several batch experiments designed to determine the best operating conditions. Various factors affecting the performance of electrocoagulation, such as applied current density, electrode type, and pH, were studied. The results indicate that, under ideal conditions, electrocoagulation worked very well. The best results were obtained by involving an applied current density of 6 mA/cm2, a mild steel anode, and a pH of 6.7. Under these conditions, the process removed 94% of the chemical oxygen demand (COD) from the oily wastewater. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectrometry (EDAX) were used to characterize the residual sludge left in the reactor. The characterization results show that the oily pollutants were successfully removed through electroflotation. Additionally, oil precipitate particles were easily coated during the electrocoagulation operation. The findings show that electrocoagulation is an effective method for treating oil-contaminated wastewater.
1. Introduction
Due to demand, the increase in the opetroleum industry has simply led to its proliferation everywhere. As a consequence, these increases in service often produce large amounts of oily wastewater containing a variety of pollutants [1,2,3,4,5]. Wastewater containing oily pollutants produced by industrial plants is one of the major sources of contamination of natural resources because of its chemical nature, consisting of organic and inorganic materials [6,7,8,9,10]. The United States Environmental Protection Agency (USEPA) classifies the majority of these pollutants as hazardous aqueous chemicals due to their ability to cause adverse effects on both the environment and human health. Exposure to such pollutants can damage vital organs such as the kidneys, liver, central nervous system, and blood cells [11]. They can also inhibit plant and animal growth and are classified as probably carcinogenic to humans. Oily wastewater dispersal creates substantial emulsions, which significantly increases treatment costs and pose health risks. Unfortunately, these emulsions exacerbate the situation, requiring advanced techniques to address the contamination effectively. Inaction would severely affect public health and environmental integrity [12,13].
It should be noted that oily wastewater contains high amounts of BOD and COD, making it a major source of environmental pollution [3]. Therefore, to deal with such extensive quantities of BOD and COD, it is necessary to take oil waste seriously and carefully treat it before being discharged into the environment. Therefore, it is necessary in essence to carry out efficient treatment of wastewater generated from petroleum-related industries and from the pollution and detritus that precede those industries [13,14,15,16,17].
Several treatment methods, such as chemical precipitation [18], ion exchange [19], flotation [20,21], adsorption [22], coagulation-flocculation [23], and electro-oxidation [24], have proven to have drawbacks in removing or extracting hydrocarbons one way or another [3,13,17,25]. Industrial pollution control has therefore become common because it is served well by new advanced technologies [26,27,28,29,30,31].
A comprehensive exploration of the potential of the electrochemical treatment process and its diverse applications has been recently achieved [25,26]. The growing research guarantees the hot electrochemistry technology as a new and innovative method for processing industrial wastewater with appreciable prospects. Its multiple advantages—especially high selectivity, low cost, and environmental friendly nature—makes it well-suited to modern wastewater treatment methods. Also, the significant influence of factors such as pH, current density, and operating time on the effectiveness of electrochemical water treatment [4] further enhances its potential. The promising results from numerous studies on electro-Fenton, electrocoagulation, electrochemical oxidation, and electrochemical membrane reactors, whether implemented as single-stage or multi-stage treatment processes, instill optimism about their potential applicability and feasibility in addressing wastewater contaminations in the petroleum industry [11,15,25].
Electrocoagulation is one of the electrochemical treatment techniques used in oily wastewater treatment, which is considered a simple and efficient treatment method that involves the electrode solution of sacrificial anodes and the formation of hydroxo-metal as coagulant products [32,33,34]. At the same time, the simultaneous production of hydrogen at the cathode eases the pollutant removal by flotation. The electrocoagulation system is completely characterized and addresses the prospective application of the technique in the following studies [35,36,37,38,39,40].
Electrocoagulation has numerous advantages, including its high capacity to accommodate diverse wastewater compositions, high efficiency in treating colloidal and dissolved pollutants, and its potential to mitigate reliance on chemical coagulants. However, this technology does include several limitations, such as energy consumption and regular maintenance of the electrode materials required [41,42,43,44].
Omar et al. (2020) [39] thermally evaluated the performance of the electrocoagulation process with and without aeration for treating oily wastewater. The study indicated that aeration can improve the process performance. The maximum COD removal with aerated cells was 93.3%, while the non-aerated cell at 120 A m−2 current density resulted in 84% COD removal.
The parameters influencing the design of a continuous electrocoagulation reactor used to remove oily pollutants were investigated statistically by F. Farzam and H. Mehri [45]. The statistical analysis of the experimental results shows that when the residence duration is increased from 16 to 28 min, the operational expenses are lowered by 37%. Moreover, the findings of this study could be used to develop an effective and affordable industrial electrocoagulation unit for the treatment of oily wastewater.
All research and studies on electrocoagulation technology’s application to the treatment of oily wastewater from 2018 to 2022 are listed in the Jasim et al. review article from 2023 [28].
The current study focuses on the design and optimization of electrochemical cells, the identification of appropriate electrode materials, and the evaluation of treatment efficiency in removing pollutants from oily wastewater, alongside enhancing the understanding of the process by sludge analysis. Such efforts are essential in addressing environmental concerns and meeting regulatory requirements in the petroleum industry while reducing the impact of its wastewater on ecosystems.
2. Materials and Methods
The experimental procedures were conducted within a cylindrical batch electrochemical reactor, fabricated using glass, with a total volume of one liter. The reactor was outfitted with a temperature control system to maintain a stable operational temperature of 27 ± 0.5 °C.
Figure 1 presents a visual representation of the experimental configuration. The electrode configuration utilized in the experiment involved using anodes fabricated from mild steel (AISI 1018) and aluminum (Al 6061) used in distinct experiments. The cathode utilized in the investigation was constructed from stainless steel, namely (SS 316). The electrode had an effective surface area of 20 cm2, and the anode and cathode had a uniform thickness of 0.5 cm. The electrodes were placed at a distance of 2.5 cm, measured from one edge to the other. The synthetic oily wastewater was created in the laboratory using tap water, engine oil, and surfactants. To achieve uniformity of the wastewater sample, a magnetic stirrer (Brand XYZ, Model 123) operated at a constant speed of 100 rpm was employed.
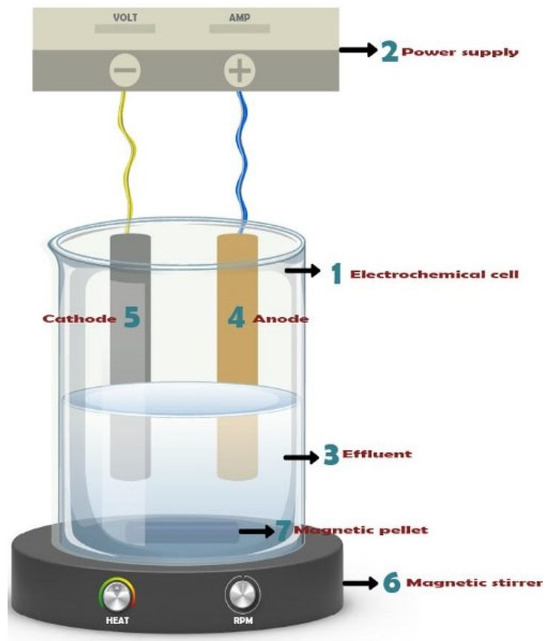
Figure 1.
Illustration of the electrocoagulation process setup [46,47].
A controlled direct current (DC) power source was employed, with a predetermined voltage setting of 5 volts (V) and a predetermined current setting of 1 ampere (A).
The samples were collected at 10, 20, 30, 45, and 60 min. Parameters of COD, pH, specific energy consumption (SEC), and anode dissolution were assessed utilizing established methodologies [3,36]. The treatment efficiency was evaluated by determining the COD as a target parameter for removing all organic pollutants. In addition, COD is a critical factor that reflects the reduction in organic concentration in an industrial wastewater. COD was measured using the open reflux method, and the concentration of contaminants was afterward evaluated by applying the COD percentage [46,47]. Following each experiment, the electrodes were thoroughly cleaned with a 1 M hydrochloric acid solution (HCl(aq)) to effectively eliminate any potential surface impurities.
The initial characteristics of the oily wastewater exhibited comparable values in all experimental tests, with a standard deviation of less than 5% [48], as shown in the Table 1. The findings indicate that no supporting electrolytes were required due to the high conductivity of the effluent. All the features of oily wastewater were identified using established techniques for analyzing water and wastewater [49]. Regarding the process’s economics, specific energy consumption (SEC) and anode dissolution were also investigated.

Table 1.
Oily wastewater characterization [49].
3. Results and Discussion
3.1. Current Density’s Impact on COD Removal Efficiency
The remaining percentage of COD during the electrolysis process was evaluated a function of three different current densities (CD), viz. 3, 6, and 9 mA/cm2. Utilizing two different anodes, mild steel and aluminum, the reliance of % COD remaining with time is demonstrated in Figure 2 and Figure 3, respectively.
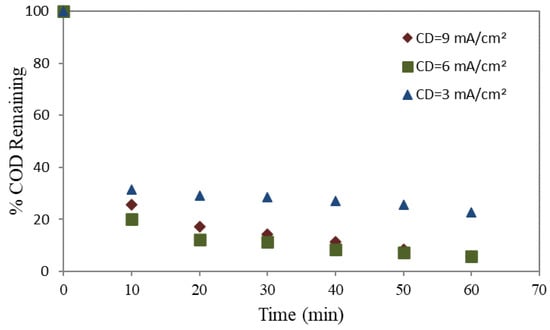
Figure 2.
% COD remaining utilizing a mild steel anode.
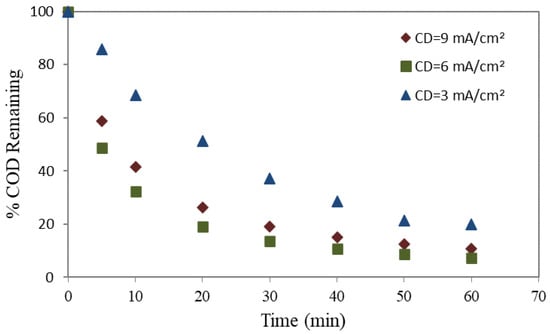
Figure 3.
% COD remaining utilizing an aluminum anode.
For all applied CD values, a decrease in the remaining COD percentage was observed with increasing electrolysis time, regardless of whether mild steel or aluminum was employed as the anode. The results further indicate that the elimination of COD was more efficient when using a mild steel anode. Elevating the CD from 3 to 6 mA/cm2 enhanced the COD elimination percentage. Nevertheless, no substantial enhancement in the percentage of COD elimination was observed when the current density was increased to 9 mA/cm2.
The concept of CD in electrocoagulation pertains to the quantity of electrical current administered to the surface area of the electrode. The determination of the rate of metal dissolution from the electrodes is of utmost importance in the electrocoagulation process, as it plays a critical role in the synthesis of coagulants. These results show that anodic dissolution in mild steel or aluminum remained dependent on the applied current density (CD). Consequently, the formation of hydroxo-cationic complexes occurs, leading to COD removal. Furthermore, applying the CD with a higher rate than the optimal value may influence the process efficiency, since it governs the rate of coagulant production and bubble creation. Increased gas bubble density with smaller bubble sizes leads to greater upward flow, which eventually causing increased degradation of pollutants and flotation of sludge [50].
Also, as the CD value exceeds the optimal value, it may increase the pH level in the wastewater. The alteration in pH could impact the electrocoagulation process and disrupt the removal of pollutants. Negative effects were associated with excessive solubility of the anode and changes in pH [51].
Consequently, the abovementioned process can generate metal flocs, which may not yield substantial benefits regarding COD elimination, albeit it escalates treatment expenditures. The occurrence of metal flocs is attributed to increased CD higher than 6 mA/cm2. Although metal flocs can contribute to coagulation, their excessive development without a proportional improvement in COD elimination may not be economically viable; hence, balancing treatment efficacy and the corresponding operating expenditures is crucial.
3.2. Effect of Electrocoagulation on pH
Figure 4 and Figure 5 illustrate the variation in pH over time (minutes) at various CDs in the reaction using a mild steel and aluminum anode, respectively. During the electrochemical experiment, a time-dependent inverse pH response was seen at all studied CD values for both used anodes.
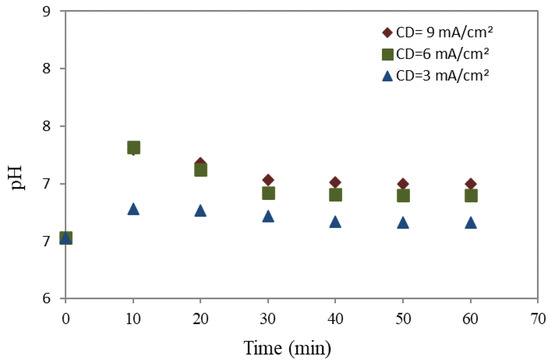
Figure 4.
The pH of wastewater utilizing a mild steel anode.
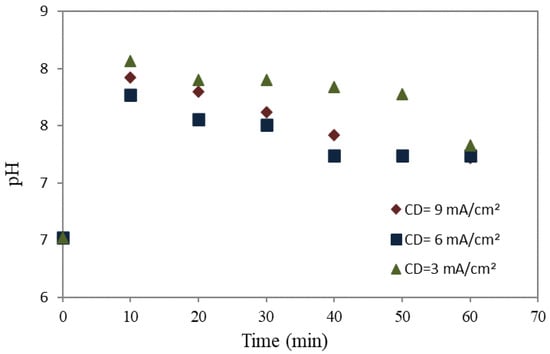
Figure 5.
The pH of wastewater utilizing the aluminum anode.
This characteristic feature is commonly observed in complex electrochemical systems and can be explained by the increase in pH during the electrocoagulation process, which occurs due to the hydrogen evolution during the cathodic reduction of water (Equation (1)). Conversely, Equations (2)–(4) indicate that the pH decreases due to the generation of separate iron and aluminum species at the anode, resulting from the combination of electro-dissolved ions, hydroxyl ions, and water oxidation [52].
2H2O(l)+ 2e− → H2(g)+ 2OH−(aq)
Fe+2(aq)+ 2OH−(aq) → Fe(OH)2(s)
Fe+2(aq) + 10H2O(l)+ O2(g) → Fe(OH)3(s)+ 8H+(aq)
Al+3(aq)+ 3H2O(l) → Al(OH)3(s)+ 3H+(aq)
The observed outcome was an increase in pH levels with time, from pH 7–7.5. The removal rates increased dramatically with the reduction in the acidity of the solution to neutral. The aluminum anode, as presented in Figure 6, was generally lower and highly stable than the mild steel anode (in Figure 4) due to the generation of OH− ions that govern in pH bulk in the cathode, while the hydrolysis of Al+3 ions opposes this, acting as a lightening force to reduce the increasing pH. The electrocoagulation process requires a longer duration to reach a stable state for aluminum (more than 40 min) than mild steel (around 30 min), owing to the equilibrium reactions involved. This phenomenon arises due to the tendency of the primary constituent of mild steel, Fe(OH)3, to maintain a pH level within the neutral range [53,54]. In this system, the pH was stable because the generation of OH− at the cathode side led to an increase the pH, which remained opposite on the anode side due to the hydrolysis of Al+3, causing a decrease in pH [55]. Moreover, comparing current densities revealed that the treated wastewater generated at a current density of 6 mA/cm2 displayed a higher pH level than other current densities.
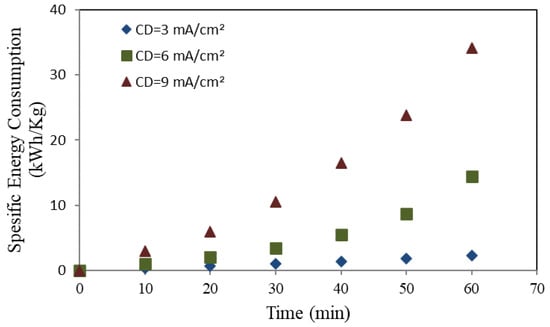
Figure 6.
Specific energy consumption utilizing a mild steel anode.
3.3. Specific Energy Consumption
Energy management plays a crucial role in the enhancement of energy efficiency. One of the most significant tools in energy management is using specific energy consumption (SEC) to identify possible areas for efficiency enhancements. SEC is commonly employed as an energy performance metric in literary works and international standards to assess and quantify energy efficiency performance. It is a widely utilized metric to evaluate and quantify the energy efficiency of various processes, industries, or individual operational activities. By employing SEC, it become possible to measuring energy performance, identify inefficiencies, and evaluate the effects of energy-efficient technologies. Nevertheless, it is imperative to consider regional and process-specific variables while employing SEC for research. The calculation of specific energy consumption (kWh/kg of COD eliminated) was performed by applying the following equation [56]:
The variables in Equation (5) include voltage (expressed in volts), electrolysis time (measured in hours), the volume of effluent (in m3), chemical oxygen demand (COD) initial concentration, and concentration at a given time (t) (in kg/m3).
Figure 6 and Figure 7 illustrate an increasing correlation between the electrolysis time and the SEC under different applied current densities (3, 6, and 9 mA/cm2). The SEC exhibited similar values and elevating trends with time for mild steel and aluminum when modest applied charges were utilized (3 and 6 mA/cm2). At high CD (9 mA/cm2), the mild steel anode has an exponentially increasing rate, as illustrated in Figure 7.
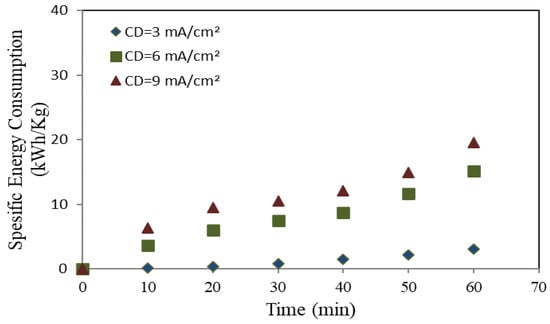
Figure 7.
Specific energy consumption utilizing an aluminum anode.
Furthermore, Figure 7 and Figure 8 demonstrate the relation between metal ions generation and energy consumption during the electrolysis process. When the electrolysis process exceeds 40 min for both types of anodes, the rate of metal ion generation decreases in proportion to the time elapsed, leading to additional current requirements to raise the solution’s temperature.
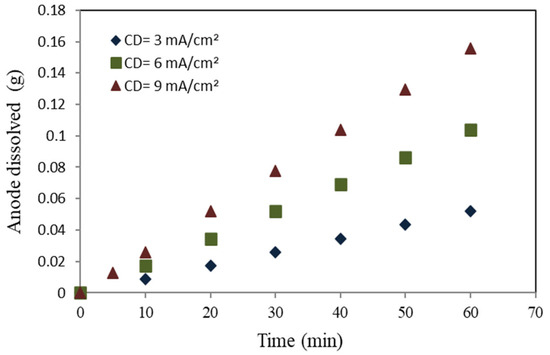
Figure 8.
Mild steel anode dissolving rate.
Consequently, the proportion of COD removed decreases with time, leading to an increase in the SEC per kilogram of COD removed. This observation corroborates our previous findings (depicted in Figure 4 and Figure 5), indicating that the rate of COD removal exhibited an initial increase followed by a substantial decrease over time as the electrolysis process advanced. On the other hand, with aluminum as the electrode material, the results shown in Figure 8 indicate that specific energy consumption (SEC) per kilogram of COD removed increases linearly over time [53].
3.4. Anode Dissolution
The estimation of electrode usage can be determined by utilizing equations in the following:
Equation (6) includes the variables mild steel and aluminum atomic weights (expressed in g/mol), current (expressed in Ambir), and electrolysis time (measured in sec).
The relationship between the electrolysis duration, applied charge, and the production of metal ions can be seen in Figure 8 and Figure 9 for mild steel and aluminum anodes, respectively. These figures illustrate a linear increase in metal ion generation with an increase in the applied current density and electrolysis duration. The dissolution rate for the mild steel anode, at a specified equivalent current density, was three times greater than aluminum. Equation (6) demonstrates that the dissolution of the anode will exhibit an upward trend with an increase in molecular weight (M) and a decrease in the number of free electrons, as observed in the context of mild steel. The lower aluminum molecular weight, combined with its higher number of free electrons compared to mild steel, reduces the dissolution of the anode. The electrode erosion rates of mild steel and aluminum, at current densities of 3, 6, and 9 mA/cm2, were found to be around 0.9, 1.7, and 2.6 mg/min, respectively, for mild steel, whereas for aluminum, they were found to be 0.3, 0.6, and 0.8 mg/min at the same current densities.
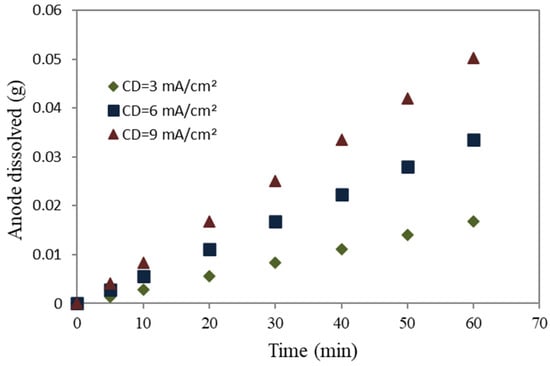
Figure 9.
Aluminum anode dissolving rate.
3.5. Scanning Electron Microscope (SEM) with Energy-Dispersive X-Ray Analysis (EDAX)
After the effluent stood for an hour, it was found that sludge from the mild steel anode had collected at the reactor’s top and bottom. However, the aluminum anode sludge was collected at the reactor’s bottom. The sludge samples was analyzed by energy-dispersive X-ray analysis (EDAX), and the results are shown in Figure 10 and Figure 11 and Table 2 and Table 3 for mild steel and aluminum as anodes, respectively.
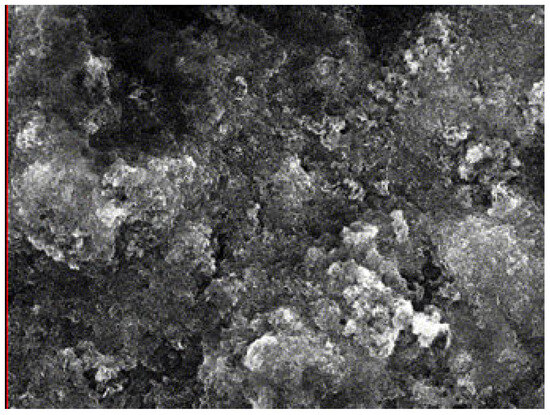
Figure 10.
SEM analysis for mild steel anode.
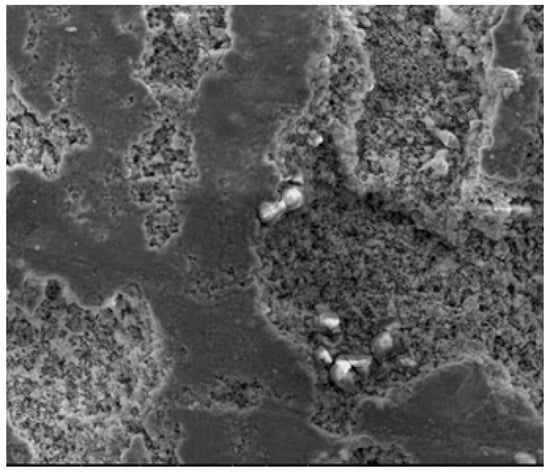
Figure 11.
SEM analysis for aluminum anode.

Table 2.
EDAX analysis mild steel anode.

Table 3.
EDAX analysis for aluminum anode.
According to EDAX analysis, the sludge contained both organic and inorganic contaminants. The elements such as C, S, Ca, Na, and Si were adsorbed, destabilized, and precipitated on the surface of Fe(OH)2(s), 4Fe(OH)3(s), and Al(OH)3(s). During the electrocoagulation process, gases, including H2, O2, and CO2, evolved, which eventually helped to remove contaminants through electroflotation (to a certain extent). According to the weight percentage of pollutants (i.e., carbon), electroflotation of the upper part of the sludge removed around 48.8% of the oily contaminants. In comparison, floc precipitation of the lower part of the sludge removed 51.2% of the oily contaminants. However, aluminum was 23.5% less efficient than mild steel at removing oily contaminants, as it was influenced by the weight percentage of pollutants. The scrap impurities of mild steel and aluminum electrodes were the source of additional elements in the sludge [57]. The SEM images of the electrodes show visible dents on the mild steel surface (a), which are rougher and thus have more surface-active sites available compared to the surface of the aluminum (b). Thus, more surface-active sites are available on mild steel than on aluminum. When the electrode dissolves, iron and aluminum hydroxides are produced. Due to the simultaneous formation of oxygen at its surface, the adsorption of organic and inorganic contaminants leads to the consumption of anode material increasingly in active areas, resulting in better adsorption efficiency [57].
Unpredictably, the EDAX analysis of generating sludge at a mild steel anode showed the presence of zinc (identified by a peak at 314 eV), related to the following:
- The commercial mild steel is an alloy that may contain zinc as impurities or as a galvanizing coat, which may not dissolve through the process of electrocoagulation process.
- The industrial oily wastewater involving metalworking or corrosion inhibition may contain zinc product or residues, which is removed effectivity by iron hydroxide flocs co-precipitation.
This outcome is significant to demonstrate the electrocoagulation process to remove pollutants from industrial wastewater. Moreover, it also focuses on the importance of the sacrificial anode composition as an extra source of contamination.
3.6. Effect of Anode Material
The connection between CD (mA/cm2) and the remaining COD % for both anode materials is shown in Figure 12. The results show a significant difference in the effectiveness of mild steel and aluminum anodes in COD% remaining from oily wastewater.
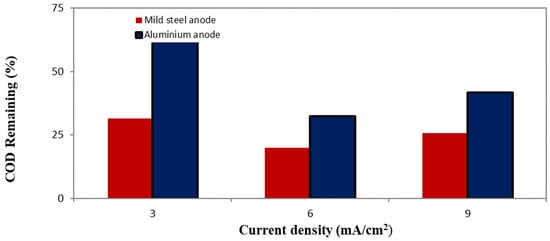
Figure 12.
Effect of electrode materials on the treatment of oily wastewater at 10 min.
The COD% remaining was constantly reduced in the presence of mild steel compared to aluminum electrodes across all applied current densities, which indicates the efficiency of using a mild steel as a sacrificial anode to degrade the organic pollutants via electrochemical coagulation and oxidation processes.
This higher presentation is clearly established after one hour of the treatment: the mild steel showed a considerably lower COD comparison to aluminum at the same current densities. The COD remaining aligns with the higher concentration of Fe2+/Fe3+ from mild steel in the solution, which improves the removal of pollutants by coagulation, flocculation, and precipitation, as shown by the EDAX results (Figure 10 and Figure 11).
While the presence of coagulant mass is important to reflect comparison of electrode materials, that should also consider to lowering of operating costs and characteristics of sludge.
The high removal efficiency of pollutants achieved in a recent study is related to the effect of synergistic interplay among three primary mechanisms: electrocoagulation, electroflotation, and electro-oxidation. The electro-dissolution of the sacrificial anode (Fe or Al) forms hydroxide metal coagulants that destabilize with entrapment of oily droplets [1,2], that enhanced by simultaneous side reactions. At the cathode side, the water reduction causes the generation of hydrogen bubbles (i.e., 2H2O + 2e− → H2(g) + 2OH−), which enhances the coagulated flocs separation through electroflotation and carries it to the water surface [3]. At the same time, at the anode surface, the water oxidation can generate reactive oxygen species (ROS) may increase the degradation of organic compounds and the breakdown of emulsifying agents via electro-oxidation [4,5].
3.7. Residual Metal Ions and Assessment of Secondary Contamination
The environmental sustainability of electrocoagulation needs to improve the ability of the process to highly remove pollutants via separation of metal ion residues in the treated wastewater, as presented in Table 2.
The residual iron concentrations varied from 0.85 to 3.45 mg/L at the mild steel anode, while the standard value of regulatory should did not exceed over 0.3 mg/L of iron [58].
In contrast, the residual aluminum concentrations ranged between 0.45 and 2.85 mg/L due to its toxicity in the effluent at the aluminum anode, and the regulatory value in water ranged between 0.1 and 0.2 mg/L [58].
When the concentration of metals was increased with current density, the higher currents encourage the dissolution of the anode. These outcomes highlight the importance of optimizing the operation parameters to efficiently remove and reduce contamination.
The electrocoagulation process successfully transfers pollutants from a precipitate as a solid phase, generating a metal hydroxide sludge containing the removed oils and organics, as shown in Table 4.

Table 4.
Anode analysis.
4. Conclusions
The findings of the outcomes of the removal of organic pollutants from industrial wastewater using electrocoagulation allow for higher removal efficiency and a deeper understanding of the relationship between remaining COD and multiple sources of pollutants.
A comprehensive investigation of the electrocoagulation process for treating oily wastewater using a batch reactor with two different anodes, mild steel and aluminum, was conducted to identify the critical parameters that influence the elimination of chemical oxygen demand (COD). The COD reduction percentage was represented by the effect of varying the operational conditions, the duration of the electrolysis process, the electrode material selection, and the applied current density on treatment process efficiency.
Under ideal conditions, a COD removal rate of 94% was achieved when using mild steel as an anode, with a pH value of 6.7, a current density of 6 mA/cm2, and an initial COD concentration of 700 ppm. In general, electrocoagulation demonstrates versatility and promise as a technique for oily wastewater treatment. It is considered a highly promising alternative for treating and recycling such waste, particularly in sectors and regions where the preservation of the environment necessitates adherence to rigorous effluent quality regulations.
Author Contributions
Conceptualization, Q.A.-O. and M.K.A.M.; Methodology, M.D.; Validation, A.W.S. and M.S.; Formal analysis, K.B.A.; Investigation, M.D.; Resources, M.K.A.M.; Data curation, Q.A.-O.; Writing—original draft preparation, A.W.S.; Writing—review and editing, M.S.; Visualization, K.B.A.; Supervision, Q.A.-O.; Project administration, M.D.; Funding acquisition, M.K.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Central Labs at King Khalid University, grant number CL/CO/B/6, and the APC was funded by CL/CO/B/6.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors extend their appreciation to University Higher Education Fund for funding this research work under Research Support Program for Central labs at King Khalid University through the project number CL/CO/B/6 and University of Technology, Baghdad, Iraq.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohammed, M.N.; Abdullah, O.I.; Jweeg, M.J.; Aljibori, H.S.S.; Abdullah, T.A.; Alawi, N.M.; Rasheed, R.T.; Meharban, F.; Hamzah, H.T.; Al-Obaidi, Q. Comprehensive Review on Wastewater Treatment using Nanoparticles: Synthesis of Iron Oxide Magnetic Nanoparticles, Publication Trends via Bibliometric Analysis, Applications, Enhanced Support Strategies, and Future Perspectives. ASEAN J. Sci. Eng. 2025, 5, 1–30. [Google Scholar] [CrossRef]
- Kriipsalu, M.; Marques, M.; Maastik, A. Characterization of oily sludge from a wastewater treatment plant flocculation-flotation unit in a petroleum refinery and its treatment implications. J. Mater. Cycles Waste Manag. 2008, 10, 79–86. [Google Scholar] [CrossRef]
- Mahdy, S.A.; Al-Naseri, H. Effect of Magnesium Oxide Nanoparticles (MgO) on Wastewater Treatment and Electric Current Generation Using Microbial Fuel Cell Technology. Tikrit J. Eng. Sci. 2024, 31, 219–228. [Google Scholar] [CrossRef]
- Kundu, P.; Mishra, I.M. Treatment and reclamation of hydrocarbon-bearing oily wastewater as a hazardous pollutant by different processes and technologies: A state-of-the-art review. Rev. Chem. Eng. 2018, 35, 73–108. [Google Scholar] [CrossRef]
- Wu, B. Human health hazards of wastewater. In High-risk Pollutants Wastewate; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–139. [Google Scholar]
- Mokif, L.A.; Jasim, H.K.; Abdulhusain, N.A. Petroleum and oily wastewater treatment methods: A mini review. Mater. Today Proc. 2022, 49, 2671–2674. [Google Scholar] [CrossRef]
- Ansam, A.M.; Thamer, A.A.; Aljibori, H.S.S.; Mohammed, M.N.; Abdullah, O. Effect of Nanoparticles Flow to Improve the Oil Refineries Wastewater. Int. Rev. Mech. Eng. 2024, 18, 351. [Google Scholar]
- Mahdy, S.A. Biodegradability enhancement of oily wastewater by an SBR treatment methods. In Proceedings of the AIP Conference Proceedings, Al-Amarah, Iraq, 1–2 February 2022; AIP Publishing: Melville, NY, USA; Volume 2809. [Google Scholar]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Waheed Khafaji, S.O.; Diab, M.A.; El-Sabban, H.A.; Jumanazarov, D.; Atamurotov, F.; Almehizia, A.A. Intensified wastewater treatment using In 2 S 3 on activated carbon derived from waste tire: Peroxydisulfate activation via visible-light, characterization, composition, pH influence, and mechanism. Chem. Eng. Process Process Intensif. 2025, 214, 110352. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily wastewater treatment: Overview of conventional and modern methods, challenges, and future opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Al-Obaidi, Q.; Ibrahim, D.S.; Mohammed, M.N.; Abdullah, O.; Selem, N.Y. A Comprehensive Analysis of the Hydrogen Generation Technology Through Electrochemical Water and Industrial Wastewater Electrolysis. Pol. J. Chem. Technol. 2024, 26, 39–50. [Google Scholar] [CrossRef]
- Ansam, A.; Al-Obaidi, Q.; Rand, N. Study of prototypes for biofuel production Extraction from biodegradation in oxygen-free environments Processing Wastewater. Sol. Energy Sustain. Dev. J. 2025, 14, 522–539. [Google Scholar]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Waqed, H.H.; Naglaa, F.; Soliman, P.K.S.; Mohammad, I.K.; Ibrahm, M.; Farruh, A.; Ahmadjon, A.; Diab, M.A. Enhanced wastewater purification and photocatalytic green energy production via a novel CaIn2S4-coupled BiFeO3 nanocomposite: Characterization and mechanistic insights. J. Water Process Eng. 2025, 75, 108048. [Google Scholar]
- El-Gohary, F.; Tawfik, A.; Mahmoud, U. Comparative study between chemical coagulation/precipitation (C/P) versus coagulation/dissolved air flotation (C/DAF) for pre-treatment of personal care products (PCPs) wastewater. Desalination 2020, 252, 106–112. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Vellido-Pérez, J.A.; González-Hernández, R.; Martínez-Férez, A. Optimization and modeling of two-phase olive-oil washing wastewater integral treatment and phenolic compounds recovery by novel weak-base ion exchange resins. Sep. Purif. Technol. 2020, 249, 117084. [Google Scholar] [CrossRef]
- Shen, W.; Mukherjee, D.; Koirala, N.; Hu, G.; Lee, K.; Zhao, M.; Li, J. Microbubble and nanobubble-based gas flotation for oily wastewater treatment: A review. Environ. Rev. 2022, 30, 359–379. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Liu, Z. Air flotation techniques for oily wastewater treatment, in Advan. Technol. In Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 153–172. [Google Scholar]
- Yeit Haan, T.; Isma Nordin, P.M.; Ahmad Juanda, N.I.; Mohd Shafi, M.A.; Krishnan, P. A Review on Adsorption Process for the Treatment of Oily Wastewater. Adv. Environ. Eng. Res. 2023, 4, 1–30. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Salman, R.H.; Abbar, A.H. Optimization of a combined electrocoagulation-electro-oxidation process for the treatment of Al-Basra Majnoon Oil field wastewater: Adopting a new strategy. Chem. Eng. Process.-Process Intensif. 2023, 183, 109227. [Google Scholar] [CrossRef]
- Al-Obaidi, Q.; Aljibori, H.S.S.; Abdullah, T.A.; Mohammed, M.N.; Abdullah, O.I. Performance Investigation of Surface Modified Ceramic Microfilitration Membranes of Ionic Water Treatment. Environ. Res. Eng. Manag. 2024, 80, 49–55. [Google Scholar] [CrossRef]
- Al-Obaidi, Q.; Selem, N.Y.; Al-Dahhan, M.H. Emulsion liquid membrane (ELM) enhanced by nanoparticles and ionic liquid for extracting vanadium ions from wastewater. Environ. Sci. Pollut. Res. 2024, 31, 48576–48589. [Google Scholar] [CrossRef]
- Khorram, A.G.; Fallah, N.; Nasernejad, B.; Afsham, N.; Esmaelzadeh, M.; Vatanpour, V. Electrochemical-based processes for produced water and oily wastewater treatment: A review. Chemosphere 2023, 338, 139565. [Google Scholar] [CrossRef] [PubMed]
- Druskovic, M.; Vouk, D.; Posavcic, H.; Halkijevic, I.; Nad, K. The application of electrochemical processes in oily wastewater treatment: A review. J. Environ. Sci. Health Part A 2021, 56, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef]
- Jasim, M.A.; AlJaberi, F.Y.A. Treatment of oily wastewater by electrocoagulation technology: A general review (2018–2022). J. Electrochem. Sci. Eng. 2023, 13, 361–372. [Google Scholar] [CrossRef]
- Chaturvedi, S.I. Mercury removal using Fe–Fe electrodes by electrocoagulation. Int. J. Mod. Eng. Res. 2013, 3, 101–108. [Google Scholar]
- Merma, A.G.; Santos, B.F.; Rego, A.S.; Hacha, R.R.; Torem, M.L. Treatment of oily wastewater from mining industry using electrocoagulation: Fundamentals and process optimization. J. Mater. Res. Technol. 2020, 9, 15164–15176. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Olson, N.; Ives, R.; Nshimyimana, J.P.; Rusinek, C.A.; Rose, J.B.; Liao, W. Techno-economic analysis of electrocoagulation on water reclamation and bacterial/viral indicator reductions of a high-strength organic wastewater—Anaerobic digestion effluent. Sustainability 2020, 12, 2697. [Google Scholar] [CrossRef]
- Al-Rubaiey, N.A.; Al-Barazanjy, M.G. Electrocoagulation Treatment of Oily Wastewater in the Oil Industry. J. Pet. Res. Stud. 2018, 8, 274–289. [Google Scholar] [CrossRef]
- Khalifa, O.; Banat, F.; Srinivasakannan, C.; Radjenovic, J.; Hasan, S.W. Performance tests and removal mechanisms of aerated electrocoagulation in the treatment of oily wastewater. J. Water Process Eng. 2020, 36, 101290. [Google Scholar] [CrossRef]
- Fotovat, F.; Hosseini, M. Treatment of oily wastewater by electrocoagulation: Simultaneous optimization of oil removal efficiency and specific energy consumption. J. Water Process Eng. 2023, 55, 104221. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Ghernaout, D.; Alghamdi, A.; Ghernaout, B. Electrocoagulation process: A mechanistic review at the dawn of its modeling. J. Environ. Sci. Allied Res. 2019, 2, 23–31. [Google Scholar] [CrossRef]
- Bajpai, M.; Katoch, S.S.; Kadier, A.; Singh, A. A review on electrocoagulation process for the removal of emerging contaminants: Theory, fundamentals, and applications. Environ. Sci. Pollut. Res. 2022, 29, 15252–15281. [Google Scholar] [CrossRef] [PubMed]
- Shahedi, A.; Darban, A.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Magnisali, E.; Yan, Q.; Vayenas, D.V. Electrocoagulation as a revived wastewater treatment method–practical approaches: A review. J. Chem. Technol. Biotechnol. 2022, 97, 9–25. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Alardhi, S.M.; Ahmed, S.A.; Salman, A.D.; Juzsakova, T.; Cretescu, I.; Le, P.-C.; Chung, W.J.; Chang, S.W.; Nguyen, D.D. Can electrocoagulation technology be integrated with wastewater treatment systems to improve treatment efficiency? Environ. Res. 2022, 214, 113890. [Google Scholar] [CrossRef]
- Tran, L.H.; Drogui, P.; Mercier, G.; Blais, J.F. Electrochemical degradation of polycyclic aromatic hydrocarbons in creosote solution using ruthenium oxide on titanium expanded mesh anode. J. Hazard. Mater. 2009, 164, 1118–1129. [Google Scholar] [CrossRef]
- Swain, K.; Abbassi, B.; Kinsley, C. Combined electrocoagulation and chemical coagulation in treating brewery wastewater. Water 2020, 12, 726. [Google Scholar] [CrossRef]
- Ali, M.A.; Mahdy, S.A.; Hussein, N.N. Synthesis of Titanium Dioxide Nanoparticles (TiO2) and Application for Reduction of Bacterial Growth. J. Nanostruct. 2024, 14, 1046–1057. [Google Scholar]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- Vidal, J.; Villegas, L.; Peralta-Hernández, J.M.; Salazar González, R. Removal of Acid Black 194 dye from water by electrocoagulation with aluminum anode. J. Environ. Sci. Health Part A 2016, 51, 289–2966. [Google Scholar] [CrossRef] [PubMed]
- Abbar, A.H.; Alkurdi, S.S. Performance evaluation of a combined electrocoagulation–electrooxidation process for the treatment of petroleum refinery wastewater. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Diyala, Iraq, 16–17 December 2020; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Şengil, İ.A. Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J. Hazar. Mater. 2016, 137, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Canizares, P.; Martinez, F.; Jiménez, C.; Sáez, C.; Rodrigo, M.A. Coagulation and electrocoagulation of oil-in-water emulsions. J. Hazar. Mater. 2008, 151, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, J.; Wang, Q.; Meng, H.; Wang, L. Research trends in electrochemical technology for water and wastewater treatment. Appl. Water Sci. 2017, 7, 13–30. [Google Scholar] [CrossRef]
- Ali, M.A.; Mahdy, S.A.; Hussein, N.N. Anticancer properties of titanium dioxide (TiO2) nanoparticles obtained from Quercus infectoria plant extract. J. Nanostruct. 2024, 14, 492–504. [Google Scholar]
- Weiss, S.F.; Christensen, M.L.; Jørgensen, M.K. Mechanisms behind pH changes during electrocoagulation. AIChE J. 2021, 67, e17384. [Google Scholar] [CrossRef]
- Tetteh, E.; Rathilal, S. Evaluation of different polymeric coagulants for the treatment of oil refinery wastewater. Cogent Eng. 2020, 7, 1785756. [Google Scholar] [CrossRef]
- Godart, P.; Fischman, J.; Seto, K.; Hart, D. Hydrogen production from aluminum-water reactions subject to varied pressures and temperatures. Int. J. Hydrogen Energy 2019, 44, 11448–11458. [Google Scholar] [CrossRef]
- Kawai, M.; Nagao, N.; Kawasaki, N.; Imai, A.; Toda, T. Improvement of COD removal by controlling the substrate degradability during the anaerobic digestion of recalcitrant wastewater. J. Environ. Manag. 2016, 181, 838–846. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Zuhair, S.; Al-Lobaney, A.; Makhlouf, S. Assessment of electrocoagulation for the treatment of petroleum refinery wastewater. J. Environ. Manag. 2009, 91, 180–185. [Google Scholar] [CrossRef]
- Huang, J.; Ali, A.B.; Samad, S.; Abdulrahman, A.; Osman, H.; Jumanazarov, D.; Atamurotov, F.; Mahariq, I. Sustainable and Electrostatically Engineered MXene-Based composite for environmental pollution remediation of Congo red dye and Cefixime from wastewater. Sep. Purif. Technol. 2025, 369, 133164. [Google Scholar] [CrossRef]
- Krupińska, I. Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef]
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 3500-fe iron. In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2022; Available online: https://www.standardmethods.org/doi/abs/10.2105/SMWW.2882.055 (accessed on 30 November 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).