Choice of Primer Pairs Affects the eDNA-Based Detection of Eukaryotic Phytoplankton Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Bioinformatic Analysis
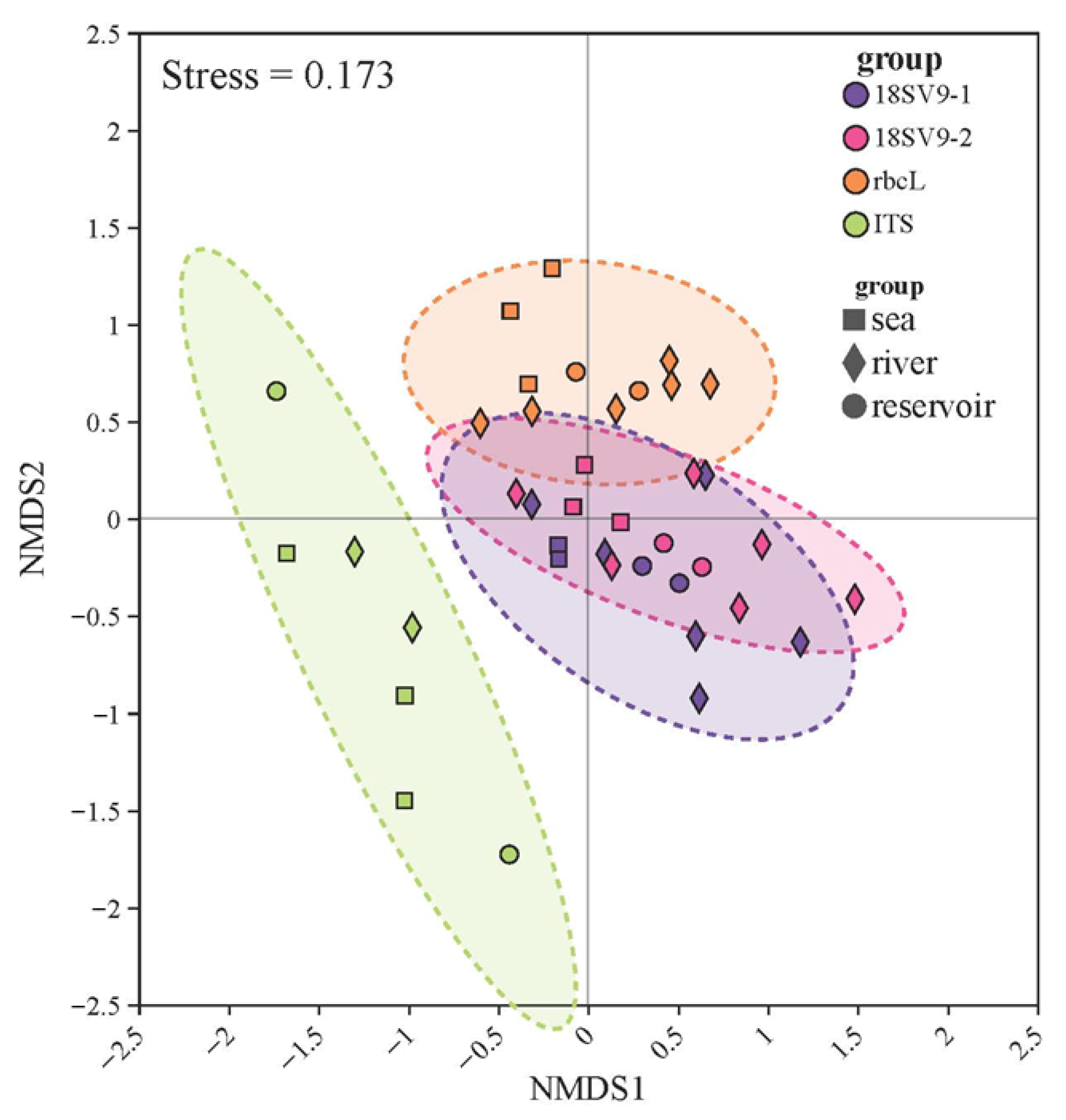
2.4. Statistical Analysis
3. Results
3.1. Analysis of Eukaryotic Phytoplankton eDNA Sequence Specificity
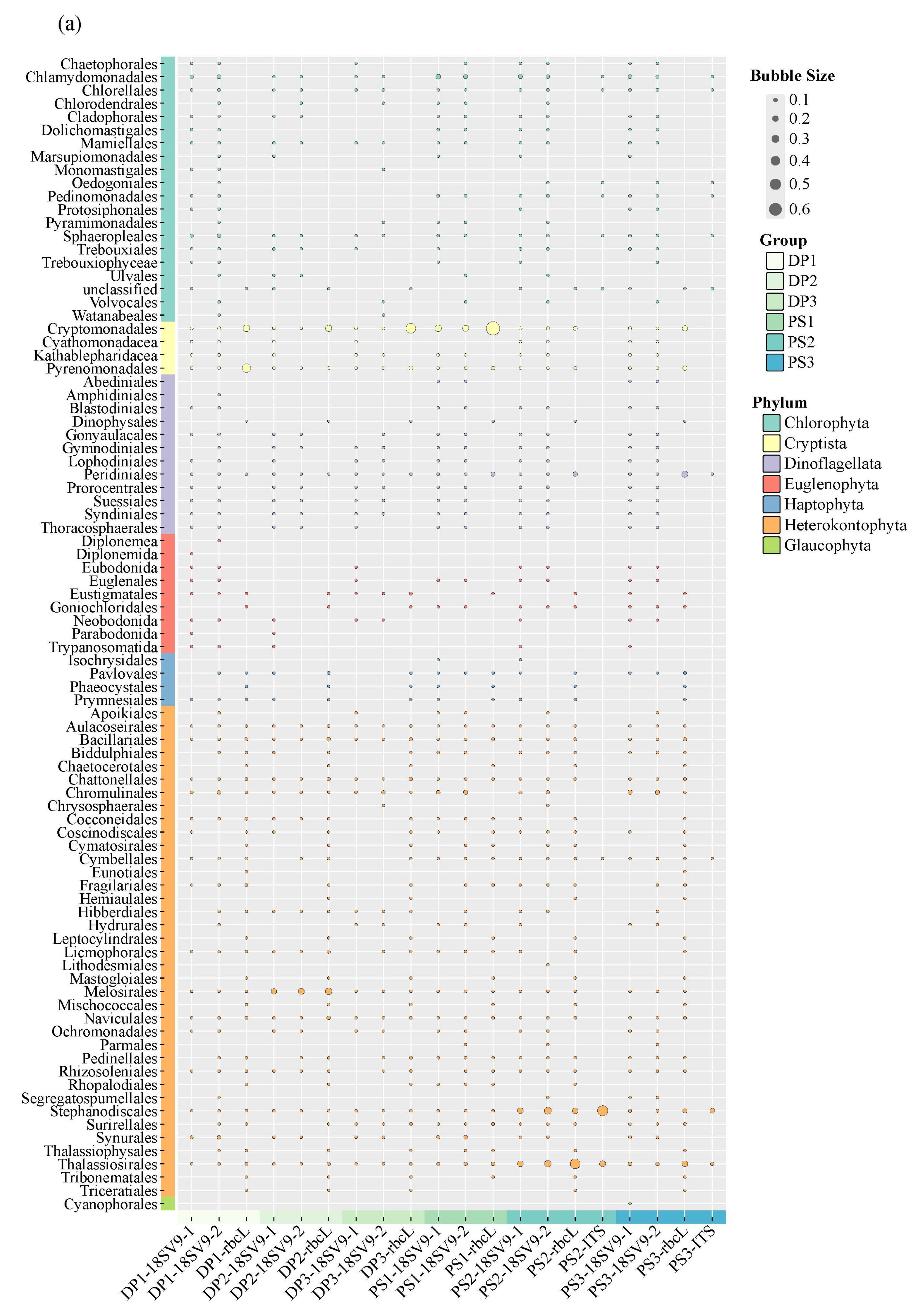
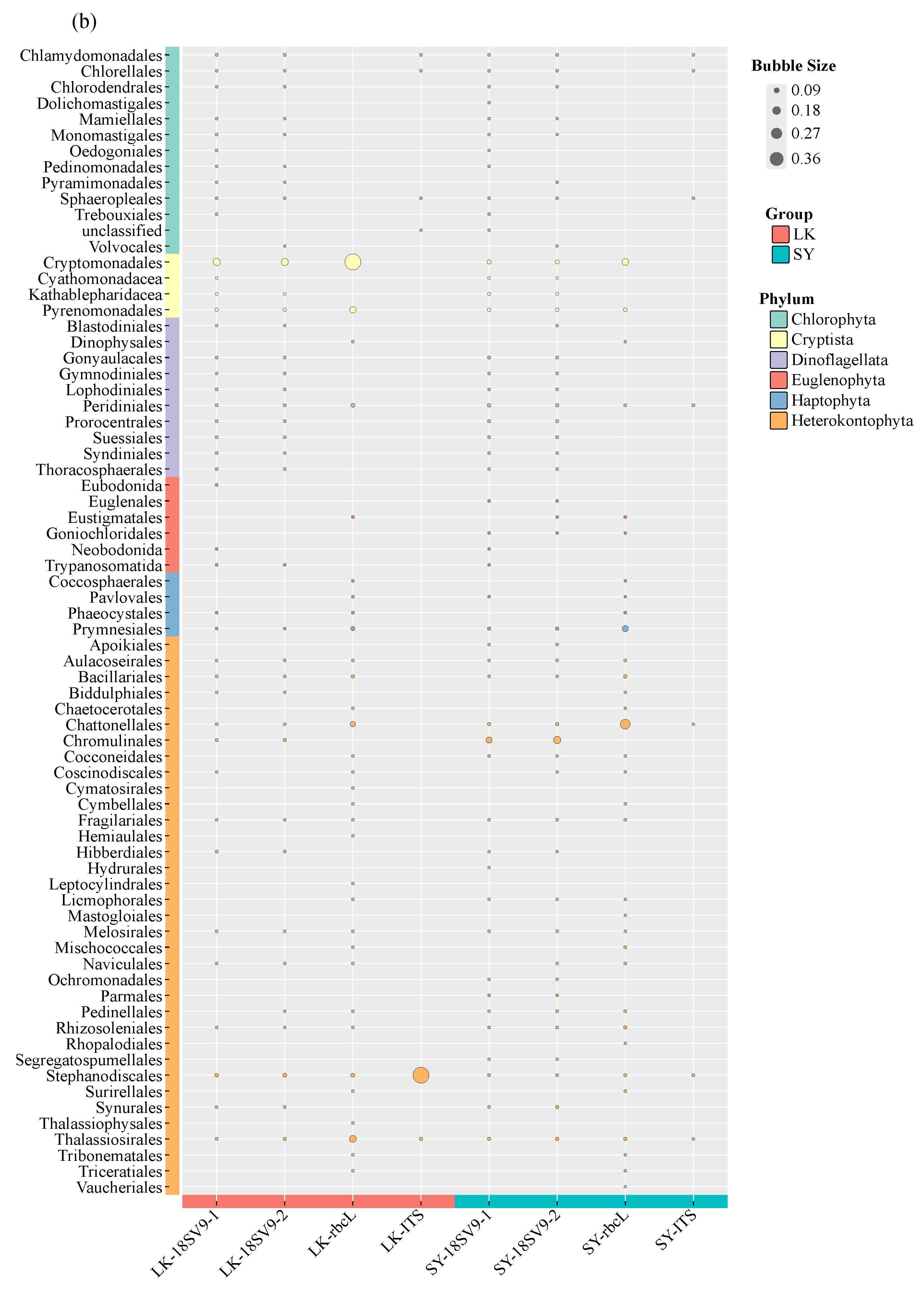
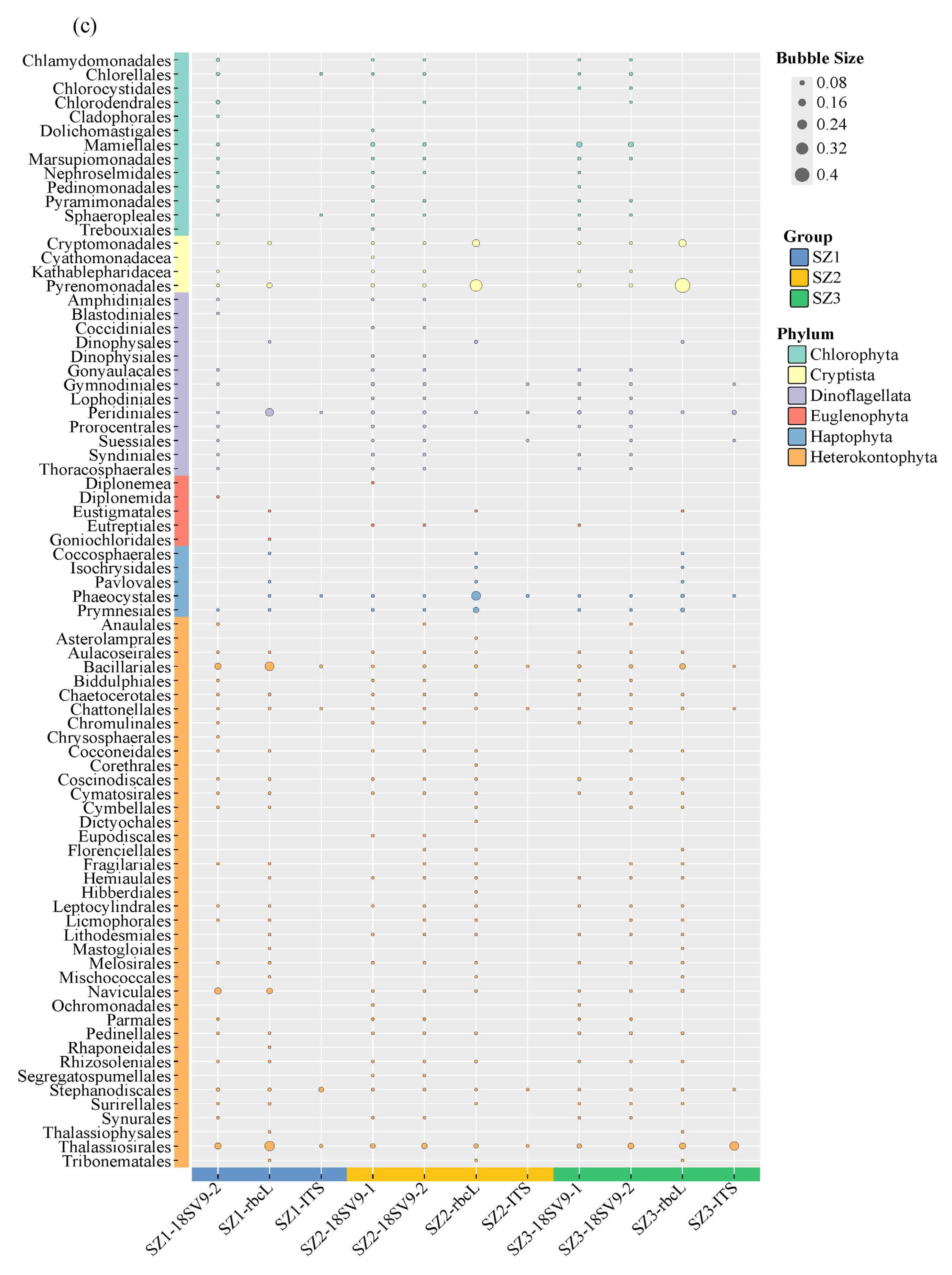
3.2. Species Composition and Taxonomic Richness
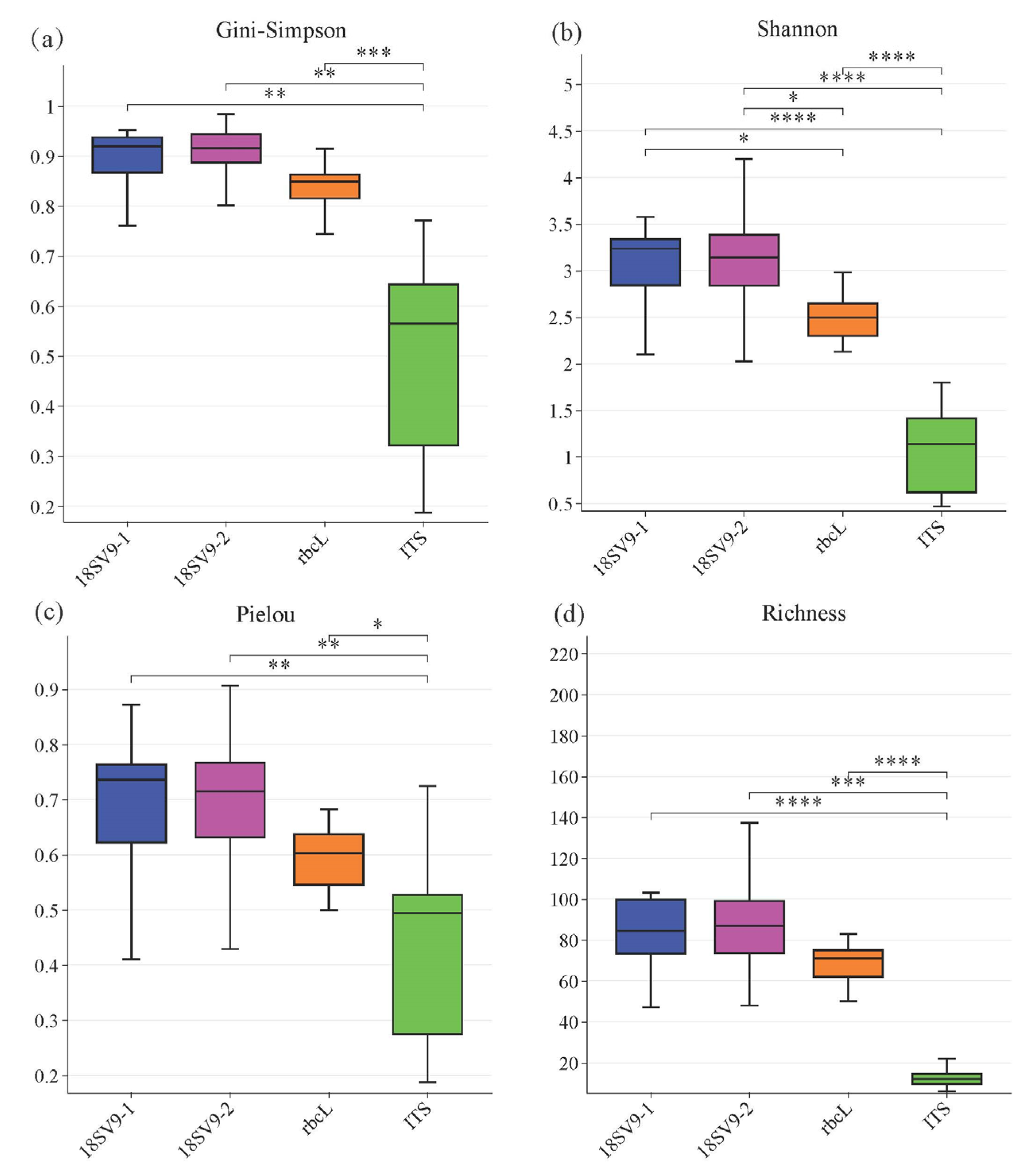
3.3. The Alpha Diversity
3.4. Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henson, S.A.; Cael, B.B.; Allen, S.R.; Dutkiewicz, S. Future phytoplankton diversity in a changing climate. Nat. Commun. 2021, 12, 5372. [Google Scholar] [CrossRef] [PubMed]
- Domis, L.N.D.; Van de Waal, D.B.; Helmsing, N.R.; Van Donk, E.; Mooij, W.M. Community stoichiometry in a changing world: Combined effects of warming and eutrophication on phytoplankton dynamics. Ecology 2014, 95, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.T.; Jin, X.L.; Feng, J.; Wu, S.H.; Wu, J.J.; Liu, Y.; Xie, Z.X.; Li, Z.; Chen, C.X. Spatial and Temporal Characteristics of Phytoplankton Communities in Drinking Water Source Reservoirs in Shenzhen, China. Plants 2023, 12, 3933. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.J.; Xu, F.; Xia, J.; Yang, X.; Zhang, F.B.; Liu, S.Y.; Zhang, T. Evaluation of the current status and risks of aquatic ecology in the Jialing River Basin based on the characteristics and succession trends of phytoplankton communities. Ecol. Indic. 2025, 170, 113121. [Google Scholar] [CrossRef]
- Li, X.X.; Chen, K.; Wang, C.; Zhuo, T.Y.; Li, H.T.; Wu, Y.; Lei, X.H.; Li, M.; Chen, B.; Chai, B.B. Deciphering environmental factors influencing phytoplankton community structure in a polluted urban river. J. Environ. Sci. 2025, 148, 375–386. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, J.H.; Zhang, Y.; Shi, J.Z.; Yu, H.X.; Zhang, X.W. eDNA biomonitoring revealed the ecological effects of water diversion projects between Yangtze River and Tai Lake. Water Res. 2022, 210, 117994. [Google Scholar] [CrossRef]
- Wu, D.; Li, R.P.; Zhang, F.Y.; Liu, J. A review on drone-based harmful algae blooms monitoring. Environ. Monit. Assess. 2019, 191, 211. [Google Scholar] [CrossRef]
- Zeng, L.P.; Wen, J.; Huang, B.J.; Yang, Y.; Huang, Z.W.; Zeng, F.T.; Fang, H.Y.; Du, H.W. Environmental DNA metabarcoding reveals the effect of environmental selection on phytoplankton community structure along a subtropical river. Environ. Res. 2024, 243, 117708. [Google Scholar] [CrossRef]
- Gibson, T.I.; Baillie, C.; Collins, R.A.; Wangensteen, O.S.; Corrigan, L.; Ellison, A.; Heddell-Cowie, M.; Westoby, H.; Byatt, B.; Lawson-Handley, L.; et al. Environmental DNA reveals ecologically relevant spatial and temporal variation in fish assemblages between estuaries and seasons. Ecol. Indic. 2024, 165, 112215. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Bao, Y.C.; Fang, X.Y.; Ruan, Y.L.; Rong, Y.; Yang, G. A circumpolar study of surface zooplankton biodiversity of the Southern Ocean based on eDNA metabarcoding. Environ. Res. 2024, 255, 119183. [Google Scholar] [CrossRef]
- Hu, H.; Wei, X.Y.; Liu, L.; Wang, Y.B.; Bu, L.K.; Jia, H.J.; Pei, D.S. Biogeographic patterns of meio- and micro-eukaryotic communities in dam-induced river-reservoir systems. Appl. Microbiol. Biotechnol. 2024, 108, 130. [Google Scholar] [CrossRef]
- Buxton, A.; Diana, A.; Matechou, E.; Griffin, J.; Griffiths, R.A. Reliability of environmental DNA surveys to detect pond occupancy by newts at a national scale. Sci. Rep. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Specchia, V.; Zangaro, F.; Tzafesta, E.; Saccomanno, B.; Vadrucci, M.R.; Pinna, M. Environmental DNA detects biodiversity and ecological features of phytoplankton communities in Mediterranean transitional waters. Sci. Rep. 2023, 13, 15192. [Google Scholar] [CrossRef]
- Hou, T.Y.; Lu, S.C.; Shao, J.; Dong, X.H.; Yang, Z.C.; Yang, Y.W.; Yao, D.D.; Lin, Y.H. Assessment of planktonic community diversity and stability in lakes and reservoirs based on eDNA metabarcoding--A case study of Minghu National Wetland Park, China. Environ. Res. 2025, 271, 121025. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, C.C.; Campbell, L. Metabarcoding reveals high genetic diversity of harmful algae in the coastal waters of Texas, Gulf of Mexico. Harmful Algae 2023, 121, 102368. [Google Scholar] [CrossRef] [PubMed]
- Orberg, S.B.; Krause-Jensen, D.; Geraldi, N.R.; Ortega, A.; Díaz-Rúa, R.; Duarte, C.M. Fingerprinting Arctic and North Atlantic Macroalgae with eDNA—Application and perspectives. Environ. DNA 2022, 4, 385–401. [Google Scholar] [CrossRef]
- Zhang, G.L.; Guo, Z.L.; Ke, Y.; Li, H.Y.; Xiao, X.L.; Lin, D.; Lin, L.J.; Wang, Y.H.; Liu, J.C.; Lu, H.L.; et al. Comparative analysis of size-fractional eukaryotic microbes in subtropical riverine systems inferred from 18S rRNA gene V4 and V9 regions. Sci. Total Environ. 2024, 953, 175972. [Google Scholar] [CrossRef]
- Zimmermann, H.H.; Haròardóttir, S.; Ribeiro, S. Assessing the performance of short 18S rDNA markers for environmental DNA metabarcoding of marine protists. Environ. DNA 2024, 6, e580. [Google Scholar] [CrossRef]
- Min, X.Y.; Li, F.L.; Zhang, X.F.; Guo, F.; Zhang, F.; Zhang, Y. Choice of primer pairs and PCR polymerase affect the detection of fish eDNA. Environ. Sci. Eur. 2023, 35, 103. [Google Scholar] [CrossRef]
- Kezlya, E.; Tseplik, N.; Kulikovskiy, M. Genetic Markers for Metabarcoding of Freshwater Microalgae: Review. Biology 2023, 12, 1038. [Google Scholar] [CrossRef]
- Nolte, V.; Pandey, R.V.; Jost, S.; Medinger, R.; Ottenwälder, B.; Boenigk, J.; Schlötterer, C. Contrasting seasonal niche separation between rare and abundant taxa conceals the extent of protist diversity. Mol. Ecol. 2010, 19, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Câmara, P.; Menezes, G.C.A.; Pinto, O.H.B.; Silva, M.C.; Convey, P.; Rosa, L.H. Using metabarcoding to assess Viridiplantae sequence diversity present in Antarctic glacial ice. An. Da Acad. Bras. De Cienc. 2022, 94, e20201736. [Google Scholar] [CrossRef]
- Fawley, M.W.; Fawley, K.P.; Cahoon, A.B. Finding needles in a haystack-Extensive diversity in the eustigmatophyceae revealed by community metabarcode analysis targeting the rbcL gene using lineage-directed primers. J. Phycol. 2021, 57, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A Method for Studying Protistan Diversity Using Massively Parallel Sequencing of V9 Hypervariable Regions of Small-Subunit Ribosomal RNA Genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Wang, X.C.; Liu, C.; Huang, L.; Bengtsson-Palme, J.; Chen, H.M.; Zhang, J.H.; Cai, D.Y.; Li, J.Q. ITS1: A DNA barcode better than ITS2 in eukaryotes? Mol. Ecol. Resour. 2015, 15, 573–586. [Google Scholar] [CrossRef]
- Sipos, R.; Székely, A.J.; Palatinszky, M.; Révész, S.; Márialigeti, K.; Nikolausz, M. Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. Fems Microbiol. Ecol. 2007, 60, 341–350. [Google Scholar] [CrossRef]
- Tanabe, A.S.; Nagai, S.; Hida, K.; Yasuike, M.; Fujiwara, A.; Nakamura, Y.; Takano, Y.; Katakura, S. Comparative study of the validity of three regions of the 18S-rRNA gene for massively parallel sequencing-based monitoring of the planktonic eukaryote community. Mol. Ecol. Resour. 2016, 16, 402–414. [Google Scholar] [CrossRef]
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.M.; Breiner, H.W.; Richards, T.A. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef]
- Zou, S.M.; Fei, C.; Yang, W.N.; Huang, Z.; He, M.L.; Wang, C.H. High-efficiency 18S microalgae barcoding by coalescent, distance and character-based approaches: A test in Chlorella and Scenedesmus. J. Oceanol. Limnol. 2018, 36, 1771–1777. [Google Scholar] [CrossRef]
- Tragin, M.; Zingone, A.; Vaulot, D. Comparison of coastal phytoplankton composition estimated from the V4 and V9 regions of the 18S rRNA gene with a focus on photosynthetic groups and especially Chlorophyta. Environ. Microbiol. 2018, 20, 506–520. [Google Scholar] [CrossRef]
- Hall, J.D.; Fucíková, K.; Lo, C.; Lewis, L.A.; Karol, K.G. An assessment of proposed DNA barcodes in freshwater green algae. Cryptogam. Algol. 2010, 31, 529–555. [Google Scholar]
- Li, X.W.; Yang, Y.; Henry, R.J.; Rossetto, M.; Wang, Y.T.; Chen, S.L. Plant DNA barcoding: From gene to genome. Biol. Rev. 2015, 90, 157–166. [Google Scholar] [CrossRef]
- Apothéloz-Perret-Gentil, L.; Bouchez, A.; Cordier, T.; Cordonier, A.; Guéguen, J.; Rimet, F.; Vasselon, V.; Pawlowski, J. Monitoring the ecological status of rivers with diatom eDNA metabarcoding: A comparison of taxonomic markers and analytical approaches for the inference of a molecular diatom index. Mol. Ecol. 2021, 30, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Li, R.R.; Lan, X.; Kong, D.N.; Liu, X.D.; Xie, S.L. Benthic diatom eDNA metabarcoding for ecological assessment of an urban river: A comparison with morphological method. Ecol. Indic. 2024, 166, 112302. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Kibler, S.R.; Reece, K.S.; Stokes, N.A.; Lutzoni, F.M.; Yonish, B.A.; West, M.A.; Black, M.N.D.; Tester, P.A. Recognizing dinoflagellate species using ITS rDNA sequences. J. Phycol. 2007, 43, 344–355. [Google Scholar] [CrossRef]
- Tzafesta, E.; Saccomanno, B.; Zangaro, F.; Vadrucci, M.R.; Specchia, V.; Pinna, M. DNA Barcode Gap Analysis for Multiple Marker Genes for Phytoplankton Species Biodiversity in Mediterranean Aquatic Ecosystems. Biology 2022, 11, 1277. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.g.; Tian, F.; Li, Y.t.; Zhang, L.b.; Zhang, Z.; Wang, X.F.; Cai, W.G. Community structure of phytoplankton and its relationship to environmental factors in Shenzhen Bay. Ecol. Sci. 2021, 40, 9–16. [Google Scholar]

| Habitat | Sampling Site | Latitude and Longitude | |
|---|---|---|---|
| River | DP1 | 22.6271° N | 114.4376° E |
| DP2 | 25.2239° N | 114.2483° E | |
| DP3 | 22.6136° N | 114.3227° E | |
| PS1 | 22.6722° N | 114.3025° E | |
| PS2 | 22.6947° N | 114.3710° E | |
| PS3 | 22.7110° N | 114.4116° E | |
| Reservoir | LK | 22.6786° N | 114.1914° E |
| SY | 22.6997° N | 113.9031° E | |
| Sea | SZ1 | 22.5210° N | 113.9951° E |
| SZ2 | 22.4673° N | 113.9226° E | |
| SZ3 | 22.4950° N | 113.9658° E | |
| Primer Name | Primer Sequence (5′→3′) | Target Gene | Amplicon Size (bp) | References | |
|---|---|---|---|---|---|
| 18SV9-1 | 1380F | TTGTACACACCGCCC | 18S | 130 | [24] |
| 1510R | CCTTCYGCAGGTTCACCTAC | ||||
| 18SV9-2 | 1389F | TCCCTGCCHTTTGTACACAC | 18S | 121 | [24] |
| 1510R | CCTTCYGCAGGTTCACCTAC | ||||
| rbcL | EU-rbcL500FA | GGNCGYGTWGTDTWYGAAGGT | rbcL | 370 | [23] |
| Eustig-rbcL-R900 | CACCWGCCATACGCATCC | ||||
| ITS | ITS1F | TCCGTAGGTGAACCTGCGG | ITS | 280 | [25] |
| b-ITS1R | CGCTGCGTTCTTCATCG | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Liu, Y.; Wu, S.; Chen, J.; Feng, J.; Wu, J.; Chen, C. Choice of Primer Pairs Affects the eDNA-Based Detection of Eukaryotic Phytoplankton Communities. Water 2025, 17, 3173. https://doi.org/10.3390/w17213173
Liang Q, Liu Y, Wu S, Chen J, Feng J, Wu J, Chen C. Choice of Primer Pairs Affects the eDNA-Based Detection of Eukaryotic Phytoplankton Communities. Water. 2025; 17(21):3173. https://doi.org/10.3390/w17213173
Chicago/Turabian StyleLiang, Qiting, Ying Liu, Shenhao Wu, Jianyi Chen, Jie Feng, Jiajia Wu, and Chunxing Chen. 2025. "Choice of Primer Pairs Affects the eDNA-Based Detection of Eukaryotic Phytoplankton Communities" Water 17, no. 21: 3173. https://doi.org/10.3390/w17213173
APA StyleLiang, Q., Liu, Y., Wu, S., Chen, J., Feng, J., Wu, J., & Chen, C. (2025). Choice of Primer Pairs Affects the eDNA-Based Detection of Eukaryotic Phytoplankton Communities. Water, 17(21), 3173. https://doi.org/10.3390/w17213173






