Synergistic Nitrogen and Phosphorus Elimination via Iron–Carbon Micro-Electrolysis in Constructed Wetlands Treating Low-Pollution Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Setup
2.3. Water Sampling and Analysis
2.4. Analysis of Microbial Community Composition and Diversity
3. Results and Discussion
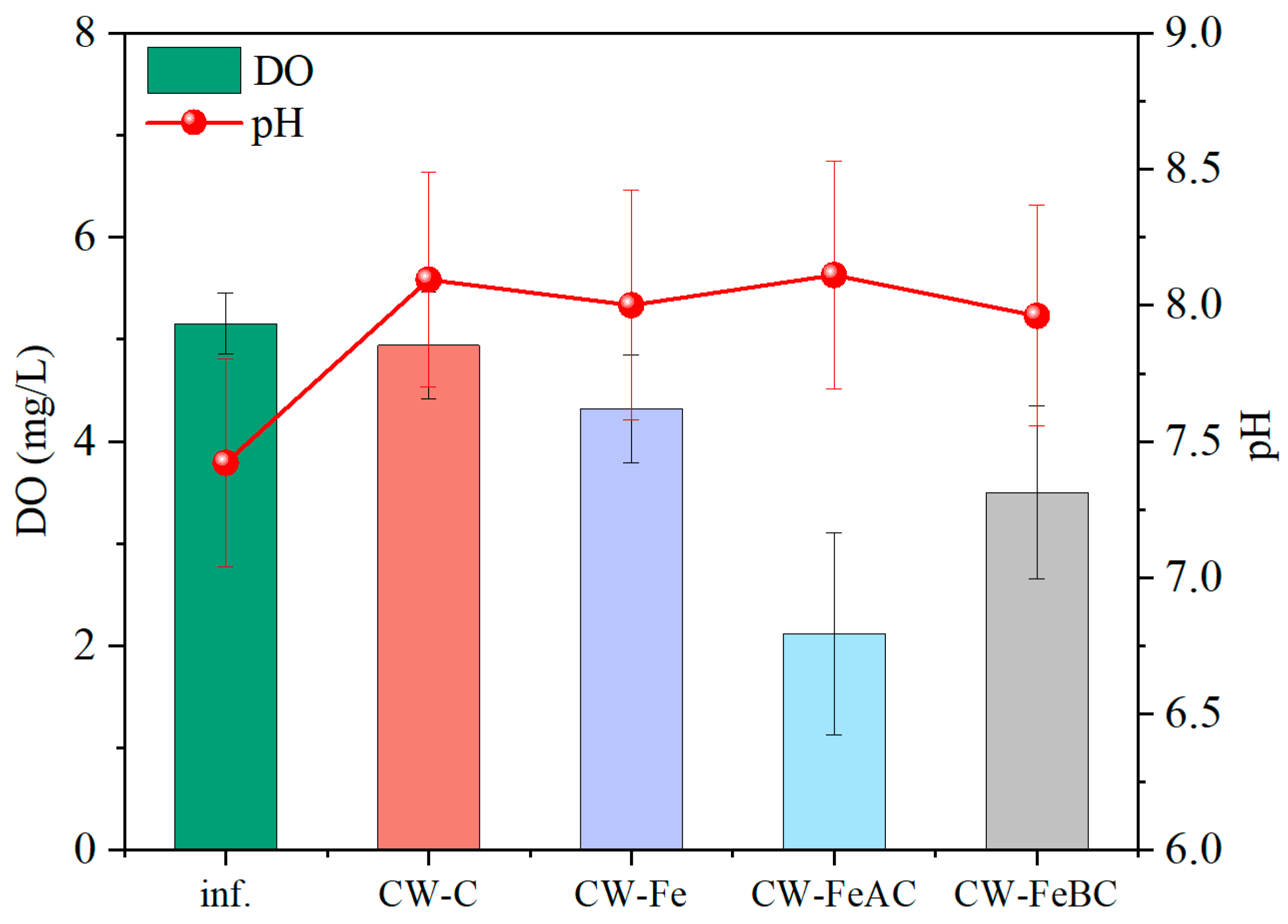
3.1. Changes in Physical and Chemical Properties
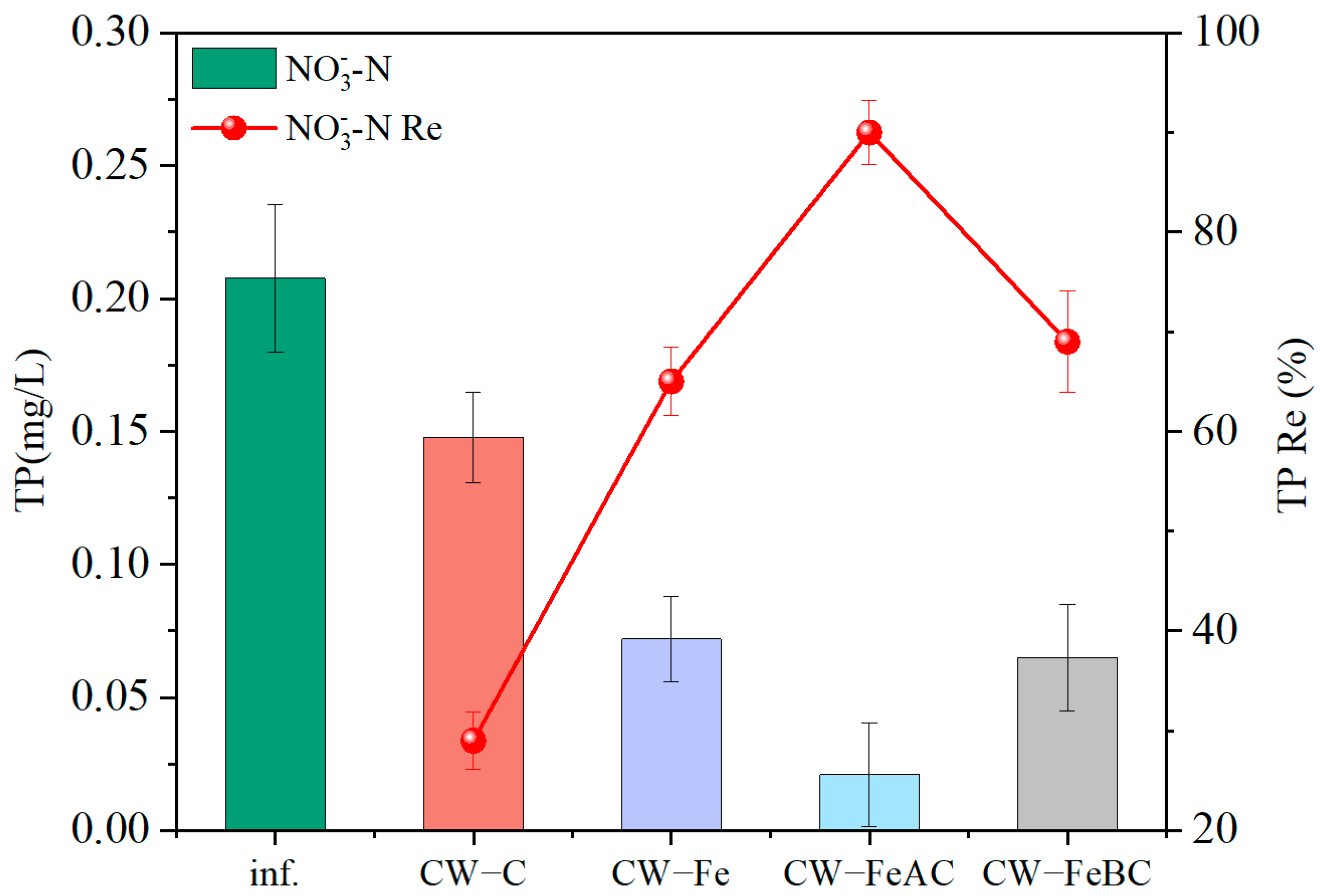
3.2. The Nitrogen Removal Performance
3.3. Phosphorus Removal Performance
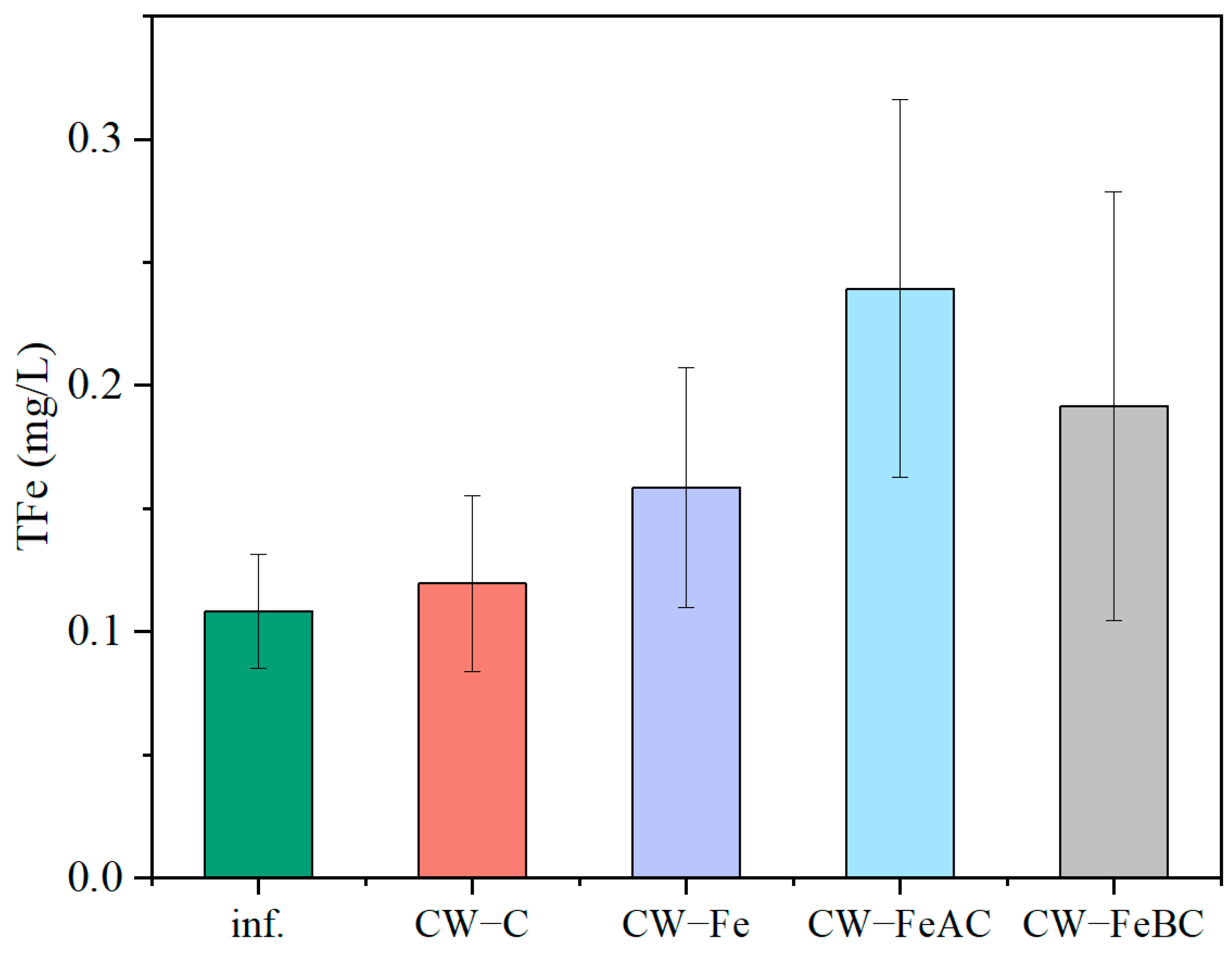
3.4. The Changes in the TFe Content
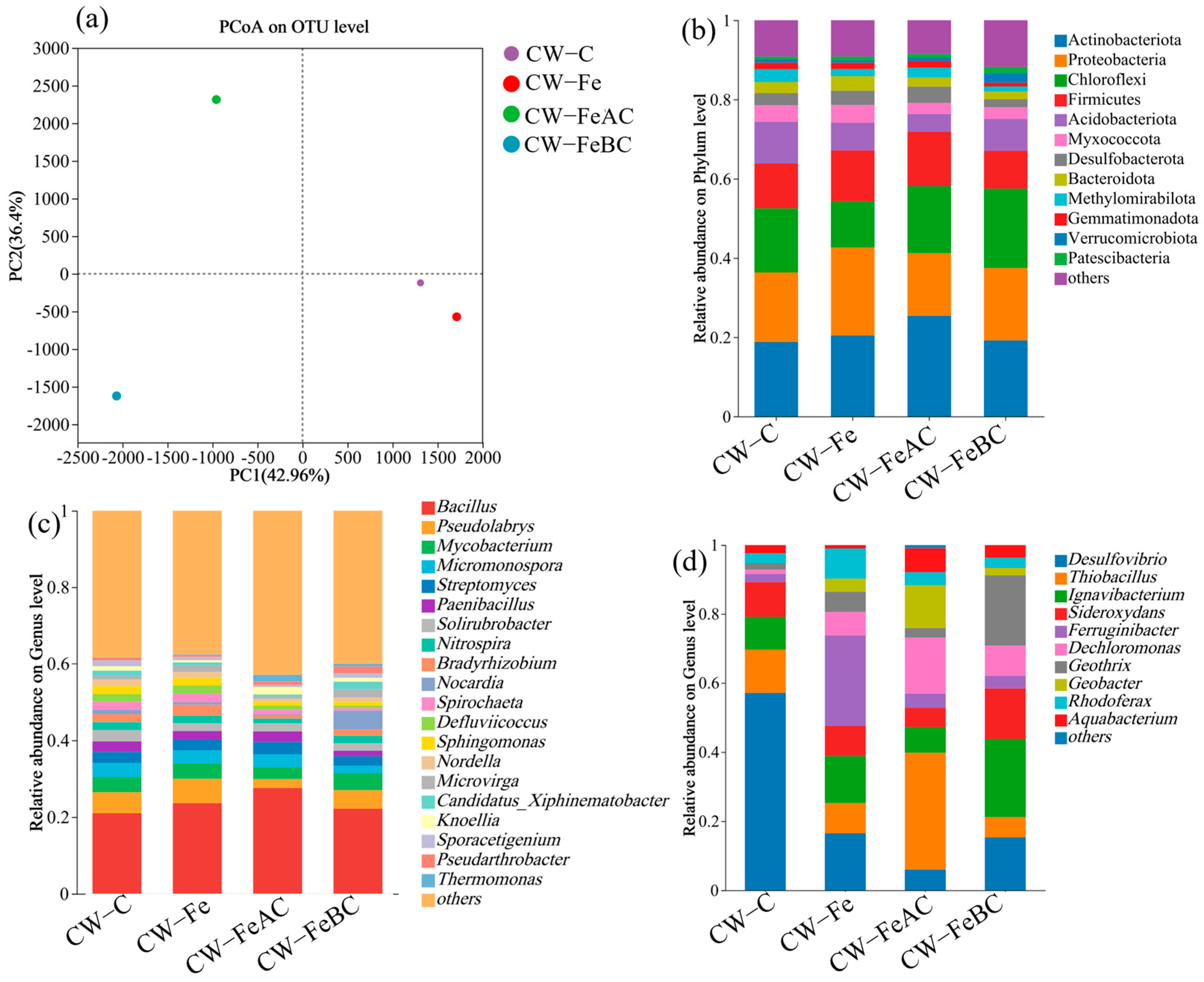
3.5. Analysis of Microbial Community Structure
3.6. Function Prediction
3.7. Limitations and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Cui, B.; Yuan, B.; Zhang, A.; Feng, J.; Zhang, J.; Han, X.; Pan, L.; Li, L. Denitrification Mechanism and Artificial Neural Networks Modeling for Low-Pollution Water Purification Using a Denitrification Biological Filter Process. Sep. Purif. Technol. 2021, 257, 117918. [Google Scholar] [CrossRef]
- Feng, J.; Cui, B.; Yuan, B.; Zhang, L.; Zhang, J.; Zhang, A.; Han, X.; Pan, L. Purification mechanism of low-pollution water in three submerged plants and analysis of bacterial community structure in plant rhizospheres. Environ. Eng. Sci. 2019, 37, 560–571. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Sun, S.; Yan, P.; Fan, Y.; Xi, Y.; He, S. Trade-off between electrochemical and microbial nutrient eliminations in iron anode-assisted constructed wetlands: The specificity of voltage level. J. Environ. Manag. 2025, 377, 124623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Wang, Y.; Huang, W.; Wang, J.; Zhou, J.; He, Q. Sulfur and iron cycles promoted nitrogen and phosphorus removal in electrochemically assisted vertical flow constructed wetland treating wastewater treatment plant effluent with high S/N ratio. Water Res. 2019, 151, 20–30. [Google Scholar] [CrossRef]
- Guo, H.; Zhai, X.; Hu, M.; Chang, J.; Lee, D. Atypical removals of nitrogen and phosphorus with biochar-pyrite vertical flow constructed wetlands treating wastewater at low C/N ratio. Bioresour. Technol. 2025, 422, 132219. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, Q.; Li, Y.; Wu, W. Integrated Treatment of Suburb Diffuse Pollution Using Large-Scale Multistage Constructed Wetlands Based on Novel Solid Carbon: Nutrients Removal and Microbial Interactions. J. Environ. Manag. 2023, 326, 116709. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Li, K.; Lu, S.; Guo, X.; Zhang, J.; Xi, B. Removal of Nitrogen from Low Pollution Water by Long-Term Operation of an Integrated Vertical-Flow Constructed Wetland: Performance and Mechanism. Sci. Total Environ. 2019, 652, 977–988. [Google Scholar] [CrossRef]
- Hou, M.; Gu, X.; Lai, W.; Fan, Y.; Sun, S.; Yan, P.; Zhang, Y.; Zheng, X.; He, S. Sulfur-iron interactions forming activated FexSy pool in-situ to synergistically improve nitrogen removal in denitrification system. J. Environ. Manag. 2025, 388, 126047. [Google Scholar] [CrossRef]
- Deng, S.; Li, D.; Yang, X.; Zhu, S.; Li, J. Process of Nitrogen Transformation and Microbial Community Structure in the Fe(0)–Carbon-Based Bio-Carrier Filled in Biological Aerated Filter. Environ. Sci. Pollut. Res. 2016, 23, 6621–6630. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, D.; Yang, X.; Xing, W.; Li, J.; Zhang, Q. Iron [Fe(0)]-Rich Substrate Based on Iron–Carbon Micro–Electrolysis for Phosphorus Adsorption in Aqueous Solutions. Chemosphere 2017, 168, 1486–1493. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, W.; Zheng, P.; Zheng, X.; He, S.; Zhao, M. Iron Scraps Enhance Simultaneous Nitrogen and Phosphorus Removal in Subsurface Flow Constructed Wetlands. J. Hazard. Mater. 2020, 395, 122612. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ou, Y.; Yan, B.; Zhu, H.; Liu, H.; Cheng, L.; Jiao, P. Synergistic Improvement of Nitrogen and Phosphorus Removal in Constructed Wetlands by the Addition of Solid Iron Substrates and Ferrous Irons. Fundam. Res. 2023, 3, 890–897. [Google Scholar] [CrossRef]
- He, C.-S.; Ding, R.-R.; Chen, J.Q.; Li, W.Q.; Li, Q.; Mu, Y. Interactions between Nanoscale Zero Valent Iron and Extracellular Polymeric Substances of Anaerobic Sludge. Water Res. 2020, 178, 115817. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Wu, P.; Yang, F.; Chen, B.; Wang, Y.; Meng, G.; Kong, Q.; Fang, J.; Wu, H. Enhanced Nitrogen Removal and Greenhouse Gas Reduction via Activated Carbon Coupled Iron-Based Constructed Wetlands. J. Water Process Eng. 2024, 66, 106098. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Liu, M.; Niu, J. Numerical Simulation of the Hydrodynamic Behavior and the Synchronistic Oxidation and Reduction in an Internal Circulation Micro-Electrolysis Reactor. Chem. Eng. J. 2020, 381, 122709. [Google Scholar] [CrossRef]
- Xing, W.; Li, D.; Li, J.; Hu, Q.; Deng, S. Nitrate Removal and Microbial Analysis by Combined Micro-Electrolysis and Autotrophic Denitrification. Bioresour. Technol. 2016, 211, 240–247. [Google Scholar] [CrossRef]
- Easton, Z.M.; Rogers, M.; Davis, M.; Wade, J.; Eick, M.; Bock, E. Mitigation of Sulfate Reduction and Nitrous Oxide Emission in Denitrifying Environments with Amorphous Iron Oxide and Biochar. Ecol. Eng. 2015, 82, 605–613. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, F.; Yang, C.; Su, X.; Guo, F.; Xu, Q.; Peng, G.; He, Q.; Chen, Y. Highly efficient nitrate removal in a heterotrophic denitrification system amended with redox-active biochar: A molecular and electrochemical mechanism. Bioresour. Technol. 2019, 275, 297–306. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of Pyrolysis Temperature on Soybean Stover- and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Pullammanappallil, P.; Ding, W.; Zimmerman, A.R. Biochar from Anaerobically Digested Sugarcane Bagasse. Bioresour. Technol. 2010, 101, 8868–8872. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, S.; Wang, H.; Chu, Y.; Zheng, L.; Song, Y.; Fang, C. Responses of nitrogen removal, microbial community and antibiotic resistance genes to biodegradable microplastics during biological wastewater treatment. Biochem. Eng. J. 2025, 219, 109732. [Google Scholar] [CrossRef]
- Tang, X.; Chen, L.; Liu, H.; Tao, R.; Zhang, X.; Dao, Y.; Yang, Y. Micropollutant removal efficiency and microbial community of different hybrid constructed wetland systems. J. Environ. Manag. 2025, 389, 126143. [Google Scholar] [CrossRef]
- Hocaoglu, S.M.; Insel, G.; Cokgor, E.U.; Orhon, D. Effect of Low Dissolved Oxygen on Simultaneous Nitrification and Denitrification in a Membrane Bioreactor Treating Black Water. Bioresour. Technol. 2011, 102, 4333–4340. [Google Scholar] [CrossRef]
- Tang, H.; Ma, Z.; Qin, Y.; Wu, H.; Xu, X.; Xin, L.; Wu, W. Pilot-Scale Study of Step-Feed Anaerobic Coupled Four-Stage Micro-Oxygen Gradient Aeration Process for Treating Digested Swine Wastewater with Low Carbon/Nitrogen Ratios. Bioresour. Technol. 2023, 380, 129087. [Google Scholar] [CrossRef]
- Molina-Balmaceda, A.; Rojas-Candia, V.; Arismendi, D.; Richter, P. Activated carbon from avocado seed as sorbent phase for microextraction technologies: Activation, characterization, and analytical performance. Anal. Bioanal. Chem. 2024, 416, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yin, M.; Nie, S.; Xiao, X.; Chu, C.; Chen, B. Pyrogenic Carbon Enhances Hydroxyl Radical Generation during Microbial Transformation of Ferrihydrite at Redox Interfaces. Environ. Sci. Technol. 2025, 59, 12692–12702. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, Y.; Li, H.; Yang, Y.; Wu, R. Research Progress of Phosphorus Adsorption by Attapulgite and Its Prospect as a Filler of Constructed Wetlands to Enhance Phosphorus Removal from Mariculture Wastewater. J. Environ. Chem. Eng. 2022, 10, 108748. [Google Scholar] [CrossRef]
- Su, M.; Liang, J.; Zhang, J.; Meng, F.; Li, S.; Jia, Q.; Liu, H.; Chen, X.; Jiang, H. Advanced Nitrogen and Phosphorus Removal from Municipal Tailwater in Sulfur-Based Constructed Wetland Strengthened by Boron Oxide and Magnesium Oxide at Low Temperatures: Role of Multipath Autotrophic Pathways. Process Saf. Environ. Prot. 2025, 200, 107384. [Google Scholar] [CrossRef]
- Li, M.; Fu, S.; Han, Y.; Zheng, J.; Wang, C.; Xu, X.; Zhu, L. Synergistic Removal of Carbon and Phosphorus by Modified Carbon-Based Magnetic Materials. Chem. Eng. J. 2024, 491, 151244. [Google Scholar] [CrossRef]
- Mansour, S.; Basiouny, M.E.; Abosiada, O.A. Activated Carbon and Biochar Prepared from Date Palm Fiber as Adsorbents of Phosphorus from Wastewater. Desalination Water Treat. 2025, 321, 100925. [Google Scholar] [CrossRef]
- Wu, R.; Jeyakumar, P.; Bolan, N.; Zhai, X.; Wang, H.; Pan, M.; Lian, J.; Cheng, L.; Li, J.; Hou, M.; et al. Enhanced Denitrification Driven by a Novel Iron-Carbon Coupled Primary Cell: Chemical and Mixotrophic Denitrification. Biochar 2024, 6, 5. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, S.; Gu, X.; Fan, Y.; He, S. Mechanistic insights into microplastic-mediated shifts in nitrogen metabolism and sensory quality across emergent and submerged-plant wetlands: Evidence from metagenomics and physiological indicators. J. Hazard. Mater. 2025, 498, 139937. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Wang, Y.; Liu, B. Effect of Temperature on Simultaneous Nitrogen and Phosphorus Removal and Microbial Community in Anaerobic-Aerobic-Anoxic Sequencing Batch Reactor. Desalination Water Treat. 2022, 253, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Li, W.; Shi, Z.; Lin, Y.; Zhou, R.; Meng, J.; Tang, J.; Hou, P. Coupled Process of In-Situ Sludge Fermentation and Riboflavin-Mediated Nitrogen Removal for Low Carbon Wastewater Treatment. Bioresour. Technol. 2022, 363, 127928. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Wei, C.; Yang, Z.; Yang, L. The Enhancement of Autotrophic Denitrification by Manganese Carbonate on Sulfur-Pyrite Composite Filler and Its Biochemical Mechanism. J. Water Process Eng. 2025, 76, 108156. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Xu, L.; Su, J.; Feng, J.; Zhang, Y.; Cheng, W.; Bai, J. Mechanistic insights and performance of Mn redox cycling in a dual-bacteria bioreactor for ammonium and Cr(VI) removal. Water Res. 2025, 268, 123713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, W.; Liu, G.; Yu, X.; Huang, J.; Wang, F.; Meng, X.; Cao, J. Metabolic Characteristics and the Cross-Feeding of Bacillus and Ca. Brocadia in an Integrated Partial Denitrification-Anammox Reactor Driven by Glycerol. J. Environ. Chem. Eng. 2024, 12, 111859. [Google Scholar]
- Zhang, X.; Song, X.; Cheng, X.; Huang, Z.; Dong, D.; Li, X. Enhanced Denitrification of Biodegradable Polymers Using Bacillus Pumilus in Aerobic Denitrification Bioreactors: Performance and Mechanism. Bioresour. Technol. 2024, 394, 130240. [Google Scholar] [CrossRef]
- Tan, C.; Chen, S.; Zhang, H.; Ma, Y.; Qu, Z.; Yan, N.; Zhang, Y.; Rittmann, B.E. The Roles of Rhodococcus Ruber in Denitrification with Quinoline as the Electron Donor. Sci. Total Environ. 2023, 902, 166128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Dong, H.; Min, J.; Xu, H.; Sun, D.; Liu, X.; Dang, Y.; Qiu, B.; Mennella, T.; et al. Evidence of autotrophic direct electron transfer denitrification (DETD) by thiobacillus species enriched on biocathodes during deep polishing of effluent from a municipal wastewater treatment plant. Chem. Eng. J. 2024, 495, 153389. [Google Scholar] [CrossRef]
- Sestok, A.E.; Brown, J.B.; Obi, J.O.; O’Sullivan, S.M.; Garcin, E.D.; Deredge, D.J.; Smith, A.T. A Fusion of the Bacteroides Fragilis Ferrous Iron Import Proteins Reveals a Role for FeoA in Stabilizing GTP-Bound FeoB. J. Biol. Chem. 2022, 298, 101808. [Google Scholar] [CrossRef]
- Chen, X.; Sheng, Y.; Wang, G.; Zhou, P.; Liao, F.; Mao, H.; Zhang, H.; Qiao, Z.; Wei, Y. Spatiotemporal Successions of N, S, C, Fe, and As Cycling Genes in Groundwater of a Wetland Ecosystem: Enhanced Heterogeneity in Wet Season. Water Res. 2024, 251, 121105. [Google Scholar] [CrossRef]
- Guo, H.; Su, Y.; Xue, S.; Li, N.; Li, R.; Wang, S.; Bu, J.; Zhang, H.; Huang, T. Breaking through the Bottleneck of Nitrogen Pollution Control in Micropolluted Water Sources: Enhancement of Aerobic Denitrification Efficacy Driven by Microbial Community Enrichment and Its Sustainable Application. Water Res. 2025, 285, 124070. [Google Scholar] [CrossRef] [PubMed]



| Sample | ACE | Chao | Coverage | Shannon | Simpson | Sobs |
|---|---|---|---|---|---|---|
| CW-C | 4344.35 | 4271.80 | 0.9628 | 6.99 | 0.0022 | 3366 |
| CW-Fe | 4484.55 | 4366.41 | 0.9618 | 7.02 | 0.0024 | 3456 |
| CW-FeAC | 3647.61 | 3633.34 | 0.9698 | 6.69 | 0.0039 | 2895 |
| CW-FeBC | 4772.06 | 4659.33 | 0.9591 | 7.14 | 0.0020 | 3689 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Ren, X.; Shen, J.; Zhou, X.; Wu, D.; He, S. Synergistic Nitrogen and Phosphorus Elimination via Iron–Carbon Micro-Electrolysis in Constructed Wetlands Treating Low-Pollution Water. Water 2025, 17, 3139. https://doi.org/10.3390/w17213139
Sun S, Ren X, Shen J, Zhou X, Wu D, He S. Synergistic Nitrogen and Phosphorus Elimination via Iron–Carbon Micro-Electrolysis in Constructed Wetlands Treating Low-Pollution Water. Water. 2025; 17(21):3139. https://doi.org/10.3390/w17213139
Chicago/Turabian StyleSun, Shanshan, Xiaojiao Ren, Jian Shen, Xuejin Zhou, Di Wu, and Shengbing He. 2025. "Synergistic Nitrogen and Phosphorus Elimination via Iron–Carbon Micro-Electrolysis in Constructed Wetlands Treating Low-Pollution Water" Water 17, no. 21: 3139. https://doi.org/10.3390/w17213139
APA StyleSun, S., Ren, X., Shen, J., Zhou, X., Wu, D., & He, S. (2025). Synergistic Nitrogen and Phosphorus Elimination via Iron–Carbon Micro-Electrolysis in Constructed Wetlands Treating Low-Pollution Water. Water, 17(21), 3139. https://doi.org/10.3390/w17213139







