Abstract
Although the NFT (nutrient film technique) solution application rate for cilantro is known for fresh water, the application rate is still debatable when using brackish water. The application rate alone influences flow velocity dynamics, which, when associated with nutrient solution salinity, can impact plant development when saline water is used. Knowledge of how to best combine solution salinity and application rates will help decide if brackish water can be used to produce cilantro under hydroponic conditions. Thus, two trials were conducted in sequence from November 2019 to February 2020 under a protected environment. Cilantro cv. Verdão was submitted to four levels of electrical conductivity of nutrient solutions (ECns of 1.7, 3.0, 4.5, and 6.0 dS m−1) combined with four flow rates (1.0, 2.0, 3.0, and 4.0 L min−1). Because Na+ and Ca2+ are predominant ions in brackish waters in the crystalline and sedimentary regions in the Brazilian Semiarid region, the first study used brackish waters dominated by NaCl and the second study used waters dominated by CaCl2. We measured gas exchange and other photosynthetic parameters in plants cultivated with nutrient solutions high in Cl− and prevalent in Na+ or Ca2+, each combined with different application rates. We concluded that the increment in salinity decreased the gas exchange of cilantro plants, especially when the brackish waters were dominant in Ca2+ and Cl−. Up to an ECns of 4.5 dS m−1, plants maintained their leaf chlorophyll concentrations, although with reduced gas exchange. Salt stress compromised chlorophyll a fluorescence, affecting important parameters such as initial, maximum, and variable fluorescence. Besides the effects of salinity on chlorophyll a and b concentrations, the quantum and maximum yields of photosystem II remained stable, indicating that photosystem II may have adapted to the saline conditions applied in this study. The variation in application rates was unable to attenuate the deleterious effects of salinity, regardless of the ionic prevalence. We conclude that cilantro plants can be cultivated under hydroponic conditions, using currently accepted flow rates, with nutrient solutions of up to 3.0 dS m−1 without severe damage to plant photosynthetic parameters.
1. Introduction
Although fresh water becomes scarcer as you move into the Brazilian semiarid region, away from the coast, brackish water becomes more available, but its high salt concentrations are unsuited for the irrigation of most crops. As freshwater resources have become more limited, brackish water potential increases as the main resource for irrigated agriculture in the interior of the Brazilian semiarid region [,]. Brackish water is saltier than freshwater (less than 1.0 dS m−1) but not as salty as seawater (approximately 53 dS m−1 or 35 ppt of dissolved salts). Brackish waters with salinity higher than 2.0 dS m−1 can be tolerated but will cause losses in the yield of salt-sensitive crops, such as cilantro []. It can result from the mixture of seawater with freshwater, but it can also occur naturally as groundwater.
The main soluble salts found in the groundwater of the Crystalline Region of the Brazilian semiarid region are the anions Cl−, CO3−2, and HCO3− and the cations Na+, Ca2+, and Mg2+ [], with the water’s chemical composition being influenced by the material’s origin, as well as the weathering degree of that material. A potential use for this water in agriculture is hydroponics, which is also an important system in which to evaluate groundwater when growing different crops. Because there is no soil in the hydroponic system, the matrix potential is minimized, making the total water potential dependent on the osmotic potential. This minimizes the adverse consequence of salinity because there is no salt build-up around the root zone as occurs when crops are cultivated in soil [].
In glycophytes, salt toxicity may occur when excessive salt ions (e.g., Na+ and Cl−) absorbed from brackish irrigation waters accumulate in plant tissues, causing a reduction in crop yield. This excess, at first, promotes a cell osmotic imbalance and, subsequently, causes ionic toxicity that causes damage to the cytoplasm, resulting in visible damage, mainly at the edges of mature leaves, where salt accumulation is greater than in young leaves [,].
Depending on the salinity level to which the plants are exposed, and the water’s chemical composition, the damage to the photosynthetic apparatus may vary []. Severe reductions in gas exchange in okra were reported from exposure to a nutrient solution prepared with waters dominant in Na+ []. Similarly, references [,,] also reported reductions in the physiological parameter of melons irrigated with waters predominant in Na+.
In the literature, research with vegetables in hydroponics, using brackish water, has provided comprehensive results in terms of the ionic nature of the nutrient solution. As verified for cilantro [] and lettuce [], there is a necessity for more detailed studies testing different ionic compositions of brackish waters, nutrient solutions, and the flows in which these solutions are offered to plants in hydroponic channels. Within this line of research, in a previous publication with cilantro [], we reported that the increment in the flow rate compromised plant yield parameters and that the prevalence of Ca2+ on brackish water caused lower dry mass accumulation, plant height, water consumption, and instantaneous and intrinsic water use efficiency than waters prevalent in Na+.
Cilantro (Coriandrum sativum L.) is an annual vegetable belonging to the family Apiaceae, like parsley (Petroselinum crispum Mill.). Its seeds (sold as a spice) are called coriander. The plant is cultivated worldwide due to its culinary uses but is native to the Mediterranean and Middle East regions. The Romans used it to flavor bread and its use dates to 5000 BCE []. There are reports that the plant is adaptable to different edaphic and climatic conditions []. Although salinity hinders the growth and development of most crops, some crops have various levels of salinity tolerance depending on the cultivars evaluated [,]. Although the cilantro plant has been reported to have a tolerance of up to 120 mM NaCl regarding irrigation water salinity, with no loss of shoot biomass up to 80 mM NaCl (ca. 12 and 8 dS m−1, respectively), the authors did not report the cultivar’s name. Although the authors reported significant losses in seed yield at every salinity level, starting from 40 mM NaCl, the use of seeds inoculated with Azospirillum Brasiliense and Azobacter chroococcum significantly improved total leaf chlorophyll and K concentrations while helping the plants restrict Na leaf accumulation []. Our literature review on the salinity response of cilantro brought limited results because the reports did not name the cultivar used or establish the salinity threshold of the crop based on either soil paste salinity or irrigation water salinity.
In this work, we propose to evaluate (1) the influence of the flow rate as a mitigator of salinity’s negative effects on the physiological parameters of cilantro and (2) the effects of nutrient solution ionic compositions on the physiological parameters of cilantro cultivated in a hydroponic NFT system.
2. Materials and Methods
2.1. Experimental Conditions
Between November 2019 and February 2020, two successive experiments were performed in the greenhouses of the Rural Federal University of Pernambuco (UFRPE) in Recife-PE, Brazil—Universal Transverse Mercator (World Geodetic System WGS84), Zone 25L N: 9113024.33, E: 285297.82.
Climatic parameters were monitored daily for the first (Figure 1a) and second (Figure 1b) experiments. The data were obtained with the aid of an automatic weather station—model CR1000 (Campbell Scientific, Ltd., Logan, UT, USA).
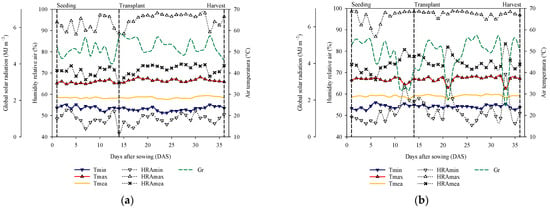
Figure 1.
Dynamics of the temperature (°C) and relative humidity (%) of air and global solar radiation (MJ m−2) inside the greenhouse. The first graph reflects the weather conditions from 8 November 2019 to 13 December 2019, EXP I (a), and the second graph reflects the weather data from 14 January 2020 to 18 February 2020, EXP II (b). Tmin = minimal temperatures, Tmax = maximal temperatures, Tmea = mean temperatures. HRAmin = minimal relative humidity (RH), HRAmax = maximal RH, HRAmea = mean RH, Gr = global solar radiation.
2.2. Experimental Treatments
The same experimental arrangement and treatments were used in both experiments. The experiments were arranged in a completely randomized design with four replicates using a 4 × 4 factorial scheme. The experiments evaluated the effects of four initial levels of electric conductivity of the nutrient solution, ECns of 1.7, 3.0, 4.5, and 6.0 dS m−1, applied at different flow rates (1.0, 2.0, 3.0, and 4.0 L min−1). The ECns and application flow rates were defined based on other studies [,].
In the first experiment, salinized waters were obtained through the dilution of different concentrations of NaCl, while in the second experiment, CaCl2·2H2O was used to salinize the nutrient solution.
2.3. Hydroponic System
The NFT (nutrient film technique) hydroponic system was installed with every experimental unit and made up of four independent trapezoidal gully channels (75 mm) that were 3 m long each and arranged over an easel, with spacing of 0.20 m among plants and 0.30 between gully channels. The average height of the gully channels to the soil was 1.10 m, with three footholds and a 3.33% slope.
In each gully channel, one 220 V circulating electric pump was installed, with 34 W of power, a maximum flow of 14 L min−1, a main reservoir for nutrient solution storage with a capacity of 40 L, and a secondary 15 L reservoir for immediate automatic water replenishment, connected to the main reservoir (Figure 2) by gravity to replenish the volume used in plant evapotranspiration.
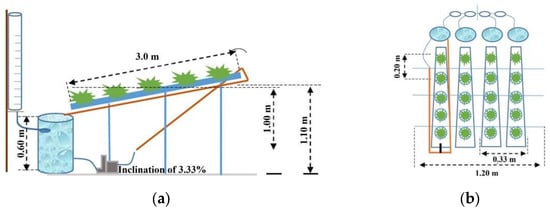
Figure 2.
Schematic drawing with side (a) and top (b) views of the hydroponic system used.
2.4. Preparation and Management of the Nutrient Solution
The preparation took place according to the following steps. First, 1000 L of water from the local UFRPE municipal water (ECw = 0.12 dS m−1) was placed in the reservoir. Then, the recommended quantity of fertilizers [] was solubilized to provide calcium nitrate (750 g m−3), potassium nitrate (500 g m−3), monoammonium phosphate (MAP) (150 g m−3), magnesium sulfate (400 g m−3), copper sulfate (0.150 g m−3), zinc sulfate (0.300 g m−3), manganese sulfate (1.5 g m−3), boric acid (1.8 g m−3), sodium molybdate (0.150 g m−3), and iron Fe-EDTA-13% (16 g m−3) (Table 1).

Table 1.
Concentrations of ions and the electroconductivity (dS m−1) of the nutrient solutions (ECns).
Once this was done, the reservoirs for all treatments were filled with this same nutrient solution. From this stage, in the first experiment, the target salinity levels (ECns) planned for each specific treatment were achieved through the addition of NaCl. In the second experiment, target salinities were achieved by adding CaCl2·2H2O (Table 1).
Regarding the management of the nutrient solution, in both tests, the first storage reservoir level had its volume restored with brackish water when it was at reduced volume due to evapotranspiration, with ECw values corresponding to each treatment. The application rate in the gully channels was programmed with the support of an electric timer to inject the solution into the system. During the day, from 6 a.m. to 6 p.m., 15 min application and 15 min rest periods were adopted; at night, the injection of the solution occurred every 2 h for 15 min.
The electrical conductivity (ECns), hydrogen potential (pHns), dissolved oxygen (DOns), and temperature (Tns) of the nutrient solution were monitored on alternate days and, when necessary, the pHns was corrected to an ideal range of 5.0–6.5 using NaOH.
2.5. Description and Management of the Culture
The vegetable grown was cilantro (Coriandrum sativum L.), cv. ‘Verdão’. The seeds were germinated in disposable cups, that were adapted with small holes for drainage. The cultivation substrate was composed of washed coconut fiber. Each disposable cup received 10 seeds. The cups had dimensions compatible with the hydroponic system used.
Until the sixth Day After Sowing (DAS), the plants were irrigated with low-salinity municipal water. From the seventh DAS to the 13th DAS, control nutrient solution (ECns = 1.7 dS m−1), diluted to 50%-strength, was applied. At 13th DAS, thinning was conducted-leaving 10 plants per cup before transferring the cups to the hydroponic system for treatment initiation.
2.6. Analyzed Variables and Data Analysis
The variables analyzed at 30 DAS were (gs) stomatal conductance [mol H2O m−2 s−1], (E) the transpiration rate [mmol H2O m−2 s−1], (A) the photosynthetic rate [μmol CO2 m−2 m−1], (Ci) the internal concentration of CO2 [μmol CO2 mol−1], and (CEi) instantaneous carboxylation efficiency [µmol CO2 m−2 s−1].
In all experiments, measurements started at 8:30 am and finished at 10:30 am. The variables were evaluated on the mid-section of one healthy leaf limb, making use of one portable infrared gas analyzer (IRGA, model ADC—LCpro—SD by ADC/England), using 1200 μmol m−2 s−1 intensity of active photosynthetic radiation as external light.
An earlier method [] was used to measure photosynthetic pigments. First, leaf discs were collected to prepare the plant extract, then this material was subjected to spectrophotometer readings at absorbance wavelengths (ABS) of 470, 646, and 663 nm. Chlorophyll concentrations were determined using the following equations: Chlorophyll a (Chl a) = (12.21 × ABS663) − (2.81 × ABS646); Chlorophyll b (Chl b) = (20.13 × ABS64) − (5.03 × ABS663); e Carotenoids (Car) = [(1000 × ABS470) − (1.82 × Chl a) − (85.02 × Chl b)]/198. The concentrations of Chl a, Chl b, and Car were expressed as µg g−1 of fresh matter.
Initial fluorescence (Fo), maximum fluorescence (Fm), and variable fluorescence (Fv = Fm − Fo) were measured 30 DAS; the quantum yield of photosystem II (Fv/Fm), the maximum yield of photosystem II (Fv/Fo), the absorption flow per reaction center (ABS/RC), the electron transport flow per reaction center (ETo/RC), and the dissipated energy per reaction center (DIo/RC) were also measured.
The measurements were taken in the morning for both experiments, using one FluorPen, model FP 100 (Photon Systems Instruments, Drásov, Czech Republic), on a physiologically developed leaf from plants’ mid-section that was physiologically mature and healthy. To obtain complete oxidation of the photosynthetic electron transport system, clips were placed on the sheets for adaptation to the dark for 30 min.
The normality of the data was confirmed by the Shapiro–Wilk test. We used the F test with a probability level of 0.05 and the data were subjected to analysis of variance. The data passed the normality test before submission to ANOVA. In the case of a significant effect for treatment, the ECns levels and application flow rates were compared using regression analysis, testing linear and quadratic adjustments. Statistical analyses were performed using the SISVAR 5.2 method [].
3. Results
3.1. Nutrient Solution Parameters
Considering the initial values for the electrical conductivity of nutrient solutions (EC ns) for EXP I (water salinized with NaCl), all initial ECns, except for the control (ECns = 1.7 dS m−1) increased during the experiment. For the control salinity of 1.7 dS m−1, ECns decreased when flow rates increased, the decrease being of 6.67% for 1 L min−1 and 15.63% for 4 L min−1. When the ECns was the highest (ECns = 6 dS m−1), for all the flow rates tested, the increase in ECns was higher than 30% during the experiment. The variation in pHns, DOns, and Tns during the experiment was between 5.42 and 6.75, 5.3 and 7.0 mg L−1, and 28.8 and 32.7 °C, respectively.
Under salinized waters with CaCl2 (Experiment II), a reduction in the initial value for the ECns = 1.7 dS m−1 was observed. For the flow rates of 1 and 4 L min−1, the reductions in ECns were 21.25% and 15.74%, respectively, indicating that an increase in flow rate minimized the reduction in ECns in relation to the initial value. However, the ECns of the nutrient solutions increased, mainly for the ECns of 6.0 dS m−1, in 35.13, 29.70, 14.73, and 38.97% according to the flow rates of 1, 2, 3, and 4 L min−1, respectively. Regarding the pHns, the variation occurred between 5.54 and 6.50; the mean DOns content was 6.26 mg L−1 and Tns ranged between 27.7 and 33.3 °C.
3.2. Photosynthetic Parameters
The increase in ECns significantly affected (p ≤ 0.01) the photosynthetic rate (A), stomatal conductance (gs), and internal concentration of CO2 (Ci), as well as the instantaneous carboxylation efficiency (CEi) of cilantro plants in both experiments (Supplementary Table S1). The application flow rate affected (p ≤ 0.01) the A and Ci of the plants in Experiment I (NaCl), as well as the A and transpiration (E) in Experiment II (CaCl2) (Supplementary Table S1). Increases in ECns linearly reduced stomatal conductance in both experiments, with decreasing rates of 0.015 and 0.010 mol H2O m−2 s−1 when the water was salinized with NaCl or CaCl2, respectively (Figure 3a,b). Notwithstanding, increases in the application flow only affected (p ≤ 0.01) the photosynthetic rate (A) and the internal CO2 concentration (Ci) (Supplementary Table S1). Stomatal conductance (gs) maintained average values of 0.219 mol H2O m−2 s−1 (Experiment I) and 0.206 mol H2O m−2 s−1 (Experiment II).
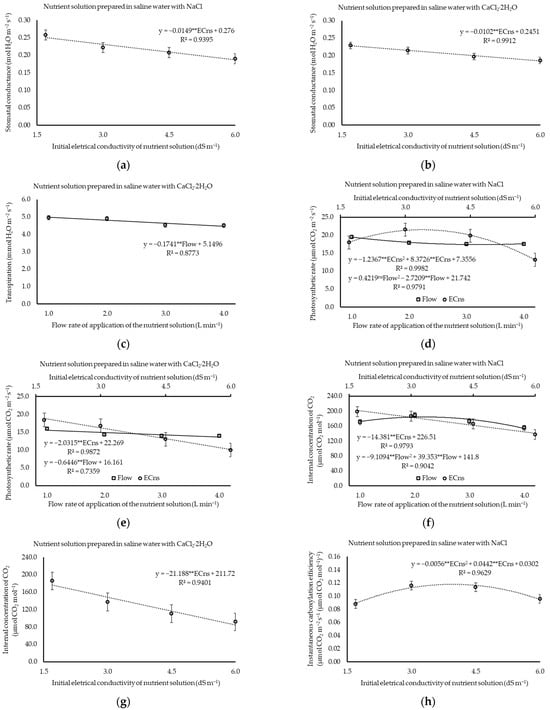
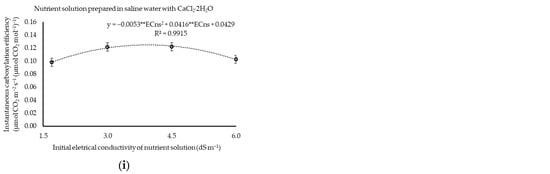
Figure 3.
Stomatal conductance (a,b), transpiration (c), photosynthetic rate (d,e), internal concentration of CO2 (f,g), and instantaneous carboxylation efficiency (h,i) of cilantro cv. Verdão exposed to nutrient solutions prepared in brackish waters dominant in NaCl or CaCl2, each combined with crescent flow rates. * or ** significant at p ≤ 0.05 or p ≤ 0.01 levels of probability, respectively; ns = not significant.
Cilantro leaf transpiration was not affected by the treatments in Experiment I (NaCl), with an average value of 4.78 mmol H2O m−2 s−1. However, under the predominance of CaCl2, it was observed that E reduced at a rate of 0.1741 mmol H2O m−2 s−1, with a unitary increase in the application flow rate (Figure 3c); however, transpiration was not influenced by the increase in ECns, which averaged 4.71 mmol H2O m−2 s−1.
By salinizing the water with NaCl, the maximum photosynthetic rate (21.53 µmol CO2 m−2 s−1) was obtained at an estimated ECns of 3.4 dS m−1, which is a 64.7% increase in A in relation to that observed in plants under 6.0 dS m−1. Nevertheless, the photosynthetic rate reached its minimum value (17.36 µmol CO2 m−2 s−1) when the estimated application flow rate was 3.2 L min−1 (Figure 3d). In waters predominant in CaCl2, the maximum photosynthetic rate (18.81 µmol CO2 m−2 s−1) was estimated for an ECns of 1.7 dS m−1, and an estimated loss of 2.03 µmol CO2 m−2 s−1 was observed for each unit increase in dS m−1 in the photosynthetic rate of the plant, covering a variation of 86.7% from the smallest to the largest ECns. Similarly, the unit increase in the application flow rate resulted in a decrease in A of 0.64 µmol CO2 m−2 s−1 (Figure 3e).
The internal concentration of CO2 decreased by 14.38 and 21.19 µmol CO2 mol−1 when the plants were exposed to nutrient solutions with a prevalence of NaCl or CaCl2, respectively. At the lowest salinity level (1.7 dS m−1), the Ci values were 202.06 µmol CO2 mol−1 (Experiment I) and 175.70 µmol CO2 mol−1 (Experiment II), surpassing the Ci of plants subjected to the highest salinity level (6.0 dS m−1) under the prevalence of NaCl and CaCl2 by 61.84 and 91.11 µmol CO2 mol−1, respectively (Figure 3f,g). This results in a maximum variation of 44.1% (Experiment I) and 107.7% (Experiment II) in this variable in response to increased salinity. On the other hand, with the increase in the application flow rate, the maximum Ci was 184.30 µmol CO2 mol−1 for an estimated flow rate of 2.20 L min−1 when there was a prevalence of NaCl (Figure 3f), while it remained stable under the prevalence of CaCl2, with an average of 131.20 µmol CO2 mol−1.
The maximum instantaneous efficiency of plant carboxylation (0.117 and 0.125 µmol CO2 m−2 s−1) was verified for the estimated ECns of 3.95 and 3.92 dS m−1 under the predominance of NaCl or CaCl2 in the water, respectively (Figure 3h,i). The application flow rate also had no effect (p > 0.05) on the maximum instantaneous efficiency of plant carboxylation, with CEi values of 0.103 or 0.111 [µmol CO2 m−2 s−1] under waters prevalent in either NaCl or CaCl2, respectively.
3.3. Photosynthetic Pigments
When isolated, the increase in ECns significantly influenced (p ≤ 0.01) the levels of chlorophyll a and b and carotenoids in both experiments, while total chlorophyll was only affected (p ≤ 0.01) when salinization occurred with NaCl. Under NaCl prevalence, the application flow rate had a significant effect on Chl a at the level of p ≤ 0.05, as well as on Chl b and total Chl at the level of p ≤ 0.01. On the other hand, under the prevalence of CaCl2, the application flow rate affected (p ≤ 0.01) Chl a and carotenoids. A significant interaction (p ≤ 0.01) was observed only for carotenoids in plants irrigated with nutrient solutions dominant in CaCl2 (Supplementary Table S2).
The maximum values of Chl a, Chl b, and total Chl (1.76, 1.50, and 3.08 µg g−1) were verified at estimated ECns levels of 3.7, 3.7, and 2.9 dS m−1, respectively, under NaCl prevalence (Figure 4a,c,e). Likewise, except for total Chl, which was not significantly influenced (p ≥ 0.05) by ECns, when plants were exposed to the prevalence of CaCl2, chlorophyll a and b concentrations were at their maximum (2.14 and 2.12 µg g−1) under estimated ECns values of 3.5 and 3.0 dS m−1, respectively (Figure 4b,d).
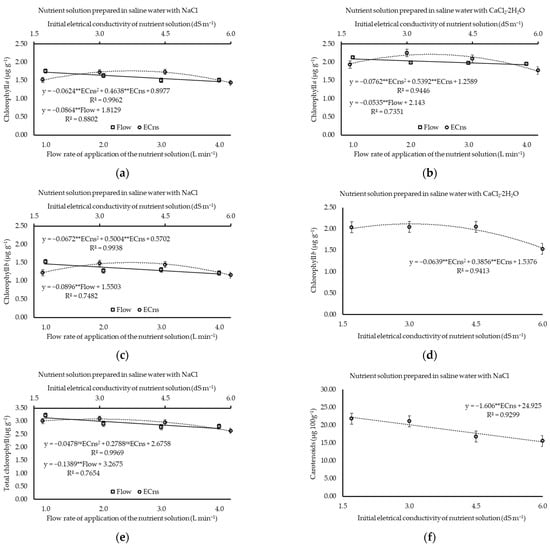
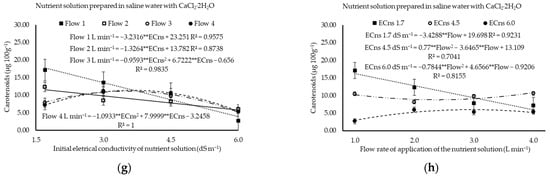
Figure 4.
Chlorophyll a (a,b), chlorophyll b (c,d), total chlorophyll (e), and total carotenoids (f–h) of water use of cilantro cv. Verdão exposed to nutrient solutions prepared with brackish waters dominant in NaCl or CaCl2, each applied at increasing flow rates of 1 (Flow 1), 2 (Flow 2), 3 (Flow 3), and 4 (Flow 4) liters per minute. * or ** significant at p ≤ 0.05 or p ≤ 0.01 levels of probability, respectively; ns = not significant.
For each unit increment in application flow, there were reductions of 0.0864, 0.0896, and 0.1389 µg g−1 in the content of Chl a, Chl b, and total Chl in plants, respectively, when saline water prevalent in NaCl was used (Figure 4a,c,e). On the other hand, under the prevalence of CaCl2, Chl a reduced at a rate of 0.0535 µg g−1 with unit increases in the nutrient solution flow rate (Figure 4b), while Chl b and total Chl were not significantly affected (p > 0.05), with mean values of 1.91 and 3.99 µg g−1, respectively.
When the water was dominant in NaCl, the concentration of carotenoids decreased at a rate of 1.61 µg 100 g−1 per unit increment of ECns, while the carotenoid concentration was not affected (p > 0.05) by flow rate, with a mean of 1.90 µg 100 g−1 (Figure 4f).
Under the prevalence of CaCl2, an analysis of each flow rate within the proposed range of ECns (Figure 4g) determined that there were estimated reductions of 3.23 and 1.33 µg 100 g−1 in the carotenoid concentration at each increment (in dS m−1) regarding the salinity of the nutrient solution when the solution was applied at flow rates of 1.0 and 2.0 L min−1, respectively. However, at flow rates of 3.0 and 4.0 L min−1, carotenoid levels were at their maximum (11.10 and 11.43 µg 100 g−1) under estimated ECns values of 3.5 and 3.7 dS m−1, respectively (Figure 4g). It is important to highlight that the lowest concentrations of carotenoids (2.96, 5.28, 6.04, and 5.24 µg 100 g−1 for flows of 1.0, 2.0, 3.0, and 4.0 L min−1, respectively) were recorded when the plants were cultivated at the highest salinity level (6.2 dS m−1).
Regarding the predominance of CaCl2, after analysis of each ECns within the proposed range of flow rates (Figure 4h), it was found that the concentration of carotenoids reduced at a rate of 3.43 µg 100 g−1 with each unit of increment in flow rate in the plants exposed to an ECns of 1.7 dS m−1 (Figure 4h). Regarding ECns values of 4.5 and 6.0 dS m−1, minimum and maximum values of 8.78 and 6.04 µg 100 g−1, respectively, were observed (Figure 4h). At an ECns = 3.0 dS m−1, even with a quadratic influence on the levels of carotenoids, the data were not presented in any graphs or tables as the low value of the coefficient of determination was verified (R2 = 0.5311).
3.4. Chlorophyll a Fluorescence
In both experiments, the salinity of the nutrient solution (ECns) affected, in isolation, the initial fluorescence (Fo) at p ≤ 0.01, as well as the maximum (Fm) and variable (Fv) fluorescence levels at p ≤ 0.05 (Experiment I) and p ≤ 0.01 (Experiment II). Furthermore, ECns levels affected (p ≤ 0.05) the absorption flux per reaction center (ABS/RC) when the water was salinized with CaCl2 (Supplementary Table S3).
With the prevalence of NaCl in water, a linear reduction in Fo, Fm, and Fv was observed at rates of 127.21, 584.47, and 457.26 electrons quantum−1, respectively, per unit increment of ECns, with variances of 8, 8, 8.5, and 8.4% from the lowest to highest electrical conductivity (Figure 5a,c,e). The application flow rate did not affect (p > 0.05) the Fo, Fm, and Fv values, whose averages were 6516.72, 30,879.39, and 24,362.67 electrons quantum−1, respectively.
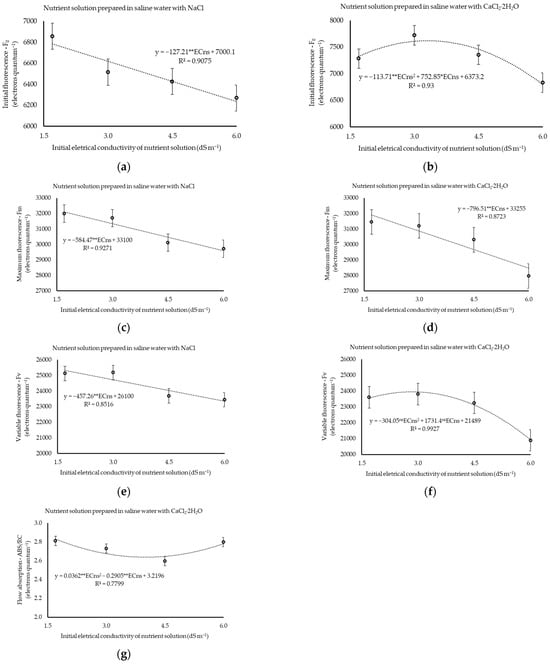
Figure 5.
Initial fluorescence (a,b), maximum fluorescence (c,d), variable fluorescence (e,f), and absorption flux per reaction center (g) of cilantro cv. Verdão exposed to nutrient solutions prepared in brackish waters dominant in NaCl and CaCl2, each applied in crescent flow rates. * or ** significant at p ≤ 0.05 or p ≤ 0.01 levels of probability, respectively; ns = not significant.
Under CaCl2 dominance in the water, it was observed that Fo and Fv reached their maximum values (7619.32 and 23,953.85 electrons quantum−1) under estimated ECns values of 3.3 and 2.8 dS m−1, respectively (Figure 5b,f). On the other hand, Fm reduced at a rate of 796.51 quantum electrons−1 for each increased dS m−1 (Figure 5d). Like the results verified under NaCl prevalence, a significant effect (p > 0.05) of increasing the flow rate on the measurements of Fo, Fm, and Fv cannot be verified, resulting in mean values of 7296.89, 30,227.94, and 22,888.66 electrons quantum−1, respectively.
When the water was salinized with CaCl2, the ABS/RC was minimal (2.64 electrons quantum−1) at the estimated ECns of 4.0 dS m−1 (Figure 5g). This variable was not affected (p > 0.05) by either the increase in flow under a prevalence of CaCl2, with a mean value of 2.73 electrons quantum−1, or by the treatments when the plants were exposed to waters salinized with NaCl, resulting in an average value of 2.31 electrons quantum−1.
The variables Fv/Fm, Fv/Fo, ETo/RC, and DIo/RC were not affected (p > 0.05) by treatments in any of the experiments, resulting in relative values equal to 0.79, 3.74, 1, 16, and 0.48 electrons quantum−1 in the first experiment, respectively. Similarly, in the second experiment, the mean values recorded for Fv/Fm, Fv/Fo, ETo/RC, and DIo/RC were 0.76, 3.16, 1.26, and 0.67 electrons quantum−1, respectively, remaining stable in response to the applied treatments.
4. Discussion
The reduction in the initial levels of ECns verified in both experiments, specifically for 1.7 dS m−1 can be partly attributed to the steady absorption of ions dissolved in the water by the plants plus the strategy adopted to replenish the level of the stock reservoir. This reduction was reported by other researchers who worked with leafy vegetables, such as parsley [] and chicory []. At the lowest salinity treatment, nutrient solutions in reservoirs had their levels automatically replenished by low-salinity municipal water (ECw ≈ 0.12 dS m−1), used in the preparing of nutrient solution, but devoid of salts and mineral nutrients.
For treatments other than the control, the levels of the stock reservoirs for nutrient solutions were replaced with the brackish waters of higher salinities, used for initial nutrient solutions. For instance, for the ECns of 3.0 dS m−1, the replacement was done with water of 1.5 dS m−1, while for levels of 4.5 and 6.0 dS m−1 of ECns, the nutrient solution replacement was done with water of 3.0 and 4.5 dS m−1, respectively. In these replacements, there was no addition of fertilizers. So, at the salinity levels of 3.0, 4.5, and 6.0 dS m−1, there was an increase in ECns compared to their initial value in both experiments.
Increased concentrations of Na+ or Ca2+ from waters salinized with NaCl or CaCl2 may have limited the growth of celery plants by limiting their absorption of nutrients, leading to a nutritional imbalance in the ratios of Na+/K+, Na+/Ca2+, and Na+/Mg2+ [] intensifying salt toxicity in plants []. These results imply that the salt concentration or the rate of mineral/salt absorption by plants is not correlated to the volume of the nutrient solution used. Thus, every time the solution volume is replaced by water of the same salt concentration the ECns may increase while plant growth decreases. For a better interpretation of this phenomenon, nutrient solutions should be periodically analyzed for their ion composition. Then, only nutrients and salts depleted from nutrient solutions by plants should be replaced. These are mostly NO3− and K+, but also include Na+ and Cl− based on previous long-term hydroponic experiments with alfalfa and passion fruit at the US Salinity Laboratory [].
In both experiments, the pHns were maintained within the ideal range of 5.5 to 6.5 [,], which was also followed by others working with leafy vegetables irrigated with water predominant in Na+ [,]. Likewise, DOns levels were also above the minimum limit of 5 mg L−1 recommended for most crops [], and the Tns range was considered adequate for plant growth between 20 and 30 °C [].
Under saline stress, stomatal closure is one of the first plant defense mechanisms []. Under these conditions, plants adopt the strategy of partially or completely closing their stomata to reduce losses and maintain a high water status, which contributes to minimizing the absorption of toxic ions such as Na+ and Cl− []. The reduction in stomatal conductance implies a reduction in intercellular CO2 and is detrimental to the activity of several enzymes, including RuBisCO, thus limiting carboxylation, and reducing the net photosynthetic rate []. Therefore, the reduction rate in stomatal conductance (gs) in response to saline stress can be linked to the salinity tolerance of plants, as verified by other authors [,,].
The reduction in the transpiration rate was related to the decrease in stomatal conductance, as a smaller stomatal opening results in restricted transpiration that limits the exit of water from the leaf to the atmosphere []. However, it is important to note that, even though this relationship has been discussed in previous studies [,], in our research, the decrease in the transpiration rate cannot be attributed exclusively to the reduction in stomatal opening. This is because, although the stomatal conductance was significantly affected in both experiments by the increase in salt concentration, it was observed that E was negatively influenced by the increase in the application flow only in the second experiment, when the water was salinized with CaCl2. Therefore, the restriction in the transpiration rate may have been triggered by limitations not related to the stomates. Furthermore, it is worth considering that the prevalence of Ca2+ in brackish water, and the fact that CaCl2 provides more Cl− than NaCl, may have impacted the transpiration rate of cilantro plants, considering that chloride acts on osmotic regulation, transpiration, and the movement of nutrients with positive charges (cations) inside and outside plant cells. In practice, hydroponic vegetable producers who use waters high in Ca2+ and Cl− must adjust the application rate of the nutrient solution so that it does not exceed 2 L min−1.
Nevertheless, the increase in salinity in our study did not result in a reduction in leaf transpiration, which is different from the findings reported for other leafy vegetables, such as cilantro [] and lettuce []. In addition, although Na is not considered a nutrient, some crops (such as spinach) may require small concentrations of Na for osmotic balance, mainly when K is deficient [].
Photosynthesis is intrinsically dependent on the availability of carbon dioxide (CO2) at carboxylation sites and is strongly influenced by gs []. In this sense, the availability of water for plants plays a fundamental role in photosynthesis. Water limitation, common in situations of salt stress, triggers a series of adjustments that directly impact the photosynthetic rate of plants []. Under salinity conditions, photosynthesis tends to decrease due to stomatal closure, resulting in reduced CO2 diffusion, as well as due to decreased photochemical and carbon metabolism []. In the present work, it was noted that the presence of Ca2+ and Cl− in brackish waters intensified reductions in Ci with the increase in saline stress.
Evidence of such effects of salinity on the photosynthetic rate was observed in studies with okra [], cucumber [], and arugula []. In addition to the significant effect exerted by ECns on A, it was identified that this variable was also sensitive to variations in the flow rate. Specifically, the highest rates of net photosynthesis were obtained under the lowest flow rate (1.0 L min−1). The increment in the circulation flow of the solution promotes a large amount of salts in the rhizosphere, causing greater contact between the solution and the roots, which can reduce the absorption of nutrients by the roots, leading to a mineral imbalance that directly affects the physiology of the crop []. For producers using brackish water to prepare the nutrient solution, it is clear from these results that increasing the application rate of the nutrient solution beyond the recommended range for fresh water (1 to 2 L min−1) does not mitigate saline damage or result in other practical advantages.
The decrease in the internal concentration of CO2 in response to the increase in ECns is a plant reaction to the effect of salinity and, in the present work, is also due to the toxicity caused by the increment in chloride that is probably a result of the restriction of the lower diffusion of CO2 in the substomatal chamber due to the closure of the stomata []. This scenario can cause changes in the activity of the enzyme RuBisCO carboxylase (ribulose-1,5-bisphosphate carboxylase oxygenase), favoring oxygenase to the detriment of carboxylase and resulting in an increase in photorespiration and a decrease in the net rate of CO2 assimilation [], as observed in this study. Other researchers have also reported a reduction in Ci due to salinity [].
The instantaneous efficiency of carboxylation (CEi) is linked to the availability of ATP, NADPH, and the substrate of the enzyme RuBisCO []. Factors such as the availability of CO2 in the leaf mesophyll, light intensity, temperature, and ideal conditions for enzymatic activity play decisive roles in obtaining a higher CEi []. When analyzing the behavior of CEi concerning the increment in the concentration of salts, it is suggested that there was a tolerance limit of the crop (3.9 dS m−1) concerning this variable in both experiments. This implies that, from that point on, RuBisCO may have been affected by salinity. As stress intensifies, dehydration of mesophyll cells tends to inhibit photosynthesis, compromising metabolism and, consequently, impairing the instantaneous efficiency of carboxylation []. In a study with cilantro cv. Verdão in hydroponic conditions [], the CEi values were at their maximum (0.086, 0.073, and 0.068 8 µmol CO2 m−2 s−1 (µmol CO2 mol−1)−1) at estimated ECns values of 2.98, 2.99, and 3.10 dS m−1, respectively, when the nutrient solution was prepared with water predominantly containing Na+, Ca2+, and Mg2+. The results were lower than the results presented here.
It was found in the present study that salt stress inhibited the accumulation of leaf photosynthetic pigments, including Chl a, Chl b, total Chl (mostly in Experiment I), and carotenoids. In general, the reduction of these parameters in response to saline stress can be interpreted as a plant acclimatization strategy aimed at energy conservation and limiting the absorption of light energy [], which was also limited by the reduction in carotenoids observed at every increase in ECns. In addition, according to these authors, this adjustment seeks to reduce the flow of electrons to the electron transfer chain to mitigate damage caused by photo-oxidative stress.
The concentration of chlorophyll present in the leaves serves as an indicator of plant tolerance to saline stress []. As previously observed in the CEi analysis, the chlorophyll concentration (a and b) had a pattern of increases, followed by a decrease after reaching a maximum point, suggesting a salinity tolerance threshold for the crop. This decline in the concentration of photosynthetic pigments triggered by salinity may be related to changes in metabolic activities, damage to the cell membrane, and possible inhibition in the development and differentiation of tissues inside chloroplasts []. Other probable reasons that contribute to the reduction in chlorophyll concentration in response to ECns levels include increased activity of the chlorophyllase enzyme, which is responsible for the degradation of chlorophyll molecules [], and the possible instability of protein complexes [].
With the reduction in the concentration of Chl a, the crucial role of auxiliary pigments (Chl b and carotenoids) is evidenced by the transfer of energy to Chl b and carotenoids to compensate for the Chl a reduction, as suggested by several studies [,]. Carotenoids can function as antioxidant agents, playing a key role in protecting lipid membranes against the oxidative stress generated in plants exposed to salinity []. In addition, the action of carotenoids as competitive inhibitors in the formation of oxygen radicals is vital for plants, particularly under conditions of high light intensity [,]. On the other hand, the reduction in the concentration of carotenoids may be associated with several causes, including the diminution in the production of photosynthetic pigments due to salinity stress, which leads to the degradation of β-carotene and, consequently, to the decrease in the levels of carotenoids that play an integrated role in the integrity of thylakoid membranes and act in absorbing and transferring light energy to chlorophyll []. In the present work evaluating the effect of the flow rate, although it was observed that at lower ECns levels, the increase in flow resulted in a reduction in the carotenoid content, it was noted that with the increment in salinity to 4.5 and 6.0 dS m−1, increasing the flow rate to 3 or 4 L min−1 provided carotenoid contents similar to those found at lower flow rates.
The analysis of chlorophyll a-fluorescence, which reflects the intensity of the energetic excitation that drives photosynthesis and allows us to estimate the inhibition in the electron transfer process of photosystem II (PSII) [], represents a fundamental parameter to analyze the integrity of the photosynthetic apparatus [] and to help identify salinity-tolerant plants []. In the context of this research, the negative influence of salt stress on the fluorescence parameters (Fo, Fm, and Fv) was notable, which suffered significant reductions with the increase in the concentration of salts in the nutrient solution. Furthermore, based on the results obtained, it is possible to infer that the variation in the application rate of the nutrient solution was not capable of mitigating the deleterious effects of salinity on such parameters. In addition to chlorophyll a fluorescence, other physiological and photosynthetic aspects that have already been mentioned in this work also corroborate the inefficiency of increasing the application rate of the nutrient solution to mitigate saline damage, especially for producers who work with brackish waters prevalent in Ca2+ and Cl−.
Initial fluorescence (Fo) is indicative of the oxidizing capacity of quinone (QA), the primary electron acceptor in the PSII reaction center, and is determined when QA is completely oxidized and the PSII reaction center is open, which anticipates the activation of photochemical reactions []. Therefore, the decrease in Fo in response to salt stress may derive from problems in the PSII reaction center or from a limitation in the transfer of excitatory energy from the light-collecting system to the reaction center []. The limitation caused by salinity on Fo was also noted in other hydroponic crops, such as parsley [] and cilantro [].
When quinone (QA) is reduced and the reaction centers reach their full capacity for photochemical reactions, the fluorescence is at its maximum (Fm) []. The reduction of this parameter in response to increased salt concentrations has been documented in several studies [,,] related to photoinhibition induced by salinity stress [].
The variable fluorescence (Fv) reflects the flow of electrons from the PSII reaction center to plastoquinone, to form NADPH, ATP, and reduced ferredoxin proteins (Fdr), contributing to a greater capacity for CO2 assimilation in the biochemical phase of photosynthesis [,]. Given that Fv is directly influenced by Fo and Fm, any changes in these parameters result in a decrease in the PSII quinone primary electron acceptor []. Reductions in Fv according to increased salinity have also been identified in other studies [,], suggesting the occurrence of damage to the photosynthetic apparatus, compromising the PSII and, consequently, promoting a reduction in the assimilation of CO2 in the plant [].
The quantum yield (Fv/Fm) and the maximum yield of photosystem II (Fv/Fo) were not impacted by the concentration of salts in the nutrient solution, contrasting with the findings in other studies involving leafy vegetables, such as parsley [] and cucumber []. The Fv/Fm ratio, which indicates the state and efficiency of the electron transport chain [], maintained average values within the ideal range (between 0.75 and 0.85) [] in both experiments. This suggests that saline stress and variation in nutrient solution flow did not compromise this relationship, indicating that the photosynthetic apparatus of cilantro plants was intact []. The Fv/Fo ratio, a sensitive indicator of photosynthetic activity, in both healthy and stressed plants [], amplifies the analysis of small variations detected by the Fv/Fm ratio [].
The results regarding the absorption flux per reaction center (ABS/RC) suggest that when using saline water predominant in CaCl2 in the preparation of the nutrient solution, there was a decrease in the ABS/RC up to a minimum point due to the reduction of photosynthetic pigments, especially chlorophyll a concentration, in response to the increased salinity [], possibly because of the chloride concentration in the nutrient solution. Biochemically, chlorine is required for the photosynthetic reactions involved in the evolution of O2. Chlorine is necessary for water breakdown to occur during photosynthesis and for oxygen to be produced. It may also be necessary for cell division in leaves and roots [].
However, from the ECns estimated at 4.0 dS m−1, this relationship increased. This rise can be attributed to the possible preservation of the antenna that supplies excitation energy to the active reaction centers, as well as the integrity of the quinone reduction centers at higher ECns values, and a greater active amount of absorbing chlorophyll per reaction center []. On the other hand, despite the increase in salt concentration resulting in a reduction in the flow of electrons, it is likely that, under the experimental conditions under which plants were tested, the PSII electron acceptor site has not been stressed [], since salt stress did not affect the electron transport flux per reaction center (ETo/RC). Because of this stability in electron transport, the dissipation of energy in the form of heat per reaction center (DIo/RC) was also not compromised by salinity [].
5. Conclusions
Increasing the concentration of salts in the nutrient solution resulted in damage to leaf gas exchange in cilantro plants, regardless of the prevalence of Na+ or Ca2+ in saline water. The variation in the flow rates of the nutrient solution did not attenuate the deleterious effects of salinity stress on leaf gas exchange. In addition, when cilantro plants were cultivated in nutrient solutions with a predominance of Ca2+, increased water salinity compromised the photosynthetic rate (A) and leaf transpiration (E), which may have been caused by the higher concentration of Cl− in brackish waters salinized with CaCl2. Although the concentrations of chlorophyll a and b were affected by salinity, nutrient solutions up to 3.0 dS m−1, whether dominated by NaCl or CaCl2, resulted in higher concentrations of these photosynthetic pigments. Increased salinity negatively impacted chlorophyll a fluorescence and affected parameters such as the initial (Fo), maximum (Fm), and variable (Fv) fluorescence, while neither of the applied nutrient solution flow rates was able to mitigate the deleterious effects of salinity. The quantum yield (Fv/Fm) and the maximum yield of photosystem II (Fv/Fo) remained stable in response to salinity treatments. The absorption flux per reaction center (ABS/RC) was compromised by the increase in salinity, particularly when CaCl2 was predominant in nutrient solutions up to an estimated value of 4.0 dS m−1. Although Na+ and Ca2+ were present in the same equivalent amounts in both waters, Cl− equivalents were twice as high in waters dominant in CaCl2 than in water dominant in NaCl. Thus, our work suggests that the deleterious effects of salinity on the photosynthetic parameters of cilantro may have been triggered not only by the two-fold increase in Cl− (compared to Na+) during increases in the salinity of the nutrient solution, but also due to the two-fold increase in Ca2+ when comparing irrigation waters of similar salinities. Thus, hydroponic vegetable producers must be aware that crop growth can be reduced by both increases in Na or Cl but also in Ca, as excessive Ca can lead to imbalances in K and Mg. However, the flow of the nutritive solution will have no significant effect on crop yield under the salinities tested in this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17111640/s1, Table S1. Mean squares (MS) analysis of variance (ANOVA) for stomatal conductance (gs), transpiration (E), photosynthetic rate (A), internal concentration of CO2 (Ci) and instantaneous carboxylation efficiency (CEi) of cilantro plants, cv. Verdão, exposed to different application flow levels of the nutrient solution, which was prepared with brackish water with a prevalence of Na+ and Ca2+. Table S2. Mean squares (MS) analysis of variance (ANOVA) for chlorophyll a (Chl a) chlorophyll b (Chl b), total chlorophyll (Chl total) and total carotenoids (Car) of cilantro plants, cv. Verdão, exposed to different application flow levels of the nutrient solution, which was prepared with brackish water with a prevalence of Na+ and Ca2+. Table S3. Mean squares (MS) analysis of variance (ANOVA) for initial fluorescence (Fo); maximum fluorescence (Fm); variable fluorescence (Fv); quantum yield (Fv/Fm); maximum yield of photosystem II yield (Fv/Fo); absorption flux per reaction center (ABS/RC); electron transport flux per reaction center (ETo/RC), and energy dissipated per reaction center (DIo/RC) of cilantro plants, cv. Verdão, exposed to different application flow levels of the nutrient solution, which was prepared with brackish water with a prevalence of Na+ and Ca2+.
Author Contributions
Conception and execution of the project, U.C.M.P.; conception and guidance of the project, Ê.F.d.F.e.S. and J.A.S.J.; data analysis and support in the drafting of the manuscript, T.F.d.O. and M.M.R.; revision of the manuscript J.F.S.F., Ê.F.d.F.e.S. and J.A.S.J.; consultant for experimental data analysis, E.R.d.S. and A.O.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) and the research project by the National Council for Scientific and Technological Development (CNPq) 409312/2021-5.
Data Availability Statement
Data is contained within the article (and Supplementary Materials).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sá, F.V.d.S.; Brito, M.E.B.; Silva, L.d.A.; Moreira, R.C.L.; Fernandes, P.D.; Figueiredo, L.C.d. Fisiologia da percepção do estresse salino em híbridos de tangerineira “Sunki Comum” sob solução hidropônica salinizada. Comun. Sci. 2015, 6, 463. [Google Scholar] [CrossRef]
- Medeiros, J.F.d.; Terceiro Neto, C.P.C.; Dias, N.d.S.; Gheyi, H.R.; Silva, M.V.T.d.; Loiola, A.T. Salinidade e pH de um argissolo irrigado com água salina sob estratégias de manejo. Rev. Bras. Agric. Irrig. 2017, 11, 1407–1419. [Google Scholar] [CrossRef]
- Silva, M.G.d.; Soares, T.M.; Gheyi, H.R.; Santos, C.C.d.; Oliveira, M.G.B.d. Hydroponic cultivation of coriander intercropped with rocket subjected to saline and thermal stresses in the root-zone. Rev. Ceres 2022, 69, 148–157. [Google Scholar] [CrossRef]
- Gheyi, H.R.; Dias, N.S.; Lacerda, C.F.; Gomes Filho, E. Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados; INCTSal: Fortaleza, Brazil, 2016; p. 504. [Google Scholar]
- Silva, M.G.d.; Soares, T.M.; Gheyi, H.R.; Costa, I.P.; Vasconcelos, R.S. Ghowth, production and water consumption of coriander grown under different recirculation intervals and nutrient solution depths in hydroponic channels. Emir. J. Food Agric. 2020, 34, 281–294. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.J.; Rengasamy, P.; McDonald, G.K. Additive effects of Na⁺ and Cl⁻ ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef]
- Martins, J.B.; Santos Júnior, J.A.; Leal, L.Y.d.C.; Paulino, M.K.S.S.; Souza, E.R.d.; Gheyi, H.R. Fluorescence emission and photochemical yield of parsley under saline waters of different cationic nature. Sci. Hortic. 2020, 273, 109574. [Google Scholar] [CrossRef]
- Mendonça, A.J.; Lima, G.S.d.; Soares, L.A.d.A.; Sá, V.K.d.; Silva, S.S.d.; Torres, R.A.; Ferreira, J.T.; Gheyi, H.R. Gas exchange, photochemical efficiency and growth of hydroponic okra under salt stress and salicylic acid. Rev. Caatinga 2024, 37, e12143. [Google Scholar] [CrossRef]
- Morais, P.L.D.d.; Dias, N.d.S.; Oliveira, A.M.d.; Sousa Neto, O.N.d.; Sarmento, J.D.A.; Gonzaga, M.I.S. Effects of Nutrient Solution Salinity on the Physiological Performance of Melon Cultivated in Coconut Fiber. Rev. Caatinga 2018, 31, 713–718. [Google Scholar] [CrossRef]
- Sousa, V.F.d.O.; Costa, C.C.; Diniz, G.L.; Santos, J.B.d.; Bomfim, M.P. Physiological behavior of melon cultivars submitted to soil salinity. Pesqui. Agropecu. Trop. 2018, 48, 271–279. [Google Scholar] [CrossRef]
- Sousa, V.F.d.O.; Costa, C.C.; Diniz, G.L.; Santos, J.B.d.; Bomfim, M.P.; Lopes, K.P. Growth and gas changes of melon seedlings submitted to water salinity. Rev. Bras. Eng. Agric. Ambient. 2019, 23, 90–96. [Google Scholar] [CrossRef]
- Silva, M.G.d.; Oliveira, I.d.S.; Soares, T.M.; Gheyi, H.R.; Santana, G.d.O.; Pinho, J.d.S. Growth, production and water consumption of coriander in hydroponic system using brackish waters. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 547–552. [Google Scholar] [CrossRef]
- Viana, P.C.; Freitas, F.T.O.d.; Silva, N.D.d.; Soares, T.M.; Paz, M.G.F. da Estatística multivariada como ferramenta. descritiva na análise sensorial de alface hidropônica produzida com águas salobras. Rev. Bras. Agric. Irrig. 2018, 12, 2725–2730. [Google Scholar] [CrossRef][Green Version]
- Bezerra, R.R.; Santos Júnior, J.A.; Pessoa, U.C.; Silva, Ê.F.d.F.e.; Oliveira, T.F.d.; Nogueira, K.F.; Souza, E.R.d. Water Efficiency of Coriander under Flows of Application of Nutritive Solutions Prepared in Brackish Waters. Water 2022, 14, 4005. [Google Scholar] [CrossRef]
- Encyclopaedia Britannica. Available online: https://www.britannica.com/plant/coriander (accessed on 11 December 2024).
- Rabiei, Z.; Hosseini, S.J.; Pirdashti, H.; Hazrati, S. Physiological and Biochemical Traits in Coriander Affected by Plant Growth-Promoting Rhizobacteria under Salt Stress. Heliyon 2020, 6, e05321. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Fruit Yield and Survival of Five Commercial Strawberry Cultivars under Field Cultivation and Salinity Stress. Sci. Hortic. 2019, 243, 401–410. [Google Scholar] [CrossRef]
- Sandhu, D.; Pudussery, M.V.; William, M.; Kaundal, A.; Ferreira, J.F.S. Divergent Gene Expression Responses to Salinity Stress in 16 Geographically Diverse Spinach Genotypes. ACS Agric. Sci. Technol. 2023, 3, 795–804. [Google Scholar] [CrossRef]
- Furlani, P.R. Cultivo Hidropônico de Plantas; Instituto Agronômico: Campinas, Brazil, 1999. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Martins, J.B.; Santos Júnior, J.A.; Bartusch, V.P.; Gheyi, H.R.; Bezerra Neto, E.; Silva, M.M. Water relations in parsley plants cultivated in brackish nutrient solutions of different cationic natures. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 662–668. [Google Scholar] [CrossRef]
- Oliveira, T.F.d.; Santos Júnior, J.A.; Silva, M.G.d.; Gheyi, H.R.; Almeida, J.C.d.; Guiselini, C. Cultivation of chicory under nutrient solutions prepared in brackish waters and applied at different temperatures. Rev. Bras. Eng. Agric. Ambient. 2023, 27, 719–728. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Ferreira, J.F.S. (US Salinity Laboratory (USDA-ARS), Riverside, CA, USA). Personal communication, 2024. [Google Scholar]
- Campos Júnior, J.E.; Santos Júnior, J.A.; Martins, J.B.; Silva, Ê.F.d.F.e; Almeida, C.D.G.C.d. Rocket production in a low cost hydroponic system using brackish water. Rev. Caatinga 2018, 31, 1008–1016. [Google Scholar] [CrossRef]
- Jensen, M.H. Hydroponics. HortScience 1997, 32, 1018–1021. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of root cooling on plant growth and fruit quality of cocktail tomato during two consecutive seasons. J. Food Qual. 2019, 2019, 3598172. [Google Scholar] [CrossRef]
- Navarro, F.E.C.; Silva, I.A.C.E.; Santos Júnior, J.A.; Oliveira, T.F.d.; Silva, G.F.d.; Silva, Ê.F.d.F.e. Hydroponic coriander grown under nutritional solutions prepared with brackish water of different cation prevalence. Rev. Bras. Eng. Agric. Ambient. 2023, 27, 736–745. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, G.S.d.; Pinheiro, F.W.A.; Gheyi, H.R.; Soares, L.A.d.A. Gas exchanges, quantum yield and photosynthetic pigments of west indian cherry under salt stress and potassium fertilization. Rev. Caatinga 2019, 32, 429–439. [Google Scholar] [CrossRef]
- Hniličková, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Guimarães, R.F.B.; Maia Júnior, S.d.O.; Nascimento, R.D.; Melo, D.F.D.; Ramos, J.G.; Andrade, J.R.D. Trocas gasosas em cultivares de alface crespa em cultivo hidropônico com água salina. Rev. Bras. Agric. Irrig. 2019, 13, 3599–3609. [Google Scholar] [CrossRef]
- Lima, G.S.d.; Dias, A.S.; Gheyi, H.R.; Soares, L.A.d.A.; Nobre, R.G.; Pinheiro, F.W.A.; Silva, A.A.R.d. Gas exchanges and production of colored cotton under salt sress and nitrogen fertilization. Biosci. J. 2017, 33, 1495–1505. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; da Silva Filho, J.B.; Liu, X.; Sandhu, D. Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ when K+ is Deficient in the Soil. Plants 2020, 9, 507. [Google Scholar] [CrossRef]
- Gago, J.; Daloso, D.M.; Carriquí, M.; Nada, L.M.; Morales, M.; Araujo, W.L.; Nunes-Nesi, A.; Perera-Castro, A.; Clemente-Moreno, M.J.; Flexas, J. The photosynthesis game is in the ‘inter-play’: Mechanisms underlying CO2 diffusion in leaves. Environ. Exp. Bot. 2020, 178, 104174. [Google Scholar] [CrossRef]
- Dantas, M.V.; Sá, V.K.N.O.d.; Lima, G.S.d.; Soares, L.A.d.A.; Gheyi, H.R.; Silva, L.d.A.; Nobre, R.G.; Sousa, A.A.d.; Azevedo, C.A.V.d. Morphophysiology of cucumber under saline nutrient solutions and salicylic acid application in hydroponic system. Braz. J. Agric. Environ. Eng. 2025, 29, e287640. [Google Scholar] [CrossRef]
- Maia, F.M.A.; Costa, A.C.; Castro, J.N.; Megguer, C.A.; Soares, F.A.L. Photosynthesis and water relations of sunflower cultivars under salinity conditions. Afr. J. Agric. Res. 2016, 11, 2817–2824. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Dalastra, C.; Teixeira Filho, M.C.M.; Silva, M.R.d.; Nogueira, T.A.R.; Fernandes, G.C. Head lettuce production and nutrition in relation to nutrient solution flow. Hortic. Bras. 2020, 38, 21–26. [Google Scholar] [CrossRef]
- Marenco, R.A.; Antezana-Vera, S.A.; Gouvêa, P.R.d.S.; Camargo, M.A.B.; Oliveira, M.F.d.; Santos, J.K.d.S. Fisiologia de espécies florestais da Amazônia: Fotossíntese, respiração e relações hídricas. Rev. Ceres 2014, 61, 786–799. [Google Scholar] [CrossRef]
- Dantas, M.V.; Lima, G.S.d.; Gheyi, H.R.; Pinheiro, F.W.A.; Silva, P.C.C.; Soares, L.A.d.A. Gas exchange and hydroponic production of zucchini under salt stress and H2O2 application. Rev. Caatinga 2022, 35, 436–449. [Google Scholar] [CrossRef]
- Silva, F.G.d.; Dutra, W.F.; Dutra, A.F.; Oliveira, I.M.d.; Filgueiras, L.M.B.; Melo, A.S.d. Trocas gasosas e fluorescência da clorofila em plantas de berinjela sob lâminas de irrigação. Rev. Bras. Eng. Agric. Ambient. 2015, 19, 946–952. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 888. [Google Scholar]
- Silva, A.R.A.d.; Bezerra, F.M.L.; Lacerda, C.F.d.; Sousa, C.H.C.d.; Chagas, K.L. Pigmentos fotossintéticos e potencial hídrico foliar em plantas jovens de coqueiro sob estresses hídrico e salino. Rev. Agroamb. Line 2016, 10, 317–325. [Google Scholar] [CrossRef]
- Christen, D.; Schönmann, S.; Jermini, M.; Strasser, R.J.; Défago, G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Ali, Y.; Aslam, Z.; Ashraf, M.Y.; Tahir, G.R. Effect of salinity on chlorophyll concentration, leaf area, yield and yield components of rice genotypes grown under saline environment. Int. J. Environ. Sci. Technol. 2004, 1, 221–225. [Google Scholar] [CrossRef]
- Freire, J.L.d.O.; Cavalcante, L.F.; Nascimento, R.d.; Rebequi, A.M. Teores de clorofila e composição mineral foliar do maracujazeiro irrigado com águas salinas e biofertilizante. Rev. Ciênc. Agrár. 2013, 36, 57–70. [Google Scholar] [CrossRef]
- Houimli, S.I.M.; Denden, M.; Mouhandes, B.D. Effects of 24-epibrassinolide on growth, chlorophyll, electrolyte leakage and proline by pepper plants under NaCl-stress. EurAsian J. Biosci. 2010, 4, 96–104. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. Subcell. Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef]
- Hosseini, S.J.; Tahmasebi-Sarvestani, Z.; Pirdashti, H.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Nicola, S. Investigation of yield, phytochemical composition, and photosynthetic pigments in different mint ecotypes under salinity stress. Food Sci. Nutr. 2021, 9, 2620–2643. [Google Scholar] [CrossRef]
- Falk, J.; Munné-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef]
- Santos, C.M.d.; Gonçalves, E.R.; Endres, L.; Gomes, T.C.d.A.; Jadoski, C.J.; Nascimento, L.A.d.; Santos, E.D.d. Photosynthetic measurements in lettuce submitted to different agroindustrial residue composting. Pesq. Aplic. Agrotec. 2010, 3, 103–112. [Google Scholar]
- Sá, F.V.d.S.; Gheyi, H.R.; Lima, G.S.d.; Paiva, E.P.d.; Moreira, R.C.L.; Silva, L.d.A. Water salinity, nitrogen and phosphorus on photochemical efficiency and growth of west indian cherry. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 158–163. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Tatagiba, S.D.; Moraes, G.A.B.K.; Nascimento, K.J.T.; Peloso, A.F. Limitações fotossintéticas em folhas de plantas de tomateiro submetidas a crescente concentrações salinas. Rev. Eng. Agric. 2014, 22, 138–149. [Google Scholar] [CrossRef]
- Nabati, J.; Kafi, M.; Masoumi, A.; Mehrjerdi, M.Z. Effect of salinity and silicon application on photosynthetic characteristics of sorghum (Sorghum bicolor L.). Int. J. Agric. Sci. 2013, 3, 483–492. [Google Scholar]
- Zanandrea, I.; Nassi, F.d.L.; Turchetto, A.C.; Braga, E.J.B.; Peters, J.A.; Bacarin, M.A. Efeito da salinidade sob parametros de fluorescencia em Phaseolus vulgaris. Rev. Bras. Agrocienc. 2006, 12, 157–161. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Time course analysis of ABA and non-ionic osmotic stress-induced changes in water status, chlorophyll fluorescence and osmotic adjustment in Arabidopsis thaliana wild-type (Columbia) and ABA-deficient mutant (aba2). Environ. Exp. Bot. 2013, 86, 44–51. [Google Scholar] [CrossRef]
- Azevedo Neto, A.D.d.; Pereira, P.P.A.; Costa, D.P.; Santos, A.C.C.d. Fluorescência da clorofila como ferramenta possível para a seleção de tolerância à salinidade em girassol. Rev. Cienc. Agron. 2011, 42, 893–897. [Google Scholar] [CrossRef]
- Sayyad-Amin, P.; Jahansooz, M.-R.; Borzouei, A.; Ajili, F. Changes in photosynthetic pigments and chlorophyll-a fluorescence attributes of sweet-forage and grain sorghum cultivars under salt stress. J. Biol. Phys. 2016, 42, 601–620. [Google Scholar] [CrossRef]
- Jafarinia, M.; Shariati, M. Effects of salt stress on photosystem II of canola plant (Barassica napus L.) probing by chlorophyll a fluorescence measurement. Iran. J. Sci. 2012, 36, 73–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).